Open Access Article
Open Access ArticleEngineering gold nanoparticles-infused silica aerogel composite for trace mercury adsorption
Subhash Kumar Sharma†
 a,
Eitan Yosef†b,
Hadas Mamaneb and
Rajnish Kumar
a,
Eitan Yosef†b,
Hadas Mamaneb and
Rajnish Kumar *ac
*ac
aDepartment of Chemical Engineering, Indian Institute of Technology Madras, Chennai-600036, India. E-mail: rajnish@iitm.ac.in
bSchool of Environmental Engineering, Faculty of Mechanical Engineering, Tel Aviv University, Israel
cSchool of Sustainability, Indian Institute of Technology Madras, Chennai-600036, India
First published on 8th July 2025
Abstract
Mercury (Hg) contamination in water poses severe environmental and health risks, necessitating efficient and scalable remediation technologies. We report a novel gold nanoparticle-doped silica aerogel (Au-AG) composite synthesized via supercritical fluid impregnation, designed to harness the high surface area and porosity of silica aerogels alongside the strong Hg-binding affinity of gold nanoparticles (Au-NPs). The resulting composite exhibits robust structural integrity and a characteristic purple hue, indicating uniform dispersion of Au-NPs. Adsorption experiments at environmentally relevant Hg concentrations show that uptake follows Langmuir isotherm behavior and pseudo-second-order kinetics, indicative of monolayer chemisorption and rapid adsorption rates. The Au-AG composite achieves up to 85% Hg removal within 24 hours and a maximum adsorption capacity of 12.82 mg g−1 at ppb-level Hg concentrations, outperforming conventional materials such as activated carbon, thiol-functionalized resins, and undoped silica aerogels under similar conditions. The composite's structural integrity and chemical stability indicate potential for regeneration and reuse in cyclic adsorption processes, making it a promising candidate for sustainable water purification technologies. Moreover, the synthesis approach is compatible with scalable and sustainable production, reinforcing its applicability in real-world water purification systems. These findings position the Au-AG composite as a high-performance and scalable solution for trace-level mercury remediation in contaminated water, advancing sustainable environmental technologies.
1. Introduction
Mercury (Hg) is a highly toxic, persistent contaminant with a strong tendency to bioaccumulate, posing global environmental and public health risks.1 Geogenic sources like volcanic eruptions and oceanic degassing release 500–800 metric tons of mercury annually.2 However, human activities, especially industrial processes,3 have increased emissions significantly, with the UNEP estimating over 2000 metric tons released yearly, largely from coal combustion. Mercury exposure mainly occurs through methylmercury in contaminated fish, impacting human health with severe neurological, cardiovascular, and immune effects.4 High-risk groups include fetal development and communities consuming fish high in mercury, like tuna. Mercury emissions travel globally, affecting remote regions like the Arctic, harming ecosystems and species such as bald eagles, polar bears, and panthers. In terrestrial environments, mercury also accumulates, impacting species like bats through contaminated prey. Economically, mercury exposure leads to costly cognitive impairments, and fishing communities face food insecurity and cultural loss due to dietary mercury restrictions.5Mercury exists as elemental (Hg0), inorganic (Hg2+), and organic (methylmercury, MeHg) forms, each with distinct properties and removal challenges.6,7 Elemental mercury disperses easily in the air, complicating containment; inorganic mercury dissolves in water, binding with other compounds; and methylmercury, the most toxic, biomagnifies in food chains, impacting wildlife and human health.8 Removal techniques must be tailored to each form, with elemental mercury removal (e.g., activated carbon adsorption) often ineffective for methylmercury, complicating removal efforts and driving up costs, especially in resource-limited areas. Traditional methods like chemical precipitation, membrane filtration, ion exchange, and adsorption offer varied benefits and drawbacks: chemical precipitation forms insoluble mercury compounds but produces challenging sludge, membrane filtration can remove mercury ions but is energy-intensive and prone to fouling, ion exchange captures ions effectively but requires costly regeneration, and adsorption, while affordable for elemental mercury, is less efficient for methylmercury and requires large adsorbent quantities, making comprehensive mercury removal both costly and complex.9
Meeting strict mercury limits (e.g., 2 ppb per EPA, 1 ppb per WHO) is difficult for traditional removal methods, which often struggle with trace levels and require costly multistage processes to meet compliance.10 Effective systems demand continuous operation and maintenance, as they are sensitive to pH, temperature, and contaminant variability, particularly in complex industrial effluents. Worker safety in high-mercury environments is a concern, with OSHA (Occupational Safety and Health Administration) reporting mercury levels five times above safe limits, necessitating strict safety protocols. Advances in nanotechnology, like aerogels11 and gold nanoparticles (Au-NPs), show promise for mercury removal due to high surface areas and strong mercury-binding efficiency.12 Au-NPs impregnated silica aerogels, for instance, merge aerogel durability with the adsorption capacity of Au-NPs, achieving over 90% mercury removal and up to 200 mg g−1 adsorption capacity, though challenges in production costs, scalability, and nanoparticle agglomeration remain.13 Other composites, like MoS2/graphene oxide aerogels with Fe3O4 magnetic NPs, have achieved 1527 mg g−1 adsorption but still face scalability issues.14 However, these results were obtained at significantly higher initial mercury concentrations (100–500 ppm), which are not typical of natural water systems. In contrast, this study focuses on adsorption performance at trace-level mercury concentrations (10–100 ppb), which are more relevant to real-world contamination scenarios. Au-NPs silica aerogels also have potential in catalysis,15 sensing, and shielding applications, with supercritical CO2 impregnation aiding in versatile metal incorporation.16
Silica aerogels impregnated with gold nanoparticles17 (Au-NPs) using supercritical carbon dioxide (scCO2) offer an effective solution for mercury (Hg) adsorption from water, addressing mercury's environmental risks due to toxicity and bioaccumulation. The high surface area and porosity of silica aerogels, combined with gold's affinity for mercury ions, enable efficient adsorption in industrial and natural water sources.18 scCO2 uniformly disperses Au-NPs within the aerogel matrix, preserving structure and maximizing mercury interaction.19 Studies show that silica-based hybrids, like silica–carbon nanotube composites,20–22 offer high Hg2+ adsorption capacity, surpassing traditional methods. Gold-enhanced aerogels exhibit robust adsorption kinetics and monolayer adsorption, following pseudo-second-order models, indicating chemisorption. Additional innovations, such as sulfur-functionalized silica for vapor-phase remediation and mercaptoamine-functionalized23 silica for wastewater, highlight the adaptability of these materials.24 Hydrophobic silica aerogels25 also adsorb organic compounds, while modified silicas can capture CO2, suggesting broad applications beyond metal remediation, including in environmental and analytical applications.
This research aims to develop a highly efficient mercury adsorbent by synthesizing a silica aerogel impregnated with gold nanoparticles (Au-NPs) using supercritical CO2 (scCO2). It focuses on characterizing the composite's properties, analyzing adsorption kinetics, and evaluating mercury-binding mechanisms to deliver a scalable solution for industrial mercury removal and environmental remediation. By offering a sustainable alternative to traditional methods, the study advances heavy metal adsorption and supports next-generation adsorbent development for diverse applications.
2. Materials and methods
2.1. Chemicals
Cabot P400 silica aerogel was provided as a gift by Cabot. Gold chloride (AuCl2) was obtained from SRL Chemicals, India. Carbon dioxide (CO2) gas, with a purity of 99.995%, was sourced from Indogas, India, for the supercritical impregnation. The ethanol (EtOH), hydrochloric acid (HCl), and nitric acid (HNO3) reagents were obtained from SRL Chemicals, India. Merck, India supplied mercury chloride (HgCl2).2.2. Procedure for Au-AG aerogel synthesis
The synthesis protocol was designed with scalability in mind, using industrial-grade silica aerogel and supercritical CO2 a process already used at commercial scale in other sectors. The Cabot P400 silica aerogel (4 g) was impregnated by spraying it with AuCl2 (160 mg) dissolved in EtOH (3.2 mL) on a metal sieve inside a custom-built high-pressure supercritical reactor (SS-316), as detailed in Fig. 1. The reactor was sealed and flooded with CO2 at 5 °C and 5 MPa. The temperature was then raised to 80 °C, and the pressure reached 15.3 MPa, where it was maintained for 6 hours. After a 45 minute depressurization, the resulting gold-doped aerogel was calcined at 300 °C for 1.5 hours, yielding an Au-impregnated aerogel (Au-AG, 3.3 g) with a purple color, indicating the presence of AuAl2, consistent with the aluminum content reported in the raw aerogel.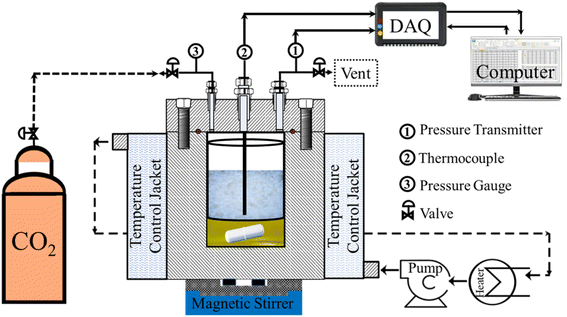 | ||
| Fig. 1 Schematic diagram of the experimental setup used for the supercritical deposition of gold nanoparticles into the aerogel matrix. | ||
The SS-316 reactor contains a magnetic stirrer placed beneath the metal sieve to facilitate mixing. A cooling jacket circulated a mixture of ethylene glycol and water from external circulator (Siskin Profichill RCC1200-ST40). Pressure within the reactor was monitored using a Baumer pressure transducer in conjunction with an analogue pressure gauge, while temperature was measured utilizing an RTD thermocouple. Both instruments were interfaced with a Data Acquisition (DAQ) system (Manufacturer: PPI, Mumbai, India), which was further connected to a computer running “ProLog” software for real-time recording and display of pressure and temperature readings at one-second intervals.
2.3. Analysis
FTIR spectra were acquired using IC-Agilent Cary 630 FTIR analyser. A surface characterization analysis was performed with a Brunauer–Emmett–Teller (BET) analyzer to measure the pore volume, specific surface area, and pore radius of the adsorbent. This analysis involved studying N2 adsorption–desorption isotherms at low pressures within the 0 to 1 bar range, utilizing the JWGB Micro 122W equipment from China, installed at IIT Madras, India. Powder X-ray Diffraction (PXRD) analysis was conducted with a RIGAKU instrument, specifically the SUPERMINI FLEX 6 G BENCHTOP model. The PXRD setup was equipped with a Cu detector and D/tex ultra 2 high-speed silicon strip detectors, enabling coverage of 2θ ranges from −3° to +145°. Scan speeds varied from 0.01 to 100° per minute during data acquisition. SEM was conducted at Tel Aviv University using a Thermo Fisher, Quanta 200 FEG ESEM. Concentrations of Hg were determined with an Agilent 7500 ICP-MS; samples were filtered with 0.22 μm nylon filters to remove any remaining particles, then digested with 2% HCl, 5% HNO3, and 1 ppm Au, and diluted to an expected Hg concentration below 40 ppb.2.4. Sorption experiments
Adsorption experiments were carried out in ddH2O spiked with HgCl2 in volumes of 100 mL, using nickel foam to contain the Au-AG composite and maintain its position submerged beneath the surface of the water. Concentrations of Hg ranged between 10 ppb and 10 ppm.A Langmuir model of adsorption was operated using eqn (1) below:
 | (1) |
To model the kinetics, adsorption experiments were taken over an hour at 100 ppb Hg, and the results were analyzed according to pseudo-first and pseudo-second-order kinetic models, shown below in eqn (2) and (3), respectively.
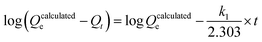 | (2) |
 | (3) |
3. Results and discussion
3.1. Materials characterization
SEM analysis revealed a broad, uniform distribution of gold nanoparticles throughout the silica aerogel matrix, with minimal aggregation. High-magnification images showed these nanoparticles were embedded within the aerogel's porous framework, often located along pore walls and bridging branches within the silica structure. The particles exhibited an average diameter of 20–40 nm, aligning well with synthesis specifications. This size range, verified through particle size distribution analysis, indicates controlled nucleation and growth during the doping process. In areas where deposition was heavier (as seen in Fig. 2a–c), the nanoparticles appeared relatively monodisperse and formed small clusters within the aerogel particles. Importantly, the gold nanoparticles were evenly dispersed without significant correlation to color variations, ruling out effects of AuAl2 formation. The integration of these nanoparticles appears to enhance the aerogel's textural stability, likely due to their stabilizing influence on pore walls, thus reducing potential collapse. This high concentration of gold nanoparticles within the porous structure of the aerogel provides an ideal medium for applications such as mercury adsorption, leveraging the unique properties of the doped aerogel for advanced environmental and catalytic applications.
The SEM analysis confirmed that the SFD method was effective in retaining the mechanical integrity and homogeneity of the doped aerogel structure.26 There were no observable cracks or fractures in the silica network, and the gold nanoparticles did not induce any structural disintegration. This integrity is likely due to the compatibility between the silica and gold components and the SFD technique's capability of producing an aerogel with low density and high mechanical strength. The surface texture of the silica aerogel doped with gold nanoparticles appeared smooth and consistent under SEM observation. The interface between the silica matrix and the gold nanoparticles was well-defined, indicating the successful incorporation of the nanoparticles into the aerogel network without compromising the silica matrix (Fig. 2a–c). This smooth texture suggests that the gold nanoparticles are intimately integrated within the silica framework, likely contributing to enhanced catalytic and adsorption properties by creating accessible active sites throughout the porous structure. EDS characterization of samples analyzed in SEM (Fig. 2d) indicates an elemental concentration of Au of between 13 and 20%, and the absence of Cl, thereby verifying the metallization of Au nanoparticles within the aerogel matrix. Significant concentrations of Si and O were also determined (as well as a smaller concentration of C and Al), consistent with the atomic content of the raw aerogel material.
Using the Scherrer equation, the crystallite size of the gold nanoparticles was estimated based on the width of the prominent (111) diffraction peak at 2θ = 38.2°. Calculations indicate an average crystallite size within the range of 20–40 nm, which aligns with particle sizes observed through SEM analysis. This consistency in size estimation further verifies that the gold nanoparticles achieved during the SFD process maintained controlled growth and retained the desired nanometer scale. The XRD analysis did not reveal any additional peaks that would indicate alloy formation or intermetallic compounds, such as Au–Si or AuAl2, within the doped aerogel. The lack of such secondary phases suggests that the SFD method successfully maintained phase purity, with gold nanoparticles remaining distinct from the silica matrix. This phase purity is advantageous, as it preserves the chemical and physical properties of both the gold and the silica, essential for applications like catalysis and adsorption.
The crystallinity of gold nanoparticles embedded in the amorphous silica matrix potentially contributes to enhanced stability and durability of the aerogel structure. Gold's fcc lattice structure is inherently stable, and the strong interfacial interaction between gold and silica (observed from XRD data and corroborated by SEM analysis) suggests that the nanoparticles are well-integrated into the matrix without disrupting the aerogel's porous nature. This structural integrity further supports the aerogel's suitability for use in adsorption or catalysis applications, where stability under various conditions is critical.
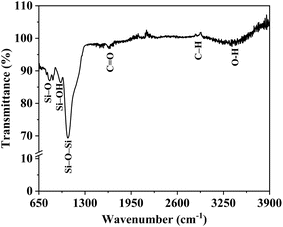
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations, suggesting the presence of carbonyl groups within the aerogel network. The presence of residual organic precursors or reaction byproducts containing carbonyl groups may contribute to these peaks. A strong, broad peak around 1050 cm−1, attributed to the asymmetric stretching of Si–O–Si bonds, which is typical of silica aerogels. A peak around 800 cm−1, indicating symmetric Si–O bending vibrations within the silica framework. A minor band near 950 cm−1, representing the Si–OH surface hydroxyl groups, which are essential for maintaining the aerogel's hydrophobicity nature and surface reactivity.
O stretching vibrations, suggesting the presence of carbonyl groups within the aerogel network. The presence of residual organic precursors or reaction byproducts containing carbonyl groups may contribute to these peaks. A strong, broad peak around 1050 cm−1, attributed to the asymmetric stretching of Si–O–Si bonds, which is typical of silica aerogels. A peak around 800 cm−1, indicating symmetric Si–O bending vibrations within the silica framework. A minor band near 950 cm−1, representing the Si–OH surface hydroxyl groups, which are essential for maintaining the aerogel's hydrophobicity nature and surface reactivity.
Interestingly, the FTIR analysis did not reveal any new peaks associated with direct chemical bonding between the gold nanoparticles and the silica framework. This absence suggests that the gold nanoparticles were physically incorporated within the silica matrix rather than chemically bonded, consistent with their uniform dispersion and retention of phase purity observed in XRD analysis. The lack of Au–O–Si bands further indicates that the SFD method successfully retained the chemical properties of both components without inducing unintended interactions. The FTIR spectrum's consistent peaks in the silica framework and hydroxyl regions suggest that the gold nanoparticles did not disrupt the aerogel's structural or chemical stability. The presence of surface hydroxyl groups could enhance the stability of gold nanoparticles within the matrix by providing sites for hydrogen bonding or weak physical interactions, thereby maintaining nanoparticle dispersion and preventing aggregation.
SEM imaging confirms that the open-pore architecture remains intact post-impregnation, while EDS mapping and spectral analysis reveal a uniform dispersion of gold nanoparticles (∼18.5 wt%) throughout the aerogel. The scCO2 environment supports controlled nucleation, reducing agglomeration and suppressing uncontrolled particle growth often seen in liquid-phase syntheses due to supersaturation or solubility gradients. The absence of large gold clusters suggests effective suppression of Ostwald ripening, commonly promoted by prolonged residence times in aqueous or alcoholic media. Crucially, preserving the aerogel's mesostructure is not just a structural benefit, it is essential for performance. High surface area and interconnected mesoporosity facilitate short diffusion paths and improved access to active sites, which is especially important for trace-level mercury adsorption where transport limitations prevail. By maintaining both nanostructural integrity and chemical functionality, scCO2 impregnation ensures a high density of accessible chemisorptive sites and consistent adsorption performance. These advantages are further supported by previous studies,27,28 which show that conventional deposition methods often lead to poor nanoparticle dispersion, pore blockage, and loss of structural uniformity, thereby compromising sorbent efficiency and reproducibility. Complementary BJH (Barrett–Joyner–Halenda) analysis in Fig. 5 confirms that the SFD process does not significantly alter pore volume or size distribution. The Au-AG composite exhibits a pore volume of 3.66 cm3 g−1, similar to that of the undoped silica aerogel. This indicates that the SFD method effectively integrates Au nanoparticles without causing substantial filling or reduction of pore volume. The average pore size remains stable at 21.8 nm, which is beneficial for applications that require consistent pore accessibility and favorable fluid dynamics.
The isotherms of the Au-AG composite are classified as type IV, characteristic of mesoporous materials, and show minimal deviation from the undoped aerogel. This indicates that capillary condensation and pore connectivity are maintained, reflecting an intact porous structure. The presence of a consistent H1 hysteresis loop further supports the conclusion that SFD does not lead to significant structural disruption or pore blockage.
Overall, the BET analysis in Fig. 5 reveals no significant deviations in surface area, pore volume, or pore size distribution, confirming that the SFD process enables a well-dispersed integration of Au nanoparticles without obstructing the pore structure. The controlled dispersion facilitated by SFD enhances the aerogel's properties while retaining a uniform pore size distribution, which is crucial for high-performance applications. Moreover, the use of supercritical CO2 in the SFD process allows for efficient deposition of Au nanoparticles with high penetrability into the aerogel matrix, ensuring even distribution without pore obstruction. Careful selection of precursor concentration during SFD is critical to achieving optimal Au loading while preventing excessive aggregation, thereby maintaining the integrity of the aerogel's pore structure.
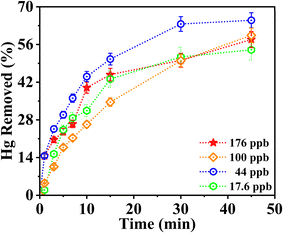 | ||
| Fig. 6 Percentage removal of mercury (Hg) spiked in deionized water (ddH2O) by the Au-AG composite at varying concentrations, illustrating the composite's effectiveness in Hg adsorption over time. | ||
The Au-AG composite exhibits rapid adsorption kinetics, with approximately 60% of the initial Hg content removed within the first 45 minutes. This suggests that the composite contains highly accessible active sites, likely due to chemisorption interactions between Hg ions and the Au and Ag nanoparticles embedded in the porous aerogel matrix. The large surface area and reactive sites contribute to the fast initial uptake, which is advantageous for batch purification systems requiring shorter treatment times. Despite the strong initial adsorption, equilibrium was reached only after 24 hours, with approximately 85% of the initial Hg removed and ∼15% remaining in solution. In comparison, control experiments using undoped pristine silica aerogel under identical conditions showed only ∼28% Hg removal, indicating primarily weak physisorption. This stark contrast highlights the critical role of gold nanoparticles in enhancing mercury capture, likely via Hg–Au amalgamation and chemisorption mechanisms. These results confirm that bare silica aerogel lacks sufficient affinity for effective trace-level Hg adsorption, whereas the incorporation of Au provides the necessary active sites for selective and efficient binding. The gradual approach to equilibrium suggests that while the composite has high initial capacity, complete removal requires extended contact time—possibly due to pore diffusion limitations or surface saturation effects. This behavior supports the potential for multi-stage purification processes, enabling high-purity water treatment within shorter contact durations.
The structure of the Au-AG composite likely plays a significant role in these observed adsorption dynamics. The aerogel's highly porous structure enables rapid mass transport and access to a large surface area, while the distribution of Au nanoparticles within AG matrix ensures high site availability for Hg binding. However, the slower approach to full adsorption equilibrium may indicate that some binding sites are less accessible or that the adsorption process involves multiple mechanisms (e.g., surface adsorption followed by possible diffusion into micropores over time). These characteristics highlight the composite's efficiency in fast adsorption but also suggest that adjustments, such as increasing dosage or contact time, may enhance its overall effectiveness in applications where maximum Hg removal is required.
The equilibrium adsorption data were analyzed using the Langmuir isotherm model shown in Fig. 7b, which assumes monolayer adsorption onto a surface with a finite number of energetically identical binding sites. This model is particularly suited for systems where chemisorption dominates and where each adsorbate molecule binds to a single adsorption site. The linearized Langmuir plot (Fig. 7b) yielded a slope of 7.799 × 10−5 and an intercept of 6.531 × 10−2, from which the maximum adsorption capacity (Qmax) and the Langmuir constant (KL) were calculated as 12.82 mg g−1 and 1.191 × 10−3 L μg−1, respectively shown in Table 1. Unlike some literature reports of higher capacities obtained under artificially elevated mercury concentrations (e.g., >100 ppm), the Qmax reported here (12.82 mg g−1) reflects performance under environmentally relevant Hg levels (17.6–176 ppb), where most conventional materials fail to achieve high removal efficiency. The relatively high Qmax indicates the significant capacity of the Au-AG composite to adsorb mercury ions at the trace level, reflecting the contribution of well-dispersed gold nanoparticles and preserved mesoporosity. The KL value, though moderate, suggests that the surface sites have a consistent and meaningful affinity for mercury, consistent with the strong known interaction between Hg and Au, likely driven by amalgamation or orbital hybridization effects.
| Qmax (mg g−1) | KL (L μg−1) | RL | R2 |
|---|---|---|---|
| 12.82 | 0.0012 | 0.807 | 0.999 |
To further assess the favorability of the adsorption process, the dimensionless separation factor (RL) was calculated for trace level mercury concentration of 17.6 ppb, 44 ppb, 100pp, 176 ppb, the resulting RL were 0.979,0.950, 0.893, and 0.826 respectively, which lies in the favorable range of 0 < RL< 1, indicating that the adsorption is thermodynamically feasible and efficient under the tested conditions. These results confirm that the Langmuir model provides a good description of the adsorption mechanism of Hg on the Au-AG composite, consistent with monolayer chemisorption occurring on uniformly distributed and energetically similar sites, primarily on the Au nanoparticle surfaces embedded within the silica aerogel matrix. The close model fit also suggests that the composite offers a homogeneous distribution of active binding sites, a key factor in achieving reproducible and high-efficiency adsorption, particularly at trace concentration levels. While the moderate KL implies room for improvement in affinity, possibly through increasing Au nanoparticle loading, the current performance demonstrates a balance between capacity, accessibility, and material economy suitable for environmental mercury removal applications.
| Initial Hg(II) (ppb) | Qe (exp) (μg g−1) | Pseudo-first-order Qe (cal) | K1 (min−1) | R2 | Pseudo-second-order Qe (cal) | K2 (g μg−1 min−1) | R2 |
|---|---|---|---|---|---|---|---|
| 17.6 | 48 | 46.05 | 0.00426 | 0.864 | 45.79 | 0.0025 | 0.994 |
| 44 | 136 | 111.57 | 0.00562 | 0.879 | 134.06 | 0.0012 | 0.993 |
| 100 | 340 | 325.84 | 0.00458 | 0.985 | 338.98 | 0.000145 | 0.989 |
| 176 | 460 | 510.56 | 0.00375 | 0.856 | 456.62 | 0.000313 | 0.978 |
Moreover, the extremely high adsorption capacities observed, even at ultralow mercury concentrations, are particularly noteworthy. At just 176 ppb, the adsorbent achieved a Qe of 460 μg g−1, underscoring its exceptional performance in trace-level applications. Such behavior suggests a high density of accessible active sites and strong binding energy between the mercury ions and the Au-AG composite. The kinetic rate constants (K2) show a decreasing trend with increasing initial Hg(II) concentrations, which may be attributed to the progressive occupation of active sites and the onset of diffusion limitations at higher loadings. Nevertheless, even at the highest concentration tested, the kinetic behavior adhered closely to the pseudo-second-order model, further affirming the robustness of the chemisorption mechanism across a range of trace-level contaminant concentrations.
4. Conclusions
This study presents a novel gold nanoparticle-doped silica aerogel (Au-AG) composite synthesized via supercritical CO2 impregnation, demonstrating rapid and selective mercury (Hg) adsorption at environmentally relevant concentrations. The composite achieves 60% Hg removal within 45 minutes and reaches an adsorption capacity of 12.82 mg g−1, following Langmuir isotherm behavior and pseudo-second-order kinetics, indicative of monolayer chemisorption on uniformly distributed active sites. Notably, its high performance at trace Hg concentrations, where many conventional adsorbents fail, highlights its practical relevance for environmental and drinking water applications.Beyond efficacy, the use of commercially available Cabot P400 silica and a solvent-free, scalable supercritical process underscores the composite's potential for industrial deployment. The material's tunable Au loading capacity and high internal porosity further suggest opportunities to enhance selectivity and kinetics in future designs. These findings establish the Au-AG composite as a promising, scalable platform for trace-level mercury remediation and illustrate the broader potential of functionalized aerogels in advancing sustainable water purification technologies.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.Author contributions
All authors contributed significantly to this work. Co-first author Eitan Yosef led the conceptualization, methodology development, data collection, analysis, and drafting of the manuscript. Co-first author Subhash Kumar Sharma led methodology development, analysis, provided supervision, validated the results, wrote the original manuscript, and contributed to manuscript review and editing. Rajnish Kumar secured funding, supplied resources, reviewed the manuscript, and oversaw project administration. Hadas Mamane supported data curation, visualization, manuscript review, and formal analysis. All authors reviewed and approved the final manuscript, adhering to the journal's authorship criteria.Conflicts of interest
There is no conflicts to declare.Acknowledgements
This research was funded by the SPARC Programme and the Israeli Science Fund (ISF), for which we are very grateful.References
- C. T. Driscoll, R. P. Mason, H. M. Chan, D. J. Jacob and N. Pirrone, Environ. Sci. Technol., 2013, 47(10), 4967–4983 CrossRef CAS PubMed.
- A. Casselman, Nature, 2014 DOI:10.1038/nature.2014.15680.
- S. Kabiri, D. N. H. Tran, S. Azari and D. Losic, ACS Appl. Mater. Interfaces, 2015, 7, 11815–11823 CrossRef CAS PubMed.
- S. Wu, L. Kong and J. Liu, Res. Chem. Intermed., 2016, 42, 4513–4530 CrossRef CAS.
- F. Tadayon, M. Saber-Tehrani and S. Motahar, Korean J. Chem. Eng., 2013, 30, 642–648 CrossRef CAS.
- D. Liu, C. Lu and J. Wu, Colloids Interfaces, 2018, 2, 66, DOI:10.3390/colloids2040066.
- K. Johari, N. Saman and H. Mat, Environ. Technol., 2014, 35, 629–636 CrossRef CAS PubMed.
- L. Zhi, W. Zuo, F. Chen and B. Wang, ACS Sustain. Chem. Eng., 2016, 4, 3398–3408 CrossRef CAS.
- T. A. Saleh, Environ. Sci. Pollut. Res., 2015, 22, 16721–16731 CrossRef CAS PubMed.
- K. P. Lisha and T. Pradeep, Gold Bull., 2009, 42, 144–152 CrossRef CAS.
- R. Ganesamoorthy, V. K. Vadivel, R. Kumar, O. S. Kushwaha and H. Mamane, J. Clean. Prod., 2021, 329, 129713 CrossRef CAS.
- N. Katagiri, M. Ishikawa, N. Adachi, M. Fuji and T. Ota, J. Asian Ceram. Soc., 2015, 3, 151–155 CrossRef.
- G. Lazovski, G. Bar, B. Ji, N. Atar, U. Banin and R. Gvishi, J. Supercrit. Fluids, 2020, 159, 104496 CrossRef CAS.
- K. S. Subrahmanyam, C. D. Malliakas, D. Sarma, G. S. Armatas, J. Wu and M. G. Kanatzidis, J. Am. Chem. Soc., 2015, 137, 13943–13948 CrossRef CAS PubMed.
- Y. Zhang, D. Kang, M. Aindow and C. Erkey, J. Phys. Chem. B, 2005, 109, 2617–2624 CrossRef CAS PubMed.
- M. Pantić, Ž. Knez and Z. Novak, J. Non-Cryst. Solids, 2016, 432, 519–526 CrossRef.
- K. L. Solis, G. U. Nam and Y. Hong, Environ. Eng. Res., 2016, 21, 99–107 CrossRef.
- H. Gunes, Y. Özbakir, S. B. Barim, H. Yousefzadeh, S. E. Bozbag and C. Erkey, Front. Mater., 2020, 7 DOI:10.3389/fmats.2020.00018.
- P. Franco, E. Pessolano, R. Belvedere, A. Petrella and I. De Marco, J. Supercrit. Fluids, 2023, 24, 9269 Search PubMed.
- Y. Ling, S. Tan, D. Wang, J. Wu, F. Luo, Q. Liu, Y. an Zhang, F. Wang, Z. Zhang and Y. Cao, Chem. Eng. J., 2022, 101(8), 4640–4647 Search PubMed.
- J. Goel, K. Kadirvelu, C. Rajagopal and V. K. Garg, J. Chem. Technol. Biotechnol., 2005, 80, 469–476 CrossRef CAS.
- Z. Huang, Y. Wan, J. Liang, Y. Xiao, X. Li, X. Cui, S. Tian, Q. Zhao, S. Li and C. S. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 31624–31634 CrossRef CAS PubMed.
- S. Bao, K. Li, P. Ning, J. Peng, X. Jin and L. Tang, Appl. Surf. Sci., 2017, 393, 457–466 CrossRef CAS.
- E. Kokkinos, A. Lampou, I. Kellartzis, D. Karfaridis and A. Zouboulis, Water, 2022, 14, 49 CrossRef CAS.
- S. K. Sharma, P. Ranjani, H. Mamane and R. Kumar, Sci. Rep., 2023, 13, 1–19 CrossRef PubMed.
- A. Huerta, M. J. Torralvo, M. J. Tenorio, E. Pérez, J. Bermúdez, L. Calvo and A. Cabañas, J. Supercrit. Fluids, 2022, 184, 105582 CrossRef CAS.
- B. Zhou, Z. Chen, Q. Cheng, M. Xiao, G. Bae, D. Liang and T. Hasan, npj 2D Mater. Appl., 2022, 6, 34 CrossRef CAS.
- P. D. Brown, S. K. Gill and L. J. Hope-Weeks, J. Mater. Chem., 2011, 21, 4204–4208 RSC.
Footnote |
| † These authors contributed equally and are co-first authors. |
| This journal is © The Royal Society of Chemistry 2025 |