Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cinchona alkaloid copolymers as fluorimetric INHIBIT and colorimetric AND logic gates for detection of iodide†
Nicola' Agius a,
Catherine J. Ashton
a,
Catherine J. Ashton b,
Helen Willcock
b,
Helen Willcock b and
David C. Magri
b and
David C. Magri *a
*a
aDepartment of Chemistry, Faculty of Science, University of Malta, Msida, MSD 2080, Malta. E-mail: david.magri@um.edu.mt
bDepartment of Materials, Loughborough University, Leicestershire, LE11 3TU, England, UK
First published on 8th April 2025
Abstract
Four cinchona alkaloid-acrylamide water soluble copolymers with a mean hydrodynamic diameter of 3 nm were synthesised by free radical polymerization. The copolymers were characterised by 1H NMR, FTIR, GPC, DLS, UV-vis and fluorescence spectroscopy. A blue emission is observed with H+ switching of 185 and 175-fold for the quinidine and quinine copolymers, and 21 and 11-fold for the cinchonine and cinchonidine copolymers, while the presence of Cl−, Br− or I− causes fluorescence quenching. In emission mode, the copolymers function as fluorescent H+, X−-driven INHIBIT logic gates (where X = Cl−, Br− or I−). In absorbance mode, the copolymers function as colorimetric H+, I−-driven AND logic gates in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/water with a 76-fold enhancement. The solution colour changes from colourless to yellow with formation of new absorbance bands at 288 nm and 353 nm due to a π-anion non-covalent charge transfer interaction. The copolymers may be useful as selective iodide sensors for medical and analytical diagnostics.
1 (v/v) THF/water with a 76-fold enhancement. The solution colour changes from colourless to yellow with formation of new absorbance bands at 288 nm and 353 nm due to a π-anion non-covalent charge transfer interaction. The copolymers may be useful as selective iodide sensors for medical and analytical diagnostics.
Introduction
Fluorescent natural products play a central role in many biological and medicinal processes, of which a mere 300 are intrinsically fluorescent.1 This exclusive group of compounds is extraordinary with oversized applications in theranostic nanomedicine, tissue imaging and photodynamic therapy.2,3 Another ambition is the development of optical probes derived from fluorescent natural products.4–7 Arguably, the oldest of these fluorescent natural products are the cinchona alkaloids, in particular the diastereomeric pair, quinine (QN) and quinidine (QD), which have medicinal uses as an antimalarial and antiarrhythmia, respectively.8 Quinine is also a prominent fluorescence quantum yield standard.3 Their less popular diastereomeric relatives, cinchonine (CN) and cinchonidine (CD), are near duplicates, except for the replacement of the methoxy group with a hydrogen atom (Fig. 1). The fascination with the cinchona alkaloids remains a topical subject due to their potential utility.9–16 For example, Reineke developed copolymers of quinine13 and hydroquinine14 for gene delivery and diagnostic imaging of polymer-pDNA binding. Yang reported a quinine-based quaternised copolymer as a drug-resistant antibacterial.15 Chen demonstrated cinchona alkaloid copolymers as circularly polarized organic afterglow smart materials for anticounterfeiting applications.16 | ||
| Fig. 1 The cinchona alkaloid copolymers 1–4 (two-letter abbreviation in parentheses): QD = quinidine, QN = quinine, CN = cinchonine, CD = cinchonidine. The dashed and solid lines indicate the 3-dimensional stereochemistry of the OH and H groups. The colour-coded box diagram highlights the modular design within the polymeric logic gates. The copolymer ratios for x and y are given in Table 1. | ||
An offshoot of fluorescent probes and luminescent sensors17,18 is the field of molecular logic-based computation.19–25 While small molecule entities with logic functions are common, polymeric logic gates are scarce.26–33 The first example by de Silva was a polymeric AND logic gate for pH and T (temperature) with N-alkylacrylamides.28 This study effectively expanded the field of molecular logic to polymers. Soon after, a structurally related T, H+-driven INHIBIT logic gate polymer was reported by Pasparakis with glucosyloxyethyl moieties,29 while an enzyme-driven lipase, β-galactosidase INHIBIT gate with a poly(phenyl) fluorene backbone was reported by Xing and Wang.30 Other examples include a poly(vinyl) alcohol Fe3+, F−-driven IMPLICATION (IMP) logic gate by Chowdhury,31 a methylmethacrylate F−, HSO4−-driven INHIBIT/IMP logic gate by Jiang,32 and a three-input T, H+, Cu2+-driven inverted enabled-OR gate by Tian.33 However, up to now, polymeric logic gates derived from fluorescent natural products are almost non-existent.34 Herein we demonstrate fluorescent natural product-based polymers as sustainable sourced intelligent materials.
In our earlier communication,35 we reported a fluorescent polymeric INHIBIT logic gate derived from quinidine 1 in water. We exploited the vinyl moiety to prepare an acrylamide copolymer by free radical polymerization.36 The copolymer was designed as a macromolecular logic gate with a receptor1–fluorophore–spacer–receptor2–linker–backbone blueprint (Fig. 1). The cinchona alkaloids remarkably incorporate an internal charge transfer (ICT) and photoinduced electron transfer (PET) mechanisms.35,37 Briefly, the modules within 1 are a 6-methoxyquinoline as the fluorophore, the quinoline nitrogen atom as receptor1, the hydroxylated ethane as the spacer, and the azabicyclic amine as receptor2. A satisfying outcome of our initial study was that the quinidine monomer and copolymer displayed identical photophysical properties, notably a bright fluorescent emission, indicating the optical functionality is conserved within the copolymer.35 We subsequently reported the logic-based fluorescent properties of the four cinchona alkaloids, quinidine, quinine, cinchonine and cinchonidine.37 In this study, we proceeded to study the logic properties of copolymers 1–4 (Fig. 1).
Herein we report the cinchona acrylamide copolymers 1–4 as dual sensing fluorimetric H+, X−-driven INHIBIT logic gates (where X = Cl−, Br− or I−) and colorimetric H+, I−-driven AND logic gates. In emission mode, the copolymers operate in water with H+ as the enabling input, and Cl− or another halide anion (Br− or I−) as the disabling input. In absorbance mode, the copolymers cooperatively detect H+ and I− in water, and optimally in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/water, the copolymers provide a rapid, selective method for iodide detection.38–47 Being an essential dietary mineral, iodide is required for good health, notably for the prevention of thyroid diseases, such as goiter. Governmental health departments could find this technology useful for ensuring food and beverage producers adhere to the strict guidelines for iodide content in drinking water and food stuffs.
1 (v/v) THF/water, the copolymers provide a rapid, selective method for iodide detection.38–47 Being an essential dietary mineral, iodide is required for good health, notably for the prevention of thyroid diseases, such as goiter. Governmental health departments could find this technology useful for ensuring food and beverage producers adhere to the strict guidelines for iodide content in drinking water and food stuffs.
Results & discussion
Synthesis
The copolymers 1–4 were synthesised as shown in Scheme 1. A cinchona alkaloid and an excess of acrylamide were reacted in the presence of the radical initiator 2,2′-azobis[2-(2-imidazolin-2-yl)propane] dihydrochloride (VA-044) in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) H2O/EtOH. The synthesis of copolymer 1, initially prepared using ammonium persulfate as the free radical initiator,35 was repeated with VA-044 and found to provide a higher purity of the copolymer and improved synthetic reproducibility. Copolymers 1, 2 and 4 were reacted for 24 hours while 3 required 120 hours due to solubility issues. The copolymers were precipitated from solution with cold ethanol. The identity of the copolymers was accessed by 1H NMR and FTIR. Detailed characterisation data are available in the ESI (Fig. S1–S8†).
1 (v/v) H2O/EtOH. The synthesis of copolymer 1, initially prepared using ammonium persulfate as the free radical initiator,35 was repeated with VA-044 and found to provide a higher purity of the copolymer and improved synthetic reproducibility. Copolymers 1, 2 and 4 were reacted for 24 hours while 3 required 120 hours due to solubility issues. The copolymers were precipitated from solution with cold ethanol. The identity of the copolymers was accessed by 1H NMR and FTIR. Detailed characterisation data are available in the ESI (Fig. S1–S8†).
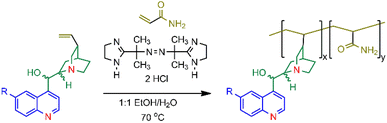 | ||
| Scheme 1 The synthesis of the cinchona alkaloid/acrylamide copolymers 1–4 using VA-044 (2,2′-azobis[2-(2-imidazolin-2-yl) propane]dihydrochloride) where R = OCH3 in 1 and 2 and R = H in 3 and 4. Refer to Fig. 1 for the stereochemistry. | ||
1H NMR was used to confirm the formation of the copolymers and to calculate the ratio of alkaloid to acrylamide units. The 1H NMR spectra of 1–4 in DMSO-d6 are noticeably broader than the cinchona monomers. While the proton chemical shifts and coupling constants are easily determined for the alkaloid monomers, such information was difficult to decipher for the copolymers as the peaks are broad due to the slower correlation time for rotational diffusion of polymers.48 The absence of the vinyl ABX pattern at 5.0–6.2 ppm in the 1H NMR spectra (Fig. S1–S4†) is evidence that the radical polymerisation reaction was successful. The FTIR spectra of the copolymers (Fig. S5–S8†) are noticeably broader than the monomers in the fingerprint region, and the appearance of an intense, broad peak at 1680 cm−1 is indicative of the primary amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch from acrylamide in the polymer backbone.49 The composition ratios of the copolymers were estimated from the 1H NMR spectra by integrating the area of the quinoline proton at 8.70 ppm and the amide band at 6.80 ppm. The percentage of alkaloid is 17 ± 6% (acrylamide 82 ± 7%) in agreement with related quinine copolymers.13 We observe a stereochemical preference for a greater amount of acrylamide in the 1 and 3 copolymers. A comparison is provided in Table 1.
O stretch from acrylamide in the polymer backbone.49 The composition ratios of the copolymers were estimated from the 1H NMR spectra by integrating the area of the quinoline proton at 8.70 ppm and the amide band at 6.80 ppm. The percentage of alkaloid is 17 ± 6% (acrylamide 82 ± 7%) in agreement with related quinine copolymers.13 We observe a stereochemical preference for a greater amount of acrylamide in the 1 and 3 copolymers. A comparison is provided in Table 1.
Gel permeation chromatography (GPC) and dynamic light scattering (DLS) analysis
The molecular number (Mn), molecular weight (Mw) and polydispersity index (Đ) were determined by GPC in water (Fig. S9 and Table S1†). Quinidine copolymer 1 has a Mn of 2400 Da with a Đ of 1.45. The Mn of 3 is similar at 2700 Da but has a larger Đ of 3.1 attributed to the longer reaction time. The diastereomeric copolymers 2 and 4 have a Mn of 1800 Da and 1900 Da, and a high Đ of 1.56 and 1.81. Using the ratios from the NMR data (Table 1) and the GPC data (Table S1†), the mean composition of the copolymers is three cinchona alkaloids and 18, 12, 27 and 15 AM repeat units for 1–4, respectively.From dynamic light scattering (DLS),50 the hydrodynamic diameter of the copolymers is 3.0 nm in water. The polydispersity (PDI) of the diameters typically ranges between 0.186 and 0.539 indicating the copolymers are non-uniform in size (Table S2†). The correlation coefficients were good in all cases except for 3. To screen for the possibility of aggregate formation, concentration dependent studies were performed at pH 6 and pH 2. Samples of 5 mg mL−1 of copolymer 2 and 4 at pH 6 revealed the presence of particles with diameters of 87 nm and 58 nm, both with a PDI of 0.27. Dilution by 10-fold resulted in disassembly to 2 (PDI = 0.48), while particles remained for 4, but with narrower dispersity (PDI = 0.22). No assembly was observed at pH 6 with 1 and 3 at 5 mg mL−1. At pH 2 the copolymers showed no evidence of assembly at 5 mg mL−1, except in the case of 4, with particles of 72 nm, but on a two-fold dilution, they disassemble to 3.71 nm. At pH 6 the copolymers are monoprotonated at the quinuclidine N atom, while at pH 2 they are also protonated on the quinoline (vide infra).37 Hence, in acidic aqueous solution the copolymers are solvated by water molecules. These results are excellent considering that the photophysical studies were performed at concentration ≤0.12 g L−1 (Table 2). The DLS results clearly indicate that the UV-vis absorbance and fluorescence results (vide infra) are due to the intrinsic properties of copolymers 1–4 and not to self-assembled aggregates. DLS graphs are available in the ESI (Fig. S10–S14†).
| 1 QD | 2 QN | 3 CN | 4 CD | |
|---|---|---|---|---|
| a 0.078 g L−1 1, 0.12 g L−1 2, 0.063 g L−1 3, 0.11 g L−1 4.b pH adjusted with 0.10 M TMAH.c absorptivity units/cm−1 g−1 L.d pH adjusted with 0.10 M CH3SO3H.e Excited state pKas determined by log[(Imax − I)/(I − Imin)] = −log[H+] + log Ka from emission spectra in water buffered with 0.1 μM Na2EDTA. Fluorescence emission spectra obtained by excitation at λIsos.f Only one inflection point is observed from I–pH plot.g H+-induced fluorescence enhancement (FE) IFpH 2/IFpH 11. | ||||
| λAbs pH 11/nmb | 332 | 320 | 273 | 273 |
| Log apH 11c | 2.86 | 0.89 | 3.49 | 1.17 |
| λAbs pH 2/nmd | 352 | 345 | 316 | 315 |
| Log apH 2c | 1.59 | 0.54 | 1.90 | 0.84 |
| λAbs(isos)/nm | 261, 294, 329 | 263, 306 | 257, 304 | 305, 328 |
| λFlu pH 11/nm | 389 | 382 | 381 | 400 |
| λFlu pH 2/nm | 450 | 450 | 436 | 411 |
| λFlu(isos)/nm | 393 | 390 | 381 | 349 |
| pKa*e | 4.08, 8.26 | 3.86, 8.20 | 3.80, 8.55 | 4.12f |
| FEg | 185 | 175 | 21 | 11 |
Spectroscopic results
The UV-visible absorption and emission properties of the copolymers 1–4 and the monomers37 were studied in water (Fig. 2, Table 2). Copolymers 1 and 2 have maximum absorbance bands (λAbs) at 352 nm and 345 nm at 10−2 M H+, and 3 and 4 have peaks at 316 nm and 315 nm. These values are similar to the monomers confirming the acrylamide units do not contribute to the UV-vis spectrum. The monomers strongly absorb light with log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε (molar extinction coefficient) between 3.3-4.1 consistent with a π → π* electronic transition.37 The UV-vis properties of the copolymers were measured using absorptivity (a) in units of cm−1 g−1 L and determined to be 1.59, 0.54, 1.90 and 0.84 for 1–4, respectively. At 10−11 M H+, the log
ε (molar extinction coefficient) between 3.3-4.1 consistent with a π → π* electronic transition.37 The UV-vis properties of the copolymers were measured using absorptivity (a) in units of cm−1 g−1 L and determined to be 1.59, 0.54, 1.90 and 0.84 for 1–4, respectively. At 10−11 M H+, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a increases to 2.86, 0.89, 3.49 and 1.17. A hypsochromic shift of ca. 30 nm reveals peak maxima at 332 nm, 320 nm, 273 nm and 273 nm. Isosbestic points are observed (listed in Table 2) consistent with protonation equilibria alluding to an ICT mechanism on protonation of the quinoline fragment. Fig. 3 highlights the emission spectra of copolymers 1–4. The spectra are broad ranging from 350–580 nm with a peak maximum at 450 nm, 450 nm, 436 nm and 411 nm for 1–4, respectively, in the presence of 10−2 M H+ consistent with monomer emission.37 At 10−11 M H+ the emission spectra of 1–4 are weak but accompanied by a bathochromic shift of 55 nm to 68 nm. As the titration proceeds, an isoemissive point appears at 393 nm, 390 nm, 381 nm and 349 nm. The fluorescence enhancement (FE) ratios from IFpH 2/IFpH 11 for 1 and 2 are 186 and 197, while those of 3 and 4 are an order of magnitude lower at 21 and 11. The greater enhancement for the methoxy cinchona derivatives suggests a stronger charge transfer character.
a increases to 2.86, 0.89, 3.49 and 1.17. A hypsochromic shift of ca. 30 nm reveals peak maxima at 332 nm, 320 nm, 273 nm and 273 nm. Isosbestic points are observed (listed in Table 2) consistent with protonation equilibria alluding to an ICT mechanism on protonation of the quinoline fragment. Fig. 3 highlights the emission spectra of copolymers 1–4. The spectra are broad ranging from 350–580 nm with a peak maximum at 450 nm, 450 nm, 436 nm and 411 nm for 1–4, respectively, in the presence of 10−2 M H+ consistent with monomer emission.37 At 10−11 M H+ the emission spectra of 1–4 are weak but accompanied by a bathochromic shift of 55 nm to 68 nm. As the titration proceeds, an isoemissive point appears at 393 nm, 390 nm, 381 nm and 349 nm. The fluorescence enhancement (FE) ratios from IFpH 2/IFpH 11 for 1 and 2 are 186 and 197, while those of 3 and 4 are an order of magnitude lower at 21 and 11. The greater enhancement for the methoxy cinchona derivatives suggests a stronger charge transfer character.
 | ||
| Fig. 3 The emission spectra of copolymers 1–4 in water excited at 352 nm, 345 nm, 316 nm and 315 nm, respectively, with increasing H+. | ||
Fig. 4 illustrates the fluorescence intensity–pH plots for 1–4 based on the peak maximum. Copolymers 1–3 have a two-step sigmoidal curve whilst copolymer 4 has only a single-step sigmoidal curve. This same trend was observed with the monomers, due to the much lower fluorescence quantum yield (ΦF) of cinchonidine (vide infra).37 The excited state pKas (pKa*s) of the quinoline and azabicyclic nitrogen atoms were evaluated to be 3.91 ± 0.17 and 8.35 ± 0.20 by fitting the data to the Henderson–Hasselbalch equation adapted for spectroscopic studies (Table 2, see footnote). These values are lower than the cinchona alkaloid monomers by 0.5–1.0 log unit. This observation suggests a less polar microenvironment about the alkaloids within the copolymer.
 | ||
| Fig. 4 Maximum peak emission intensity–pH plots of copolymers 1–4 in water excited at 352 nm, 345 nm, 316 nm and 315 nm. | ||
Molecular logic by fluorescence
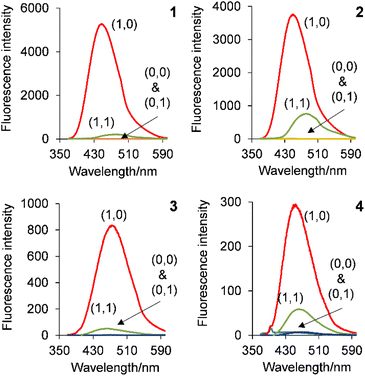
Solutions of the copolymers 1–4 irradiated with 365 nm UV light are shown in Fig. 5 and the emission spectra are shown in Fig. 6. The inputs are H+ and Cl− whilst the output is fluorescence. In all four cases, the copolymers function as H+, Cl−-driven INHIBIT logic gates. Initially at low H+ and Cl−, corresponding to the input state (0, 0), the emission is low due to PET from the azabicyclic amine. On addition of 10−2 M H+, the (1,0) input state, the emission significantly increases with FE ratios for 1 and 2 of 185 and 175-fold. The FE ratios of 3 and 4 are respectably good at 21 and 11-fold. The ΦF of 1 and 2 are 0.553 and 0.549 resulting in a bright blue emission, while those of 3 and 4 are 0.046 and 0.025. In the absence of Cl− (or even the presence of 1 mM Cl−), the (0,1) input state is an off state due to PET from the azabicyclic amine. The addition of 10−2 M H+ and 100 mM Cl−, the (1,1) input state, has a detrimental effect on the emission resulting in a similar output as the (0,0) input state. In all cases, the copolymers are non-fluorescent in the absence of H+ and Cl−, in the presence of Cl−, or in the presence of both H+ and Cl−. A high emission output is observed only when H+ is high. The truth tables for copolymers 1–4 are provided in Table 3.| Label | Input1 (H+)c | Input2 (Cl−)d | Output 1 (ΦF)e | Output 2 (ΦF)e | Output 3 (ΦF)e | Output 4 (ΦF)e |
|---|---|---|---|---|---|---|
| a 0.078 g L−1 1, 0.12 g L−1 μM 2, 0.063 g L−1 3, 0.11 g L−1 4.b Excited at 352 nm, 343 nm 315 nm, and 312 nm.c High input1 10−2 M H+ and low input1 H+ 10−11 M adjusted with CH3SO3H and TMAH.d High input2 100 mM Cl− and low input2 1 mM Cl− added as NaCl.e Relative ΦF versus 10−6 M quinine sulfate in aerated 0.1 M H2SO (ΦF = 0.55). High threshold output level set at ΦF max/2. | ||||||
| A | 0 (low) | 0 (low) | 0 (0.003) | 0 (0.004) | 0 (0.004) | 0 (0.001) |
| B | 1 (high) | 0 (low) | 1 (0.553) | 1 (0.549) | 1 (0.046) | 1 (0.025) |
| C | 0 (low) | 1 (high) | 0 (0.003) | 0 (0.003) | 0 (0.004) | 0 (0.002) |
| D | 1 (high) | 1 (high) | 0 (0.003) | 0 (0.006) | 0 (0.004) | 0 (0.004) |
Besides Cl−, Br− or I− anions also act as disabling inputs. The presence of 100 mM chloride, bromide or iodide anions, whether in acidic or basic media, causes a low emission output. Hence, all three halide anions disable input1 (H+). Therefore, the copolymers 1–4 can also be regarded as a three-input disabled OR logic gate feeding into the disabling input of INHIBIT logic gate. The logic behavior of the polymers remained identical to those of the monomer cinchona alkaloids. Therefore, modularity is conserved upon polymerisation with the acrylamide unit. The disabling ability of Cl−, Br− or I− anions has historically been explained by collisional quenching of the singlet excited state.51 The disabling power of the halides is according to the heavy atom effect whereby the order is I− > Br− > Cl−. However, an alternative rationale for the quenching process may be due to an intermolecular charge transfer (vide infra).
Molecular logic by absorbance
A serendipitous finding during our study was that the copolymers slowly undergo a colour change in water in the presence of I− at 10−2 M H+. After 48 hours the aqueous solutions of 1–4 changed from colourless to a faint yellow in the presence of 200 mM iodide (Fig. S17†). Inspection by UV-vis absorbance spectrometry revealed significant spectral changes as a function of time with new peaks at 288 nm and 353 nm (Fig. S15†). The monomer cinchona alkaloids under identical conditions also resulted in similar colour changes, although higher concentrations of I− were needed. A review47 of the literature reveals precedence for this phenomenon with nitrogen-rich chemosensors containing pyridine,38 tripodal benzimidazole,39 acridine,40 fluorene-dipyridine,41 o-phenylenediamine42 and fluorenone-imine-catechol.43 Copolymers of 1,5-naph thyridine,44 carbazole45 and phenylene vinylenes46 are reported as both colorimetric and fluorimetric I− sensors. A commonality in these literature sources is that iodide sensing is typically demonstrated in aqueous THF solutions.38–47 Thus, we proceeded to test the response of copolymers 1–4 in THF/water compositions.Copolymers 1–4 display colorimetric logic characteristics dependent on the solvent conditions. In 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/water, a yellow colour was observed with the naked eye only in the presence of 10−2 M H+ and 1 mM I− after 10 min (Fig. 7 inset and Fig. S16†). No colour change was observed with 10−9 M H+ and 1 mM I−, or in the presence of only 10−9 M H+, or only 1 mM I−. Satisfyingly, these results in water (Fig. S19†) and 1
1 (v/v) THF/water, a yellow colour was observed with the naked eye only in the presence of 10−2 M H+ and 1 mM I− after 10 min (Fig. 7 inset and Fig. S16†). No colour change was observed with 10−9 M H+ and 1 mM I−, or in the presence of only 10−9 M H+, or only 1 mM I−. Satisfyingly, these results in water (Fig. S19†) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/water (Fig. 7) are consistent with AND logic.52 The AND truth table with absorbance outputs are given in Table 4. Remarkably, the absorbance increases 76-fold.
1 (v/v) THF/water (Fig. 7) are consistent with AND logic.52 The AND truth table with absorbance outputs are given in Table 4. Remarkably, the absorbance increases 76-fold.
| Label | Input1 (H+)b | Input2 (I−)c | Output abs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/H2O)d 1 THF/H2O)d |
Output abs (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/H2O)d 1 THF/H2O)d |
|---|---|---|---|---|
a 0.12 g L−1 μM 2.b High and low input1 is 10−2 M and 10−9 M H+ adjusted with CH3SO3H and TMAH.c High input2 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/H2O is 1 mM I−. High input2 in 9 1 THF/H2O is 1 mM I−. High input2 in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/H2O is 10 μM. I− added as KI.d High threshold output level set at abs >0.6. 1 THF/H2O is 10 μM. I− added as KI.d High threshold output level set at abs >0.6. |
||||
| A | 0 (low) | 0 (low) | 0 (low, 0.010) | 0 (low, 0.013) |
| B | 1 (high) | 0 (low) | 0 (low, 0.011) | 0 (low, 0.010) |
| C | 0 (low) | 1 (high) | 0 (low, 0.014) | 1 (high, 1.05) |
| D | 1 (high) | 1 (high) | 1 (high, 1.06) | 1 (high, 3.94) |
By comparison, in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/water, a yellow colour change was observed with 10 μM I− after 1 min. With 10 μM I− and 10−2 M H+ the solution colour is even darker (Fig. S18†). The resulting UV-vis spectra is so absorbent on addition of both inputs that the detector becomes saturated (Fig. S20,† and Table 4). In THF the colour change on addition of I− is immediate. From a Boolean perspective, the copolymers now function as H+, I−-driven TRANSFER logic gates where I− is the enabling input.53 Tuning of the solvent polarity, reconfigures the copolymers 1–4 from rapid colorimetric H+, I−-driven AND logic gates in 1
1 (v/v) THF/water, a yellow colour change was observed with 10 μM I− after 1 min. With 10 μM I− and 10−2 M H+ the solution colour is even darker (Fig. S18†). The resulting UV-vis spectra is so absorbent on addition of both inputs that the detector becomes saturated (Fig. S20,† and Table 4). In THF the colour change on addition of I− is immediate. From a Boolean perspective, the copolymers now function as H+, I−-driven TRANSFER logic gates where I− is the enabling input.53 Tuning of the solvent polarity, reconfigures the copolymers 1–4 from rapid colorimetric H+, I−-driven AND logic gates in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/water to H+, I−-driven TRANSFER logic gates in 9
1 (v/v) THF/water to H+, I−-driven TRANSFER logic gates in 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) THF/water.54,55 We also tested the response of the copolymers to F− and observed no change in either the absorbance or emission output. Thus, the copolymers are selective colorimetric indicators for differentiating I− from F−, Cl− and Br−.
1 (v/v) THF/water.54,55 We also tested the response of the copolymers to F− and observed no change in either the absorbance or emission output. Thus, the copolymers are selective colorimetric indicators for differentiating I− from F−, Cl− and Br−.
These observations are rationalized by an intermolecular charge transfer mechanism56 – more specifically, to a π-anion non-covalent interaction.57,58 Iodide is an electron-rich halide, more so than F−, Cl− and Br−, which hold onto their electron density tighter. Hence, I− ions are more able to share electron density with electron-deficient π-systems, such as the positively charged quinolinium units, of the cinchona alkaloids. The supramolecular interaction is faciliated by the electrostatic attraction between I− and the positively charged π-system, and by an anion-induced polarization of the π-system.59 Furthermore, the cationic polymer environment amplifies an electric field effect due to the multiple quinoliniums units such that the iodide counter ions cause the copolymers to condense into a compact structure (similar to cation condensation with DNA).60 This explains the higher sensitivity (lower I− concentrations) detected by the copolymers compared to the monomers.
Conclusions
Four copolymers of cinchona alkaloids and acrylamide with a mean hydrodynamic diameter of 3 nm were synthesised by free radical polymerization. The copolymers are illustrative examples of H+, X−-driven INHIBIT fluorescent logic gates (where X = Cl−, Br− or I−) in aqueous solution. A blue fluorescence is observed in the presence of high H+, while the presence of Cl−, Br− or I− disables the emission, irrespective of whether H+ is present. The QD and QN copolymers exhibit fluorescence quantum yields (ΦF = 0.55) equivalent to the monomers,3,37 which are more intense than the CN (ΦF = 0.046) and CD (ΦF = 0.025) copolymers. The assimilation of the cinchona alkaloids into the acrylamide backbone does not interfere with the intrinsic fluorescence of the alkaloids. Hence, the optical properties of the cinchona alkaloids are conserved within the copolymer environment. Additionally, the copolymers function as rapid and selective colorimetric H+, I−-driven AND logic gates in absorbance mode in aqueous THF solution. Iodide is detected with the naked eye by a solution colour change from colourless to yellow due to a π-anion non-covalent interaction between iodide and the quinolinium fragment.56,57 Studies are currently underway to better understand this supramolecular interaction.Author contributions
Conceptualization (DCM), investigation (NA, CJA), methodology (DCM, HW), formal analysis (NA, CJA, DCM), supervision (DCM, HW), writing – original draft (DCM, NA), writing – review & editing (NA, CJA, HW, DCM).Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the FUSION R&I: Research Excellence Programme, grant agreement no. REP-2023-023, administered by Xjenza Malta; the University of Malta for financial support, Dr Duncan Micallef for NMR support, Kairen Zhao for the GPC analysis and the Engineering and Physical Sciences Research Council (EP/R513088/1) for supporting the DLS study.Notes and references
- R. Duval and C. Duplais, Nat. Prod. Rep., 2017, 34, 161–193 RSC.
- H. Yuan, A. Jiang, H. B. Fang, Y. C. Chen and Z. J. Guo, Adv. Drug Delivery Rev., 2021, 179, 113917 CrossRef CAS PubMed.
- B. Valeur and M. N. Berberan-Santos, Autofluorescence and Fluorescence Labeling in Biology and Medicine, in Molecular Fluorescence: Principles and Applications, John Wiley & Sons, Weinheim, 2012, ch. 15, pp. 479–505 Search PubMed.
- T. Hirano, H. Yokoo, A. Ohsaki and H. Kagechika, Development of Novel Fluorescent Sensors Based on Fluorescent Natural Products, Biomed. Eng., 2021, 6, 103–116 Search PubMed.
- D.-B. Sung and J. Seok Lee, RSC Med. Chem., 2023, 14, 412–432 Search PubMed.
- S. B. Wagh, V. A. Maslivetc, J. J. La Clair and A. Kornienko, ChemBioChem, 2021, 22, 3109–3139 CAS.
- M. D. Alexander, M. D. Burkart and M. S. Leonard, et al., ChemBioChem, 2006, 7, 409–416 CrossRef CAS PubMed.
- M. J. O'Neil, P. E. Heckelman, P. H. Dobbelaar and K. J. Roman, The Merck Index: an Encyclopedia of Chemical, Drugs, and Biologicals, The Royal Society of Chemistry, Cambridge, 15th edn, 2013 Search PubMed.
- J. G. Woodland, R. Hunter, P. J. Smith and T. J. Egan, Org. Biomol. Chem., 2017, 15, 589–597 RSC.
- F. Iftikhar, H. Ali and S. G. Musharraf, RSC Adv., 2019, 9, 17551–17559 CAS.
- S. Snyder, S. Murundi, L. Crawford and D. Putnam, J. Controlled Release, 2020, 317, 291–299 CAS.
- N. Dey, J. Mol. Liq., 2021, 327, 114799 CrossRef CAS.
- C. Van Bruggen, D. Punihaole, A. R. Keith, A. J. Schmitz, J. Tolar, R. R. Frontiera and T. M. Reineke, Proc. Natl. Acad. Sci. U.S.A., 2020, 117, 32919–32928 CrossRef CAS PubMed.
- P. Roy, N. W. Kreofsky, M. E. Brown, C. Van Bruggen and T. M. Reineke, J. Am. Chem. Soc., 2023, 3, 1876–1889 CAS.
- H.-H. Ding, M.-H. Zhao, L. Zhai, J.-B. Zhen, L.-Y. Sun, J.-Z. Chigan, C. Chen, J.-Q. Li, H. Gao and K.-W. Yang, Polym. Chem., 2021, 12, 2397–2403 RSC.
- L. Zhang, Y. Jin, Y. Wang, W. Li, Z. Guo, J. Zhang, L. Yuan, C. Zheng, Y. Zheng and R. Chen, ACS Appl. Mater. Interfaces, 2023, 15, 49623–49632 Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 Search PubMed.
- J. F. Callan, A. P. de Silva and D. C. Magri, Tetrahedron, 2005, 61, 8551–8588 CrossRef CAS.
- A. P. de Silva, Molecular Logic-Based Computation, The Royal Society of Chemistry, Cambridge, 2013 Search PubMed.
- Z. Qin, H. Guo, X. Cheng, Z. Dou, H. Lian, X. Li, W.-Y. Wong and Q. Dong, ACS Mater. Lett., 2024, 6, 1504–1521 CrossRef CAS.
- F. T. Souto and G. G. Dias, Analytica, 2023, 4, 456–499 CrossRef CAS.
- S. Zanella, M. A. Hernández-Rodríguez, R. A. S. Ferreira and C. D. S. Brites, Chem. Commun., 2023, 59, 7863–7874 RSC.
- D. C. Magri, Coord. Chem. Rev., 2021, 426, 213598 CrossRef CAS.
- C.-Y. Yao, H.-Y. Lin, H. S. N. Cory and A. P. de Silva, Mol. Syst. Des. Eng., 2020, 5, 1325–1353 RSC.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 Search PubMed.
- C. Y. Yao, S. Uchiyama and A. P. de Silva, Polymers, 2019, 11, 1351 CrossRef CAS PubMed.
- A. S. Oshchepkov, M. S. Oshchepkov, M. V. Oshchepkova, A. Al-Hamry, O. Kanoun and E. A. Kataev, Adv. Opt. Mater., 2021, 9, 2001913 Search PubMed.
- S. Uchiyama, N. Kawai, A. P. de Silva and K. Iwai, J. Am. Chem. Soc., 2004, 126, 3032–3033 CrossRef CAS PubMed.
- G. Pasparakis, M. Vamvakaki, N. Krasnogor and C. Alexander, Soft Matter, 2009, 5, 3839–3841 RSC.
- Y. J. Wang, B. J. Xin, X. R. Duan, G. W. Xing and S. Wang, Macromol. Rapid Commun., 2010, 31, 1473–1478 CrossRef CAS PubMed.
- D. Biswas, P. Chowdhury, N. Bar, A. Ray and G. K. Das, Dyes Pigm., 2023, 219, 111559 CrossRef CAS.
- W. Lu, D. Chen, H. Jiang, L. Jiang and Z. Shen, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 590–598 CrossRef CAS.
- Z. Q. Guo, W. H. Zhu, Y. Y. Xiong and H. Tian, Macromolecules, 2009, 42, 1448–1453 CrossRef CAS.
- N. Agius and D. C. Magri, Nat. Prod. Commun., 2024, 19, 1–11 Search PubMed.
- N. Agius and D. C. Magri, New J. Chem., 2021, 45, 14360–14363 Search PubMed.
- G. J. Scerri, M. Caruana, N. Agius, G. Agius, T. J. Farrugia, J. C. Spiteri, A. D. Johnson and D. C. Magri, Molecules, 2022, 27, 5939 Search PubMed.
- N. Agius and D. C. Magri, RSC Adv., 2023, 13, 13505–13510 CAS.
- X. J. Wang, C. H. Zhang, L. H. Feng and L. W. Zhang, Sens. Actuators, B, 2011, 156, 463–466 CAS.
- D. Y. Lee, N. Singh, M. J. Kim and D. O. Jang, Org. Lett., 2011, 13, 3024–3027 CAS.
- M. Yu, L. Jiang, L. Mou, X. Zeng, R. X. Wang, T. Peng, F. Y. Wu and T. Z. Shi, Molecules, 2024, 29, 1355 CAS.
- M. Jafari, A. Badiei, A. Shayesteh and A. Amiri, J. Photochem. Photobiol., A, 2018, 364, 336–343 CAS.
- H. Wang, Q. Lu, Y. Liu, H. Li, Y. Zhang and S. Yao, Sens. Actuators, B, 2017, 250, 429–435 CrossRef CAS.
- H. M. Junaid, M. T. Waseem, Z. A. Khan, F. Munir, S. Sohail, U. Farooq and S. A. Shahzad, ACS Omega, 2022, 7, 9730–9742 CrossRef CAS PubMed.
- A. A. Boali, M. Mansha, A. Waheed and N. Ullah, J. Taiwan Inst. Chem. Eng., 2018, 91, 420–426 Search PubMed.
- M. Vetrichelvan, R. Nagarajan and S. Valiyaveettil, Macromolecules, 2006, 39, 8303–8310 CrossRef CAS.
- M. Mansha, M. Sohail and N. Ullah, J. Appl. Polym. Sci., 2017, 134, 44948 CrossRef.
- M. Mansha, S. Akram Khan, M. A. Aziz, A. Zeeshan Khan, S. Ali and M. Khan, Chem. Rec., 2022, 22, e202200059 CrossRef CAS PubMed.
- F. Bovey and P. Mirau, NMR of Polymers, Academic Press, San Diego, 1996, pp. 358–360 Search PubMed.
- K. G. Uranta, S. Rezaei-Gomari, P. Russell and F. Hamad, Energies, 2018, 11, 2201 CrossRef.
- C. J. Marsden, C. Breen, J. D. Tinkler, T. R. Berki, D. W. Lester, J. Martinelli, L. Tei, S. J. Butler and H. Willcock, Polym. Chem., 2022, 13, 4124–4135 RSC.
- N. Kumar Joshi, N. Tewari, P. Arora, R. Rautela, S. Pant and H. C. Joshi, J. Lumin., 2015, 158, 412–416 Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42–44 Search PubMed.
- A two-input TRANSFER(input1) logic gate functions as a YES gate acting on input1 whereas input2 (H+) is not involved (as if the input2 channel is disconnected).
- G. Gauci and D. C. Magri, RSC Adv., 2022, 12, 35270–35278 CAS.
- A. Diacono, M. C. Aquilina, A. Calleja, G. Agius, G. Gauci, K. Szaciłowski and D. C. Magri, Org. Biomol. Chem., 2020, 18, 4773–4782 CAS.
- M. Kosower, J. Am. Chem. Soc., 1958, 80, 3253–3260 Search PubMed.
- P. Gamez, T. J. Mooibroek, S. J. Teat and J. Reedijk, Acc. Chem. Res., 2007, 40, 435–444 CAS.
- G. Balamurugan, W. Zhang, Y. W. Choi, J. M. Park, S. Velmathi and J. S. Park, Dyes Pigm., 2025, 232, 112498 CAS.
- A. Frontera, D. Quiñonero and P. M. Deya, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 1, 440–459 CAS.
- V. A. Bloomfield, Biopolymers, 1997, 44, 269–282 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, NMR, FTIR, UV-vis spectra, GPC and DLS. See DOI: https://doi.org/10.1039/d5ra01281c |
| This journal is © The Royal Society of Chemistry 2025 |