DOI:
10.1039/D5RA01430A
(Paper)
RSC Adv., 2025,
15, 16708-16723
Zinc-encapsulating covalent organic frameworks for enhanced chemiresistive NH3 sensing at room temperature†
Received
27th February 2025
, Accepted 5th May 2025
First published on 19th May 2025
Abstract
Ammonia (NH3) is a hazardous gas used in industry, agriculture, and biomedical applications, and the development of efficient room-temperature and low-concentration ammonia detection sensors is essential. However, conventional sensors, including metal oxides, nanocomposites, and MOFs, require highly elevated temperatures (200–500 °C), leading to high energy consumption and less durability. To overcome these challenges, we developed functionalized zinc-encapsulated covalent organic frameworks (Zn@COFs) using a facile metal-doping approach. COFs doped with zinc have a modulated electronic environment, increased active sites, efficient charge transfer, and enhanced gas interactions. The incorporation of Zn2+ into the COF frameworks was confirmed by IR, TEM-EDAX, 13C CP MAS NMR spectra (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at ∼183 ppm, and imine C
O peak at ∼183 ppm, and imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peaks at ∼148 and ∼146 ppm) and XPS (C
N peaks at ∼148 and ∼146 ppm) and XPS (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 527.84 eV, C
O peak at 527.84 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at 399.2 eV; Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized frameworks, Zn@COF-3 exhibited exceptional NH3 sensing at a concentration of 1 ppm at room temperature, with a rapid response time (26 s) and recovery time (18 s), outperforming pristine COFs and Zn@COFs. This superior performance is attributed to its rich active sites (C
N at 399.2 eV; Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized frameworks, Zn@COF-3 exhibited exceptional NH3 sensing at a concentration of 1 ppm at room temperature, with a rapid response time (26 s) and recovery time (18 s), outperforming pristine COFs and Zn@COFs. This superior performance is attributed to its rich active sites (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), high surface area (335 m2 g−1), porosity, strong NH3 adsorption energy (−282 kJ mol−1), and low energy gap (2.65 eV), as confirmed by DFT calculations. Additionally, Zn@COF-3 shows excellent selectivity and long-term stability over 30 days. This Zn@COF-based approach yields next-generation ammonia sensors, featuring energy-efficient, highly selective, and room-temperature chemiresistive sensors for industrial, environmental, and biomedical applications.
O), high surface area (335 m2 g−1), porosity, strong NH3 adsorption energy (−282 kJ mol−1), and low energy gap (2.65 eV), as confirmed by DFT calculations. Additionally, Zn@COF-3 shows excellent selectivity and long-term stability over 30 days. This Zn@COF-based approach yields next-generation ammonia sensors, featuring energy-efficient, highly selective, and room-temperature chemiresistive sensors for industrial, environmental, and biomedical applications.
1. Introduction
Gas sensors are gaining significance in contemporary society for detecting harmful gasses in various conditions, including the environment, industrial facilities, residential areas, and public spaces.1–3 These sensors are essential in various applications, such as toxic gas monitoring in public places, air quality control, safety systems, and biomedical diagnostics.4,5 Chemiresistive gas sensors are becoming increasingly popular due to their simple design, capacity to monitor continuously, flexibility, and ease of connection with ordinary electrical systems.6,7
Ammonia (NH3) is a significant industrial chemical with widespread applications, but it poses severe health and environmental risks. Exposure to NH3 at concentrations exceeding 300 ppm can result in significant irritation to the eyes and skin, along with burning sensations in the nasal passages, throat, and respiratory tract, potentially causing cellular damage within the body. Ammonia leaks in industrial production indeed pose significant hazards, with concentrations between 15% and 28% by volume being capable of creating flammable and potentially explosive environments. The Occupational Safety and Health Administration (OSHA) has established allowable exposure limits of 25 ppm for an 8 h duration and 35 ppm for a 15 min interval, which is above the olfactory detection threshold of roughly 20 ppm.8,9 Moreover, ammonia functions as a crucial biomarker in breath diagnostics for identifying renal disorders, with increased concentrations (exceeding 800 ppb) signifying health complications. Despite thorough investigation, metal-oxide-based sensors, which are frequently employed for NH3 detection, have been constrained by their limited detection range and generally necessitate elevated operating temperatures (100 °C to 300 °C).10–12 Two-dimensional materials like carbon nanotubes (CNTs) and metal oxides such as TiO2, ZnO, and WO3 have gained considerable attention in gas sensor technology due to their adaptable structural, physical, and chemical properties.13–17 These materials, which are categorized as either n-type or p-type semiconductors, play a crucial role in gas sensing applications by facilitating charger transfer interactions upon exposure to target gases. Notably, they exhibit optimal gas sensing performance within the temperature range of 250–550 °C.18–20 Hence, it is essential to study new sensing materials that can effectively detect ammonia at lower temperatures and with enhanced sensitivity and selectivity. Therefore, the development of excellent sensors for NH3 detection at ambient temperature remains a significant challenge.
COFs are a promising new class of materials for next-generation gas sensors and have attracted a great deal of attention since the groundbreaking investigation due to their unique characteristics, which include adjustable porosity, an organic backbone, and remarkable stability.21 Compared to metal–organic frameworks (MOFs), COFs exhibit superior chemical and thermal stability, making them more suitable for long-term sensing under humid conditions.22–24 MOFs often suffer from framework collapse due to weak metal–ligand interactions in humid or high-temperature environments due to the metals present in the framework being converted into metal oxides at high temperature, whereas COFs, which are built on strong covalent linkages, provide greater long-term stability and reusability in gas sensing applications. In comparison to MOFs, COFs have shown potential in diverse chemical sensing applications25–30 such as explosive detection,31,32 detection of metal ions,33,34 humidity detection,35,36 pH levels,37,38 and gases.39,40 However, pristine COF-based sensors still face some limitations, such as insolubility, weak conductivity, less active sites, more aggregation, weak electronic environment, and poor dispersion of microcrystalline nature.41–49 To overcome these challenges, adding metal or metal oxide NPs into COFs is a key way to create a strong synergistic interaction between the framework and the metal, which results in better gas-sensing results.50–54 Metal covalent organic frameworks (MCOFs), which are formed by incorporating metal ions into COF structures, present a promising advancement in material design, as the metal centers act as catalytic and adsorption sites, significantly improving sensitivity and selectivity for various gases.55
While MCOFs provide enhanced gas-sensing properties, the choice of the dopant metal significantly influences their performance. Among the various metal dopants, Zn2+ has been selected for COF modification due to its ability to enhance charge transfer, improve conductivity, and maintain structural integrity.56 Unlike other transition metals, the incorporation of Zn2+ preserves the porosity of the COF, ensuring efficient gas diffusion while introducing Lewis acid sites that strengthen interactions with NH3.57 Its superior chemical stability, non-toxic nature, and proven effectiveness in gas sensing make it an ideal choice for enhancing COF-based NH3 and VOC detection.58 Zn2+ has been selected over precious metals such as Pt, Au, or Pd due to its low cost and non-toxic nature. Additionally, it facilitates strong charge transfer, offers a high surface area, introduces Lewis acid sites for NH3 adsorption, and enables effective operation at ambient conditions. Furthermore, Zn2+ exhibits fast response and recovery times along with long-term chemical stability, making it highly suitable for gas-sensing applications.59 COFs' uniform and stable porous structure also makes them ideal for single-atom catalysts (SACs), as they can be tailored through a bottom-up synthesis approach and post-metalation to introduce specific catalytic sites.60–62
In this work, we focus on the synthesis of a series of covalent organic frameworks via the controlled increase of hydroxyl (–OH) groups in Ar(CHO)3, which directs the formation of β-keto-enamine COFs (COF-1, COF-2, and COF-3). This step-wise functionalization results in COF-1 with a single β-keto-enamine group, COF-2 with two β-keto-enamine groups, and COF-3 with three β-keto-enamine groups. Following the successful synthesis of the COFs, they are subsequently doped with Zn2+ ions to enhance their gas-sensing performance (Zn@COF-3 at 1 ppm, Tres = 26 s and Trec = 16 s in NH3 gas sensing). Additionally, density functional theory (DFT) calculations using the B3LYP method were performed to analyze the electrostatic potential (ESP), highest occupied molecular orbital (HOMO), and lowest unoccupied molecular orbital (LUMO) of the COFs and MCOFs, providing insights into their interactions with NH3 molecules. The findings from this study highlight the effectiveness of Zn2+ doping and progressive functionalization in enhancing the sensing performance of COFs, demonstrating their potential for real-world gas detection applications.
2. Experimental section
2.1 Synthesis of parent COFs
The synthesis of COF-1, COF-2, and COF-3 was carried out by Schiff-base condensation of 2-hydroxy-1,3,5-benzenetricarbaldehyde, 2,4-hydroxy-1,3,5-benzenetricarbaldehyde, or 2,4,6-hydroxybenzene-1,3,5-tricarbaldehyde with 1,4-benzenediamine to form the respective COFs (Fig. 1).
 |
| | Fig. 1 Synthetic procedure for covalent organic frameworks (COF-1, 2, and 3) and metal-encapsulating COFs (Zn@COF-1, Zn@COF-2, and Zn@COF-3). | |
2.1.1 Synthesis of COF-1. COF-1 was synthesized using the procedures stated in the literature, with a few modifications.62–64 In a round bottom flask, 2-hydroxy-1,3,5-benzenetricarbaldehyde (73 mg), 1,4 benzenediamine (58 mg), 1.5 mL of 1,4-dioxane, 2.0 mL of 1,3,5-trimethylbenzene, and 0.3 mL of 6 M aqueous acetic acid solution were mixed uniformly. The solution was subjected to sonication for 30 minutes under a nitrogen (N2) atmosphere. Subsequently, the mixture was moved to an autoclave, placed in a hot air oven at 130 °C, and maintained for 3 days. After cooling, the final product was washed with acetone, hexane, and anhydrous THF.65 Subsequently, vacuum-drying at a temperature of 100 °C for 24 h was carried out, resulting in the formation of COF-1.
2.1.2 Synthesis of COF-2. Similarly, COF-2 was synthesized using a similar procedure, with slight modifications to the aldehyde precursor. Initially, the round bottom flask contained 2,4,-hydroxy-1,3,5-benzenetricarbaldehyde (73 mg), 1,4-benzenediamine (58 mg), 1.5 mL of 1,4-dioxane, 2.0 mL of 1,3,5-trimethylbenzene, and 0.3 mL of 6 M aqueous acetic acid. The solution was sonicated under a nitrogen atmosphere and transferred into a stainless-steel Teflon autoclave container maintained at 130 °C for three days and then cooled to room temperature. Purification and drying processes were carried out, similar to the COF-1 process, leading to the formation of COF-2.
2.1.3 Synthesis of COF-3. In a round bottom flask, 73 mg of 2,4,6-hydroxybenzene-1,3,5-tricarbaldehyde, 58 mg of 1,4-benzenediamine, 1.5 mL of 1,4-dioxane, 2.0 mL of 1,3,5-trimethylbenzene, and 0.3 mL of 6 M aqueous acetic acid were mixed and sonicated in a nitrogen atmosphere. The solution was transferred into a stainless-steel Teflon autoclave container, heated at 130 °C for three days, and cooled to room temperature slowly. The final product was washed with acetone, hexane, and anhydrous THF and dried under vacuum at 100 °C for 24 h, forming COF-3.
2.2 Synthesis of zinc-encapsulating COFs (Zn@COFs)
The COFs (COF-1, COF-2, and COF-3) were modified with Zn2+ ions through a facile solution method to enhance their stability and gas-sensing capabilities. The synthesis procedures for the metal-modified frameworks Zn@COF-1, Zn@COF-2, and Zn@COF-3, are given below.
2.2.1 Synthesis of Zn@COF-1. Initially, COF-1 (30 mg) was dissolved in 50 mL of distilled water to form a COF-1 suspension and transferred into the round bottom flask. In another beaker, 60 mg of ZnCl2 was dissolved in 50 mL of distilled water. A two-channel syringe pump was used to add ZnCl2 solution at a steady rate of 1 mL h−1 to the round bottom flask containing the COF-1 suspension. Simultaneously, the mixture was agitated using a magnetic stirrer at ambient temperature for 24 h. Afterward, the product was filtered and rinsed sequentially with hexane, acetone, and anhydrous THF to remove any unreacted precursors and by-products.65 The sample was further dried at a temperature of 120 °C in a vacuum for 12 h, forming Zn@COF-1.
2.2.2 Synthesis of Zn@COF-2. The previously obtained COF-2 (30 mg) was dissolved in 50 mL of distilled water, Then, 50 mL of ZnCl2 (60 mg) aqueous solution was slowly added using a two-channel syringe pump at a steady rate of 1 mL h−1. Simultaneously, the mixture was agitated using a magnetic stirrer at ambient temperature for 24 h. Afterward, the product was filtered and rinsed sequentially with hexane, acetone, and anhydrous THF to remove any unreacted precursors and by-products. The sample was further dried at a temperature of 120 °C in a vacuum for 12 h, forming Zn@COF-2.
2.2.3 Synthesis of Zn@COF-3. The COF-3 (30 mg) was suspended in 50 mL of distilled water. Then, 50 mL of ZnCl2 (60 mg) aqueous solution was slowly added using a two-channel syringe pump at a steady rate of 1 mL h−1. Simultaneously, the mixture was stirred for 24 h with a magnetic stirrer at ambient temperature. Afterward, the product was filtered and rinsed sequentially with hexane, acetone, and anhydrous THF to remove any unreacted precursors and by-products. The sample was further dried at a temperature of 120 °C in a vacuum for 12 h, forming Zn@COF-3. Using solvents with very low surface tension, such as hexane, makes it possible to activate the COF easily while minimizing pore collapse.66
2.3 Gas sensor fabrication and measurement
To establish the reproducibility of the pristine COF and Zn@COF-based gas sensors, multiple independent sensors were fabricated and tested under identical conditions. The fabrication of the Zn@COFs and pristine COFs was followed by their dispersion in an agate mortar, to which polyvinylidene difluoride (PVDF) was added as a binding agent. To ensure uniform dispersion, a small amount of anhydrous ethanol was added dropwise while continuously grinding the mixture for 10 min. The obtained homogeneous paste was then carefully coated onto 1 cm × 2 cm glass substrates to form thin sensing films. The coated films were dried at room temperature for 30 min to remove residual solvent and enhance film adhesion. The fabricated Zn@COFs and COF-based sensors underwent an aging process for 72 h using Winsen's TS-64B sensor gas-sensitive aging platform to enhance sensor stability and achieve a consistent response. This process is crucial for stabilizing the sensing material, ensuring minimal variations in response during subsequent measurements (Fig. 2).
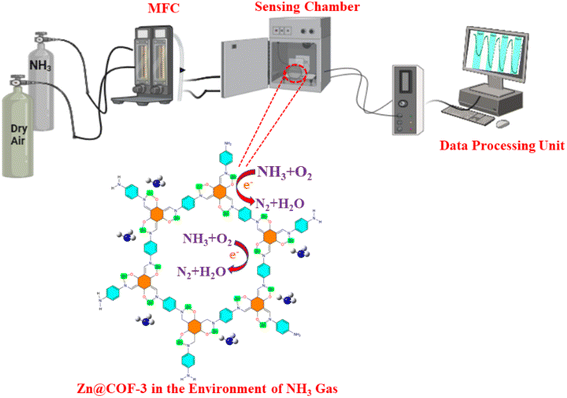 |
| | Fig. 2 Schematic illustration of ammonia gas sensing instrument setup. | |
Gas sensing tests were conducted at room temperature (25 ± 2 °C) under a controlled humidity of 0–75% RH with a gas flow rate of 100 mL min−1. Sensors were pre-exposed to dry air for 30 min before testing. NH3 concentrations ranged from 1 to 50 ppm, and the response was determined by the change in electrical resistance. Reproducibility was evaluated by performing multiple independent gas sensing measurements on identical Zn@COF and COF-based sensors. The response (S) of the sensors was calculated using the equation:
where
Ra is the baseline resistance in air and
Rg is the resistance upon exposure to different concentrations of ammonia gas. To validate reproducibility, four consecutive sensing cycles were conducted at an ammonia concentration of 1 ppm, and the response of each cycle was recorded. The results demonstrate minimal deviation between cycles, confirming the high reproducibility of the sensor's performance.
67
Additionally, the long-term stability of the fabricated sensors was assessed over 30 days by exposing the sensors to 50 ppm ammonia at regular intervals. The response was monitored, and the results indicate that the sensors maintained a stable response with negligible fluctuations, highlighting the robustness and durability of the Zn@COF and COF-based materials for extended applications. As environmental humidity can influence gas sensing performance, the impact of relative humidity (RH) on the sensing response was also examined. The sensors were tested at varying humidity levels ranging from 0% to 75% RH while maintaining a constant ammonia gas concentration of 1 ppm.
2.4 Density functional theory calculations
With the B3LYP functional level and the 6-31G basis set, the DFT approach was utilized to optimise the geometry of Zn@COFs and perform energy calculations both with and without the NH3 molecule. For self-consistent field and geometry optimisation computations involving organic molecules, the 6-31G basis set is popular and efficient.68,69 Before computing the energy of the systems, the atoms underwent relaxation. Eqn (2) can be used to calculate the ammonia adsorption energy and binding energy on the Zn@COFs.70–72| | |
Eads(Zn@COF) = ENH3/Zn@COF − (EZn@COFs + ENH3)
| (2) |
Eqn (2) represents the adsorption energy of Zn@COF in the presence of NH3. The energies of the Zn@COF complexes are identified as ENH3/Zn@COF, where ENH3 indicates the energy of a single NH3 molecule and E represents the total energy of the Zn@COF. The Gaussian 16 W program for electronic structure was used for all calculations after building the basic structure with ChemDraw.
3. Results and discussion
3.1 Structural and morphological analysis
3.1.1 PXRD analysis. The PXRD patterns are shown in Fig. 3(a). The peaks at 4.8°, 8.3°, and 26.7° for the COFs (COF-1, COF-2, and COF-3) are also present in the MCOFs (Zn@COF-1, Zn@COF-2, and Zn@COF-3), and correspond to the (100), (110), and (001) planes, respectively.62,73 These peaks are present in both the parent and Zn-incorporating COFs, indicating that the incorporation of Zn2+ ions does not significantly disrupt the polycrystalline structure. The persistence of these peaks indicates that only slight alterations occur.74 The peak at 26.7° is caused by π–π stacking interactions between the COF molecular layers,75,76 and the peaks at 4.8° and 8.3° indicate a highly ordered porous structure. This means that the crystalline structure of the original COFs is maintained after the incorporation of Zn2+, with only minor shifts in the peak positions, likely resulting from interactions between the Zn2+ ions and the COF active site network.77 We used the d-spacing values from the PXRD data to determine the unit cell parameters of both the parent and Zn-modified COFs (Tables S1 and S2 ESI†). This was further supported by the X-ray diffraction (XRD) patterns of the parent COFs, which are similar to the simulated pattern.63,78
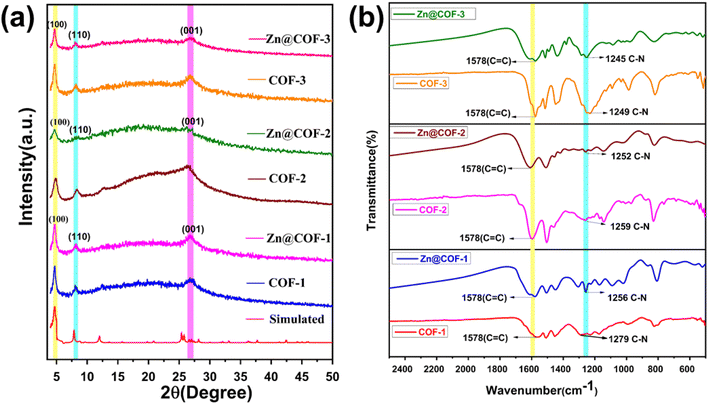 |
| | Fig. 3 (a) Powder X-ray diffraction (PXRD) patterns of the COFs and MCOFs. (b) FTIR spectra of all COFs and MCOFs. | |
3.1.2 FT-IR spectral analysis. Fig. 3(b) shows the FT-IR spectra of the COFs and Zn@COFs. These spectra display a significant shoulder peak at 1622 cm−1, which indicates the stretching of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and a notable peak at 1578 cm−1.79 The peak detected at 1256 cm−1 is caused by the stretching vibration of the C–N bond. These results indicate that the –OH group of the phloroglucinol entity is in the keto form, while the imine bond resulting from Schiff base condensation transforms into an enamine group.80–82 The peak corresponding to the C–N bond shifted from 1279 cm−1 in COF-1 to 1256 cm−1 in Zn@COF-1; from 1259 cm−1 in COF-2 to 1252 cm−1 in Zn@COF-2, and from 1249 cm−1 in COF-3 to 1245 cm−1 in Zn@COF-3. These changes demonstrate the coordination of Zn2+ to N sites.
O bond, and a notable peak at 1578 cm−1.79 The peak detected at 1256 cm−1 is caused by the stretching vibration of the C–N bond. These results indicate that the –OH group of the phloroglucinol entity is in the keto form, while the imine bond resulting from Schiff base condensation transforms into an enamine group.80–82 The peak corresponding to the C–N bond shifted from 1279 cm−1 in COF-1 to 1256 cm−1 in Zn@COF-1; from 1259 cm−1 in COF-2 to 1252 cm−1 in Zn@COF-2, and from 1249 cm−1 in COF-3 to 1245 cm−1 in Zn@COF-3. These changes demonstrate the coordination of Zn2+ to N sites.
3.1.3 13C CP-MAS SS NMR spectra. The 13C CP-MAS SS NMR spectra confirmed the presence of imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and keto (–C
N–) and keto (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–) bonds in both the COFs and Zn@COFs, as shown in Fig. 4(a–c) and S1(a–c) (ESI†). These spectra provide insight into the chemical environment of the carbon atoms in the COF structures before and after Zn incorporation. In the pristine COFs, the characteristic peaks in the 110–150 ppm region correspond to aromatic carbon atoms, while the peaks at around 180–183 ppm (182 ppm for COF-1, 182 ppm for COF-2, and 183 ppm for COF-3) are attributed to carbonyl (–C
O–) bonds in both the COFs and Zn@COFs, as shown in Fig. 4(a–c) and S1(a–c) (ESI†). These spectra provide insight into the chemical environment of the carbon atoms in the COF structures before and after Zn incorporation. In the pristine COFs, the characteristic peaks in the 110–150 ppm region correspond to aromatic carbon atoms, while the peaks at around 180–183 ppm (182 ppm for COF-1, 182 ppm for COF-2, and 183 ppm for COF-3) are attributed to carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–) and imine (–C
O–) and imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) groups, which play a crucial role in the framework stability and electronic properties of the COFs.83 Upon the incorporation of Zn, the overall peak positions remain largely unchanged, which suggests that the Zn coordination does not directly affect the carbon atoms. For Zn@COF-1 and Zn@COF-2, the peaks at 121–135 ppm exhibit slight broadening and shifting, which may indicate Zn coordination through the nitrogen atoms of the imine (–C
N–) groups, which play a crucial role in the framework stability and electronic properties of the COFs.83 Upon the incorporation of Zn, the overall peak positions remain largely unchanged, which suggests that the Zn coordination does not directly affect the carbon atoms. For Zn@COF-1 and Zn@COF-2, the peaks at 121–135 ppm exhibit slight broadening and shifting, which may indicate Zn coordination through the nitrogen atoms of the imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) or amine (–NH2) functional groups. A similar trend is observed for Zn@COF-3, for which shifts in the 105–134 ppm range further support the possibility of the interaction of Zn with nitrogen-rich sites. The absence of significant changes in the carbonyl (∼183 ppm) and aromatic (∼120–150 ppm) regions suggests that the incorporation of Zn does not strongly perturb the overall electronic structure of the COFs.62,84 Instead, Zn coordination likely occurs through weak interactions with nitrogen/oxygen sites, leading to localized electronic modifications rather than major structural reorganization. The lack of significant chemical shift changes after Zn incorporation is due to the non-covalent nature of Zn coordination with the COF framework. Unlike covalent modifications that strongly alter the electronic environment of carbon atoms, Zn coordination occurs through weak interactions with imine (–C
N–) or amine (–NH2) functional groups. A similar trend is observed for Zn@COF-3, for which shifts in the 105–134 ppm range further support the possibility of the interaction of Zn with nitrogen-rich sites. The absence of significant changes in the carbonyl (∼183 ppm) and aromatic (∼120–150 ppm) regions suggests that the incorporation of Zn does not strongly perturb the overall electronic structure of the COFs.62,84 Instead, Zn coordination likely occurs through weak interactions with nitrogen/oxygen sites, leading to localized electronic modifications rather than major structural reorganization. The lack of significant chemical shift changes after Zn incorporation is due to the non-covalent nature of Zn coordination with the COF framework. Unlike covalent modifications that strongly alter the electronic environment of carbon atoms, Zn coordination occurs through weak interactions with imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and (–C
N–) and (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–), leading to minimal perturbation in the carbon chemical shifts.
O–), leading to minimal perturbation in the carbon chemical shifts.
 |
| | Fig. 4 13C CP-MAS solid-state NMR spectra of (a) COF-1, (b) COF-2, and (c) COF-3. | |
3.1.4 FE-SEM & TEM analysis. The SEM images of COF-1 and COF-2 (Fig. 5(a) and (b)) displayed a thread-like network morphology, which enhances the fast diffusion of gas and decreases the interaction between NH3 and the sensing material, while the high porosity and few active sites result in low adsorption of NH3. COF-3 (Fig. 5(c)) exhibited a micro-flower morphology, with increased surface area and a greater number of NH3 adsorption sites due to its hierarchical roughness and layered nanosheets, which helps to increase charge transfer interaction and NH3 interaction. The morphology of the MCOFs is similar to that of the COFs, except in the case of COF-1. Zn@COF-1 and Zn@COF-2 (Fig. 5(d) and (e)) exhibited a porous and fibrous network morphology, facilitating rapid gas diffusion, whereas Zn@COF-3 (Fig. 5(f)) showed a clustered, micro-flower-like structure with increased surface roughness, contributing to enhanced NH3 adsorption and charge transfer efficiency. The pore size distribution histograms (Fig. 5(g–l)) provide further insights into the porosity of the synthesized COFs and Zn@COFs. The pristine COFs (Fig. 5(g–i)) exhibit relatively uniform pore size distributions, with COF-1 and COF-2 exhibiting well-defined porosity, which promotes gas diffusion. COF-3 shows a broader pore distribution, which could be attributed to its hierarchical nanosheet arrangement. The changes in pore size distribution upon Zn incorporation (Fig. 5(j–l)) suggest partial pore occupation or structural rearrangement due to metal coordination. Zn@COF-1 and Zn@COF-2 retain significant porosity, ensuring efficient gas transport, while Zn@COF-3 shows a slight reduction in pore size, which might enhance adsorption and charge transfer efficiency for NH3 sensing. High-resolution TEM analysis of Zn@COF-3 (Fig. 6(a–c)) reveals well-defined lattice fringes with a spacing of 0.31 nm, corresponding to the d(110) value of Zn@COF-3, which enhances electronic conductivity and sensing response. The FFT pattern (Fig. 6(d)) confirms the crystalline nature, which facilitates efficient electron transfer upon NH3 interaction. Additionally, elemental mapping (Fig. 6(e–i)) and EDAX images Fig. S2 (ESI†) confirm the homogenous distribution of C, N, O, and Zn, ensuring uniform active sites for NH3 adsorption and charge transfer. The porous and fibrous network of Zn@COF-1 and Zn@COF-2 facilitates rapid gas diffusion, while the micro-flower-like structure of Zn@COF-3 provides a large surface area for enhanced NH3 adsorption and improved charge transfer efficiency. Furthermore, the high crystallinity and well-structured Zn sites improve conductivity and selectivity, making Zn@COFs more effective NH3 sensing materials than the pristine COFs.
 |
| | Fig. 5 FE SEM images of (a) COF-1, (b) COF-2, (c) COF-3, (d) Zn@COF-1, (e) Zn@COF-2, (f) and Zn@COF-3. (g–i) Pore size distribution histograms of the pristine COFs ((g) COF-1, (h) COF-2, and (i) COF-3). (j–l) Pore size distribution histograms of the Zn@COFs ((j) Zn@COF-1, (k) Zn@COF-2, and (l) Zn@COF-3). | |
 |
| | Fig. 6 (a and b) Low resolution TEM images of Zn@COF-3. (c) High resolution TEM image of Zn@COF-3. (d) AFM 3D image of Zn@COF-3. (e–i) EDAX images of Zn@COF-3. | |
3.1.5 Atomic force microscopy (AFM) analysis. Similarly, Fig. S3(a–c) (ESI†) presents the atomic force microscopy (AFM) surface morphology of the Zn@COF samples with magnifications of 10 μm2 × 10 μm2. The AFM contact mode was employed to obtain the area roughness parameters of the MCOFs. The mean surface roughness (Sa) for Zn@COF-1 is 260.16 nm, that of Zn@COF-2 is 292.33 nm, and that of Zn@COF-3 is 471.75 nm. The root mean square (Sq) values of the Zn@COF-1, 2 and 3 samples are 562.36 nm, 671.06 nm, and 903.45 nm, respectively. High surface roughness was found in the Zn@COF-3 sample; this roughness of the films provides effective surface area, adsorption sites, and gas reactivity.71
3.1.6 X-ray photoelectron spectroscopy (XPS). Analysis of the XPS survey spectra confirmed the presence of C, O, N, and Zn (Fig. S4, ESI†). The C 1s spectra with high resolution reveal the existence of four distinct carbon species on the surface of the samples: C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. 7(a), 8(a), and Fig. S5–S8, ESI†). The N 1s peak of COF-1 shifted from 399.44 eV to 399.2 eV in Zn@COF-1 (Fig. S7(b) ESI†), indicating the successful coordination between Zn2+ and the N atom. In COF-2 and COF-3, similar coordination was observed between Zn2+ and N atoms (Fig. 8(b) and S8(b), ESI†).85 COF-1 exhibits energy peaks at 528.0 eV and 529.8 eV in its high-resolution O 1s XPS spectrum (Fig. S5(c), ESI†). COF-2 exhibits energy peaks at 528.4 eV and 530.4 eV (Fig. S6(c), ESI†). Similarly, COF-3 exhibits peaks at 527.84 eV and 530.5 eV (Fig. 7(c)).86,87 The Zn2p spectra in Fig. 8(d), S7(d) and S8(d) (ESI†) for Zn@COF-1, 2, and 3, respectively, show two distinct peaks at 1042.8 eV and 1019.9 eV. The binding energy for Zn 2p3/2 is 1042 eV, while the binding energy for Zn 2p1/2 is 1019 eV.88 Following the treatment of COF-1 with Zn2+, a new Zn–O peak at 528.3 eV emerged, and the COF-1 peak at 528.6 eV was reduced to a certain degree, indicating that a portion of the oxygen atoms of C
O (Fig. 7(a), 8(a), and Fig. S5–S8, ESI†). The N 1s peak of COF-1 shifted from 399.44 eV to 399.2 eV in Zn@COF-1 (Fig. S7(b) ESI†), indicating the successful coordination between Zn2+ and the N atom. In COF-2 and COF-3, similar coordination was observed between Zn2+ and N atoms (Fig. 8(b) and S8(b), ESI†).85 COF-1 exhibits energy peaks at 528.0 eV and 529.8 eV in its high-resolution O 1s XPS spectrum (Fig. S5(c), ESI†). COF-2 exhibits energy peaks at 528.4 eV and 530.4 eV (Fig. S6(c), ESI†). Similarly, COF-3 exhibits peaks at 527.84 eV and 530.5 eV (Fig. 7(c)).86,87 The Zn2p spectra in Fig. 8(d), S7(d) and S8(d) (ESI†) for Zn@COF-1, 2, and 3, respectively, show two distinct peaks at 1042.8 eV and 1019.9 eV. The binding energy for Zn 2p3/2 is 1042 eV, while the binding energy for Zn 2p1/2 is 1019 eV.88 Following the treatment of COF-1 with Zn2+, a new Zn–O peak at 528.3 eV emerged, and the COF-1 peak at 528.6 eV was reduced to a certain degree, indicating that a portion of the oxygen atoms of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were coordinated with Zn2+. A new peak corresponding to Zn–O bonding was seen in the cases of COF-2 and COF-3 treated with Zn2+. The C
O were coordinated with Zn2+. A new peak corresponding to Zn–O bonding was seen in the cases of COF-2 and COF-3 treated with Zn2+. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding in COF-2 and COF-3 corresponded to the peaks at 528.4 eV and 527.84 eV, respectively. These peaks show decreased intensity in Zn@COF-1, 2 and 3. This may be due to the coordination of Zn2+ with the oxygen atoms of the C
O bonding in COF-2 and COF-3 corresponded to the peaks at 528.4 eV and 527.84 eV, respectively. These peaks show decreased intensity in Zn@COF-1, 2 and 3. This may be due to the coordination of Zn2+ with the oxygen atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of COFs.89 This confirms the successful incorporation of zinc into the COF framework, as well as the characteristic zinc peaks and variations in binding energies.
O groups of COFs.89 This confirms the successful incorporation of zinc into the COF framework, as well as the characteristic zinc peaks and variations in binding energies.
 |
| | Fig. 7 High-resolution XPS spectra of COF-3: (a) carbon atom, (b) nitrogen atom, and (c) oxygen atom. | |
 |
| | Fig. 8 High-resolution XPS spectra of Zn@COF-3: (a) carbon atom, (b) nitrogen atom, (c) oxygen atom, and (d) Zn atom. | |
3.1.7 Brunauer–Emmett–Teller (BET) analysis. The N2 adsorption–desorption isotherms of COF-1, COF-2, and COF-3 (Fig. S9(a–c), ESI†) exhibit type-IV hysteresis loops, which are indicative of mesoporous frameworks with well-defined pore channels. Similarly, Zn@COF-1, Zn@COF-2, and Zn@COF-3 (Fig. S9(d–f), ESI†) also showed type-IV behavior, confirming that the mesoporous nature of the parent COFs was retained after Zn2+ incorporation. Notably, a significant enhancement in BET surface area was observed upon zinc doping: COF-1, COF-2, and COF-3 exhibited surface areas of 122 m2 g, 191 m2 g, and 253 m2 g, respectively (Table 1), whereas Zn@COF-1, Zn@COF-2, and Zn@COF-3 showed increased values of 273 m2 g−1, 320 m2 g−1, and 335 m2 g−1, respectively (Table 1). This increase in surface area after zinc incorporation is likely due to improved framework ordering and pore accessibility, which facilitate enhanced gas interactions. These findings indicate that all the Zn@COF materials can adsorb, making them effective gas sensors.90–92
Table 1 BET surface area values of pristine COFs and Zn@COFs
| S. no. |
Material |
Surface area |
| 1 |
COF-1 |
122 m2 g−1 |
| 2 |
COF-2 |
191 m2 g−1 |
| 3 |
COF-3 |
253 m2 g−1 |
| 4 |
Zn@COF-1 |
273 m2 g−1 |
| 5 |
Zn@COF-2 |
320 m2 g−1 |
| 6 |
Zn@COF-3 |
335 m2 g−1 |
3.1.8 Thermogravimetric analysis (TGA). Thermogravimetric analysis (TGA) revealed that the COFs and MCOFs possess good thermal stability up to 350–400 °C, as indicated by the less than 10% weight loss when the temperature was increased from room temperature to 350 °C·As shown in Fig. S10(a–c) (ESI†) the TGA graphs of all the COFs and MCOFs gave pyrolysis temperatures in the approximate range of 350 °C to 450 °C with approximately 35% pyrolysis residue.93
3.2 Gas sensing performance
Ammonia gas sensing investigations at ambient temperature using Schiff-based COFs and MCOFs have not been reported, although they have been provided in NO2 and H2 sensing research in the literature.94,95 The presence of keto–enol tautomerism in the present COFs and MCOFs significantly enhances the gas sensing performance owing to their structural flexibility and dynamic behaviour. The ability to switch between keto and enol forms can create different binding sites, electronic states, and the potential to alter the charge distribution within the COFs, which is useful for enhancing the gas sensing and selectivity of ammonia gas.96
Therefore, in the current investigation, a quantitative-based sensor for ammonia, acetic acid, formaldehyde, and ethanol gas detection was fabricated with the COFs and MCOFs at room temperature. The current study focused on four representative gas analytes, namely, ammonia, formaldehyde, ethanol, and acetic acid, at concentrations of 1, 10, 25, and 50 ppm to demonstrate the chemiresistive gas sensing capability of the parent COFs (COF-1, 2, and 3) and the MCOFs (Zn@COF-1, 2 and 3).
3.2.1 Gas sensing performance of parent COFs (COF-1, 2, and 3). The COF-1 materials demonstrate gas-sensing responses of 1.02, 1.63, 4.45, and 11.65 to ammonia at concentrations of 1, 10, 25, and 50 ppm, respectively. For ethyl alcohol, the corresponding responses are 1.12, 1.50, 1.63, and 1.89. The response times for ammonia at concentrations of 1, 10, 25, and 50 ppm are 118 s, 175 s, 163 s, and 116 s, respectively. The recovery times for ammonia are 82 s, 64 s, 58 s, and 48 s. For ethyl alcohol, the response times are 42 s, 59 s, 58 s, and 48 s, with recovery times of 34 s, 37 s, 38 s, and 36 s. Additionally, this compound does not detect acetic acid or formaldehyde gas at ambient temperatures; these values are summarized in Table S3 (ESI†). Similarly, the COF-2 materials demonstrate gas-sensing responses of 1.2, 9.8, 14.14, and 23.88 for ammonia at concentrations 1, 10, 25, and 50 ppm, respectively. For acetic acid, the responses are 1.35, 4.2, 8.1, and 17.24. The response times for ammonia at different concentrations (1, 10, 25, and 50 ppm) are 110 s, 177 s, 136 s, and 79 s, respectively. The recovery times for ammonia are 40 s, 61 s, 49 s, and 46 s. For acetic acid, the response times are 30 s, 37 s, 43 s, and 41 s with recovery times of 23 s, 69 s, 56 s, and 51 s. Additionally, this compound does not detect ethyl alcohol or formaldehyde gas at ambient temperature; these values are summarized in Table S3 (ESI†).The COF-3 materials demonstrated gas-sensing responses of 1.5, 10.5, 21.6, and 55.8 for ammonia at different concentrations of 1, 10, 25, and 50 ppm, respectively. For acetic acid, the response values were 1.34, 4.08, 7.64, and 15.80 at the same concentrations. It showed 1.2, 1.26, 1.29, and 1.47 responses to ethyl alcohol, while those for formaldehyde were 1.13, 1.16, 1.19, and 1.26. The response times of COF-3 to ammonia at concentrations of 1, 10, 25, and 50 ppm were 38 s, 43 s, 39 s, and 34 s respectively, while the recovery times for these concentrations were 22 s, 36 s, 33 s, and 29 s. Similarly, the response times were 36 s, 54 s, 30 s, and 27 s, and the recovery times 23 s, 29 s, 13 s, and 15 s for acetic acid. The response values and graphs of ammonia, acetic acid, ethyl alcohol, and formaldehyde gas are shown in Fig. S11(a–d) and Table S3 (ESI†). Comparing the gas sensing performance of the COFs, COF-3 detects all gases and shows superior responses, response times, and recovery times than COF-1 and COF-2. COF-1 could not detect the gases acetic acid and formaldehyde, while COF-2 failed to detect ethyl alcohol and formaldehyde. The enhanced sensing activity of COF-3 is attributed to its highly crystalline nature, spongy flower morphology, and an increase in keto–enol active sites.
3.2.2 Gas sensing performance of Zn@COFs. Similarly, the response values of the Zn@COF-1 material to ammonia at concentrations of 1, 10, 25, and 50 ppm are 1.65, 2.83, 5.54, and 20.24, respectively; the response times are 105 s, 163 s, 121 s, and 53 s, with recovery times of 31 s, 24 s, 19 s, and 28 s. For acetic acid, the response values were 1.24, 1.58, 5.1, and 10; the response times were 37 s, 39 s, 65 s, and 60 s, with recovery times of 28 s, 36 s, 75 s, and 43 s. Ethyl alcohol gave response values of 1.42, 1.58, 1.69, and 1.76; the response times were 30 s, 39 s, 41 s, and 57 s with recovery times of 24 s, 26 s, 29 s, and 40 s. For formaldehyde, the response values were 1.13, 1.37, 1.45, and 1.59, and the response times were 17 s, 37 s, 41 s, and 49 s with recovery times of 13 s, 14 s, 26 s, and 29 s. The response graphs for ammonia, acetic acid, ethyl alcohol, and formaldehyde gas are presented in Fig. S12(a–d) and Table S4 (ESI†).The response values of the Zn@COF-2 material for ammonia at different concentrations of 1, 10, 25, and 50 ppm were 1.65, 11.83, 23.18, 72.85, and the response times were 100 s, 167 s, 125 s, and 66 s with recovery times of 29 s, 34 s, 31 s, and 41 s, respectively. For acetic acid, the response values were 1.44, 4.34, 8.84, and 10; the response times were 26 s, 38 s, 39 s, and 40 s with recovery times of 17 s, 65 s, 55 s, and 62 s. For ethyl alcohol, the response values were 1.46, 2.87, 3.21, and 4.78, and the response times were 30 s, 43 s, 45 s, and 58 s with recovery times of 23 s, 32 s, 34 s, and 42 s. For formaldehyde, the response values were 1.13, 1.36, 1.45, and 1.56; the response times were 18 s, 31 s, 22 s, and 30 s with recovery times of 17 s, 25 s, 30 s, and 22 s, and the response values and graphs for ammonia, acetic acid, ethyl alcohol, and formaldehyde gas are presented in Fig. S13(a–d) and Table S4 (ESI†).
The Zn@COF-3 material exhibited response values of 2.54, 11.58, 36.13, and 94.90 at ammonia concentrations of 1, 10, 25, and 50 ppm; the response times were 26 s, 28 s, 25 s, and 17 s with recovery times of 18 s, 12 s, 8 s, and 6 s, showing enhanced results compared to the other COFs and Zn@COFs. For acetic acid, the response values were 1.54, 4.28, 8.54, and 17.80; the response times were 32 s, 46 s, 27 s, and 23 s with recovery times of 17 s, 22 s, 10 s, and 8 s. For ethyl alcohol, the response values were 1.42, 1.47, 1.57, and 1.9, and response times were 42 s, 57 s, 48 s, and 50 s with recovery times of 12 s, 24 s, 24 s, and 31 s. For formaldehyde, the response values were 1.11, 1.23, 1.28, and 1.36; the response times were 25 s, 41 s, 20 s, and 39 s with recovery times 21 s, 24 s, 53 s, and 31 s. The response values and graphs for ammonia, acetic acid, ethyl alcohol, and formaldehyde gas are presented in Fig. 9(a–d) and Table S4 (ESI†).
 |
| | Fig. 9 Resistance curves of Zn@COF-3 at concentrations of 1, 10, 25, and 50 ppm for: (a) ammonia, (b) acetic acid, (c) ethyl alcohol, and (d) formaldehyde. | |
Comparing the gas sensing performance of COFs and MCOFs, the MCOFs demonstrate superior results. The response time and recovery time of the COFs at 1 ppm were as follows: COF-1 (tres = 118 s, trec = 42 s at 1 ppm), COF-2 (tres = 110 s, trec = 40 s at 1 ppm), and COF-3 (tres = 38 s, trec = 22 s at 1 ppm). Similarly, the response times and recovery times of the MCOFs at 1 ppm were as follows: Zn@COF-1 (tres = 105 s, trec = 31 s at 1 ppm), Zn@COF-2 (tres = 100 s, trec = 29 s at 1 ppm), and Zn@COF-3 (tres = 26 s, trec = 18 s at 1 ppm). Fig. 10 and Table 2 present a comparison of the ammonia sensor responses with those of previous reported sensors. The zinc-doped COFs (Zn@COF-3) show superior detection responses and recovery times due to the incorporation of zinc metal, which increases the active sites and enhances the interaction with ammonia gas. Additionally, it enhances the stability, selectivity, electrical conductivity, surface area, and response times, making Zn@COFs superior for NH3 sensing compared to traditional COFs. Comparing the MCOFs, Zn@COF-3 shows superior results to Zn@COF-1 and 2, which this may be attributed to Zn@COF-3 having abundant active sites, keto–enol tautomerism, high surface area, and pore volume, which have a substantial impact on gas sensors.109–112
 |
| | Fig. 10 Sensing comparison of ammonia, acetic acid, ethanol, and formaldehyde using the parent COFs (COF-1, 2 and 3) and MCOFs (Zn@COF-1, 2 and 3). | |
Table 2 Comparison of ammonia sensing performance of the present sensor with previously reported studies at ambient temperaturea
| Sensor |
LOD |
Tres |
Trec |
T °C |
Ref. |
| LOD – limit of detection, Tres – response time, Trec – recovery time, T °C – temperature, and Ref. – references. |
| ZnCo(NA) |
1 ppm |
39 s |
40 s |
RT |
97 |
| ZnO/SiO2 |
10 ppm |
65 s |
60 s |
RT |
98 |
| TiO2 |
5 ppm |
34 s |
90 s |
RT |
99 |
| ZnO/Pd |
30 ppm |
198 s |
334 s |
RT |
100 |
| Cu-BTC@GO |
500 ppm |
20 s |
30 s |
RT |
101 |
| CuHITP2 |
100 ppm |
1.7 s |
3.34 s |
RT |
102 |
| CuPc@IRMOF-3 |
0–50 ppm |
NA |
NA |
RT |
103 |
| COF-DC-8 |
NA |
NA |
NA |
RT |
104 |
| pCTF |
100 ppm |
100 s |
400 s |
RT |
105 |
| TAPB-BPDA COF |
100 ppm |
8–40 s |
100–120 s |
RT |
106 |
| CTF-1-A |
100 ppm |
100 s |
420 s |
NA |
107 |
| HMP-TAPB-1 |
500 ppm |
65 s |
100–9 s |
NA |
108 |
| COF-1 |
1 ppm |
118 s |
42 s |
RT |
This work |
| COF-2 |
1 ppm |
110 s |
40 s |
RT |
This work |
| COF-3 |
1 ppm |
38 s |
22 s |
RT |
This work |
| Zn@COF-1 |
1 ppm |
105 s |
31 s |
RT |
This work |
| Zn@COF-2 |
1 ppm |
100 s |
29 s |
RT |
This work |
| Zn@COF-3 |
1 ppm |
26 s |
18 s |
RT |
This work |
One of the most important aspects of sensing materials is their long-term stability, reproducibility, and relative humidity sensing. Fig. 11(a and b) presents a reproducibility analysis of the consistent resistance variations and stable response across four cycles, confirming the reliability of Zn@COF-3 at 1 ppm of NH3. Fig. 11(c) reveals a decreasing response with increasing relative humidity, suggesting that water molecule adsorption competes with ammonia adsorption, leading to reduced sensitivity. However, the Zn@COF-3 sensor maintains a detectable response, indicating its practical applicability. The term stability refers to the extent to which a sensor's properties remain consistent over time. To evaluate the performance of the Zn@COFs as sensing materials, their stability and repeatability were assessed over 30 days at a concentration of 50 parts per million (ppm) as shown in Fig. 11 (d–f), which present the stability response graphs over the entire 30-day period for the three Zn@COFs, confirming their stability.
 |
| | Fig. 11 (a and b) Reproducibility of Zn@COF-3 at 1 ppm. (c) Response (Ra/Rg) of the Zn@COF-3 sensor to 1 ppm of ammonia gas under varying relative humidity (RH) conditions. (d–f) Long-term stability of the responses of Zn@COF-1, Zn@COF-2, and Zn@COF-3 towards ammonia gas at 50 ppm. | |
3.3 Gas sensing mechanism
The gas sensors exhibited n-type sensing characteristics, rendering them less resistant to reducing gases such as ammonia, acetic acid, ethyl alcohol, and formaldehyde due to the surface-related chemical reactions of the target gas molecules. The sensing materials performance is significantly affected by their composition, specific surface area, and valence states. The sensing mechanism of the MCOFs is the creation of a charge depletion layer on the MCOF surface as a result of electron trapping on adsorbed oxygen species.109 The overall gas sensing process includes gas adsorption, surface reactions, and desorption processes. The chemosensor undergoes a process in which oxygen molecules attach to its surface. This results in the creation of more active ionized oxygen species (O and O2−) and the formation of holes as they take electrons from the Schiff-based Zn@COF-1, 2, and 3 (eqn (3) and (4)). Due to the capture of conduction band electrons and the expansion of the surface electron depletion layer in Zn@COF-1, 2 and 3, the conductance of the sensor decreases, leading to an increase in resistance.113–115| | |
O2(ads) + e− → O2(ads)−
| (4) |
| | |
O2(ads)− + e− → 2O(ads)−
| (5) |
| | |
O(ads)− + e− → O2(ads)−
| (6) |
When the resistance of the sensor decreases, trapped electrons are released back into the conduction band. This happens when the sensor is exposed to target gases such as ammonia and interacts with adsorbed oxygen ions (Fig. 12). The above equations (eqn (5) and (6)) provide a brief overview of the oxygen adsorption depletion layer and subsequent reactions for ammonia sensing, and the subsequent equations (eqn (7)–(10)) involve the air and targeted gases.| | |
NH3 + O2(ads) → N2 + H2O (ammonia)
| (7) |
| | |
HCHO + O2(ads)− → CO2 + H2O + e− (formaldehyde)
| (8) |
| | |
CH3CH2OH + 6O(ads)− → 2CO2(g) + 3H2O(g) + 6e− (ethyl alcohol)
| (9) |
| | |
CH3COOH + 4O(ads)− → 2CO2(g) + 2H2O(g) + 4e− (acetic acid)
| (10) |
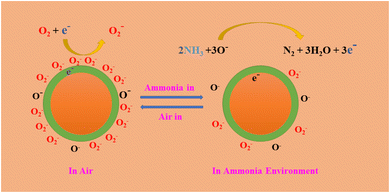 |
| | Fig. 12 Mechanism diagrams of 2D-MCOF sensors in the air and targeted gas. | |
Further, to support the above mechanism, DFT simulation studies were performed. The electrostatic distributions of Zn@COF and NH3 show potential adsorption areas where NH3 might interact with Zn2+. The study also simulated potential interactions between NH3 gas molecules and the surfaces of Zn@COF-1, 2, and 3. Fig. 13(a–c) shows the original and geometrically optimised arrangements of NH3 on these surfaces, while Fig. S16(a–c) (ESI†) illustrates the optimised arrangements of Zn@COF-1, 2, and 3. It was also observed that the Zn2+ interlayer functions as a trap site for NH3 gas molecules. The binding energy of Zn@COF-3 is −281.77 kJ mol−1, which is higher than that of Zn@COF-1 (−56.51 kJ mol−1) and Zn@COF-2 (−171.1 kJ mol−1). These findings suggest that Zn@COF-3 has a stronger affinity for NH3 due to its extensive surface area. The HOMO is localised on the phenylenediamine unit (donor) and LUMO on the 1,3,5-hydroxybenzene-2,4,6-tricarbaldehyde unit (acceptor) (Fig. 13(e), S17(a) and (b) (ESI†)); the band gap energy values are listed in Table S5 (ESI†). The gas adsorption energy values indicated by the density of states (DOS) graphs are presented in Fig. 13(d), S18(a), (b) and Table S6 (ESI†).
 |
| | Fig. 13 Adsorption energies for (a) Zn@COF-1@NH3, (b) Zn@COF-2@NH3, and (c) Zn@COF-3@NH3. (d) Density of states for Zn@COF-3, and (e) HOMO–LUMO plots of Zn@COF-3. | |
4. Conclusion
In this study, we successfully synthesized COFs and Zn-encapsulated COFs (Zn@COFs) for highly efficient NH3 sensing at room temperature. The incorporation of Zn2+ into the COF framework was confirmed by 13C CP-MAS NMR spectroscopy (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, peak at ∼183 ppm, and imine C
O, peak at ∼183 ppm, and imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peaks at ∼148 and ∼146 ppm) and XPS (C
N peaks at ∼148 and ∼146 ppm) and XPS (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 527.84 eV, C
O peak at 527.84 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at 399.2 eV, Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized materials, Zn@COF-3 exhibited superior NH3 sensing performance, with a low detection limit of 1 ppm, a rapid response time of 26 s, and a fast recovery time of 18 s. These remarkable properties can be attributed to its micro-flower-like morphology, high crystallinity, and large surface area (335 m2 g−1), which facilitate efficient gas interaction and charge transfer. DFT calculations further confirmed a strong interaction between NH3 molecules and Zn@COF-3, with a high adsorption energy of −281.77 kJ mol−1 and a low energy gap of 2.651 eV, leading to enhanced charge transfer efficiency. These characteristics make the Zn@COFs significantly more effective than the pristine COFs for NH3 sensing at room temperature. Given their exceptional sensing properties, Zn@COFs hold great potential for applications in environmental monitoring, industrial safety, and medical diagnostics. Future research can explore their integration into flexible and wearable sensors, portable miniaturized devices, multi-gas sensing applications, and IoT-based environmental monitoring systems.
N at 399.2 eV, Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized materials, Zn@COF-3 exhibited superior NH3 sensing performance, with a low detection limit of 1 ppm, a rapid response time of 26 s, and a fast recovery time of 18 s. These remarkable properties can be attributed to its micro-flower-like morphology, high crystallinity, and large surface area (335 m2 g−1), which facilitate efficient gas interaction and charge transfer. DFT calculations further confirmed a strong interaction between NH3 molecules and Zn@COF-3, with a high adsorption energy of −281.77 kJ mol−1 and a low energy gap of 2.651 eV, leading to enhanced charge transfer efficiency. These characteristics make the Zn@COFs significantly more effective than the pristine COFs for NH3 sensing at room temperature. Given their exceptional sensing properties, Zn@COFs hold great potential for applications in environmental monitoring, industrial safety, and medical diagnostics. Future research can explore their integration into flexible and wearable sensors, portable miniaturized devices, multi-gas sensing applications, and IoT-based environmental monitoring systems.
Data availability
The data that supports the findings of this study are available in the ESI† of this article.
Author contributions
Sujith Benarzee Nallamalla – experimental, methodology, data acquisition, formal analysis, and writing – original draft. Naresh Kumar Katari – visualisation, data curation, and resources. A. Jagan Mohan Reddy – data curation, Sreekantha Babu Jonnalagadda – formal analysis and data curation. Surendra Babu Manabolu Surya – conceptualisation, methodology, supervision, review and editing.
Conflicts of interest
The authors report no declarations of interest.
Acknowledgements
Mr Sujith Benarzee Nallamalla acknowledges the financial support from the Department of Science and Technology (DST), Government of India for the INSPIRE fellowship (INSPIRE code: IF210570) and thanks the CIF, GITAM Deemed to be University for the facilities. The authors also would like to acknowledge the SAIF, DST-supported Institute NMR Facility at IISc Bangalore for providing the solid-state NMR spectra. Jonnalagadda S. B. and Katari N. K. acknowledge the University of KwaZulu-Natal for funding.
Notes and references
- S. Panda, S. Mehlawat, N. Dhariwal, A. Sanger and A. Kumar, Mater. Sci. Eng., B, 2024, 117616, DOI:10.1016/j.mseb.2024.117616.
- B. Zong, S. Wu, Y. Yang, Q. Li, T. Tao and S. Mao, Nano-Micro Lett., 2025, 54, DOI:10.1007/s40820-024-01543-w.
- C. Mahapatra, Med. Gas Res., 2025, 15, 318–326 CrossRef CAS.
- M. Yang, C. Au, G. Deng, S. Mathur, Q. Huang, X. Luo, G. Xie, H. Tai, Y. Jiang, C. Chen, Z. Cui, X. Liu, C. He, Y. Su and J. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 52850–52860 CrossRef CAS.
- I. D. Weiner and J. W. Verlander, Compr. Physiol., 2013, 3, 201–220 CrossRef.
- S. M. Majhi, A. Mirzaei, H. W. Kim, S. S. Kim and T. W. Kim, Nano Energy, 2021, 105369, DOI:10.1016/j.nanoen.2020.105369.
- V. R. Naganaboina, M. Anandkumar, A. S. Deshpande and S. G. Singh, ACS Appl. Nano Mater., 2022, 5, 4524–4536 CrossRef CAS.
- S. Alwarappan, N. Nesakumar, D. Sun, T. Y. Hu and C. Z. Li, Biosens. Bioelectron., 2022, 113943, DOI:10.1016/j.bios.2021.113943.
- L. Chen, Q. Yu, C. Pan, Y. Song, H. Dong, X. Xie, Y. Li, J. Liu, D. Wang and X. Chen, Talanta, 2022, 123527, DOI:10.1016/j.talanta.2022.123527.
- F. R. Juang, C. H. Hsieh, I. Y. Huang, W. Y. Wang, W. B. Lin and L. Yen, Solid-State Electron., 2022, 108224, DOI:10.1016/j.sse.2021.108224.
- N. Chakraborty, A. Sanyal, S. Das, D. Saha, S. K. Medda and S. Mondal, ACS Appl. Nano Mater., 2020, 3, 7572–7579 CrossRef CAS.
- X. Tian, L. Yao, X. Cui, R. Zhao, T. Chen, X. Xiao and Y. Wang, J. Mater. Chem. A, 2022, 10, 5505–5519 RSC.
- F. J. Maldonado-Hódar, C. Moreno-Castilla, J. Rivera-Utrilla and M. A. Ferro García, Stud. Surf. Sci. Catal., 2000, 130B, 1007–1012 CrossRef.
- Y. Masuda, Sens. Actuators, B, 2022, 131876, DOI:10.1016/j.snb.2022.131876.
- C. Moreno-Castilla and F. J. Maldonado-Hodar, Phys. Chem. Chem. Phys., 2000, 2, 4818–4822 RSC.
- X. Liu, K. Zhao, X. Sun, C. Zhang, X. Duan, P. Hou, G. Zhao, S. Zhang, H. Yang, R. Cao and X. Xu, Sens. Actuators, B, 2019, 285, 1–10 CrossRef CAS.
- Y. Zeng, Z. Hua, X. Tian, X. Li, Z. Qiu, C. Zhang, M. Wang and E. ping Li, Sens. Actuators, B, 2018, 273, 1291–1299 CrossRef CAS.
- D. Kwak, Y. Lei and R. Maric, Talanta, 2019, 204, 713–730 CrossRef CAS.
- X. Xu, Z. Wang, K. Zhan, C. Bao, Z. Zhu, B. Chang, Q. Chen, X. Jing and L. Tao, 2021 IEEE 16th Nanotechnology Materials and Devices Conference, NMDC, 2021, DOI:10.1109/NMDC50713.2021.9677543.
- J. Stulik, J. Slauf, R. Polansky, M. Mergl and M. Kalbac, IEEE Sens. J., 2022, 22, 3024–3032 CAS.
- A. P. Côté, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355(6328), eaal1585, DOI:10.1126/science.aal1585.
- S. Liu, M. Wang, Y. He, Q. Cheng, T. Qian and C. Yan, Coord. Chem. Rev., 2023, 214882, DOI:10.1016/j.ccr.2022.214882.
- E. L. Spitler, B. T. Koo, J. L. Novotney, J. W. Colson, F. J. Uribe-Romo, G. D. Gutierrez, P. Clancy and W. R. Dichtel, J. Am. Chem. Soc., 2011, 133, 19416–19421 CrossRef CAS PubMed.
- J. W. Crowe, L. A. Baldwin and P. L. McGrier, J. Am. Chem. Soc., 2016, 138, 10120–10123 CrossRef CAS.
- S. Dalapati, C. Gu and D. Jiang, Small, 2016, 12, 6513–6527 CrossRef CAS.
- J. Dong, X. Li, S. B. Peh, Y. Di Yuan, Y. Wang, D. Ji, S. Peng, G. Liu, S. Ying, D. Yuan, J. Jiang, S. Ramakrishna and D. Zhao, Chem. Mater., 2019, 31, 146–160 CrossRef CAS.
- P. Albacete, A. López-Moreno, S. Mena-Hernando, A. E. Platero-Prats, E. M. Pérez and F. Zamora, Chem. Commun., 2019, 55, 1382–1385 RSC.
- Y. Kumar, I. Ahmad, A. Rawat, R. K. Pandey, P. Mohanty and R. Pandey, ACS Appl. Mater. Interfaces, 2024, 16, 11605–11616 CrossRef CAS.
- P. Das and S. K. Mandal, J. Mater. Chem. A, 2018, 6, 16246–16256 RSC.
- Z. Li, Y. Zhang, H. Xia, Y. Mu and X. Liu, Chem. Commun., 2016, 52, 6613–6616 RSC.
- Y. Cai, Y. Jiang, L. Feng, Y. Hua, H. Liu, C. Fan, M. Yin, S. Li, X. Lv and H. Wang, Anal. Chim. Acta, 2019, 1057, 88–97 CAS.
- H. Singh, V. K. Tomer, N. Jena, I. Bala, N. Sharma, D. Nepak, A. De Sarkar, K. Kailasam and S. K. Pal, J. Mater. Chem. A, 2017, 5, 21820–21827 RSC.
- H. L. Qian, C. Dai, C. X. Yang and X. P. Yan, ACS Appl. Mater. Interfaces, 2017, 9, 24999–25005 CrossRef CAS.
- L. Chen, L. He, F. Ma, W. Liu, Y. Wang, M. A. Silver, L. Chen, L. Zhu, D. Gui, J. Diwu, Z. Chai and S. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 15364–15368 CrossRef CAS PubMed.
- M. Xu, L. Wang, Y. Xie, Y. Song and L. Wang, Sens. Actuators, B, 2019, 281, 1009–1015 CrossRef CAS.
- N. B. Shustova, A. F. Cozzolino, S. Reineke, M. Baldo and M. Dincă, J. Am. Chem. Soc., 2013, 135, 13326–13329 CrossRef CAS.
- S. Dalapati, E. Jin, M. Addicoat, T. Heine and D. Jiang, J. Am. Chem. Soc., 2016, 138, 5797–5800 CrossRef CAS.
- S. M. J. Rogge, A. Bavykina, J. Hajek, H. Garcia, A. I. Olivos-Suarez, A. Sepúlveda-Escribano, A. Vimont, G. Clet, P. Bazin, F. Kapteijn, M. Daturi, E. V. Ramos-Fernandez, F. X. I. Llabrés Xamena, V. Van Speybroeck and J. Gascon, Chem. Soc. Rev., 2017, 46, 3134–3184 RSC.
- J. Wang, J. Li, M. Gao and X. Zhang, Nanoscale, 2017, 9, 10750–10756 RSC.
- X. Wang, Y. Wang and Y. Ying, TrAC, Trends Anal. Chem., 2021, 116395, DOI:10.1016/j.trac.2021.116395.
- C. Guo, F. Duan, S. Zhang, L. He, M. Wang, J. Chen, J. Zhang, Q. Jia, Z. Zhang and M. Du, J. Mater. Chem. A, 2022, 10, 475–507 RSC.
- X. Ma, J. Kang, Y. Wu, C. Pang, S. Li, J. Li, Y. Xiong, J. Luo, M. Wang and Z. Xu, TrAC, Trends Anal. Chem., 2022, 116793, DOI:10.1016/j.trac.2022.116793.
- C. Zhang, B. H. Wu, M. Q. Ma, Z. Wang and Z. K. Xu, Chem. Soc. Rev., 2019, 48, 3811–3841 RSC.
- K. Vikrant, V. Kumar, K. H. Kim and D. Kukkar, J. Mater. Chem. A, 2017, 5, 22877–22896 RSC.
- X. Liu, L. Zhang and J. Wang, J. Materiomics, 2021, 7, 440–459 CrossRef.
- H. Sun, Y. Li, S. Yu and J. Liu, Nano Today, 2020, 100985, DOI:10.1016/j.nantod.2020.100985.
- Y. Liu, W. Zhou, W. L. Teo, K. Wang, L. Zhang, Y. Zeng and Y. Zhao, Chem, 2020, 6, 3172–3202 CAS.
- J. Wang, Y. Feng and B. Zhang, Prog. Chem., 2022, 34, 1308–1320 CAS.
- G. Li, S. Zhao, Y. Zhang and Z. Tang, Adv. Mater., 2018, 1800702, DOI:10.1002/adma.201800702.
- S. Lu, Y. Hu, S. Wan, R. McCaffrey, Y. Jin, H. Gu and W. Zhang, J. Am. Chem. Soc., 2017, 139, 17082–17088 CrossRef CAS PubMed.
- S. Yang, W. Hu, X. Zhang, P. He, B. Pattengale, C. Liu, M. Cendejas, I. Hermans, X. Zhang, J. Zhang and J. Huang, J. Am. Chem. Soc., 2018, 140, 14614–14618 CrossRef CAS.
- H. C. Ma, C. C. Zhao, G. J. Chen and Y. Bin Dong, Nat. Commun., 2019, 3368, DOI:10.1038/s41467-019-11355-x.
- B. Sun, X. Li, T. Feng, S. Cai, T. Chen, C. Zhu, J. Zhang, D. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 51837–51845 CrossRef CAS.
- S. Zhao, Y. Shen, X. Yan, P. Zhou, Y. Yin, R. Lu, C. Han, B. Cui and D. Wei, Sens. Actuators, B, 2019, 286, 501–511 CrossRef CAS.
- I. Taha, Z. M. Abdulhamid, R. Straubinger, A. H. Emwas, K. Polychronopoulou and D. H. Anjum, Sci. Rep., 2024, 29712, DOI:10.1038/s41598-024-81279-0.
- M. T. Noman, N. Amor and M. Petru, Crit. Rev. Solid State Mater. Sci., 2022, 47, 99–141 CrossRef CAS.
- Z. M. Ali, M. E. El Sayed, A. Samir and M. N. Murshed, J. Electron. Mater., 2025, 262–272, DOI:10.1007/s11664-024-11506-x.
- S. Xu and Q. Zhang, Mater. Today Energy, 2021, 100635, DOI:10.1016/j.mtener.2020.100635.
- R. Qin, K. Liu, Q. Wu and N. Zheng, Chem. Rev., 2020, 120, 11810–11899 CrossRef CAS.
- Q. Cao, L. L. Zhang, C. Zhou, J. H. He, A. Marcomini and J. M. Lu, Appl. Catal., B, 2021, 294, 120238, DOI:10.1016/j.apcatb.2021.120238.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS.
- S. Kandambeth, B. P. Biswal, H. D. Chaudhari, K. C. Rout, S. H. Kunjattu, S. Mitra, S. Karak, A. Das, R. Mukherjee, U. K. Kharul and R. Banerjee, Adv. Mater., 2017, 29, 1603945, DOI:10.1002/adma.201603945.
- D. Zhu and R. Verduzco, ACS Appl. Mater. Interfaces, 2020, 12, 33121–33127 CrossRef CAS.
- T. Ni, D. Zhang, J. Wang, S. Wang, H. Liu and B. Sun, Sens. Actuators, B, 2018, 269, 340–345 CrossRef CAS.
- Y. Hu, T. Li, J. Zhang, J. Guo, W. Wang and D. Zhang, Sens. Actuators, B, 2022, 352, 130912, DOI:10.1016/j.snb.2021.130912.
- A. S. Rad, Surf. Sci., 2016, 645, 6–12 CrossRef CAS.
- M. Yoosefian, H. Raissi and A. Mola, Sens. Actuators, B, 2015, 212, 55–62 CrossRef CAS.
- A. M. Khudhair, M. H. Mohammed, F. N. Ajeel and S. H. Mohammed, Chem. Phys. Impact, 2023, 6, 100154, DOI:10.1016/j.chphi.2022.100154.
- A. M. Khudhair and A. Ben Ahmed, Opt. Quantum Electron., 2023, 55, 812, DOI:10.1007/s11082-023-05069-0.
- M. H. Mohammed, F. N. Ajeel and A. M. Khudhair, J. Electron Spectrosc. Relat. Phenom., 2018, 228, 20–24 CrossRef CAS.
- D. Deng, S. Wen, Y. Wang, J. Liu, F. Li and X. Yang, Microchem. J., 2022, 107924, DOI:10.1016/j.microc.2022.107924.
- J. Wang, H. Li, S. Liu, Y. Hu, J. Zhang, M. Xia, Y. Hou, J. Tse, J. Zhang and Y. Zhao, Angew. Chem., Int. Ed., 2021, 60, 181–185 CrossRef CAS.
- H. Guo, L. Sun, M. Yang, M. Wang, N. Wu, T. Zhang, J. Zhang, F. Yang and W. Yang, Anal. Methods, 2021, 13, 4994–5002 RSC.
- S. Ghosh, R. A. Molla, U. Kayal, A. Bhaumik and S. M. Islam, Dalton Trans., 2019, 48, 4657–4666 RSC.
- H. Jiang, X. Shen, F. Wang, J. Zhang, Y. Du and R. Chen, Ind. Eng. Chem. Res., 2021, 60, 13523–13533 CrossRef CAS.
- B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328–5331 CrossRef CAS PubMed.
- Y. Zhu, D. Zhu, Q. Yan, G. Gao, J. Xu, Y. Liu, S. B. Alahakoon, M. M. Rahman, P. M. Ajayan, E. Egap and R. Verduzco, Chem. Mater., 2021, 33, 6158–6165 CrossRef CAS.
- A. Akthakul, N. Maklakov and J. White, Anal. Chem., 2010, 82, 6487–6494 CrossRef CAS.
- C. Yuan, B. Liu, F. Liu, M. Y. Han and Z. Zhang, Anal. Chem., 2014, 86, 1123–1130 CrossRef CAS.
- Y. Wang, J. T. Chen and X. P. Yan, Anal. Chem., 2013, 85, 2529–2535 CrossRef CAS.
- R. Paul, S. C. Shit, A. Singh, R. J. Wong, D. Q. Dao, B. Joseph, W. Liu, S. Bhattacharya and J. Mondal, Nanoscale, 2022, 14, 1505–1519 RSC.
- W. Zhou, L. Yang, X. Wang, W. Zhao, J. Yang, D. Zhai, L. Sun and W. Deng, JACS Au, 2021, 1, 1497–1505 CrossRef CAS.
- Y. Chen, B. Shan, C. Yang, J. Yang, J. Li and B. Mu, J. Mater. Chem. A, 2018, 6, 9922–9929 RSC.
- G. Li, B. Yuan, S. Chen, L. Gan and C. Xu, Nanomaterials, 2022, 2953, DOI:10.3390/nano12172953.
- J. Wang, L. Zhao and B. Yan, ACS Appl. Mater. Interfaces, 2020, 12, 12990–12997 CrossRef CAS PubMed.
- V. S. Ganesha Krishna and M. G. Mahesha, J. Electron Spectrosc. Relat. Phenom., 2021, 147072, DOI:10.1016/j.elspec.2021.147072.
- G. Yergaliuly, B. Soltabayev, S. Kalybekkyzy, Z. Bakenov and A. Mentbayeva, Sci. Rep., 2022, 851, DOI:10.1038/s41598-022-04782-2.
- Y. Yang, L. Ge, V. Rudolph and Z. Zhu, Dalton Trans., 2014, 43, 7028–7036 RSC.
- Y. V. Kaneti, S. Dutta, M. S. A. Hossain, M. J. A. Shiddiky, K. L. Tung, F. K. Shieh, C. K. Tsung, K. C. W. Wu and Y. Yamauchi, Adv. Mater., 2017, 1700213, DOI:10.1002/adma.201700213.
- S. Y. Cho, S. J. Kim, Y. Lee, J. S. Kim, W. Bin Jung, H. W. Yoo, J. Kim and H. T. Jung, ACS Nano, 2015, 9, 9314–9321 CrossRef PubMed.
- C. Zhu, S. Pang, Z. Chen, L. Bi, S. Wang, C. Liang and C. Qin, Polymers, 2022, 3158, DOI:10.3390/polym14153158.
- N. Dhariwal, P. Yadav, A. Sanger, S. B. Kang, M. S. Goyat, Y. K. Mishra and V. Kumar, Mater. Adv., 2024, 5, 4187–4199 RSC.
- N. Thokala, K. Vankayala, K. Basavaiah and S. B. Kalidindi, Int. J. Hydrogen Energy, 2024, 81, 270–279 CrossRef CAS.
- Y. Li, M. Liu, J. Wu, J. Li, X. Yu and Q. Zhang, Front. Optoelectron., 2022, 38, DOI:10.1007/s12200-022-00032-5.
- A. Jagan Mohan Reddy, K. Suresh, N. Sujith benarzee and M. S. Surendra Babu, Inorg. Chem. Commun., 2023, 111612, DOI:10.1016/j.inoche.2023.111612.
- S. Y. Wang, J. Y. Ma, Z. J. Li, H. Q. Su, N. R. Alkurd, W. L. Zhou, L. Wang, B. Du, Y. L. Tang, D. Y. Ao, S. C. Zhang, Q. K. Yu and X. T. Zu, J. Hazard. Mater., 2015, 285, 368–374 CrossRef CAS.
- M. Y. Kuo, H. Y. Chen and I. Chao, Chem.–Eur. J., 2007, 13, 4750–4758 CrossRef CAS.
- G. H. Mhlongo, D. E. Motaung and H. C. Swart, Mater. Lett., 2015, 160, 200–205 CrossRef CAS.
- N. A. Travlou, K. Singh, E. Rodríguez-Castellón and T. J. Bandosz, J. Mater. Chem. A, 2015, 3, 11417–11429 RSC.
- M. G. Campbell, D. Sheberla, S. F. Liu, T. M. Swager and M. Dincə, Angew. Chem., Int. Ed., 2015, 54, 4349–4352 CrossRef CAS PubMed.
- J. Zheng, K. Pang, X. Liu, S. Li, R. Song, Y. Liu and Z. Tang, Adv. Funct. Mater., 2020, 2005727, DOI:10.1002/adfm.202005727.
- Z. Meng, R. M. Stolz and K. A. Mirica, J. Am. Chem. Soc., 2019, 141, 11929–11937 CrossRef CAS.
- F. Niu, Z. W. Shao, L. M. Tao and Y. Ding, Sens. Actuators, B, 2020, 128513, DOI:10.1016/j.snb.2020.128513.
- F. Niu, Z. W. Shao, J. L. Zhu, L. M. Tao and Y. Ding, J. Mater. Chem. C, 2021, 9, 8562–8569 RSC.
- L. M. Tao, F. Niu, D. Zhang, T. M. Wang and Q. H. Wang, New J. Chem., 2014, 38, 2774–2777 RSC.
- N. Sharma, N. Sharma, P. Srinivasan, S. Kumar, J. B. Balaguru Rayappan and K. Kailasam, J. Mater. Chem. A, 2018, 6, 18389–18395 RSC.
- Y. H. Choi, D. H. Kim and S. E. Hong, ACS Appl. Mater. Interfaces, 2018, 10(17), 14901–14913, DOI:10.1021/acsami.8b02439.
- W. Li, X. Wu, N. Han, J. Chen, X. Qian, Y. Deng, W. Tang and Y. Chen, Sens. Actuators, B, 2016, 225, 158–166 CrossRef CAS.
- Q. Ma, S. Chu, Y. Liu, Y. Chen, J. Song, H. Li, J. Wang, Q. Che, G. Wang and Y. Fang, Mater. Lett., 2019, 236, 452–455 CrossRef CAS.
- W. Ling, S. Zhang, S. Cao, Y. Pu and D. Zhu, Sens. Actuators, B, 2023, 133057, DOI:10.1016/j.snb.2022.133057.
- A. J. Mohan Reddy, M. S. Surendra Babu and P. Nagaraju, Solid State Sci., 2022, 106819, DOI:10.1016/j.solidstatesciences.2022.106819.
- M. M. Gomaa, G. RezaYazdi, M. Rodner, G. Greczynski, M. Boshta, M. B. S. Osman, V. Khranovskyy, J. Eriksson and R. Yakimova, J. Mater. Sci.: Mater. Electron., 2018, 29, 11870–11877 CrossRef CAS.
- C. S. Rout, M. Hegde, A. Govindara and C. N. R. Rao, Trends in Chemistry of Materials: Selected Research Papers of C. N. R. Rao, 2008, pp. 595–603 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Chemicals and reagents used for the synthesis of COFs, and characterization data such as 13C CP-MAS solid-state NMR spectra, energy dispersive X-ray images, atomic force microscopy, X-ray photoelectron spectroscopy, Brunauer–Emmett–Teller (BET) analysis, and thermogravimetric analysis (TGA). Gas sensing responses of graphs for different analytes, while theoretical studies and results. See DOI: https://doi.org/10.1039/d5ra01430a |
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article b,
A. Jagan Mohan Reddyc,
Sreekantha Babu Jonnalagadda
b,
A. Jagan Mohan Reddyc,
Sreekantha Babu Jonnalagadda b and
Surendra Babu Manabolu Surya
b and
Surendra Babu Manabolu Surya *a
*a
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at ∼183 ppm, and imine C
O peak at ∼183 ppm, and imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peaks at ∼148 and ∼146 ppm) and XPS (C
N peaks at ∼148 and ∼146 ppm) and XPS (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 527.84 eV, C
O peak at 527.84 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at 399.2 eV; Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized frameworks, Zn@COF-3 exhibited exceptional NH3 sensing at a concentration of 1 ppm at room temperature, with a rapid response time (26 s) and recovery time (18 s), outperforming pristine COFs and Zn@COFs. This superior performance is attributed to its rich active sites (C
N at 399.2 eV; Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized frameworks, Zn@COF-3 exhibited exceptional NH3 sensing at a concentration of 1 ppm at room temperature, with a rapid response time (26 s) and recovery time (18 s), outperforming pristine COFs and Zn@COFs. This superior performance is attributed to its rich active sites (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), high surface area (335 m2 g−1), porosity, strong NH3 adsorption energy (−282 kJ mol−1), and low energy gap (2.65 eV), as confirmed by DFT calculations. Additionally, Zn@COF-3 shows excellent selectivity and long-term stability over 30 days. This Zn@COF-based approach yields next-generation ammonia sensors, featuring energy-efficient, highly selective, and room-temperature chemiresistive sensors for industrial, environmental, and biomedical applications.
O), high surface area (335 m2 g−1), porosity, strong NH3 adsorption energy (−282 kJ mol−1), and low energy gap (2.65 eV), as confirmed by DFT calculations. Additionally, Zn@COF-3 shows excellent selectivity and long-term stability over 30 days. This Zn@COF-based approach yields next-generation ammonia sensors, featuring energy-efficient, highly selective, and room-temperature chemiresistive sensors for industrial, environmental, and biomedical applications.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, and a notable peak at 1578 cm−1.79 The peak detected at 1256 cm−1 is caused by the stretching vibration of the C–N bond. These results indicate that the –OH group of the phloroglucinol entity is in the keto form, while the imine bond resulting from Schiff base condensation transforms into an enamine group.80–82 The peak corresponding to the C–N bond shifted from 1279 cm−1 in COF-1 to 1256 cm−1 in Zn@COF-1; from 1259 cm−1 in COF-2 to 1252 cm−1 in Zn@COF-2, and from 1249 cm−1 in COF-3 to 1245 cm−1 in Zn@COF-3. These changes demonstrate the coordination of Zn2+ to N sites.
O bond, and a notable peak at 1578 cm−1.79 The peak detected at 1256 cm−1 is caused by the stretching vibration of the C–N bond. These results indicate that the –OH group of the phloroglucinol entity is in the keto form, while the imine bond resulting from Schiff base condensation transforms into an enamine group.80–82 The peak corresponding to the C–N bond shifted from 1279 cm−1 in COF-1 to 1256 cm−1 in Zn@COF-1; from 1259 cm−1 in COF-2 to 1252 cm−1 in Zn@COF-2, and from 1249 cm−1 in COF-3 to 1245 cm−1 in Zn@COF-3. These changes demonstrate the coordination of Zn2+ to N sites.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and keto (–C
N–) and keto (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–) bonds in both the COFs and Zn@COFs, as shown in Fig. 4(a–c) and S1(a–c) (ESI†). These spectra provide insight into the chemical environment of the carbon atoms in the COF structures before and after Zn incorporation. In the pristine COFs, the characteristic peaks in the 110–150 ppm region correspond to aromatic carbon atoms, while the peaks at around 180–183 ppm (182 ppm for COF-1, 182 ppm for COF-2, and 183 ppm for COF-3) are attributed to carbonyl (–C
O–) bonds in both the COFs and Zn@COFs, as shown in Fig. 4(a–c) and S1(a–c) (ESI†). These spectra provide insight into the chemical environment of the carbon atoms in the COF structures before and after Zn incorporation. In the pristine COFs, the characteristic peaks in the 110–150 ppm region correspond to aromatic carbon atoms, while the peaks at around 180–183 ppm (182 ppm for COF-1, 182 ppm for COF-2, and 183 ppm for COF-3) are attributed to carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–) and imine (–C
O–) and imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) groups, which play a crucial role in the framework stability and electronic properties of the COFs.83 Upon the incorporation of Zn, the overall peak positions remain largely unchanged, which suggests that the Zn coordination does not directly affect the carbon atoms. For Zn@COF-1 and Zn@COF-2, the peaks at 121–135 ppm exhibit slight broadening and shifting, which may indicate Zn coordination through the nitrogen atoms of the imine (–C
N–) groups, which play a crucial role in the framework stability and electronic properties of the COFs.83 Upon the incorporation of Zn, the overall peak positions remain largely unchanged, which suggests that the Zn coordination does not directly affect the carbon atoms. For Zn@COF-1 and Zn@COF-2, the peaks at 121–135 ppm exhibit slight broadening and shifting, which may indicate Zn coordination through the nitrogen atoms of the imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) or amine (–NH2) functional groups. A similar trend is observed for Zn@COF-3, for which shifts in the 105–134 ppm range further support the possibility of the interaction of Zn with nitrogen-rich sites. The absence of significant changes in the carbonyl (∼183 ppm) and aromatic (∼120–150 ppm) regions suggests that the incorporation of Zn does not strongly perturb the overall electronic structure of the COFs.62,84 Instead, Zn coordination likely occurs through weak interactions with nitrogen/oxygen sites, leading to localized electronic modifications rather than major structural reorganization. The lack of significant chemical shift changes after Zn incorporation is due to the non-covalent nature of Zn coordination with the COF framework. Unlike covalent modifications that strongly alter the electronic environment of carbon atoms, Zn coordination occurs through weak interactions with imine (–C
N–) or amine (–NH2) functional groups. A similar trend is observed for Zn@COF-3, for which shifts in the 105–134 ppm range further support the possibility of the interaction of Zn with nitrogen-rich sites. The absence of significant changes in the carbonyl (∼183 ppm) and aromatic (∼120–150 ppm) regions suggests that the incorporation of Zn does not strongly perturb the overall electronic structure of the COFs.62,84 Instead, Zn coordination likely occurs through weak interactions with nitrogen/oxygen sites, leading to localized electronic modifications rather than major structural reorganization. The lack of significant chemical shift changes after Zn incorporation is due to the non-covalent nature of Zn coordination with the COF framework. Unlike covalent modifications that strongly alter the electronic environment of carbon atoms, Zn coordination occurs through weak interactions with imine (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) and (–C
N–) and (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O–), leading to minimal perturbation in the carbon chemical shifts.
O–), leading to minimal perturbation in the carbon chemical shifts.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, and C
C, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (Fig. 7(a), 8(a), and Fig. S5–S8, ESI†). The N 1s peak of COF-1 shifted from 399.44 eV to 399.2 eV in Zn@COF-1 (Fig. S7(b) ESI†), indicating the successful coordination between Zn2+ and the N atom. In COF-2 and COF-3, similar coordination was observed between Zn2+ and N atoms (Fig. 8(b) and S8(b), ESI†).85 COF-1 exhibits energy peaks at 528.0 eV and 529.8 eV in its high-resolution O 1s XPS spectrum (Fig. S5(c), ESI†). COF-2 exhibits energy peaks at 528.4 eV and 530.4 eV (Fig. S6(c), ESI†). Similarly, COF-3 exhibits peaks at 527.84 eV and 530.5 eV (Fig. 7(c)).86,87 The Zn2p spectra in Fig. 8(d), S7(d) and S8(d) (ESI†) for Zn@COF-1, 2, and 3, respectively, show two distinct peaks at 1042.8 eV and 1019.9 eV. The binding energy for Zn 2p3/2 is 1042 eV, while the binding energy for Zn 2p1/2 is 1019 eV.88 Following the treatment of COF-1 with Zn2+, a new Zn–O peak at 528.3 eV emerged, and the COF-1 peak at 528.6 eV was reduced to a certain degree, indicating that a portion of the oxygen atoms of C
O (Fig. 7(a), 8(a), and Fig. S5–S8, ESI†). The N 1s peak of COF-1 shifted from 399.44 eV to 399.2 eV in Zn@COF-1 (Fig. S7(b) ESI†), indicating the successful coordination between Zn2+ and the N atom. In COF-2 and COF-3, similar coordination was observed between Zn2+ and N atoms (Fig. 8(b) and S8(b), ESI†).85 COF-1 exhibits energy peaks at 528.0 eV and 529.8 eV in its high-resolution O 1s XPS spectrum (Fig. S5(c), ESI†). COF-2 exhibits energy peaks at 528.4 eV and 530.4 eV (Fig. S6(c), ESI†). Similarly, COF-3 exhibits peaks at 527.84 eV and 530.5 eV (Fig. 7(c)).86,87 The Zn2p spectra in Fig. 8(d), S7(d) and S8(d) (ESI†) for Zn@COF-1, 2, and 3, respectively, show two distinct peaks at 1042.8 eV and 1019.9 eV. The binding energy for Zn 2p3/2 is 1042 eV, while the binding energy for Zn 2p1/2 is 1019 eV.88 Following the treatment of COF-1 with Zn2+, a new Zn–O peak at 528.3 eV emerged, and the COF-1 peak at 528.6 eV was reduced to a certain degree, indicating that a portion of the oxygen atoms of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O were coordinated with Zn2+. A new peak corresponding to Zn–O bonding was seen in the cases of COF-2 and COF-3 treated with Zn2+. The C
O were coordinated with Zn2+. A new peak corresponding to Zn–O bonding was seen in the cases of COF-2 and COF-3 treated with Zn2+. The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonding in COF-2 and COF-3 corresponded to the peaks at 528.4 eV and 527.84 eV, respectively. These peaks show decreased intensity in Zn@COF-1, 2 and 3. This may be due to the coordination of Zn2+ with the oxygen atoms of the C
O bonding in COF-2 and COF-3 corresponded to the peaks at 528.4 eV and 527.84 eV, respectively. These peaks show decreased intensity in Zn@COF-1, 2 and 3. This may be due to the coordination of Zn2+ with the oxygen atoms of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of COFs.89 This confirms the successful incorporation of zinc into the COF framework, as well as the characteristic zinc peaks and variations in binding energies.
O groups of COFs.89 This confirms the successful incorporation of zinc into the COF framework, as well as the characteristic zinc peaks and variations in binding energies.




![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, peak at ∼183 ppm, and imine C
O, peak at ∼183 ppm, and imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N peaks at ∼148 and ∼146 ppm) and XPS (C
N peaks at ∼148 and ∼146 ppm) and XPS (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peak at 527.84 eV, C
O peak at 527.84 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N at 399.2 eV, Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized materials, Zn@COF-3 exhibited superior NH3 sensing performance, with a low detection limit of 1 ppm, a rapid response time of 26 s, and a fast recovery time of 18 s. These remarkable properties can be attributed to its micro-flower-like morphology, high crystallinity, and large surface area (335 m2 g−1), which facilitate efficient gas interaction and charge transfer. DFT calculations further confirmed a strong interaction between NH3 molecules and Zn@COF-3, with a high adsorption energy of −281.77 kJ mol−1 and a low energy gap of 2.651 eV, leading to enhanced charge transfer efficiency. These characteristics make the Zn@COFs significantly more effective than the pristine COFs for NH3 sensing at room temperature. Given their exceptional sensing properties, Zn@COFs hold great potential for applications in environmental monitoring, industrial safety, and medical diagnostics. Future research can explore their integration into flexible and wearable sensors, portable miniaturized devices, multi-gas sensing applications, and IoT-based environmental monitoring systems.
N at 399.2 eV, Zn 2p3/2 peak at 1042 eV, and Zn 2p1/2 at 1019 eV). Among the synthesized materials, Zn@COF-3 exhibited superior NH3 sensing performance, with a low detection limit of 1 ppm, a rapid response time of 26 s, and a fast recovery time of 18 s. These remarkable properties can be attributed to its micro-flower-like morphology, high crystallinity, and large surface area (335 m2 g−1), which facilitate efficient gas interaction and charge transfer. DFT calculations further confirmed a strong interaction between NH3 molecules and Zn@COF-3, with a high adsorption energy of −281.77 kJ mol−1 and a low energy gap of 2.651 eV, leading to enhanced charge transfer efficiency. These characteristics make the Zn@COFs significantly more effective than the pristine COFs for NH3 sensing at room temperature. Given their exceptional sensing properties, Zn@COFs hold great potential for applications in environmental monitoring, industrial safety, and medical diagnostics. Future research can explore their integration into flexible and wearable sensors, portable miniaturized devices, multi-gas sensing applications, and IoT-based environmental monitoring systems.








