Open Access Article
Open Access ArticleDetermination of gold content in rock gold ore samples based on closed water bath aqua regia digestion-polyurethane foam enrichment and using flame atomic absorption spectrometry
Shunxiang Wanga,
Cang Gong *a,
Hongcheng Wanga,
Xiang Xiaa,
Jiufen Liub and
Haichuan Lu*a
*a,
Hongcheng Wanga,
Xiang Xiaa,
Jiufen Liub and
Haichuan Lu*a
aResearch Center of Applied Geology of China Geological Survey, Chengdu, 610036, China. E-mail: dugufengxue@yeah.net; 962149558@qq.com
bNatural Resources Comprehensive Survey Command Center of China Geological Survey, Beijing, 100055, China
First published on 18th June 2025
Abstract
At present, due to the uneven distribution and low content of gold (Au) in rock gold ore samples, the separation, enrichment and accurate determination of Au have become daunting tasks in the analysis of rock gold samples. This study established a method for determining the Au content in rock gold ore samples with the advantages of high efficiency, low cost, safety and more environment-friendliness. It was based on closed water bath digestion-polyurethane foam (PUF) enrichment and thiourea release, and then, flame atomic absorption spectrometry was used to determine the Au content. By eliminating interference and optimizing the PUF pretreatment method, aqua regia dosage, water bath dissolution time, oscillation frequency and time, thiourea concentration, release time and temperature and other working conditions, technical problems such as the low adsorption efficiency of the PUF and interference from tungsten, antimony and iron elements were solved. The Au detection limit of this method was 0.03 μg g−1, the lower limit of determination was 0.1 μg g−1, and the optimal determination range was 0.1–40 μg g−1. This method was used to determine 7 national standard materials of gold ores with different high and low contents (GBW07808b, GBW07809b, GBW07297a, GBW07298, GBW07300a, GBW(E)070012a and GBW07807a); the relative error (RE) was ≤3.06% and the relative standard deviation (RSD) was ≤8.35%. This method was performed in 9 laboratories to compare and verify the 7 national standard materials of gold ores with different high and low contents (GBW(E)070067, GBW(E)070262, GBW(E)070138a, GBW07300a, GBW07809b, GBW(E)070141 and GBW07298a); the RE was ≤10.55% and the RSD was ≤3.93%, achieving good results. A total of 85 rock gold ore samples were selected for determination using this method and compared with the results of activated carbon enrichment-flame atomic absorption spectrometry. The qualification rate was 97.65%, and the analysis and testing efficiencies were improved by nearly 10 times.
1 Introduction
Gold (Au) is a precious metal with high ductility, corrosion resistance and chemical stability. Owing to its special properties, it plays a vital role in many fields, such as economic development, national defense, geology, materials science, and biology.1–3 According to the China Mineral Resources Report 2024, China's Au mine reserves in 2023 were 3203.77 t, with an increase of 2.4% over 2022. As of 2023, global Au reserves were 193000 t, of which 167000 t are on the surface and 26000 t are under the sea. With the development of economy, the demand for Au is still increasing, and hence, rational development and utilization of Au mines are of great significance for the sustainable development of Au industry. Rapid, sensitive and accurate determination of Au has been a long-term priority in earth science, analytical chemistry and spectroscopy.4–7 However, the non-uniform distribution of Au in various geological samples and low Au content make the separation, enrichment and accurate determination of Au difficult in geological analysis.There are two main methods for decomposing rock Au ores: dry method and wet method. The dry method is mainly a fire assay, which has always been the most important method in Au analysis owing to its large sampling volume, good sampling representativeness, high enrichment efficiency, wide application range, and accurate and stable test results, and it can also be combined with other detection methods. It is recognized by domestic and foreign laboratories as the most authoritative analysis method for Au detection.8–12 Despite its advantages, fire assay is accompanied by several limitations, including prolonged processing times, high labor demands, significant blank values, unfavorable working conditions, and substantial environmental pollution.13–16 The most common wet method is the aqua regia dissolution method, which uses an open hot plate to dissolve the sample. Due to the temperature gradient across the hot plate surface, the elevated temperature at the center often causes sample splashing and evaporation, while the peripheral samples may not dissolve adequately, leading to poor precision in the measurement results.17–19 In addition, the aqua regia dissolution method uses an electric heating plate to heat the aqua regia, which volatilizes and produces a large amount of nitrogen oxides, causing environmental pollution. The number of samples dissolved each time is limited by the surface area of the electric heating plate, resulting in low efficiency.6,16 The closed sample dissolution method is a special wet sample dissolution method. This method uses the solvent to increase the temperature and pressure in a closed space to digest the sample. Compared with the open sample dissolution of the electric hot plate, it can greatly eliminate the pollution of aqua regia to the environment, and the temperature is constant and uniform, the acidity is easy to control, and the number of samples dissolved in a single time can reach hundreds, which greatly saves production and labor costs and greatly improves work efficiency.20,21
In addition to the decomposition of rock Au ore, enrichment and separation are necessary, often for sensitive and accurate measurement of the Au content. The main methods used for the separation and enrichment of Au include polyurethane foam adsorption, fire assay coprecipitation, solvent extraction, activated carbon adsorption, ion exchange, hanging droplet microextraction, on-line separation and enrichment by micro-column, sulfhydryl cotton adsorption, extraction chromatography, and liquid membrane separation and enrichment.22–29 In recent years, some emerging technologies have been applied to the separation and enrichment of gold. Yu30 established a CIP-MS method for the determination of gold in geological samples with 5-mercapt-3-phenyl-1,3,4-thiadiazole-2-thione potassium salt as a complexing agent and polyethylene glycol octyl phenyl ether as an extractant. Chen et al.31 added Cu2+ to an alkaline Na2S2O3 solution to conduct experimental research on the leaching of gold in waste-integrated circuit chips, with a gold leaching rate as high as 92.25%. Zan32 used 717 strong alkaline styrene anions to pre-separate and enrich copper tailings, and studied the effects of the flow rate of the upper column liquid, the acidity of the upper column liquid, and the sample volume on the adsorption rate. Hao et al.33 used the cloud point phenomenon of the nonionic surfactant TritonX-114 to extract gold complexes and used spectrophotometry to determine trace gold in geological samples. The spiking recovery rate was 97.8–103.0%. He et al.28 used a P350 microchromatography column to establish online separation enrichment-flame atom absorption spectrometry to determine trace gold in ores, and the adsorption rate of gold reached almost 100%. Xiao et al.29 established a method of ashing, acid dissolution, and suspended droplet microextraction of tributyl phosphate in platinum dishes for ultra-trace gold analysis in high-purity graphite. The relative standard deviation of the measurement results is 1.5–4.9%, and the spiking recovery rate is 94.9–105.3%. Considering comprehensive separation and enrichment efficiency, operational complexity, cost and other factors, the activated carbon adsorption method has gained extensive application in gold ore laboratories. Fire assay has the advantages of high enrichment rate and good stability, which is called the classic method. The PUF adsorption method has become the most common method for the separation and enrichment of geological samples because of its batch operation.19,34 Bowen first applied PUFs to adsorb Au in an acidic medium in 1970.19 The PUF is a sponge-like foam plastic with certain cross-links made of toluene diisocyanate and polyether polyol. When Au in the solution meets the foam, adsorption and exchange occur on the foam membrane.19 The PUF has been widely used in the determination of Au in geological samples due to its strong adsorption performance and low price.
The purpose of this work is to select the best PUF and discuss the pretreatment method of PUFs. Reasonable sample dissolving time and aqua regia dosage were chosen, and the traditional open digestion was replaced with more efficient closed water bath digestion. Furthermore, the elimination of interfering elements was optimized, the best oscillation frequency and time were determined, and the best thiourea concentration, desorption time and desorption temperature were selected to establish closed water bath aqua regia digestion-polyurethane foam enrichment thiourea elution-flame atomic absorption spectrometry to realize the accurate determination of gold content in rock gold ores.
2 Materials and methods
2.1 Instrumentation
The samples were crushed into coarse medium-sized particles using a jaw crusher (XPC-100 × 60, Guiyang Prospecting, China) and a disc mill (MP-ø250, Guiyang Prospecting, China) and then finely ground using a multifunctional rod mill (ZN-II, Huangshi Angtai, China). The sample powder was weighed using an electronic balance (JJ500, Changshu Shuangjie, China) and turned into ashes in a muffle furnace (SX-10-12PII, Taister, China). A self-made closed sample dissolving water bath with a lid was used for sample digestion. A cyclotron oscillator (DC-400, Chengdu Haiguang, China) was used to ensure that Au was completely adsorbed by the PUF. A constant temperature water bath (SY-100, Chengdu Haiguang, China) was used to desorb Au. A flame atomic absorption spectrometer (AAS) (GG-910, Beijing Haiguang, China) was used to determine Au. AAS working conditions: wavelength = 242.8 nm, lamp current = 10 mA, acetylene gas flow rate = 1.5 L min−1, spectral bandwidth = 0.2 nm, integration time = 1 s, air-acetylene flame, and Au hollow cathode lamp.2.2 Reagents and standards
Hydrochloric acid (HCl), nitric acid (HNO3), ferric chloride (FeCl3·6H2O), ammonium fluoride (NH4F) and thiourea (CH4N2S) used in sample digestion were all of analytical grade, and ultrapure deionized water with a resistivity of 18.2 MΩ cm at 25 °C was used throughout the experiment. The main reagents prepared in the experiment were 250 g L−1 FeCl3·6H2O solution, 20% NH4F solution, 10 g L−1 CH4N2S solution and aqua regia. The national standard Au single-element solution (GSB04-1715-2004, 1000 μg mL−1) dissolved in 1.5 mol L−1 HCl was provided by the National Nonferrous Metals and Electronic Materials Analysis and Testing Center. The Au standard solution required for the experiment was prepared by a stepwise dilution method. Gold's 17 Certified Reference Substances (CRMs): GBW07802b purchased from Yantai Zhaoyuan Inspection and Testing Center (Yantai, China); GBW(E)070067 purchased from Shaanxi Experimental Institute of Geology and Mineral Resources (Xi 'an, China); GBW(E)070138a, GBW(E)070140 and GBW(E)070141 purchased from Changchun Au Research Institute Co., LTD (Changchun, China); GBW(E)070262 and GBW(E)070266 purchased from Shandong Metallurgical Research Institute Co., LTD (Jinan, China); GBW07856 and GBW07857 purchased from the Armed Police Au Research (Langfang, China); and GBW07297a, GBW07298, GBW07298a, GBW07300a, GBW(E)070012a, GBW07807a, GBW07808b and GBW07809b purchased from the Institute of Geophysical and Geochemical Exploration, Chinese Academy of Geological Sciences (Langfang, China). Actual samples were collected from Yangshan Gold Mine, Gansu Province, China. Actual samples were collected from Yangshan Gold Mine, Gansu Province, China.2.3 PUF treatment
First, the PUF was cut into small cuboids (30 mm × 10 mm × 10 mm, 0.2 g), boiled in water for 2 h, dried, and put into clean plastic bottles for later use.2.4 Sample digestion
First, 20.0 g sample (accurate to 0.1 g) was weighed in a 50 mL porcelain crucible, placed in a muffle furnace from low temperature to 700 °C, and roasted for 2 h. The sample was removed and placed at room temperature, and the roasted sample was transferred to a 250 mL polyethylene plastic bottle, followed by the addition of 50 mL aqua regia (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 1 mL 250 g L−1 FeCl3·6H2O solution, and 5 mL 20% NH4F solution The sample was covered, heated in a water bath for 120 min and then cooled.
1), 1 mL 250 g L−1 FeCl3·6H2O solution, and 5 mL 20% NH4F solution The sample was covered, heated in a water bath for 120 min and then cooled.
2.5 Proposed experimental procedure for Au enrichment and desorption
About 100 mL of water was added to a polyethylene plastic bottle, a piece of PUF was placed, and the plastic cup was tightened and placed on an oscillator, followed by oscillation for 60 minutes at an amplitude of 2 cm and a frequency of 180 Hz. After the oscillation is completed, the PUF in the sample was taken out, and the remaining acid and residue were rinsed with tap water and then with deionized water. The PUF was squeezed to dryness and completely soaked in a colorimetric tube containing 20 mL thiourea solution using a foam hook, and then the colorimetric tube was put into a boiling water bath for 30 minutes. After the liberation was completed, the boiling water state was maintained and the foam was removed while it is hot, cooled, and waited for measurements.At the same time, a blank control experimental sample was prepared in the same manner.
2.6 Calibration merit
For plotting the calibration curves, 0 mL, 0.50 mL, 1.00 mL, 2.00 mL, 4.00 mL, and 6.00 mL of 10 μg mL−1 Au standard working solution were pipetted into a 250 mL polyethylene plastic bottle, and 50 mL of aqua regia, 1 mL of FeCl3·6H2O solution, 100 mL of water, and 1 piece of PUF were added. Then, it was placed \in an oscillator for 60 min of synchronous oscillation adsorption with the sample, as discussed in section 2.5. The subsequent treatment process is the same as mentioned in section 2.5. After the obtained solution was cooled to room temperature, it was measured, and the calibration curve was drawn.Twelve replicates of a 0.5 μg mL−1 gold standard solution were prepared and analyzed following the experimental methods described in section 2.5. The method detection limit (LOD) was calculated as three times the standard deviation of the blank, and the method quantification limit (LOQ) was determined as three times the standard deviation.
3 Results and discussion
3.1 Comparison of pretreatment methods for PUFs
Two types of PUFs were treated under three methods: no treatment, treatment with boiling water for 2 h, and treatment by soaking in 10% hydrochloric acid for 6 h; the PUFs were then washed to become neutral and squeezed to dryness for use. A treated PUF was added into a polyethylene plastic bottle containing 30 mL Au standard solution (4 μg g−1), enriched and released according to the experimental method described in section 2.5, and the Au content was determined by AAS. The PUFs of the three treatments were tested in parallel for 3 times, respectively. The Au recovery rates obtained by the three PUF treatment methods are shown in Table 1. The results indicated that both PUF treatment in boiling water and 10% hydrochloric acid soaking could obtain good Au recoveries, both of which were above 95%. The recovery rate of Au obtained by the PUF without treatment is lower than 90%. Considering the simple and quick operation of boiling water, this method determines to use boiling water for 2 h to treat PUFs.| No. | PUF features | Au recovery (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Colour | Density (kg m−3) | 25% Hardness (N) | 40% Hardness (N) | Resilience (%) | No treatment | Boiling water | 10% HCl soaking | |||||||
| 1 | White | 40 | 105 | 130 | 64 | 89.7 | 80.3 | 73.1 | 99.1 | 96.6 | 95.2 | 99.2 | 96.7 | 97.0 |
| 2 | White | 35 | 140 | 175 | 54 | 89.9 | 82.9 | 81.6 | 99.6 | 96.6 | 95.5 | 99.4 | 95.6 | 96.5 |
In order to explore the effect of different foam sizes on Au adsorption, PUFs with specifications of 30 mm × 10 mm × 10 mm and 40 mm × 20 mm × 20 mm were selected. After treatment with boiling water, they were placed in 10 μg g−1 and 25 μg g−1 Au standard solutions and GBW07807a, GBW07809B and GBW07298a sample solutions. Duplicate experiments were conducted according to Sections 2.4 and 2.5. The results are shown in Table 2. The results show that the two sizes of PUFs have no significant effect on the determination of Au. Therefore, this method selects PUFs with specifications of 30 mm × 10 mm × 10 mm.
| Sample | Recommended value (μg g−1) | PUF size (30 × 10 × 10) mm | PUF size (40 × 20 × 20) mm | ||
|---|---|---|---|---|---|
| Measured value 1 (μg g−1) | Measured value 2 (μg g−1) | Measured value 1 (μg g−1) | Measured value 2 (μg g−1) | ||
| 1 | 10 | 10.26 | 10.12 | 10.57 | 10.67 |
| 2 | 25 | 24.96 | 24.98 | 26.08 | 26.38 |
| GAu-16b | 1.1 | 1.18 | 1.18 | 1.12 | 1.19 |
| GAu-18b | 10.4 | 10.03 | 9.97 | 9.95 | 9.98 |
| GAu-20a | 31.9 | 31.12 | 31.01 | 31.43 | 31.42 |
3.2 Elimination of interfering elements
Through PUF adsorption, Au and symbiotic elements in rock Au ores are effectively separated and enriched. Although Fe, Cu, Ag, Pb, Zn, Ni, Cd, Sb and other elements will be adsorbed to a certain extent, the content is low, which generally does not interfere with the determination of Au.35 If the sample contains extremely high amounts of C, As, S, organic matter, W, Sb, Fe and Si, the determination of Au will be seriously low.36 When the sample contains high levels of C, As, S and organic matter, the sample can be calcined at 700 °C for 2 h to eliminate their interference.This is due to the fact that W forms H2WO4 gel-like substances during the sample pretreatment process, which captures a portion of AuCl4−, resulting in reduced ion-exchange efficiency between AuCl4− in the solution and the active groups (–RNH3+) on the foam plastic.37 The interference of Sb with Au primarily arises because, during the sample pretreatment process, Sb compounds are prone to hydrolysis when acidity or temperature decreases, and their products adsorb Au, preventing complete adsorption of AuCl4− onto the foam plastic.38 By controlling the acidity of the sample decomposition solution and adding proper amounts of tartaric acid to complex with tungsten and antimony, the stability of Au(III) in the solution can be ensured, converting potential H2WO4 gel-like substances into soluble salts and preventing the hydrolysis of Sb compounds when the acidity or temperature decreases.37,38
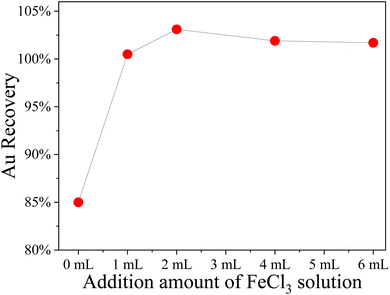
Interestingly, the addition of a small amount of iron salt helps the PUF to adsorb Au. In five 250 mL polyethylene bottles, 500 μg of Au standard solution was added to each, and then 0, 1, 2, 4, and 6 mL of 250 g L−1 FeCl3·6H2O solution were added, respectively, and the volume was fixed to 20 mL. The test was carried out according to Section 2.5, and the results are shown in Fig. 1. The results show that the addition of 1 mL of 250 g L−1 FeCl3·6H2O solution can help the foam to adsorb Au. Therefore, in this method, 1 mL of 250 g L−1 FeCl3·6H2O solution was added during sample digestion.
3.3 Selection of aqua regia dosage
Five 250 mL polyethylene bottles were selected and 500 μg Au standard solution was added to each bottle. Then, 20 mL, 30 mL, 40 mL, 50 mL, and 60 mL of aqua regia (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added, respectively, heated in a hot water bath for 30 min, removed and cooled. After that, 80 mL of water and 1 PUF were added, placed on an oscillator and operated according to Section 2.5. The results are shown in Table 3. The results show that when the aqua regia concentration (volume ratio) is 16–20%, the adsorption rate of PUF on Au is above 100%; when the aqua regia concentration is greater than 20%, it is easy to destroy the structure of PUF, making the performance of PUF adsorbing Au worse. In this method, the concentration of aqua regia was chosen as 16.7% (1
1) were added, respectively, heated in a hot water bath for 30 min, removed and cooled. After that, 80 mL of water and 1 PUF were added, placed on an oscillator and operated according to Section 2.5. The results are shown in Table 3. The results show that when the aqua regia concentration (volume ratio) is 16–20%, the adsorption rate of PUF on Au is above 100%; when the aqua regia concentration is greater than 20%, it is easy to destroy the structure of PUF, making the performance of PUF adsorbing Au worse. In this method, the concentration of aqua regia was chosen as 16.7% (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Aqua regia dosage (mL) 1 Aqua regia dosage (mL) |
Aqua regia concentration (v/v) during PUF enrichment (%) | Au standard solution concentration (μg mL−1) | Measured value (μg mL−1) | Average adsorption rate of Au (%) |
|---|---|---|---|---|
| 20 | 10 | 25 | 21.37 | 87.1 |
| 22.16 | ||||
| 30 | 13.6 | 25 | 23.13 | 92.8 |
| 23.28 | ||||
| 40 | 16.7 | 25 | 24.92 | 101.6 |
| 25.88 | ||||
| 50 | 19.2 | 25 | 25.24 | 100.4 |
| 24.96 | ||||
| 60 | 21.4 | 25 | 23.69 | 94.0 |
| 23.31 |
3.4 Selection of water bath dissolution time
Three different types of Au ore reference materials (GBW07807a, GBW07809B and GBW07298a) were selected, and 4 portions of each were weighed. The closed water bath dissolution time was set to 0.5 h, 1 h, 2 h and 3 h, respectively, and the operation was carried out according to Sections 2.4 and 2.5. The results are shown in Fig. 2. When the closed dissolution time was not higher than 1 h, the Au recovery in all the samples was less than 80%; when the dissolution time reached 2 h, the Au recovery in all the samples was more than 95%. Through observations in daily routine analysis, the closed dissolution of 2 h can basically meet the decomposition of Au in common rock Au ore samples. The dissolution time determined by this method is 2 h.3.5 Selection of oscillation frequency and oscillation time
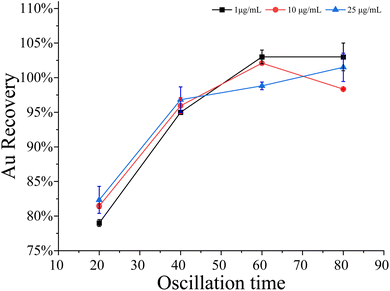
The oscillation frequency is an adjustable variable for the adsorption of gold by PUFs. Different oscillation frequencies may affect the adsorption of gold. According to the experimental method discussed in Section 2.5, the recovery rate test of gold standard solutions with concentrations of 1, 10, and 25 μg mL−1 was carried out with only the oscillation frequency changed. The oscillation frequencies were 140, 180, 220, and 260 times/min, respectively. The results are shown in Fig. 3. When the oscillation frequency is 140 times/min, the gold recovery rate is significantly low, which may be due to the fact that Au cannot be completely adsorbed; when the oscillation frequency is 180 times/min, the gold recovery rate is close to 100%, the frequency continues to increase, and the gold recovery rate does not change much. Through observation, it was found that when the oscillator frequency is 180 times/min, the solution in the bottle shakes exactly once, so the oscillation frequency of this method is 180 times/min.According to the experimental method discussed in Section 2.5, under the condition of oscillation frequency of 180 times/min, the oscillation time was set to 20, 40, 60 and 80 times/min to conduct the recovery test on gold standard solutions with concentrations of 1, 10 and 25 μg mL−1, and the results are shown in Fig. 4. The results show that when the oscillation time is 20 minutes, the gold recovery rate is less than 85%; when the oscillation time is 40 minutes or more, the gold recovery rate is above 95%. This method selects an oscillation time of not less than 40 minutes.
3.6 Selection of thiourea concentration
The selection of thiourea concentration in the desorption solution is an important factor to determine whether the gold desorption is complete. According to the experimental method, thiourea desorption solutions with different concentrations of 1, 3 and 10 g L−1 were selected to conduct parallel sample experiments on the national primary standard material GBW07809b of gold ore, and the results are shown in Table 4. The results show that when the thiourea concentration is 10 g L−1, the gold recovery rate is the best. Therefore, this method selects the thiourea concentration of the desorption solution to be 10 g L−1.| Thiourea concentration | GBW07809b recommended value (μg g−1) | Measured value (μg g−1) | Recovery (%) |
|---|---|---|---|
| 1 g L−1 | 10.4 | 8.05 | 77.4 |
| 7.95 | 76.4 | ||
| 3 g L−1 | 9.23 | 88.8 | |
| 9.23 | 88.8 | ||
| 10 g L−1 | 10.6 | 102.1 | |
| 10.4 | 100.0 |
3.7 Selection of release time and temperature
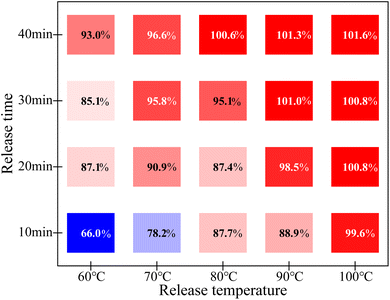
The PUF that adsorbs 100.0 μg of gold was selected and placed in a thiourea desorption solution with a concentration of 10 g L−1. The gold recovery rates under different desorption temperatures and times were compared, as shown in Fig. 5. The results indicate that different release temperatures and release times have a significant impact on the release of gold by thiourea. When the temperature is low, it takes enough time for the gold adsorbed by PUF to be completely released. When the temperature is high, the gold adsorbed by PUF can be released in a short period of time. Considering that it can completely release without causing boiling water vapor droplets to splash into the test tube and interfere with the gold test, this method selects a release temperature of 90 °C and a time greater than 20 minutes.3.8 Method detection limit and measurement range
According to the experimental methods in Sections 2.4 and 2.5, the blank solutions of 12 sample processes were measured. The LOD of gold was calculated as 0.03 μg g−1 by 3 times the standard deviation, and the LOQ of the determination was calculated as 0.1 μg g−1 by 3 times the standard deviation. In the actual sample enrichment, it was found that a large amount of slag would occupy the PUF adsorption sites, and there would also be competition in adsorption with iron, gallium, molybdenum, thallium, rhenium, etc. Considering comprehensively, under the experimental conditions, the optimal determination range of gold is 0.1–40 μg g−1.3.9 Method accuracy and precision
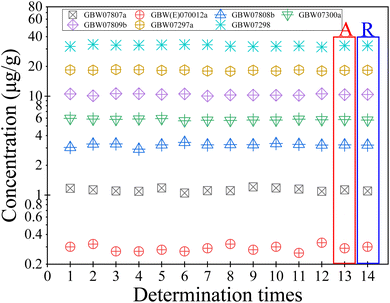
The 7 national standard materials of gold ores with different high and low contents (GBW07808b, GBW07809b, GBW07297a, GBW07298, GBW07300a, GBW(E)070012a and GBW07807a) were selected and analyzed in parallel 12 times according to the experimental method, and the relative standard deviation (RSD) and relative error (RE) of the Au determination results were calculated. The results are shown in Fig. 6. The results show that the determination results of Au in each standard material are consistent with the recommended value, RE ≤ 3.06%. The RSD of Au in each standard material is ≤8.35%, which meets the requirements of the Quality Management Specification for Laboratory Testing of Geological Mineral Resources (DZ/T0130-2006).3.10 Validation results from different laboratories
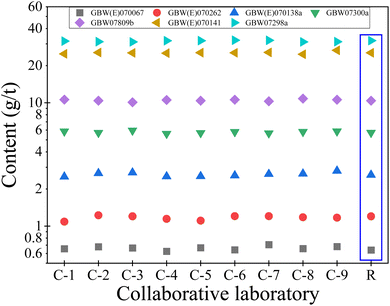
The 7 national standard materials of gold ores with different high and low contents (GBW(E)070067, GBW(E)070262, GBW(E)070138a, GBW07300a, GBW07809b, GBW(E)070141 and GBW07298a) were selected, and the method validation test was carried out in 9 laboratories according to the established method. The results are shown in Fig. 7. The results show that the determination results of Au of the 7 standard materials in the 9 laboratories are consistent with the recommended values, RE ≤ 10.55% and RSD ≤ 3.93%. It can be seen that the method established in this paper has also been used very well in different laboratories.3.11 Actual samples
The 85 rock gold ore samples were selected, and the gold content was determined according to the experimental method established in this paper. At the same time, use the activated carbon enrichment flame atomic absorption spectroscopy method in GB/T 20899.1-2019, Part 1 Determination of Gold Content, to determine the gold content of these 85 samples. Compare and verify the results of the two methods. The results are shown in Fig. 8. The results show that among the 85 samples, only 2 samples had a relative deviation of gold results exceeding the allowable limit, and the qualified rate was 97.65%. It can be seen that the determination of gold by the method established in this paper is consistent with the determination results of GB/T20899.1-2019, and the efficiency is increased by nearly 10 times. | ||
| Fig. 8 Comparison of the results of Au determination in actual rock Au ore samples using different methods. | ||
4 Conclusions
The optimal conditions for sample digestion, adsorption and desorption were determined through the selection of PUF pre-treatment methods, elimination of interfering elements, selection of aqua regia dosage, water bath dissolution time, oscillation frequency and time, thiourea concentration, release time, and temperature. A closed water bath aqua regia digestion-PUF enrichment thiourea elution-flame atomic absorption spectrometry method was established to determine the Au content in rock gold ores, by measuring the Au content of a series of national standard reference materials for rock gold ores and actual rock gold ore samples, and pushing the established method to other laboratories for verification. The results showed that key technical indicators such as detection limit, accuracy and precision of the established method meet the relevant national standards for rock gold ore determination, and meet the requirements of geological laboratories for the determination of Au content in rock gold ores. Compared with the methods such as fire assay and activated carbon adsorption, it is safer, more environmentally friendly, time-saving, labor-saving, easy to master, efficient and cost-effective.Data availability
All data generated or analyzed during this study are included in this published article.Author contributions
Shunxiang Wang: conceptualization, methodology, formal analysis, data curation, writing – original draft, visualization. Hongcheng Wang and Xiang Xia: methodology, software, formal analysis, investigation, data curation. Cang Gong: conceptualization, writing – review & editing, supervision. Jiufen Liu: conceptualization, investigation, supervision, writing – review & editing. Haichuan Lu: conceptualization, investigation, supervision, writing – review & editing. All authors have approved the final version of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was supported by the Geological Survey Project of China Geological Survey (DD20251135, DD20250209113, DD20250009, DD20243098 and DD20242769) and the Open Foundation of the Key Laboratory of Natural Resource Coupling Process and Effects (No. 2023KFKTB011).References
- C. I. Yeo, K. K. Ooi and E. R. T. Tiekink, Gold-Based Medicine: A Paradigm Shift in Anti-Cancer Therapy?, Molecules, 2018, 23(6), 1410, DOI:10.3390/molecules23061410.
- C. H. Wang, D. H. Wang, J. Xu, L. J. Ying, L. J. Liu and S. B. Liu, A Preliminary Review of Metallogenic Regularity of Gold Deposits in China, Acta Geol. Sin. (Engl. Ed.), 2015, 89(2), 632–651, DOI:10.1111/1755-6724.12452.
- S. Alim, J. Vejayan, M. M. Yusoff and A. K. M. Kafi, Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review, Biosens. Bioelectron., 2018, 121, 125–136, DOI:10.1016/j.bios.2018.08.051.
- N. Zari, J. Hassan, K. Tabar-Heydar and S. H. Ahmadi, Ion-association dispersive liquid-liquid microextraction of trace amount of gold in water samples and ore using Aliquat 336 prior to inductivity coupled plasma atomic emission spectrometry determination, J. Ind. Eng. Chem., 2020, 86, 47–52, DOI:10.1016/j.jiec.2017.01.038.
- A. V. Volzhenin, N. I. Petrova, N. S. Medvedev, D. S. Irisov and A. I. Saprykin, Determination of gold and palladium in rocks and ores by atomic absorption spectrometry using two-stage probe atomization, J. Anal. Chem., 2017, 72(2), 156–162, DOI:10.1134/s1061934817020150.
- W. Helmeczi, E. Helmeczi, L. A. Baker, Y. Wang and I. D. Brindle, Development of a general acid method for the digestion of gold ore samples together with a comparison of extraction solvents for gold and determination by microwave-induced plasma-atomic emission spectrometry (MIP-AES), J. Anal. At. Spectrom., 2018, 33(8), 1336–1344, 10.1039/c8ja00136g.
- L. Daniel, D. W. Laird and G. T. Hefter, Sodium peroxide fusion for reliable determination of gold in ores and metallurgical samples, Int. J. Miner. Process., 2017, 168, 35–39, DOI:10.1016/j.minpro.2017.09.001.
- M. Balcerzak, Sample digestion methods for the determination of traces of precious metals by spectrometric techniques, Anal. Sci., 2002, 18(7), 737–750, DOI:10.2116/analsci.18.737.
- V. Balaram, Recent Advances in the Determination of (PGE) in Exploration Studies - A Review, J. Geol. Soc. India, 2008, 72(5), 661–677 CAS.
- V. A. Shvetsov and N. V. Adel'shina, Improvements in the grinding of gold-bearing geochemical samples and their sampling for fire assay, J. Anal. Chem., 2004, 59(3), 202–205, DOI:10.1023/b:Janc.0000018958.46729.59.
- V. A. Shvetsov and N. V. Adel'shina, Improvement of fine-grained gold collection in fire assay analysis, J. Anal. Chem., 2004, 59(9), 834–840, DOI:10.1023/b:Janc.0000040697.33757.18.
- P. C. Santos-Munguía, F. Nava-Alonso, V. M. Rodríguez-Chávez and O. Alonso-González, Hidden gold in fire assay of gold telluride ores, Miner. Eng., 2019, 141, 105844, DOI:10.1016/j.mineng.2019.105844.
- N. Wang, X. D. Sun and D. Huo, Determination of Gold in Mineral Samples by Flame Atomic Absorption Spectrometry after the Separation and Preconcentration with Small Fire Assay, Spectrosc. Spectral Anal., 2019, 39(8), 2614–2617, DOI:10.3964/j.issn.1000-0593(2019)08-2614-04.
- A. Masasire, F. Rwere, P. Dzomba and M. Mupa, A new preconcentration technique for the determination of PGMs and gold by fire assay and ICP-OES, J. South. Afr. Inst. Min. Metall., 2022, 122(1), 29–36, DOI:10.17159/2411-9717/1638/2022.
- Y. H. Liu, B. Wan and D. S. Xue, Sample Digestion and Combined Preconcentration Methods for the Determination of Ultra-Low Gold Levels in Rocks, Molecules, 2019, 24(9), 1778, DOI:10.3390/molecules24091778.
- P. Ghosh, H. Mandal, B. Sirisha, U. Sen, S. Goswami and N. V. R. Kiran, A Modified Acid Digestion Method for Analysis of Gold in Geological Samples: A Comparative Study, Mapan - J. Metrol. Soc. India, 2019, 34(4), 551–558, DOI:10.1007/s12647-019-00326-8.
- The Study and Application of Hydrometallurgical Gold Leaching in the Analysis of Refractory Precious Metals, International Conference on Environmental and Energy Engineering (IC3E), ed. M. Yang, X. Geng, Y. L. Wang and D. X. Li, Suzhou, Peoples R China2017, 2017 Search PubMed.
- Y. Wang, L. A. Baker and I. D. Brindle, Determination of gold and silver in geological samples by focused infrared digestion: a re-investigation of aqua regia digestion, Talanta, 2016, 148, 419–426, DOI:10.1016/j.talanta.2015.11.019.
- Y. R. Hou, J. L. Lu, M. Li, Q. Q. Wei, Y. C. Fan and Y. Z. Wang, A new desorption method of polyurethane foam for the determination of gold and a comparative study on four desorption methods based on meta-analysis, RSC Adv., 2023, 13(18), 12355–12360, 10.1039/d3ra00118k.
- K. Shao, J. X. Fan, H. Zheng and H. Yu, Determination of gold in ore samples by flame atomic absorption spectrometry of sealed dissolution after adsorption using polyurethane foam, Multipurp. Util. Miner. Resour., 2023,(04), 182–187 Search PubMed.
- J. Liu, H. L. Yan, W. L. Lian, H. F. Chen, L. Wang and Y. H. Yu, Determination of gold, silver, platinum and palladium in geological samples by inductively coupled plasma mass spectrometry with sealed dissolution, Metall. Anal., 2016, 36(07), 25–33, DOI:10.13228/j.boyuan.issn1000-7571.009902.
- T. Itagaki, T. Ashino and K. Takada, Determination of trace amounts of gold and silver in high-purity iron and steel by electrothermal atomic absorption spectrometry after reductive coprecipitation, Fresenius' J. Anal. Chem., 2000, 368(4), 344–349, DOI:10.1007/s002160000455.
- A. U. Oya, Y. G. Zeynep, D. Sabahattin, K. Y. Ece and A. Adnan, A novel ligand for cloud point extraction to determine gold content in ore samples, Environ. Chem. Lett., 2014, 12(3), 449–453, DOI:10.1007/s10311-014-0471-5.
- J. Hassan, M. Shamsipur and M. H. Karbasi, Single granular activated carbon microextraction and graphite furnace atomic absorption spectrometry determination for trace amount of gold in aqueous and geological samples, Microchem. J., 2011, 99(1), 93–96, DOI:10.1016/j.microc.2011.04.003.
- D. Afzali, A. Mostafavi and M. Mirzaei, Preconcentration of gold ions from water samples by modified organo-nanoclay sorbent prior to flame atomic absorption spectrometry determination, J. Hazard. Mater., 2010, 181(1–3), 957–961, DOI:10.1016/j.jhazmat.2010.05.106.
- A. S. Bashammakh, S. O. Bahaffi, F. M. Al-Shareef and M. S. El-Shahawi, Development of an Analytical Method for Trace Gold in Aqueous Solution Using Polyurethane Foam Sorbents: Kinetic and Thermodynamic Characteristic of Gold(III) Sorption, Anal. Sci., 2009, 25(3), 413–418, DOI:10.2116/analsci.25.413.
- R. Al-Merey, Z. Hariri and J. Abu Hilal, Selective separation of gold from iron ore samples using ion exchange resin, Microchem. J., 2003, 75(3), 169–177, DOI:10.1016/s0026-265x(03)00092-4.
- P. H. He, Y. Rong and Z. X. Gong, Quantification of trace gold in ores by flame atomic absorption spectrometry with P350 micro chromatographic column online separation and enrichment, Rock Miner. Anal., 2011, 30(04), 457–460, DOI:10.15898/j.cnki.11-2131/td.2011.04.009.
- F. Xiao, T. Y. Zhang, L. P. Zhang, L. Liu, X. J. Mao and W. S. Ni, Ultratrace gold in high-purity graphite by high-resolution continuous light source graphite furnace atomic absorption spectrometry with hanging droplet microextraction, Rock Miner. Anal., 2024, 43(05), 734–743, DOI:10.15898/j.ykcs.202406210137.
- T. Yu, K. Shao and J. K. Li, Determination of gold in geological sample by inductively coupled plasma mass spectrometry with cloud point extraction, Metall. Anal., 2015, 35(03), 32–36, DOI:10.13228/j.boyuan.issn1000-7571.009493.
- L. L. Chen, J. W. Wang, J. F. Bai, P. C. W ang and X. Zhao, Experimental study of gold leaching from waste IC with thiosulfate, Chem. Ind. Eng. Prog., 2015, 34(03), 884–890, DOI:10.16085/j.issn.1000-6613.2015.03.046.
- M. J. Zan, Determination of gold content in copper tailings by ion exchange resin separation enrichment-spectrophotometry, Shanxi Chem. Ind., 2021, 41(02), 49–51, DOI:10.16525/j.cnki.cn14-1109/tq.2021.02.15.
- Y. P. Hao, J. Y. Shi and Y. M. Fan, Determination of trace gold in geological samples by spectrophotometry after cloud point extraction, Chem. Ind. Times, 2022, 36(10), 17–19, DOI:10.16597/j.cnki.issn.1002-154x.2022.10.004.
- X. D. Tang, B. Li, J. L. Lu, H. Y. Liu and Y. Y. Zhao, Gold determination in soil by ICP-MS: comparison of sample pretreatment methods, J. Anal. Sci. Technol., 2020, 11(1), 45, DOI:10.1186/s40543-020-00245-3.
- J. W. Chen, Y. M. Li, S. X. Song, J. T. Song and L. Chu, Determination of gold in gold ores by inductively coupled plasma-optical emission spectrometry with carbon-loaded foam plastic adsorption, Rock Miner. Anal., 2015, 34(03), 314–318, DOI:10.15898/j.cnki.11-2131/td.2015.03.009.
- J. Y. Li, Determination of low-grade associated gold in minerals by flame atomic absorption spectrometry with foam-enriched, Chin. J. Inorg. Anal. Chem., 2021, 11(03), 60–65 CAS.
- L. Xie, Determination of gold in tungsten-bearing molybdenum ores by foam plastics-AAS, Gold, 2015, 36(05), 76–79 CAS.
- Z. G. Zhang, K. Liu, H. Chen, R. Feng, J. Huang and J. J. Wei, et al., Determination of gold in antimony ores by hydroquinone volumetric method with antimony tartrate as complexing and masking agent, Rock Miner. Anal., 2015, 34(04), 454–458, DOI:10.15898/j.cnki.11-2131/td.2015.04.013.
| This journal is © The Royal Society of Chemistry 2025 |