Open Access Article
Open Access ArticleEfficient photocatalytic degradation of methylene blue using 3D urchin-like TiO2@rGO-hBN architecture†
Nguyen Thi Huyenab,
Tran Ai Suong Suongc,
Cao Thi Thanha,
Nguyen Van Tua,
Pham Van Trinh a,
Tran Van Tan
a,
Tran Van Tan d,
Le Thi Quynh Xuana,
Bui Hung Thanga,
Tran Van Hau
d,
Le Thi Quynh Xuana,
Bui Hung Thanga,
Tran Van Hau a,
Do Tuana,
Pham Duy Longa,
Phan Ngoc Minhb,
Hiroya Abee and
Nguyen Van Chuc
a,
Do Tuana,
Pham Duy Longa,
Phan Ngoc Minhb,
Hiroya Abee and
Nguyen Van Chuc *ab
*ab
aInstitute of Materials Science, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam. E-mail: chucnv@ims.vast.vn
bGraduate University of Science and Technology, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
cVNU University of Engineering and Technology, 144 Xuan Thuy, Cau Giay, Hanoi, Vietnam
dFaculty of Physics, University of Science, Vietnam National University, 334 Nguyen Trai, Thanh Xuan, Hanoi, Vietnam
eJoining and Welding Research Institute, Osaka University, Osaka 5670047, Japan
First published on 7th April 2025
Abstract
3D urchin-like TiO2@rGO-hBN architectures were produced by a hydrothermal method, followed by a cold plasma jet process. The morphology, crystal structure, composition and photocatalytic performance of the 3D urchin-like TiO2@rGO-hBN architectures towards methylene blue (MB) were evaluated using SEM, Raman, XRD, EDS and UV-vis-NIR spectrophotometry. The obtained results indicated that under the same conditions, the MB degradation efficiencies were ∼72%, ∼81%, ∼87%, and ∼98% for 3D urchin-like TiO2, 3D urchin-like TiO2@rGO, 3D urchin-like TiO2@hBN, and 3D urchin-like TiO2@rGO-hBN, respectively, within 70 min under ultraviolet (UV) light irradiation with a wavelength of 365 nm. The significantly improved MB degradation efficiency was attributed to the effective separation of electron–hole pairs and the formation of ternary heterojunctions based on TiO2, rGO and hBN. The results show the promising potential of 3D urchin-like TiO2@rGO-hBN for applications in the photocatalytic degradation of organic pollutants.
1. Introduction
The escalating issue of water pollution, especially from industrial effluents, has drawn global attention due to its severe impact on ecosystems and public health. Among the various pollutants, synthetic dyes, such as methylene blue (MB), are particularly problematic because of their toxic, carcinogenic, and non-biodegradable nature.1 These dyes are widely used in industries, such as textiles, plastics, and cosmetics, where they often end up in water bodies, causing significant environmental hazards.1–3 As traditional water treatment methods are not fully effective in removing dyes, there is an urgent need for innovative solutions to tackle these pollutants in a sustainable and efficient manner.Recent advancements in photocatalysis have opened new avenues for treating dye-contaminated wastewater by utilizing sunlight or artificial light to degrade harmful organic compounds.4 Photocatalysis is classified into two types: homogeneous and heterogeneous.5 In homogeneous photocatalysis, the catalyst and reactants are in the same phase, typically in a solution. In contrast, heterogeneous photocatalysis uses a solid catalyst in a different phase from the reactants, usually in a liquid or gas mixture.6 This type offers the advantage of allowing the catalyst to be easily recovered and reused, making it more cost-effective and sustainable.5 Titanium dioxide (TiO2) is a widely used heterogeneous photocatalyst due to its non-toxic nature, abundance, strong oxidative capabilities, chemical stability, high redox potential, and low cost.2,7–9 Its ability to generate reactive oxygen species (ROS) under ultraviolet (UV) irradiation makes it highly effective for degrading organic pollutants, including dyes.10 Despite these advantages, TiO2's practical application in environmental remediation is limited by its wide bandgap (3.2 eV), restricting its absorption to the UV region, which accounts for only a small fraction of the solar spectrum.2,3,8 Additionally, rapid recombination of photogenerated electron–hole pairs reduces its overall efficiency.11 As a result, there has been substantial interest in modifying TiO2 or combining it with other materials to overcome these limitations and enhance its photocatalytic performance. TiO2 has been combined with one or more materials to improve its photocatalytic ability.
With high surface area, suitable band gap, superior chemical and physical properties, high carrier mobility and a two-dimensional (2D) structure, materials like graphene, hexagonal boron nitride (hBN) and molybdenum disulfide (MoS2) have received special attention from scientists in photocatalytic applications for environmental treatment.3,12–15 When combined with TiO2, 2D materials act as electron acceptors, helping to minimize the recombination of electron–hole pairs and supporting active sites for the reaction. R. Jain et al. found that TiO2 nanoparticles supported on rGO sheets display enhanced photocatalytic features due to the rapid molecular diffusion of water and MB molecules within the mesoporous structure of crystalline/amorphous heterojunctions in the TiO2/Ex-rGO composite.3 Compared to bare TiO2, TiO2/Ex-rGO composite has a photodegradation rate for MB that is 2.38 times higher.3 L. Chen et al. showed that, compared with bare TiO2, the MoS2/TiO2 nanocomposite fabricated by the hydrothermal method increased MB photocatalytic degradation by 3.8 times.13 D. Liu et al. reported that the porous BN/TiO2 composite nanosheet exhibits enhanced photocatalytic degradation of rhodamine B (RhB) compared to pure TiO2 due to the large number of highly chemically active Ti–O–B chemical bonds formed between B and TiO2 in the composite. The Ti–O–B bonds in the nanocomposites facilitate electron transfer from TiO2 to hBN, leading to a narrower bandgap of h-BN/TiO2.14 Recently, V. Yadav et al. showed that the insertion of GO into hBN nanosheets enhances the photocatalytic degradation of methyl orange (MO) compared to bare hBN or GO due to the formation of a potential barrier at the GO-hBN interface and the reduced band gap of the GO-hBN nanocomposite. These factors help enhance two-photon absorption, separate charge carriers and reduce electron–hole recombination.16 S. Al-Kandari et al. synthesized a BNrGO-TiO2 nanocomposite by the hydrothermal method and used it as a high-performance photocatalyst in the photodegradation of phenol.4 Therefore, the combination of TiO2 with the rGO-hBN composite can be considered as one of the photocatalyst starting materials for the degradation of organic pollutants. However, most of the synthesized samples are in powder form, which limits the ability to recover the product after photocatalytic degradation.
Motivated by the above analysis, this study aims to develop a novel 3D urchin-like TiO2@rGO-hBN composite architecture on silicon substrates with enhanced photocatalytic properties and good recoverability for the degradation of MB. The unique 3D structure is expected to increase the active surface area for photocatalysis, while the integration of rGO and hBN will improve charge transfer and reduce the recombination of photogenerated electron–hole pairs. This combination seeks to address the challenges faced by conventional TiO2-based photocatalysts and achieve efficient degradation of organic pollutants under UV light irradiation.
2. Materials and methods
2.1. Materials
Titanium tetrachloride (TiCl4, 99%), hydrochloric acid (HCl, 37%), methylene blue (MB, 99%), isopropyl alcohol (IPA, >99%), benzoquinone (BQ, 99%) and ethylenediaminetetraacetic acid disodium (EDTA, 99%) were purchased from Macklin Co. Ltd (China). Graphene oxide (GO) with sizes of several micrometers (Fig. S1†) and boron nitride (BN) sheets with sizes of several tens of nanometers (Fig. S2†) were fabricated by the Hummers' method17 and ball milling method,18 respectively.2.2. Fabrication of 3D urchin-like TiO2, TiO2@rGO and TiO2@rGO-hBN architectures
3D urchin-like TiO2 architectures were synthesized on silicon substrates via a hydrothermal process. Firstly, 8 mL of 37% HCl was mixed with 4 mL of deionized water, followed by the addition of 0.3 mL titanium tetrachloride (TiCl4, 99%) under continuous stirring for 20 min at a temperature of 7–10 °C using an ice bath. Next, the resulting solution was transferred to a 50 mL stainless steel autoclave, with the silicon substrates (measuring 0.5 cm × 0.5 cm) placed horizontally at the bottom of the autoclave. The autoclave was then heated to 160 °C and maintained for 6 h, before being allowed to cool naturally to room temperature. After that, the obtained samples were collected, washed with deionized water, dried at 50 °C for 60 min, and calcined at 400 °C for 1 h in air to obtain 3D urchin-like TiO2 samples. For the preparation of 3D urchin-like TiO2 @rGO architectures, 10 μL of GO solution (0.25 mg mL−1), prepared by the Hummers' method, was dropped onto the surface of the TiO2 at room temperature, followed by cold plasma jet treatment for 3 min in Ar gas with a flow rate of 0.5 L min−1. The detailed information about the configuration of the cold plasma jet was presented by Xuan et al. (2022).19 The as-prepared samples were dried naturally for 24 h and annealed at 200 °C in air atmosphere. For the preparation of 3D urchin-like TiO2@rGO-hBN architectures, the fabrication steps were performed similarly to those of the 3D urchin-like TiO2@rGO architectures, with 10 μL of GO solution replaced by 10 μL of GO/hBN mixture solution with a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
2.3. Photocatalytic activity measurement
The absorption and photocatalytic activity of 3D urchin-like TiO2, TiO2@rGO and TiO2@rGO-hBN architectures on silicon substrates were evaluated by measuring the degradation rate of MB solution under ultraviolet (UV) light irradiation with a wavelength of 365 nm. The obtained photocatalyst substrates were immersed in 20 mL of MB solution (10−5 M) for 1 h in the dark to reach the absorption/desorption equilibrium. The samples were then irradiated at fixed time intervals, and a volume of 3 mL of the reaction solution was withdrawn for each measurement of MB degradation.2.4. Apparatus
The morphological features, presence of elements and structures of 3D urchin-like TiO2, TiO2@rGO and TiO2@rGO-hBN architectures were characterized using scanning electron microscopy (FESEM, Hitachi S-4800, Japan), energy dispersive X-ray spectroscopy (EDS) attached to SEM (JSM-IT800, JOEL, Japan), Raman microscopy and X-ray diffraction (XRD D8 Advance, Bruker, Germany). The absorption and photocatalytic activities of the samples were measured at room temperature using a UV-vis spectrophotometer (UV-2450, Shimadzu, Japan).3. Results and discussions
Fig. 1a shows the SEM image of 3D urchin-like TiO2 synthesized after hydrothermal process. Fig. 1b and c show the SEM images of TiO2@rGO and TiO2@rGO-hBN nanocomposites synthesized after cold plasma treatment process, respectively. The SEM image of the TiO2 sample (Fig. 1a) showed a 3D urchin shape with an average diameter of about 10 μm to 12 μm. The mechanism for the formation of the 3D urchin-like TiO2 structure may follow a process of nucleation, dissolution, recrystallization and self-assembly, as presented in previous reports.20,21 In the initial stage, amorphous or rutile TiO2 nuclei were formed, which then coalesced into crinkly spherical units. As the reaction time increased, these TiO2 spherical units dissolved and recrystallized to form TiO2 nanoneedles on the surface of the microspheres. Subsequently, the TiO2 nanoneedles began to directly self-assemble to form 3D urchin-like TiO2 structures. In the case of TiO2@rGO (Fig. 1b), the rGO sheets were homogeneously dispersed between the TiO2 nanoneedles. It could be clearly seen that the restacking phenomenon of the rGO sheets was less pronounced. The rGO sheets appeared as thin ribbons that interconnected the TiO2 nanoneedles, forming a composite material of rGO-wrapped 3D urchin-like TiO2. In the case of TiO2@rGO-hBN (Fig. 1c), the hBN nanosheets were also uniformly dispersed within the rGO matrix and coated onto the surface of TiO2 after the cold plasma jet process. To further confirm the successful decoration of the rGO-hBN nanocomposite on TiO2, Raman spectra of the hBN, GO, TiO2, TiO2@rGO and TiO2@rGO-hBN samples were measured. | ||
| Fig. 1 SEM images of (a) TiO2, (b) TiO2@rGO and (c) TiO2@rGO-hBN. Raman spectra (d) and XRD pattern (e) of hBN, GO, TiO2, TiO2@rGO and TiO2@rGO-hBN. | ||
Fig. 1d shows the Raman spectra of hBN, GO, TiO2, TiO2@rGO and TiO2@rGO-hBN. For hBN nanosheets, a band at ∼1368 cm−1 was assigned to the in-plane vibration of the nitrogen and boron atoms (E2g).22 For GO nanosheets, two bands at ∼1348 cm−1 and ∼1600 cm−1 were assigned to the disorder band (D) and the graphite band (G).17 For 3D urchin-like TiO2, two characteristic peaks were observed at ∼443 cm−1 and ∼608 cm−1, corresponding to the Eg and A1g active modes of the rutile phase.23,24 The Raman spectrum of the 3D urchin-like TiO2@rGO nanocomposite showed the characteristic peaks of the TiO2 rutile phase (at ∼443 cm−1 and ∼608 cm−1) and graphene (at ∼1348 cm−1 and ∼1600 cm−1). The defect levels of graphene could be estimated via the intensity ratio between the D and G bands (ID/IG).25,26 Compared to pure GO, the ID/IG ratio of TiO2@rGO increased from 0.95 to 1.32, demonstrating that GO had been reduced to rGO during the jet plasma process.27 For the 3D urchin-like TiO2@rGO-hBN, it could be clearly seen that all the characteristic Raman peaks of the TiO2 rutile phase (at ∼443 cm−1 and ∼608 cm−1) and graphene (at ∼1348 cm−1 and ∼1600 cm−1) were observed. The characteristic peak of hBN at ∼1368 cm−1 was not distinctly discernible in TiO2@rGO-hBN, possibly due to its blending with the D band of rGO.
The XRD patterns were obtained to determine the crystal structure and phase of the samples. As shown in Fig. 1e, for hBN, diffraction peaks were observed at 2θ angles of 26.6°, 41.6°, 44.0°, 50.1° and 55.1°, corresponding to the (002), (100), (101), (102) and (004) crystal planes of hBN (JCPDS 01-073-2095).28 GO nanosheets showed a characteristic diffraction peak at 2θ = 10.6°, corresponding to the (001) plane.29 For 3D urchin-like TiO2, the diffraction peaks at 2θ values of 27.4°, 36.1°, 39.2°, 41.3°, 44.1°, 54.4°, 56.7° and 62.8° were indexed to the (110), (101), (200), (111), (210), (211), (220), and (002) planes of rutile TiO2 crystal (JCPDS No. 01-087-0710).30 For the 3D urchin-like TiO2@rGO and TiO2@rGO-hBN nanocomposites, all the characteristic diffraction peaks of rutile TiO2 were clearly observed. Meanwhile, the characteristic diffraction peaks of rGO and hBN were not observed due to the high density, large size and crystallinity of the 3D urchin-like TiO2. The architectural shape of the 3D urchin-like TiO2@rGO-hBN and the uniform distribution of the elements Ti, O, C, B and N were confirmed by SEM and EDS mapping. The SEM-EDS mapping of 3D urchin-like TiO2@rGO-hBN showed the elements distribution of Ti, O, C, B, and N, corresponding to the sky blue, green, red, purple, and orange colors, as shown in Fig. 2.
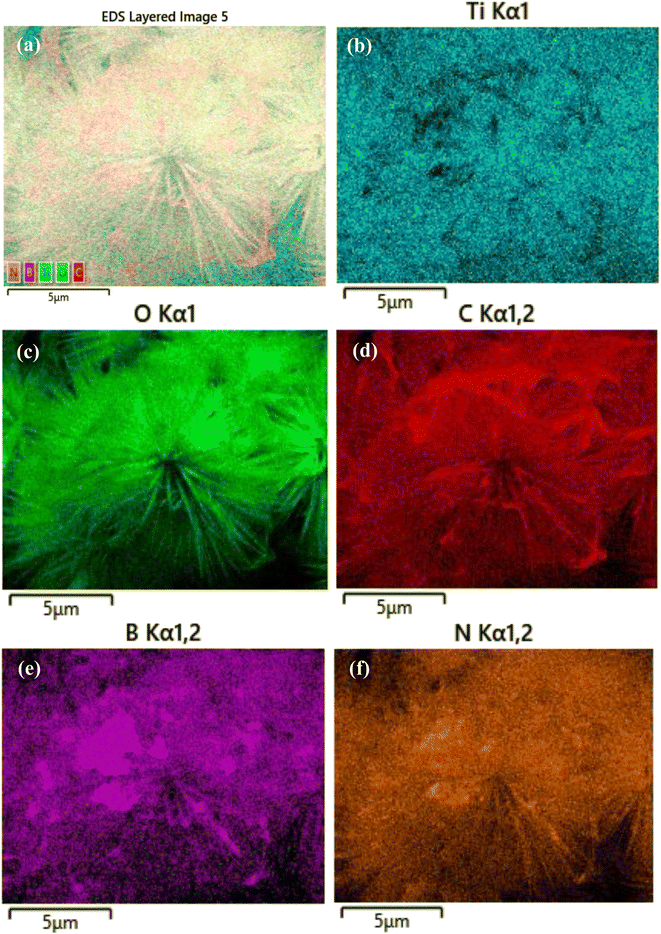 | ||
| Fig. 2 (a) SEM mapping image and elemental mapping images of (b) Ti, (c) O, (d) C, (e) B and (f) N elements of TiO2@rGO-hBN. | ||
Fig. 3a shows the UV-visible absorption spectra of TiO2, TiO2@rGO and TiO2@rGO-hBN samples recorded in the range of 280 nm to 900 nm. Compared to pure TiO2 and TiO2@rGO, the visible light absorption of TiO2@rGO-hBN nanocomposites was increased. The increased visible light absorption of the TiO2@rGO-hBN nanocomposites could improve the generation of more electron–hole pairs, contributing to the enhancement of photocatalytic activities. The optical band gaps of bare TiO2, TiO2@rGO and TiO2@rGO-hBN composites were estimated to be about 2.84, 2.78 and 2.72 eV, respectively, from a plot of (αhν)2 vs. phonon energy (eV), as shown in Fig. 3b. The reduced band gap energy and improved light absorption in the visible light region were beneficial for the generation of photoexcited charge carriers, thus improving efficient photocatalytic reactions under visible light irradiation.
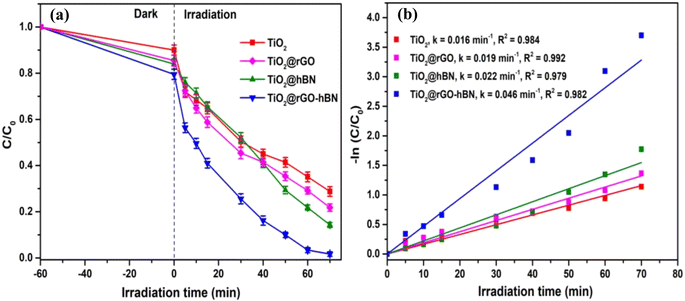
The photocatalytic degradation of MB over TiO2, TiO2@rGO, TiO2@hBN and TiO2@rGO-hBN samples under UV light irradiation (365 nm) was evaluated by measuring the UV-vis absorption spectrum at different UV irradiation times. As shown in Fig. 4, two characteristic absorption peaks of MB for four samples were observed at about 663 nm and 613 nm, and these peaks were related to the monomer and dimer of MB, respectively. The longer the UV irradiation time, the lower the UV-vis signal intensity of the MB dye. The decrease in absorption intensity indicated the degradation of MB. Since the intensity of the MB absorption spectrum peak was proportional to MB concentration,31–33 the relative change in MB concentration (C/C0) due to photocatalytic degradation could be monitored over time by measuring the main peak intensity at 663 nm after TiO2, TiO2@rG, TiO2@hBN and TiO2@rGO-hBN samples were immersed in the MB solution. Fig. 5a shows that TiO2@rGO-hBN exhibited a photocatalytic degradation efficiency of about ∼98%, which was higher than that of TiO2@hBN (∼87%), TiO2@rGO (∼81%) and TiO2 (∼72%). The degradation rate constants (k) of MB were calculated from the pseudo-first-order reaction using the graph of −ln(C/C0) vs. irradiation time. As shown in Fig. 5b, the rate constants for TiO2, TiO2@rGO, TiO2@hBN and TiO2@rGO-hBN were estimated to be 0.016 min−1, 0.019 min−1, 0.022 min−1, and 0.046 min−1 for MB dye. The degradation efficiency of TiO2@rGO-hBN under UV lamp irradiation was also carefully evaluated over five cycles, as shown in Fig. 6a. The degradation percentages of TiO2@rGO-hBN for MB molecules after the 1st, 2nd, 3rd, 4th and 5th cycles were estimated to be ∼98%, ∼96%, ∼95%, ∼91% and ∼88%, respectively. Additionally, the recoverability of TiO2@rGO-hBN after photocatalytic degradation was close to 100%. These results demonstrated the high recycling performance of the TiO2@rGO-hBN material. Compared with previously reported materials for the photocatalytic degradation of MB under UV irradiation sources, as shown in Table 1, the 3D urchin-like TiO2@rGO-hBN architectures demonstrated promising potential for applications in the photocatalytic degradation of organic pollutants.
 | ||
| Fig. 4 Photodegradation absorption spectra of MB solution in (a) TiO2, (b) TiO2@rGO, (c) TiO2@hBN and (d) TiO2@rGO-hBN. | ||
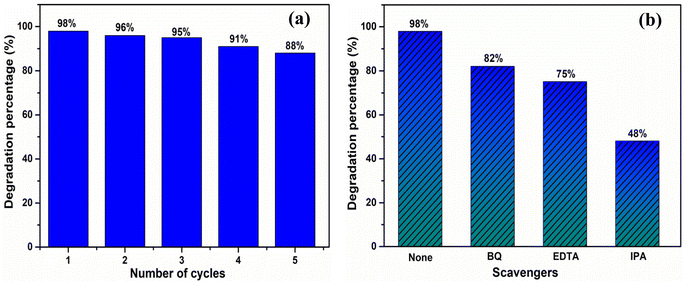 | ||
| Fig. 6 (a) Recycling performance and (b) trapping experiment for photocatalytic degradation of MB solution over TiO2@rGO-hBN under UV light irradiation. | ||
| Catalyst | Irradiation source | Evaluation method | Time (min) | Degradation efficiency (%) | Degradation rate (min−1) | Ref. |
|---|---|---|---|---|---|---|
| a AuNPs, gold nanoparticles; NiCd, nickel cadmium; F-BN, fluorinated hexagonal boron nitride; PL, photoluminescence; SA, sodium alginate; PAM, polyacrylamide; ZT: ZnO–TiO2; Ex-rGO, exfoliated rGO; BNMT, boron nitride micron square tubes; BNNS, boron nitride nanosheets. | ||||||
| AuNPs-TiO2 NTs | UV (254 nm, 15 W) | Raman | 80 | 100 | 5.1 × 10−2 | 36 |
| TiO2/BNNS NRs | UV (254 nm, 66 W) | Raman | 50 | 100 | 8.5 × 10−2 | 18 |
| F–TiO2 | UV (>400 nm, 400 W) | PL | 240 | 91.0 | — | 37 |
| TiO2 particles | UV (254 nm, 360 W) | UV-vis | 40 | 54.69 | 2.3 × 10−2 | 38 |
| F-BN/TiO2 | UV (420 nm) | UV-vis | 120 | 90.5 | — | 39 |
| ZnO–TiO2/rGO | UV | UV-vis | 63.5 | 99.4 | 7.07 × 10−2 | 40 |
| NiCd TiO2 | UV (254 nm, 15 W) | UV-vis | 100 | 86.0 | — | 41 |
| hBN/TiO2 powder | UV (365 nm) | UV-vis | 50 | 92.0 | 4.98 × 10−2 | 42 |
| rGO-TiO2/SA/PAM | Mercury lamp (200 W) | UV-vis | 100 | 97.14 | 3.628 × 10−2 | 43 |
| ZT-rGO | UV (365 nm) | UV-vis | 30 | 98.5 | 1.49 × 10−1 | 44 |
| TiO2/Ex-rGO | UV lamp (750 W) | UV-vis | 160 | 92.1 | 6.455 × 10−2 | 3 |
| TiO2 film | UV (254 nm) | UV-vis | 480 | 97.0 | 7.8 × 10−3 | 45 |
| NiO/TiO2 | UV (365 nm) | UV-vis | 60 | 25.3 | 4.93 × 10−3 | 46 |
| BNMT-BNNS/TiO2 | UV (<400 nm) | UV-vis | 115 | 99.62 | — | 47 |
| TiO2@rGO-hBN | UV (365 nm) | UV-vis | 70 | ∼98 | 4.6 × 10−2 | This work |
The significant improvement in the photocatalytic performance of TiO2@rGO-hBN for MB molecules could be explained by the existence of a heterogeneous structure and the large surface area of the rGO-hBN nanocomposite on the surface of TiO2. The large surface area and numerous interfaces of TiO2@rGO-hBN were beneficial for the adhesion of MB molecules, enhancing the photocatalytic performance. To identify the radical scavengers, three different capturing agents, including isopropyl alcohol (IPA),1 ethylenediaminetetraacetic acid disodium (EDTA),1 and benzoquinone (BQ),34 were introduced into the reaction mixture. IPA, EDTA, and BQ were used to trap hydroxyl radicals (*OH), holes (h+), and superoxide radicals (*O2−), respectively. As shown in Fig. 6b, in the presence of BQ, EDTA, and IPA, the degradation of MB under UV light irradiation decreased from 98% to 82%, 75%, and 48%, respectively. These results suggested that *OH radicals played an important role in the decomposition of MB, while h+ and *O2− played a relatively minor role in MB photodecomposition.
The photocatalytic mechanism of TiO2@rGO-hBN for MB degradation is shown in Fig. 7. Under ultraviolet light irradiation, excited electrons (e−) from the surface of hBN were rapidly transferred from the valence band (VB) of hBN to the conduction band (CB), generating electron–hole (e−–h+) pairs. The photogenerated e− in the CB of hBN could move from hBN to rGO, and then these e− would continue to move to the CB of TiO2. The rGO nanosheets acted as “transport bridges” between the photocatalysts and improved the ability to effectively separate photogenerated e−–h+ pairs due to their superior conductivity.12,35 Meanwhile, the large surface area of hBN was also beneficial for dye attachment.16 As a result, the electrons and holes reacted with dissolved oxygen (O2) and water (H2O) molecules to generate superoxide anions (*O2−) and hydroxyl radicals (*OH). The h+, *O2− and *OH activated radicals degraded the MB molecules adsorbed on the surface of the photocatalyst into CO2 and H2O under UV light irradiation.
4. Conclusions
In this work, 3D urchin-like TiO2@rGO-hBN architectures on silicon substrates were fabricated by a hydrothermal method, followed by the deposition of rGO-hBN nanocomposites onto the surface of 3D urchin-like TiO2 using a cold plasma jet process. The 3D urchin-like TiO2@rGO-hBN architectures were used as candidates for photocatalytic degradation of MB. The obtained results indicated that the MB degradation efficiency was about 98% within 70 min under UV light irradiation (365 nm). The significantly improved MB degradation efficiency was attributed to the effective separation of electron–hole pairs and the formation of ternary heterojunctions based on TiO2, rGO and hBN.Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request. In addition, all the data generated or analyzed during this study are included in this article.Author contributions
Nguyen Thi Huyen: conceptualization, methodology, investigation, formal analysis. Tran Ai Suong Suong: conceptualization, methodology, investigation. Cao Thi Thanh: formal analysis, investigation. Nguyen Van Tu: formal analysis, investigation. Pham Van Trinh: formal analysis, investigation. Tran Van Tan: formal analysis, investigation. Le Thi Quynh Xuan: formal analysis, investigation. Bui Hung Thang: formal analysis, investigation. Tran Van Hau: formal analysis, investigation. Do Tuan: formal analysis, investigation. Pham Duy Long: investigation. Phan Ngoc Minh: supervision, investigation. Hiroya Abe: supervision, investigation. Nguyen Van Chuc: supervision, conceptualization, methodology, formal analysis, project administration, writing – original draft preparation, validation, writing – reviewing and editing. All authors discussed and approved the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Vietnam Academy of Science and Technology (VAST) under Project NCXS02.03/24-25.References
- R. Nilmadhab, T. Nivedya, P. Priyankar and C. Rinku, RSC Adv., 2025, 15, 3008–3025 Search PubMed.
- M. Kocijan, L. Curkovíc, D. Ljubas, K. Mužina, I. Băcíc, T. Radoševíc, M. Podlogar, I. Bdikin, G. O. Irurueta, M. J. Hortigüela and G. Gonçalves, Appl. Sci., 2021, 11, 3966 CAS.
- R. Jain, M. Upadhyaya, A. Singh and R. P. Jaiswal, J. Environ. Chem. Eng., 2024, 12, 113881 CrossRef CAS.
- S. Al-Kandari, A. M. Abdullah, H. Al-Kandari, G. K. Nasrallah, M. A. Sharaf, D. S. Al-Marzouq, A. M. Mohamed, N. Younes, N. Kafour and T. Al-Tahtamouni, Environ. Sci. Pollut. Res., 2021, 28, 62771–62781 CrossRef CAS PubMed.
- S. Khan, T. Noor, N. Iqbal and L. Yaqoob, ACS Omega, 2024, 9, 21751–21767 Search PubMed.
- N. Akechatree, R. Rajendran, T. Rojviroon, P. Arumugam, V. Vasudevan, S. Sirivithayapakorn, A. Dhayalan, P. Wongpipun, N. Phetyim and O. Rojviroon, Mater. Res. Bull., 2025, 181, 113119 CrossRef CAS.
- G. Ali, I. Ali, M. A. Marwat, J. Khan, M. A. Basit and T. J. Park, Chem. Phys. Lett., 2024, 848, 141372 Search PubMed.
- H. Atout, M. G. Álvarez, D. Chebli, A. Bouguettoucha, D. Tichit, J. Llorca and F. Medina, Mater. Res. Bull., 2017, 95, 578–587 CAS.
- L. Jiang, W. Wei, S. Liu, S. A. Haruna, M. Zareef, W. Ahmad, Md. M. Hassan, H. Li and Q. Chen, J. Food Meas. Charact., 2022, 16, 2890–2898 Search PubMed.
- H. Y. Ma, L. Zhao, L. H. Guo, H. Zhang, F. J. Chen and W. C. Yu, J. Hazard. Mater., 2019, 369, 719–726 CAS.
- T. Gupta, Samriti, J. Cho and J. Prakash, Mater. Today Chem., 2021, 20, 100428 CAS.
- Y. Ren, Y. Dong, Y. Feng and J. Xu, Catalysts, 2018, 8, 590 Search PubMed.
- L. Chen, S. F. Ou, T. B. Nguyen, Y. Chuang, C. W. Chen and C. D. Dong, J. Taiwan Inst. Chem. Eng., 2025, 166, 105436 CAS.
- D. Liu, M. Zhang, W. Xie, L. Sun, Y. Chen and W. Lei, Appl. Catal., B, 2017, 207, 72–78 CAS.
- W. Zhang, Y. Xu and X. Zou, Food Chem., 2018, 261, 1–7 CAS.
- V. Yadav, M. K. Kumawat, S. Tiwari, A. K. Singh and T. Mohanty, FlatChem, 2024, 45, 100659 CAS.
- C. T. Thanh, N. H. Binh, P. N. D. Duoc, V. T. Thu, P. V. Trinh, N. N. Anh, N. V. Tu, N. V. Tuyen, N. V. Quynh, V. C. Tu, B. T. P. Thao, P. D. Thang, H. Abe and N. V. Chuc, Bull. Environ. Contam. Toxicol., 2021, 106, 1017–1023 CAS.
- N. T. Huyen, N. V. Tu, T. V. Hau, P. V. Trinh, C. T. Thanh, N. T. Loan, T. V. Tan, N. V. Tuyen, V. D. Chinh, V. X. Hoa, C. T. Anh and N. V. Chuc, Mater. Lett., 2023, 340, 134213 Search PubMed.
- L. T. Q. Xuan, L. N. Nguyen and N. T. Dao, Nanotechnology, 2022, 33, 105603 Search PubMed.
- L. Xiang, X. Zhao, J. Yin and B. Fan, J. Mater. Sci., 2012, 47, 1436–1445 CAS.
- C. Shi, M. Eqi, J. Shi, Z. Huang and H. Qi, J. Colloid Interface Sci., 2023, 650, 1736–1748 CrossRef CAS PubMed.
- B. Yu, J. Fan, J. He, Y. Liu, R. Wang, K. Qi, P. Han and Z. Luo, J. Alloys Compd., 2023, 930, 167303 CrossRef CAS.
- D. Nunes, A. Pimentel, L. Santos, P. Barquinha, E. Fortunato and R. Martins, Catalysts, 2017, 7, 60 CrossRef.
- E. Perevedentseva, Y. C. Lin, A. Karmenyan, K. T. Wu, A. Lugovtsov, E. Shirshin, A. Priezzhev and C. L. Cheng, Materials, 2021, 14, 5920 CrossRef CAS PubMed.
- P. N. D. Duoc, N. H. Binh, T. V. Hau, C. T. Thanh, P. V. Trinh, N. V. Tuyen, N. V. Quynh, N. V. Tu, V. D. Chinh, V. T. Thu, P. D. Thang, P. N. Minh and N. V. Chuc, J. Hazard. Mater., 2020, 400, 123185 CrossRef CAS.
- C. T. Thanh, N. T. Huyen, P. V. Trinh, N. V. Tu, V. T. Thu, V. C. Tu, D. N. Nhiem, P. T. Binh, N. N. Anh, V. X. Hoa, P. N. Minh, H. Abe and N. V. Chuc, Diamond Relat. Mater., 2025, 152, 111889 CrossRef CAS.
- N. T. Huyen, L. T. Q. Ngan, L. T. Q. Xuan, T. A. S. Suong, C. T. Thanh, N. V. Tu, P. T. Binh, T. V. Tan, N. V. Tuyen, D. T. Cao, P. V. Hai, V. X. Hoa and N. V. Chuc, Opt. Mater., 2025, 162, 116935 CrossRef CAS.
- N. K. Mahto, K. Shafali, R. Tyagi, O. P. Sharma, O. P. Khatri and S. K. Sinha, Wear, 2023, 530–531, 205065 CrossRef CAS.
- F. Zhu, X. She, Z. Zhang, X. Yu, H. Huang, J. Ji, L. Li and S. Li, J. Phys. Chem. Solids, 2024, 195, 112269 CrossRef CAS.
- S. Wannapop and A. Somdee, Ceram. Int., 2020, 46, 25758–25765 Search PubMed.
- S. Y. Lee, H. T. Do and J. H. Kim, Appl. Surf. Sci., 2022, 573, 151383 CrossRef CAS.
- S. A. Haladu, K. A. Elsayed, A. A. Manda, U. I. Gaya, M. A. Almessiere and M. A. Hafez, Opt. Laser Technol., 2025, 180, 111498 Search PubMed.
- N. T. K. Ngan, F. Grasset, S. Ishii, H. Fudouzi and T. Uchikoshi, Mater. Adv., 2024, 5, 8615–8628 Search PubMed.
- Z. Zhu, J. Ye, X. Tang, Z. Chen, J. Yang, P. Huo, Y. H. Ng and J. Crittenden, Environ. Sci. Technol., 2023, 57, 16131–16140 Search PubMed.
- Z. Zhu, Q. Han, D. Yu, J. Sun and B. Liu, Mater. Lett., 2017, 209, 379–383 CrossRef CAS.
- R. Li, A. Zhou, Q. Lu, C. Yang and J. Zhang, Colloids Surf., A, 2013, 436, 270–278 CrossRef CAS.
- W. Yu, X. Liu, L. Pan, J. Li, J. Liu, J. Zhang, P. Li, C. Chen and Z. Sun, Appl. Surf. Sci., 2014, 319, 107–112 Search PubMed.
- A. T. Le, S. Y. Pung, S. L. Chiam, N. A. H. BT, N. Josoh, T. Y. Koay, J. S. Lee and N. B. Mustar, AIP Conf. Proc., 2020, 2267, 020017 CrossRef.
- A. ul Ahmad, A. Abbas, S. Ali, M. F. e-alam, Z. Farroq, Q. Abbas, M. Ahmad, A. Farid, A. M. afzal, H. M. U. Arshad, M. Javid and M. Iqbal, Ceram. Int., 2021, 47, 10089–10095 CrossRef.
- T. Q. Q. Viet, V. H. Khoi, N. T. H. Giang, H. T. V. Anh, N. M. Dat, M. T. Phong and N. H. Hieu, Colloids Surf., A, 2021, 629, 127464 Search PubMed.
- S. Khan, A. Noor, I. Khan, M. Muhammad, M. Sadiq and N. Muhammad, Catalysts, 2023, 13, 44 Search PubMed.
- Y. Sheng, J. Yang, F. Wang, L. Liu, H. Liu, C. Yan and Z. Guo, Appl. Surf. Sci., 2019, 465, 154–163 CrossRef CAS.
- A. Sawut, T. Wu, R. Simayi, T. Wu, X. Gong and Z. Wang, Colloids Surf., A, 2023, 678, 132531 CrossRef CAS.
- A. A. Manda, S. A. Haladu, K. A. Elsayed, U. I. Gaya, M. Alheshibri, A. A. Baroot, E. Çevik, I. Ercan, F. Ercan, T. S. Kayed, S. M. Magami and N. A. Altamini, Opt. Laser Technol., 2023, 160, 109105 CrossRef CAS.
- I. J. Badovinac, R. Peter, A. Omerzu, K. Salamon, I. Šarić, A. Samaržija, M. Perčić, I. K. Piltaver, G. Ambrožić and M. Petravić, Thin Solid Films, 2020, 709, 138215 CrossRef.
- A. Villamayor, T. Pomone, S. Perero, M. Ferraris, V. L. Barrio, E. G. Berasategui and P. Kelly, Ceram. Int., 2023, 49, 19309–19317 CrossRef CAS.
- Y. Ji, R. Xiao, X. Tang, W. Chen, J. Wang, F. Long and Z. Zou, Opt. Mater., 2024, 150, 115335 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00845j |
| This journal is © The Royal Society of Chemistry 2025 |