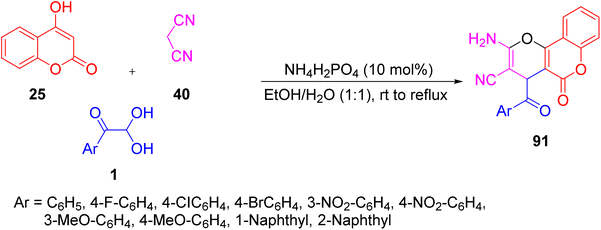Open Access Article
Open Access ArticleAryl glyoxal: a prime synthetic equivalent for multicomponent reactions in the designing of oxygen heterocycles
Abdur Rehman Sheikh
 ,
Anam Arif
and
Md. Musawwer Khan
,
Anam Arif
and
Md. Musawwer Khan
 *
*
Department of Chemistry, Aligarh Muslim University, Aligarh, 202002, India. E-mail: musawwer@gmail.com
First published on 30th April 2025
Abstract
The category of bifunctional building blocks overrides many others because of their fascinating wide applicability in synthetic chemistry. Aryl glyoxal is one of the key molecules used extensively in heterocyclic chemistry to afford nearly all types of five- and six-membered heterocycles, which are the structural constituents of many natural products. The multicomponent reaction is a practical strategy to utilize this wonderful moiety with different types of starting materials to obtain numerous diverse oxygen heterocycles. This review covers the advancement of aryl glyoxal as a prime synthetic equivalent in recent years for the synthesis of oxygen heterocycles.
1. Introduction
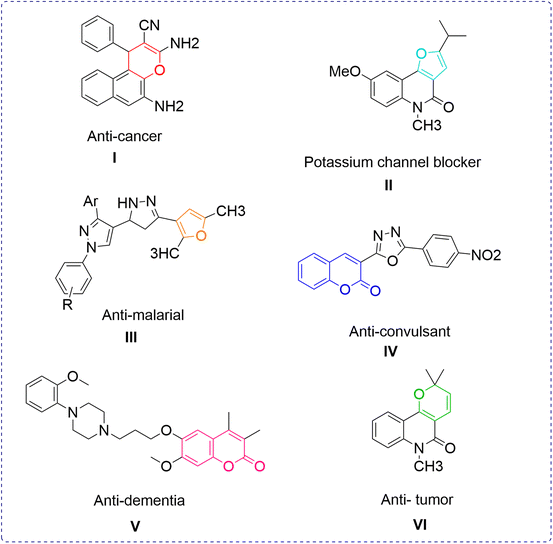
Organic synthesis has served as an all-time interesting topic for researchers from the very beginning. There have been many changes in synthesis paradigms since the first synthesis of an organic compound by Wöhler.1 The quest for an easy and efficient methodology brought about the concept of the one-pot multicomponent reaction approach. Multicomponent reactions (MCRs) are a type of reaction where at least three different starting materials are made to react in a single reaction vessel.2–5 The availability of various reacting sites in the reaction mixture increases the chances of probable novelty in the desired product. Therefore, MCRs have expedited organic synthesis to a significant level of novel compound formation. The pronounced benefits of multicomponent reactions, like efficient atom economy, minimization of extra time consumption, and reduced environmental waste over traditional methods have revolutionized the work of synthetic chemists to produce a chemical library of novel moieties.6 MCRs are the only way to meet the intermittent demands of advanced technology-driven research, such as in medicines, drugs, agrochemicals, polymers and cosmetic industries. Many important building blocks and manifolds are the results of rationally designed multicomponent named reactions. Owing to their significance, reagent- and substrate-based multicomponent reactions have recently been reported in high numbers.7,8Heterocycles occupy a key functional position in many living processes and therefore they provide an in-line sustained research area to be worked upon. Among the heterocycles, hydrocarbons with oxygen as their constituent element in the ring exhibit a special place in the category owing to their several benefits to life. In addition to their occurrence in natural products and living systems, including the human body, scaffolds belonging to each class are known to show distinctive biological and pharmacological properties. Pyran derivatives are screened for antianaphylatic, diuretic, spasmolytic, anticoagulant and anticancer properties, etc.9–11 Alkaloids possessing pyranoquinoline as the structural unit feature anti-microbial characteristics.12 Also, a number of them act as photoactive enhancers in the treatment of neurodegenerative diseases.13,14 Another oxygen heterocycle, namely the furan skeleton, is available in kallolides,15 in fragrances, and dye-like commercial products. The furan derivative furoquinolinone blocks the potassium channel Kv1.3, which is the target for immunosuppressive therapy in the treatment of auto-immune diseases and transplantation.16 Likewise, the pyrazoline derivative containing a furan moiety shows anti-malarial activity against Plasmodium falciparum.17 Isoxazoles, as oxygen–nitrogen heterocycles, add to the synthetic utility of oxygen heterocycles as they offer anti-tubercular,18 anti-inflammatory,19 and COX-2 inhibitor properties,20 and are thus a constituent of many therapeutic drugs. The newly synthesized chroman derivative was tested and found to show potential anti-convulsant21 activity. One of the benzopyranone derivatives, Enasculin, is a pharmacologically tested neuronal activator KA 672-HCl,22 which works as an antagonist by simultaneously activating several neurotransmitters that are deactivated in dementia. Furthermore, the structural presence of benzo[g]chromene in many useful natural products with reported anticancer activity and the synergistic effect of β-lapachone with taxol against tumour growth,23–25 etc., are driving the search for newer strategies to synthesize oxygen heterocyclic moieties in economical and environmentally friendly conditions. Therapeutic functions of some O-heterocycles are illustrated in Fig. 1.
Revolutionary advancements in the physiological studies of living beings for the sake of mankind and the environment are due to the organic biomimetic pathways adopted by synthetic chemists to produce optimum results. So, the challenging process of synthesizing heterocyclic moieties can be facilitated by employing bifunctional building blocks in the reaction design, in which the multiple reactive centres lead to product diversity. Aryl glyoxal is one of the aldo-ketone bifunctional building block molecules used by synthetic chemists to produce a diverse library of molecules.26 The presence of a reactive aldehyde group adjacent to the carbonyl group is the peculiar structural feature that makes aryl glyoxal a distinctive building block for the synthesis of heterocyclic and carbocyclic compounds. The electron-withdrawing ketone group makes aryl glyoxal more reactive than benzaldehyde and allows the site to be open to nucleophilic attack followed by cyclisation in various ways. Furthermore, the non-enolizability of the ketone group under acidic or basic conditions is responsible for its sufficient stability and for making aryl glyoxal monohydrate commercially available. Interestingly, besides the numerous applications of all types of oxygen heterocycles as drugs and therapeutic agents, there are some biologically potent molecules with a phenyl glyoxal unit embedded in their structure (as shown in Fig. 2), such as 4-aroyl chromene derived from phenyl glyoxal hydrate and naphthyl glyoxal, which has shown anti-bacterial properties by inhibiting Escherichia coli growth with a minimum inhibitory concentration (MIC) of 32 μg cm−3.27 A furan-substituted guaiazulene moiety synthesized through a simple route by utilizing phenyl glyoxal exhibited significant in vitro anti-oxidant activity against lipid peroxidation with a minimum IC50 value of 3.9 μg mL−1.28 Guaiazulene, a derivative of azulene, acts therapeutically against skin and asthma-like diseases due to allergy or inflammation reactions.29 Coumarin targets the α-glucosidase enzyme, which is responsible for the hydrolysis of starch and higher carbohydrates into simple sugars.30 Dihydrochromeno[4,3-b]pyrrol-3-yl obtained by using phenyl glyoxal as one of the starting materials exhibits α-glucosidase inhibitory activity,31 thus aiding the drug-development process for hyperglycemia and Type 2 diabetes mellitus (T2DM). This in vitro evaluation was further extended by in silico docking studies with reference to the standard drug acarbose.32 Also, attempts are being made to synthesize the C-4 aroyl group-substituted pyrano[3,2-c]chromene and benzo[g]chromene by employing phenyl glyoxal due to their divergent properties.33 A new HIV integrase inhibitor, namely a pyrano[2,3-d:6,5-d′]dipyrimidine (V-165)-like framework of pyrano[2,3-d:6,5-d′]dipyrimidines, constructed through phenyl glyoxal showed increased chances for extensive use in drug precursor development.34 Phenyl glyoxal was further used in the synthesis of a furo(2,3-b)furan moiety35 mimicking drug candidates like Brecanavir (GW640385), an HIV inhibitor,36 and Darunavir (TMC-114),37,38 another HIV-1 protease inhibitor. A coumarin-glyoxal hybrid, namely the tartrate salt of a Mannich base bearing coumarin derivatives39, was found to be an efficient contraceptive as it shows activity as both a spermicide and a microbicide. The compound was tested for spermicidal activity against nonoxynol (N-9),40 a contraceptive. The two compounds of the series showed activity better than N-9. For anti-microbial activity, again the two compounds showed activity better than the Metronidazole41 chosen as the standard for the study. For this reason, from many years in the past up to recent years, aryl glyoxal has been extensively used as a key building block in multicomponent single-pot reactions.42
In this review article, we intend to highlight the extensive use of the bifunctional building block known as aryl glyoxal monohydrate as the starting material in the construction of novel, and mimics of, naturally found heterocyclic moieties. Here, is a brief account of the reactivity pattern of aryl glyoxal with different substrates leading to the synthesis of various derivatives and fused oxygen heterocycles.
2. Use of aryl glyoxal in designing O-heterocycles using multicomponent reactions
Aryl glyoxal is unique in having two adjacent carbonyl groups, but it mostly exists in the hydrated form. It forms different types of five-membered and six-membered heterocyclic rings depending on the type of other substrates, attacking nucleophiles, and cyclisation pattern in the reaction. The application of aryl glyoxal in the synthesis of five- and six-membered O-heterocycles is discussed in the following sections.2.1. Synthesis of five-membered furan derivatives
Li et al. efficiently utilized aryl glyoxal 1 in the gold-catalysed three-component reaction with amine 2 and a terminal alkyne 3 to obtain substituted furans 4 through cyclisation in a nitrogen atmosphere with methanol as a solvent. This reaction provides an effective protocol for the preparation of synthetic and pharmacological derivatives of furan (Scheme 1).43 The plausible mechanism of the protocol starts with the coupling of aryl glyoxal 1, amine 2 and alkyne 3 in a Mannich–Grignard pattern to give a propargyl intermediate aa, which is followed by the attack of an oxygen lone pair on the electrophilic triple bond, forming a cation ab, leading to the final product indolizines 4 through deprotonation and demetallation of ac (Scheme 2).For the synthesis of isoxazolyl amino furo[3,2-c]quinolinone scaffolds 7, Modugu and co-workers developed an efficient one-pot, three-component reaction of aryl glyoxal monohydrate, 4-hydroxy-1-methyl-2-quinilinone 6, and 4-amino-3-methyl-5-styrylisoxazoles 5 under reflux conditions in an aqueous medium with a 5 mol% catalytic amount of p-TSA. The scheme was tested with different Lewis acids and organic acids, but p-TSA in water as the solvent was found to give the best results in terms of the time, yield and environmentally friendly protocol (Scheme 3).44 The reaction mechanism (as shown in Scheme 4) starts from the p-TSA-catalysed condensation of compound 5 and 1 yielding the iminium ion intermediate ba, which serves as an electrophilic site for the nucleophile 6. The nucleophilic addition reaction between ba and 6 produces the bb intermediate, which is in tautomeric isomerisation with bc. The intermediate bd undergoes intramolecular cyclisation initiated by the acid to give the final product 7 through dehydration of be (Scheme 4).
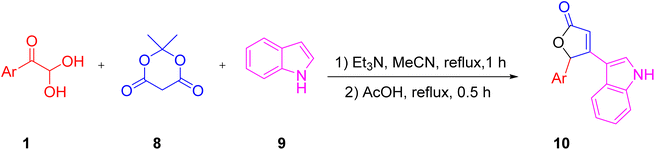
For the synthesis of the furan-2(5H)-one derivative 10 with indole as a structural fragment, Komogortsev and his group established a facile and one-pot novel methodology, which was completed in two steps starting with the interaction of aryl glyoxal 1, indole 9, and Meldrum’s acid 8 in acetonitrile with triethylamine at reflux, followed by an acidic reflux using acetic acid (Scheme 5).45 The formation of the new oxygen heterocycle is considered to go through the aryl glyoxal 1 condensation with Meldrum’s acid to generate intermediate ca followed by Michael addition with the indole to give cb and finally cyclisation with the elimination of CO2 to give 10 (Scheme 6).
 | ||
| Scheme 6 Mechanism to explain the synthesis of furan-2(5H)-one 10 using indole 9, Meldrum’s acid 8, and aryl glyoxal 1 as starting materials. | ||
Shahbazi-Alavi and co-workers demonstrated an environmentally friendly nano-catalysed synthesis of substituted furans. The target molecule 13 was afforded through the heterogeneous catalysis of aryl glyoxal 1, dimethylacetylenedicarboxylate 11, and primary amine 12 at room temperature using the HPA-ZSM-5 nano-catalyst in dichloromethane (Scheme 7).46 The mechanistic pathway of the reaction is initiated by the nucleophilic attack of the amine lone pair 12 on the electrophilic site of dimethyl acetylenedicarboxylate 11 forming an enaminone, namely aminobutenedioate da. This aminobutenedioate acts as a C-nucleophile and attacks the electron-deficient carbon of phenyl glyoxal 1, thereby generating the second intermediate iminium-oxoanion db, which tautomerizes to the intermediate dc. Intermediate dc then undergoes γ-lactonization to give 5-oxo-2,5-dihydro-3-furancarboxylate 13 as the final product (Scheme 8). In 2021, the same protocol was also followed by Ebrahimi and co-workers,47 this time using nano-CuO. This work gained popularity due to its ease of operation, facile and quick extraction of the product, quick response time, high yield, and low loading and re-utilisation of the catalyst.
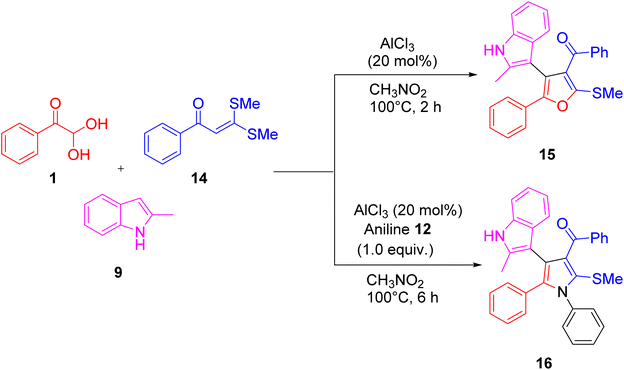
Phenyl glyoxal 1 and α-oxoketene dithioacetals 14 with indoles 9 as the precursors were utilized by Liu et al. to create six multicomponent reactions, which were used to synthesize a variety of heterocycles, including quinolines, dihydrocoumarins, furans 15, and pyrroles 16. The discovery of these multicomponent reactions was made feasible by the coupling of bifunctional aldo-X reagents and α-oxoketene dithioacetals since these reagents contain a minimum of two reactive sites, therefore diverse substrates can be put together in different ways (Scheme 9).48
Dehghanzadeh and co-workers applied aryl glyoxal 1 for the fabrication of 5-(furan-yl)barbiturate and 5-(furan-3-yl)thiobarbiturate 19 via a one-pot assembly of 1 with acetylacetone 17 and barbituric acid and thiobarbituric acid 18, respectively, in water at 60 °C (Scheme 10).49 The plausible mechanism starts with the Knoevenagel reaction between 1 and 17 to give intermediate ea, which undergoes a 1,4-conjugated addition with barbiturate 18 yielding 1,4-diketone eb, followed by a Paal–Knorr cyclisation ec to finally give 5-(furan-3-yl)barbiturate/thiobarbiturate 19 (Scheme 11).
 | ||
| Scheme 10 Three-component synthesis of 5-(furan-yl)barbiturate and 5-(furan-3-yl)thiobarbiturate 19 from acetylacetone 17 and barbituric acid 18. | ||
 | ||
| Scheme 11 Mechanism proposed to explain the formation of 5-(furan-3-yl)barbiturate/thiobarbiturate 19. | ||
A further synthesis was done by Khoeiniha and his group using aryl glyoxal 1 as a building block for obtaining novel 4H-indeno[1,2-b]furan-4-ones 23 through its one-pot condensation with 2-aminopyridines 21 and 1,3-indandione 22 in water in the absence of any catalyst. In addition, by employing aryl glyoxal 1, 21 and barbituric acid 18 under the same green conditions, they obtained furo[2,3-d]pyrimidine 24 derivatives in high yield (Scheme 12).50 The reaction is believed to start from the aldol condensation of 1 and 1,3-indanedione 22 generating intermediate fa. Amine 21 is added to this intermediate through a Michael addition fb, followed by intramolecular cyclisation fc, with subsequent dehydration to produce the final product 23. When 1,3-indandione is replaced with barbituric acid following the same methodology, the furo-pyrimidine derivative 24 is obtained as the product (Scheme 13).
In 2009, Mosslemin’s group reported the isocyanide-based formation of furo[3,2-c]coumarin 27 through a condensation-cycloaddition of the easily available 4-hydroxy coumarin 25 with the extensively used aryl glyoxal and alkyl isocyanides 26 in a neutral acetonitrile medium. The reaction also gave the same result when 4-hydroxycoumarin was substituted with dimethylcyclohexandione (Scheme 14).51 The underlying mechanism for the synthesis begins with the generation of the Knoevenagel adduct ga of 4-hydoxycoumarin 25 and 1 with a subsequent [4 + 1] cycloaddition reaction between the resulting hetero-diene ga and isocyanide 26 to form iminolactone gb as an intermediate. Lastly, a [1,3]-H shift in this iminolactone leads to furo[3,2-c]coumarin 27 (Scheme 15).
 | ||
| Scheme 15 Mechanism to explain the synthesis of furo[3,2-c]coumarin 27 via a cycloaddition reaction. | ||
Chang et al. developed an effective method for synthesising furo[3,2-c]coumarins utilising 1 in multicomponent tandem reactions driven by FeCl3 or ZnCl2. As per the reports, this reaction between 4-hydroxycoumarin 25 and allyltrimethylsilane 28 or toluene 29 produced two C–C bonds and one C–O bond in compounds 30 and 31, respectively. This approach provided the desired furo[3,2-c]coumarin structures in good to outstanding yields. Some notable traits of the method include the ease with which the starting materials may be obtained, the great functional group tolerance, and the outstanding atom economy (Scheme 16).52
 | ||
| Scheme 16 Tandem reactions leading to diverse furo[3,2-c]coumarin compounds 30 and 31 using allyltrimethylsilane 28 and toluene 29, respectively. | ||
The use of aryl glyoxal 1 by Salari and co-workers set an efficient protocol for the synthesis of functionalized trans-tetrahydrobenzofuran-4-ones 34 via the condensation reaction of aryl glyoxal, N-(4-halophenacyl)-pyridinium bromide 33, and cyclic 1,3-diketone 32 utilizing DABCO in a catalytic amount with water as the solvent under reflux conditions. Furthermore, optimization of the protocol was accomplished by using different derivatives of aryl glyoxal, showing that p-nitroaryl glyoxal and o-bromoaryl glyoxal gave the highest yields under the same conditions (Scheme 17).53
 | ||
| Scheme 17 Three-component DABCO-catalysed synthetic protocol for the synthesis of trans-tetrahydrobenzofuran-4-ones 34. | ||
Next, in 2019, the synthetic application of aryl glyoxal was further explored to construct novel moieties of biological importance. Komogortsev et al. devised a new convenient method to obtain 7-oxo-7H-furo[3,2-b]pyran-3-ylacetic acid 36 through the reaction of pyranone 35, Meldrum’s acid 8 and aryl glyoxal 1 as the carbonyl compound in one pot. The reaction was accomplished by the catalysis of Et3N in MeCN under reflux conditions (Scheme 18).54 The distinguishing feature of the protocol is the application of the Kojic acid analogue 3-hydroxy-pyran-4-one 35.
 | ||
| Scheme 18 Synthesis of 7-oxo-7H-furo[3,2-b]pyran-3-ylacetic acid 36 through a condensation reaction. | ||
Lichitsky et al. in 2020 developed a systematic telescoping-synthesis protocol to synthesise substituted furan-2(5H)-one derivatives 39 containing a 4H-chromen-4-one fragment via the multicomponent reaction of 3-(dimethylamino)-1-(2-hydroxyaryl)prop-2-en-1-one 38, aryl glyoxal 1, and Meldrum’s acid 8. The simultaneous production of 4H-chromen-4-one and furan-2(5H)-one fragments 39 in one synthetic stage is a distinguishing aspect of the suggested methodology. This approach is atom economical, and has other virtues, such as mild reaction conditions and a simple workup approach, which eliminates the need for chromatographic purifications (Scheme 19).55
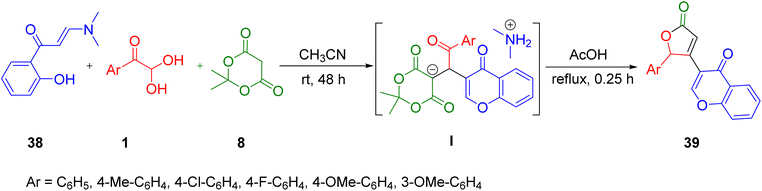 | ||
| Scheme 19 3-(Dimethylamino)-1-(2-hydroxyaryl)prop-2-en-1-one 38-derived formation of the substituted furan-2(5H)-one derivative 39. | ||
In 2020, Komogortsev et al. explored a new one-pot protocol for the synthesis of various substituted 2-aminofuran moieties 41 based on the multicomponent reaction of 3-hydroxy-4H-pyran-4-ones 35, aryl glyoxal 1 and methylene active nitriles 40. The formation of 2-aminofuran is a distinguishing aspect of this protocol. The excellent yield, high atom economy, simple workup procedure and maintenance of mild reaction conditions for the reaction to proceed are all major benefits of this protocol (Scheme 20).56 The suggested mechanism (Scheme 21) begins with the generation of a Michael acceptor from the reaction of the C–H active malononitrile 40 with 1. The allomaltol anion (deprotonated by triethylamine) adds to the intermediate, thus forming the adduct ha. This adduct undergoes deprotonation to form an oxoanion hb followed by cyclisation at the nitriles hc, finally leading to furo-pyran as the final product 41.
In 2013, Karami and co-workers regiospecifically synthesized amido-substituted furo[4,5-c]coumarins 43 by reacting aryl glyoxal 1, 4-hydroxycoumarin 25 and benzamide 42 in a single pot through coupling, followed by cyclisation in acetic acid at reflux (Scheme 22).57 The product showed selectivity over isoxazolo-substituted coumarin. The plausible pathway for the reaction is generation of the intermediate in the condensation reaction, followed by its intramolecular cyclisation and then dehydration to give the final product.
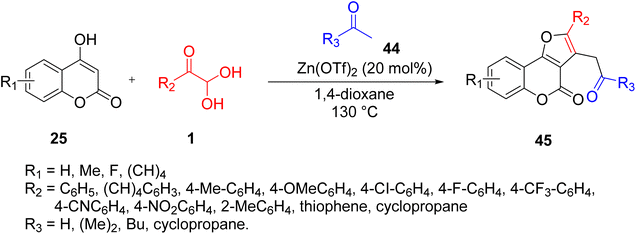
Huang and co-workers reported an efficient Lewis-acid-catalysed reaction for synthesizing diverse furo[3,2-c]coumarins 45 by exploiting aryl glyoxal, 4-hydroxycoumarins 25, and methylketone 44 in 1,4-dioxane at 130 °C using 20 mol% Zn(OTf)2. The substrate scope of the protocol showed that both electron-rich and electron-neutral aryl glyoxal favoured an annulation reaction, while that with an electron-withdrawing group, such as the NO2-bearing aryl ring of aryl glyoxal, did not yield any product (Scheme 23).58 The proposed reaction design (Scheme 24) follows the pathway initially from the loss of a water molecule through the condensation reaction of 1 and 25 to give the intermediate ia, which reacts with the enol form of acetone in a Michael-type addition to produce the second intermediate ib. The electrophilicity of the condensed adduct ia is enhanced by the Zn(OTf)2. Finally, dehydration preceded by an intramolecular cyclisation leads to the formation of furo[3,2-c]coumarin 45.
 | ||
| Scheme 24 Mechanism showing furo[3,2-c]coumarin 45 synthesis through a condensation reaction followed by intramolecular cyclisation. | ||
After this, Lichitsky et al. used the Meldrum's acid 8 with hydroxycoumarin derivative 46 to access the novel furylacetic acid moiety 47. The pronounced advantage of the biologically important furo-coumarin derivative synthesis is that the formation was possible through a single-pot approach using easily available starting materials with no harsh reaction conditions (Scheme 25).59
In 2022, Jana et al. developed a straightforward, facile and efficient approach for the synthesis of novel thioether-linked coumarin-fused furans 49 via the Sc(OTf)3-catalysed one-pot combination of aryl glyoxal 1, 4-hydroxycoumarin 25 and different aromatic thiols 48. This approach can yield either a three-component thioether-linked coumarin-fused furan 49 or a two-component furo-coumarin product 50, depending on the thiols. The key attributes of this approach are its broad substrate range, good to exceptional yields, and products with multiple pharmaceutically significant motifs (Scheme 26).60
 | ||
| Scheme 26 Synthesis of the thioether-linked coumarin-fused furan 49 and the furo-coumarin product 50 through reaction with aromatic thiols 48. | ||
In 2020, Palanivel explored the combination of 1 and benzimidazole acetonitrile 51 with malononitrile 40 at room temperature in a trifilic acid/acetonitrile system to afford tricyclic aza-cyclopenta(cd)diindene 54. The above combination was also executed with benzoyl acetonitrile 53 at room temperature in ethanol/water to access the furo[2,3-b]furan derivative 55 in the presence of the organic base DABCO. Furthermore, he obtained the pyrrolo-pyridine carboxamide 57 and furo-pyrrolo imidazole carboxamide 56 when the aryl glyoxal 1 was made to react with two equivalents of benzimidazole acetonitrile 52 in a pseudo-three-component reaction employing DABCO and NaOtBu as additives, respectively, in ethanol/water at room temperature (Scheme 27).61
2.2. Synthesis of benzofuran derivatives
In 2019, El-Harairy et al. used aryl glyoxal for synthesizing functionalized benzofuran 59 through an ionic liquid catalysis. The desired product 59 was obtained by the assembly of aryl glyoxal hydrate 1, sesamol 58, and indole 9 in an imidazolium-based Brønsted acid ionic liquid/butyl acetate green system. The above catalyst was recovered and used for further reactions (Scheme 28).62In 2020, Lichitskii and co-workers effectively used aryl glyoxal for the preparation of terarylenes 60, a starting material for the synthesis of naphtho[1,2-b]benzofuran-7(8H)-ones 61 via a green photochemical rearrangement reaction of 4H-chromen-4-one derivatives. Aryl glyoxal 1 underwent a three-component tandem reaction with 3-(dimethylamino)-1-(2-hydroxyaryl)prop-2-en-1-one 38 and cyclic 1,3-diketone 32 in the presence of an inert solvent and base at room temperature, followed by the subsequent addition of a hydrochloric acid and acetic acid mixture. The model reaction was optimized by using different solvents and varying the system temperature. Terarylenes were best obtained in good yield at room temperature with acetonitrile and triethylamine as the solvent/base system. The limitation of the novel multicomponent methodology to cyclic diketones was also confirmed by the unsuccessful test reaction of 2,4-pentanedione (Scheme 29).63 The plausible mechanism for this reaction includes the expected base-catalysed condensation reaction of 1,3-diketone 32 with 1, followed by the subsequent addition of enaminone 38 to the generated Michael acceptor ja, forming the adduct jb. Intramolecular cyclisation through nucleophilic attack of the hydroxyl group to the iminium ion generates an intermediate jc, which finally undergoes acid-catalysed dehydration to form the desired terarylene 60 with a new furan moiety in the structure. This terarylene 60 was exposed to UV light of 365 nm for 30 hours and yielded the desired benzofuran derivative (Scheme 30).
 | ||
| Scheme 29 Synthesis of naphtho[1,2-b]benzofuran-7(8H)-ones 61 via photochemical rearrangement reactions of the 4H-chromen-4-one derivative (terarylene) 60. | ||
 | ||
| Scheme 30 Mechanistic pathway representing the synthesis of naphtho[1,2-b]benzofuran-7(8H)-ones 61 via an acid-catalysed dehydration reaction. | ||
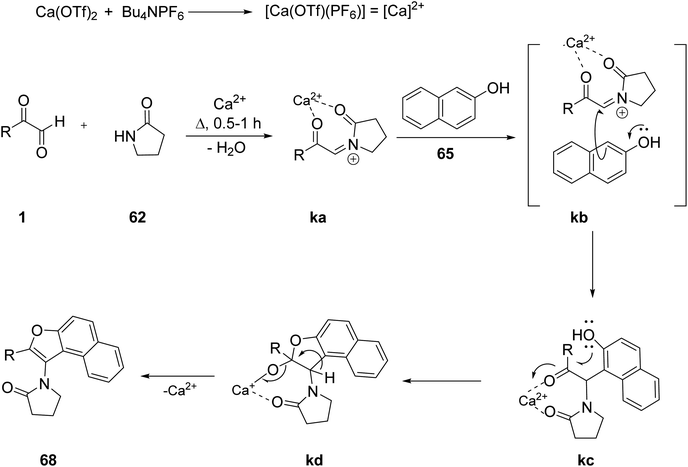
Recently, Rajesh P. et al. successfully demonstrated a three-component calcium-catalysed approach for obtaining naphthofuran (3-aminofurans) 68 in good yield via annulation of the in situ intermediate C,N-diacyliminium ion generated as a result of a three-component reaction between a variety of aryl glyoxal 1 and lactams 62 with 2-naphthols 65 under solvent-free conditions at 100 °C (Scheme 31).64 The proposed mechanism includes formation of the Ca2+-catalysed imine ka in the initial step followed by ligand metathesis between Ca2+ and the hydroxyl group of β-naphthol to form a C–C bond kc, and then an intermediate kd with a new C–O bond. Finally, kd undergoes aromatisation yielding the naphthofuran 68 (Scheme 32). The reaction was also tested against different solvents and Lewis acid catalysts, but none of them gave a yield of more than 50%. The ligand metathesis between Ca(OTf)2 and Bu4NPF6 increases the acidity of the catalyst, making it more efficient and able to provide excellent yields. Interestingly, they also repeated the same protocol by changing the nucleophile source to mequinol (substituted phenol) 63 to access the corresponding benzofuran 66 and later glyoxal with the 9H-fluoren-3-ol derivative 64 and lactam-furnished fluorenofuran 67 in a good yield of 84%. Furthermore, the annulation reaction of glyoxal and lactam with 4-hydroxycoumarin as a nucleophile did not prove efficient under solvent-free conditions. The products were studied for their photophysical properties and a broad substrate scope was established for all of these protocols.
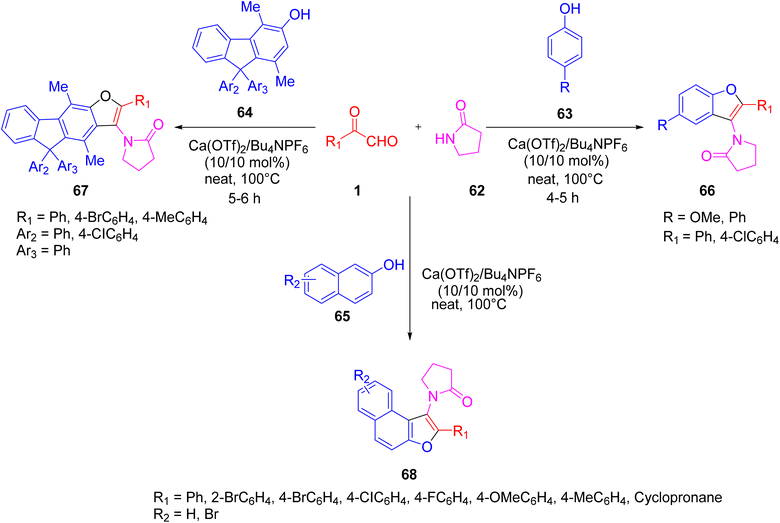 | ||
| Scheme 31 Three-component calcium-catalysed protocol for syntheses of 3-aminofurans 68, fluorenofuran 67 and benzofuran 66. | ||
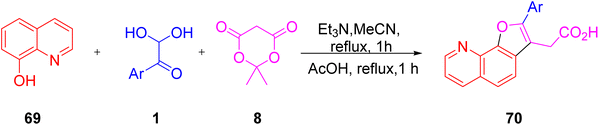
The utility of aryl glyoxal as a synthetic building block was explored by Lichitsky et al. through the triethylamine-promoted reaction of the 4-methoxy derivative of 1 with Meldrum’s acid 8 and 8-hydroxyquinoline 69 in acetonitrile followed by cyclisation in refluxing acetic acid to afford furylacetic acid 70. Advantages of this protocol include a simple work-up route and high atom economy (Scheme 33).65
In 2021, Lichitsky and co-workers described a simple one-pot approach for synthesizing a regiospecific 4H-furo[2,3-h]chromene 72 core using aryl glyoxal. The proposed method was based on a multicomponent reaction of aryl glyoxal, flavones 71, and Meldrum’s acid 8. The mild reaction conditions, atom economy, and simple workup procedure are all advantages of this method, which eliminates the need for chromatographic purification (Scheme 34).66
 | ||
| Scheme 34 Regiospecific synthesis of 4H-furo[2,3-h]chromene 72 from flavone 71, Meldrum’s acid 8 and aryl glyoxal 1. | ||
In 2021, Komogortsev and co-workers performed a reaction involving 1, Meldrum’s acid 8, and colchiceine 73, which led to a practical one-pot method to synthesize colchicine derivatives 74 with different substitutions. One of the distinctive aspects of the method is the production of the 2-(7-acetamido-1,2,3-trimethoxy-9-oxo-11-phenyl-5,6,7,9-tetrahydrobenzo[9,10]heptaleno[3,2-b]furan-12-yl)acetic acid. 74 moiety. Some of the key features of this method include the use of easily available precursors, facile execution of the protocol, and the ease with which the target products were separated. Two-dimensional NMR spectroscopy verified the structure of one of the furotropolone products (Scheme 35).67
 | ||
| Scheme 35 Synthesis of 2-(7-acetamido-1,2,3-trimethoxy-9-oxo-11-phenyl-5,6,7,9-tetrahydrobenzo[9,10]heptaleno[3,2-b]furan-12-yl)acetic acid 74 using Meldrum’s acid 8 and colchiceine 73. | ||
A multicomponent reaction protocol was developed by Chen et al. for the formation of 2-aryl-3-aminobenzofuran 75 derivatives by reacting aryl glyoxal monohydrates 1, phenols 63, and para-toluenesulfonamide 12, catalysed by 10 mol% of indium trichloride in the presence of dichloromethane as the solvent to obtain an excellent yield (Scheme 36).68
 | ||
| Scheme 36 Indium-catalysed multicomponent protocol for the synthesis of 2-aryl-3-aminobenzofuran 75. | ||
In 2018, Chang et al. reported the formation of the highly functionalized furan derivatives 76 and 77 via the reaction of aryl glyoxal, phenols 63 and 4-hydroxycoumarin 25 in the presence of MeSO3H or FeCl3 as a catalyst. By altering the reaction media, a range of furan derivatives with various substitution patterns were produced. This method’s atom-economical traits and moderate conditions are consistent with the principles of contemporary green chemistry. In a short time, a substantial number of heterocycles of biological importance can be created using this protocol (Scheme 37).69
Khodabakhshi and Hashemi demonstrated the application of aryl glyoxal in the construction of 2-aryl-3-benzamido-benzofurans 78 via a solvent-free three-component condensation reaction of 1 benzamide 42 and the phenolic substrate 63 with a catalytic amount of tungstate sulfuric acid (TSA) at 120 °C (Scheme 38).70 The plausible mechanism for the formation of benzofuran consists of three steps, starting with the generation of the intermediate la through an in situ condensation of amide 42 and 1, followed by the regiospecific formation of the oxygen-containing five-membered ring lc via the intramolecular cyclisation of lb, and finally dehydration leading to the final product 78 (Scheme 39).
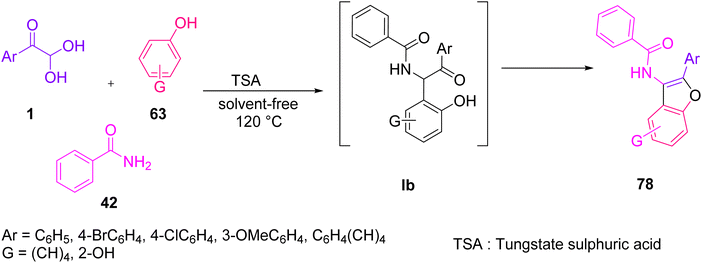 | ||
| Scheme 38 Three-component solvent-free TSA-catalysed protocol for the synthesis of 2-aryl-3-benzamido-benzofuran 78. | ||
A pentacyclic product named as (7-benzoyl-6,7-dihydro-[1,3]dioxolo[4,5-f]benzofuran-6-yl)(4-chloroaryl)methanone 80 was afforded by Reza Salari et al. via a one-pot 1,4-diaza-bicyclo[2.2.2]octane (DABCO)-catalysed condensation of three components, namely 2-(2-(4-chloroaryl)-2-oxoethyl))isoquinolinium bromide 79, benzo(1,3)dioxol-5-ol 58, and aryl glyoxal 1 in water at reflux. Furthermore, a library of substrates was constructed by using different halo-, nitro- and hydroxyl-substituted aryl glyoxals (Scheme 40).71
 | ||
| Scheme 40 Synthesis of (7-benzoyl-6,7-dihydro-[1,3]dioxolo[4,5-f]benzofuran-6-yl)(4-chloroaryl)methanone 80 in a one-pot approach using DABCO. | ||
In 2020, Zhang et al. reported a simple and straight-forward protocol for the quick synthesis of the benzofuran derivative 82, that involved an FeCl3-mediated intermolecular tandem reaction between anisole 81 and 1 via a Friedel–Crafts alkylation and oxidative annulation. This reaction offers a lot of benefits, such as readily available starting materials, high atom economy, and strong functional group tolerance (Scheme 41).72 The mechanistic steps for the annulation reaction include the Friedel–Crafts alkylation of 1 with anisole 81 forming the intermediate ma, which in turn is oxidised by FeCl3 to generate the second radical cation intermediate mb. FeCl3 is then reduced a second time to further oxidise mc with a subsequent intramolecular cyclisation followed by deprotonation to yield the annulated benzofuran 82 (Scheme 42).
By employing microwave irradiation in the presence of the H3PW12O40@Fe3O4–ZnO catalyst, Taheri and co-workers in 2021 developed an effective and convenient single-pot reaction of benzo[a]phenazine 85 (synthesized from 2-hydroxynaphthalene-1,4-dione 83 and benzene 1,2-diamine 84)a , aryl glyoxal 1, and indoles 9 to produce benzo[a]furo[2,3-c]phenazine 86 derivatives with excellent yields. The facile and quick extraction of the products, short reaction time, mild reaction conditions, high product yield, solvent-free conditions, low energy demand and economically affordable chemicals are the major advantages of this reaction protocol (Scheme 43).73
 | ||
| Scheme 43 Synthesis of benzo[a]furo[2,3-c]phenazine 86 via microwave irradiation in the presence of H3PW12O40@Fe3O4–ZnO as a catalyst. | ||
Taheri and colleagues performed the same reaction in 2021 to develop an easy, efficient and straight-forward protocol for the synthesis of benzo[a]furo[2,3-c]phenazine 86 derivatives in excellent yields by the reaction of benzo[a]phenazin-5-ol 85, 1, and 9 utilising the Fe3O4@rGO@ZnO-HPA catalyst and keeping other the conditions similar to those in the previous reaction (Scheme 44).74
A one-pot multicomponent reaction leading to the synthesis of urea-substituted 2-arylfurans 88 was reported for the first time by Komogortsev’s group, and involved the reaction of numerous carbocyclic and heterocyclic enols 25 with cyanamide 87 and aryl glyoxal 1 under reflux conditions in the presence of the solvent acetonitrile, and the base triethylamine (Scheme 45).75
 | ||
| Scheme 45 One-pot protocol for the synthesis of urea-substituted 2-arylfurans 88 by employing cyanamide 87 and heterocyclic enols 25. | ||
For the first time, the synthesis of substituted 2-aminooxazoles containing the 3-hydroxy-4H-pyran-4-one moiety 89 was accomplished by Komogortsev’s group in a one-step method via the multicomponent condensation of allomaltol derivatives 35 with aryl glyoxal 1 and cyanamide 87, followed by an acid-catalysed recyclisation into substituted furo[3,2-b]pyrans 90. The formation of the 2-aminooxozole core as opposed to a urea-containing condensed furan is the distinctive feature of this approach (Scheme 46).76
2.3. Synthesis of pyran derivatives
A green nanoparticle-catalysed strategy for the production of dihydropyrano[c]chromenes 91 was demonstrated by Khodabakhshi’s group using aryl glyoxal 1, 4-hydroxycoumarin 25, and malononitrile 40 in a single pot with an ethanol–water solvent system at reflux. The same reaction was tested against p-TSA, Na2CO3, FeCl3, ZnCl2, and AcOH, and gave no significant yields of the desired products, except for the magnetically recyclable Fe3O4 nanoparticles (Scheme 47).77 | ||
| Scheme 47 Nano-catalysed synthesis of dihydropyrano[c]chromenes 91 by reacting, 4-hydroxycoumarin 25 and malononitrile 40. | ||
Also in 2014, Khodabakhshi and co-workers explored the use of nanosheets of graphene oxide with the same one-pot three-component combination under reflux conditions in an ethanol/water system with a low loading of GO catalyst. The advantages of the above protocol were marked by the recyclable catalyst with a minimum loading of 0.005 g, a high yield and a simple work-up procedure (Scheme 48).78
 | ||
| Scheme 48 Graphene-oxide-catalysed synthesis of dihydropyrano[c]chromenes 91 by the reaction of 4-hydroxycoumarin and malononitrile. | ||
A synthetic library of multifunctional pyrano[c]chromenes 91 was developed by Khodabakhshi et al., this time in the presence of ammonium dihydrogen phosphate at room temperature for the first 30–40 min, and then at reflux in an ethanol/water system (Scheme 49).79
In 2014, Khodbakhshi’s group successfully executed the same reaction in excellent yield with a high degree of purity in the presence of Mohr’s salt (Scheme 50).80
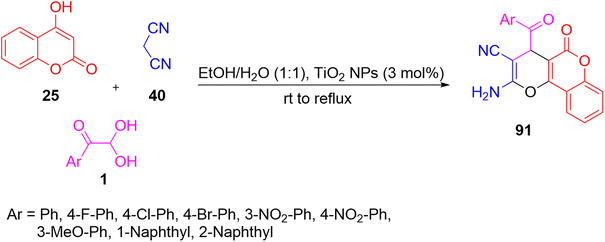
Afterwards, they reported a similar three-component reaction protocol where they replaced Mohr’s salt with TiO2 nanoparticles. Their process involved the coupling of 1 with malononitrile 40, and 25 in the presence of a catalytic amount of TiO2 nanoparticles to give a novel family of pyrano[3,2-c]chromenes 91 in excellent yield. Their reaction was both environmentally feasible and economically cost-effective since it utilised a greener solvent, and a reusable and safer catalyst (Scheme 51).81
Then in 2016, Mishra and Choudhury investigated the possibility of using microwave irradiation as one of the synthetic routes for the reaction of aryl glyoxal monohydrate 1, malononitrile 40, and cyclic 1,3-dicarbonyls 6 to afford several pyrans fused with many functionalities, such as coumarins, quinolones, naphthoquinones, and pyrones 92. This approach is a basic and simple way of obtaining functionalized pyrans without the need for a catalyst or column chromatographic purification (Scheme 52).82
In addition, Mishra and Choudhury also explored the scope of this technique to obtain the fused pyrans 91 and 94 by changing 1,3-dicarbonyls to other cyclic functionalities, such as 4-hydroxycoumarin 25 and 4-hydroxy-6-methyl-2H-pyran-2-one 93. The corresponding products were isolated in high yields (Scheme 53).82 The reaction pathway is outlined in Scheme 54.
Furthermore, Khalafy’s group reported the nano-catalysed synthesis of 2-amino-4-aroyl-5-oxo-5,6-dihydro-2H-pyrano[3,2-c]quinoline-3-carbonitrile 92 through the same combination of aryl glyoxal 1, active methylene group 40 and 4-hydroxyquinolin-2(1H)-one 6 in a single-pot approach with the SBA-15 nanocatalyst in a green solvent. The simplicity of the workup procedure, the use of an ethanol/water system as the green medium, and the good to extraordinary product yields represent the key benefits of this synthetic technique (Scheme 55).83
 | ||
| Scheme 55 SBA-15-catalysed green synthesis of 2-amino-4-aroyl-5-oxo-5,6-dihydro-2H-pyrano[3,2-c]quinoline-3-carbonitrile 92. | ||
Then Khalafy et al. extended the library of oxygen heterocycles in 2018 through 4-aroyl-4H-benzo[g]chromene 95 synthesis using 1, 2-hydroxy-1,4-naphtoquinone(lawsone) 83, and one active methylene species 40 with the effective use of a Zn(L-Pr)2 metal–amino acid complex. Zn(L-Pr)2 is a water-soluble catalyst that shows Lewis acid behaviour together with significant reusability. The protocol was also tested with different acid catalysts, namely sulfanilic acid, p-toluenesulfonic acid, the phase-transfer catalyst tetrabutylammonium bromide, and L-cystein obtaining the target molecule 95 in a high yield with 20% L-proline in ethanol/water at 50 °C (Scheme 56).25
Next, in 2018, Nasri et al. successfully employed aryl glyoxal to obtain the biologically important oxygen heterocycle and useful chemical synthon chromene. A chemical library of pyrano[3,2-c]chromene 91 and benzo[g]chromene95 was constructed through a catalyst-free one-pot assembly of aryl glyoxal monohydrate 1, malononitrile as the active methylene group 40, and 4-hydroxycoumarin 25/2-hydroxy-1,4-nephthaquinone 83, respectively, under reflux conditions with ethanol as the solvent. Ethyl cyanoacetate, methyl cyanoacetate and cyanoacetamide favoured the enol product 95a (Scheme 57).33 This assembly is also considered to follow the same mechanistic pathway as explained in the case of other equivalent functionalities, starting from a Knoevenagel condensation to form the Michael acceptor oa, followed by 1-4 addition, thereby generating the open-chain intermediates ob and od, which subsequently undergo intramolecular cyclisation to yield the desired products 91 and 95 (Scheme 58).
 | ||
| Scheme 58 Mechanisms for 91 and 95 formation through Knoevenagel condensation with a subsequent intramolecular cyclisation. | ||
In 2018, Khaligh synthesised 2-amino-4H-benzo[g]chromenes 95 using the same tactic of a condensation–Michael addition reaction by stirring aryl glyoxal 1, malononitrile 40, and 2 hydroxynephthaquinone 83 with the catalyst poly(N-vinylimidazole) (PVIm) for two hours in refluxing ethanol. The resultant product 95 showed antibacterial activity against Escherichia coli at 32 mg cm−3 (Scheme 59).27
Aryl glyoxal monohydrate as the same starting material was explored by Poursattar Marjani et al. in 2018, who reacted it with malononitrile 40 and 1,3-diketones 32 in the presence of the catalyst L-proline in ethanol solvent for the facile construction of 4H-chromenes, namely 2-amino-4-aroyl-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile 96 (Scheme 60).84
Poursattar Marjani and co-workers revealed the synthetic utility of aryl glyoxal by bringing together substituted aryl glyoxal 1 in a reaction vessel with 4-hydroxyquinolin-2(1H)-one 6 and ethyl cyanoacetate 40 in the presence of the catalyst TPAB in a water/ethanol system at reflux. This assembly yielded a series of ethyl 2-amino-4-benzoyl-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3- carboxylate compounds 92 (Scheme 61).85
 | ||
| Scheme 61 Synthesis of ethyl 2-amino-4-benzoyl-5-oxo-5,6-dihydro-4H-pyrano[3,2-c]quinoline-3-carboxylate 92 from 4-hydroxyquinoline-2(1H)-one 6. | ||
A convenient method for the synthesis of a novel series of 2-amino-4-aroyl-4H-benzo[h]chromene-3-carbonitriles 98 was developed by Poursattar Marjani et al. under solvent-free microwave (MW) conditions in high yields by using a multicomponent condensation reaction of aryl glyoxals 1, 1-naphthol 97, and malononitrile 40 in the presence of Mg–Al hydrotalcite (Scheme 62).86 A plausible outline of the scheme starts with the Mg–Al hydrotalcite-accelerated Knoevenagel reaction of 1 with C–H acid 40 to form an intermediate, namely 2-(2-oxo-2-arylethylidene)malononitrile pa. In the second step, α-naphthol is added as a C-nucleophile to the pa, forming a new species with the C–C bond pb. The intermediate pc undergoes intramolecular cyclisation to generate the species pd, which tautomerizes to give the product 98 (Scheme 63).
 | ||
| Scheme 62 Microwave-assisted reaction to afford 2-amino-4-aroyl-4H-benzo[h]chromene-3-carbonitriles 98 using Mg–Al hydrotalcite. | ||
In 2015, a novel synthetic route was developed by Rimaz et al. to afford a library of biologically active substituted pyrano[2,3-d]pyrimidines 99. They obtained the desired products through an excess ammonium acetate-catalysed one-pot condensation of aryl glyoxal monohydrate 1 with barbituric acid 18 using the greenest solvent water at room temperature (Scheme 64).87
 | ||
| Scheme 64 Synthesis of pyrano[2,3-d]pyrimidines 99 via an ammonium acetate-catalysed reaction in the presence of a green solvent. | ||
Rimaz and co-workers also reported the synthesis of pyrano-fused pyrimidine derivatives 99 through the regioselective pseudo-three-component condensation reaction of aryl glyoxal monohydrate 1 and 1-ethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione 18 in a catalytic system of DABCO or ZrOCl2·8H2O in ethanol at 50 °C. The pyrano-fused-pyrimidine scaffold in the synthesized compound was found to possess HIV integrase inhibitor activity (Scheme 65).34
 | ||
| Scheme 65 Three-component reaction to yield pyrano-fused pyrimidine derivatives 99 using a catalytic system of DABCO or ZrOCl2·8H2O. | ||
The potassium phthalimide-N-oxyl(PPINO)-organocatalysed one-pot green reaction of aryl glyoxal monohydrate 1 with barbituric acid 18 or thiobarbituric acid and β-naphthol 65 at reflux in water yielding benzo[5,6]chromene 100 and derivatives was single-handedly reported by Etivand et al. The distinguishing features of the protocol as highlighted were the use of green solvents, a short reaction time of only 30 minutes, and the product contained more than one heterocycle centre (Scheme 66).88
 | ||
| Scheme 66 Organocatalysed reaction of (PPINO) to yield benzo[5,6]chromene 100 using a green solvent. | ||
In 2020, Taheri and Mohebat proposed a unique greener and environmentally friendly one-pot four-component protocol for the synthesis of (1-methyl-3-phenyl-3,15-dihydrobenzo[a]pyrazolo[4′,3′:5,6]pyrano[2,3-c]phenazin-15-yl)methanone 102 scaffolds in a solvent-free medium using nano-Fe3O4@TiO2–SO3H as the catalyst under microwave monitoring at 75 °C. The major benefits of this green synthetic protocol were the mild reaction conditions, a high product yield, fast reaction time, solvent-free condition, low energy demand and economic affordability (Scheme 67).89
 | ||
| Scheme 67 Nano-Fe3O4@TiO2–SO3H catalysed formation of (1-methyl-3-phenyl-3,15-dihydrobenzo[a]pyrazolo[4′,3′:5,6]pyrano[2,3-c]phenazin-15-yl)methanone 102. | ||
2.4. Miscellaneous reactions
Babu’s group reported the simple and efficient synthesis of oxazoles 105 and furocoumarins 106. The essential step in these transformations comprises the in situ formation of N-acyliminium ion (NAI) precursors 103 from aryl glyoxal and 2-pyrrolidinone 62 in the absence of a catalyst or solvent, followed by their further transformations aided by trifilic acid in the same vessel. It was demonstrated experimentally that the special exocyclic proto-solvated N-acyliminium ion is involved in the reaction. Additionally, the results of the fluorescence and UV-visible experiments revealed that a limited number of the compounds emit blue light when exposed to light in EtOH in the 404–422 nm wavelength range (Scheme 68).90 An insight into the mechanism revealed that, at first, formation of the N-acyliminium ion (NAI) precursor qa from 1 and 2-pyrrolodinone 62 occurs under acid catalysis. There are two probable cyclisation routes. In route ‘a’, the attack of acetonitrile on the iminium intermediate qb leads to the formation of the nitrilium ion qc, which undergoes cyclisation to form the final product 105 through the involvement of an adjacent carbonyl group. While in route ‘b’, neutralisation of the nitrilium ion by a water molecule generates the bisamide qd, which subsequently undergoes cyclisation, losing a water molecule to yield the desired product 105 (Scheme 69).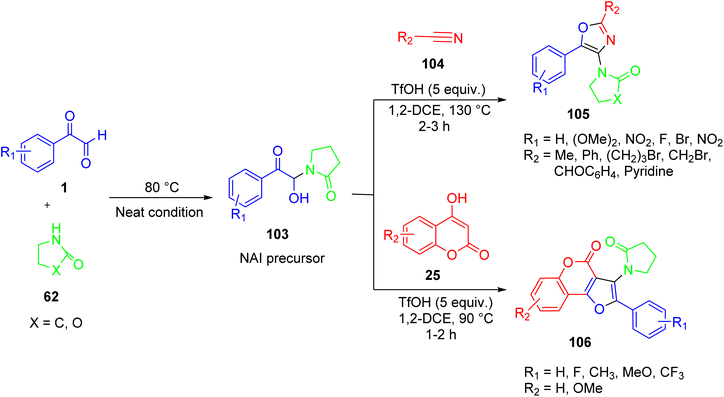 | ||
| Scheme 68 Syntheses of oxazoles 105 and furocoumarins 106 via an in situ N-acyliminium ion (NAI) precursor. | ||
In 2008, García-Valverde et al. reported the reaction of aryl glyoxal 1 with aniline 12, cyclohexyl-isocyanide 107, and trichloroacetic acid 108 in dichloromethane, in the presence of a 3 Å molecular sieve, to afford the oxazolone derivative 109 through the Ugi multicomponent reaction pathway group. The remarkable feature of the strategy is the carbonic acid function of the trichloroacetyl group (Scheme 70).91
2.5. In situ domino reactions of aryl glyoxal to afford oxygen heterocycles
A novel method was developed by Pogaku et al. for the production of oxazoles 112 from methyl ketones 110 and TosMIC (toluenesulfonylmethyl isocyanide) 111 using a self-sorting domino reaction approach. TosMIC was used as an ammonium surrogate in contrast to its typical reactivity as a C–N C synthon in the production of oxazoles 112 (Scheme 71).92 The technique is appealing due to its ease of operation, the wide availability of the starting materials, absence of bases and metals, and the production of C–N and C–O bonds with excellent yields.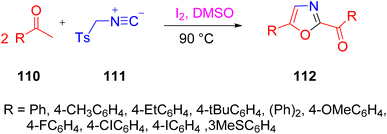 | ||
| Scheme 71 Synthesis of oxazole 112 through a domino reaction using toluenesulfonylmethyl isocyanide 111. | ||
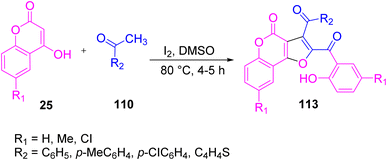
A facile, straightforward novel, and highly efficient protocol was reported by Kolita et al. in 2016 to obtain functionalized furo[3,2-c]coumarins 113 from 4-hydroxycoumarins 25. The reaction was allowed to proceed by reacting aldehyde/aryl methyl ketone 110 with 25 in the presence of molecular iodine in DMSO at 80 °C. DMSO here works as a solvent as well as an oxidizing agent to recycle the iodine during the reaction process (Scheme 72).93 The plausible mechanism of the reaction is that initially phenylglyoxal 1 is formed from phenyl methyl ketone 110 via sp3-CH activation and oxidation, and is subsequently allowed to react with 4-hydroxycoumarin 25 in the presence of iodine to form the intermediate ra. Species ra loses a water molecule, thereby generating rb. Nucleophilic attack by the second molecule of 4-hydroxycoumarin on the intermediate rb yields the final product 113 (Scheme 73).
Cheng’s group prepared the highly dense benzofuran 59 by in situ-generating aryl glyoxal from methyl ketone 110, phenol derivatives 58, and indole 9 together in a reaction vessel with molecular iodine in a catalytic amount and DMSO as a solvent. Later, the nucleophile was changed to thiophenol and 1,2,4-trimethoxybenzene, etc., in place of indole (Scheme 74).7
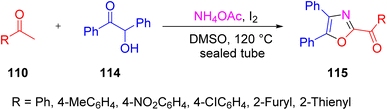
In 2012, Xue et al. proposed a novel and highly efficient protocol to synthesize polysubstituted oxazole derivatives 115 from simple and easily available starting materials, that is by reacting methyl ketone 110, benzoin 114, and ammonium acetate via the convergent integration of two “self-labour domino sequences”. This reaction has wide application in medicinal and life science chemistry (Scheme 75).94
In 2018, Zhao and co-workers proposed a novel path for the synthesis of fused heterocycles by employing an iodine-promoted fragment assembly method. They formulated pyrazolone-oxepine-pyrazoles 118 by the reaction of phenylhydrazine 116, aryl methyl ketones 110, and acetoacetate ester 117 using molecular iodine at 140 °C in the presence of TfOH, and also explored the application of a five-component reaction. Acetoacetate ester engages in two crucial steps, which are the formation of the 3-methyl-5-pyrazolone skeleton and the formation of the C(sp3)–O bond (Scheme 76).95
 | ||
| Scheme 76 Synthesis of pyrazolone-oxepine-pyrazoles 118 from phenylhydrazine 116, acetoacetate ester 117 and in situ-generated phenyl glyoxal 1. | ||
In 2011, Yang et al. performed a convergent and linear domino reaction for the first time from easily available inexpensive substrates, like methyl ketone 110, indole 9, and 1,3-dicarbonyl 17 to afford the 3-(furan-3-yl or 4-yl) indole derivative 119 via a direct two-step process without the need for purification of the intermediate. This reaction has a wide range of applications in synthetic and medicinal chemistry due to its operational simplicity (Scheme 77).96
 | ||
| Scheme 77 Synthesis of 3-(furan-3-yl or 4-yl) indole derivative 119 from 1,3-diketone 17, indole 9 and methyl ketone 110. | ||
3. Conclusions
As a unique molecule with robust applications in organic synthesis, ranging from C–C bond formation reactions to the synthesis of heterocycle libraries, aryl glyoxal is an efficient and easily accessible building block that has been extensively used in the past decade. Exploitation of this commercially available precursor all over the world by chemists has produced many novel functionalized moieties that are both pharmacologically and biologically beneficial to mankind. The different synthetic protocols of aryl glyoxal along with amines and nitrogen heterocycles in multicomponent reaction systems give rise to highly complex synthetic diversity in the products, inspiring researchers to think beyond the current scope towards new horizons.After the gigantic number of nitrogen heterocycles, oxygen heterocycles are the second category approved for clinical use as medicinal drugs. Their synthesis through different resources has raised the attention of researchers to a significant level, but there is still a huge gap between the synthetic methodologies and application patterns of nitrogen and oxygen scaffolds. The lower reactivity and somewhat complicated biomimicking process compared to nitrogen members are the possible reasons behind their limited number of applications in biosystems. Furthermore, researchers have brought phenyl glyoxal into action using it either as a substitute for mono-functional aldehydes for forming a structural backbone by adding carbon to the target structure, or as an oxygen donor in the product molecule. The optimum use of phenyl glyoxal with new combinations of reagents possessing multiple functionalities is still awaited. The mounting up of oxygen heterocycles’ applications is conditional on the proper execution of advanced tools and techniques for their strategic biosynthesis followed by extensive tests and trials.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.References
- F. Wöhler, Ann. Phys. Chem., 1828, 88, 253–256 Search PubMed.
- A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083–3135 Search PubMed.
- S. Banerjee, A. Horn, H. Khatri and G. Sereda, Tetrahedron Lett., 2011, 52, 1878–1881 Search PubMed.
- I. Ugi, A. Dömling and W. Hörl, Endeavour, 1994, 18, 115–122 Search PubMed.
- M. M. Khan, R. Yousuf, S. Khan and Shafiullah, RSC Adv., 2015, 5, 57883–57905 Search PubMed.
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958–2975 Search PubMed.
- C. Cheng, C. Liu and Y. Gu, Tetrahedron, 2015, 71, 8009–8017 Search PubMed.
- M. M. Khan, S. Khan, S. Saigal and S. Iqbal, RSC Adv., 2016, 6, 42045–42061 Search PubMed.
- A. M. El-Agrody, A. M. Fouda and A.-A. M. Al-Dies, Med. Chem. Res., 2014, 23, 3187–3199 Search PubMed.
- A. Zonouzi, R. Mirzazadeh, M. Safavi, S. Kabudanian Ardestani, S. Emami and A. Foroumadi, Iran. J. Pharm. Res., 2013, 12, 679–685 Search PubMed.
- A. Poursattar Marjani, J. Khalafy and A. Farajollahi, J. Heterocycl. Chem., 2019, 56, 268–274 Search PubMed.
- P. Prasad, P. G. Shobhashana and M. P. Patel, R. Soc. Open Sci., 2017, 4, 170764 Search PubMed.
- D. Armesto, W. M. Horspool, N. Martin, A. Ramos and C. Seoane, J. Org. Chem., 1989, 54, 3069–3072 Search PubMed.
- M. Eghtedari, Y. Sarrafi, H. Nadri, M. Mahdavi, A. Moradi, F. Homayouni Moghadam, S. Emami, L. Firoozpour, A. Asadipour, O. Sabzevari and A. Foroumadi, Eur. J. Med. Chem., 2017, 128, 237–246 Search PubMed.
- S. A. Look, M. T. Burch, W. Fenical, Q. Zheng and J. Clardy, J. Org. Chem., 1985, 50, 5741–5746 Search PubMed.
- I. Butenschön, K. Möller and W. Hänsel, J. Med. Chem., 2001, 44, 1249–1256 Search PubMed.
- H. N. Akolkar, S. G. Dengale, K. K. Deshmukh, B. K. Karale, N. R. Darekar, V. M. Khedkar and M. H. Shaikh, Polycyclic Aromat. Compd., 2022, 42, 1959–1971 Search PubMed.
- A. Lilienkampf, M. Pieroni, S. G. Franzblau, W. R. Bishai and A. P. Kozikowski, Curr. Top. Med. Chem., 2012, 12, 729–734 Search PubMed.
- G. Daidone, D. Raffa, B. Maggio, F. Plescia, V. M. C. Cutuli, N. G. Mangano and A. Caruso, Arch. Pharm., 1999, 332, 50–54 Search PubMed.
- J. J. Talley, D. L. Brown, J. S. Carter, M. J. Graneto, C. M. Koboldt, J. L. Masferrer, W. E. Perkins, R. S. Rogers, A. F. Shaffer, Y. Y. Zhang, B. S. Zweifel and K. Seibert, J. Med. Chem., 2000, 43, 775–777 Search PubMed.
- P. Rawat and S. M. Verma, Drug Des., Dev. Ther., 2016, 10, 2779–2788 Search PubMed.
- H. Sourgens, R. Hoerr, A. Biber, H. Steinbrede and H. Derendorf, J. Clin. Pharmacol., 1998, 38, 373–381 Search PubMed.
- A. B. Pardee, Y. Li and C. J. Li, Curr. Cancer Drug Targets, 2002, 2, 227–242 Search PubMed.
- C. J. Li, Y.-Z. Li, A. N. Pinto and A. B. Pardee, Proc. Natl. Acad. Sci. U. S. A., 1999, 96, 13369–13374 Search PubMed.
- J. Khalafy, S. Ilkhanizadeh and M. Ranjbar, J. Heterocycl. Chem., 2018, 55, 951–956 Search PubMed.
- P. Ravichandiran, B. Lai and Y. Gu, Chem. Rec., 2017, 17, 142–183 Search PubMed.
- N. G. Khaligh, Monatsh. Chem., 2018, 149, 33–38 Search PubMed.
- S.-T. Guo, J. Xu, X.-L. Zhang, X.-S. Xiong, L. Zhang, D.-L. Wang and S.-Q. Zhang, Heterocycles, 2021, 102, 105 Search PubMed.
- M. Guarrera, L. Turbino and A. Rebora, J. Eur. Acad. Dermatol. Venereol., 2001, 15, 486–487 Search PubMed.
- A. S. Alqahtani, S. Hidayathulla, M. T. Rehman, A. A. ElGamal, S. Al-Massarani, V. Razmovski-Naumovski, M. S. Alqahtani, R. A. El Dib and M. F. AlAjmi, Biomolecules, 2019, 10, 61 Search PubMed.
- M. Karami, A. Hasaninejad, H. Mahdavi, A. Iraji, S. Mojtabavi, M. A. Faramarzi and M. Mahdavi, Mol. Diversity, 2022, 26, 2393–2405 Search PubMed.
- M. Hanefeld and F. Schaper, Expert Rev. Cardiovasc. Ther., 2008, 6, 153–163 Search PubMed.
- S. Nasri and M. Bayat, J. Mol. Struct., 2018, 1164, 77–88 Search PubMed.
- M. Rimaz, H. Mousavi, B. Khalili and L. Sarvari, J. Iran. Chem. Soc., 2019, 16, 1687–1701 Search PubMed.
- L. Palanivel and V. Gnanasambandam, Org. Biomol. Chem., 2020, 18, 3082–3092 Search PubMed.
- R. Hazen, R. Harvey, R. Ferris, C. Craig, P. Yates, P. Griffin, J. Miller, I. Kaldor, J. Ray, V. Samano, E. Furfine, A. Spaltenstein, M. Hale, R. Tung, M. St. Clair, M. Hanlon and L. Boone, Antimicrob. Agents Chemother., 2007, 51, 3147–3154 Search PubMed.
- A. K. Ghosh, Z. L. Dawson and H. Mitsuya, Bioorg. Med. Chem., 2007, 15, 7576–7580 Search PubMed.
- K. McKeage, C. M. Perry and S. J. Keam, Drugs, 2009, 69, 477–503 Search PubMed.
- S. Gupta, B. Kushwaha, A. Srivastava, J. P. Maikhuri, S. N. Sankhwar, G. Gupta and A. K. Dwivedi, RSC Adv., 2016, 6, 76288–76297 Search PubMed.
- H. S. Whang, M. A. Hunt, N. Wrench, J. E. Hockney, C. E. Farin and A. E. Tonelli, J. Appl. Polym. Sci., 2007, 106, 4104–4109 Search PubMed.
- S. A. Dingsdag and N. Hunter, J. Antimicrob. Chemother., 2018, 73, 265–279 Search PubMed.
- B. Eftekhari-Sis, M. Zirak and A. Akbari, Chem. Rev., 2013, 113, 2958–3043 Search PubMed.
- J. Li, L. Liu, D. Ding, J. Sun, Y. Ji and J. Dong, Org. Lett., 2013, 15, 2884–2887 Search PubMed.
- N. R. Modugu and P. K. Pittala, Tetrahedron Lett., 2017, 58, 3859–3863 Search PubMed.
- A. N. Komogortsev, B. V. Lichitsky and V. G. Melekhina, Molbank, 2021, 2021, M1292 Search PubMed.
- H. Shahbazi-Alavi, R. Teymuri and J. Safaei-Ghomi, Nanochem. Res., 2021, 6, 135–142 Search PubMed.
- S. M. Ebrahimi, B. Hamah-Ameen, A. Kareem Abbas, H. Shahbazi-Alavi, H. Gholamzadeh and J. Safaei-Ghomi, Polycyclic Aromat. Compd., 2022, 42, 6389–6397 Search PubMed.
- C. Liu, L. Zhou, D. Jiang and Y. Gu, Asian J. Org. Chem., 2016, 5, 367–372 Search PubMed.
- F. Dehghanzadeh, F. Shahrokhabadi and M. Anary-Abbasinejad, Arkivoc, 2019, 2019, 133–141 Search PubMed.
- R. Khoeiniha, A. Olyaei and M. Saraei, Synth. Commun., 2018, 48, 155–160 Search PubMed.
- M. H. Mosslemin, M. Anary-Abbasinejad, A. F. Nia, S. Bakhtiari and H. Anaraki-Ardakani, J. Chem. Res., 2009, 2009, 599–601 Search PubMed.
- X. Chang, P. Zeng and Z. Chen, Eur. J. Org. Chem., 2019, 2019, 6478–6485 Search PubMed.
- M. R. Salari, M. H. Mosslemin and A. Hassanabadi, J. Chem. Res., 2017, 41, 657–660 Search PubMed.
- A. N. Komogortsev, B. V. Lichitsky, A. D. Tretyakov, A. A. Dudinov and M. M. Krayushkin, Chem. Heterocycl. Compd., 2019, 55, 818–822 Search PubMed.
- B. V. Lichitsky, V. G. Melekhina, A. N. Komogortsev and M. E. Minyaev, Tetrahedron Lett., 2020, 61, 152602 Search PubMed.
- A. N. Komogortsev, V. G. Melekhina, B. V. Lichitsky and M. E. Minyaev, Tetrahedron Lett., 2020, 61, 152384 Search PubMed.
- B. Karami, S. Khodabakhshi and K. Eskandari, Synlett, 2013, 24, 998–1000 Search PubMed.
- Z. Chen, P. Zeng, S. Zhang and X. Huang, ChemistrySelect, 2021, 6, 4539–4543 Search PubMed.
- B. V Lichitsky, A. N. Komogortsev and V. G. Melekhina, Molbank, 2021, 2021, M1304 Search PubMed.
- A. Jana, D. Ali, P. Bhaumick and L. H. Choudhury, J. Org. Chem., 2022, 87, 7763–7777 Search PubMed.
- L. Palanivel and V. Gnanasambandam, Org. Biomol. Chem., 2020, 18, 3082–3092 Search PubMed.
- A. El-Harairy, Yiliqi, M. Yue, W. Fan, F. Popowycz, Y. Queneau, M. Li and Y. Gu, ChemCatChem, 2019, 11, 4403–4410 Search PubMed.
- B. V. Lichitskii, V. G. Melekhina, A. N. Komogortsev, C. V. Milyutin, A. N. Fakhrutdinov, Y. O. Gorbunov and M. M. Krayushkin, Org. Biomol. Chem., 2020, 18, 2501–2509 Search PubMed.
- P. Rajesh, A. I. Almansour, N. Arumugam and S. Yaragorla, Org. Biomol. Chem., 2021, 19, 1060–1065 Search PubMed.
- B. V. Lichitsky, A. N. Komogortsev and V. G. Melekhina, Molbank, 2022, 2022, M1315 Search PubMed.
- B. V. Lichitsky, V. G. Melekhina, A. N. Komogortsev, V. A. Migulin, Y. V. Nelyubina, A. N. Fakhrutdinov, E. D. Daeva and A. A. Dudinov, Tetrahedron, 2021, 83, 131980 Search PubMed.
- A. N. Komogortsev, B. V. Lichitsky and V. G. Melekhina, Tetrahedron Lett., 2021, 78, 153292 Search PubMed.
- C. X. Chen, L. Liu, D. P. Yang, D. Wang and Y. J. Chen, Synlett, 2005, 13, 2047–2051 Search PubMed.
- X. Chang, X. Zhang and Z. Chen, Org. Biomol. Chem., 2018, 16, 4279–4287 Search PubMed.
- B. Karami, S. Khodabakhshi and F. Hashemi, Tetrahedron Lett., 2013, 54, 3583–3585 Search PubMed.
- M. R. Salari, M. H. Mosslemin and A. Hassanabadi, J. Chem. Res., 2019, 43, 86–89 Search PubMed.
- X. Zhang, P. Zeng, S. Zhang and Z. Chen, ChemistrySelect, 2020, 5, 3934–3938 Search PubMed.
- M. Taheri, R. Mohebat and M. H. Moslemin, Artif. Cells, Nanomed., Biotechnol., 2021, 49, 250–260 Search PubMed.
- M. Taheri, R. Mohebat and M. H. Moslemin, Polycyclic Aromat. Compd., 2023, 43, 586–596 Search PubMed.
- A. N. Komogortsev, V. G. Melekhina, B. V. Lichitsky, V. A. Migulin, T. T. Karibov and M. E. Minyaev, Tetrahedron, 2022, 111, 132716 Search PubMed.
- A. N. Komogortsev, B. V. Lichitsky, T. T. Karibov and V. G. Melekhina, Tetrahedron, 2022, 117–118, 132836 Search PubMed.
- S. Khodabakhshi, B. Karami and M. Baghernejad, Monatsh. Chem., 2014, 145, 1839–1843 Search PubMed.
- S. Khodabakhshi and B. Karami, New J. Chem., 2014, 38, 3586–3590 Search PubMed.
- S. Khodabakhshi, B. Karami, K. Eskandari and M. Farahi, Tetrahedron Lett., 2014, 55, 3753–3755 Search PubMed.
- S. Khodabakhshi, F. Jafari, F. Marahel and M. Baghernejad, Heterocycl. Commun., 2014, 20, 285–288 Search PubMed.
- S. Khodabakhshi, M. Shahamirian and M. Baghernejad, J. Chem. Res., 2014, 38, 473–476 Search PubMed.
- R. Mishra and L. H. Choudhury, RSC Adv., 2016, 6, 24464–24469 Search PubMed.
- J. Khalafy, F. M. Arlan and S. S. Chalanchi, J. Heterocycl. Chem., 2018, 55, 149–153 Search PubMed.
- A. Poursattar Marjani, B. E. Saatluo and F. Nouri, Iran. J. Chem. Chem. Eng., 2018, 37, 149–157 Search PubMed.
- A. Poursattar Marjani, J. Khalafy and A. Farajollahi, J. Heterocycl. Chem., 2019, 56, 268–274 Search PubMed.
- A. Poursattar Marjani, J. Khalafy, P. Eslamipour and M. A. Sabegh, Iran. J. Chem. Chem. Eng., 2019, 38, 51–57 Search PubMed.
- M. Rimaz, A. Mirshokraie, B. Khalili and P. Motiee, Arkivoc, 2015, 2015, 88–98 Search PubMed.
- N. Etivand, J. Khalafy and M. G. Dekamin, Synthesis, 2020, 52, 1707–1718 Search PubMed.
- M. Taheri and R. Mohebat, Green Chem. Lett. Rev., 2020, 13, 165–178 Search PubMed.
- V. N. Babu, A. Murugan, N. Katta, S. Devatha and D. S. Sharada, J. Org. Chem., 2019, 84, 6631–6641 Search PubMed.
- M. García-Valverde, S. Macho, S. Marcaccini, T. Rodríguez, J. Rojo and T. Torroba, Synlett, 2008, 1, 33–36 Search PubMed.
- N. Pogaku, P. R. Krishna and Y. L. Prapurna, Synth. Commun., 2018, 48, 1986–1993 Search PubMed.
- S. Kolita, P. Borah, P. S. Naidu and P. J. Bhuyan, Tetrahedron, 2016, 72, 532–538 Search PubMed.
- W. J. Xue, Q. Li, Y. P. Zhu, J. G. Wang and A. X. Wu, Chem. Commun., 2012, 48, 3485–3487 Search PubMed.
- P. Zhao, X. Wu, X. Geng, C. Wang, Y. D. Wu and A. X. Wu, Tetrahedron, 2018, 74, 4323–4330 Search PubMed.
- Y. Yang, M. Gao, L. M. Wu, C. Deng, D. X. Zhang, Y. Gao, Y. P. Zhu and A. X. Wu, Tetrahedron, 2011, 67, 5142–5149 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |