Open Access Article
Open Access ArticleSynergetic interplay of a nitrogen- and sulfur-rich copper bi-linker 2D cubic-layered MOF composite with MXene for improved hybrid supercapacitor application†
Maham Saeeda,
Shahzad Sharif *a,
Javed Hussain Shah
*a,
Javed Hussain Shah a,
Tayyaba Tur Rehman Afzala,
Muhammad Shahbaz
a,
Tayyaba Tur Rehman Afzala,
Muhammad Shahbaz a,
Azhar Mehmood Shehzada,
Ayesha Shahzada,
Onur Şahinb and
Sundas Shahzada
a,
Azhar Mehmood Shehzada,
Ayesha Shahzada,
Onur Şahinb and
Sundas Shahzada
aMaterials Chemistry Laboratory, Institute of Chemical Sciences, Government College University Lahore, 54000, Pakistan. E-mail: mssharif@gcu.edu.pk
bDepartment of Occupational Health & Safety, Faculty of Health Sciences, Sinop University, TR-57000, Sinop, Turkey
First published on 21st May 2025
Abstract
To combine the properties of batteries and capacitors in a single hybrid device, metal–organic frameworks have emerged as promising materials. In this study, by incorporation of a heteroatom (N, O and S)-based bi-linker, a novel copper-based two-dimensional metal–organic framework (Cu-SIP-MOF), derived from 5-sulfoisophathalic acid monosodium salt (SIP sodium salt) and 4,4-bipyridine was synthesized and characterized using different techniques. The conductivity of the extended 2D MOF was attributed to π-d orbital contribution, which was further enhanced by fabricating its composite with MXene. The synthesized MOFs and its composites were electrochemically evaluated using different electroanalytical techniques such as cyclic voltammetry, galvanostatic charge–discharge and electrochemical impedance spectroscopy via three-electrode assembly. The composites of Cu-SIP-MOF with MXene (CM-200) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio possessed the highest specific capacity of 683.69 C g−1, highlighting the potential for their practical implementation in asymmetric hybrid devices. The material demonstrated an energy density and a power density of 62 W h kg−1 and 2330.4 W kg−1, respectively. It also expressed 98.3% coulombic efficiency after 5000 galvanic charge–discharge cycles. The significant values of specific capacity, energy density and power density of CM-200 make it a promising electrode material for a futuristic hybrid device.
2 ratio possessed the highest specific capacity of 683.69 C g−1, highlighting the potential for their practical implementation in asymmetric hybrid devices. The material demonstrated an energy density and a power density of 62 W h kg−1 and 2330.4 W kg−1, respectively. It also expressed 98.3% coulombic efficiency after 5000 galvanic charge–discharge cycles. The significant values of specific capacity, energy density and power density of CM-200 make it a promising electrode material for a futuristic hybrid device.
1. Introduction
To cater to the growing demand for stable energy sources, scientists are seeking to design potent and advanced energy storage devices.1–4 Nowadays, batteries and supercapacitors are frequently used in various electronic devices, such as smartphones, laptops and home appliances.5–7 Owing to the huge number of faradaic reactions occurring at the electrode, batteries show greater energy density; however, the extraction of high power density from them remains a challenging task so far.8–10 In contrast, electrochemical capacitors exhibit superior cyclability and power density but have limited energy density compared to traditional batteries.11–13 Hence, there is a dire need to design a system that exhibits the characteristics of both batteries and supercapacitors. A hybrid supercapacitor has one electrode with pseudocapacitive nature, responsible for high energy density, and the other electrode possesses capacitive nature with long duration cyclability.14,15 For efficient electrochemical energy storage devices, the quality of the electrode materials should be very promising. The higher capacitance of the electric double-layer capacitor (EDLC) is due to charge separation between the electrolyte and electrode interface.16 Many materials such as metal sulfides, oxides and phosphides are considered good for pseudocapacitive and battery-grade electrode materials. However, the development of new porous materials with rich electrochemical properties is a prerequisite to meet the requirements of advanced storage devices.17The intrinsic characteristics of metal–organic frameworks (MOFs), such as shape, porous structure, greater surface area and durability, can help in customizing the structure of electrode materials to optimize electrochemical performance.18–21 The highly porous structure of MOFs can be shaped by combining specific ratios of different metals and organic linkers.18 Moreover, the presence of conjugation in the linkers constituting MOFs assists in electron transfer, while the rich porosity facilitates ion diffusion to promote redox reactions at the redox-active metal sites, resulting in improved electrochemical performance of supercapacitors. However, the high stability and conductivity of MOFs have remained a challenging task for researchers.
Hydrogen bonding occurs in MOFs because of the presence of hydrophilic centers in organic ligands and metals. Various functional groups have been employed to enhance the structural stability of MOFs. The sulfonic acid ligand possesses two hydroxyl groups that can coordinate with the metal and have multiple sites to form a stable chelate structure.22,23 Moreover, the presence of hetero atoms (N, O, S) in the aromatic linkers plays a vital role in the enhancement of surface properties like wettability for the efficient faradaic reactions with electrolyte ions. Because of lone pair electrons, they can cause extended pi-conjugation for better overlap of orbitals and can also donate electrons to metal nodes, thus increasing charge transportation and conductivity. Moreover, heteroatom-based linkers maintain structural stability after long cyclability, thereby sustaining their remarkable electrochemical performance.24–26 Pyridine 2, 4, 6-tricarboxylic acid and 2, 6-pyridinedicarboxylic acid ligands containing N and O were used to construct cerium- and copper-based MOFs, respectively, which resulted in better charge storage and high capacitance.27 In addition, doping of N, S and P in MOFs features a larger number of electrons/holes, causing a significant increase in conductivity.28 5-Sulfoisophathalic acid monosodium linkers contain phthalate groups having a high chance of binding with metals to form complexes, especially in the presence of N-donor-based co-ligands like pyridine. The hydrophilic nature of the sulfo-group facilitates hydrogen bonding to stabilize complexes. Among transition metals, copper is considered one of the most electron-rich and efficient redox-active agents.29 Thus, the idea of utilizing SIP as well as a co-ligand such as bipyridine with copper metal to construct MOFs is very interesting for tuning the electrochemical properties of supercapacitors. Electrical conductivity in MOFs can be achieved by (1) the involvement of metal and ligand orbitals, (2) participation of π–d conjugation, (3) contribution of π–π stacking of organic ligands. 2D MOFs responsible for charge delocalization as well as π–π interaction promote layer stacking, which ultimately enhances the mobility and conductivity in the form of one-dimensional (1D) channels. The fascinating characteristics such as tunable topologies, high conductivity and porosity enable 2D MOFs to be promising candidates for excellent electrochemical energy applications.30–33
Although MOFs exhibit properties such as tunable pore size and high surface area but their lower conductivity may reduce their use in energy storage devices. For this purpose, conductive materials like graphene oxide (GO), reduced graphene oxide (rGO), carbon nanotubes (CNT) and MXenes have been widely used in the synthesis of MOF composites to enhance their electrochemical performance in energy storage devices, particularly in hybrid supercapacitors.34–36 Singh et al. (2024) synthesized an MOF composite (MC) by integrating CNTs with Cu-MOF to enhance the conductivity of the MOF. It was found that the formation of a solid composite structure is possible by the coordination between Cu(II) of the MOF and the carboxylate groups of the CNTs. However, they also faced challenges in obtaining robust interfacial bonds and even dispersion between MOFs and CNTs, which are crucial elements for a dependable electrochemical performance. However, the results revealed a specific capacitance of 348.6 F g−1 at 1 A g−1, energy density and power density of 27.7 W h kg−1 and 1640 W kg−1.37 Singh et al. (2021) manufactured a new Cu-based MOF composite with graphene oxide. The composite exhibits a specific capacitance of 366.6 F g−1 at 1 A g−1. The device has an energy density of 57.2 W h kg−1 and power density of 4.38 kW kg−1.38 Azadfalah et al. (2019) combined the synergic effects of Cu-MOF with GO by synthesizing Cu-MOF/GO composites that exhibited 34.5 W h kg−1, 1350 W kg−1 energy density and power density, respectively, at 0.5 A g−1.39 MXene is a class of 2D nitrides and carbides that is used as a conductive material for the synthesis of MOF composites. It exhibits distinct features that make it superior to other conductive materials, such as metallic conductivity, easy functionalization and hydrophilic nature.40 In the MXene structure, the transition metal plays a vital role in the conductivity by providing conductive channels for electron transport. The other contributing factor to conductivity is the morphology of the MXene. The 2D structure of MXene facilitates in free transportation of electrons with scattering. Conductivity can also be controlled by changeable functional groups like hydroxyl (–OH) and oxygen (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) on the terminations of MXene.41 Thus, the incorporation of MXene can control the resistivity and conductivity of the electrode, thereby affecting the thermal stability of the battery.42 Titanium carbide-based MXenes (Ti3C2Tx), display high conductivity because of their exceptional structural and electronic properties. The metallic conductivity owing to delocalized electrons within the 2D layers enables effective charge transport. Additionally, the functional groups (Tx) on the surface affect the conductivity because, by varying the surface terminations, the density of electronic states near the Fermi level changes, thus affecting electrical properties.43 Hassan et al. (2024) explored the potential of a Zn-based MOF (MOF-5) composite with MXene (V2CTx), which showed a significant specific capacity of 961 C g−1 in three electrode assembly and 234 C g−1 in two electrode assembly. The hybrid assembly of MOF-5/V2CTx//AC, demonstrated 48.75 W h kg−1 as energy density and 920 W kg−1 as power density.44 Hassan et al. (2024) also explored another Cu-BTC-based MOF composite with nitrogen-doped titanium-based MXene (Ti3C2Tx) which exhibited energy density of 65.23 W h kg−1 and power density of 923 W kg−1. The composite showed 99% capacity retention after 8000 cycles. However, the MXene content ratio for composite preparation is very crucial for optimal results such as capacitance and cyclability. An appropriate amount of MXene in MOFs for composite synthesis prevents the re-stacking of MXene layers, which can reduce the number of electroactive sites.45–47
O) on the terminations of MXene.41 Thus, the incorporation of MXene can control the resistivity and conductivity of the electrode, thereby affecting the thermal stability of the battery.42 Titanium carbide-based MXenes (Ti3C2Tx), display high conductivity because of their exceptional structural and electronic properties. The metallic conductivity owing to delocalized electrons within the 2D layers enables effective charge transport. Additionally, the functional groups (Tx) on the surface affect the conductivity because, by varying the surface terminations, the density of electronic states near the Fermi level changes, thus affecting electrical properties.43 Hassan et al. (2024) explored the potential of a Zn-based MOF (MOF-5) composite with MXene (V2CTx), which showed a significant specific capacity of 961 C g−1 in three electrode assembly and 234 C g−1 in two electrode assembly. The hybrid assembly of MOF-5/V2CTx//AC, demonstrated 48.75 W h kg−1 as energy density and 920 W kg−1 as power density.44 Hassan et al. (2024) also explored another Cu-BTC-based MOF composite with nitrogen-doped titanium-based MXene (Ti3C2Tx) which exhibited energy density of 65.23 W h kg−1 and power density of 923 W kg−1. The composite showed 99% capacity retention after 8000 cycles. However, the MXene content ratio for composite preparation is very crucial for optimal results such as capacitance and cyclability. An appropriate amount of MXene in MOFs for composite synthesis prevents the re-stacking of MXene layers, which can reduce the number of electroactive sites.45–47
In this study, we report Cu-SIP-2D-MOFs derived from 5-sulfoisophathalic acid monosodium, 4,4-bipyridine and copper metal and their composites with MXene. Structural and morphological characterization was carried out with the help of thermogravimetric analysis (TGA), single-crystal X-ray diffraction (XRD), elemental analysis and Fourier-transform infrared (FT-IR) spectroscopy. The electrochemical performance of Cu-SIP-MOF and its composites CM-100, CM-200 and CM-300 was also determined by cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) and electrochemical impedance spectroscopy (EIS) via three electrode assembly, and the practical implementation of CM-200 in an asymmetric hybrid device was explored. In contrast to traditional electrode materials, the CM-200 offers significant potential for further exploration as an energy storage material. The novelty of this study revolves around the incorporation of N, S, and O (heteroatoms) in MOFs through careful selection of bi-linkers for tuning the pore size, porosity of MOFs and the integration of MXene to improve the performance of the electrode material.
2. Experimental
2.1 Materials and methods
Copper chloride dehydrate (CuCl2·2H2O) was purchased from Alfa Acer Company, USA. 4,4-bipyridine, 5-sulfoisophthalic acid monosodium salt and MXene (Ti3C2) with 99.9% purity were provided by Merck Chemical Company, Germany. Distilled H2O, ethanol, and other solvents were used as received. Overall, high analytical-grade chemicals were utilized, which are also easily accessible in the market.2.2 Equipment
The FTIR spectrum ranged from 4000 to 400 cm−1, comparing the spectra of the complex and ligand using an infrared spectrophotometer made by IR Spirit, SHIMADZU, Japan. Elementar, Vario Micro Cube elemental analyzer was employed to check the elemental % age results. Thermal analysis was conducted on TA Instrument TGA, SDT Q 600 at 10 °C min−1 ramp-rate. Scanning electron microscope (SEM) of Cu-SIP-MOF was performed using a JEOLJSM-6480 SEM.2.3 Synthesis of Cu-SIP-MOF
116 mg of copper chloride dihydrate (0.68 mmol), 91 mg of SIP (0.34 mmol) and 8 mg of co-ligand, 4,4-bipyridine (0.05 mmol), were separately sonicated for 1 minute in 3 mL, 4 mL and 3 mL of distilled water, respectively. Solutions of copper chloride dihydrate and 4,4-bipyridine were added to the SIP solution. The resultant mixture was further sonicated (MSE Sanyo, Soni prep 150) for 15 minutes at a frequency of 23 kHz and an amplitude of 15 microns. The obtained solution was filtered and stored at room temperature. After 10 days, dark blue crystals appeared. Yield ca. 69%. Elemental analysis (%) calc. for C108H114Cu3N12O60S6 (2923.09): N, 5.75; C, 44.34; H, 3.90; S, 6.57; found: N, 5.61; C, 44.58; H, 4.12; S, 6.89. The synthesis scheme is illustrated in Fig. 1.2.4 Fabrication of the electrode
Owing to its good conductivity, nickel foam (NF) was used as a current collector in electrochemical cells. To remove any oxide present in the NF (1.5 × 1.5 cm2), it was washed with hydrochloric acid, ethanol and acetone, followed by drying in an oven. A normal consistency-based slurry was formed by mixing Cu-SIP-MOF (80 wt%), acetylene black (10 wt%), and polyvinylidene fluoride (10 wt%) in 50 μl of N-methyl-2-pyrrolidone solvent. Cu-SIP-MOF was also mixed with MXene in altered ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and 1
2, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to prepare composites, CM-100, CM-200 and CM-300, respectively. After 12 h of thorough mixing through magnetic stirring, the MOF and its composites were deposited separately onto the nickel foam via the drop casting technique and dried at 60 °C for 4 h. To evaluate the performance of the best sample in the hybrid supercapacitor, an activated carbon (AC) slurry was prepared using the same material ratio and procedure. Eqn (1) was used to maintain the charge-to-mass ratio so that the maximum performance of the hybrid supercapacitor could be attained.
3 to prepare composites, CM-100, CM-200 and CM-300, respectively. After 12 h of thorough mixing through magnetic stirring, the MOF and its composites were deposited separately onto the nickel foam via the drop casting technique and dried at 60 °C for 4 h. To evaluate the performance of the best sample in the hybrid supercapacitor, an activated carbon (AC) slurry was prepared using the same material ratio and procedure. Eqn (1) was used to maintain the charge-to-mass ratio so that the maximum performance of the hybrid supercapacitor could be attained.
 | (1) |
3. Characterization techniques
3.1 FTIR, TGA and SEM analysis
FTIR, TGA further confirmed the metal–ligand binding. SEM analysis confirmed the crystalline morphology of Cu-SIP-MOF. Details are provided in the ESI (Fig. S1–S4).†3.2 Single crystal structure of Cu-SIP-MOF
A single-crystal X-ray analysis demonstrated that the complex Cu-SIP-2D-MOF is a 2D coordination polymer. The asymmetric unit of the complex Cu-SIP-2D-MOF consists of one half and one full Cu(II) ions, two full and two half 4,4-bipyridineligands, three 5-sulfoisophthalic acid ligands and nine water molecules (Fig. 2(A)). The Cu1 atom is coordinated by four nitrogen atoms [Cu1–N bond lengths ranged between 2.009(2)–0.046(2) Å] from 4,4-bipyridine ligands and two oxygen atoms [Cu1–O1 = 2.505(2) Å and Cu1–O8 = 2.687(2) Å] from 5-sulfoisophthalic acid ligands, giving an octahedral coordination geometry, while the Cu2 atom is coordinated by four nitrogen atoms[Cu1–N3 = 2.041(2) Å, Cu1–N4 = 2.000(3) Å and Cu1–N5iv = 2.014(3) Å] from 4,4-bipyridine ligands, giving a square planar coordination geometry [(iv) x, y + 1, z]. The Cu(II) ions were bridged by 4,4-bipyridine ligands to form rectangular [Cu4(bpy)4] clusters, with the Cu⋯Cu separations are 11.065 and 11.180 Å. The combination of [Cu4(bpy)4] clusters produces a 2-D coordination polymer moving parallel to the bc plane (Fig. 2 (B)). Adjacent 2D coordination polymers were then connected by O–H⋯O, O–H⋯S and C–H⋯O hydrogen bonds (Table S3†), generating a 3D supramolecular network (Fig. S5†). The simulated powder XRD diffractogram is shown in Fig. S6.† The improved electron delocalization in 2D MOFs is attributed to the combination of metal node d orbitals and conjugated π-orbitals of the ligand.32,33 A polyhedral view of a 2D MOF along the 100-direction showing cubic-shaped pores of 1.2 nm and metal-to-ligand charge transfer in the 2D MOF is presented in Fig. 3. | ||
| Fig. 2 (A) Molecular structure of Cu-SIP-MOF. (B) Ball and stick overview of an infinite 2D coordination polymer in Cu-SIP-MOF. | ||
 | ||
| Fig. 3 Infinite polyhedral view of 2D MOF grown along the 100-direction showing cubic-shaped pores and a schematic view of the charge transport pathway through metal-to-ligand bonds. | ||
4. Results and discussion
4.1 Half-cell electrochemical study
For the electrochemical study, an Origalys electrochemical workstation (including OrigaFlex-OGFO1A and OrigaFlex-OGFEIS) was used.4.2 Hybrid supercapacitor assembly
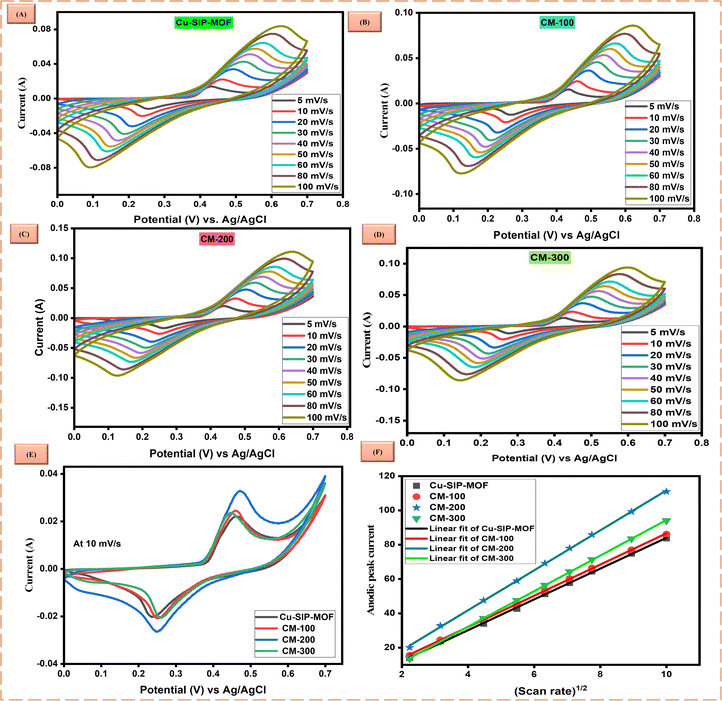
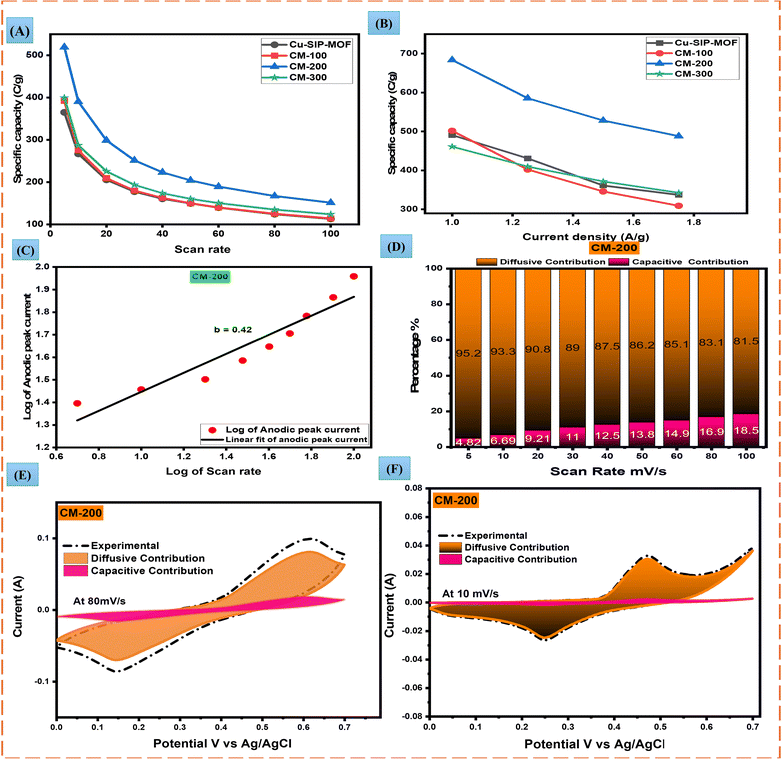
To understand the practicability of CM-200 for battery–supercapacitors hybrid, a device was constructed using activated carbon (CM-200//AC) in 1 M KOH as the electrolyte, as shown in Fig. 7(A). The electrochemical testing was performed, and Fig. 7(B) shows a CV comparison for AC and CM-200 in a two-electrode assembly.CV was performed at various scan rates (10, 30, 50 and 80 mV s−1) by adjusting a potential window of 0–1.7 V. Results depicted both pseudo-capacitive and EDLC phenomenon exhibited in the asymmetric device, as shown in Fig. 7(C). The value of the regression parameter was 0.99, which confirmed the reversibility of the current to scan rate in the device (Fig. S9(A)†). The b-value of 0.62 for the anodic peak (Fig. S9(B)†) also indicates capacitive-predominance in the device. Dunn's method also confirmed the pseudocapacitive predominance because of the higher capacitive contribution of the current. Fig. 7(D) illustrates the continuous increase in capacitive contribution with increasing scan rate. At 10 and 80 mV s−1 scan rates, the diffusion and capacitive contributions of current are shown for the experimental value in Fig. 7(E) and (F). An increase in the capacitive contribution is attributed to the occurrence of the EDLC phenomenon at higher scan rates, as less time is required for the ions to intercalate.
GCD was performed at various current densities (1–6 Ag−1) with a potential window of 0–1.7 V (Fig. 8(A)). As the current density increased, the discharging time decreased. The CM-200//AC electrode material showed a high energy density of 62 W h kg−1 and a power density of 2330.4 W kg−1 using eqn (S9) and (S10)† are also shown in Fig. 8(B). The device demonstrated specific capacity and capacitance of 307 C g−1 and 212 F g−1, respectively, as depicted in Fig. 8(C) and (D). Through stability tests, the hybrid material was found to exhibit high stability and efficiency. After 5000 GCD cycles, the coulombic efficiency was maintained at 98.3% (Fig. 8(F)). The conductivity of the device was assessed by EIS in the frequency range of 0.1 to 100 kHz. Through circuit fitting on the Nyquist plot, the complete circuit of the electrode was obtained. After stability, the ESR value was 1.6539 Ω, whereas the value of Rct came out as 3.6142 Ω. A Nyquist plot with a smaller semicircle shows low resistance for charge transfer and high conductivity. The straight line represents the Warburg impedance (Zw), which is related to the mass transfer resistance (Fig. 8 (E)). The results of the CM-200//AC electrode are promising for electrode usage in forthcoming hybrid supercapacitors.
5. Conclusions
In summary, a new copper(II) MOF, Cu–SIP-MOF, was synthesized, characterized and evaluated for electrochemical performance with its composites with MXene (CM-100, CM-200 and CM-300). The electrochemical properties of the fabricated electrode materials were investigated through a three-electrode assembly process using CV, GCD and EIS. CM-200 was evaluated as the best sample because it exhibited a specific capacity of 683.6 C g−1 through GCD. Due to the significant results of CM-200, a hybrid supercapacitor assembly was constructed as CM-200//AC, which showed energy and power density of 62 W h kg−1 and 2330.4 W kg−1, respectively. The capacitive and diffusive contributions of the current were determined by Dunn's method. This study provides a valuable perspective that using N, S, and O heteroatom-based bi-linkers, the pore size, shape and porosity of MOFs can be controlled. 2D MOF facilitates the charge transport and optimization in the ratio of conductive materials, resulting in improved characteristics for utilization in advanced battery, supercapacitor hybrid devices. A comparison of the MOF synthesized in this study with other MOFs using a Ragone plot is presented in Fig. 9 and Table S4.†Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Maham: writing original draft, Shahzad: supervision, Javed: characterization, Tayyaba: investigation, Muhammad: formal analysis, Azhar: visualization, Ayesha: validation, Onur: XRD software, Sundas: methodology.Conflicts of interest
All authors declare that there are no competing interests.Acknowledgements
We gratefully acknowledge financial assistance from the Higher Education Commission of Pakistan, HEC-NRPU project no. 20-17612/NRPU/R&D/HEC/2021, and the Office of Research Innovation & Commercialization (ORIC), Govt. College University Lahore, Project No. 378/ORIC/24.References
- H. D. Yoo, E. Markevich, G. Salitra, D. Sharon and D. Aurbach, On the challenge of developing advanced technologies for electrochemical energy storage and conversion, Mater. Today, 2014, 17(3), 110–121 CrossRef CAS.
- D. Larcher and J.-M. Tarascon, Towards greener and more sustainable batteries for electrical energy storage, Nat. Chem., 2015, 7(1), 19–29 CrossRef CAS PubMed.
- M. S. Javed, et al., The emergence of 2D MXenes based Zn-ion batteries: recent development and prospects, Small, 2022, 18(26), 2201989 CrossRef CAS PubMed.
- S. Dai, et al., In situ Raman study of nickel bicarbonate for high-performance energy storage device, Nano Energy, 2019, 64, 103919 CrossRef CAS.
- M. Z. Iqbal, U. Aziz, M. W. Khan, S. Siddique, M. Alzaid and S. Aftab, Strategies to enhance the electrochemical performance of strontium-based electrode materials for battery-supercapacitor applications, J. Electroanal. Chem., 2022, 924, 116868 CrossRef CAS.
- M. S. Javed, et al., An ultra-high energy density flexible asymmetric supercapacitor based on hierarchical fabric decorated with 2D bimetallic oxide nanosheets and MOF-derived porous carbon polyhedra, J. Mater. Chem. A, 2019, 7(3), 946–957 RSC.
- M. Zahir Iqbal, N. Amjad and M. Waqas Khan, Metal-organic-framework as novel electrode materials for hybrid battery-supercapacitor applications, ChemElectroChem, 2022, 9(17), e202200036 CrossRef CAS.
- L. Yang, et al., Anthraquinone-modified nitrogen-doped graphene aerogel for boosting energy density of supercapacitors by self-matching of capacity, Electrochim. Acta, 2021, 393, 139057 CrossRef CAS.
- S. Sagadevan et al., Fundamental electrochemical energy storage systems, in Advances in Supercapacitor and Supercapattery, Elsevier, 2021, pp. 27–43 Search PubMed.
- M. S. Javed, H. Lei, Z. Wang, B. Liu, X. Cai and W. Mai, 2D V2O5 nanosheets as a binder-free high-energy cathode for ultrafast aqueous and flexible Zn-ion batteries, Nano Energy, 2020, 70, 104573 CrossRef CAS.
- Y. Ma, et al., Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors, Adv. Compos. Hybrid Mater., 2021, 1–19 Search PubMed.
- S. Manoharan, K. Krishnamoorthy, A. Sathyaseelan and S.-J. Kim, High-power graphene supercapacitors for the effective storage of regenerative energy during the braking and deceleration process in electric vehicles, Mater. Chem. Front., 2021, 5(16), 6200–6211 RSC.
- M. S. Javed, et al., High performance solid state flexible supercapacitor based on molybdenum sulfide hierarchical nanospheres, J. Power Sources, 2015, 285, 63–69 CrossRef CAS.
- M. Z. Iqbal and U. Aziz, Supercapattery: Merging of battery-supercapacitor electrodes for hybrid energy storage devices, J. Energy Storage, 2022, 46, 103823 CrossRef.
- W. J. Basirun, I. M. Saeed, M. S. Rahman and S. A. Mazari, Nickel oxides/hydroxides-graphene as hybrid supercapattery nanocomposites for advanced charge storage materials–a review, Crit. Rev. Solid State Mater. Sci., 2021, 46(6), 553–586 CrossRef CAS.
- E. Dhandapani, S. Thangarasu, S. Ramesh, K. Ramesh, R. Vasudevan and N. Duraisamy, Recent development and prospective of carbonaceous material, conducting polymer and their composite electrode materials for supercapacitor—A review, J. Energy Storage, 2022, 52, 104937 CrossRef.
- M. M. Faisal, S. R. Ali, K. C. Sanal, M. W. Iqbal, M. Z. Iqbal and A. Saeed, Highly porous terpolymer-MOF composite electrode material for high performance supercapattery devices, J. Electroanal. Chem., 2021, 893, 115321 CrossRef CAS.
- C. Liu, Y. Bai, W. Li, F. Yang, G. Zhang and H. Pang, In situ growth of three-dimensional MXene/metal–organic framework composites for high-performance supercapacitors, Angew. Chem., Int. Ed., 2022, 61(11), e202116282 CrossRef CAS PubMed.
- J. Pokharel, et al., MOF-derived hierarchical carbon network as an extremely-high-performance supercapacitor electrode, Electrochim. Acta, 2021, 394, 139058 CrossRef CAS.
- S. Chuhadiya, D. Suthar, S. L. Patel and M. S. Dhaka, Metal organic frameworks as hybrid porous materials for energy storage and conversion devices: A review, Coord. Chem. Rev., 2021, 446, 214115 CrossRef CAS.
- T. Chen, et al., In situ synthesis of MOF-74 family for high areal energy density of aqueous nickel–zinc batteries, Adv. Mater., 2022, 34(30), 2201779 CrossRef CAS PubMed.
- X. Zhao, K. Tao and L. Han, Self-supported metal–organic framework-based nanostructures as binder-free electrodes for supercapacitors, Nanoscale, 2022, 14(6), 2155–2166 RSC.
- Q. Bi, Q. Ma, K. Tao and L. Han, Hierarchical core–shell 2D MOF nanosheet hybrid arrays for high-performance hybrid supercapacitors, Dalton Trans., 2021, 50(23), 8179–8188 RSC.
- X. Yang, et al., Tailoring ion-accessible pores of robust nitrogen heteroatomic carbon nanoparticles for high-capacity and long-life Zn-ion storage, J. Energy Storage, 2024, 104, 114509 CrossRef.
- J. H. Shah, et al., Redox active cobalt based bi-linker metal organic frameworks derived from 5-sulfoisopthalic acid and 4, 4-bipyridine for supercapacitor, Mater. Res. Bull., 2025, 181, 113123 CrossRef CAS.
- M. Shahbaz, S. Sharif, A. Shahzad, Z. S. Şahin, B. Riaz and S. Shahzad, Enhanced electrochemical performance of cerium-based metal organic frameworks derived from pyridine-2, 4, 6-tricarboxylic acid for energy storage devices, J. Energy Storage, 2024, 88, 111463 CrossRef.
- J. H. Shah, et al., Electrochemical investigation of copper 1D conductive polymer for hybrid supercapacitor applications, J. Energy Storage, 2024, 102, 114058 CrossRef.
- Y. Peng, Y. Bai, C. Liu, S. Cao, Q. Kong and H. Pang, Applications of metal–organic framework-derived N, P, S doped materials in electrochemical energy conversion and storage, Coord. Chem. Rev., 2022, 466, 214602 CrossRef CAS.
- Q. Bi, Q. Ma, K. Tao and L. Han, Hierarchical core–shell 2D MOF nanosheet hybrid arrays for high-performance hybrid supercapacitors, Dalton Trans., 2021, 50(23), 8179–8188 RSC.
- Z. Ye, Y. Jiang, L. Li, F. Wu and R. Chen, Rational design of MOF-based materials for next-generation rechargeable batteries, Nano-Micro Lett., 2021, 13, 1–37 CrossRef PubMed.
- L. S. Xie, G. Skorupskii and M. Dinca, Electrically conductive metal–organic frameworks, Chem. Rev., 2020, 120(16), 8536–8580 CrossRef CAS PubMed.
- R. Li, X. Yan and L. Chen, 2D Conductive Metal–Organic Frameworks for Electrochemical Energy Application, Org. Mater., 2024, 6(02), 45–65 CrossRef CAS.
- J. Liu, G. Xing and L. Chen, 2D Conjugated Metal–Organic Frameworks: Defined Synthesis and Tailor-Made Functions, Acc. Chem. Res., 2024, 57(7), 1032–1045 CrossRef CAS PubMed.
- K. W. Nam, et al., Conductive 2D metal-organic framework for high-performance cathodes in aqueous rechargeable zinc batteries, Nat. Commun., 2019, 10(1), 4948 CrossRef PubMed.
- L. Guo, J. Sun, J. Wei, Y. Liu, L. Hou and C. Yuan, Conductive metal-organic frameworks: recent advances in electrochemical energy-related applications and perspectives, Carbon Energy, 2020, 2(2), 203–222 CrossRef CAS.
- J. Ren, et al., Twisting carbon nanotube fibers for both wire-shaped micro-supercapacitor and micro-battery, Adv. Mater., 2013, 25(8), 1155–1159 CrossRef CAS PubMed.
- M. K. Singh, S. Krishnan, K. Singh and D. K. Rai, CNT Interwoven Cu-MOF: A Synergistic Electrochemical Approach for Solid-State Supercapacitor and Hydrogen Evolution Reaction, Energy Fuels, 2024, 38, 12098–12110 CrossRef CAS.
- M. K. Singh, A. K. Gupta, S. Krishnan, N. Guha, S. Marimuthu and D. K. Rai, A new hierarchically porous Cu-MOF composited with rGO as an efficient hybrid supercapacitor electrode material, J. Energy Storage, 2021, 43, 103301 CrossRef.
- M. Azadfalah, A. Sedghi and H. Hosseini, Synthesis of nano-flower metal–organic framework/graphene composites as a high-performance electrode material for supercapacitors, J. Electron. Mater., 2019, 48(11), 7011–7024 CrossRef CAS.
- M. Safarkhani, et al., Engineering MXene@ MOF composites for a wide range of applications: a perspective, ACS Appl. Eng. Mater., 2023, 1(11), 3080–3098 CrossRef CAS.
- M. Sun, W. Ye, J. Zhang and K. Zheng, Structure, Properties, and Preparation of MXene and the Application of Its Composites in Supercapacitors, Inorganics, 2024, 12(4), 112 CrossRef CAS.
- J. L. Hart, et al., Control of MXenes' electronic properties through termination and intercalation, Nat. Commun., 2019, 10(1), 522 CrossRef CAS PubMed.
- N. Boussadoune, O. Nadeau and G. Antonius, Electronic transport in titanium carbide MXenes from first principles, Phys. Rev. B, 2023, 108(12), 125124 CrossRef CAS.
- H. Hassan, et al., Innovative MOF-5/V2CTx composite for high-performance, and ultra-fast supercapacitors and hydrogen evolution reaction, Electrochim. Acta, 2024, 489, 144277 CrossRef CAS.
- H. Hassan, et al., Effect of electrolyte optimization on nitrogen-doped MXene (Ti 3 C 2 T x) coupled with Cu–BTC MOF for a supercapattery and the hydrogen evolution reaction, New J. Chem., 2024, 48(14), 6277–6295 RSC.
- D. Xu, Z. Zhang, K. Tao and L. Han, A heterostructure of a 2D bimetallic metal–organic framework assembled on an MXene for high-performance supercapacitors, Dalton Trans., 2023, 52(8), 2455–2462 RSC.
- X. Chen, Z. Ding, H. Yu, H. Ge, W. Liu and S. Sun, Facile fabrication of CuCo 2 S 4 nanoparticles/MXene composite as anode for high-performance asymmetric supercapacitor, Mater. Chem. Front., 2021, 5(20), 7606–7616 RSC.
- B. M. Omkaramurthy, G. Krishnamurthy and S. Foro, Synthesis and characterization of mesoporous crystalline copper metal–organic frameworks for electrochemical energy storage application, SN Appl. Sci., 2020, 2(3), 342 CrossRef CAS.
- R. Zhu, Y. Gu, L. Liu, J. Lu and H. Pang, Integration of conductive MOF and MXene for high-performance supercapacitor, New J. Chem., 2024, 48(23), 10593–10598 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2311394. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra02060c |
| This journal is © The Royal Society of Chemistry 2025 |