Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and resolution of a 1,1′-biazulene analogue of BINOL†
Anthony P. Gee‡
 a,
Tiberiu-M. Gianga§
a,
Tiberiu-M. Gianga§
 a,
Gabriele Kociok-Köhn
a,
Gabriele Kociok-Köhn b,
G. Dan Pantoş
b,
G. Dan Pantoş a and
Simon E. Lewis
a and
Simon E. Lewis *ac
*ac
aDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: S.E.Lewis@bath.ac.uk
bPhysical Structure Characterization Facility, University of Bath, Bath, BA2 7AY, UK
cInstitute of Sustainability and Climate Change, University of Bath, Bath, BA2 7AY, UK
First published on 16th May 2025
Abstract
Biaryls exhibiting axial chirality have been extensively exploited in fields such as asymmetric catalysis, but the biaryl linkage typically consists of benzenoid aromatic rings, with non-benzenoid biaryls being scarce. Here we report the first preparation of a (non-benzenoid) 1,1′-biazulene-2,2′-diol (“1,1′-BAzOL”) in enantiopure form and determine its barrier to racemisation. Furthermore we transformed a 1,1′-biazulene-2,2′-diol into the corresponding 2,2′-bis(phosphonate), thereby demonstrating functional group interconversion through cross coupling and highlighting the potential for diversification.
Introduction
Azulene 1 is a non-benzenoid 10π bicyclic aromatic compound, known for its blue colour,1 large dipole2 and anomalous fluorescence.3 Each of these properties differ from those of the corresponding benzenoid isomer naphthalene 2 (Fig. 1a).4 Azulene derivatives have been used in multiple applications, including in fluorescence imaging,5 colorimetric sensing,6 solar cells,7 photothermal therapy,8 dyestuffs,9 organic field-effect transistors (OFETs),10 and other optoelectronics.11Biazulenes are a group of biaryls for which 15 different positional isomers can be envisaged (Fig. 1b) whose structural and electronic properties can vary significantly depending on the position of the biaryl linkage as well as the substituents.12 The first examples of biazulene synthesis, reported in 1968,13 were of 1,1′- and 2,2′-biazulenes formed by (multistep) dimerisation of the natural product guaiazulene (this can also undergo direct oxidative dimerisation to give 1,1′-,14,15 1,2′-15,16 and 1,5′-16 biazulenes). Another early report describes the synthesis of 1,1′- and 2,2′-biazulenes by Ullmann coupling of the corresponding haloazulene monomers17 (1,2′- and 2,6′-biazulenes were also isolated from mixtures arising from coupling of two different monomers). As of now, 2,4-, 4,5′- and 5,6′-biazulenes remain unknown to the best of our knowledge, but examples of all other positional isomers have been reported. Most extensively studied are the 1,1′-biazulenes, which have been synthesised by approaches including reductive coupling,18 oxidative dimerisation either electrochemically19 or using FeCl3,20,21 MnO2,22 (NH4)2S2O8,23 CuBr/O2,24 DDQ25 or electrophilic halide sources,26 as well as by photochemical methods,27 sulfide/sulfoxide activation,28 C–H activation,29 Suzuki coupling30 and aromatisation of a partially saturated precursor.31 Less common are the 1,2′-,32 1,4′-,33 1,5′-16 and 1,6′-34,35 biazulenes. The “linear” biazulenes (i.e. the 2,2′-,36 2,6′-34,37 and 6,6′-38 isomers) have found diverse applications in organic functional materials, e.g. in surface modifiers,39 OFETs,40,41 supercapacitors,42 molecular rectifiers,43 hole-transport materials for perovskite solar cells,44 enhancers of π-π stacking45 and memristors.46 The remaining known biazulene isomers (2,5′-,47 4,4′-,12c,33,48 4,6′-,12c,49 and 5,5-50) have been reported only rarely.
Axial chirality is a form of stereoisomerism which arises in molecules comprising two pairs of (inequivalent) substituents oriented in a non-planar manner about a chiral axis. Atropisomers exhibit axial chirality arising from restricted rotation around a σ-bond, with the most well-known examples being biaryl systems where the presence of substituents ortho to the biaryl bond imposes a steric barrier to racemisation. In particular binaphthyl is a privileged motif in asymmetric catalysis, with the archetypal BINOL (3)51 and BINAP (4)52 chiral ligands and their derivatives53 imparting high levels of enantioselectivity in diverse transition metal-catalysed reactions (Fig. 2a). Chiral Brønsted acid organocatalysts based on the BINOL scaffold are also well developed.54
In contrast to binaphthyl, axial chirality in biazulenyl systems has been much less studied. Whereas any biazulene positional isomer could potentially exhibit atropisomerism if appropriately substituted, the few published reports mostly concern 1,1′-biazulenes. In 1983 Tajiri was the first to disclose the resolution of a biazulene, using preparative chiral stationary phase HPLC to separate the enantiomers of 2,2′-dimethyl-1,1′-biazulene 6 and 2,2′-dimethoxy-1,1′-biazulene 7 (Fig. 2b);55 6 was reported have greater configurational stability than 7. Subsequently Daub studied chiral annulated 1,1′-biazulene quinones 11 as electron-transfer mediators, resolving their enantiomers by HPLC56 as well as using a chiral auxiliary to attempt diastereoselective azulene dimerisation, giving the 1,1′-biazulene product in moderate diastereoisomeric excess.57 Chen described the synthesis of a 2,2′-diamino-1,1′-biazulene 12, resolution of the racemate by HPLC and attempted enantioselective oxidative dimerisation of the 2-aminoazulene precursor, employing various chiral ligands and achieving modest enantiomeric excess.21 Ito, Itami and co-workers reported π-extended 1,1′-biazulenes (8 and its cyclised derivative) which they resolved by HPLC.58 Tsuchiya, Mazaki and co-workers reported 2,2′-diphenyl-1,1′-biazulene 9 and 2,2′-bis(4-pyridyl)-1,1′-biazulene 10 and their resolution through crystal picking.59 Tani, Murafuji and co-workers reported 4,4′-biazulene 5 and its resolution by HPLC.60 Most recently the Clever group reported 2,2′-diamino-3,3′-bis(3-pyridyl)-1,1′-biazulene 13 and 2,2′-diamino-3,3′-bis(6-quinolinyl)-1,1′-biazulene 14, their resolution by HPLC and their chiral self-sorting phenomena in Pd2L4 coordination cages.61 Biazulenes exhibiting helical chirality have also been reported.62
Here we report the design, synthesis, resolution and characterisation of a biazulene analogue of BINOL, i.e. a 1,1′-biazulene-2,2′-diol, which we have termed “1,1′-BAzOL” (Fig. 2c). Whereas Tajiri had reported 2,2′-dimethoxy-1,1′-biazulene 7, we specifically targeted the free hydroxyl groups to facilitate potential applications of 1,1′-BAzOL, e.g. as a chiral ligand or in chiral Brønsted acid catalysis. Our design incorporated two further motifs with specific functions. Firstly, we introduced “flanking” groups at the 8- and 8′-positions, intended to increase the barrier to racemisation. Secondly, we appended ester groups at the 3- and 3′-positions, anticipating that these would enhance the chemical stability of 1,1′-BAzOL. 2-Hydroxyazulene is only moderately stable in solution since the substituent renders the azulene core sufficiently electron-rich that it may undergo oxidative degradation. Furthermore, depending on the solvent, 2-hydroxyazulene can tautomerise to the corresponding keto-form to an appreciable degree.63 This may then undergo aldol-type self-condensation reactions, ultimately leading to decomposition, and we were mindful that this decomposition pathway might also be operative for a 1,1′-biazulene-2,2′-diol. However, 2-hydroxyazulenes bearing an electron-withdrawing ester group at the adjacent position are less electron-rich and generally stable, with the tautomeric equilibrium seemingly favouring the enol form to a much greater degree. We therefore sought to introduce ester groups at the BAzOL 3- and 3′-positions, in the hope this would suppress decomposition via the keto tautomer. The realisation of our BAzOL design concept is reported in this paper. Of note, a 1,1′-biazulene-2,2′-diol has never been isolated in enantiopure form. Chen and co-workers prepared a 1,1′-biazulene-2,2′-diol by electrochemical oxidative dimerisation, but chirality was not considered.64 Yang, Nozoe and co-workers prepared a 1,1′-biazulene crown ether, in which the chirality of the system was recognised, but resolution was not attempted.65
Results and discussion
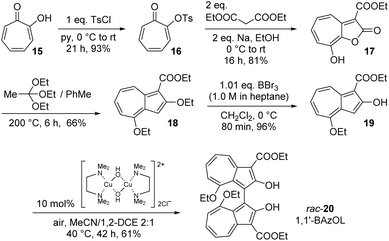
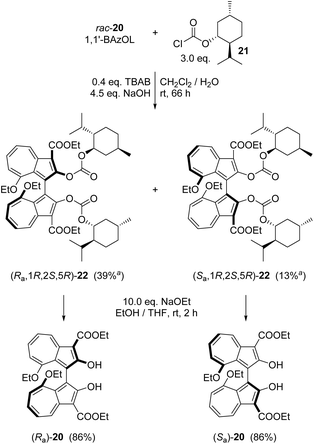
The synthesis of 1,1′-BAzOL began with commercially available tropolone 15, which was tosylated to give 16, then reacted with ethyl cyanoacetate to give bicyclic hydroxylactone 17, as described previously (Scheme 1).66 Heating of 17 with triethyl orthoacetate in a sealed tube gave bis(ethoxy)azulene 18. The reaction proceeds by in situ generation of a ketene acetal which undergoes an [8 + 2] cycloaddition with 17, followed by extrusion of CO2 to give 18.67 Deethylation with boron tribromide proceeded entirely regioselectively to give 19, which was of sufficient purity to be used in the next step without purification. The 8-ethoxy group was inert under these reaction conditions since this ether oxygen is less Lewis basic, being attached to the more electron-poor position on azulene 18. Then, oxidative dimerisation of 19 was effected using [Cu(OH)(TMEDA)]2Cl2 under air, which has previously been reported to be an effective catalyst system for dimerisation of 2-naphthols to BINOLs.68 In this case, the reaction gave rac-1,1′-BAzOL 20 in 62% yield (5 step synthesis from tropolone, 29% overall yield).To isolate 1,1′-BAzOL 20 in enantiopure form, we attempted to develop an enantioselective variant of the dimerisation of 19. A procedure reported for enantioselective dimerisation of 2-naphthols using Cu-BINAM complexes69 was adapted for reaction of 19, but 1,1′-BAzOL 20 was obtained in only low e.e., and in low yield, with various copper sources. We therefore sought to resolve rac-1,1′-BAzOL 20 instead, through derivatisation with a chiral pool-derived auxiliary and separation of the resultant diastereoisomers. Commercially-available (−)-menthyl chloroformate 21 has previously been used successfully for the derivatisation and separation of enantiomers of BINOL and related chiral diols,70 and we applied this approach to 1,1′-BAzOL (Scheme 2). Thus, reaction of an excess of 21 with rac-1,1′-BAzOL 20 in a biphasic dichloromethane–water medium, in the presence of TBAB (tetra-n-butylammonium bromide) as phase-transfer catalyst and NaOH as base gave bis(menthyl carbonate) 22 as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereoisomers. In the original reports on the resolution of BINOL by this method, fractional crystallisation of the diastereoisomeric mixture afforded one diastereoisomer (100% d.e.) in pure crystalline form, whereas the motherliquor contained the other diastereoisomer in ≈90% d.e., that could be further purified to higher d.e. through subsequent operations. In the case of 1,1′-BAzOL, the bis(carbonate) 22 derived from (Ra)-1,1′-BAzOL 20 could indeed be isolated as a single diastereoisomer through careful crystallisation, albeit in more moderate yield. The motherliquor was concentrated and then underwent further recrystallisations from a different solvent, giving the bis(carbonate) 22 derived from (Sa)-1,1′-BAzOL 20 as a single diastereoisomer, in low yield. (Subsequent chromatography and recrystallisation provided additional material; see ESI† for details) Both of the diastereoisomers of 22 isolated in this way were then separately subjected to ethanolysis to cleave the menthol auxiliary and regenerate 1,1-BAzOL 20. As shown in Scheme 2, this was achieved in the same high yield for both diastereoisomers of 22, thus allowing the isolation of both enantiomers of 1,1′-BAzOL 20 in enantiopure form.
1 mixture of diastereoisomers. In the original reports on the resolution of BINOL by this method, fractional crystallisation of the diastereoisomeric mixture afforded one diastereoisomer (100% d.e.) in pure crystalline form, whereas the motherliquor contained the other diastereoisomer in ≈90% d.e., that could be further purified to higher d.e. through subsequent operations. In the case of 1,1′-BAzOL, the bis(carbonate) 22 derived from (Ra)-1,1′-BAzOL 20 could indeed be isolated as a single diastereoisomer through careful crystallisation, albeit in more moderate yield. The motherliquor was concentrated and then underwent further recrystallisations from a different solvent, giving the bis(carbonate) 22 derived from (Sa)-1,1′-BAzOL 20 as a single diastereoisomer, in low yield. (Subsequent chromatography and recrystallisation provided additional material; see ESI† for details) Both of the diastereoisomers of 22 isolated in this way were then separately subjected to ethanolysis to cleave the menthol auxiliary and regenerate 1,1-BAzOL 20. As shown in Scheme 2, this was achieved in the same high yield for both diastereoisomers of 22, thus allowing the isolation of both enantiomers of 1,1′-BAzOL 20 in enantiopure form.
The 1H-NMR spectra for the two diastereoisomers of 22 are very similar in the aromatic region, but exhibit significant chemical shift differences in the upfield region (Fig. 3). Thus, the methyl groups of the menthyl auxiliary are clearly discernible as three doublets between 0 and 1 ppm (since each isopropyl group comprises two inequivalent methyl groups). We ascribe the chemical shift differences between the two isomers for these signals to differing degrees of anisotropic shielding by the azulene seven-membered rings. Further structural information for the diastereoisomers of 22 was obtained through X-ray crystallography, with the structures so acquired shown in Fig. 4 (for the (Ra) diastereoisomer) and Fig. 5 (for the (Sa) diastereoisomer). Additionally, an X-ray crystal structure for (Ra)-1,1′-BAzOL 20 itself was also acquired (Fig. 6). Selected crystallographic parameters are shown in Table 1.
 | ||
| Fig. 3 Overlaid 1H-NMR Spectra (in CDCl3) of the separated diastereoisomers of BAzOL-bis(menthyl carbonate) 22: (Ra) isomer (purple) and (Sa) isomer (green). | ||
 | ||
| Fig. 4 ORTEP representations of the X-ray structure of (Ra,1R,2S,5R)-22. Ellipsoids are shown at 30% probability. A molecule of ethanol has been omitted for clarity. Only hydrogens on stereogenic centres are shown (as spheres of arbitrary radius). CCDC #2421193. | ||
 | ||
| Fig. 5 ORTEP representations of the X-ray structure of (Sa,1R,2S,5R)-22. Ellipsoids are shown at 30% probability. Disorder in the ethyl esters and menthyl isopropyl group has been omitted for clarity. Only hydrogens on stereogenic centres are shown (as spheres of arbitrary radius). CCDC #2421194. | ||
 | ||
| Fig. 6 ORTEP representations of the X-ray structure of 1,1′-BAzOL (Ra)-20. Ellipsoids are shown at 50% probability. Hydrogens are shown as spheres of arbitrary radius. CCDC #2421195. | ||
| Structure | C1-C1′ biaryl bond length (Å) | C2-C1-C1′-C2′ dihedral angle (°) |
|---|---|---|
| (Ra,1R,2S,5R)-22 | 1.476(2) | 71.4(2) |
| (Sa,1R,2S,5R)-22 | 1.450(8) | 99.0(8) |
| 1,1′-BAzOL (Ra)-20 | 1.460(7) | 111.8(6) |
Circular dichroism spectra for the enantiomers of 1,1′-BAzOL 20 were acquired and are shown in Fig. 7. The superimposable mirror image spectra indicate that 1,1′-BAzOL 20 is configurationally stable at room temperature and confirm the enantiopurity. The configurational stability was then investigated at elevated temperatures. As shown in Fig. 8, partial racemisation was observed when a solution of (Sa)-1,1′-BAzOL 20 was maintained at 60 °C for 14 h, whereas near-complete racemisation was observed at 80 °C for the same period. Using data acquired at these and other temperatures, the barrier to racemisation was calculated (see ESI† for details). The key parameters are summarised in Table 2.
 | ||
| Fig. 7 Circular dichroism plots of (Ra)-20 (black) and (Sa)-20 (red), recorded as 0.01 mM solutions in CHCl3. | ||
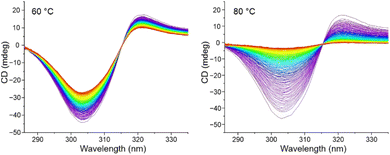 | ||
| Fig. 8 Circular dichroism plots of (Sa)-20, recorded as 0.01 mM solutions in 1,1,2,2-tetrachloroethane. Spectra recorded at 5 minutes intervals (left) at 60 °C; (right) at 80 °C. | ||
| Ea/kJ mol−1 | ΔH‡/kJ mol−1 | ΔS‡/J mol−1 K−1 | ΔG‡293.15 K/kJ mol−1 | t1/2 293.15 K/h |
|---|---|---|---|---|
| 84.90 | 82.06 | −96.06 | 110.2 | 1389 |
Table 3 presents a comparison of the barrier to racemisation determined for 1,1′-BAzOL 20 with all other biazulenes for which data on racemisation have been reported. The measured Ea value for 1,1′-BAzOL 20 is similar to that for 4,4′-biazulene 5. The value for 2,2′-dimethoxy-1,1′-biazulene 7 is appreciably lower than for 20, which may be due to the fact that 7 lacks the flanking groups in the 8,8′-positions. On the other hand, the values for 9 and 10 are appreciably higher than the value we have measured for 20, implying that a sufficiently bulky group at C2 can hinder rotation around the biaryl axis regardless of the presence or absence of flanking groups on the seven-membered rings. The Ea value for the racemisation of 1,1′-binaphthyl 23 has also been included for comparison; it is higher than for 20 but lower than for 9 and 10.
We next sought to apply the biazulene synthesis we had developed to produce a 1,1′-biazulene bearing different functional groups, through derivatisation of the diol. To this end, lactone 17 was reacted with trimethyl orthoacetate to give dimethoxyazulene 24 (Scheme 3), this reaction proceeding in higher yield (79%) than for the diethoxy homologue 18 (66%, see Scheme 1). Dealkylation was again selective for the 2-position, giving hydroxyazulene 25, which underwent oxidative dimerisation to give rac-26. At this point, we sought to convert this biazulene diol into the corresponding bis(triflate) in order to be able to derivatise it by cross-coupling approaches. In the event, the potential cross-coupling partner 27 was formed in moderate yield upon use of excess triflic anhydride. At this point we considered the necessity of 3,3′-diester substituents in the present synthesis. As explained above, they were considered essential in the 1,1′-BAzOL design strategy in order to impart stability by suppressing keto–enol tautomerism and also to block over-oxidation/oligomerisation in the dimerisation of 19 to 1,1′-BAzOL 20. However, in 27 the triflate groups are non-enolisable (and less electron-rich), so we reasoned the ester functionalities could be considered to have served their purpose at this point in the synthetic sequence. As such, we aimed to demonstrate their removal upon treatment with phosphoric acid, which is often employed for hydrolysis/decarboxylation of esters at the azulene 1- and 3-positions.23,72 In this case, treatment of 27 with H3PO4/P2O5 gave expected bis(triflate) 28 only in low yield. Much more satisfactory was reversing the order of events, with acid-mediated ester removal from 26 giving 29, which if used immediately (and without purification) could be doubly sulfonylated to give 28 in a greatly improved 52% yield over two steps. Then a representative twofold cross-coupling was demonstrated for 28, with the synthesis of 2,2′-bis(phosphonate)-1,1′-biazulene 30 according to the method of Stawinski et al.73 (Subsequently, an attempt to couple 27 under the same conditions gave only the mono-coupled product).
Conclusions
We have prepared an axially chiral 1,1′-biazulenyl-2,2′-diol in enantiopure form and determined the barrier to its racemisation. The synthetic access to 1,1′-BAzOL 20 is concise (5 steps from commercial materials to the racemate; 7 steps to the single enantiomers) and there is scope for diversification of the substituents. We have demonstrated this by carrying out an exemplary cross-coupling using a variant of 20 – transformation to bis(triflate) 28 gave a suitable electrophilic coupling partner, which underwent a double cross-coupling to give 2,2′-bis(phosphonate) 30. Analogous cross-couplings to introduce many other substituents or functional groups at the 2-positions may be envisaged. In addition, functionalisation at the 3-positions should be possible either by functional group interconversions of the esters, or by their removal (as per the transformation of 27 to 28) followed by electrophilic aromatic substitution (since the unsubstituted 3-position may be anticipated to be the most reactive for SEAr). For these reasons we anticipate that the BAzOL synthesis described here may find varied applications in synthesis and catalysis.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for both diastereoisomers of 22 and for 1,1′-BAzOL (Ra)-20 have been deposited at the CCDC under #2421193–2421195 and can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures.Author contributions
S. E. L. conceived the project. A. P. G. carried out all synthetic work. T. M. G. and G. D. P. carried out circular dichroism analysis. G. K. K. carried out X-ray crystallography. S. E. L. wrote the manuscript with input from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank EPSRC for a DTP PhD studentship to A. P. G. We acknowledge the research facilities at the University of Bath (https://doi.org/10.15125/mx6j-3r54).Notes and references
- R. S. H. Liu, Colorful azulene and its equally colorful derivatives, J. Chem. Educ., 2002, 79, 183–185 CrossRef CAS.
- A. G. Anderson Jr and B. M. Steckler, Azulene. VIII. A study of the visible absorption spectra and dipole moments of some 1- and 1,3-substituted azulenes, J. Am. Chem. Soc., 1959, 81, 4941–4946 CrossRef.
- (a) M. Beer and H. C. Longuet-Higgins, Anomalous light emission of azulene, J. Chem. Phys., 1955, 23, 1390–1391 CrossRef CAS; (b) B. D. Wagner, D. Tittelbach-Helmrich and R. P. Steer, Radiationless decay of the S2 states of azulene and related compounds: solvent dependence and the energy gap law, J. Phys. Chem., 1992, 96, 7904–7908 CrossRef CAS; (c) K. Veys and D. Escudero, Computational protocol to predict anti-Kasha emissions: the case of azulene derivatives, J. Phys. Chem. A, 2020, 124, 7228–7237 CrossRef CAS; (d) D. Dunlop, L. Ludvíková, A. Banerjee, H. Ottosson and T. Slanina, Excited-state (anti)aromaticity explains why azulene disobeys Kasha's rule, J. Am. Chem. Soc., 2023, 145, 21569–21575 CrossRef CAS.
- F. Bayrakceken, Radiative electronic energy transfer-time studies of naphthalene-biacetyl system by 1 and 2-photon excitation and optical antenna mechanism, Spectrochim. Acta, Part A, 2005, 61, 1069–1074 CrossRef.
- (a) L. C. Murfin and S. E. Lewis, Azulene—A Bright core for sensing and imaging, Molecules, 2021, 26, 353 CrossRef CAS; (b) Z. Cui, Y. Wang, G. Wang, B. Feng, S. E. Lewis, K. Wang, K. Jiang, T. D. James and H. Zhang, Amphiphilic azulene-based fluorescent probe for simultaneous monitoring of fluctuations in carboxylesterase activity in diverse biological samples from a single organism, Anal. Chem., 2024, 96, 19732–19739 CrossRef CAS PubMed; (c) C. M. López-Alled, S. J. Park, D. J. Lee, L. C. Murfin, G. Kociok-Köhn, J. L. Hann, J. Wenk, T. D. James, H. M. Kim and S. E. Lewis, Azulene-based fluorescent chemosensor for adenosine diphosphate, Chem. Commun., 2021, 57, 10608–10611 RSC; (d) L. C. Murfin, M. Weber, S. J. Park, W. T. Kim, C. M. López-Alled, C. L. McMullin, F. Pradaux-Caggiano, C. L. Lyall, G. Kociok-Köhn, J. Wenk, S. D. Bull, J. Yoon, H. M. Kim, T. D. James and S. E. Lewis, Azulene-derived fluorescent probe for bioimaging: detection of reactive oxygen and nitrogen species by two-photon microscopy, J. Am. Chem. Soc., 2019, 141, 19389–19396 CrossRef CAS; (e) Y. Zhou, Y. Zhuang, X. Li, H. Ågren, L. Yu, J. Ding and L. Zhu, Selective dual-channel imaging on cyanostyryl-modified azulene systems with unimolecularly tunable visible–near infrared luminescence, Chem.–Eur. J., 2017, 23, 7642–7647 CrossRef CAS PubMed.
- (a) N. Bektas, A. Daştan, M. Erdoğan and A. Aydogan, A guaiazulene-based highly selective colorimetric sensor for cyanide ion detection in aqueous medium and food samples, J. Mol. Liq., 2025, 423, 127038 CrossRef CAS; (b) L. C. Murfin, K. Chiang, G. T. Williams, C. L. Lyall, A. T. A. Jenkins, J. Wenk, T. D. James and S. E. Lewis, A Colorimetric chemosensor based on a Nozoe azulene that detects fluoride in aqueous/alcoholic media, Front. Chem., 2020, 8, 10 CrossRef CAS PubMed; (c) L. C. Murfin, C. M. López-Alled, A. C. Sedgwick, J. Wenk, T. D. James and S. E. Lewis, A simple, azulene-based colorimetric probe for the detection of nitrite in water, Front. Chem. Sci. Eng., 2020, 14, 90–96 CrossRef CAS; (d) C. M. López-Alled, A. Sanchez-Fernandez, K. J. Edler, A. C. Sedgwick, S. D. Bull, C. L. McMullin, G. Kociok-Köhn, T. D. James, J. Wenk and S. E. Lewis, Azulene–boronate esters: colorimetric indicators for fluoride in drinking water, Chem. Commun., 2017, 53, 12580–12583 RSC; (e) L. Birzan, M. Cristea, C. C. Draghici, V. Tecuceanu, A. Hanganu, E.-M. Ungureanu and A. C. Razus, Tetrahedron, 2017, 73, 2488–2500 CrossRef CAS; (f) D. Lichosyt, P. Dydio and J. Jurczak, Azulene-based macrocyclic receptors for recognition and sensing of phosphate anions, Chem.–Eur. J., 2016, 22, 17673–17680 CrossRef CAS; (g) S. Wakabayashi, M. Uchida, R. Tanaka, Y. Habata and M. Shimizu, Synthesis of azulene derivatives that have an azathiacrown ether moiety and their selective color reaction towards silver ions, Asian J. Org. Chem., 2013, 2, 786–791 CrossRef CAS; (h) H. Salman, Y. Abraham, S. Tal, S. Meltzman, M. Kapon, N. Tessler, S. Speiser and Y. Eichen, 1,3-Di(2-pyrrolyl)azulene: an efficient luminescent probe for fluoride, Eur. J. Org Chem., 2005, 2207–2212 CrossRef CAS; (i) H.-G. Löhr, F. Vögtle, W. Schuh and H. Puff, Chromoionophore mit azulen-einheiten als farbträger und π-donor-baustein, Chem. Ber., 1984, 117, 2839–2849 CrossRef.
- (a) H. Li, Z. Wang, Y. Sun, Y. Su, Z. Zhao, Y. Tian, H. Li and M. Cheng, Peripheral substituent regulation of bias structured azulene-based hole transport materials for perovskite solar cells, New J. Chem., 2023, 47, 19057–19062 RSC; (b) N. Tzoganakis, B. Feng, M. Loizos, K. Chatzimanolis, M. Krassas, D. Tsikritzis, X. Zhuang and E. Kymakis, Performance and stability improvement of inverted perovskite solar cells by interface modification of charge transport layers using an azulene–pyridine molecule, Energy Tech., 2023, 11, 2201017 CrossRef CAS; (c) Y. Su, H. Li, Y. Miao, Y. Tian and M. Cheng, Azulene-based hole transport materials with proper electronic properties for perovskite solar cells, Asian J. Org. Chem., 2022, 11, e202200441 CrossRef CAS; (d) A. A. Raheem, P. Murugan, R. Shanmugam and C. Praveen, Azulene bridged π-distorted chromophores: the influence of structural symmetry on optoelectrochemical and photovoltaic parameters, ChemPlusChem, 2021, 86, 1451–1460 CrossRef CAS PubMed; (e) L. Yang, Y. Zhu, J. Liu, Y. Chen, J. Wu, Z. Pang, Z. Lu, S. Zhao and Y. Huang, Marked effects of azulenyl vs. naphthyl groups on donor-π-acceptor-π-donor small molecules for organic photovoltaic cells, Dyes Pigm., 2021, 187, 109079 CrossRef CAS; (f) M. A. Truong, J. Lee, T. Nakamura, J.-Y. Seo, M. Jung, M. Ozaki, A. Shimazaki, N. Shioya, T. Hasegawa, Y. Murata, S. M. Zakeeruddin, M. Grätzel, R. Murdey and A. Wakamiya, Influence of alkoxy chain length on the properties of two-dimensionally expanded azulene-core-based hole-transporting materials for efficient perovskite solar cells, Chem.–Eur. J., 2019, 25, 6741–6752 CrossRef CAS; (g) P. Cowper, A. Pockett, G. Kociok-Köhn, P. J. Cameron and S. E. Lewis, Azulene–Thiophene–Cyanoacrylic acid dyes with donor-π-acceptor structures. Synthesis, characterisation and evaluation in dye-sensitized solar cells, Tetrahedron, 2018, 74, 2775–2786 CrossRef CAS; (h) H. Xin, C. Ge, X. Jiao, X. Yang, K. Rundel, C. R. McNeill and X. Gao, Incorporation of 2,6-connected azulene units into the backbone of conjugated polymers: towards high-performance organic optoelectronic materials, Angew. Chem., Int. Ed., 2017, 57, 1322–1326 CrossRef PubMed; (i) Y. Chen, Y. Zhu, D. Yang, S. Zhao, L. Zhang, L. Yang, J. Wu, Y. Huang, Z. Xu and Z. Lu, An azulene-containing low bandgap small molecule for organic photovoltaics with high open-circuit voltage, Chem.–Eur. J., 2016, 22, 14527–14530 CrossRef CAS; (j) T. Umeyama, Y. Watanabe, T. Miyata and H. Imahori, Electron-rich five-membered ring of azulene as a donor unit in donor–acceptor alternating copolymers for polymer solar cell applications, Chem. Lett., 2015, 44, 47–49 CrossRef; (k) J. Yao, Z. Cai, Z. Liu, C. Yu, H. Luo, Y. Yang, S. Yang, G. Zhang and D. Zhang, Tuning the semiconducting behaviors of new alternating dithienyldiketopyrrolopyrrole–azulene conjugated polymers by varying the linking positions of azulene, Macromolecules, 2015, 48, 2039–2047 CrossRef CAS; (l) H. Nishimura, N. Ishida, A. Shimazaki, A. Wakamiya, A. Saeki, L. T. Scott and Y. Murata, hole-transporting materials with a two-dimensionally expanded π-system around an azulene core for efficient perovskite solar cells, J. Am. Chem. Soc., 2015, 137, 15656–15659 CrossRef CAS; (m) E. Puodziukynaite, H.-W. Wang, J. Lawrence, A. J. Wise, T. P. Russell, M. D. Barnes and T. Emrick, Azulene methacrylate polymers: synthesis, electronic properties, and solar cell fabrication, J. Am. Chem. Soc., 2014, 136, 11043–11049 CrossRef CAS PubMed.
- (a) H. Gao, Y. Yao, C. Li, J. Zhang, H. Yu, X. Yang, J. Shen, Q. Liu, R. Xu, X. Gao and D. Ding, Fused azulenyl squaraine derivatives improve phototheranostics in the second near-infrared window by concentrating excited state energy on non-radiative decay pathways, Angew. Chem., Int. Ed., 2024, 63, e202400372 CrossRef CAS PubMed; (b) Y. Yao, Y. Zhang, J. Zhang, X. Yang, D. Ding, Y. Shi, H. Xu and X. Gao, Azulene-containing squaraines for photoacoustic imaging and photothermal therapy, ACS Appl. Mater. Interfaces, 2022, 14, 19192–19203 CrossRef CAS PubMed.
- (a) K. Maruoka, R. Suzuki, T. Kamishima, Y. Koseki, A. T. N. Dao, T. Murafuji and H. Kasai, Total synthesis of azulene derivative, a blue pigment isolated from Lactarius indigo, and colorant application of its aqueous dispersion, J. Agric. Food Chem., 2023, 71, 11607–11614 CrossRef CAS; (b) C. Harabajiu, J. L. Hann, L. C. Murfin, G. Kociok-Köhn and S. E. Lewis, Persistent azulene α-carbocations: synthesis from aldehydes, spectroscopic and crystallographic properties, Org. Biomol. Chem., 2023, 21, 858–866 RSC; (c) B. Hou, J. Li, X. Yang, J. Zhang, H. Xin, C. Ge and X. Gao, Azulenoisoindigo: a building block for π-functional materials with reversible redox behavior and proton responsiveness, Chin. Chem. Lett., 2022, 33, 2147–2150 CrossRef CAS; (d) E. H. Ghazvini Zadeh, S. Tang, A. W. Woodward, T. Liu, M. V. Bondar and K. D. Belfield, Chromophoric materials derived from a natural azulene: syntheses, halochromism and one-photon and two-photon microlithography, J. Mater. Chem. C, 2015, 3, 8495–8503 RSC; (e) L. Cristian, I. Sasaki, P. G. Lacroix, B. Donnadieu, I. Asselberghs, K. Clays and A. C. Razus, Donating strength of azulene in various azulen-1-yl-substituted cationic dyes: application in nonlinear optics, Chem. Mater., 2004, 16, 3543–3551 CrossRef CAS; (f) R. S. H. Liu and A. E. Asato, Tuning the color and excited state properties of the azulenic chromophore: NIR absorbing pigments and materials, J. Photochem. Photobiol., C, 2003, 4, 179–194 CrossRef CAS.
- (a) Y. Yao, X. Sun, Z. Zhang, H. Yu, X. Yang, D. Ding and X. Gao, Azulene-containing bis(squaraine) dyes: design, synthesis and aggregation behaviors, Chem.–Eur. J., 2024, 30, e202400474 CrossRef CAS; (b) Y. Xu, D. Liu and M. Wang, Enhancing gating performance in organic molecular field-effect transistors by introducing polar azulene components, Chem.–Eur. J., 2023, 29, e202301294 CrossRef CAS; (c) H. Fu, C. Zhao, J. Cheng, S. Zhou, P. Peng, J. Hao, Z. Liu, X. Gao, C. Jia and X. Guo, Dipole-improved gating of azulene-based single-molecule transistors, J. Mater. Chem. C, 2022, 10, 7803–7809 RSC; (d) Y. Shibuya, A. Matsunaga, D. Kumaki, S. Tokito and H. Katagiri, Azulene end-capped 1,3,4-thiadiazole as an n-type organic semiconductor with a herringbone–brickwork cooperative 2D layered structure, Cryst. Growth Des., 2022, 22, 6554–6563 CrossRef CAS; (e) F. Schulz, S. Takamaru, T. Bens, J.-i. Hanna, B. Sarkar, S. Laschat and H. Iino, Liquid crystalline self-assembly of azulene–thiophene hybrids and their applications as OFET materials, Phys. Chem. Chem. Phys., 2022, 24, 23481–23489 RSC; (f) H. Ran, F. Li, R. Zheng, H. Zhang, F. Xie, P. Jin, Z. Lei, X.-T. Wang and J.-Y. Hu, Polarity change of OFETs based on dithienocoronene diimide (DTCDI)-derived isomeric triads end-capped with azulene, Dyes Pigm., 2022, 203, 110311 CrossRef CAS; (g) H. Ran, X. Duan, R. Zheng, F. Xie, L. Chen, Z. Zhao, R. Han, Z. Lei and J.-Y. Hu, Two isomeric azulene-decorated naphthodithiophene diimide-based triads: molecular orbital distribution controls polarity change of OFETs through connection position, ACS Appl. Mater. Interfaces, 2020, 12, 23225–23235 CrossRef CAS; (h) Y. Shibuya, K. Aonuma, T. Kimura, T. Kaneko, W. Fujiwara, Y. Yamaguchi, D. Kumaki, S. Tokito and H. Katagiri, Linear biazulene isomers: effects of molecular and packing structure on optoelectronic and charge-transport properties, J. Phys. Chem. C, 2020, 124, 4738–4746 CrossRef CAS; (i) H. Xin, J. Li, C. Ge, X. Yang, T. Xue and X. Gao, 6,6′-Diaryl-substituted biazulene diimides for solution-processable high-performance n-type organic semiconductors, Mater. Chem. Front., 2018, 2, 975–985 RSC; (j) Y. Yamaguchi, M. Takubo, K. Ogawa, K.-i. Nakayama, T. Koganezawa and H. Katagiri, Terazulene isomers: polarity change of OFETs through molecular orbital distribution contrast, J. Am. Chem. Soc., 2016, 138, 11335–11343 CrossRef CAS; (k) Y. Yamaguchi, K. Ogawa, K.-i. Nakayama, Y. Ohba and H. Katagiri, Terazulene: a high-performance n-type organic field-effect transistor based on molecular orbital distribution control, J. Am. Chem. Soc., 2013, 135, 19095–19098 CrossRef CAS.
- (a) M. Li, H. Fu, B. Wang, J. Cheng, W. Hu, B. Yin, P. Peng, S. Zhou, X. Gao, C. Jia and X. Guo, Dipole-modulated charge transport through PNP-type single-molecule junctions, J. Am. Chem. Soc., 2022, 144, 20797–20803 CrossRef CAS PubMed; (b) H. Xin and X. Gao, Application of azulene in constructing organic optoelectronic materials: new tricks for an old dog, ChemPlusChem, 2017, 82, 945–956 CrossRef CAS; (c) H. Xin, C. Ge, X. Yang, H. Gao, X. Yang and X. Gao, Biazulene diimides: a new building block for organic electronic materials, Chem. Sci., 2016, 7, 6701–6705 RSC.
- (a) A. H. M. Elwahy, I. A. Abdelhamid and M. R. Shaaban, Recent advances in the functionalization of azulene through Pd-catalyzed cross-coupling reactions, ChemistrySelect, 2021, 6, 13664–13723 CrossRef CAS; (b) J. R. Dias, Electronic and structural properties of biazulene, terazulene, and polyazulene isomers, J. Phys. Org. Chem., 2007, 20, 395–409 CrossRef CAS; (c) S. Hünig and B. Ort, Multistep reversible redox systems, XXXVII. Biazulenes and ω,ω'-diazulenylpolyenes: syntheses and spectroscopic properties, Liebigs Ann. Chem., 1984, 1905–1935 CrossRef; (d) S. Hünig and B. Ort, Multistep reversible redox systems, XXXVIII. Biazulenes and ω,ω'-diazulenylpolyenes: reactions with protic acids, nucleophiles, and reducing and oxidizing agents, Liebigs Ann. Chem., 1984, 1936–1951 CrossRef; (e) S. Hünig, B. Ort, M. Hanke, C. Jutz, T. Morita, K. Takase, Y. Fukazawa, M. Aoyagi and S. Itô, Multistep reversible redox systems, XXXIX. Isomeric biazulenes: UV/VIS spectroscopy, voltammetry, and HMO energies, Liebigs Ann. Chem., 1984, 1952–1958 CrossRef; (f) S. Hünig and B. Ort, Multistep reversible redox systems, XL. ω,ω'-Biazulene polyenes: voltammetry and HMO energies, Liebigs Ann. Chem., 1984, 1959–1971 CrossRef.
- R. Hagen, E. Heilbronner and P. A. Straub, Spektroskopische Eigenschaften alkylsubstituierter Diazulyle (Die photochemische Darstellung des 3,3′ und des 2,2′-di-guaj-azulyls), Helv. Chim. Acta, 1968, 51, 45–67 CrossRef CAS.
- (a) M. Pailer and H. Lobenwein, Autoxydationsprodukte des Guaiazulens, Monatsh. Chem., 1971, 102, 1558–1570 CrossRef CAS; (b) Y. Matsubara, S.-i. Takekuma, K. Yokoi, H. Yamamoto and T. Nozoe, Autoxidation of guaiazulene and 4,6,8-trimethylazulene in polar aprotic solvent: structural proof for products, Bull. Chem. Soc. Jpn., 1987, 60, 1415–1428 CrossRef CAS.
- S.-i. Takekuma, Y. Matsubara, H. Yamamoto and T. Nozoe, Autoxidation of solid gualazulene and of the solution in DMF in the presence of base or acid: a comparative study of the product distribution, Bull. Chem. Soc. Jpn., 1988, 61, 475–481 CrossRef CAS.
- Y. Matsubara, M. Morita, S.-i. Takekuma, T. Nakano, H. Yamamoto and T. Nozoe, Oxidation of 4,6,8-trimethylazulene and guaiazulene with hydrogen peroxide in pyridine, Bull. Chem. Soc. Jpn., 1991, 64, 3497–3499 CrossRef CAS.
- T. Morita and K. Takase, Synthesis of 1,1′-, 2,2′-, 1,2′-, and 2,6′-biazulenes and their derivatives by Ullmann reaction, Bull. Chem. Soc. Jpn., 1982, 55, 1144–1152 CrossRef CAS.
- M. Iyoda, K. Sato and M. Oda, Nickel-catalyzed coupling of bromides of 1,6-methano[10]annulene and azulene. A facile synthesis of biannulene and bi-, ter-, quater-, and polyazulenes, Tetrahedron Lett., 1985, 26, 3829–3832 CrossRef CAS.
- A.-H. Chen, C.-H. Yang and T. Morita, Electrochemical oxidation of ethyl 2-hydroxyazulene-1-carboxylate, Huà xué, 1995, 53, 115–124, DOI:10.6623/chem.1995010.
- (a) J. Bindl, P. Seitz, U. Seitz, E. Salbeck, J. Salbeck and J. Daub, Elektronentransferreaktionen nichtalternierender chinoider und hydrochinoider Verbindungen Verhalten von 5,12-Dimethoxynaphth[2,3-a]azulen bei der Oxidation, Chem. Ber., 1987, 120, 1747–1756 CrossRef CAS; (b) A. C. Razus, Syntheses of polycyclic compounds by oxidative coupling of azulene-1-azoarenes, J. Chem. Soc., Perkin Trans. 1, 2000, 981–988 RSC; (c) A. C. Razus, C. Nitu, S. Carvaci, L. Birzan, S. A. Razus, M. Pop and L. Tarko, J. Chem. Soc., Perkin Trans. 1, 2001, 1227–1233 RSC; (d) A. C. Razus, L. Birzan, S. Nae, C. Nitu, V. Tecuceanu and V. Cimpeanu, ARKIVOC, 2002, 142–153, DOI:10.3998/ark.5550190.0003.215; (e) R. Ostaszewski and H.-J. Hansen, Oxidative 1,1′-coupling of highly alkylated 2-methoxycarbonylazulenes, Heterocycles, 2015, 90, 1135–1141 CrossRef CAS; (f) J. Wang, F. Gordillo Gámez, J. Marín-Beloqui, A. Diaz-Andres, X. Miao, D. Casanova, J. Casado and J. Liu, Synthesis of a dicyclohepta[a,g]heptalene-containing polycyclic conjugated hydrocarbon and the impact of non-alternant topologies, Angew. Chem., Int. Ed., 2023, 62, e202217124 CrossRef CAS PubMed; (g) A. Diaz-Andres, J. Marín-Beloqui, J. Wang, J. Liu, J. Casado and D. Casanova, Rational design of anti-Kasha photoemission from a biazulene core embedded in an antiaromatic/aromatic hybrid, Chem. Sci., 2023, 14, 6420–6429 RSC.
- A.-H. Chen, H.-H. Yen, Y.-C. Kuo and W.-Z. Chen, Asymmetric synthesis and characterization of chiral 2,2′-diamino-3,3′-diethoxycarbonyl-8,8′-diphenyl-1,1′-biazulene, Synth. Commun., 2007, 37, 2975–2987 CrossRef CAS.
- R. Sigrist and H.-J. Hansen, Benzo[a]azulenediones and 10,10′-bibenzo[a]azulene, Helv. Chim. Acta, 2014, 97, 1165–1175 CrossRef CAS.
- K. Yamamoto, R. Nakamae, H. Suemune and K. Usui, 10,10’-Dimethoxy-9,9’-biazuleno[2,1-c]phenanthrene, Molbank, 2015, M843 CrossRef.
- T. Shoji, A. Maruyama, A. Yamamoto, Y. Fujiwara, S. Ito, T. Okujima and N. Morita, Synthesis of 2,2′-diamino-1,1′-biazulenes by the copper-catalyzed homocoupling reaction of 2-aminoazulenes, Chem. Lett., 2014, 43, 1122–1124 CrossRef CAS.
- M. Narita, T. Teraoka, T. Murafuji, Y. Shiota, K. Yoshizawa, S. Mori, H. Uno, S. Kanegawa, O. Sato, K. Goto and F. Tani, An Azulene-based chiral helicene and its air-stable cation radical, Bull. Chem. Soc. Jpn., 2019, 92, 1867–1873 CrossRef CAS.
- (a) M. Hyoudou, H. Nakagawa, T. Gunji, Y. Ito, Y. Kawai, R. Ikeda, T. Konakahara and N. Abe, Synthesis, cyclization, and evaluation of the anticancer activity against HeLa S-3 cells of ethyl 2-acetylamino-3-ethynylazulene-1-carboxylates, Heterocycles, 2012, 86, 233–244 CrossRef CAS; (b) T. Shoji, E. Shimomura, Y. Inoue, M. Maruyama, A. Yamamoto, K. Fujimori, S. Ito, M. Yasunami and N. Morita, Synthesis of novel thiophene-fused 1,1′-biazulene derivative by the reaction of azuleno[1,2-b]thiophene with N-iodosuccinimide, Heterocycles, 2013, 87, 303–306 CrossRef CAS; (c) H. Nakagawa, S. Tsukada, N. Abe and T. Gunji, unexpected formation of 2-amino-(1-(2-nitrophenylsulfinyl))azulene by the reaction of 2-aminoazulene with 2-nitrobenzenesulfonyl chloride, Heteroat. Chem., 2014, 25, 389–395 CrossRef CAS.
- T.-I. Ho, C.-K. Ku and R. S. H. Liu, Preparation of 1-arylazulenes through regioselective photoarylation of azulene with aryl iodides, Tetrahedron Lett., 2001, 42, 715–717 CrossRef CAS.
- (a) T. Shoji, S. Ito, K. Toyota, M. Yasunami and N. Morita, The novel transition metal free synthesis of 1,1′-biazulene, Tetrahedron Lett., 2007, 48, 4999–5002 CrossRef CAS; (b) T. Shoji, J. Higashi, S. Ito, K. Toyota, T. Asao, M. Yasunami, K. Fujimori and N. Morita, Synthesis and redox behavior of 1-azulenyl sulfides and efficient synthesis of 1,1′-biazulenes, Eur. J. Org Chem., 2008, 1242–1252 CrossRef CAS; (c) T. Shoji, A. Maruyama, M. Maruyama, S. Ito, T. Okujima, J. Higashi, K. Toyota and N. Morita, Synthesis and properties of 6-methoxy- and 6-dimethylamino-1-methylthio- and 1,3-bis(methylthio)azulenes and triflic anhydride-mediated synthesis of their biaryl derivatives, Bull. Chem. Soc. Jpn., 2014, 87, 141–154 CrossRef CAS.
- M. Murai, M. Yanagawa, M. Nakamura and K. Takai, Palladium-catalyzed direct arylation of azulene based on regioselective C–H bond activation, Asian J. Org. Chem., 2016, 5, 629–635 CrossRef CAS.
- K. Ninomiya, Y. Harada, T. Kanetou, Y. Suenaga, T. Murafuji and R. Tsunashima, Synthesis and acid–base properties of a proton-bridged biaryl compound based on pyridylazulene, New J. Chem., 2015, 39, 9079–9085 RSC.
- K. Tanino, T. Yamada, F. Yoshimura and T. Suzuki, Cyanoazulene-based multistage redox systems prepared from vinylcyclopropanecarbonitrile and cyclopentenone via divinylcyclopropane-rearrangement approach, Chem. Lett., 2014, 43, 607–609 CrossRef CAS.
- (a) T. Morita, H. Kanzawa and K. Takase, The Synthesis and some properties of 2,6-dihydroxyazulene, Chem. Lett., 1977, 6, 753–756 CrossRef; (b) G. Dyker, S. Borowski, J. Heiermann, J. Körning, K. Opwis, G. Henkel and M. Köckerling, First intermolecular palladium catalyzed arylation of an unfunctionalized aromatic hydrocarbon, J. Organomet. Chem., 2000, 606, 108–111 CrossRef CAS; (c) T. Shoji, K. Miyashita, T. Araki, M. Tanaka, A. Maruyama, R. Sekiguchi, S. Ito and T. Okujima, Synthesis of 1,2′-biazulenes by palladium-catalyzed unusual homocoupling reaction of 1-haloazulenes in the presence of ferrocene, Synthesis, 2016, 48, 2438–2448 CrossRef CAS; (d) C. Wang, Z. Deng, D. L. Phillips and J. Liu, Extension of non-alternant nanographenes containing nitrogen-doped Stone-Thrower-Wales defects, Angew. Chem., Int. Ed., 2023, 62, e202306890 CrossRef CAS.
- A. L. Crombie, J. L. Kane Jr, K. M. Shea and R. L. Danheiser, Ring expansion-annulation strategy for the synthesis of substituted azulenes and oligoazulenes. 2. Synthesis of azulenyl halides, sulfonates, and azulenylmetal compounds and their application in transition-metal-mediated coupling reactions, J. Org. Chem., 2004, 69, 8652–8667 CrossRef CAS PubMed.
- K. Kurotobi, H. Tabata, M. Miyauchi, T. Murafuji and Y. Sugihara, Coupling reaction of azulenyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolanes with haloazulenes, Synthesis, 2002, 1013–1016 CrossRef CAS.
- (a) A. C. Razus, C. Pavel, O. Lehadus, S. Nica and L. Birzan, Synthesis and properties of [1,6′]biazulene compounds, Tetrahedron, 2008, 64, 1792–1797 CrossRef CAS; (b) T. Shoji, A. Yamamoto, E. Shimomura, M. Maruyama, S. Ito, T. Okujima, K. Toyota and N. Morita, Synthesis of 1,6′-bi- and 1,6′:3,6′′-terazulenes from 1-pyridyl- and 1,3-di(pyridyl)azulenes by the Ziegler–Hafner method, Chem. Lett., 2013, 42, 638–640 CrossRef CAS; (c) T. Shoji, A. Yamazaki, Y. Ariga, M. Uda, D. Ando, N. Sasahara, N. Kai and S. Ito, Azulene-substituted donor-acceptor polymethines and 1,6’-bi-, 1,6′;3,6′′-ter-, and quinqueazulenes via Zincke salts: Synthesis, and structural, optical, and electrochemical properties, ChemPlusChem, 2021, 86, 946–966 CrossRef CAS PubMed.
- (a) Y. Fukazawa, M. Aoyagi and S. ltô, Naphtho[1,8-ab:4,5-a′b′]diazulene, the first nonalternant isomer of dibenzopyrene, Tetrahedron Lett., 1981, 22, 3879–3882 CrossRef CAS; (b) S. Ito, T. Terazono, T. Kubo, T. Okujima, N. Morita, T. Murafuji, Y. Sugihara, K. Fujimori, J. Kawakamia and A. Tajiri, Efficient preparation of 2-azulenylboronate and Miyaura–Suzuki cross-coupling reaction with aryl bromides for easy access to poly(2-azulenyl)benzenes, Tetrahedron, 2004, 60, 5357–5366 CrossRef CAS; (c) T. Shibasaki, T. Ooishi, N. Yamanouchi, T. Murafuji, K. Kurotobi and Y. Sugihara, A New efficient route to 2-substituted azulenes based on sulfonyl group directed lithiation, J. Org. Chem., 2008, 73, 7971–7977 CrossRef CAS; (d) T. Nakae, T. Kikuchi, S. Mori, T. Okujima, T. Murafuji and H. Uno, Bisarylation of 1,1′,3,3′-tetrahalo-2,2′-biazulene under Suzuki–Miyaura cross-coupling conditions, Chem. Lett., 2014, 43, 504–506 CrossRef CAS; (e) T. Shoji, A. Maruyama, S. Ito, T. Okujima, M. Yasunami, J. Higashi and N. Morita, Synthesis of 2-aryl- and 6-heteroaryl-1,3-di(4-pyridyl)azulenes by Katritzky's pyridylation of 2-aryl- and 6-heteroarylazulenes, Heterocycles, 2014, 89, 2588–2603 CrossRef CAS; (f) M. Narita, T. Murafuji, S. Yamashita, M. Fujinaga, K. Hiyama, Y. Oka, F. Tani, S. Kamijo and K. Ishiguro, Synthesis of 2-iodoazulenes by the iododeboronation of azulen-2-ylboronic acid pinacol esters with copper(I) iodide, J. Org. Chem., 2018, 83, 1298–1303 CrossRef CAS; (g) O. Sato and R. Sakai, Synthesis and electrochemical properties of 2,2′-biguaiazulene-based 1,2-dithiin and thiophene, Heterocycles, 2018, 96, 1259–1265 CrossRef CAS; (h) T. Shoji, N. Sakata, Y. Ariga, A. Yamazaki, R. Katoh, T. Okujima, R. Sekiguchi and S. Ito, Construction of a 2,2′-biazulene framework via Brønsted acid-promoted annulation of 2,3-di(1-azulenyl)benzofurans, Chem. Commun., 2023, 59, 3447–3450 RSC; (i) B. Yu, P. Du, J. Guo, H. Xin and J. Zhang, Nonalternant isomer of pentacene fusing two azulene units, Chin. Chem. Lett., 2024, 35, 109321 CrossRef CAS.
- (a) T. Okujima, S. Ito and N. Morita, Preparation and Stille cross-coupling reaction of the first organotin reagents of azulenes. An efficient Pd(0)-catalyzed synthesis of 6-aryl- and biazulenes, Tetrahedron Lett., 2002, 43, 1261–1264 CrossRef CAS; (b) S. Ito, T. Okujima and N. Morita, Preparation and Stille cross-coupling reaction of the first organotin reagents of azulenes. Easy access to poly(azulen-6-yl)benzene derivatives, J. Chem. Soc., Perkin Trans. 1, 2002, 1896–1905 RSC.
- (a) M. Hanke and C. Jutz, Synthese von 6,6′-Biazulene, Synthesis, 1980, 31–32 CrossRef CAS; (b) D. Balschukat and E. V. Dehmlow, Neuartige 2,6-disubstituierte Azulene, Chem. Ber., 1986, 119, 2272–2288 CrossRef CAS; (c) T. Iwashina, H. Nakagawa, Y. Sato, R. Hayami, K. Yamamoto and T. Gunji, Formation and characterization of palladium ethyl 2-aminoazulene-carboxylate complexes, Polyhedron, 2024, 247, 116740 CrossRef CAS.
- (a) T. R. Maher, A. D. Spaeth, B. M. Neal, C. L. Berrie, W. H. Thompson, V. W. Day and M. V. Barybin, Linear 6,6′-biazulene framework featuring isocyanide termini: synthesis, structure, redox behavior, complexation, and self-assembly on Au(111), J. Am. Chem. Soc., 2010, 132, 15924–15926 CrossRef CAS PubMed; (b) P. T. Connelly, J. C. Applegate, D. A. Maldonado, M. K. Okeowo, W. C. Henke, A. G. Oliver, C. L. Berrie and M. V. Barybin, Homoleptic complexes of isocyano- and diisocyanobiazulenes with a 12-electron, ligand-based redox capacity, Dalton Trans., 2023, 52, 11419–11426 RSC; (c) S. R. Kelsey, G. Griaznov, A. D. Spaeth, D. E. Janzen, J. T. Douglas, W. H. Thompson and M. V. Barybin, Tuning the redox profile of the 6,6′-biazulenic platform through functionalization along its molecular axis, Chem. Commun., 2024, 60, 52135216 RSC.
- (a) H. Xin, C. Ge, X. Yang, H. Gao, X. Yang and X. Gao, Biazulene diimides: a new building block for organic electronic materials, Chem. Sci., 2016, 7, 6701–6705 RSC; (b) H. Xin, C. Ge, X. Jiao, X. Yang, K. Rundel, C. R. McNeill and X. Gao, Incorporation of 2,6-connected azulene units into the backbone of conjugated polymers: towards high-performance organic optoelectronic materials, Angew. Chem., Int. Ed., 2018, 57, 1322–1326 CrossRef CAS.
- (a) Y. Yamaguchi, K. Ogawa, K.-i. Nakayama, Y. Ohba and H. Katagiri, Terazulene: a high-performance n-type organic field-effect transistor based on molecular orbital distribution control, J. Am. Chem. Soc., 2013, 135, 19095–19098 CrossRef CAS PubMed; (b) Y. Yamaguchi, M. Takubo, K. Ogawa, K.-i. Nakayama, T. Koganezawa and H. Katagiri, Terazulene isomers: polarity change of OFETs through molecular orbital distribution contrast, J. Am. Chem. Soc., 2016, 138, 11335–11343 CrossRef CAS PubMed.
- C. Yang, K. S. Schellhammer, F. Ortman, S. Sun, R. Dong, M. Karakus, Z. Mics, M. Löffler, F. Zhang, X. Zhuang, E. Cánovas, G. Cuniberti, M. Bonn and X. Feng, Coordination polymer framework based on-chip micro-supercapacitors with AC line-filtering performance, Angew. Chem., Int. Ed., 2017, 56, 3920–3924 CrossRef CAS.
- S. Sun, X. Zhuang, L. Wang, B. Zhang, J. Ding, F. Zhang and Y. Chen, Azulene-bridged coordinated framework based quasi-molecular rectifier, J. Mater. Chem. C, 2017, 5, 2223–2229 RSC.
- N. Tzoganakis, B. Feng, M. Loizos, M. Krassas, D. Tsikritzis, X. Zhuang and E. Kymakis, Ultrathin PTAA interlayer in conjunction with azulene derivatives for the fabrication of inverted perovskite solar cells, J. Mater. Chem. C, 2021, 9, 14709–14719 RSC.
- C. Zhang, J. Cheng, Q. Wu, S. Hou, S. Feng, B. Jiang, C. J. Lambert, X. Gao, Y. Li and J. Li, Enhanced π–π stacking between dipole-bearing single molecules revealed by conductance measurement, J. Am. Chem. Soc., 2023, 145, 1617–1630 CrossRef CAS PubMed.
- Q. Zhang, D. Wu, Y. Fu, J. Li, Y. Chen and B. Zhang, Molecular-potential and redox coregulated cathodic electrosynthesis toward ionic azulene-based thin films for organic memristors, ACS Appl. Mater. Interfaces, 2024, 16, 22217–22228 CrossRef CAS.
- S.-J. Lin, S.-Y. Jiang, T.-C. Huang, C.-S. Dai, P.-F. Tsai, H. Takeshita, Y.-S. Lin and T. Nozoe, A new convenient synthesis of 1,2′-biazulenes by photolysis of 2-diazo-1,3-dicyanoazulen-6(2H)-one with azulene derivatives, Bull. Chem. Soc. Jpn., 1997, 70, 3071–3074 CrossRef CAS.
- R. Hatakenaka, N. Nishikawa, Y. Mikata, H. Aoyama, K. Yamashita, Y. Shiota, K. Yoshizawa, Y. Kawasaki, K. Tomooka, S. Kamijo, F. Tani and T. Murafuji, Efficient synthesis and structural analysis of chiral 4,4′-biazulene, Chem.–Eur. J., 2024, 30, e202400098 CrossRef CAS PubMed.
- J. Voss, T. Pesel and D. B. J. Lehtivarjo, EPR studies on carboxylic esters, 22. Preparation of new alkyl azulenecarboxylates and EPR-spectroscopic study of their radical anions, Z. Naturforsch., B:J. Chem. Sci., 2014, 69, 466–480 CrossRef CAS.
- M. Hanke and C. Jutz, Synthesis of 5,5′-biazulene, Angew. Chem., Int. Ed., 1979, 18, 214–215 CrossRef.
- J. M. Brunel, BINOL: a versatile chiral reagent, Chem. Rev., 2005, 105, 857–898 CrossRef CAS.
- (a) A. Miyashita, A. Yasuda, H. Takaya, K. Toriumi, T. Ito, T. Souchi and R. Noyori, Synthesis of 2,2′-bis(diphenylphosphino)-1,1′-binaphthyl (BINAP), an atropisomeric chiral bis(triaryl)phosphine, and its use in the rhodium(I)-catalyzed asymmetric hydrogenation of α-(acylamino)acrylic acids, J. Am. Chem. Soc., 1980, 102, 7932–7934 CrossRef CAS; (b) R. Noyori and H. Takaya, BINAP: an efficient chiral element for asymmetric catalysis, Acc. Chem. Res., 1990, 23, 345–350 CrossRef CAS.
- (a) L. Pu, Regioselective Substitution of BINOL, Chem. Rev., 2024, 124, 6643–6689 CrossRef CAS; (b) G. Li, F. Liu and M. Wu, BINOLs modified at 3,3′-positions: chemists' preferred choice in asymmetric catalysis, ARKIVOC, 2015, 140–174, DOI:10.3998/ark.5550190.p009.060; (c) M. Berthod, G. Mignani, G. Woodward and M. Lemaire, Modified BINAP: the how and the why, Chem. Rev., 2005, 105, 1801–1836 CrossRef CAS; (d) Y. Chen, S. Yekta and A. K. Yudin, Modified BINOL ligands in asymmetric catalysis, Chem. Rev., 2003, 103, 3155–3212 CrossRef CAS PubMed.
- (a) L. Schreyer, R. Properzi and B. List, IDPi Catalysis, Angew. Chem., Int. Ed., 2019, 58, 12761–12777 CrossRef CAS; (b) M. R. Monaco, G. Pupo and B. List, Phosphoric acid based heterodimers in asymmetric catalysis, Synlett, 2016, 27, 1027–1040 CrossRef CAS; (c) D. Parmar, E. Sugiono, S. Raja and M. Rueping, Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen bonding, ion pairing, and metal phosphates, Chem. Rev., 2014, 114, 9047–9153 CrossRef CAS PubMed; (d) M. Terada, Chiral phosphoric acids as versatile catalysts for enantioselective transformations, Synthesis, 2010, 1929–1982 CrossRef CAS.
- A. Tajiri, M. Fukuda, M. Hatano, T. Morita and K. Takase, Resolution, circular dichroism and absolute configuration of 1,1′-bis-azulenes, Angew. Chem., Int. Ed., 1983, 2, 870–871 CrossRef.
- (a) J. Daub, L. Jakob, J. Salbeck and Y. Okamoto, Model compounds for electron transfer in spatially ordered systems: chiral and achiral triptycenoazulenequinones, Chimia, 1985, 39, 393–395 CrossRef CAS; (b) J. Daub, L. Jakob and J. Salbeck, Chiral electron-transfer-active quinones with triptycene substructures: Synthesis concept and properties, Chem. Ber., 1988, 121, 2187–2194 CrossRef CAS.
- J. Bindl, G. Pilidis and J. Daub, Chiral electron transfer compounds: binaphtho[2,3-a]azulenequinone and binaphtho[2,3-a]azulene-hydroquinone derivatives by oxidative coupling, Angew. Chem., 1984, 96, 294–296 CrossRef CAS.
- Chaolumen, H. Ito and K. Itami, An axially chiral 1,1′-biazulene and its π-extended derivative: synthesis, structures and properties, Chem. Commun., 2019, 55, 9606–9609 RSC.
- T. Tsuchiya, Y. Katsuoka, K. Yoza, H. Sato and Y. Mazaki, Stereochemistry, stereodynamics and redox and complexation behaviors of 2,2′-diaryl-1,1′-biazulenes, ChemPlusChem, 2019, 84, 1659–1667 CrossRef CAS PubMed.
- R. Hatakenaka, N. Nishikawa, Y. Mikata, H. Aoyama, K. Yamashita, Y. Shiota, K. Yoshizawa, Y. Kawasaki, K. Tomooka, S. Kamijo, F. Tani and T. Murafuji, Efficient synthesis and structural analysis of chiral 4,4′-biazulene, Chem.–Eur. J., 2024, 30, e202400098 CrossRef CAS PubMed.
- A. Walther, G. Tusha, B. Schmidt, J. J. Holstein, L. V. Schäfer and G. H. Clever, Solvent-directed social chiral self-sorting in Pd2L4 coordination cages, J. Am. Chem. Soc., 2024, 146, 32748–32756 CrossRef CAS.
- R. Hatakenaka, K. Urabe, S. Ueno, M. Yamauchi, Y. Mizuhata, H. Yamada, Y. Mikata, S. Kamijo, F. Tani and T. Murafuji, Doubly linked azulene dimer: a novel non-benzenoid isomer of perylene, Chem.–Eur. J., 2025, 31, e202404679 CrossRef CAS.
- K. Takase, T. Asao, Y. Takagi and T. Nozoe, Syntheses and some properties of 2- and 6-hydroxyazulenes, Chem. Commun., 1968, 368–370 RSC.
- A.-H. Chen, Electrochemical oxidation of 1,2-disubstituted azulenes, Proc. Natl. Sci. Counc., Repub. China, Part A, 1999, 23, 437–442 CAS.
- D.-S. Lee, P.-W. Yang, T. Morita and T. Nozoe, Synthesis and 1H NMR complexation study of a novel macrocyclic polyether containing 1,1′-biazulene unit, Heterocycles, 1995, 41, 249–253 CrossRef CAS.
- (a) T. Nozoe, K. Takase, M. Kato and T. Nogi, Reaction of 2-arylsulfonyloxytropones and active methylene compounds. Formation of 8-hydroxy-2H-cyclohepta[b]furan-2-one and 2-amino-8H-cyclohepta[b]furan-8-one derivatives, Tetrahedron, 1971, 27, 6023–6035 CrossRef CAS; (b) T. Nozoe, K. Imafuku, B. Z. Yin, M. Honda, Y. Goto, Y. Hara, T. Andoh and H. Yamamoto, Synthesis and reactions of 2-(arylhydrazino)tropones. I. Preparation of 2-(2-arylhydrazino)tropones and the 4-substituted derivatives, Bull. Chem. Soc. Jpn., 1988, 61, 2531–2539 CrossRef.
- T. Nozoe, H. Wakabayashi, K. Shindo, S. Ishikawa, C. P. Wu and P. W. Yang, A convenient, one-pot azulene synthesis from 2H-cyclohepta[b]furan-2-ones with vinyl ether and its analogues. III. Orthoesters as a reagent, Heterocycles, 1991, 32, 213–220 CrossRef CAS.
- (a) H. C. Meinders, F. van Bolhuis and G. Challa, The role of μ-hydroxo-ligands in the catalytic properties of binuclear copper—tertiary amine complexes, J. Mol. Catal., 1979, 5, 225–233 CrossRef CAS; (b) M. Noji, M. Nakajima and K. Koga, A new catalytic system for aerobic oxidative coupling of 2-naphthol derivatives by the use of CuCl-amine complex: a practical synthesis of binaphthol derivatives, Tetrahedron Lett., 1994, 35, 7983–7984 CrossRef CAS; (c) M. Nakajima, I. Miyoshi, K. Kanayama, S.-i. Hashimoto, M. Noji and K. Koga, Enantioselective synthesis of binaphthol derivatives by oxidative coupling of naphthol derivatives catalyzed by chiral diamine·copper complexes, J. Org. Chem., 1999, 64, 2264–2271 CrossRef CAS; (d) E. E. Podlesny and M. C. Kozlowski, Divergent approach to the bisanthraquinone natural products: total synthesis of (S)-bisoranjidiol and derivatives from binaphthol-para-quinones, J. Org. Chem., 2013, 78, 466–476 CrossRef CAS.
- S. K. Alamsetti, E. Poonguzhali, D. Ganapathy and G. Sekar, Enantioselective oxidative coupling of 2-naphthol derivatives by copper-(R)-1,1′-binaphthyl-2,2′-diamine-TEMPO catalyst, Adv. Synth. Catal., 2013, 355, 2803–2808 CrossRef CAS.
- (a) D. Fabbri, G. Delogu and O. De Lucchi, A widely applicable method of resolution of binaphthyls: preparation of enantiomerically pure 1,1′-binaphthalene-2,2′-diol, 1,1′-binaphthalene-2,2′-dithiol, 2′-mercapto-1,1′-binaphthalen-2-ol, and 1,1′-binaphthalene-8,8′-diol, J. Org. Chem., 1995, 60, 6599–6601 CrossRef CAS; (b) Z. Li, X. Liang, F. Wu and B. Wan, A convenient resolution method for 1,1′-bi-2-naphthol and 4,4′-dibromo-1,1′-spirobiindane-7,7′-diol with menthyl chloroformate in the presence of TBAB, Tetrahedron: Asymmetry, 2004, 15, 665–669 CrossRef CAS.
- A. S. Cooke and M. M. Harris, Ground-state strain and other factors influencing optical stability in the 1,1′-binaphthyl series, J. Chem. Soc., 1963, 2365–2373 RSC.
- T. Nozoe, K. Takase, T. Nakazawa and S. Fukuda, The formation of azulene derivatives from 2H-cyclohepta[b]furan-2-one derivatives, Tetrahedron, 1971, 27, 3357–3368 CrossRef.
- M. Kalek, M. Jezowska and J. Stawinski, Preparation of arylphosphonates by palladium(0)-catalyzed cross-coupling in the presence of acetate additives: synthetic and mechanistic studies, Adv. Synth. Catal., 2009, 351, 3207–3216 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra, X-ray data. CCDC 2421193–2421195. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra02520f |
| ‡ Present address: Evotec (UK) Ltd., 95 Park Drive, Abingdon, OX14 4RY, UK. |
| § Present address: B23 Beamline, Diamond Light Source, Didcot OX11 0DE, UK. |
| This journal is © The Royal Society of Chemistry 2025 |