Open Access Article
Open Access ArticleA review of the developments in adsorbents for the efficient adsorption of ibuprofen from wastewater
Ahmer Ali Siyal *a,
Radin Maya Saphira Radin Mohamed
*a,
Radin Maya Saphira Radin Mohamed *ab,
Faizan Ahmad
*ab,
Faizan Ahmad c,
Marlinda Abdul Malek
c,
Marlinda Abdul Malek d,
Majed Alsubih
d,
Majed Alsubih e,
Rashid Shamsuddin
e,
Rashid Shamsuddin f,
Sajid Hussaing and
Sabariah Musa
f,
Sajid Hussaing and
Sabariah Musa b
b
aMicropollutant Research Centre (MPRC), Institute for Integrated Engineering (I2E), Universiti Tun Hussein Onn Malaysia, 86400 Parit Raja, Batu Pahat, Johor, Malaysia. E-mail: maya@uthm.edu.my
bFaculty of Civil Engineering and Built Environment, Universiti Tun Hussein Onn Malaysia, 86400 Parit Raja, Batu Pahat, Johor, Malaysia
cSchool of Computing, Engineering and Digital Technologies, Teesside University, Middlesbrough, UK
dWater and Environmental Engineering Department, Faculty of Civil Engineering, Universiti Teknologi Malaysia, 81310, Johor, Malaysia
eDepartment of Civil Engineering, King Khalid University, Abha, Saudi Arabia
fDepartment of Chemical Engineering, Faculty of Engineering, Islamic University of Madinah, 42311 Madinah, Saudi Arabia
gDepartment of Civil, Environmental, and Mechanical Engineering, University of Trento, ViaMesiano 77, Trento, Italy
First published on 29th May 2025
Abstract
This paper critically evaluates the recent advancements in developing adsorbents to remove ibuprofen (IBU) from wastewater. Adsorbent characteristics, their performance in removing IBU from wastewater in batch and column studies, the adsorption kinetics, isotherms, thermodynamics, and mechanisms, adsorbent regeneration, continuous adsorption, and future challenges are included in this paper. Activated carbons, nanomaterials, metal–organic frameworks, biochar, and other adsorbents have been developed to remove IBU from wastewater. Most adsorbents were mesoporous, while some were macro- and microporous, and they contained acidic and basic functional groups. Adsorbents' surface areas range from 2.38 to 2900 m2 g−1, pore sizes from 0.0195 to 87.3 nm, and pore volumes from 0.006 to 14.48 cm3 g−1. The adsorption capacity ranged between 0.220 mg g−1 to 497.3 mg g−1, with Cu-doped Mil-101(Fe) and Albizia lebbeck seed pods activated carbon (MSAC) adsorbents achieving the highest and lowest adsorption capacities. The optimal pH of 2–8, dose of 0.012–10 g L−1, IBU concentration of 0.07–200 mg L−1, and the equilibrium time of 0.083–120 h were obtained. The pseudo-second order and Langmuir isotherm models generally fit the data, showing that IBU was adsorbed through the chemisorption process by producing a monolayer of IBU onto the adsorbent, and the thermodynamics described the adsorption of IBU as a spontaneous and endothermic or exothermic process. The IBU was adsorbed through various mechanisms such as electrostatic interactions, π–π interactions, pore filling, pore diffusion, π–π EDA interactions, hydrogen bonding, and Yoshida interactions. More focus should be put on developing highly efficient, economical, green, and regenerable adsorbents that can adsorb multiple drugs from wastewater. Mass transfer adsorption kinetics should be studied to better understand adsorption processes, and artificial intelligence technologies should be utilized in IBU removal from wastewater to anticipate the adsorption capacity of adsorbents. This review serves as a guide in enhancing the performance of adsorbents in removing pharmaceuticals from wastewater.
1. Introduction
The condition of water resources is deteriorating, as almost 4000 emerging micropollutants (MPs) have been detected in surface waters.1 MPs such as pharmaceuticals, fluorinated surfactants, and personal care products (PCPs) have been detected in wastewater. The main sources of MPs are households, agriculture, industries, and transport activities.2 Pharmaceuticals contribute to the contamination of water resources, as many of these chemicals cannot be absorbed by humans and are released into the wastewater.3 They enter water resources by throwing unused and expired pharmaceuticals into the drainage system and through the excretion of humans and animals.4 The presence of pharmaceuticals in wastewater treatment plant (WWTP) effluents suggests that these substances cannot be eliminated from wastewater by the current WWTPs.5 Pharmaceuticals reach humans through the food chain by consuming foods obtained from contaminated water resources (Fig. 1).About 32 drugs have been found in German WWTPs. The highest levels of ibuprofen (IBU) were found in the sewage (3.4 μg L−1) and the river stream (0.53 μg L−1).6 In Portugal, the pharmaceuticals were higher in hospital wastewater, ranging from 5.82 μg L−1 to 38.15 μg L−1 depending on the type of hospital (university hospital, general hospital, maternity hospital, and pediatric hospital), but the effluents after treatment contained a maximum IBU concentration of 0.37 μg L−1.7 In Norway and Italy, IBU in hospital wastewater was 8.96 μg L−1 and 3.20 μg L−1.8,9 The IBU in groundwater was around 3 to 395 ng L−1 in Europe in 2014.10 Pharmaceutical substances affect aquatic creatures in various ways, including changing their behavior, upsetting their hormonal balance, and reducing their reproduction ability.11,12 Some drugs can also disturb the growth of algae and other aquatic plants, potentially affecting the food chain.13 They also cause aquatic toxicity and produce antibiotic-resistant bacteria.14
IBU is a non-steroidal anti-inflammatory drug that relieves pain and fever.15 It's extensively utilized globally, with annual production rates of approximately 200 tons.16 It is commonly detected in wastewater due to usage in large quantities and incomplete removal by wastewater treatment systems.17 Its chemical name is 2-(4-isobutylphenyl)propanoic acid, which has an acid–base constant or dissociation constant (pKa) of 4.91, showing that it is a weak acid with a low adsorption tendency on wastewater treatment sludge18 (Table 1). The carboxyl group in IBU makes it ionizable and present in an anionic form when its pH is above pKa, and in a neutral form if pH is below its pKa. Electrostatic interactions depend on the charges on the adsorbent and IBU, while the carboxyl group in IBU acts as a hydrogen bond donor and hydrogen bond acceptor during the hydrogen bonding of IBU onto the adsorbent. The phenyl rings in the structure of IBU are responsible for pi–stacking interactions with aromatic rings if available on the adsorbent. It has the main functional groups of benzene and carboxylic acids, which make it more movable and less soluble in water.19 The lower solubility of IBU in water, high lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow), and low adsorption coefficient (log
Kow), and low adsorption coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc) show that it has low mobility and no adsorption tendency in the soil.20 The log
Koc) show that it has low mobility and no adsorption tendency in the soil.20 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow is a physicochemical parameter inversely proportional to the compound's solubility. The compounds with log
Kow is a physicochemical parameter inversely proportional to the compound's solubility. The compounds with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow above 4 are hydrophobic, and those with log
Kow above 4 are hydrophobic, and those with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow below 2.5 are considered low hydrophobic. Hydrophobicity is directly proportional to adsorption. IBU can be found in wastewater in its original form or as hydroxyl-IBU or carboxyl-IBU metabolites1 (Fig. 2). It has been noticed that the adsorbents with acidic surface properties possess a higher affinity for relatively hydrophilic IBU sodium salt molecules than more basic adsorbents.18 Although IBU is present in low concentrations in drinking water, the continuous uptake of IBU-contaminated water can seriously impact human health.21 The IBU in drinking water deteriorates the taste and odor of water and decreases its appeal to consumers. It severely damages the liver and kidneys and causes gastrointestinal issues.22
Kow below 2.5 are considered low hydrophobic. Hydrophobicity is directly proportional to adsorption. IBU can be found in wastewater in its original form or as hydroxyl-IBU or carboxyl-IBU metabolites1 (Fig. 2). It has been noticed that the adsorbents with acidic surface properties possess a higher affinity for relatively hydrophilic IBU sodium salt molecules than more basic adsorbents.18 Although IBU is present in low concentrations in drinking water, the continuous uptake of IBU-contaminated water can seriously impact human health.21 The IBU in drinking water deteriorates the taste and odor of water and decreases its appeal to consumers. It severely damages the liver and kidneys and causes gastrointestinal issues.22
| Molecular formula | C13H18O2 |
| pKa (acid–base constant) | 4.91 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kow (Kow–octanol water coefficient) Kow (Kow–octanol water coefficient) |
3.97 |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Koc (Koc adsorption coefficient) Koc (Koc adsorption coefficient) |
2.5 |
| Molar mass | 206.29 g mol−1 |
| Density | 1.03 cm3 g−1 |
| Solubility in water | 0.021 mg cm−3 (20 °C) |
| Specific gravity | 1.03 |
| Boiling point | 157 °C |
| Melting point | 75–78 °C |
| Molecular dimensions | 0.43–1.03 nm |
Various techniques, such as membranes, advanced oxidation processes (AOPs), and hybrid methods, are used for removing IBU from wastewater. These techniques are difficult, require significant maintenance costs, and generate more waste. Adsorption is a preferred method due to its efficiency, simplicity, and environmental friendliness.23 Various adsorbents such as activated carbons, nanomaterials, Metal–Organic Framework (MOF), biochar, and others have been used to remove IBU from wastewater. Several reviews have been published on IBU removal from wastewater. Chopra and Kumar22 published a review paper on the IBU removal methods from wastewater. Show et al.24 conducted a comprehensive review on eliminating IBU from wastewater, specifically emphasizing adsorption and bioremediation. Oba et al.25 wrote a review paper about how to adsorb IBU from wastewater, focusing on how well different adsorbents developed during 2010–2020 removed IBU. Ahmed26 published a review paper discussing the elimination of IBU and carbamazepine from water using adsorbents derived from agricultural waste. Wu et al.27 published a review paper on IBU and acetaminophen removal from municipal wastewater treatment plants. Ayati et al.28 reviewed the adsorption of IBU using porous carbonaceous materials. Segovia et al.29 conducted a bibliometric statistical analysis on eliminating triclosan, ibuprofen, amoxicillin, and paracetamol utilizing organic residues. Different organic residues, such as activated carbons, shells, and husks, were studied. No review paper on recent advancements in adsorbent development for IBU removal from wastewater has recently been published.
This paper critically reviews the performance of recently developed adsorbents in removing IBU from wastewater. It includes the characteristics and performance of adsorbents in batch studies, the adsorption kinetics, isotherms, thermodynamics, mechanism, and regeneration of adsorbents. The adsorbent's performance in continuous studies and the future challenges in IBU adsorption are also discussed.
2. Adsorption of ibuprofen
2.1 Batch adsorption
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were mainly involved in the adsorption of IBU, and the adsorption capacity increased due to an increase in the mesoporosity of the adsorbent.31 This work developed a good adsorbent for IBU removal from wastewater; however, its IBU adsorption performance is lower than most other adsorbents used to remove IBU. Modifying its surface with appropriate materials to enhance its surface area and active adsorption sites can enhance its performance. Two activated carbons prepared from cork waste by chemical activation with K2CO3 (CAC) and chemical activation with K2CO3 and steam activation (CPAC) showed changes in their point of zero charge (PZC) due to the changes in the surface chemistry. CAC had a more acidic surface (PZC-7.5) than CPAC (PZC-9.9), which was due to the presence of higher oxygen functional groups on CAC compared to CPAC. Steam activation decreased the contents of R-OH and removed R-COOH and R-OCO functional groups and increased the R
O were mainly involved in the adsorption of IBU, and the adsorption capacity increased due to an increase in the mesoporosity of the adsorbent.31 This work developed a good adsorbent for IBU removal from wastewater; however, its IBU adsorption performance is lower than most other adsorbents used to remove IBU. Modifying its surface with appropriate materials to enhance its surface area and active adsorption sites can enhance its performance. Two activated carbons prepared from cork waste by chemical activation with K2CO3 (CAC) and chemical activation with K2CO3 and steam activation (CPAC) showed changes in their point of zero charge (PZC) due to the changes in the surface chemistry. CAC had a more acidic surface (PZC-7.5) than CPAC (PZC-9.9), which was due to the presence of higher oxygen functional groups on CAC compared to CPAC. Steam activation decreased the contents of R-OH and removed R-COOH and R-OCO functional groups and increased the R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups in CPAC. The CAC and CPAC achieved the qm of 139.2 mg g−1 and 393.4 mg g−1, respectively, at ambient temperature. The higher adsorption affinity of CPAC was due to its highly developed super microporous structure, which makes it a viable and economical adsorbent for removing IBU from wastewater.19 Two adsorbents of beverage sludge-activated carbon (BSAC) and acid-treated beverage sludge-activated carbon (ABSAC) showed that ABSAC contained smaller particles and more surface roughness as compared to BSAC, which was due to the leaching of impurities during acid treatment, as shown in Fig. 3. The microstructure of BSAC (Fig. 3(a and b)) is disorganized, having low roughness and low porosity due to the presence of impurities in the material. The microstructure of ABSAC (Fig. 3(c and d)) is more organized and shows small particles with higher roughness and higher porosity due to the removal of impurities. It helped the ABSAC adsorbent to adsorb more IBU molecules than BSAC. The ABSAC obtained a qt of 105.91 mg g−1 at the best parameters of pH 4, room temperature, and equilibrium time of 180 min. The ABSAC was also tested on pharmaceutical effluent containing ibuprofen, ketoprofen, and paracetamol, and it removed 85.16% of pharmaceuticals.32 This study developed an efficient and sustainable adsorbent from waste material, and the increase in adsorption capacity compared to its raw form was associated with the increase in porosity, roughness, and the presence of small particles. Six activated carbons prepared from red mombin seeds (RMS), corn cobs (CC), external sections of mango seeds (MSEP), coffee husk (CH), ice cream bean seeds (GS), and mango seeds internal parts (MSIP) showed differing IBU adsorption performances with RMS, CC, and MSEP adsorbents obtained the qt of 69.88, 88.03, and 52.60 mg g−1, respectively. The rapid rate of graphitization, greater abundance of oxygen groups, and significant micropore volume all contributed to the enhanced adsorption capabilities of the adsorbents. The adsorption of IBU was more advantageous in micropores with pore diameters less than 1.2 nm. The micropore volume of activated carbon adsorbents in the micropore size range of <1.2 nm ranged between 64–74% of the total volume. The micropore volume of these RMS, CC, and MSEP was 73% (0.574 cmliq3 g−1), 74% (0.494 cmliq3 g−1), and 69% (0.489 cmliq3 g−1), respectively.33 It shows that micropore volume also contributes to enhancing the adsorption capacities of the studied adsorbents. The lower adsorption capacities of CH, GS, and MSIP may also be related to the lower porosity of these adsorbents.
O functional groups in CPAC. The CAC and CPAC achieved the qm of 139.2 mg g−1 and 393.4 mg g−1, respectively, at ambient temperature. The higher adsorption affinity of CPAC was due to its highly developed super microporous structure, which makes it a viable and economical adsorbent for removing IBU from wastewater.19 Two adsorbents of beverage sludge-activated carbon (BSAC) and acid-treated beverage sludge-activated carbon (ABSAC) showed that ABSAC contained smaller particles and more surface roughness as compared to BSAC, which was due to the leaching of impurities during acid treatment, as shown in Fig. 3. The microstructure of BSAC (Fig. 3(a and b)) is disorganized, having low roughness and low porosity due to the presence of impurities in the material. The microstructure of ABSAC (Fig. 3(c and d)) is more organized and shows small particles with higher roughness and higher porosity due to the removal of impurities. It helped the ABSAC adsorbent to adsorb more IBU molecules than BSAC. The ABSAC obtained a qt of 105.91 mg g−1 at the best parameters of pH 4, room temperature, and equilibrium time of 180 min. The ABSAC was also tested on pharmaceutical effluent containing ibuprofen, ketoprofen, and paracetamol, and it removed 85.16% of pharmaceuticals.32 This study developed an efficient and sustainable adsorbent from waste material, and the increase in adsorption capacity compared to its raw form was associated with the increase in porosity, roughness, and the presence of small particles. Six activated carbons prepared from red mombin seeds (RMS), corn cobs (CC), external sections of mango seeds (MSEP), coffee husk (CH), ice cream bean seeds (GS), and mango seeds internal parts (MSIP) showed differing IBU adsorption performances with RMS, CC, and MSEP adsorbents obtained the qt of 69.88, 88.03, and 52.60 mg g−1, respectively. The rapid rate of graphitization, greater abundance of oxygen groups, and significant micropore volume all contributed to the enhanced adsorption capabilities of the adsorbents. The adsorption of IBU was more advantageous in micropores with pore diameters less than 1.2 nm. The micropore volume of activated carbon adsorbents in the micropore size range of <1.2 nm ranged between 64–74% of the total volume. The micropore volume of these RMS, CC, and MSEP was 73% (0.574 cmliq3 g−1), 74% (0.494 cmliq3 g−1), and 69% (0.489 cmliq3 g−1), respectively.33 It shows that micropore volume also contributes to enhancing the adsorption capacities of the studied adsorbents. The lower adsorption capacities of CH, GS, and MSIP may also be related to the lower porosity of these adsorbents.
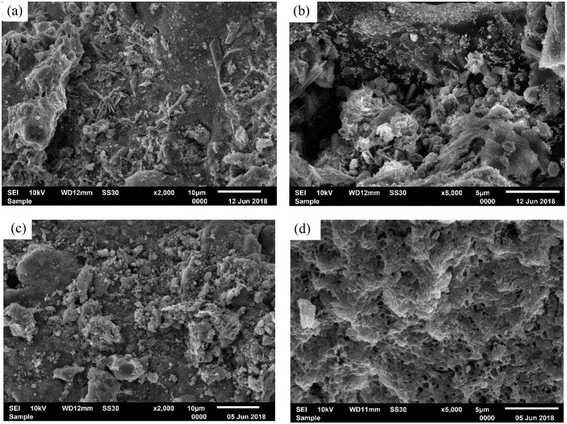 | ||
| Fig. 3 Microstructure of BSAC (a) and (b) and ABSAC (c) and (d) adsorbents.32 | ||
The porous carbon derived from MOF (zeolitic-imidazolate framework-8 (ZIF-8)) (PCDMs) through pyrolysis at 800 °C, 1000 °C, and 1200 °C indicated that the PCDM-1000 having a surface area of 1855 m2 g−1 (Table 3) achieved a qm of 320 mg g−1 that was three times higher than the activated carbon as shown in Fig. 4.34 The PCDMs showed considerable amounts of acidic (carboxyl, lactone, and phenol) and basic functional groups on the surface of PCDMS. The increase in calcination temperature decreased the amounts of carboxylic and lactone functional groups, while the phenol content decreased in the following order: PCDMS-800 < PCDMS-1200 > PCDMS-1000. The increase in temperature decreased the basic functional groups.34 This work developed an efficient and recyclable adsorbent with good adsorption capacity, but its adsorption capacity is lower than chemically and steam-activated cork waste (CPAC) and physically activated cork powder. Further enhancement in its performance can be made by improving its surface chemistry by increasing the content of phenolic groups through doping with some suitable materials. Ethylamine-modified hydrophobic activated carbon (HAC-EA) derived from date palm leaflets achieved a qt of 35.21 mg g−1, lower than the original activated carbon. The competition among methanol molecules for hydrophobic sites diminished the adsorption capability of HAC-EA. It achieved higher adsorption in deionized water compared to hospital wastewater. The adsorption capacity decreased in the following order: oxidized activated carbon (OAC) > AC > HAC-EA > ethylene diamine basic surface activated carbon (BAC-EDA), as shown in Fig. 5.23 The adsorbents' decreasing order of adsorption capacity is similar in both wastewaters. However, the adsorption capacities of adsorbents are higher in deionized water as compared to their adsorption capacities in deionized spiked hospital wastewater, which is due to the presence of dissolved organic substances in spiked hospital wastewater that create competition for active adsorption sites on the adsorbent. The adsorption equilibrium time of adsorbents was in the order of HAC-EA > OAC > BAC-EDA > AC. This work shows that functionalized activated carbon can play a better role in adsorption than unfunctionalized adsorbents. The adsorption performance of HAC-EA needs further improvement by increasing its surface area and active adsorption sites. Two activated carbons prepared from cork waste by chemical activation with K2CO3 (CAC) and chemical activation with K2CO3 and steam activation (CPAC) showed changes in their point of zero charge (PZC) due to the changes in the surface chemistry. CAC had a more acidic surface (PZC-7.5) than CPAC (PZC-9.9), which was due to the presence of higher oxygen functional groups on CAC compared to CPAC. Steam activation decreased the contents of R-OH and removed R-COOH and R-OCO functional groups, and increased the R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups in CPAC.19
O functional groups in CPAC.19
 | ||
| Fig. 4 The adsorption of IBU onto PCDM-1000, AC, and ZIF-8 (redrawn).34 | ||
 | ||
| Fig. 5 Adsorption performance of IBU in deionized and spiked HWW (drawn by using data from ref. 23). | ||
Magnetic nanoparticles incorporated on yeast-based activated carbon (NP-YC) achieved an adsorption capacity of 51 mg g−1 from deionized water, which is lower than the adsorption capacity of YC of 107 mg g−1. During competitive adsorption with caffeine (CA), NP-YC achieved removal efficiencies above 70% in deionized water and above 60% in primary sewage effluent (PE).35 This work developed an economical adsorbent using yeast, but its IBU adsorption performance needs further enhancement by improving its surface functionalization with suitable materials. A groundnut shell-activated carbon modified with titanium dioxide nanoparticles (TiO2-NPs-GNSAC) achieved a removal efficiency of 81.78% for IBU from wastewater at optimum conditions, which were optimized using BBD of RSM. Adding TiO2 NPs into activated carbon enhanced its removal performance due to the increased active adsorption sites. The addition of TiO2 NPs into the GNSAC matrix resulted in a reduction in the pore size, pore volume, and surface area of the modified adsorbent (TiO2-NPs-GNSAC).36 A magnetic composite of nickel-iron oxide nanoparticles and activated carbon (NiFe2O4/activated carbon-NiAC) obtained a qm and removal efficiency of 261.35 mg g−1 and 86.46% at optimal pH 2, respectively. It achieved a removal efficiency of 86.46% in simulated effluent containing IBU, ketoprofen, and inorganic compounds.37 Its performance is better than many adsorbents but lower than some adsorbents. The recovery of this adsorbent is very easy using external magnetic force, which reduces the process cost by removing the associated costs of centrifugation and filtration. Carbon nanospheres (CNs) achieved a qt of 356.899 mg g−1 and a removal efficiency of 94.47%, while its removal efficiency decreased to 67% in real wastewater. CNs only showed good adsorption of IBU in synthetic wastewater, while its performance in real wastewater needs further improvement.38 Erythrina speciosa (Ery-AC) showed an amorphous and porous structure with various functional groups, but the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C functional group was absent, which is involved in π–π interactions during adsorption, and it shows that the IBU will be adsorbed through electrostatic or hydrogen bonds. The Ery-AC obtained a qt and removal efficiency of 98.11 mg g−1 and 65.54% for higher IBU concentration (50–200 mg L−1), respectively, and 90% for a lower IBU concentration from a solution containing a mixture of adsorbates. The adsorption of IBU from a simulated effluent containing IBU (50 μg L−1), paracetamol (10 μg L−1), naproxen (10 μg L−1), and ketoprofen (μg L−1) showed good removal of IBU with a removal efficiency of 95.2% and higher than 90% for other pharmaceuticals.39 This work developed an efficient and sustainable adsorbent from seed pods of the forest species Erythrina speciosa, but its removal efficiency for higher IBU concentration solution needs further enhancement. Two inexpensive laboratory-developed activated carbons from rice husk (AC-RH) and peach stones (AC-PS) outperformed commercial granular activated carbon (AC-F400) and multiwalled carbon nanotubes (MWCNTs) in adsorbing tetracycline and IBU, with adsorption capacities of 845.9 mg g−1 and 239.8 mg g−1, respectively.18 The isoelectric points of AC-RH, AC-PS, AC-F400, and MWCNTs were 3.4, 3, 5, and 6.3, respectively. AC-F400 was 80% microporous, AC-PS was 30% mesoporous, and AR-CH and MWCNTs contained mesopores and macropores.18 This work developed two efficient and sustainable adsorbents for tetracycline and IBU removal. A nitrogen-doped porous carbon (NPC-2) obtained a qm of 113 mg g−1 in 1 hour of equilibrium time. Higher nitrogen concentration and the adsorbent's microporous and mesoporous structures contributed to the higher adsorption capacity. There was a small effect of the presence of NaCl and humic acid on the adsorption of IBU onto NPC-2. NPC-2 also performed well in removing IBU from lake water (94%, IBU-5 mg L−1).40 Although it can remove IBU from spiked wastewater at low IBU concentrations, the adsorption performance of NPC-2 from real wastewater at higher IBU concentrations would further shed light on its real-world applications. The composite hydrogel beads of alginate-activated carbon and carboxymethyl cellulose (Alg/AC/CMC) displayed good adsorption capacity after reswelling compared to Alg/AC without CMC (34 mg g−1 and 18 mg g−1 with adsorption capacity before reswelling of 39.6 mg g−1). The purpose of adding CMC into the composite hydrogel was to recover its surface area by soaking it in deionized water through a reswelling process. The adsorption capacity and recovery of Alg/AC/CMC after drying were influenced by the activated carbon content and the degree of saturation (DS) of CMC. The wet composite hydrogel beads of Alg/AC/CMC with high water content showed an irregular and rough surface with bumps on the surface, while the dried hydrogel showed a tight structure with disappeared pores due to shrinkage of the structure caused by drying. However, after reswelling with distilled water (DW), the hydrogel recovered to its original morphology, as shown in Fig. 6.41 This work developed an adsorbent that can be used for a longer period by recovering its before-drying properties by soaking it in distilled water.
C functional group was absent, which is involved in π–π interactions during adsorption, and it shows that the IBU will be adsorbed through electrostatic or hydrogen bonds. The Ery-AC obtained a qt and removal efficiency of 98.11 mg g−1 and 65.54% for higher IBU concentration (50–200 mg L−1), respectively, and 90% for a lower IBU concentration from a solution containing a mixture of adsorbates. The adsorption of IBU from a simulated effluent containing IBU (50 μg L−1), paracetamol (10 μg L−1), naproxen (10 μg L−1), and ketoprofen (μg L−1) showed good removal of IBU with a removal efficiency of 95.2% and higher than 90% for other pharmaceuticals.39 This work developed an efficient and sustainable adsorbent from seed pods of the forest species Erythrina speciosa, but its removal efficiency for higher IBU concentration solution needs further enhancement. Two inexpensive laboratory-developed activated carbons from rice husk (AC-RH) and peach stones (AC-PS) outperformed commercial granular activated carbon (AC-F400) and multiwalled carbon nanotubes (MWCNTs) in adsorbing tetracycline and IBU, with adsorption capacities of 845.9 mg g−1 and 239.8 mg g−1, respectively.18 The isoelectric points of AC-RH, AC-PS, AC-F400, and MWCNTs were 3.4, 3, 5, and 6.3, respectively. AC-F400 was 80% microporous, AC-PS was 30% mesoporous, and AR-CH and MWCNTs contained mesopores and macropores.18 This work developed two efficient and sustainable adsorbents for tetracycline and IBU removal. A nitrogen-doped porous carbon (NPC-2) obtained a qm of 113 mg g−1 in 1 hour of equilibrium time. Higher nitrogen concentration and the adsorbent's microporous and mesoporous structures contributed to the higher adsorption capacity. There was a small effect of the presence of NaCl and humic acid on the adsorption of IBU onto NPC-2. NPC-2 also performed well in removing IBU from lake water (94%, IBU-5 mg L−1).40 Although it can remove IBU from spiked wastewater at low IBU concentrations, the adsorption performance of NPC-2 from real wastewater at higher IBU concentrations would further shed light on its real-world applications. The composite hydrogel beads of alginate-activated carbon and carboxymethyl cellulose (Alg/AC/CMC) displayed good adsorption capacity after reswelling compared to Alg/AC without CMC (34 mg g−1 and 18 mg g−1 with adsorption capacity before reswelling of 39.6 mg g−1). The purpose of adding CMC into the composite hydrogel was to recover its surface area by soaking it in deionized water through a reswelling process. The adsorption capacity and recovery of Alg/AC/CMC after drying were influenced by the activated carbon content and the degree of saturation (DS) of CMC. The wet composite hydrogel beads of Alg/AC/CMC with high water content showed an irregular and rough surface with bumps on the surface, while the dried hydrogel showed a tight structure with disappeared pores due to shrinkage of the structure caused by drying. However, after reswelling with distilled water (DW), the hydrogel recovered to its original morphology, as shown in Fig. 6.41 This work developed an adsorbent that can be used for a longer period by recovering its before-drying properties by soaking it in distilled water.
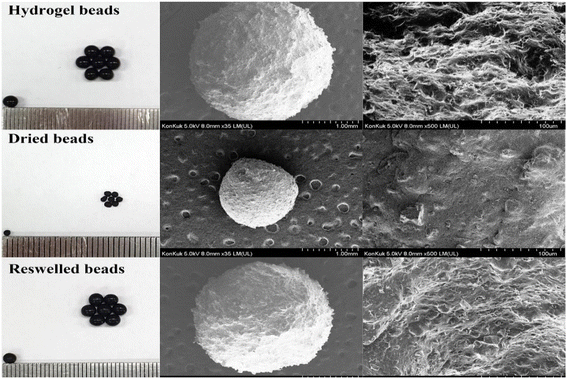 | ||
| Fig. 6 Morphology of composite beads of Alg/AC/CMC (4%, 1%, 1%).41 | ||
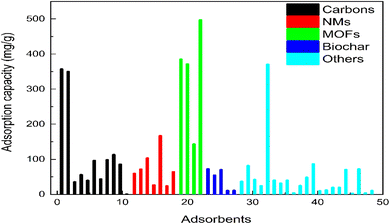
The surface area, pore size, and pore volume of activated carbons ranged between 4.4 and 1946 m2 g−1, 1.23 and 16.8 nm, and 0.0017 and 0.80 cm3 g−1, as shown in Table 3. The adsorption capacities of activated carbons range between 0.220 mg g−1 and 356.89 mg g−1. Carbon nanospheres (CNs) and Albizia lebbeck seed pods activated carbon (MSAC) achieved the highest and lowest adsorption capacities, respectively. The adsorption capacities of carbon nanospheres (CNs), waste coffee-activated carbon (WAC), and nitrogen-doped porous carbon (NPC-2) adsorbents were above 100 mg g−1, while all other activated carbon adsorbents' adsorption capacities were below 100 mg g−1, as shown in Table 2 and Fig. 7.
| Adsorbent | Performance | Optimum parameters | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Adsorption capacity (mg g−1) | R (%) | pH | Dose (g L−1) | C (mg L−1) | teq (h) | ||
| Activated carbons | |||||||
| Carbon nanospheres (CNS) | 356.89 | — | 6 | 0.8 | — | 1.66 | 38 |
| Waste coffee-derived activated carbon (WAC) | 350 | — | 6.87 | 1 | — | 0.25 | 87 |
| Ethylamine-modified hydrophobic activated carbon (HAC-EA) | 35.21 | — | 7 | — | — | 10 | 23 |
| TiO2 NPs modified groundnut shell activated carbon (TiO2 NPs-GNSAC) | 55.56 | 81.78 | — | 0.5 | — | 0.83 | 36 |
| Biomass-derived activated carbon | 96.28 | — | 3 | 0.75 | — | — | 88 |
| Nauclea diderrichii biomass-derived activated carbon (NDAC) | 43.66 | — | 6 | 0.5 | — | 1 | 31 |
| Erythrina speciosa activated carbon (Ery-AC) | 98.11 | — | 3 | 0.75 | — | 1.66 | 39 |
| Reduced graphene oxide/activated carbon composite (RGO/AC1) | 85.57 | — | 2 | 0.4 | — | 1.667 | 89 |
| Albizia lebbeck seed pods activated carbon (MSAC) | 0.220 | — | 7.82 | 0.33 | 0.0764 | — | 90 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Nanomaterials | |||||||
| NiFe2O4@SiO2@APTS | 59 | 97 | 7 | — | 12 | 0.25 | 45 |
| Corn starch nanoparticles (CSNP) | — | 86.33 | 2 | 0.33 | 10 | — | 46 |
| Silver nanoparticles modified Luffa (LF/AgNPs) | 71.3 | 92 | 5 | 2.5 | 200 | 1 | 50 |
| CNT-Fe3O4-MnO2 nanocomposite | 103.093 | — | 2 | 1 | 40 | 0.33 | 47 |
| Hemp seeds nanocomposite (HS-MnO/CuO) | 26.50 | 8 | 0.4 | 100 | 0.5 | 91 | |
| TiO2/Fe2O3/chitosan nanocomposite | 166.667 | 95.2 | 7.3 | 0.05 | — | — | 92 |
| Hydrophobic deep eutectic solvents functionalized magnetic iron oxide nanoparticles (Fe3O4@HDES-2) | 23.6 | — | 3 | 4 | — | 0.333 | 51 |
| Zn-Decorated S, P, B coped C2N (Zn-SPB@ C2N) nanosheet | — | 98 | 7 | 0.2 | 59 | — | 52 |
| Organo-silica nanosheets (G16-2-16-SiNSs) | 64.19 | — | 4 | — | — | 0.083 | 93 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Metal–organic framework (MOF) | |||||||
| Zirconium-based metal–organic framework (Zr-MOF) | 384.69 | — | — | 10 | — | 1.66 | 55 |
| Zirconium-based metal–organic framework modified with tryptophane (Zr-MOF-NH2) | 371.34 | — | — | — | — | ||
| Magnetic carboxylic multiwalled carbon nanotube metal–organic framework (MCNTs-UiO-66-NH2) | 143 | — | 1–10 | 1 | — | 2 | 58 |
| Cu-Doped Mil-101(Fe) | 497.3 | — | — | 0.2 | — | — | 57 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Biochar | |||||||
| Iron and acid-modified date palm biochar (DPAI) | 72.2 | — | 2 | — | 150 | 20 | 62 |
| Recycled textile steam-activated biochar (RT-SABC) | 54 | 50 | — | — | — | 120 | 63 |
| Walnut shell-activated biochar (WSAB) | 69.7 | 80 | 4 | 1 | 50 | — | 64 |
| Plane tree leaf-derived biochar (P-BC) | 10.41 | 96.34 | 2 | 1 | 2 | 24 | 94 |
| Pinewood biochar | 10.74 | — | 3 | — | — | — | 61 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| Zeolites, cellulose, organoclays, chitosan, polymers, and other adsorbents | |||||||
| Acid-treated maize cob (AT-MC) | 36.81 | — | 6 | 6 | 75 | 0.66 | 95 |
| Cationic surfactant cetyldimethyl benzyl ammonium chloride (HDBAC) modified montmorillonite (H-Mt-1.6) | 81.64 | — | 5 | 2 | 86 | ||
| Molecularly imprinted Fe(III) incorporated chitosan hydrogels (CS_Fe_MIP) | 41.69 | — | 5 | — | — | — | 74 |
| Biomass derived chitosan | 24.21 | — | — | 7.5 | — | — | 72 |
| Polyethyleneimine modified magnetic sugarcane bagasse cellulose film (P-SBC/Fe3O4 film) | 370.52 | 92.63 | 4 | 0.0125 | — | 8 | 69 |
| Metal–organic framework functionalized with hydrochar (MIL-53(Al)@HC) | — | 98 | 5.4 | — | — | 2 | 96 |
| Amine-grafted pumice derived silica aerogel (AMPDSA) | 39.95 | 100 | 7 | 0.5 | 6.53 | 2.5 | 85 |
| Fly ash derived zeolite modified by β-cyclodextrin (NaX-CD) | 31.3 | — | — | 0.5 | — | 0.25 | 67 |
| Iron-incorporated pomegranate husk carbon (NPH) | 39.77 | — | 8 | 100 | 1 | 97 | |
| Graphene oxide nanoplatelets (GONPs) | 3.72 | — | 6 | 1 | 6 | 1 | 98 |
| Activated bean husk (BHAA) | 24.570 | — | 4.75 | — | — | 0.66 | 99 |
| Rape straw biomass Fe3O4 treated and β-CD embedded adsorbent (RSBCDF) | 48.29 | — | 6 | 2.5 | — | 1 | 100 |
| Al/Li double layered hydroxide/polyaniline/sisal fibers composite (SF/PANI/LDH) | 86.03 | — | 5 | — | 100 | 1.5 | 101 |
| Steam activated coconut shell (CPBC) | 9.69 | — | 2 | 2.66 | 30 | 18 | 102 |
| Chemically activated (H3PO4) coconut shell (CCBC) | 12.16 | — | 2 | 3.33 | 25 | 18 | |
| Cellulosic sisal-poly (ppy-Ani) | 19.45 | 88 | 5 | 1.5 | 30 | 1 | 103 |
| Green synthesized iron oxide (Fe2O3) | 19.43 | 81.89 | 5 | 0.3 | 40 | 0.666 | 104 |
| Chitosan modified waste tire crumb rubber | 70 | — | 6 | — | — | 1 | 73 |
| Rice husk ash (RHA) | 2.321 | — | 2 | 10 | — | 4 | 105 |
| Carboxymethylcellulose/polypyrrole (CMC/PPY) composite | 72.30 | 83.17 | 7 | — | 10 | — | 106 |
| Multi-template molecularly imprinted polymer (MIP) | 3.598 | — | 4.6 | 5 | — | 0.166 | 78 |
| Porous polymer monoliths (PMLE-E) | 10.6 | 86.9 | 8 | — | — | 6 | 77 |
| Geopolymer | 5.7 | 2 | — | — | — | 107 | |
| Adsorbent | Surface properties | Adsorption capacity (mg g−1) | Ref. | ||
|---|---|---|---|---|---|
| Surface area (m2 g−1) | Pore size (nm) | Pore volume (cm3 g−1) | |||
| Activated carbons | |||||
| Erythrina speciosa (Ery-AC) | 795 | 1.23 | 0.422 | 98.11 | 39 |
| TiO2 NPs modified groundnut shell activated carbon (TiO2 NPs-GNSAC) | 39.92 | 5.353 | 0.103 | 55.56 | 36 |
| Nauclea diderrichii biomass derived activated carbon (NDAC) | 33.2 | — | 0.037 | 43.66 | 31 |
| Ethylamine-modified hydrophobic activated carbon (HAC-EA) | 9.89 | 16.8 | 0.041 | 35.21 | 23 |
| Biomass-derived activated carbon | 795.1 | 1.232 | 0.422 | 96.28 | 88 |
| Ultrasound modified activated carbon (USAC) | 731.3 | 4.15 | 0.410 | 107.1 | 108 |
| Acid treated beverage sludge activated carbon (ABSAC) | 642 | 6.18 | 0.485 | 105.91 | 32 |
| Acid and thermally treated activated carbon cloth | 1946 | — | 0.80 | 491.9 | 109 |
| Mesoporous activated carbon (MAC) honeycomb | 358.20 | 2.46 | 0.21 | 16.730 | 110 |
| Nitrogen doped porous carbon (NPC-2) | 937 | 3.6 | 0.64 | 113 | 40 |
| Surface oxidized activated carbon (AC700N2) | 809 | — | 0.55 | 160 | 111 |
| Magnetic nanoparticles incorporated on yeast-based activated carbon (NP-YC) | 644 | — | 0.41 | 51 | 35 |
| Carbon nanospheres (CNs) | 359 | — | 0.168 | 356.89 | 38 |
| Magnetic composite of nickel-iron oxide nanoparticles and activated carbon (NiAC) | 564 | 7.51 | 0.35 | 261.35 | 37 |
| Dried Alg/AC/CMC beads | 4.4 | 2.562 | 0.0017 | 34 | 41 |
| Hydrogel Alg/AC/CMC | 131.4 | 7.714 | 0.2027 | 39.6 | |
| Red mombin seeds (RMS) | 1508 | — | 0.778 | 69.88 | 33 |
| Corn cobs (CC) | 1280 | — | 0.661 | 88.03 | |
| External parts of mango seeds (MSEP) | 1279 | — | 0.700 | 52.60 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Nanomaterials | |||||
| Hemp seeds nanocomposite (HS-MnO/CuO) | 21.55 | 0.019 | — | 26.50 | 91 |
| Organo-silica nanosheets (G16-2-16-SiNSs) | 328 | 4.8 | 0.398 | 64.19 | 93 |
| Silver nanoparticles modified Luffa (LF/AgNPs) | 16.849 | — | 0.0065 | 71.3 | 50 |
| Natural piezoelectric quartz coated with green zinc oxide nanoparticles (GZnO/PQz) | 191.3 | — | 0.62 | 145.6 | 49 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| MOF | |||||
| Zirconium-based metal–organic framework modified with tryptophane (Zr-MOF-NH2) | 729.61 | — | 0.51 | 371.64 | 55 |
| Thermally activated gelatin–chitosan and amine-functionalized MOF aerogel (GGC-MOF200) | 819.6 | 2.093 | 0.430 | 5.963 | 112 |
| Cu-Doped Mil-101(Fe) | 15.48 | 14.4 | 0.056 | 497.3 | 57 |
| Zr-Based MOFs (UiO-67(Zr)-BA (10)) | 2900 | — | 1.23 | 213 | 54 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Biochar | |||||
| Iron and acid modified date palm biochar (DPAI) | 88.75 | 10.25 | 0.0591 | 72.2 | 62 |
| Recycled textile steam activated biochar (RT-SABC) | 710 | — | 0.3362 | 54 | 63 |
| Walnut shell-activated biochar (WSAB) | 686 | — | 0.57 | 69.7 | 64 |
| Biochar from pepper stem (PS-biochar) | 727.5 | 1.97 | 0.36 | 569.6 | 65 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Zeolites, cellulose, organoclays, chitosan, polymers, and other adsorbents | |||||
| Acid-treated maize cob (AT-MC) | 7.92 | 2.63 | 0.0213 | 36.81 | 95 |
| Cetyldimethyl benzyl ammonium chloride (HDBAC) modified montmorillonite (H-Mt-1.6) | 14.12 | 14.77 | 0.052 | 81.64 | 86 |
| Molecularly imprinted Fe(III) incorporated chitosan hydrogels (CS_Fe_MIP) | 30.45 | 3.71 | 0.183 | 41.69 | 74 |
| Polyethyleneimine modified magnetic sugarcane bagasse cellulose film (P-SBC/Fe3O4 film) | 2.38 | 36.86 | 14.48 | 370.52 | 69 |
| Amine-grafted pumice derived silica (AMPDSA) | 407 | 5.45 | 1.05 | 39.95 | 85 |
| Fly ash derived zeolite modified by β-cyclodextrin (NaX-CD) | 68.69 | 3.875 | 0.041 | 31.3 | 67 |
| Biomass-derived chitosan | 6.57 | 87.3 | 0.0397 | 24.21 | 72 |
| Iron-incorporated pomegranate husk carbon (NPH) | 190 | 0.088 | — | 39.77 | 97 |
| Calcined spherical hydrochar (CSH) | 257.3 | 2.27 | 0.17 | 95.6 | 84 |
| Ca2+(TAABB)Al | 208.39 | — | 0.275 | 17.54 | 113 |
| Rape straw biomass Fe3O4 treated and β-CD embedded adsorbent (RSBCDF) | 86.32 | 2.22 | — | 48.29 | 100 |
| Polydopamine imprinted polymers with fluorescent carbon dots (PIP-FCDs) | 184.09 | 3.63 | 0.094 | 209.8 | 76 |
| Acyl hydrazone covalent organic polymers (H-COP-3) | 8.558 | — | 0.069 | 242.775 | 79 |
| Versatile vermiculite modified by quinoline-based gemini surfactant (DHQU-Vt) | 2.53 | 23.51 | 0.015 | 240.69 | 83 |
The surface area, pore size, and pore volume of NMs ranged between 16.849 and 328 m2 g−1, 0.019 and 4.8 nm, and 0.006 and 0.62 cm3 g−1, as shown in Table 3. In nano-sorbents, the adsorption capacities ranged between 23.6-166.667 mg g−1. TiO2/Fe2O3/chitosan nanocomposite and hydrophobic deep eutectic solvents functionalized magnetic iron oxide nanoparticles (Fe3O4@HDES-2) achieved the highest and lowest adsorption capacities, as shown in Table 3 and Fig. 7.
The surface area, pore size, and pore volume of MOFs ranged between 15.48 and 2900 m2 g−1, 2.093 and 14.4 nm, and 0.056 and 1.23 cm3 g−1, as shown in Table 3. The adsorption capacities of MOFs ranged between 143–497.3 mg g−1. The Cu-doped MIL-101 (Fe) and MCNTs-UiO-66-NH2 showed the highest and lowest adsorption capacities, respectively, as shown in Table 3 and Fig. 7.
The adsorption capacity of iron and acid-modified date palm biochar (DPAI) was found to be 72.2 mg g−1 (optimized using Box–Behnken Design (BBD) of RSM), superior to that of pine wood biochar, chemically activated Cocos nucifera shell biochar, and methanol-modified magnetic orange peel biochar, which is due to the increased adsorption sites and improved pore structure. DPAI had the most detrimental environmental effects of all the modified biochars, emitting 10.027 kg CO2 eq kg−1 and requiring 143.22 MJ kg−1 in total energy consumption (CED) due to the modifications of biochar.62 This work developed a modified adsorbent with enhanced adsorption performance, but its performance is lower than many other adsorbents, and its recycling efficiency is low. The high environmental impacts are the main drawbacks of this adsorbent. A steam-activated recycled textile biochar (RT-SABC) achieved an adsorption performance of 53.9 mg g−1 and 50% at optimum conditions. Steam activation enhanced the surface area and micropore volume of the adsorbent. Its pHPZC was 10, indicating the abundance of carbonyl functional groups on its surface. The used adsorbent can be reused for energy production in gasification or syngas production.63 This work showed that the microporous biochar had low adsorption capacity but fast kinetics. In contrast, steam-activated microporous and mesoporous biochar increased adsorption capacity but reduced the adsorption rate or kinetics. A walnut shell-activated biochar (WSAB) achieved a qm of 69.7 mg g−1 with a maximum removal efficiency of 80%. With an estimated cost of USD 6.93 kg−1 of adsorbent, this work produced a reasonably cheap and efficient adsorbent. This adsorbent also performed well in continuous adsorption, but its regeneration work should be conducted to further shed light on its commercial value.64 A biochar derived from pepper steam achieved a maximum adsorption capacity of 569.6 mg g−1 (experimental 18.23 mg g−1).65 This work shows that PS-biochar can remove IBU from wastewater; however, its experimental adsorption capacity is very low compared to most other adsorbents. Machine learning models of LR, DT, RF, Support Vector Machines (SVM), and k-Nearest Neighbor (k-NN) were used to predict the performance of biochar produced at 600–900 °C. Biochar produced at 900 °C achieved better performance than other biochar, and the RF algorithm predicted the best performance with 90.07% accuracy.66
The surface area, pore size, and pore volume of biochar ranged between 88.75 and 727.5 m2 g−1, 1.97 and 10.25 nm, and 0.059 and 0.57 cm3 g−1, as shown in Table 3. The adsorption capacity of biochar ranged between 10.41–72.2 mg g−1. The DPAI and P-BC adsorbents achieved the highest and lowest adsorption capacities, respectively, as shown in Table 2 and Fig. 7.
The cross-linking of 2-hydroxypropyl-β-cyclodextrin polymers with poly(acrylic acid) obtained a qm of 87.5 mg g−1 at an ideal pH of 5.75 This work developed an effective modified polymer adsorbent with good recycling capability for up to ten cycles. A remarkable adsorption performance of 209.8 mg g−1 and 99.9% was attained by polydopamine imprinted polymers with fluorescent carbon dots (PIP-FCDs). PIP-FCDs showed good detection sensitivity (1.58 × 10−5 μM) and high selectivity for IBU in the presence of other drugs such as ketoprofen, aspirin, levofloxacin, and norfloxacin. PIP-FCDs also showed good performance in real wastewater samples with recoveries of 97.65–98.81% for sewage water and 98.23–99.41% for tap water samples.76 This work developed an efficient and economical bifunctional adsorbent (USD 1362.99 per ton) to detect and remove IBU from water. The removal efficiency of IBU onto porous polymer monoliths (PMLE-E) decreased in simulated waters and followed the following trend: distilled water (85.2%) > tap water (77.5%) > sea water (47.9%) > lake water (47%).77 The adsorption of IBU onto Multi-template Molecularly Imprinted Polymer (MIP) ranged between 57% and 69% in river water and influent and effluent wastewaters.78 The three-acyl hydrazone covalent organic polymers, namely H-COP-1, H-COP-2, and H-COP-3, demonstrated qm of 240.8 mg g−1, 232.25 mg g−1, and 242.775 mg g−1, respectively, with an equilibrium adsorption time of 48 h.79 The H-COP-3, which contains a higher number of acyl hydrazone bonds, showed better performance than H-COP-1 and H-COP-2 adsorbents. Though the adsorption capacity of this adsorbent is good, the higher adsorption time makes it an unfeasible adsorbent in real applications. Two adsorbents produced from cocoa shell biomass and functionalized with plasma and glycine had adsorption capacities ranging from 30.59 mg g−1 to 38.95 mg g−1. Surface functionalization enhanced the adsorbent's adsorption capability.80 The adsorption performance of both adsorbents improved compared to the raw biomass. The adsorption performance of these adsorbents needs further improvement to compete with other adsorbents. The functionalizing materials can be changed with other suitable materials to further enhance the adsorption energy and density of active adsorption sites. A maize cob treated with a base (BMC) demonstrated changes in morphology to a net-like microstructure with more cavities. Its pHPZC was also increased from 5.35 to 6.75 due to the attachment of OH functional groups. BMC demonstrated an adsorption capacity and removal efficiency of 44.92 mg g−1 and 91.07% at the best pH 8 and 80 min of equilibrium.81 Treating maize cob with sodium hydroxide (NaOH) did not significantly increase surface area and adsorption performance compared to untreated maize cob (UMC). Therefore, other chemicals such as potassium hydroxide (KOH), phosphoric acid (H3PO4), and other suitable chemicals can be tested to further enhance surface area and adsorption performance. The magnetic anion exchange resins ND-1, ND-2, and ND-3 prepared using different contents of cyclohexanol exhibited a greater capacity for adsorbing IBU at a more rapid rate. This can be attributed to the larger pore width and volume, facilitating a more efficient internal diffusion process. The increase in cyclohexane content increased the surface area, pore size, and pore volume, which resulted in an increase in adsorption capacity and absorption rate due to the increase in internal diffusion of IBU. The adsorption of IBU was hindered by chloride and sulfate ions due to their competition for the active ion exchange sites. At 1 mmol g−1 L−1 of chloride and sulfate ions, the equilibrium adsorption capacities of ND-1, ND-2, and ND-3 were reduced by 63.5%, 56.9%, 48.8%, and 93%, 91.9%, 91.8%, respectively, showing a higher effect of sulfate due to its higher negative charge.82 Although this study shared valuable information about the adsorption mechanism of IBU onto magnetic anion resins, the adsorption capacities are very low compared to other adsorbents, which need further improvements using any other suitable porogen agents. Versatile vermiculite modified by quinoline-based gemini surfactant (DHQU-Vt) showed a fluffy and rough surface, which will be helpful in the adsorption of IBU.83 The modification of Na-VT by DHQU surfactant increased hydrophobicity, interlayer spacing, and decreased surface area and total pore volume. DHQU-Vt achieved a qm of 240.69 mg g−1.83 This work developed an efficient adsorbent for IBU removal, but further study on its continuous adsorption performance and IBU adsorption from real wastewater will shed light on its real-world applications. A calcined spherical hydrochar (CSH) showed the highest adsorption capacity of 95.6 mg g−1 at 360 min of an equilibrium time.84 This work developed an effective adsorbent for IBU removal, but its adsorption capacity needs further improvement, and the equilibrium time needs further reduction to compete with other adsorbents. An aerogel of AMPDSA achieved an adsorption capacity of 39.95 mg g−1 at optimal parameters optimized using Central Composite Design (CCD) of RSM.85 The amine-grafted pumice-derived silica aerogel (AMPDSA) exhibited uniform, amorphous, and spherical particles. These particles contained silica and had a distinct pearl-like structure. Amine grafting of PDSA decreased surface area by 37%, pore volume by 63%, and pore diameter by 41% due to the filling of the pores of aerogel with 3-aminopropyltriethoxysilane (APTES) molecules.85 AMPDSA showed good adsorption capacity and 100% removal efficiency at optimum conditions, but the IBU concentration used was 2–10 mg L−1. Its testing on higher IBU concentrations would further shed light on its performance. The montmorillonite adsorbent modified with Cetyl Dimethyl Benzyl Ammonium Chloride (HDBAC) with different dosages (0.8CEC–1.8CEC (cation exchange capacity)) showed that the H-Mt-16 (qe – 81.64 mg g−1 and 13.14 mg g−1 for H-Mt-16 and Ca-Mt, respectively) performed better than other adsorbents due to higher layer spacing of Ca-Mt-16 (3.31 nm) at this HDBAC loading.86 The adsorption capacity of H-Mt-16 is better than many adsorbents, but it is lower than most adsorbents and needs further improvements using other suitable modifiers.
The adsorption performance of adsorbents is shown in Table 2 and Fig. 7, and the surface properties of adsorbents are shown in Table 3. The surface area ranges from 2.38 to 2900 m2 g−1, pore sizes from 0.0195 to 87.3 nm, and pore volumes from 0.006 to 14.48 cm3 g−1. The adsorbents exhibited adsorption capabilities ranging from 0.220 mg g−1 to 497.3 mg g−1. The removal efficiencies range between 50% to 100%. The adsorbents used to remove IBU were microporous and mesoporous. There seems to be no increasing or decreasing trend between the surface properties and the adsorption capacity of adsorbents due to the involvement of many types of adsorption forces during the adsorption of IBU. The adsorbents consisted of acidic and basic functional groups, which helped in the adsorption of IBU. Adsorbents also showed porous structure and the presence of cavities and holes in their structures, which helped in the adsorption of IBU. Cu-doped Mil-101-(Fe) achieved the best experimental adsorption capacity of 497.3 mg g−1. The optimum pH ranged between 1 to 10, the dosage between 0.0125 and 10 g L−1, the IBU concentration between 0.0764 and 200 mg L−1, and the equilibrium time between 0.083 and 120 h. The PS-biochar, Cu-doped Mil-1010 (Fe), Zr-MOF, Zr-MOF-NH2, P-SBC/Fe3O4 film, and CNs exhibited adsorption capabilities over 300 mg g−1. CNT-Fe2O3-MnO2, TiO2/Fe2O3-MnO2, TiO2/Fe2O3/chitosan nanocomposite, and MCNTs-Ui-66-NH2 all had adsorption capacities greater than 100 mg g−1, while for all other adsorbents, it was below 100 mg g−1. More work has been reported on carbon; however, MOFs performed better than carbon, which shows that they have a higher capability to adsorb IBU, so more work should be conducted on developing efficient MOFs. Although the zeta potential of adsorbents has been the subject of relatively few investigations, it is an important parameter for understanding the adsorbent–adsorbate interactions. The zeta potential of newly developed adsorbents must be determined. Overall, the adsorbents effectively eliminated IBU from wastewater, and the modifications enhanced the adsorption performance compared to unmodified adsorbents.
2.2 Adsorption kinetics, isotherms, thermodynamics, and adsorption mechanism
The adsorption mechanism helps in understanding the adsorption process and designing new adsorbents. The adsorption mechanism of IBU was studied using kinetics, isotherms, thermodynamics, and simulation techniques. The pseudo-first order, pseudo-second order, and intraparticle diffusion models are commonly used kinetics models. The pseudo-second-order model was followed by most of the studies,23,31,62 indicating IBU adsorption as a chemisorption process, while only a few studies followed other models.63 The Freundlich, Langmuir, and Temkin isotherm models are commonly used in IBU adsorption studies. Most of the studies followed the Langmuir model, while a few followed other models, indicating the formation of a monolayer of IBU onto the surface of adsorbents.23,31,62 Adsorption thermodynamics is studied to determine the Gibbs free energy (ΔG), enthalpy (ΔH), and entropy (ΔS), and it helps in understanding the nature and type of adsorption. Most of the studies described adsorption of IBU as an endothermic process,39,98,99 while some described it as an exothermic process.31,83 The adsorption is physisorption if ΔH <40 kJ mol−1 and chemisorption if ΔH is higher than 40 kJ mol−1. Most studies described the adsorption of IBU as a physisorption process,23,55,72,77,103,109 while some described it as a chemisorption process.58,99,110 Most IBU adsorption studies showed negative values of ΔG, indicating the spontaneity of the adsorption process, while the positive values of entropy showed an increase in the entropy during the interaction between IBU and adsorbents.The adsorption of IBU onto steam-activated recycled textile biochar (RT-SABC) involved ketone, amide, ester, aldehyde, carboxylic acid, aromatic ring groups, and ketones. The intraparticle diffusion kinetics model showed three adsorption phases, as shown in Fig. 8.63 The first phase was quick and occurred on the external surface of the adsorbent for a duration of up to 7 h. The second stage took place on the inner surface of the adsorbent for 25 h. The third stage was the equilibrium stage, during which the adsorbate moved from macro- and mesopores to micropores. The graph skipped the origin, indicating that the rate-determining mechanism was not controlled by intraparticle diffusion. The IBU adsorbed onto AT-MC through electrostatic interactions, π–π interactions, H-bonding, Yoshida H-bonding, hydrophobic interactions, and π-interactions.95 The adsorption of IBU onto iron-incorporated pomegranate husk carbon (NPH) occurred through π–π interactions, electrostatic interactions, Yoshida interactions, and hydrogen bonding.97 The adsorption of IBU onto magnetically activated waste-activated coffee residue biochar (MACB) occurred through hydrogen bonding, π–π EDA interaction, and electrostatic interactions.114 The IBU adsorbed onto scandium-modified oxo-triaryl methyl (Sc@oxTAM) through covalent interactions between Sc and oxygen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) in the IBU, while onto other TM@oxTAM (Ti, V, Cr, and Mn) through covalent and electrostatic interactions.115 The IBU adsorbed onto Zn-SPB@C2N by the formation of covalent bonds.116
O) in the IBU, while onto other TM@oxTAM (Ti, V, Cr, and Mn) through covalent and electrostatic interactions.115 The IBU adsorbed onto Zn-SPB@C2N by the formation of covalent bonds.116
 | ||
| Fig. 8 Intraparticle diffusion model kinetics of IBU onto RT-SABC (redrawn).63 | ||
The IBU adsorption mechanism onto activated carbon derived from tree pod biomass, determined using a double-layer model (DLM), is shown in Fig. 9. The IBU was adsorbed by forming two layers on the adsorbent. The first layer was formed through π–π interactions, hydrogen bonding, or π–anion interactions at a higher pH. The hydrogen bonding and π–π stacking were involved in forming the second layer. The first layer was deposited directly onto the adsorbent surface, whereas the subsequent layer was produced on top of the pre-existing layer on the adsorbent surface.88 The IBU adsorbed onto biomass-derived chitosan through dipole–dipole and hydrogen bonding interactions. Amine and hydroxyl groups of chitosan were the H donors, while oxygen atoms of the carbonyl groups and heterocyclic ring were the H acceptors.72 The adsorption of IBU onto PCDM-1000 occurred by hydrogen bonding through phenolic groups with PCDM and IBU as the hydrogen bond donors and acceptors, respectively. The hydrophobic and π–π interactions also participated in the adsorption.34 The adsorption of IBU onto ultrasound-modified activated carbon (USAC) also occurred through donor–acceptor interactions.108 The IBU adsorbed onto cocoa shell biomass-derived and plasma and glycine functionalized adsorbents through physical forces with adsorption energy in the range of 1.46–3.25 kJ mol−1. The adsorption was controlled by the density of adsorption sites and the adsorption energy.80 The IBU adsorbed onto CS_Fe_MIP monolith through interactions between chitosan functional groups, imprinted cavities, and iron hydroxide with IBU molecules. The electrostatic interactions between hydrogel and IBU, as well as the hydrogen bonding between the amine and hydroxyl group on chitosan and IBU, facilitated the adsorption.74 The adsorption of IBU onto zeolite-sepiolite nanoheterostructures (Zeo-Sep) and modified organo-sepiolite (O-Sep) occurred through the formation of two layers. It occurred through horizontal and non-horizontal orientations, which depended on the temperature. The adsorption of IBU onto both adsorbents was a multi-molecular and multi-docking process. The interactions between IBU/Zeo-Sep, IBU/O-Sep, and IBU/IBU displayed that it was a physisorption process.117 The IBU adsorbed onto the P-SBC/Fe3O4 film through a multilayer process, primarily occurring by electrostatic interactions between the main amine of the P-SBC/Fe3O4 film and the carboxyl groups of IBU. Other interactions of van der Waals forces and hydrogen bonding were also involved in the absorption. An average of three to four active adsorbent sites shared one molecule of IBU.69
 | ||
| Fig. 9 Adsorption mechanism of IBU onto activated carbon (redrawn).88 | ||
The adsorption of IBU onto DHQU-Vt adsorbent occurred through π–π stacking, π–π interactions, XH–π interactions, partition process, and electrostatic interactions. The density functional theory (DFT) simulations revealed that the intra-particle diffusion effect directly affected the molecular flexibility of the adsorbate, π–π stacking between isolated aromatic rings was stronger than between parallelly connected aromatic rings, and quinoline interactions like CH–π stacking, NH–π, and π–π interactions were weaker than electrostatic interactions/intraparticle diffusion.83 The IBU adsorbed onto two MOFs,-UiO-66 and UiO-66-NH2, through four interactions of hydrogen bonding, π–π EDA interactions, anion-π interactions, and Lewis acid/base complexing (LAB). The binding energies of these interactions decreased in the following order: π–π > hydrogen bonding > LAB > anion–π. The aggregation occurred at pH < pHpzc, and repulsion occurred at pH > pHpzc.118 The adsorption of IBU onto activated carbon depended on the degree of dissociation of IBU. The changes in pH and temperature dissociated IBU (ionized[A−] and non-ionized). At lower pH and higher temperatures, dissociation decreased, increasing adsorption capacity. Dissociation was high at pH > pHpzc, which caused repulsion between positively charged activated carbon species and negatively charged IBU species, and decreased the adsorption capacity.119 The adsorption of IBU onto plasma-modified biomass occurred through the formation of two layers. The adsorption temperature determined the production of dimers and trimers in the solution. The adsorption occurred on inclined positions on the biomass surface, and thermal agitation and steric hindrance could affect the adsorption process.120
The IBU adsorbed onto different adsorbents through a variety of interactions, such as electrostatic interactions, π–π interactions, pore filling, pore diffusion, π–π EDA interactions, hydrogen bonding, and Yoshida interactions. The DFT has been used for determining the adsorption mechanism. However, molecular dynamics simulations can also be used in the future to determine the adsorption mechanism of IBU.
2.3 Regeneration and recycling
The regeneration of the adsorbent makes it economical and increases its commercial value. The IBU adsorbed adsorbents were regenerated using chemical and physical methods, as shown in Table 4. The PCDM-1000 was regenerated by washing with acetone and can be used for up to three consecutive cycles without any significant loss in activity.34 GONPs showed no significant decrease in IBU removal efficiency up to eight regeneration cycles, while slightly decreased from 97.18% to 95.91% in the last two cycles (9th and 10th).98 The BHHA adsorbent regenerated using HCl (0.2 M) showed an increase of removal efficiency from 88.75% (1st cycle) to 94.68% in four cycles.99 The NP-YC can be reused consecutively for several cycles without desorption of IBU. The adsorption capacity declined gradually throughout eight cycles when the IBU concentration was 15 mg L−1. However, it abruptly increased with increasing IBU concentration to 60 mg L−1 in the ninth cycle. Subsequently, the adsorption capacity further decreased with increasing IBU concentration to 90 mg L−1.35 In the ninth cycle, the adsorption capacity was enhanced by increasing the amount of IBU molecules. However, this capacity was later diminished when the adsorbent reached saturation. The Ery-AC achieved good adsorption performance after regeneration for seven cycles. The adsorption capacity started to decrease after seven cycles.39 NPC-2 does not drop its adsorption capacity significantly up to four cycles.40 Versatile vermiculite modified by quinoline-based gemini surfactant (DHQU-Vt) showed a good adsorption capacity up to three regeneration cycles, with a decrease of 8.13 mg g−1 in adsorption capacity.83 A Cu-doped Mil-101(Fe) maintained good removal efficiency for up to five regeneration cycles.57 The UiO-67(Zr)-BA (10) can be utilized for a maximum of four cycles with a negligible decline in its adsorption capability.54 Alg/AC/CMC retained an adsorption capacity equivalent to 93% of its initial adsorption capacity after ten regeneration cycles with a desorption efficiency above 80%.41 The P-SBC/Fe3O4 film showed a very good recycling capability with 96% removal efficiency after 17 regeneration cycles.69| Adsorbent | Regeneration method | Cycles | Drop in removal efficiency (%) | Ref. |
|---|---|---|---|---|
| Carbon nanospheres (CNs) | Deionized water (DW) | 6 | 16.91 | 38 |
| BC/RF/MNPs | Mixture of water and acetone | 4 | 13.62 | 48 |
| NiFe2O4@SiO2@APTS | NaOH (0.01 M) | 4 | 5 | 45 |
| Pepper stem-derived biochar (PS-biochar) | NaOH (0.1 M) | 4 | 22.27 | 65 |
| CMC/PPY | NaOH (1 M) | 5 | 36 | 106 |
| BMC | HCl (0.2 M) | 5 | 6.4 | 81 |
| CNT-Fe3O4-MnO2 nanocomposite | HCl (0.1 M) | 5 | 6.2 | 47 |
| CPBC | Methanol | 4 | 18.6 | 102 |
| CCBC | 19 | |||
| Pinewood biochar | Methanol | 4 | 61 | |
| CS_Fe_MIP monolith | Methanol | 4 | 15 | 74 |
| GGC-MOF200 | Methanol | 5 | 28.9 | 67 |
| PMLE-E | Methanol | 8 | 22.1 | 77 |
| RSBCDF | Methanol/acetic acid | 4 | 24.56 | 100 |
| DPAI | Oven (150 °C for 2 h) | 2 | 23.7 | 62 |
| NaX-CD | Ethanol | 5 | 77 | 67 |
| P-BC | Ethanol | 5 | 25.7 | 94 |
| LF/AgNPs | — | 4 | 10.8 | 50 |
| Cellulosic sisal-poly (ppy-Ani) | — | 4 | 16.7 | 103 |
| Zr-MOF-NH2 | — | 5 | 16.8 | 55 |
| MCNTs-UiO-66-NH2 | NaHCO3 | 5 | 58 | |
| Fe3O4@HDES-2 | Acetonitrile | 5 | 5.8 | 51 |
The recycling results of adsorbents are shown in Table 4. The drop in removal efficiency was determined based on the initial and final removal efficiencies. The removal efficiency after 1st regeneration was used where the initial removal efficiency was not given. It can be noticed that some adsorbents can be recycled up to 4 or 5 cycles. The regeneration of adsorbents with their drop in removal efficiency above 10% should be improved to make them more feasible. The regeneration and recycling of only a few adsorbents were conducted, and it is recommended that the regeneration and recycling of newly developed adsorbents be conducted to know more about their commercial feasibility. Future studies should focus on efficient, sustainable, green, and economical regeneration methods for the regeneration of adsorbents to avoid further pollution of the environment.
2.4 Continuous adsorption of ibuprofen (IBU)
The adsorption performance of adsorbents in continuous columns is especially important for their commercial feasibility. The RT-SABC attained a qm of 40 mg g−1 when the bed height in the fixed-bed column was set at 30 cm. Upon increasing the bed height from 10 to 30 cm, the volume of effluent treated increased from 0.6 to 6.4 L at the breakthrough point, which occurred between 41.7 and 476.7 h, while the adsorption capacity increased from 22.09 to 39.81 mg g−1.63 The Ca2+(TAABB)Al obtained an adsorption performance of 17.54 mg g−1 and 97.48% at the best IBU concentration of 20 mg L−1, bed height of 20 cm, and flow rate of 2 mL min−1. The adsorbent displayed good IBU adsorption performance up to five regeneration cycles.113 An SF/PANI/LDH composite demonstrated that increasing IBU concentration from 40 to 80 mg L−1 and flow rate from 4 to 8 mL min−1 decreased the adsorbate's service volume in a small fixed-bed column.101 A walnut shell-activated biochar (WSAB) achieved a maximum adsorption capacity of 30.1 mg g−1.64It can be noticed that only a few studies have determined adsorbent performance in continuous mode using fixed-bed columns. It is impossible to evaluate the commercial feasibility of the adsorbent without its performance evaluation in the continuous mode. More research is needed to evaluate the commercial feasibility of adsorbents by focusing on refining the parameters and optimizing the regeneration process in continuous mode. Other continuous adsorption systems, such as moving beds, fluidized beds, and pulsed beds, should also be used to test the performance of adsorbents due to their widespread use in industries.
3. Future perspectives and challenges in the removal of IBU using adsorbents
Several adsorbents have been designed to remove IBU from wastewater, each with different levels of effectiveness. However, certain issues still need to be addressed in future studies.The adsorption performances of Nauclea diderrichii biomass-derived activated carbon (NDAC), porous carbon derived from MOF (zeolitic-imidazolate framework-8 (ZIF-8)) (PCDMs), ethylamine-modified hydrophobic activated carbon (HAC-EA), magnetic nanoparticles incorporated on yeast-based activated carbon (NP-YC), iron and acid-modified date palm biochar (DPAI), β-cyclodextrin modified and fly ash-derived zeolite (NaX-CD), modified cationic octadecylamine natural montmorillonite (C18-Mt), chitosan obtained from mud crab shells, two adsorbents produced from cocoa shell biomass and functionalized with plasma and glycine, maize cob treated with a base (BMC), calcined spherical hydrochar (CSH), and H-Mt-16 needs further improvement. Carbon nanospheres (CNs) only showed good adsorption in synthetic wastewater, while their performance in real wastewater needs further improvement. The removal efficiency of Erythrina speciosa activated carbon (Ery-AC) needs further enhancement at higher IBU concentrations. The adsorption performance of CNT-Fe3O4-MnO2 nanocomposite needs improvement in simulated pharmaceutical wastewater. The adsorption performance of molecularly imprinted Fe(III) incorporated chitosan hydrogels (CS_Fe_MIP) is quite low and needs further enhancement. The equilibrium time of acyl hydrazone covalent organic polymers (H-COP-3) is quite high, which needs to be reduced. Generally, the adsorption performance of adsorbents can be increased by increasing the porosity, surface area, and number of active adsorption sites on the surface of the adsorbent by functionalization with suitable compounds. The adsorption performance of PCDMs can be enhanced by improving their surface chemistry by increasing the content of phenolic groups through doping with some suitable materials. NaX-CD is fast in removing IBU, but its adsorption capacity needs further improvement through the surface functionalization of zeolite with suitable compounds. The performance of chitosan obtained from mud crab shells can be enhanced by increasing its surface properties and hydrogen bond acceptors by combining with suitable compounds or acid or alkaline treatment. The adsorption performance of adsorbents obtained from cocoa shell biomass and functionalized with plasma and glycine can be enhanced by functionalization with other suitable materials to further enhance the adsorption energy and density of active adsorption sites. The adsorption performance of maize cob treated with sodium hydroxide (NaOH) can be enhanced by treating it with other chemicals, such as potassium hydroxide (KOH) and phosphoric acid (H3PO4). The performance of magnetic anion exchange resins (ND-1, ND-2, and ND-3) can be enhanced using other suitable porogen agents instead of cyclohexane.
Most of the adsorbents were only tested for IBU removal in batch mode; the adsorbents must also be tested for continuous adsorption of IBU to get more insights about their commercial feasibility. More focus should be put on developing highly efficient, economical, green, and regeneratable adsorbents that can adsorb multiple drugs from wastewater. Mass transfer adsorption kinetics should be studied to better understand adsorption processes. Statistical and machine learning tools such as Response Surface Methodology (RSM) and Artificial Neural Networks (ANN), Linear Regression (LR), Decision Tree (DT), and Random Forest (RF), Support Vector Machines (SVM), and k-Nearest Neighbor (k-NN) have been used to optimize the adsorption parameters. Artificial intelligence technologies should be utilized in IBU removal from wastewater to anticipate the adsorption capacity of adsorbents. The disposal of IBU adsorbed adsorbents should also be studied. The World Health Organization (WHO) standards for IBU removal should be followed in IBU removal processes for universal environmental protection.
4. Conclusions
Various adsorbents such as activated carbons, nanomaterials, metal–organic frameworks, biochar, and others have been developed to remove IBU from wastewater. The adsorbents were mostly mesoporous and a few macro- and microporous, with a surface area of 2.38 to 2900 m2 g−1. The porous structure, the presence of cavities, and acidic and basic functional groups supported the adsorption of IBU. The Cu-doped Mil-101(Fe) adsorbent achieved the highest adsorption capacity of 497.3 mg g−1, and Albizia lebbeck seed pods activated carbon (MSAC) achieved the lowest adsorption capacity of 0.220 mg g−1. More activated carbons have been developed than other types of adsorbents. More focus has been put on batch adsorption on model wastewater with kinetics, isotherms, and thermodynamic studies. The adsorption of IBU involved electrostatic interactions, π–π interactions, pore filling, pore diffusion, π–π EDA interactions, hydrogen bonding, and Yoshida interactions. Future studies should focus more on developing highly efficient, economical, green, and regeneratable adsorbents that can adsorb multiple drugs from wastewater, adsorption of IBU and other pharmaceuticals from real wastewaters, continuous adsorption of IBU and other pharmaceuticals, mass transfer adsorption kinetics for a detailed understanding of the adsorption process, artificial intelligence technologies for predicting the adsorption capacity of adsorbents and the removal efficiency of adsorbents, and the disposal of IBU adsorbed adsorbents.Data availability
No primary research results, software, or code have been included, and no new data were generated or analyzed as part of this review.Author contributions
Ahmer Ali Siyal writing – original draft. Radin Maya Saphira Radin Mohamed supervision. Faizan Ahmed writing – review and editing. Marlinda Abdul Malek writing – review and editing. Majed Alsubih visualization. Rashid Shamsuddin writing – review and editing. Sajid Hussain writing – review and editing. Sabariah Musa resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate Studies at King Khalid University Saudi Arabia for funding this work through the Large Research Project under grant number RGP2/67/46 and to the Ministry of Higher Education (MOHE) Malaysia through the Fundamental Research Grant Scheme (FRGS/1/2024/WAS02/UTHM/02/5).References
- K. Fent, A. A. Weston and D. Caminada, Aquat. Toxicol., 2006, 76, 122–159 CrossRef CAS PubMed.
- C. Stamm, K. Räsänen, F. J. Burdon, F. Altermatt, J. Jokela, A. Joss, M. Ackermann and R. I. L. Eggen, in Advances in Ecological Research, ed. A. J. Dumbrell, R. L. Kordas and G. Woodward, Academic Press, 2016, vol. 55, pp. 183–223 Search PubMed.
- R. B. González-González, P. Sharma, S. P. Singh, J. H. P. Américo-Pinheiro, R. Parra-Saldívar, M. Bilal and H. M. N. Iqbal, Sci. Total Environ., 2022, 821, 153329 CrossRef PubMed.
- Y. Zhang, S.-U. Geißen and C. Gal, Chemosphere, 2008, 73, 1151–1161 CrossRef CAS PubMed.
- J. H. Al-Rifai, C. L. Gabelish and A. I. Schäfer, Chemosphere, 2007, 69, 803–815 CrossRef CAS PubMed.
- T. A. Ternes, Water Res., 1998, 32, 3245–3260 CrossRef CAS.
- L. H. M. L. M. Santos, M. Gros, S. Rodriguez-Mozaz, C. Delerue-Matos, A. Pena, D. Barceló and M. C. B. S. M. Montenegro, Sci. Total Environ., 2013, 461–462, 302–316 CrossRef CAS PubMed.
- K. V. Thomas, C. Dye, M. Schlabach and K. H. Langford, J. Environ. Monit., 2007, 9, 1410–1418 RSC.
- P. Verlicchi, M. Al Aukidy, A. Galletti, M. Petrovic and D. Barceló, Sci. Total Environ., 2012, 430, 109–118 CrossRef CAS PubMed.
- Y. Luo, W. Guo, H. H. Ngo, L. D. Nghiem, F. I. Hai, J. Zhang, S. Liang and X. C. Wang, Sci. Total Environ., 2014, 473–474, 619–641 CrossRef CAS PubMed.
- M. Al Sharabati, R. Abokwiek, A. Al-Othman, M. Tawalbeh, C. Karaman, Y. Orooji and F. Karimi, Environ. Res., 2021, 202, 111694 CrossRef CAS PubMed.
- K. Pazdro, M. Borecka, G. Siedlewicz, A. Biak-Bieliska and P. Stepnowski, Curr. Anal. Chem., 2016, 12, 202–226 CrossRef CAS.
- J. Sharma, M. Joshi, A. Bhatnagar, A. K. Chaurasia and S. Nigam, Environ. Res., 2022, 215, 114219 CrossRef CAS PubMed.
- E. Emmanuel, Y. Perrodin, G. Keck, J. M. Blanchard and P. Vermande, J. Hazard. Mater., 2005, 117, 1–11 CrossRef CAS PubMed.
- S. Bancos, M. P. Bernard, D. J. Topham and R. P. Phipps, Cell. Immunol., 2009, 258, 18–28 CrossRef CAS PubMed.
- S. Chopra and D. Kumar, Heliyon, 2020, 6, e04087 CrossRef PubMed.
- Y. Li, G. Zhu, W. J. Ng and S. K. Tan, Sci. Total Environ., 2014, 468–469, 908–932 CrossRef CAS PubMed.
- S. Álvarez-Torrellas, A. Rodríguez, G. Ovejero and J. García, Chem. Eng. J., 2016, 283, 936–947 CrossRef.
- A. S. Mestre, J. Pires, J. M. F. Nogueira and A. P. Carvalho, Carbon, 2007, 45, 1979–1988 CrossRef CAS.
- BASF, 2022.
- M. Caban, A. Bialk-Bielinska, P. Stepnowski and J. Kumirska, Curr. Anal. Chem., 2016, 12, 249–257 CrossRef CAS.
- S. Chopra and D. J. H. Kumar, Heliyon, 2020, 6, e04087 CrossRef PubMed.
- S. N. F. Ali, E. I. El-Shafey, S. Al-Busafi and H. A. J. Al-Lawati, J. Environ. Chem. Eng., 2019, 7, 102860 CrossRef CAS.
- S. Show, P. Chakraborty, B. Karmakar and G. Halder, Sci. Total Environ., 2021, 786, 147327 CrossRef CAS PubMed.
- S. N. Oba, J. O. Ighalo, C. O. Aniagor and C. A. Igwegbe, Sci. Total Environ., 2021, 780, 146608 CrossRef CAS PubMed.
- F. Ahmad, Heliyon, 2023, 9, e16449 CrossRef CAS PubMed.
- J.-l. Wu, Z.-h. Liu, Q.-g. Ma, L. Dai and Z. Dang, Sci. Total Environ., 2023, 891, 164600 CrossRef CAS PubMed.
- A. Ayati, B. Tanhaei, H. Beiki, P. Krivoshapkin, E. Krivoshapkina and C. Tracey, Chemosphere, 2023, 323, 138241 CrossRef CAS PubMed.
- P. Madariaga-Segovia, S. Párraga and C. A. Villamar-Ayala, Bioresour. Technol. Rep., 2023, 23, 101564 CrossRef CAS.
- M. J. Ahmed, J. Environ. Manage., 2017, 190, 274–282 CrossRef CAS PubMed.
- M. O. Omorogie, J. O. Babalola, M. O. Ismaeel, J. D. McGettrick, T. M. Watson, D. M. Dawson, M. Carta and M. F. Kuehnel, Adv. Powder Technol., 2021, 32, 866–874 CrossRef CAS.
- A. F. M. Streit, G. C. Collazzo, S. P. Druzian, R. S. Verdi, E. L. Foletto, L. F. S. Oliveira and G. L. Dotto, Chemosphere, 2021, 262, 128322 CrossRef CAS PubMed.
- L. Matějová, J. Bednárek, J. Tokarský, I. Koutník, B. Sokolová and G. J. F. Cruz, Appl. Surf. Sci., 2022, 605, 154607 CrossRef.
- B. N. Bhadra, I. Ahmed, S. Kim and S. H. Jhung, Chem. Eng. J., 2017, 314, 50–58 CrossRef CAS.
- G. Labuto, A. P. Carvalho, A. S. Mestre, M. S. dos Santos, H. R. Modesto, T. D. Martins, S. G. Lemos, H. D. T. da Silva, E. N. V. M. Carrilho and W. A. Carvalho, Sustain. Chem. Pharm., 2022, 28, 100703 CrossRef CAS.
- I. A. Olowonyo, K. K. Salam, M. O. Aremu and A. Lateef, Waste Manag. Bull., 2024, 1, 217–233 CrossRef.
- A. C. Fröhlich, E. L. Foletto and G. L. Dotto, J. Clean. Prod., 2019, 229, 828–837 CrossRef.
- A. A. Alluhaybi, A. M. Hameed, M. T. Alotaibi, A. Alharbi and A. Shahat, J. Mol. Liq., 2023, 383, 122059 CrossRef CAS.
- D. S. P. Franco, D. Pinto, J. Georgin, M. S. Netto, E. L. Foletto, C. Manera, M. Godinho, L. F. O. Silva and G. L. Dotto, J. Environ. Chem. Eng., 2022, 10, 108070 CrossRef CAS.
- X. Lei, L. Huang, K. Liu, L. Ouyang, Q. Shuai and S. Hu, J. Colloid Interface Sci., 2021, 604, 823–831 CrossRef CAS PubMed.
- J. W. Lee, J. Han, Y.-K. Choi, S. Park and S. H. Lee, Int. J. Biol. Macromol., 2023, 249, 126053 CrossRef CAS PubMed.
- A. A. Basheer, J. Mol. Liq., 2018, 261, 583–593 CrossRef CAS.
- G. Z. Kyzas and K. A. Matis, J. Mol. Liq., 2015, 203, 159–168 CrossRef CAS.
- S. C. Endres, L. C. Ciacchi and L. Mädler, J. Aerosol Sci., 2021, 153, 105719 CrossRef CAS.
- S. Chandrashekar Kollarahithlu and R. M. Balakrishnan, J. Clean. Prod., 2021, 294, 126155 CrossRef CAS.
- V. Priyan and S. Narayanasamy, Environ. Toxicol. Pharmacol., 2022, 94, 103930 CrossRef PubMed.
- I. Lung, M.-L. Soran, A. Stegarescu, O. Opris, S. Gutoiu, C. Leostean, M. D. Lazar, I. Kacso, T.-D. Silipas and A. S. Porav, J. Hazard. Mater., 2021, 403, 123528 CrossRef CAS PubMed.
- A. M. Badawy, A. A. Farghali, A. Bonilla-Petriciolet, M. K. Seliem, A. Q. Selim, M. A. Ali, M. Al-Dossari, N. S. A. El-Gawaad, M. Mobarak, E. C. Lima and H. I. Bendary, J. Taiwan Inst. Chem. Eng., 2023, 152, 105177 CrossRef CAS.
- X. Yang, J. Wang, A. M. El-Sherbeeny, A. A. AlHammadi, W.-H. Park and M. R. Abukhadra, Chem. Eng. J., 2022, 431, 134312 CrossRef CAS.
- S. Joodaki and A. Mollahosseini, Environ. Nanotechnol. Monit. Manag., 2023, 20, 100823 CAS.
- N. M. Hashim, M. Mohamad, N. N. S. N. M. Kamal, M. Y. Aziz, S. Mohamad, N. Yahaya and N. N. M. Zain, J. Mol. Liq., 2023, 383, 122082 CrossRef CAS.
- M. Khajavian and A. Haseli, J. Ind. Eng. Chem., 2024, 137, 583–592 CrossRef CAS.
- M. H. Manzoor, N. Naz, S. M. G. Naqvi, S. Ashraf, M. Z. Ashiq and F. Verpoort, Appl. Mater. Today, 2024, 40, 102358 CrossRef.
- M. M. H. Mondol, D. K. Yoo and S. H. Jhung, J. Environ. Chem. Eng., 2022, 10, 108560 CrossRef CAS.
- N. D. Alkhathami, N. A. Alamrani, A. Hameed, S. D. Al-Qahtani, R. Shah and N. M. El-Metwaly, Polyhedron, 2023, 235, 116349 CrossRef CAS.
- T. Wu, N. Prasetya and K. Li, J. Environ. Chem. Eng., 2022, 10, 107432 CrossRef CAS.
- P. Xiong, H. Zhang, G. Li, C. Liao and G. Jiang, Sci. Total Environ., 2021, 797, 149179 CrossRef CAS PubMed.
- X. Zhang, C. Gao, R. Wang and R. Han, J. Environ. Chem. Eng., 2023, 11, 111090 CrossRef CAS.
- M. M. Hassan and C. M. Carr, Chemosphere, 2021, 265, 129087 CrossRef CAS PubMed.
- M. Ahmad, A. U. Rajapaksha, J. E. Lim, M. Zhang, N. Bolan, D. Mohan, M. Vithanage, S. S. Lee and Y. S. Ok, Chemosphere, 2014, 99, 19–33 CrossRef CAS PubMed.
- M. Essandoh, B. Kunwar, C. U. Pittman, D. Mohan and T. Mlsna, Chem. Eng. J., 2015, 265, 219–227 CrossRef CAS.
- J. F. Shaheen, J. O. Eniola and B. Sizirici, Bioresour. Technol. Rep., 2024, 25, 101696 CrossRef CAS.
- C. Rabbat, A. Pinna, Y. Andres, A. Villot and S. Awad, J. Water Proc. Eng., 2023, 53, 103830 CrossRef.
- M. Patel, A. K. Chaubey, C. U. Pittman and D. Mohan, Environ. Sci.:Adv., 2022, 1, 530–545 CAS.
- A. Naima, F. Ammar, O. Abdelkader, C. Rachid, H. Lynda, A. Syafiuddin and R. Boopathy, Bioresour. Technol., 2022, 347, 126685 CrossRef CAS PubMed.
- M. R. Islam, Q. Wang, S. Sharmin and C. E. Enyoh, Water, 2024, 16, p. 3469.
- L. Bandura, M. Białoszewska, S. Malinowski and W. Franus, Appl. Surf. Sci., 2021, 562, 150160 CrossRef CAS.
- M. Obradović, A. Daković, D. Smiljanić, M. Ožegović, M. Marković, G. E. Rottinghaus and J. Krstić, Microporous Mesoporous Mater., 2022, 335, 111795 CrossRef.
- Y. Liu, Y.-S. Xiong, M.-X. Li, W. Li and K. Li, Int. J. Biol. Macromol., 2024, 265, 130969 CrossRef CAS PubMed.
- J. Martín, M. d. M. Orta, S. Medina-Carrasco, J. L. Santos, I. Aparicio and E. Alonso, Appl. Clay Sci., 2019, 171, 29–37 CrossRef.
- J. L. Malvar, J. Martín, M. d. M. Orta, S. Medina-Carrasco, J. L. Santos, I. Aparicio and E. Alonso, Appl. Clay Sci., 2020, 189, 105529 CrossRef CAS.
- E. W. E. S. Shahrin, N. A. H. Narudin, N. N. M. Shahri, M. Nur, J.-W. Lim, M. R. Bilad, A. H. Mahadi, J. Hobley and A. Usman, Emerging Contam., 2023, 9, 100199 CrossRef CAS.
- W. Phasuphan, N. Praphairaksit and A. Imyim, J. Mol. Liq., 2019, 294, 111554 CrossRef CAS.
- M. Stachowiak, M. Cegłowski and J. Kurczewska, Int. J. Biol. Macromol., 2023, 251, 126356 CrossRef CAS PubMed.
- J. Wang and F. Yang, Mater. Lett., 2021, 284, 128882 CrossRef CAS.
- I. Mohiuddin, R. Singh and V. Kaur, Int. J. Biol. Macromol., 2024, 269, 131765 CrossRef CAS PubMed.
- M. Sözbir, E. B. Simsek, H. H. Mert, B. Kekevi, M. S. Mert and E. H. Mert, Microporous Mesoporous Mater., 2023, 354, 112509 CrossRef.
- L. M. Madikizela and L. Chimuka, J. Environ. Chem. Eng., 2016, 4, 4029–4037 CrossRef CAS.
- H. Zhu, C. Ni, L. Zhou, Y. Chen and Y. Qin, J. Environ. Chem. Eng., 2023, 11, 109228 CrossRef CAS.
- H. A. Al-Yousef, B. M. Alotaibi, F. Aouaini, L. Sellaoui and A. Bonilla-Petriciolet, J. Mol. Liq., 2021, 331, 115697 CrossRef CAS.
- P. M. Thabede, N. A. H. Khumalo, P. N. Mahlambi, P. Nyamukamba and S. J. Modise, S. Afr. J. Chem. Eng., 2023, 46, 376–385 Search PubMed.
- J. Wang, H. Li, C. Shuang, A. Li, C. Wang and Y. Huang, Microporous Mesoporous Mater., 2015, 210, 94–100 CrossRef CAS.
- T. Shen, T. Han, Q. Zhao, F. Ding, S. Mao and M. Gao, Chemosphere, 2022, 295, 133846 CrossRef CAS PubMed.
- S.-H. Yoo, S.-C. Lee, H.-Y. Jang and S.-B. Kim, Chemosphere, 2023, 311, 137074 CrossRef CAS PubMed.
- A. Mohseni-Bandpei, A. Eslami, H. Kazemian, M. Zarrabi and T. J. Al-Musawi, Environ. Technol. Innovat., 2020, 18, 100642 CrossRef.
- Y. Chu, Y. Dai, M. Xia, X. Xing, F. Wang, Y. Li and H. Gao, Colloids Surf., A, 2024, 681, 132764 CrossRef CAS.
- T. Xing, Y. Wu, Q. Wang, A. Sadrnia, A. Behmaneshfar and E. N. Dragoi, Environ. Res., 2023, 231, 116223 CrossRef CAS PubMed.
- M. Bouzidi, L. Sellaoui, M. Mohamed, D. S. P. Franco, A. Erto and M. Badawi, J. Mol. Liq., 2023, 376, 121457 CrossRef CAS.
- P. Ndagijimana, X. Liu, Q. Xu, Z. Li, B. Pan and Y. Wang, Sep. Purif. Technol., 2022, 299, 121681 CrossRef CAS.
- N. Sivarajasekar, N. Mohanraj, S. Sivamani, J. Prakash Maran, I. Ganesh Moorthy and K. Balasubramani, Mater. Today: Proc., 2018, 5, 7264–7274 CAS.
- B. R. Mphuthi, P. M. Thabede, M. E. Monapathi and N. D. Shooto, Case Stud. Chem. Environ. Eng., 2023, 8, 100436 CrossRef CAS.
- M. Rajamehala, A. M. Kumara Pandian, M. Rajasimman and B. Gopalakrishnan, Environ. Res., 2023, 218, 114984 CrossRef CAS PubMed.
- H. Zeng, M. Gao, T. Shen and F. Ding, J. Taiwan Inst. Chem. Eng., 2018, 93, 329–335 CrossRef CAS.
- X. Yang, X. Zhang, H. H. Ngo, W. Guo, J. Huo, Q. Du, Y. Zhang, C. Li and F. Yang, J. Water Proc. Eng., 2022, 46, 102627 CrossRef.
- P. M. Thabede, Case Stud. Chem. Environ. Eng., 2024, 9, 100718 CrossRef CAS.
- J. M. Navia Mendoza, B. F. Rivadeneira Mendoza, J. Cevallos Mendoza, A. M. Balu, R. Luque, L. A. Zambrano Intriago and J. M. Rodríguez-Díaz, Environ. Res., 2024, 240, 117492 CrossRef CAS PubMed.
- N. D. Shooto, Heliyon, 2023, 9, e20268 CrossRef CAS PubMed.
- P. Banerjee, P. Das, A. Zaman and P. Das, Process Saf. Environ. Prot., 2016, 101, 45–53 CrossRef CAS.
- O. S. Bello, O. C. Alao, T. C. Alagbada and A. M. Olatunde, Sustain. Chem. Pharm., 2019, 13, 100151 CrossRef.
- G. Wu, Q. Liu, J. Wang, S. Xia, H. Wu, J. Zong, J. Han and W. Xing, Ind. Crops Prod., 2021, 173, 114150 CrossRef CAS.
- M. Negarestani, S. Reisi, M. Sohrabi, H. Shayesteh, H. Farimaniraad, A. Mollahosseini, M. Hosseinzadeh and S. Tavassoli, J. Water Proc. Eng., 2024, 57, 104657 CrossRef.
- P. Chakraborty, S. Show, W. Ur Rahman and G. Halder, Process Saf. Environ. Prot., 2019, 126, 193–204 CrossRef CAS.
- A. Khadir, M. Motamedi, M. Negarestani, M. Sillanpää and M. Sasani, Int. J. Biol. Macromol., 2020, 162, 663–677 CrossRef CAS PubMed.
- A. Sherazi, G. Hussain, M. Anis and S. Aurangzeb, Desalination Water Treat., 2024, 317, 100151 CrossRef CAS.
- D. D. Andi Grefa, J. E. Guevara Sánchez, L. R. Bravo Sánchez, M. S. Pomares Alfonso and M. E. Villanueva Tagle, Microchem. J., 2023, 195, 109361 CrossRef CAS.
- V. P. V., N. Kumar, H. K. Rajendran, J. Ray and S. Narayanasamy, Int. J. Biol. Macromol., 2022, 221, 547–557 CrossRef PubMed.
- R. Paparo, M. Di Serio, G. Roviello, C. Ferone, M. Trifuoggi, V. Russo and O. Tarallo, Molecules, 2024, 29, 2210 CrossRef CAS PubMed.
- A. C. Fröhlich, R. Ocampo-Pérez, V. Diaz-Blancas, N. P. G. Salau and G. L. Dotto, Chem. Eng. J., 2018, 341, 65–74 CrossRef.
- H. Guedidi, L. Reinert, Y. Soneda, N. Bellakhal and L. Duclaux, Arab. J. Chem., 2017, 10, S3584–S3594 CrossRef CAS.
- S. P. Dubey, A. D. Dwivedi, M. Sillanpää and K. Gopal, Chem. Eng. J., 2010, 165, 537–544 CrossRef CAS.
- H. Guedidi, L. Reinert, J.-M. Lévêque, Y. Soneda, N. Bellakhal and L. Duclaux, Carbon, 2013, 54, 432–443 CrossRef CAS.
- M. Kim, L. K. Njaramba, Y. Yoon, M. Jang and C. M. Park, Carbohydr. Polym., 2024, 324, 121436 CrossRef CAS PubMed.
- S. Show, R. Sarkhel and G. Halder, Sustain. Chem. Pharm., 2022, 27, 100698 CrossRef CAS.
- J. Shin, J. Kwak, S. Kim, C. Son, Y.-G. Lee, S. Baek, Y. Park, K.-J. Chae, E. Yang and K. Chon, J. Environ. Chem. Eng., 2022, 10, 107914 CrossRef CAS.
- S. Kaviani, M. Khajavian, I. Piyanzina, O. V. Nedopekin and D. A. Tayurskii, J. Mol. Graph. Model., 2024, 126, 108647 CrossRef CAS PubMed.
- M. Khajavian, S. Kaviani, I. Piyanzina, D. A. Tayurskii and O. V. Nedopekin, Colloids Surf., A, 2024, 680, 132702 CrossRef CAS.
- Z. Li, A. Gómez-Avilés, L. Sellaoui, J. Bedia, A. Bonilla-Petriciolet and C. Belver, Chem. Eng. J., 2019, 371, 868–875 CrossRef CAS.
- W. Sun, H. Li, H. Li, S. Li and X. Cao, Chem. Eng. J., 2019, 360, 645–653 CrossRef CAS.
- P. Iovino, S. Canzano, S. Capasso, A. Erto and D. Musmarra, Chem. Eng. J., 2015, 277, 360–367 CrossRef CAS.
- H. A. Al-Yousef, B. M. Alotaibi, M. M. Alanazi, F. Aouaini, L. Sellaoui and A. Bonilla-Petriciolet, J. Environ. Chem. Eng., 2021, 9, 104950 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |