 Open Access Article
Open Access ArticleA review of the combined effects of environmental and operational factors on lithium-ion battery performance: temperature, vibration, and charging/discharging cycles
Dong Shi†
ab,
Yi Cui†ab,
Xueling Shen*a,
Zhefeng Gaoab,
Xiaoli Maa,
Xu Lic,
Yanyan Fang *ab,
Shuqing Wanga and
Sheng Fanga
*ab,
Shuqing Wanga and
Sheng Fanga
aChina Automotive Battery Research Institute Co., Ltd, 11 Xingke East Street, Yanqi Economic Development Zone, Huairou District, Beijing 101407, China. E-mail: fangyy@glabat.com; shenxl@glabat.com
bGLABAT (Guangdong) Co., Ltd, Room 1608, No. 13 Huabao South Road, Zhangcun Street, Chancheng District, Foshan City, Guangdong Province 528200, China
cTesting and Technology Center for Industrial Product of Shenzhen Customs, No. 289, Gongye Ba Road, Nanshan District, Shenzhen 518067, China
First published on 25th April 2025
Abstract
The performance of lithium-ion batteries (LIBs) is influenced by the coupled effects of environmental conditions and operational scenarios, which can impact their electrochemical performance, reliability, and safety. This review examines the individual and combined effects of temperature, vibrations, and charging/discharging ratio on LIB performance. Temperature primarily affects the rate of chemical reactions and the stability of physical structures. High temperatures accelerate the aging process, while low temperatures reduce charging and discharging efficiency. Vibrations cause internal structural damage, increasing the internal resistance and capacity decay. Additionally, the charging/discharging cycle rate, especially high rates, significantly impacts cycle stability and thermal management design. The combined effects of these factors can lead to nonlinear changes in battery performance, exacerbating the aging process and potentially triggering safety issues. This review discusses the mechanisms of these combined effects and proposes corresponding mitigation strategies based on experimental data. It provides a theoretical foundation and experimental evidence for reliability research on LIBs, which has implications for battery design, usage, and maintenance. Furthermore, this work contributes to the advancement of battery technology towards higher efficiency, greater stability, and enhanced safety.
1. Introduction
Amid the escalating global drive for clean and sustainable energy solutions, battery technology has emerged as a cornerstone of modern energy systems. Batteries are indispensable in various critical applications, including energy storage,1–6 electric vehicles,7–12 and portable electronic devices,13–15 which are also pivotal in propelling the energy transition and facilitating the achievement of low-carbon economic goals.16–21 The research of multifaceted importance, conducting in-depth research on the performance, reliability, and safety of batteries, is essential and crucial for optimizing energy system designs, enhancing energy utilization efficiency, and ensuring the security and stability of the energy supply.22–24Despite significant advancements in LIB technology, the performance of these batteries in practical applications is still influenced by various environmental and operational factors, including temperature, vibration, and charging/discharging rates.25–30 Current research often examines the individual effects of these factors in isolation, as depicted in Fig. 1, while neglecting their combined impacts in real-world scenarios. (1) Temperature variations, in particular, have a profound influence on battery performance. Elevated temperatures can enhance the ionic conductivity of the electrolyte and accelerate lithium-ion migration, thereby improving charging and discharging efficiency. However, prolonged exposure to high temperatures can expedite the decomposition of the electrolyte and electrode materials. This leads to excessive growth of the solid electrolyte interphase (SEI) film, increased lithium-ion consumption, and a rise in internal resistance, ultimately reducing the battery lifespan. Conversely, low-temperature conditions increase the viscosity of the electrolyte and slow down lithium-ion migration, resulting in diminished battery capacity and charging/discharging efficiency. When the temperature decreases from 25 °C to −15 °C, the battery's State of Charge (SOC) decreases by approximately 23%; the charge transfer resistance of the LiFePO4 cathode at −20 °C is three times that at room temperature.25 (2) Vibrations, which are common during transportation or vehicular use, can induce subtle internal structural changes within the battery. These changes may include cracking of electrode materials or detachment of active substances, which can compromise the mechanical stability and long-term performance of the battery. Additionally, vibrations can exacerbate the non-uniform distribution of the electrolyte, affecting the uniform insertion and extraction of lithium ions and thereby impacting battery cycling stability. Research indicates a post-vibration average increase of 10–15% in the battery's internal resistance.28 (3) Charging/discharging rates, a high rate of charging and discharging can intensify the non-uniform deposition of lithium ions on the anode surface, leading to the formation of lithium dendrites. This not only reduces the coulombic efficiency but also increases the risk of internal short circuits. The rapid increase in internal temperature during high-rate discharging may trigger thermal runaway,31–35 potentially leading to battery combustion or explosion.36–40 These findings underscore the importance of considering a broad range of ageing conditions in battery data modelling.
In complex real-world applications, battery systems are frequently subjected to simultaneous temperature fluctuations, mechanical vibrations, and suboptimal charging/discharging ratios. The combined effects of these factors on battery performance are significantly more complex and severe than the effects of individual factors alone.41–46 However, current research often falls short in fully examining the comprehensive impact of these combined effects on battery performance, with most testing methods still focusing primarily on single-factor assessments. This approach is insufficient to accurately reflect the true performance of batteries in actual use.47–50 Consequently, this limitation has led to an inadequate understanding of the degradation mechanisms of batteries under real-world operating conditions, thereby affecting the optimization of battery designs, usage strategies, and maintenance plans.
Compared to previous reviews on battery aging, this paper not only synthesizes the individual and coupled effects of temperature, vibration, and charging/discharging conditions on battery performance but also analyzes their underlying mechanisms and compares the impacts of these factors on key performance indicators such as capacity, internal resistance, and cycling stability. It analyzes the underlying mechanisms of these factors and discusses how they collectively influence key performance indicators, such as battery capacity, internal resistance, and cycling stability. By thoroughly examining the combined effects, this review provides a robust theoretical foundation and experimental basis for reliability research on batteries, thereby promoting the development of battery technology towards higher efficiency, greater stability, and enhanced safety. Moreover, this review explores the development of new testing methods to fully assess the changes in battery performance under the combined effects of these factors. This advancement will help identify potential unknown issues with batteries in practical applications, allowing for preemptive measures to reduce unexpected failures and ensure the long-term stable operation of battery systems. Ultimately, through this comprehensive review, we aim to contribute to the sustainable development of the new energy sector, specifically by improving the performance and safety of battery systems.
2. Impact of individual factors on battery performance
2.1 Temperature effects
Temperature exerts a complex and multifaceted influence on battery performance, spanning aspects such as chemical reaction rates, the stability of physical structures, material properties, and thermodynamics. These effects can be categorized into three distinct forms: high temperature, low temperature, and rapid temperature fluctuations. | ||
| Fig. 2 Thermal runaway tests of batteries at different aging stages. (a) Thermal runaway test results of batteries under high-temperature cyclic aging conditions; (b) test results under high-temperature calendar life aging conditions. (c)–(f) Data for various characteristic temperature points related to thermal runaway, including the initial self-heating temperature (T1), thermal runaway initiation temperature (T2), maximum temperature (T3), and the maximum rate of temperature increase (max dT/dt).52 | ||
Liu et al.54 employed a range of characterization techniques, including scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), and atomic force microscopy (AFM), to investigate the degradation mechanisms of batteries at 25 °C and −20 °C. They discovered that the thermodynamic reactions of electrolyte decomposition are altered at low temperatures, resulting in an SEI layer that contains a higher proportion of intermediate organic products. This composition leads to an unstable SEI layer, which hinders the effective transport of lithium ions and increases the resistance to lithium-ion transfer through the SEI layer, as illustrated in Fig. 3. Additionally, the increased viscosity of the electrolyte at low temperatures is another factor contributing to the elevated internal resistance.55
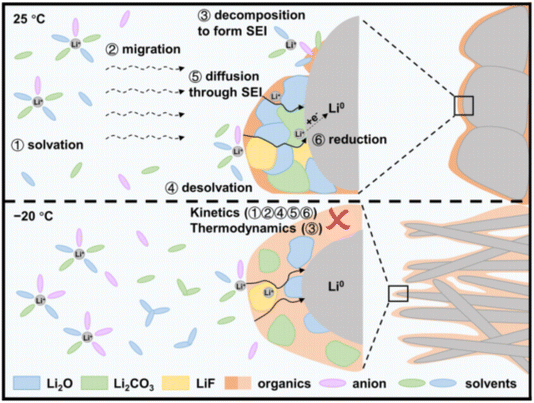 | ||
| Fig. 3 Schematic of lithium-ion diffusion and charge transfer at room and low temperature. Low temperature not only reduces the speed of Li+ through the electrolyte and the SEI layer but also leads to incomplete electrolyte decomposition, resulting in the formation of an SEI membrane composed of metastable intermediate products rich in organic matter, which is prone to lithium dendrite growth.54 | ||
In addition, the impact of temperature on solid-state batteries is similar to that observed in current liquid LIBs. Although solid-state batteries are relatively safer at high temperatures compared to their liquid counterparts, they still face risks of thermal decomposition and thermal runaway. Liquid batteries are more prone to thermal runaway due to the combustion of electrolytes and gas generation. At low temperatures, both solid-state and liquid batteries encounter issues such as reduced ionic conductivity and increased interfacial resistance. However, solid-state batteries exhibit more pronounced interfacial contact problems, with poorer mechanical properties and interfacial stability. Additionally, lithium dendrite growth is more likely to penetrate the electrolyte at low temperatures, leading to internal short circuits and a higher risk of dendrite formation.57
2.2 Vibration effects
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 batteries post-vibration via computed tomography (CT) and found that vibrations can cause internal core shaft loosening, damage to the negative electrode current collector, and crack propagation in the internal active material particles, leading to the disconnection of the active material from the current collector. As shown in Fig. 4, vibrations damaged the connection between the negative electrode and the jelly roll, creating holes in the connection tabs. In addition, some studies have dismantled batteries subjected to vibration and found that vibration causes black stripes to appear on the surface of the battery separator and cracks to form on the surface of the negative electrode.62 After cycling, the separator surface accumulates a significant amount of deposits, graphite particles on the negative electrode are stripped off, and positive electrode particles are crushed. These findings indicate that vibration exacerbates the structural degradation within the battery. This effect is intensified with increased vibration severity, especially under more rigorous conditions such as those three times the vibration severity outlined in the SAE J2380 standard.63 Changes in the battery physical properties, such as a decreased natural frequency and damping ratio with increased vibration intensity and duration, indicate a reduction in battery rigidity and internal structural damage.61,64,65
650 batteries post-vibration via computed tomography (CT) and found that vibrations can cause internal core shaft loosening, damage to the negative electrode current collector, and crack propagation in the internal active material particles, leading to the disconnection of the active material from the current collector. As shown in Fig. 4, vibrations damaged the connection between the negative electrode and the jelly roll, creating holes in the connection tabs. In addition, some studies have dismantled batteries subjected to vibration and found that vibration causes black stripes to appear on the surface of the battery separator and cracks to form on the surface of the negative electrode.62 After cycling, the separator surface accumulates a significant amount of deposits, graphite particles on the negative electrode are stripped off, and positive electrode particles are crushed. These findings indicate that vibration exacerbates the structural degradation within the battery. This effect is intensified with increased vibration severity, especially under more rigorous conditions such as those three times the vibration severity outlined in the SAE J2380 standard.63 Changes in the battery physical properties, such as a decreased natural frequency and damping ratio with increased vibration intensity and duration, indicate a reduction in battery rigidity and internal structural damage.61,64,65
 | ||
Fig. 4 CT scan of the negative electrode of an 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 battery after vibrations: (a) CT image and (b) corresponding photograph.61 650 battery after vibrations: (a) CT image and (b) corresponding photograph.61 | ||
During the initial cycles, a SEI membrane forms on the surface of the negative electrode, protecting the electrode material and enhancing battery performance. However, vibrations can induce a cycle of SEI membrane rupture and continuous regeneration, consuming the electrolyte and causing it to thicken. This process increases the SEI membrane impedance and charge transfer impedance, thereby accelerating battery aging.66
Vibration exerts a significant frequency-dependent influence on batteries, with research demonstrating that the frequency range of 5 Hz to 200 Hz effectively simulates the vibrational environment experienced by battery systems in electric vehicles during real-world operation. Low-frequency vibrations (5–18 Hz) significantly affect battery fatigue damage and shock response, potentially inducing fatigue cracks and delamination within the battery's internal structure. These mechanical degradations may consequently compromise both the structural integrity and electrochemical performance of the battery. In contrast, high-frequency vibrations (30–70 Hz) exhibit relatively minor effects on fatigue damage and impact response.67 This frequency-dependent behavior aligns with findings documented in ref. 75 regarding the correlation between vibration frequencies and battery capacity degradation.
2.3 Cycling effects
First, electrode materials degrade. Repeated lithium-ion intercalation and de-intercalation during the charging and discharging process can cause structural changes in the anode and cathode materials, leading to material fragmentation and pulverization. This degradation is primarily due to the mechanical stress and chemical reactions that occur during cycling, which can result in the loss of active material (LAM) and increased internal resistance.68 Second, the electrolyte decomposes. The electrolyte can decompose under high voltage or high-temperature conditions, forming a SEI layer. This consumption of available lithium ions results in capacity fading of the battery. The SEI layer formation and its subsequent thickening due to continuous cycling are major contributors to the irreversible capacity loss.69 Third, irreversible deposition of lithium ions occurs. Under high charging rate conditions, such as above a 2C current, lithium ions may deposit on the surface of the anode to form metallic lithium instead of intercalating into the graphite. This phenomenon, known as “lithium plating”, leads to capacity loss and safety hazards. Lithium plating is particularly problematic because it can cause internal short circuits and thermal runaway, posing significant safety risks.70
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 batteries. Training models and simulation calculations found that after 100 cycles, the capacity retention of 18
650 batteries. Training models and simulation calculations found that after 100 cycles, the capacity retention of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 LIBs at a 1C rate was approximately 80%. In contrast, at a 2C rate, the capacity retention decreased to 70%. This indicates that high-rate cycling considerably accelerates the capacity fade in batteries.
650 LIBs at a 1C rate was approximately 80%. In contrast, at a 2C rate, the capacity retention decreased to 70%. This indicates that high-rate cycling considerably accelerates the capacity fade in batteries.3. Influence of the combined effects of factors on batteries
In the context of electric vehicle operation, batteries are seldom subjected to individual factors in isolation. Instead, these factors occur in a complex and often concurrent manner, influencing the battery. The complexity of these combined effects warrants intensified research efforts to elucidate the underlying mechanisms.3.1 Vibrations coupled with charging and discharging
Compared to the effect of vibrations alone on batteries, the rate of capacity decay and the increase in internal resistance are more pronounced when vibrations are combined with charging and discharging. Lijun Zhang et al.75 investigated a 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 lithium battery and found that the discharge capacity was 2.611 A h under non-vibrating conditions, which decreased to 2.427 A h, a reduction of approximately 0.2 A h under 5 Hz vibration conditions. The internal resistance also increased from 35.691 to 44.266 mΩ, with different frequencies showing varying degrees of change. The primary cause of this phenomenon is that during charge and discharge cycles, vibrations not only induce the structural and chemical effects previously discussed but also alter the flow and distribution of the electrolyte, affecting the migration speed and path of lithium ions. Furthermore, it can exacerbate the loss of lithium inventory (LLI) and active material (LAM) compared to only cycling. This may induce delamination or loosening of the active materials of electrode, increasing the electrical resistance to current transfer within the battery.59
650 lithium battery and found that the discharge capacity was 2.611 A h under non-vibrating conditions, which decreased to 2.427 A h, a reduction of approximately 0.2 A h under 5 Hz vibration conditions. The internal resistance also increased from 35.691 to 44.266 mΩ, with different frequencies showing varying degrees of change. The primary cause of this phenomenon is that during charge and discharge cycles, vibrations not only induce the structural and chemical effects previously discussed but also alter the flow and distribution of the electrolyte, affecting the migration speed and path of lithium ions. Furthermore, it can exacerbate the loss of lithium inventory (LLI) and active material (LAM) compared to only cycling. This may induce delamination or loosening of the active materials of electrode, increasing the electrical resistance to current transfer within the battery.59
3.2 Temperature coupled with charging and discharging
The impact of temperature on battery cycle aging must be discussed in the context of both high and low temperatures. High temperatures can enhance mass transfer efficiency and electrode reaction rates within a battery, reducing polarization and internal resistance, thereby improving the charge and discharge cycling performance of the battery.76,77 However, high temperatures also accelerate reaction rates, leading to increased electrolyte decomposition, dissolution of electrode materials, accelerated growth of the SEI, and increased side reactions, which collectively accelerate battery aging. In the study of literature,76 it is shown that high temperature increases the degradation rate by 6.7–14.0 times. For example, under the condition of 0.5C, the degradation rate at room temperature is 0.005%/h, while at high temperature it is 0.07%/h, an increase of 14 times. This phenomenon aligns with Arrhenius kinetics, which states that reaction rates increase with temperature.78During low-temperature cycling, the reduced diffusion rate of lithium ions, combined with a high rate of charging and discharging, can lead to increased polarization within the battery, potentially causing a more rapid capacity fade. Zhang et al.79 conducted cycling tests on batteries at −25 °C using a 2C rate and found that under low-temperature conditions, the intercalation reaction of lithium ions at the anode slowed down, while a high rate of charging increased the deposition rate of lithium ions on the anode surface. This can lead to the formation of lithium plating and dendritic growth, as shown in Fig. 5, increasing the risk of internal short circuits.
 | ||
| Fig. 5 SEM images showing the cathode morphology. (A, a; D, d) 0 cycle; (B, b; E, e) 120 cycles; (C, c; F, f) 250 cycles; (A)–(C) show the periphery of the cathode, whereas panels (D)–(F) display the central region. The corresponding high-magnification images of (A)–(F) are presented in (a)–(f).79 | ||
In addition to the aforementioned issues, low-temperature cycling can result in a phenomenon known as “dead lithium”,80 where lithium ions may not fully intercalate into the anode material during charging at low temperatures, resulting in lithium metal deposits on the anode surface. This lithium cannot be reutilized during subsequent discharge cycles, causing a decline in battery capacity. Moreover, compared to literature findings,65 the formation of dead lithium at low temperatures can occur at much lower charge and discharge rates, as low as 0.4C.
Comparative studies on the effects of high and low temperatures on batteries have indicated81 that the combined effect of high-temperature and discharge cycling aging on batteries is less severe than that of low temperatures, primarily due to the aforementioned formation of lithium plating layers during low-temperature cycling processes.
The effects of high and low temperatures on batteries are not isolated; some factors can exacerbate adverse impacts on batteries due to alternating high and low temperatures, such as heat generation during battery cycling. The heat generated during battery cycling can be categorized into two types: reversible heat generation and irreversible heat generation. Reversible heat generation originates from the phase transitions of electrode materials and the thermodynamic effects associated with the intercalation and deintercalation of lithium ions. Irreversible heat generation is caused by increased internal resistance.
Changwei Ji et al.82 conducted research on the low-temperature aging of batteries and performed charge–discharge tests on battery samples with varying degrees of aging under different rates and temperatures, calculating entropy and enthalpy changes. The test results indicate that low-temperature aging not only significantly accelerates the aging rate of batteries but also increases reversible and irreversible heat generation due to the accumulation of polarization. This makes batteries more prone to heating during use. When such low-temperature-aged batteries operate under high-temperature conditions, the heat generated during cycling cannot be dissipated, keeping the battery in a high-temperature state, which further accelerates battery lifespan degradation.
3.3 Synergistic interactions of vibrations, temperature, and charging/discharging on battery performance
High temperatures reduce the stiffness of battery materials, making them more pliable, whereas low temperatures increase brittleness and rigidity. Vibrations under extreme temperature conditions exacerbate material damage, leading to significant degradation in overall battery performance. Although the importance of the combined effects of temperature and vibration has been acknowledged, research in this area remains insufficient, necessitating deeper exploration.83 Moreover, the effect of the simultaneous interaction of vibrations, temperature, and charging/discharging cycles on batteries has been scarcely documented in the literature. Each stressor affects battery performance, manifesting as a decline in capacity and an increase in internal resistance. The degrees of these effects are due to the specific mechanisms through which they exert their effects. For instance, under low-temperature conditions, performing high-rate charge–discharge cycles with simultaneous vibration could exacerbate battery degradation through synergistic interactions between lithium plating and mechanical stress, surpassing the damage caused by either factor in isolation, and may ultimately trigger thermal runaway. To describe these mechanisms, we present a schematic in Fig. 6 and Table 1 of how these factors interact to influence battery performance. | ||
| Fig. 6 Schematic of the mechanistic effects of various factors on lithium iron phosphate and ternary battery performance. | ||
| Factors | Impacts | Mechanism | |
|---|---|---|---|
| Temperature | High | The calendar aging accelerates, and thermal stability decreases | Thermal decomposition of the SEI layer |
| Low | Internal resistance increases, and lithium dendrites form | The lithium-ion diffusion kinetics deteriorates, inducing the formation of a complex and unstable SEI layer | |
| Rapid | Battery lifespan degrades, and cycling efficiency deteriorates | The battery's internal architecture is subjected to thermal expansion and contraction effects | |
| Vibration | Capacity fade and lifespan reduction (note: Degradation is more pronounced under low-frequency cycling) | Electrode damage, SEI layer decomposition, and stiffness reduction | |
| Cycling | Higher cycling rate | Cycle life degradation | Lithium plating and SEI layer thickening |
| Lower cycling rate | Battery maintenance | ||
| Constant-power cycling | Accelerated battery aging compared with constant-current (CC) cycling | To sustain power output, the current must be increased | |
| Temperature and cycling | High-temperature cycling | Enhanced cycling efficiency but accelerated cycling aging | Enhanced electrode reaction kinetics and charge transfer kinetics, thus accelerating SEI layer decomposition |
| Low-temperature cycling | Reduced cycling efficiency and accelerated cycling aging | Reduced electrode reaction kinetics and charge transfer kinetics, exacerbating polarization | |
| Vibration and cycling | Capacity fade and lifespan reduction | Altered ion migration pathways exacerbating inventory lithium loss | |
4. Future perspectives
Batteries are invariably subjected to a complex interplay of vibrations, temperature fluctuations, and charging/discharging cycles throughout their operational lifecycle. The combined effects of these factors result in intricate internal structural changes, altered ion transport dynamics, and complex electrochemical reactions at the electrode surfaces, which are significantly more pronounced than those observed in single-factor or dual-factor studies. Consequently, there is a critical need to investigate the synergistic interactions of influence in order to more accurately replicate the intrinsic challenges faced by batteries in real-world applications.Given that the current research landscape has primarily focused on the individual impacts of temperature, vibration, and charging/discharging, future inquiries should concentrate on the following specific areas.
4.1. Systematic research
To date, there remains a paucity of systematic studies investigating the coupled effects of temperature, vibration, and charge–discharge cycling on battery performance degradation. The fundamental distinctions between tri-factor interactions versus single-factor or dual-factor impacts remain inadequately characterized. Current understanding suggests that pairwise coupling of these stressors exacerbates their individual degradation mechanisms. However, critical knowledge gaps persist regarding whether ternary coupling induces superlinear acceleration of degradation processes, the potential nonlinearity of damage accumulation, and the identification of dominant factors governing synergistic degradation effects. Clarifying the impact of ternary coupling on batteries will facilitate research into the real-world scenarios influenced by external factors during battery usage, enhancing the understanding of mechanisms affecting battery lifespan beyond intrinsic material properties, structural design, and cell geometry.Elucidating these mechanisms is imperative for developing accurate multiphysics models that reflect real-world operating conditions, ultimately enabling optimization of battery usage protocols and operational parameters. Specifically, this research direction will provide critical insights into stressor hierarchy and interaction dynamics, essential for predictive lifetime modeling and failure mode mitigation strategies in practical energy storage applications.
4.2. Microstructural evolution of batteries
As previously described, vibrations can induce damage to electrode materials and current collectors, as well as mechanically disrupt microstructures, including the SEI membrane. High temperatures can expedite the degradation of the SEI membrane, while a high rate of charging/discharging can precipitate lithium deposition or plating. The confluence of these factors is anticipated to induce intricate microstructural transformations. Investigating these phenomena will enhance our understanding of the microstructural evolution in batteries under practical operating conditions, informing advancements in electrode material composition and manufacturing techniques.4.3. Electrode reaction kinetics
Vibrations can disrupt ion transport pathways and modulate the charging/discharging efficacy of batteries. Temperature extremes can either stimulate or retard electrode reaction rates. The amalgamation of these factors will likely intricately influence electrode reactions, exceeding the simplistic acceleration or deceleration of reaction rates. Examining these complex interactions will facilitate a deeper understanding of the internal reaction dynamics under actual usage scenarios, guiding the refinement of battery structural designs and production methodologies.In conclusion, systematically exploring the synergistic effects of vibrations, temperature, and charging/discharging is imperative for revealing the authentic operational states of batteries. Such research will be instrumental in driving continuous enhancements in battery fabrication processes and the iterative development of electrode materials, which contributes to the advancement of battery technology towards higher efficiency, greater stability, and enhanced safety.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Conceptualization, Yanyan Fang and Xueling Shen; methodology, Dong Shi and Yi Cui; investigation, Shuqing Wang; data curation, Xu Li and Zhefeng Gao; writing original draft preparation, Dong Shi; writing—review and editing, Xiaoli Ma and Sheng Fang. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was financially supported by the Beijing Natural Science Foundation of China (grant nos: L243020).Notes and references
- U. Akram, M. Nadarajah, R. Shah and F. Milano, A review on rapid responsive energy storage technologies for frequency regulation in modern power systems, Renewable Sustainable Energy Rev., 2020, 120, 109626, DOI:10.1016/j.rser.2019.109626.
- S. Nyamathulla and C. Dhanamjayulu, A review of battery energy storage systems and advanced battery management system for different applications: challenges and recommendations, J. Energy Storage., 2024, 86, 111179, DOI:10.1016/j.est.2024.111179.
- Z. K. Wang, J. Q. Zhou, H. Q. Ji, J. Liu, Y. Zhou, T. Qian and C. L. Yan, Principles and design of biphasic self-stratifying batteries toward next-generation energy Storage, Angew. Chem., Int. Ed., 2024, 63, e202320258, DOI:10.1002/anie.202320258.
- L. Tang, H. J. Peng, J. R. Kang, H. Chen, M. Y. Zhang, Y. Liu, D. H. Kim, Y. J. Liu and Z. Q. Lin, Zn-based batteries for sustainable energy storage: strategies and mechanisms, Chem. Soc. Rev., 2024, 53, 4877–4925 RSC.
- A. H. Abdelhay and A. D. Bani-Yaseen, Recent advances and perspectives of supramolecular host–guest systems for electrochemical energy storage, Mater. Today Chem., 2024, 40, 102259, DOI:10.1016/j.mtchem.2024.102259.
- Y. P. Wang, W. D. Li, R. Fang, H. H. Zhu and Q. Peng, Capacities prediction and correlation analysis for lithium-ion battery-based energy storage system, Control Eng. Pract., 2022, 125, 105224, DOI:10.1016/j.conengprac.2022.105224.
- R. Kumar and K. Das, Lithium battery prognostics and health management for electric vehicle application – a perspective review, Sustain. Energy Technol. Assess., 2024, 65, 103766, DOI:10.1016/j.seta.2024.103766.
- M. S. Reza, M. Mannan, M. Mansor, P. J. Ker, T. M. I. Mahlia and M. A. Hannan, Recent advancement of remaining useful life prediction of lithium-ion battery in electric vehicle applications: a review of modelling mechanisms, network configurations, factors, and outstanding issues, Energy Rep., 2024, 11, 4824–4848 CrossRef.
- Y. M. Ye, J. F. Zhang, S. Pilla, A. M. Rao and B. Xu, Application of a new type of lithium-sulfur battery and reinforcement learning in plug-in hybrid electric vehicle energy management, J. Energy Storage, 2023, 59, 106546, DOI:10.1016/j.est.2022.106546.
- B. Gnörich and L. Eckstein, Battery Electric Vehicles, in Fuel Cells: Data, Facts and Figures, ed. D. Stolten, R. C. Samsun, N. Garland, Wiley-VCH, 2016, pp. 3–11 Search PubMed.
- T. Iwahori, Y. Ozaki, A. Funahashi, H. Momose, I. Mitsuishi, S. Shiraga, S. Yoshitake and H. Awata, Development of lithium secondary batteries for electric vehicles and home-use load leveling systems, J. Power Sources, 1999, 81, 872–876 CrossRef.
- O. Heilmann, B. Bocho, A. Friess, S. Cortès, U. Schrade, A. C. Kulzer and M. Schlick, Driving Profiles of Light Commercial Vehicles of Craftsmen and the Potential of Battery Electric Vehicles When Charging on Company Premises, World Elec. Veh. J., 2024, 15, 211, DOI:10.3390/wevj15050211.
- Y. R. Liang, C. Z. Zhao, H. Yuan, Y. Chen, W. C. Zhang, J. Q. Huang, D. S. Yu, Y. L. Liu, M. M. Titirici, Y. L. Chueh, H. J. Yu and Q. Zhang, A review of rechargeable batteries for portable electronic devices, Infomat, 2019, 1, 6–32 CrossRef CAS.
- S. K. Kamarudin, F. Achmad and W. R. W. Daud, Overview on the application of direct methanol fuel cell (DMFC) for portable electronic devices, Int. J. Hydrogen Energy, 2009, 34, 6902–6916 CrossRef CAS.
- F. R. Xue, Z. Ling, Y. B. Yang and X. P. Miao, Design and Implementation of Novel Smart Battery Management System for FPGA Based Portable Electronic Devices, Energie, 2017, 10, 264, DOI:10.3390/en10030264.
- Z. Zhang, M. Zhou, B. Yuan, Z. Y. Wu, J. Q. Liu, S. W. Liu, Z. Guo and G. Y. Li, Decarbonizing the power system by co-planning coal-fired power plant transformation and energy storage, J. Energy Storage, 2023, 66, 107442, DOI:10.1016/j.est.2023.107442.
- Y. K. Zhou, Energy-Sharing Economy with Renewable Integration and Management in Communities-a State-of-the-Art Review, Adv. Energy Sustainability Res., 2024, 2400214, DOI:10.1002/aesr.202400214.
- N. O. Bonsu, Towards a circular and low-carbon economy: Insights from the transitioning to electric vehicles and net zero economy, J. Cleaner Prod., 2020, 256, 120659, DOI:10.1016/j.jclepro.2020.120659.
- L. Sun, X. L. Wang, Q. S. Hua and K. Y. Lee, Energy scheduling of a fuel cell based residential cogeneration system using stochastic dynamic programming, Process. Saf. Environ. Prot., 2023, 175, 272–279 CrossRef CAS.
- Q. Liu, W. M. Liang, C. Chan, Y. Q. Cao and M. T. Lu, The impact of low-carbon policy on green manufacturing development, Indoor. Built. Environ., 2023, 32, 1606–1620 CrossRef.
- G. Q. Wang, C. Chen, B. A. Beshiwork and B. Lin, Developing a low-carbon hybrid of ammonia fuel cell and internal combustion engine for carbon neutrality, Appl. Energy Combust. Sci., 2023, 16, 100214, DOI:10.1016/j.jaecs.2023.100214.
- X. Q. Zeng, P. Xiao, Y. Zhou and H. J. Li, Hybrid energy storage for the optimized configuration of integrated energy system considering battery-life attenuation, J. Eng., 2023, 2023, e12331, DOI:10.1049/tje2.12331.
- Y. F. Hu, N. Deng, X. Gong, Y. Sun and H. Chen, Energy consumption optimisation of a battery thermal management system for electric vehicles considering different cooling modes, Int. J. Veh. Des., 2023, 92, 128–148 CrossRef.
- Z. H. Lin, D. G. Li and Y. T. Zou, Energy efficiency of lithium-ion batteries: Influential factors and long-term degradation, J. Energy Storage, 2023, 74, 109386, DOI:10.1016/j.est.2023.109386.
- S. Ma, M. D. Jiang, P. Tao, C. Y. Song, J. B. Wu, J. Wang, T. Deng and W. Shang, Temperature effect and thermal impact in lithium-ion batteries: A review, Prog. Nat. Sci.: Mater. Int., 2018, 28, 653–666 CrossRef CAS.
- A. N. Singh, K. Hassan, C. Bathula and K. W. Nam, Decoding the puzzle: recent breakthroughs in understanding degradation mechanisms of Li-ion batteries, Dalton Trans., 2023, 52, 17061–17083 RSC.
- S. S. Madani, C. Ziebert, M. Marzband and S. D. Odintsov, Thermal Behavior Modeling of Lithium-Ion Batteries: A Comprehensive Review, Symmetry, 2023, 15, 1597, DOI:10.3390/sym15081597.
- X. Hua and A. Thomas, Effect of dynamic loads and vibrations on lithium-ion batteries, J. Low Freq. Noise Vib. Active Control, 2021, 40, 1927–1934, DOI:10.1177/14613484211008112.
- J. Liu, S. Yadav, M. Salman, S. Chavan and S. C. Kim, Review of thermal coupled battery models and parameter identification for lithium-ion battery heat generation in EV battery thermal management system, Int. J. Heat Mass Transfer, 2024, 218, 124748, DOI:10.1016/j.ijheatmasstransfer.2023.124748.
- S. Jie, T. Jung, S. Baek and B. Le, Review: dynamic force effects on batteries, J. Acoust. Soc. Korea, 2022, 41, 669–679 Search PubMed.
- Z. Yu, X. Shen, R. Xu, Z. Wang, Z. Wan, M. Chen, Y. Cui, Y. Fang and X. Ma, Understanding the combustion behavior of electric bicycle batteries and unveiling its relationship with fire extinguishing, J. Energy Chem., 2024, 91, 609–618 CrossRef CAS.
- S. Shahid and M. Agelin-Chaab, A review of thermal runaway prevention and mitigation strategies for lithium-ion batteries, Energy Convers. Manag.: X, 2022, 16, 100310, DOI:10.1016/j.ecmx.2022.100310.
- Y. Cui, X. L. Shen, H. Zhang, Y. P. Yin, Z. L. Yu, D. Shi, Y. Y. Fang and R. Xu, Intrinsic Safety Risk Control and Early Warning Methods for Lithium-Ion Power Batteries, Batteries, 2024, 10, 62, DOI:10.3390/batteries10020062.
- Y. Cui, D. Shi, Z. Wang, L. S. Mou, M. Ou, T. C. Fan, S. S. Bi, X. H. Zhang, Z. L. Yu and Y. Y. Fang, Thermal Runaway Early Warning and Risk Estimation Based on Gas Production Characteristics of Different Types of Lithium-Ion Batteries, Batteries, 2023, 9, 438, DOI:10.3390/batteries9090438.
- S. S. Bi, Z. L. Yu, S. Fang, X. L. Shen, Y. Cui, F. L. Yun, D. Shi, M. Gao, H. Zhang, L. Tang, X. Zhang, Y. Y. Fang and X. J. Zhang, Understanding the combustion characteristics and establishing a safety evaluation technique based on the overcharged thermal runaway of lithium-ion batteries, J. Energy Storage, 2023, 73, 109039, DOI:10.3390/batteries9090438.
- P. P. Xu, J. Q. Li, N. Lei, F. K. Zhou and C. Sun, An experimental study on the mechanical characteristics of Li-ion battery during overcharge-induced thermal runaway, Int. J. Energy Res., 2021, 45, 19985–20000, DOI:10.1002/er.7072.
- M. Doyle, t. F. Fuller and j. Newman, Modeling of Galvanostatic Charge and Discharge of the Lithium Polymer Insertion Cell, J. Electrochem. Soc., 1993, 140, 1526–1533 CrossRef CAS.
- Z. Liu, X. R. Guo, N. Meng, Z. L. Yu and H. Yang, Study of thermal runaway and the combustion behavior of lithium-ion batteries overcharged with high current rates, Thermochim. Acta, 2022, 715, 179276, DOI:10.1016/j.tca.2022.179276.
- Q. S. Zhang, J. H. Niu, Z. H. Zhao and Q. Wang, Research on the effect of thermal runaway gas components and explosion limits of lithium-ion batteries under different charge states, J. Energy Storage, 2022, 45, 103759, DOI:10.1016/j.est.2021.103759.
- L. Q. Zhao, J. X. Hou, X. N. Feng, J. Xu, C. S. Xu, H. B. Wang, H. Liu, B. W. Hou, X. Y. Rui, Y. Z. Gu, L. G. Lu, C. Bao and M. G. Ouyang, The trade-off characteristic between battery thermal runaway and combustion, Energy Storage Mater., 2024, 69, 103380, DOI:10.1016/j.est.2021.103759.
- P. J. Chacko, S. M. Krishna, R. S. S. Nuvvula, A. A. Stonier, P. P. Kumar, J. Ogale and B. Khan, Estimation of state of charge considering impact of vibrations on traction battery pack, Electr. Eng., 2024, 106, 1327–1338 CrossRef.
- S. Jie, T. Jung, S. Baek and B. Lee, Review: dynamic force effects on batteries, J. Acoust. Soc. Korea, 2022, 41, 669–679 Search PubMed.
- Z. J. Zhou, S. Chen, M. J. Luo, W. H. Du, Y. H. Wu and Y. Yu, Effect of mechanical vibration on phase change material based thermal management system for a cylindrical lithium-ion battery at high ambient temperature and high discharge rate, Int. J. Heat Mass Transfer, 2023, 211, 124255, DOI:10.1016/j.ijheatmasstransfer.2023.124255.
- Z. Wang, Q. J. Zhao, X. Y. Yu, W. G. An and B. B. Shi, Impacts of vibration and cycling on electrochemical characteristics of batteries, J. Power Sources, 2024, 601, 234274, DOI:10.1016/j.jpowsour.2024.234274.
- A. Bisht, R. Amin, M. Dixit, N. Wood, C. B. M. Kweon and I. Belharouak, Impact of cycling conditions on lithium-ion battery performance for electric vertical takeoff and landing applications, J. Power Sources, 2024, 602, 234335, DOI:10.1016/j.jpowsour.2024.234335.
- Z. Wang, Q. J. Zhao, S. J. Wang, Y. C. Song, B. B. Shi and J. J. He, Aging and post-aging thermal safety of lithium-ion batteries under complex operating conditions: a comprehensive review, J. Power Sources, 2024, 623, 235453, DOI:10.1016/j.jpowsour.2024.235453.
- L. Vichard, A. Ravey, P. Venet, F. Harel, S. Pelissier and D. Hissel, A method to estimate battery SOH indicators based on vehicle operating data only, Energy, 2021, 255, 120235, DOI:10.1016/j.energy.2021.120235.
- J. M. Tarascon and M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- S. Han and J. Lim, Performance Assessment of a Lithium-Polymer Battery for HEV Utilizing Pack-Level Battery Hardware-in-the-Loop-Simulation System, J. Electr. Eng. Technol., 2013, 8, 1431–1438 CrossRef.
- S. A. Khateeb, M. M. Farid, J. R. Selman and S. Al-Hallaj, Mechanical-electrochemical modeling of Li-ion battery designed for an electric scooter, J. Power Sources, 2006, 158, 673–678 Search PubMed.
- B. E. Hawkins, H. Asare, B. Chen, R. J. Messinger, W. West and J. P. Jones, Elucidating Failure Mechanisms in Li-ion Batteries Operating at 100 °C, J. Electrochem. Soc., 2023, 170, 100522, DOI:10.1149/1945-7111/acfc36.
- G. Zhang, X. Wei, S. Chen, G. Wei, J. Zhu, X. Wang, G. Han and H. Dai, Research on the impact of high-temperature aging on the thermal safety of lithium-ion batteries, J. Energy Chem., 2023, 87, 378–389 CrossRef CAS.
- P. Mei, Y. Zhang and W. Zhang, Low-temperature lithium-ion batteries: challenges and progress of surface/interface modifications for advanced performance, Nanoscale, 2023, 15, 987–997, 10.1039/d2nr06294a.
- S. T. Weng, X. Zhang, G. J. Yang, S. M. Zhang, B. Y. Ma, Q. Y. Liu, Y. Liu, C. X. Peng, H. X. Chen, H. L. Yu, X. L. Fan, T. Cheng, L. Q. Chen, Y. J. Li, Z. X. Wang and X. F. Wang, Temperature-dependent interphase formation and Li+ transport in lithium metal batteries, Nat. Commun., 2023, 14, 4474, DOI:10.1038/s41467-023-40221-0.
- H. X. Liu, Y. Y. Wang and M. Z. He, Decay mechanism and capacity prediction of lithium-ion batteries under low-temperature near-adiabatic condition, Inorg. Chem. Commun., 2022, 137, 109151, DOI:10.1016/j.inoche.2021.109151.
- T. Sun, T. T. Shen, Y. J. Zheng, D. S. Ren, W. K. Zhu, J. Li, Y. Wang, K. Kuang, X. Y. Rui, S. Wang, L. Wang, X. B. Han, L. G. Lu and M. G. Ouyang, Modeling the inhomogeneous lithium plating in lithium-ion batteries induced by non-uniform temperature distribution, Electrochim. Acta, 2022, 425, 140701, DOI:10.1016/j.electacta.2022.140701.
- R. Kan, Y. Xu, R. Chen, M. Jiang, B. W. Fu, C. Y. Song, P. Tao, J. Wang, T. Deng and W. Shang, Thermal effects of solid-state batteries at different temperature: Recent advances and perspectives, Energy Storage Mater., 2024, 68, 103366, DOI:10.1016/j.ensm.2024.103366.
- J. M. Hooper, D. Williams, K. Roberts-Bee, A. McGordon, P. Whiffin and J. Marco, Defining a vibration test profile for assessing the durability of electric motorcycle battery assemblies, J. Power Sources, 2023, 557, 232541, DOI:10.1016/j.jpowsour.2022.232541.
- A. B. K. Parasumanna, U. S. Karle and M. R. Saraf, Material Characterization and Analysis on the Effect of Vibration and Nail Penetration on Lithium Ion Battery, World Elec. Veh. J., 2019, 10, 69, DOI:10.3390/wevj10040069.
- T. Bruen, J. M. Hooper, J. Marco, M. Gama and G. H. Chouchelamane, Analysis of a Battery Management System (BMS) Control Strategy for Vibration Aged Nickel Manganese Cobalt Oxide (NMC) Lithium-Ion 18650 Battery Cells, Energies, 2016, 9, 255 CrossRef.
- M. J. Brand, S. F. Schuster, T. Bach, E. Fleder, M. Stelz, S. Gläser, J. Müller, G. Sextl and A. Jossen, Effects of vibrations and shocks on lithium-ion cells, J. Power Sources, 2015, 288, 62–69 CrossRef CAS.
- Z. Wang, Q. J. Zhao, X. Y. Yu, W. G. An and B. B. Shi, Impacts of vibration and cycling on electrochemical characteristics of batteries, J. Power Sources, 2024, 601, 234274, DOI:10.1016/j.jpowsour.2024.234274.
- P. Berg, M. Spielbauer, M. Tillinger, M. Merkel, M. Schoenfuss, O. Bohlen and A. Jossen, Durability of lithium-ion 18650 cells under random vibration load with respect to the inner cell design, J. Energy Storage, 2020, 31, 101499, DOI:10.1016/j.est.2020.101499.
- P. Berg, J. Soellner and A. Jossen, Structural dynamics of lithium-ion cells - Part I: Method, test bench validation and investigation of lithium-ion pouch cells, J. Energy Storage, 2019, 26, 100916, DOI:10.1016/j.est.2019.100916.
- J. M. Hooper, J. Marco, G. H. Chouchelamane, J. S. Chevalier and D. Williams, Multi-axis vibration durability testing of lithium ion 18650 NCA cylindrical cells, J. Energy Storage., 2018, 15, 103–123 CrossRef.
- L. Somerville, J. M. Hooper, J. Marco, A. McGordon, C. Lyness, M. Walker and P. Jennings, Impact of Vibration on the Surface Film of Lithium-Ion Cells, Energies, 2017, 10, 741, DOI:10.3390/en10060741.
- J. M. Hooper and J. Marco, Defining a Representative Vibration Durability Test for Electric Vehicle (EV) Rechargeable Energy Storage Systems (RESS), EVS29 Symposium, 2016, pp. 0327–0338 Search PubMed.
- R. B. Wright, J. P. Christophersen, C. G. Motloch, J. R. Belt, C. D. Ho, V. S. Battaglia, J. A. Barnes, T. Q. Duong and R. A. Sutula, Power fade and capacity fade resulting from cycle-life testing of Advanced Technology Development Program lithium-ion batteries, J. Power Sources, 2003, 119, 865–869 CrossRef.
- S. Santhanagopalan, Q. Z. Guo, P. Ramadass and R. E. White, Review of models for predicting the cycling performance of lithium ion batteries, J. Power Sources, 2006, 156, 620–628 Search PubMed.
- J. F. Song, Y. L. Li, T. Ren, J. T. Wang and Z. Y. Yang, Two strain modes and transition point of 18650 lithium-ion battery at different charging rates, J. Energy Storage, 2024, 80, 100005, DOI:10.1016/j.est.2023.110383.
- I. Bloom, B. W. Cole, J. J. Sohn, S. A. Jones, E. G. Polzin, V. S. Battaglia, G. L. Henriksen, C. Motloch, R. Richardson, T. Unkelhaeuser, D. Ingersoll and H. L. Case, An accelerated calendar and cycle life study of Li-ion cells, J. Power Sources, 2001, 101, 238–247 Search PubMed.
- X. B. Han, L. G. Lu, Y. J. Zheng, X. N. Feng, Z. Li, J. Q. Li and M. G. Ouyang, A review on the key issues of the lithium ion battery degradation among the whole life cycle, Etransportation, 2019, 1, 100005, DOI:10.1016/j.etran.2019.100005.
- A. P. Wang, S. Kadam, H. Li, S. Q. Shi and Y. Qi, Review on modeling of the anode solid electrolyte interphase (SEI) for lithium-ion batteries, npj Comput. Mater., 2018, 4, 15, DOI:10.1038/s41524-018-0064-0.
- B. Bairwa, S. Hampannavar, K. S. Vishal and K. M. Bhargavi, Modeling and Simulation for Capacity Fade Prediction of Lithium-Ion Battery, IEEE Eurocon-2021, 2021, pp. 537–541 Search PubMed.
- L. J. Zhang, Z. Q. Mu and X. Y. Gao, Coupling Analysis and Performance Study of Commercial 18650 Lithium-Ion Batteries under Conditions of Temperature and Vibration, Energies, 2018, 11, 2856, DOI:10.3390/en11102856.
- D. X. Ouyang, J. W. Weng, M. Y. Chen and J. Wang, Impact of high-temperature environment on the optimal cycle rate of lithium-ion battery, J. Energy Storage, 2020, 28, 101242, DOI:10.1016/j.est.2020.101242.
- X. Shu, Y. J. Li, K. X. Wei, W. X. Yang, B. Yang and M. Zhang, Research on the output characteristics and SOC estimation method of lithium-ion batteries over a wide range of operating temperature conditions, Energy, 2025, 317, 134726, DOI:10.1016/j.energy.2025.1347267.
- J. L. Liu, L. F. Zhou, Y. Zhang, J. L. Wang and Z. R. Wang, Aging behavior and mechanisms of lithium-ion battery under multi-aging path, J. Cleaner Prod., 2023, 423, 138678, DOI:10.1016/j.jclepro.2023.138678.
- G. X. Zhang, X. Z. Wei, S. Q. Chen, G. S. Han, J. G. Zhu and H. F. Dai, Investigation the Degradation Mechanisms of Lithium-Ion Batteries under Low-Temperature High-Rate Cycling, ACS Appl. Energy Mater., 2022, 5, 6462–6471 CrossRef CAS.
- J. L. Liu, Y. Zhang, J. L. Bai, L. F. Zhou and Z. R. Wang, Influence of lithium plating on lithium-ion battery aging at high temperature, Electrochim. Acta, 2023, 454, 142362, DOI:10.1016/j.electacta.2023.142362.
- M. Feinauer, M. Wohlfahrt-Mehrens, M. Hölzle and T. Waldmann, Temperature-driven path dependence in Li-ion battery cyclic aging, J. Power Sources, 2024, 594, 233948, DOI:10.1016/j.jpowsour.2023.233948.
- C. W. Ji, D. Q. Liu, Y. Y. Liu, S. F. Wang, Y. A. Wang, Z. Z. Zhang and B. Wang, Effect of low temperature and high-rate cyclic aging on thermal characteristics and safety of lithium-ion batteries, Process Saf. Environ., 2024, 188, 1514–1526 CrossRef CAS.
- P. Berg, J. Soellner, M. Herrmann and A. Jossen, Structural dynamics of lithium-ion cells-part II: investigation of large-format prismatic cells and method evaluation, J. Energy Storage, 2020, 28, 101246, DOI:10.1016/j.est.2020.101246.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |

