 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Research progress of silicon-based anode materials for lithium-ion batteries
Zhenjun Zhang,
Yilong Wu *,
Zuxue Mo,
Xiaoxu Lei,
Xuerui Xie,
Xiangyong Xue,
Haiqing Qin and
Haowen Jiang
*,
Zuxue Mo,
Xiaoxu Lei,
Xuerui Xie,
Xiangyong Xue,
Haiqing Qin and
Haowen Jiang *
*
Guangxi Key Laboratory of Superhard Material, National Engineering Research Center for Special Mineral Material, Guangxi Technology Innovation Center for Special Mineral Material, China Nonferrous Metal (Guilin) Geology And Mining Co., Ltd, Guilin 541004, P. R. China. E-mail: 1849095151@qq.com
First published on 7th April 2025
Abstract
In recent years, with the rapid development of fields such as portable electronic devices, electric vehicles, and energy storage systems, the performance requirements for lithium-ion batteries have been continuously rising. Among the numerous key components of lithium-ion batteries, the performance of the anode materials plays a crucial role, as it is directly related to core indicators such as the energy density, cycle life, and safety of the batteries. Among them, silicon-based anode materials have stood out among many anode materials by virtue of their extremely high theoretical specific capacity, becoming one of the hot research directions in the field of lithium-ion battery anode materials at present. However, silicon-based anode materials have problems such as severe volume expansion, poor electrical conductivity, low initial coulombic efficiency, and unstable solid electrolyte interphase during the charging and discharging process, which limit their wide application and urgently require the seeking of new solutions. This paper comprehensively and in-depth introduces the research progress of silicon-based anode materials for lithium-ion batteries in recent years, focusing on the failure mechanisms and modification methods of silicon-based anodes, and provides effective solutions to the severe challenges faced in the commercialization process of silicon-based anodes.
1 Introduction
Lithium-ion batteries, as an efficient energy storage device, play a crucial role in various fields of modern society. From portable electronic devices to electric vehicles and then to large-scale energy storage systems, the performance and application range of lithium-ion batteries have been continuously expanded. In the development process of lithium-ion batteries, searching for high-capacity and high-performance anode materials has always been one of the research focuses. Silicon-based anodes have gradually become a research hotspot due to their unique advantages. Since its commercialization in the 1990s, the lithium-ion battery has undergone multiple technological innovations. Its advantages such as high energy density, long cycle life, and low self-discharge rate1–3 have made it the preferred energy storage technology in fields such as consumer electronics, electric vehicles, and renewable energy storage. The development of lithium-ion batteries has not only promoted the miniaturization and intellectualization of electronic devices but also provided strong support for the popularization of electric vehicles and the large-scale application of renewable energy. With the continuous increase in the requirements for the energy density and performance of lithium-ion batteries, the traditional graphite anode is gradually unable to meet the demand. Silicon has an extremely high theoretical specific capacity (about 4200 mA h g−1),4,5 which is more than ten times that of graphite. However, silicon undergoes a huge volume change (about 300%),6,7 during the charging and discharging process, resulting in the destruction of the electrode structure and rapid capacity decay. Optimizing the silicon structure and compounding silicon with other materials can alleviate the volume expansion problem of silicon to a certain extent,8,9 effectively improve the cycle performance and rate performance of the silicon anode, and are expected to achieve a high-performance lithium-ion battery anode.2. Structure and principle of lithium-ion batteries
A lithium-ion battery mainly consists of five parts: a cathode, an anode, a separator, an electrolyte, and a casing. Its working principle is based on the continuous intercalation and deintercalation of lithium ions, which are combined with electrons at the same time. Taking a battery system composed of LiFePO4 (lithium iron phosphate) as the cathode and graphite as the anode as an example (Fig. 1): the charging and discharging process is achieved through the migration of lithium ions between the cathode and the anode. | ||
| Fig. 1 Schematic diagram of the working principle of lithium-ion batteries.10 | ||
During charging, an external power source is connected to the battery. An oxidation reaction occurs at the positive electrode, and lithium ions are extracted from LiFePO4, releasing electrons. The electrons flow to the negative electrode through the external circuit, while the lithium ions migrate through the electrolyte and across the separator to the negative electrode. At the negative electrode, the lithium ions are intercalated between the graphite layers to form a lithium-carbon compound (LixC6).
| LiFePO4 → Li1−xFePO4 + xLi++xe− | (1) |
| 6C + xLi+ → LixC6 | (2) |
During discharging, the battery is connected to an external load. Electrons flow from the negative electrode to the positive electrode through the external circuit. At the same time, lithium ions are extracted from the negative electrode and return to the positive electrode through the electrolyte and the separator. At the positive electrode, the lithium ions are intercalated into the LiFePO4 structure and combine with electrons, undergoing a reduction reaction.
| Li1−xFePO4 + xLi+ + xe− → LiFePO4 | (3) |
| LixC6 → 6C + xLi+ + xe− | (4) |
During this process, the LiFePO4 of the positive electrode material loses lithium ions during charging and reabsorbs them during discharging. Meanwhile, the graphite of the negative electrode material absorbs lithium ions during charging and releases them during discharging. These chemical reactions enable the migration of lithium ions between the positive and negative electrodes, thus realizing the charging and discharging of the battery and fulfilling the energy storage and release of the battery.
During the intercalation and deintercalation of lithium ions, the intercalation and deintercalation of an equivalent amount of electrons accompany lithium ions (customarily, the terms intercalation or deintercalation are used for the positive electrode, while insertion or extraction are used for the negative electrode). It is like a rocking chair movement of lithium ions back and forth between the positive and negative electrodes. Therefore, the lithium-ion battery is vividly called a rocking chair battery.11
The cathode of a lithium-ion battery has a relatively high potential and is often a lithium-intercalated transition metal oxide or a polyanionic compound, such as lithium cobalt oxide12,13 (LiCoO2), lithium manganate14,15 (LiMn2O4), ternary materials16,17 (such as lithium nickel cobalt manganese oxide LiNi1−x−yCoxMnyO2), lithium iron phosphate18 (LiFePO4), etc.; The anode is usually a carbon material, such as graphite and non-graphitized carbon. The electrolyte is mainly a non-aqueous solution composed of an organic mixed solvent and a lithium salt. The solvent is mostly an organic solvent like carbonate, and the lithium salt is mostly a monovalent polyanionic lithium salt, such as lithium hexafluorophosphate (LiPF6). The separator is generally a microporous film of polyethylene or polypropylene, which functions to separate the cathode and anode materials, prevent electrons from passing through to cause a short circuit, and allow ions in the electrolyte to pass through.
3 Introduction to anode materials of lithium-ion batteries
3.1 Intercalation reaction type anode
In lithium-ion batteries, the intercalation reaction type anode refers to the anode material that realizes charge storage and release through the intercalation and deintercalation of lithium ions between the material layers. Common intercalation reaction type anode materials include graphite, metal oxides, etc. Graphite is currently the most commercialized anode material for lithium-ion batteries. It has a layered structure, and Li+ can be relatively easily intercalated between the graphite layers and combined with 6 carbon atoms to form LiC6,19 with a corresponding theoretical specific capacity of 372 mA h g−1. This intercalation reaction is relatively stable, endowing the graphite anode with good cycling performance. However, the discharge platform of LiC6 is relatively low. This low discharge platform restricts the output voltage of the battery. For some electrical equipment or application scenarios with high voltage requirements, it may not be able to meet their normal operating needs. The lithium intercalation potential of graphite is close to that of the lithium anode. During the charging process, it is prone to causing the deposition and precipitation of metallic lithium on the surface of the anode. Lithium deposition not only leads to a rapid decline in the battery capacity but may also pierce the separator, causing an internal short circuit in the battery. At the same time, the graphite anode undergoes various side reactions with the electrolyte during the charging and discharging processes. These side reactions result in the formation of an unstable solid electrolyte interface (SEI) film on the anode surface, reducing the first coulombic efficiency of the battery.Besides graphite-based materials, lithium titanate also belongs to the intercalation-reaction-type anode materials. Its lithium-storage mechanism is the transformation between spinel-structured lithium titanate (Li4Ti5O12) and rock-salt-structured lithium titanate (Li7Ti5O12).20 During the charging and discharging processes, it has a very small volume change, which endows the battery with a long cycle life.21,22 Its relatively high potential can avoid the generation of lithium dendrites and improve the safety of the battery. However, its specific capacity is relatively low, which limits the energy density of the battery to a certain extent. Moreover, its poor electronic conductivity will affect the rate performance of the battery.
In general, intercalation reaction-type anodes have important applications in lithium-ion batteries. However, continuous research and improvement are still needed to meet the demands for higher energy density, longer cycle life, and better rate performance.
3.2 Conversion-reaction-type anodes
Conversion-reaction-type anode materials for lithium-ion batteries refer to those that store lithium with a high specific capacity through the reversible displacement redox reaction between Li and transition-metal cations. These materials include metal oxides, metal sulfides, metal hydrides, metal nitrides, metal phosphides, metal fluorides, etc. The electrochemical reaction formula is as follows:| MaXb + (b·n)Li+ + (b·n)e− → aM + bLinX | (5) |
As shown in Fig. 2, during the first lithiation of MaXb, M nanocrystalline particles and LinX coated with a solid-electrolyte-interface (SEI) film are formed. The key to the reversibility of the subsequent conversion mechanism lies in the formation of highly electroactive M nanocrystalline particles to decompose the SEI layer on the LinX matrix. Many conversion-reaction-type anodes (such as Fe3O4, FeS2, and MnO2) exist in natural forms (magnetite, pyrite, and pyrolusite), with relatively low production costs. Conversion-reaction-type anodes exhibit adjustable reaction potentials, which specifically depend on the ionic-bond strength between the transition-metal (M) cations and the anionic substances. Compared with the graphite anode with a low lithium-insertion potential, conversion-reaction-type anodes can ensure better battery safety by avoiding the problem of lithium-dendrite formation. They have higher specific capacities and better safety compared to intercalation-type materials. However, due to their inherently poor electronic and ionic conductivity, relatively large volume expansion (<200%), and continuous electrolyte decomposition, conversion-reaction-type anodes still face significant challenges.
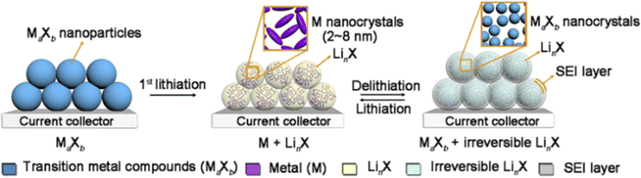 | ||
| Fig. 2 Schematic diagram of local chemical transformations of transition metal compounds during the conversion reaction process.23 | ||
3.3 Lithium metal anode
The lithium-metal anode has high conductivity and a high theoretical specific capacity (3860 mA h g−1, which is equivalent to ten times that of the graphite anode of commercial lithium-ion batteries).24 Moreover, it has the lowest anode potential and can be paired with the existing cathode systems of lithium-ion batteries, making it a highly promising anode material.In the development history of lithium-metal anodes, early research can be traced back to the last century. In 1913, Gilbert N. Lewis first proposed the concept of a lithium-metal anode.25 Harris first discovered in 1958 that lithium metal could stably exist in non-aqueous solvents. During the 1960s and 1970s, primary batteries using lithium metal as the anode and non-aqueous solvent electrolytes were extensively studied. In the late 1980s, the commercial MoS2/Li battery developed by Moli Company had a greatly enhanced energy density and occupied a large market share. However, later, due to several fire and explosion incidents of lithium-ion batteries, a large-scale product recall was forced.26 Subsequent research showed that the cause of the fire and explosion of lithium-ion batteries was the growth of lithium dendrites during charging and discharging, which pierced the separator, leading to an internal short-circuit and then thermal runaway.27 In the 1990s, due to the inability to completely solve safety hazards such as the growth of lithium dendrites, scientists turned to study other anode materials. By the 21st century, as the specific capacity of common anodes for lithium-ion batteries approached their theoretical values, and with the proposal of new battery systems such as lithium-sulfur and lithium-air batteries, researchers had to return to the study of lithium-metal anodes. After 2010, the development of solid electrolytes such as polymers,28,29 oxides, and sulfides brought new directions to the research of lithium-metal anodes. Replacing traditional liquid electrolytes with solid electrolytes can effectively suppress the growth of lithium dendrites, reduce interfacial side reactions, and increase the energy density.30 At present, certain progress has been made in improving the ionic conductivity of solid electrolytes. However, the safety issues caused by the growth of lithium dendrites still pose a huge obstacle on the path to commercial applications.
3.4 Alloy-reaction-type anode
Alloy-reaction-type anode materials for lithium-ion batteries refer to metals and their alloys that can undergo alloying reactions with lithium. Some common metals that can alloy with lithium include tin (Sn), aluminum (Al), germanium (Ge), magnesium (Mg), calcium (Ca), silicon (Si), etc. The theoretical specific capacity and charge density of this type of anode material are generally higher than those of intercalation-type anode materials. Additionally, compared with the lithium–intercalation potential of graphite (<0.1 V vs. Li/Li+), the lithium-intercalation voltage of alloying anodes is slightly higher. This is beneficial for avoiding the growth of lithium dendrites and improving the safety of the battery.31–34 Meanwhile, their relatively low operating potential (∼0.3–0.6 V vs. Li/Li+) is also conducive to matching high-voltage cathode materials, thus enabling the development of lithium-ion batteries with higher energy density.Tin and its oxides are among the materials in this type of anode that have been studied relatively early.35,36 The theoretical specific capacity of Sn37 is 990 mA h g−1,and that of SnO2 is 1494 mA h g−1, which are respectively more than 2 times and 4 times the theoretical capacity of graphite carbon.38,39 However, during the lithium-insertion and extraction processes, tin-based materials undergo phase transitions and alloying reactions, generating a huge volume expansion effect. This causes the material to be pulverized, its structure to be damaged, resulting in a sharp decline in capacity and poor cycling performance.40 In addition, as a metal oxide, SnO2 material has poor electronic conductivity, thus its rate performance is also unsatisfactory.
Silicon has received extensive attention due to its high capacity. As shown in Fig. 3, the electrochemical de-/intercalation curves of lithium in silicon at room temperature and high temperature are presented. The results show that lithium and silicon can form four intermediate-phase alloys, namely Li12Si7, Li7Si3, Li13Si4 and Li22Si5. Among them, the theoretical specific capacity corresponding to Li22Si5 is 4200 mA h g−1, which is 10 times higher than that of graphite. However, like tin-based materials, silicon-based materials undergo phase transitions and alloying reactions during the lithium-insertion and extraction processes, generating a huge volume-expansion effect. This causes the material to be pulverized and its structure to be damaged, leading to a sharp decline in capacity and poor cycling performance.42
 | ||
| Fig. 3 The delithiation and lithiation curves of silicon at room temperature and 450 °C.41 | ||
In the system of alloy-reaction-type anode materials, silicon-based anodes have become highly promising research objects in this field due to their extremely high theoretical specific capacity of up to 4200 mA h g−1, far exceeding that of most similar materials. Compared with traditional intercalation-reaction-type anodes, their theoretical specific capacity shows a significant advantage, providing broad prospects for enhancing the energy density of batteries. In comparison with conversion-reaction-type anodes, silicon-based anodes have unique advantages in terms of lithium-intercalation voltage and compatibility with high-voltage cathode materials, and play a key role in constructing high-energy-density battery systems. However, in the process of moving towards large-scale practical applications, silicon-based anodes face numerous severe challenges, which makes the modification research on them an important topic in the current lithium-battery field. The following text will elaborate in detail on the inherent disadvantages that restrict the development of silicon-based anodes while they possess outstanding advantages, as well as the cutting-edge modification work carried out to address these issues.
4 Problems existing in silicon-based anode materials
During the charging and discharging process, an alloying reaction occurs between silicon and lithium, causing a significant volume expansion of silicon. This repeated contraction and expansion can lead to the formation of cracks in the silicon-based anode material until it pulverizes.43–48 As a result, the contact between the electrode material and the current collector is disrupted, and the active material detaches from the electrode sheet, leading to a rapid decline in battery capacity.49–51 These problems severely limit the practical application of silicon-based anodes. The interfacial evolution patterns of Si can be classified, and the corresponding failure mechanisms can be briefly summarized into the following three types (Fig. 4).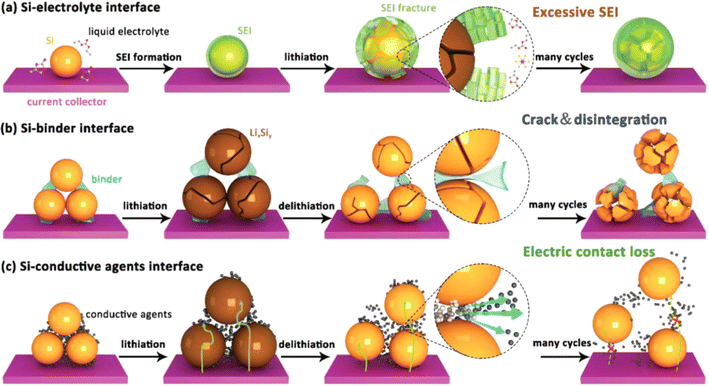 | ||
| Fig. 4 Various issues faced by silicon anodes: (a) schematic diagram of the failure process at the electrolyte interface; (b) schematic diagram of the failure process at the polymer binder interface; (c) schematic diagram of the failure process at the conductive additive interface.52 | ||
4.1 Electrolyte-interface failure
During the first charge–discharge process of silicon, a reaction occurs between the electrode material and the electrolyte at the solid–liquid interface, forming a passivation layer covering the surface of the electrode material, namely the solid-electrolyte-interface (SEI) film. The SEI film provides specific channels for the intercalation and de-intercalation of lithium ions between the electrode and the electrolyte, facilitating the transmission of lithium ions. At the same time, it can protect the electrode material from being corroded by the electrolyte and extend the service life of the electrode. The formation and subsequent repair processes of the SEI film consume a certain amount of lithium ions, resulting in a reduction in the available lithium ions in the battery, thus reducing the battery capacity. During the battery cycling process, due to factors such as the volume change of the electrode material, the SEI film will break and reform, causing the film to continuously thicken.53–56 An overly thick SEI film will further impede the transmission of lithium ions and consume a large amount of electrolyte, affecting the battery performance.57–624.2 Polymer binder interface failure
During the charging and discharging process, silicon undergoes significant volume changes. This intense expansion and contraction exert enormous pressure on the interface between silicon and the binder. Since the binder cannot effectively withstand and adapt to this continuous volume change, the bonding strength at the interface gradually weakens. This not only affects the transmission efficiency of electrons and ions at the interface but also causes silicon particles to easily detach from the binder, thus destroying the structural integrity of the electrode.63,64 As the number of cycles increases, the problem of interface instability becomes more severe, significantly affecting the battery's performance, such as capacity, rate characteristics, and cycle life.4.3 Conductive additive interface failure
During the lithium-insertion process of Si, the active particles squeeze each other due to volume expansion. When Si undergoes lithium-extraction, the volume shrinks, and the internal morphology of the electrode plate is difficult to restore. This easily leads to the separation of the active material and the conductive agent. This continuous volume change makes the contact at the interface unstable, gradually damaging the originally effective conductive channels.65 As the number of cycles increases, the bond between silicon and the conductive additive becomes less tight, and the resistance at the interface increases sharply. This failure phenomenon seriously hinders the transmission of electrons, significantly increasing the internal resistance of the battery, greatly reducing the charge–discharge efficiency, and also affecting the rate performance of the battery. In addition, interface failure increases the non-uniformity of the electrode structure. In some areas, silicon cannot fully participate in the electrochemical reaction, thus accelerating the attenuation of the battery capacity and shortening the cycle life of the battery.5 Improvement measures for silicon-based anode materials
Silicon-based anode materials have become one of the current research hotspots of anode materials for lithium-ion batteries due to their relatively high theoretical specific capacity. However, they undergo severe volume expansion during the charging and discharging process, leading to problems such as capacity fading, which restricts their further development in the battery industry. To improve the electrochemical performance of silicon, researchers have adopted a variety of modification methods. The following are some common directions of modification research.5.1 Nanostructuring
 | ||
| Fig. 5 Schematic diagram of the critical fracture size of silicon nanoparticles.66 | ||
 | ||
| Fig. 6 (a) The preparation process of SiNWs/graphite composite materials and the SEM images of the mixed powder before and after electrolysis.70 (b) The process of synthesizing UTSiNWs by the BACE method.71 | ||
Silicon nanotubes are hollow tubular structures composed of silicon atoms, featuring a unique hollow tubular morphology and a nanoscale tube diameter. Silicon nanotubes can effectively buffer the volume change of silicon during the charging and discharging process. Their hollow structure provides space for the expansion of silicon, reducing the risk of structural damage caused by the volume effect.80 Compared with other materials, silicon nanotubes have a larger specific surface area, enabling them to fully contact the electrolyte and providing more active sites for electrochemical reactions. Lithium ions can be relatively easily inserted into the surface of silicon nanotubes, and due to their nanoscale tube diameter, the diffusion distance of lithium ions is shorter, resulting in relatively less polarization and capacity decay of silicon at high rates. At the same time, silicon nanotubes can promote the penetration of the electrolyte, providing a smoother channel for charge transfer and improving the charge–discharge efficiency of the battery. However, the preparation of silicon nanotubes is difficult, usually requiring complex processes and expensive equipment, resulting in a high cost. Silicon nanotubes can accommodate large volume changes related to lithium and achieve reversible behavior during morphological changes, thereby improving the cycle retention rate and reliable operation of the battery. Wen et al.78 prepared silicon nanotubes by first preparing silica nanotubes using rod-like as Ni–N2H4 a template and then converting them into silicon nanotubes via a magnesiothermic reduction process assisted with magnesium powder, and used them as anodes for lithium-ion batteries (Fig. 7a). They found that these silicon nanotubes had better electrochemical performance compared to commercial silicon meshes. The silicon nanotubes prepared by a specific method have a one-dimensional structure, with a diameter of approximately 15 nm, a length of 50–200 nm, a good crystal structure, and certain pore characteristics (Fig. 7b and c). At 0.5C, the capacity loss of the silicon nanotube electrode was less than that of the Si-325 mesh electrode. Initially, the capacities of both decreased due to the formation of the solid electrolyte interface (SEI), irreversible lithium insertion/extraction, electrode pulverization, and defect structures in the silicon material, with the capacity decline of the Si-325 silicon mesh being more significant. After the 6th cycle, the performance of the silicon nanotubes became relatively stable, and the discharge capacity in the 10th cycle was approximately twice that of the Si-325 silicon mesh. In terms of rate performance, the capacity retention rate of silicon nanotubes at different rates was higher than that of the Si-325 mesh, and they still had a relatively high capacity after 90 cycles. Although their capacity was lower than that reported in some cases, possibly due to incomplete reduction of silica nanotubes, the nanotube structure and the amorphous matrix gave them advantages in alleviating volume changes, increasing electrolyte contact, and stabilizing the interface, making their performance superior to that of commercial silicon meshes and graphite electrodes. Park et al.79 prepared silicon nanotubes for use as anodes in lithium-ion batteries through carbon-coating deposition (Fig. 7d–f). In a half-cell, at a rate of 0.2C, the first-discharge capacity reached 3648 mA h g−1, and the charge capacity was 3247 mA h g−1, demonstrating a coulombic efficiency as high as 89%. When the charging rate was increased to 5C (i.e., 15 A g−1), the charge capacity could still reach 2878 mA h g−1. A full-cell was constructed with LiCoO2 as the cathode and silicon nanotubes as the anode, and the initial capacity was higher than 3000 mA h g−1 at both 3C and 5C rates. Notably, after 200 cycles at a 1C rate, the capacity retention rate was as high as 89%, showing significant advantages compared with commercial graphite batteries and indicating its high performance and stability in long-term cyclic use. The high capacity is attributed to the large active surface area provided by the porous SiNTs array structure, which is conducive to tolerating large volume expansions during the alloying/de-alloying reactions, thus promoting the storage of Li+ according to the pseudocapacitance mechanism.
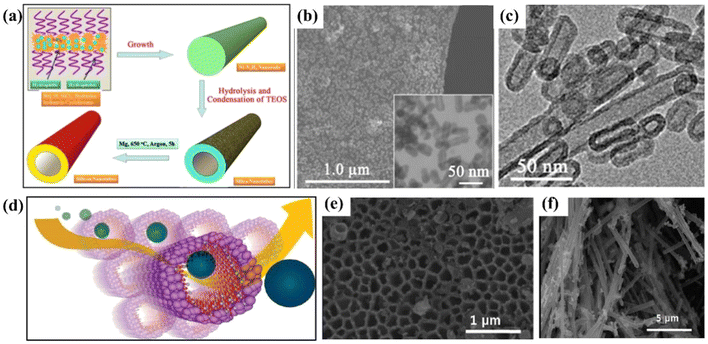 | ||
| Fig. 7 (a) Schematic diagram of the synthesis of silicon nanotubes. (b) SEM image and TEM image (inset) of silicon nanotubes. (c) SEM image of silicon nanotubes;78 (d) schematic diagram of the lithium-ion path in silicon nanotubes. (e) Top-view FE-SEM image of silicon nanotubes. (f) SEM image of cycled silicon nanotubes (silicon nanotubes extracted from a lithium-ion battery after 200 cycles).79 | ||
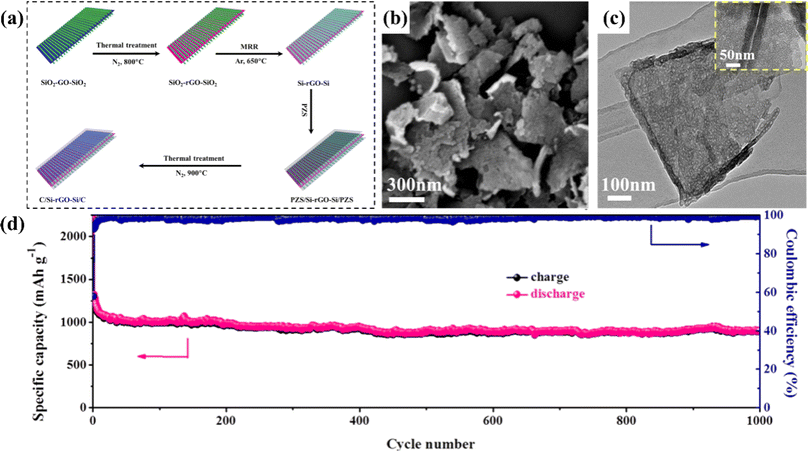 | ||
| Fig. 8 (a) For 2D sandwich-like C/Si-rGO-Si/C nanosheets: (a) schematic diagram of the manufacturing process (b) SEM (c) TEM (d) long-term cycling performance diagram of 1000 cycles at 1 A g−1.81 | ||
 | ||
| Fig. 9 (a) Schematic diagram of the synthesis of porous silicon, which involves two steps: high-energy mechanical milling of Si and P2O5, and hydrofluoric acid (HF) treatment. (b) SEM image of metallurgical silicon powder (∼98 wt%). (c) SEM image of nano-Si particles with P-dopants and SiO2 prepared after ball-milling. (d) SEM image of porous nano-Si particles with P-dopants after acid etching. (e) Cycling performance of porous nano-Si particles with P-dopants prepared from different weight ratios of Si to P2O5 at different rates ranging from 0.05C to 5C. (f) Cycling performance of the prepared carbon-coated porous nano-Si particles with P-dopants.88 | ||
5.2 Composite materials1
 | ||
| Fig. 10 (a) Schematic diagrams of the preparation of traditional yolk–shell Si@void@C and the novel core–shell yolk–shell Si@C@void@C. (b) TEM images of different Si and Si/C materials. (c) Cycling performance diagrams of Si, Si@C, Si@void@C, and Si@C@void@C at an electrode current of 0.5 A g−1.97 | ||
Although remarkable achievements have been made in the research and development of traditional carbon-coated silicon–carbon materials with core–shell structures, yolk–shell structures, etc., the exploration of more advanced silicon–carbon composites is still ongoing. New types of silicon–carbon materials are prepared through chemical vapor deposition (CVD). Resin-based or bio-based materials are used as porous carbon sources, and silanes are used as silicon sources. The deposition is carried out in two steps of adsorption and cracking inside the porous carbon. Finally, carbon-source gases such as acetylene and ethylene are introduced to uniformly coat a carbon layer on the surface of the materials. Sung et al.102 used ethylene to inhibit the growth of silicon crystals and synthesized sub-nanometer silicon anode materials embedded in a dual matrix of silicon carbide and amorphous carbon through chemical vapor deposition. This material shows excellent electrochemical performance. The C(5)Si-G electrode exhibits good performance at a rate of 0.1C, and the coulombic efficiency reaches 99.96% after 50 cycles. The 110 A h−1 full cell prepared with this material has good cycle stability (the capacity retention rate is 91% after 2875 cycles) and a long calendar life (97.6% after 365 days). This unique structure has excellent electrochemical performance. On the one hand, the carbon-based material provides a stable support framework for the sub-nanometer silicon, effectively buffering the volume changes of silicon during the lithiation and delithiation processes. At the same time, the formation of Si–C bonds slows down the growth of the silicon core and inhibits the unfavorable phase evolution, thus significantly improving the cycle stability. Despite the many advantages of CVD-deposited silicon–carbon materials, due to the limitations of technical barriers such as the selection of porous carbon, the cost of silanes, and the deposition process, large-scale and continuous production remains an industrialization challenge for enterprises producing new silicon–carbon materials. It will still take some time to improve the relevant equipment and optimize the process.
 | ||
| Fig. 11 Si/Cu NW: (a) schematic diagram of the pulsed-discharge method, and (b) preparation process of Si/Cu/Zn. (c) SEM image of Si/Cu/Zn ternary microspheres. (d) Rate performance. (e) Cycling performance.103 | ||
Metal oxides (such as titanium dioxide, iron oxide, etc.) can act as buffer layers to alleviate the volume expansion of silicon. They have certain structural stability and can accommodate part of the volume change of silicon. Some metal oxides can also react with the electrolyte to form a stable solid-electrolyte-interface film, reducing the decomposition of the electrolyte and side reactions, and improving the cycling performance of the battery. Lotfabad et al.105 coated the surface of silicon nanowires (SiNWs) with TiO2 by atomic layer deposition (ALD) to prepare a TiO2/SiNW nanocomposite as the anode of a lithium-ion battery. In this material, by changing the thickness of the TiO2 coating, it has different structures, which can improve the cycling stability and coulombic efficiency of the composite. At a rate of 0.1C, the capacity retention rate of (10)TiO2-200/SiNWs after 100 cycles reached 58%, which is higher than 30% of bare SiNWs. At 5C, (10)TiO2-200/SiNWs could maintain 34% of the initial capacity. This is attributed to the special structure formed by the TiO2 coating and its influence on the SEI layer, making the composite material perform excellently in terms of electrochemical performance.
Metal sulfides (such as molybdenum disulfide, tungsten disulfide, etc.) have a relatively high theoretical specific capacity. Their synergistic effect with silicon can increase the specific capacity of the composite material. The layered structure of some metal sulfides is conducive to the insertion and extraction of lithium ions, which can improve the rate performance of the electrode. At the same time, it can also buffer the volume change of silicon to a certain extent and enhance the cycling stability of the electrode.106 Molybdenum disulfide (MoS2) is a classic transition-metal sulfide. Its S–Mo–S interlayer structure is connected by weak van der Waals forces. Its graphite-like layered distribution allows for more Li-ion insertion and extraction. During the lithiation/delithiation process, the volume expansion rate of MoS2-based materials is only 103%. Moreover, the smaller the volume expansion rate, the better the cycling performance and lithium-storage capacity. Marriam et al.107 synthesized two-dimensional MoS2 nanosheets with a heterostructure on a water-soluble NaCl substrate. These nanosheets can not only adapt to the volume expansion of silicon particles but also possess excellent electrochemical properties. The long-term cycling stability of the heterostructured MoS2@Si electrode is much better than that of MoS2 and Si. In particular, in the 500th cycle, MoS2@Si exhibited excellent cycling stability. At a high current density of 500 mA g−1, its capacity retention rate was 60%, while that of MoS2 was 21% and that of pristine Si was 0.3%. Compared with pristine Si (about 430%), a significant reduction in volume expansion (68%) was observed in the MoS2@Si anode. The method for synthesizing two-dimensional MoS2 nanosheets and their application in the sandwich-like heterostructure add advantages to the application of MoS2 in energy storage. Kawade et al.108 successfully synthesized a flexible graphene-like layered MoS2-G structure as an ideal substrate for silicon nanoparticles. The MoS2-G nanoparticles sandwiched between the layers have sufficient space, which facilitates the transmission of lithium ions and also restricts the volume expansion during the lithiation and attenuation processes. The Si/MoS2 nanocomposite has a reversible discharge capacity of 1549 mA h g−1 in the voltage range of 0.01–3.0 V, which is higher than that of MoS2-G, and has better stability than pristine silicon. At the same time, the Si/MoS2 nanocomposite provided the highest and most stable reversible capacity of 923 mA h g−1 in 90 cycles at 200 mA g−1. More importantly, by integrating the advantages of nanostructure engineering and hybridization, the diffusion path length was shortened, better lithium-diffusion kinetics were observed, and ultimately, the reversible capacity and cycling performance were improved.
 | ||
| Fig. 12 (a) Schematic diagram of the dynamically stable interface by the molecular-zipper fixation strategy. (b) Enlarged TEM image of the dynamically interface-modified Si/MZ/CNT. (c and d) TEM images. Galvanic charge curves of Si/MZ/CNT, Si/CNT and Si/CB anodes at a current density of 1.0 A g−1 at (e) 25 °C and (f) 0 °C.109 | ||
5.3 Development of silicon-containing precursors
5.4 The prelithiation technology of silicon-based anode materials
Nanostructuring silicon materials or preparing porous materials can effectively increase their specific surface area, expand the contact interface with the electrolyte, enhance the reaction activity, shorten the lithium-ion transport distance, and facilitate lithium-ion diffusion. However, a large specific surface area will increase the interface for side reactions. In particular, it is prone to forming more solid-electrolyte-interface (SEI) films, causing problems such as low initial coulombic efficiency and poor cycling stability in practical applications. As shown in the Fig. 13a–d, prelithiation technology, as one of the effective ways to solve these problems, can compensate for the irreversible capacity loss by introducing lithium into the silicon-based anode material in advance, either before battery assembly or during the initial charging process. This improves the initial coulombic efficiency and cycling stability. There are mainly three methods of prelithiation technology for anodes: chemical prelithiation, electrochemical prelithiation, and mechanical prelithiation. | ||
| Fig. 13 Schematic diagram of the principle of pre-lithiation technology. (a) In the initial electrochemical cycles of the anode of a traditional lithium-ion battery with a low ICE, there is a permanent loss of active lithium. This leads to a net energy loss due to the insufficient utilization of the cathode capacity. (b) Achieve the maximum energy density of the full battery through direct contact prelithiation to obtain the ideal ICE. (c) Capture the irreversible lithium through electrochemical prelithiation, which promotes the formation of the SEI. (d) Conduct lithiation/delithiation through chemical prelithiation. Volume expansion and the loss of active lithium occur before the battery is assembled.120 | ||
5.5 Subsection
In the research of silicon-based anode materials, the four major strategies, namely nanostructuring, composite materials, development of silicon-containing precursors, and prelithiation technology, mainly target issues such as severe volume expansion, poor electrical conductivity, low initial coulombic efficiency, and an unstable solid–electrolyte interface of silicon during the charging and discharging processes. Table 1 shows different structures and types of silicon-based anode materials reported in the literature, classified by modification strategies. Nanostructuring alleviates volume expansion and enhances electrochemical performance by reducing the size of silicon materials and designing special structures. However, it has high preparation costs and complex processes. The composite material strategy involves combining silicon with carbon, metals/metal, oxides/metal sulfides, and conductive polymers, which significantly buffers volume expansion, enhances electrical conductivity, and improves stability. Nevertheless, it has problems such as high-temperature side reactions, reduced electrical conductivity, and high costs. The strategy of developing silicon-containing precursors utilizes natural silicon-containing minerals and organosilicon compounds. The former has a low cost, while the latter is beneficial for precisely controlling material properties. However, it faces challenges in improving the purity of natural minerals and controlling the cost of organosilicon compounds. Prelithiation technology can compensate for irreversible capacity loss and increase the initial coulombic efficiency. However, direct-contact prelithiation has issues such as high reactivity of the lithium source and uneven prelithiation; electrochemical prelithiation has a complex process and limited increase in lithium storage capacity in industrial applications; and some reagents used in chemical prelithiation pose safety risks. Future research needs to focus on solving the existing problems of these strategies, strengthen collaborative innovation among multiple strategies, comprehensively consider material performance, cost, safety, and preparation processes, and contribute to the development of energy storage technology.| Electrode | Modification strategies | Sample | ICE value (%); reversible capacity (mA h g−1) | Capacity retention (%) (cycles; rate) | Ref. |
|---|---|---|---|---|---|
| 0D Si | Nanostructuring | Si@SiO2 | 63; 1215.2 | 99.4 (50; 0.6 A g−1) | 127 |
| 0D Si | Si/C-CNFs | 53.4; 1020.7 | 51.2 (100; 0.2 A g−1) | 128 | |
| 1D Si | 30 nm UTSiNWs | —; 933.1 | 87.5 (50; 0.3 A g−1) | 129 | |
| 1D Si | SiNWs/G@C | 83.37; 608.5 | 90.04 (100; 0.5C) | 70 | |
| 1D Si | Sealed Si nanotubes | 90; 2169 | 81 (50; 2C) | 130 | |
| 1D Si | Si nanotubes | 89; 3247 | 89 (200; 1C) | 131 | |
| 2D Si | Si-NSs@rGO | 99.5; 1006.1 | 84.2 (1000; 2 A g−1) | 132 | |
| 2D Si | sC/Si-rGO-Si/C | —; 894 | 70.3 (1000; 1 A g−1) | 81 | |
| 3D Si | EPD-5s | 97; 1913 | 73 (100; 0.1C) | 133 | |
| 3D Si | pSi@NC | 71.4; 1300 | — (200; 1 A g−1) | 134 | |
| Si/C | Composite materials | R-Si/C | —; 501.4 | 90 (150; 0.5C) | 135 |
| Si/C | C@Si/C | 46.5; 1233 | 25.3 (100; 1 A g−1) | 136 | |
| Si/C | NPC/C@Si | —; 592 | 55 (450; 1C) | 137 | |
| Si/C | r-Si/4 + 2 | —; 1614 | 30 (300; 0.5C) | 138 | |
| CVD Si/C | Si-VACNF | 76; 1050 | 30.4 (100; C/1.5) | 139 | |
| CVD Si/C | SGC | 92; 517 | 96 (100; 0.5C) | 140 | |
| Si/O | (10)TiO2-200/SiNWs | —; 1600 | 58 (100; 0.1C) | 105 | |
| Si/Cu | Si/Cu NWs | 1456; 86.5 | 70 (500; 0.2 A g−1) | 103 | |
| Si/S | MoS2@Si | 92.3; – | 86 (100; 0.1C) | 107 | |
| SiOx | Prelithiation technology | PB-SiOx-60 | 93.5; 1300 | 97.7 (250; 1C) | 141 |
| SiOx | NCM622-SiOx | 89.2; 833 | 75.4 (300; 0.5C) | 123 |
6 Summary and prospects
Silicon-based anode materials for lithium-ion batteries have advantages such as high theoretical specific capacity, low lithium-insertion/extraction potential, and excellent fast-charging performance, which have attracted many researchers at home and abroad. An ideal silicon-based anode material should possess the following characteristics: (1) good structural stability: the ideal material can effectively buffer the volume expansion of silicon and prevent the electrode structure from being damaged due to volume effects. (2) Interface stability: a stable, uniform, and thin solid-electrolyte-interface (SEI) film can form on the surface of the ideal material. The stable SEI film can prevent further decomposition of the electrolyte and reduce the consumption of lithium ions. (3) High electrical conductivity: the ideal silicon-based anode material should have high electrical conductivity. By compounding with other conductive materials, an efficient electron-transfer channel can be constructed. (4) High initial coulombic efficiency: during the first charge–discharge process, the silicon-based anode material should minimize the irreversible consumption of lithium ions and improve the initial coulombic efficiency. (5) Good processability: the ideal silicon-based anode material should be easy to process into an electrode and be adaptable to the existing lithium-ion battery production process. (6) High safety performance: the silicon-based anode material should have good thermal and chemical stability. During the battery's charge–discharge process and under different usage environments (such as high temperature, over-charging, over-discharging, etc.), it should not experience thermal runaway, combustion, explosion, or other safety accidents.To promote the commercial application of silicon-based anodes, some progress has been made through years of research. However, problems such as volume expansion and interface failure still exist during the charge–discharge process. Improvement measures such as nanostructuring, composite materials, the development of silicon-containing precursors, and prelithiation technology have alleviated these problems to a certain extent and improved the material performance. In the future, further optimization of material structure and performance should be carried out, including in-depth research on the synergistic effects of nanostructures, strengthening research on the interfaces of composite materials, improving the conversion mechanism of silicon-containing precursors, and prelithiation technology. With technological progress and cost reduction, silicon-based anode materials are expected to be more widely used in fields such as electric vehicles, energy storage systems, and aerospace.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Zhenjun Zhang: writing – original draft, data curation, formal analysis. Yilong Wu: formal analysis, investigation, validation, writing – review & editing, supervision. ZuXue Mo: data curation, writing – review & editing, conceptualization. Xiaoxu Lei: visualization, validation, writing – review & editing. Xuerui Xie: supervision, writing – review & editing. Xiangyong Xue: supervision, writing – review & editing. Haiqing Qin: supervision, funding acquisition, writing – review & editing, project administration. Haowen Jiang: formal analysis, investigation, writing – review & editing, supervision, resources, methodology.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This study was financially supported by the Central Guidance on Local Science and Technology Development Fund of Guangxi Province, China (Grant No. ZY20220102), the Guangxi Key Laboratory Operation Subsidy Program (Grant No. 22-035-35), the Guilin City Innovation Platform and Talent Program (Grant No. 20220120-5), the China Nonferrous Metal Mining Group Project (Grant No. 2022KJZX04), and the China Nonferrous Metal Mining Group Youth Fund (Grant No. 2023KJZX016). We express our sincere gratitude for the support from these funding agencies.References
- M. Khan, S. Yan, M. Ali, F. Mahmood, Y. Zheng and G. Li, et al., Innovative Solutions for High-Performance Silicon Anodes in Lithium-Ion Batteries: Overcoming Challenges and Real-World Applications, Nano–Micro Lett., 2024, 16(9), 179 CAS.
- G. Wang, G. Wang, L. Fei, L. Zhao and H. Zhang, Structural Engineering of Anode Materials for Low-Temperature Lithium-Ion Batteries:Mechanisms,Strategies,and Prospects, Nano–Micro Lett., 2024, 16(8), 169–195 Search PubMed.
- D. Ji and J. Kim, Trend of Developing Aqueous Liquid and Gel Electrolytes for Sustainable,Safe,and High-Performance Li-Ion Batteries, Nano–Micro Lett., 2023, 16(1), 17–34 Search PubMed.
- M. Wang, L. Fan, M. Huang, J. Li and X. Qu, Conversion of diatomite to porous Si/C composites as promising anode materials for lithium-ion batteries, J. Power Sources, 2012, 219, 29–35 CrossRef CAS.
- F. Zhao, B. Tao, L. Yu, C. Pan, L. Ma and L. Wei, et al., Dynamic mechanical equilibrium of silicon anodes for lithium-ion batteries enabled by surface hydroxyl-rich bonding, Inorg. Chem. Front., 2024, 11(14), 4374–4386 RSC.
- Y. Pei, Y. Wang, A. Chang and Y. Liao, Nanofiber-in-microfiber carbon/silicon composite anode with high silicon content for lithium-ion batteries, Carbon, 2023, 203, 436–444 CrossRef CAS.
- B. Wang, J. Ryu, S. Choi, X. Zhang, D. Pribat and X. Li, et al., Ultrafast-Charging Silicon-Based Coral-Like Network Anodes for Lithium-Ion Batteries with High Energy and Power Densities, ACS Nano, 2019, 13(2), 2307–2315 CAS.
- X. Zuo, J. Zhu, P. Müller-Buschbaum and Y. Cheng, Silicon based lithium-ion battery anodes: A chronicle perspective review, Nano Energy, 2017, 31, 113–143 CrossRef CAS.
- R. J. Larkin, S. C. Willenberg and N. Ross, Silicon-Based Anodes towards Enhanced Cycling Efficiencies for Next-Generation Lithium-Ion Batteries, Int. J. Electrochem. Sci., 2023, 18(6), 100158 CrossRef CAS.
- H. Lee, M. Yanilmaz, O. Toprakci, K. Fu and X. Zhang, A review of recent developments in membrane separators for rechargeable lithium-ion batteries, Energy Environ. Sci., 2014, 7(12), 3857–3886 CAS.
- J. Xu, Y. Dou, Z. Wei, J. Ma, Y. Deng and Y. Li, et al., Recent Progress in Graphite Intercalation Compounds for Rechargeable Metal (Li, Na, K, Al)-Ion Batteries, Adv. Sci., 2017, 4(10), 1700146 Search PubMed.
- J. Wu, H. Qiu, J. Zhang, Z. Zhuang and X. Wang, Enhanced capacity of LiCoO2 and graphite battery by using methylene methanedisulfonate as electrolyte additive, J. Appl. Electrochem., 2024, 54(10), 2193–2203 CAS.
- N. Hamao, H. Itasaka, K. Mimura, Z. Liu and K. Hamamoto, Effect of Stirring on Particle Shape and Size in Hydrothermal Synthesis of LiCoO2, ACS Omega, 2024, 9(22), 23597–23602 CAS.
- D. Kim, P. Muralidharan, H. Lee, R. Ruffo, Y. Yang and C. Chan, et al., Spinel LiMn2O4 Nanorods as Lithium Ion Battery Cathodes, Nano Lett., 2008, 8(11), 3948–3952 CAS.
- W. Xu, B. Liu, S. Xia, L. Chen, R. Qi and Q. Li, et al., Surface reconstructed layer with bulk high-valence Mo doping to achieve long-life LiMn2O4 cathode material, Electrochim. Acta, 2024, 500, 144706 CAS.
- G. Li, Z. Zhang, Z. Huang, C. Yang, Z. Zuo and H. Zhou, Understanding the accumulated cycle capacity fade caused by the secondary particle fracture of LiNi1−x−yCoxMnyO2 cathode for lithium ion batteries, J. Solid State Electrochem., 2017, 21, 673–682 CAS.
- X. Ouyang, Z. Chen and X. Zheng, Determination of sodium in ternary cathode material (LiNi1-x-yCoxMnyO2) for lithium ion battery by inductively coupled plasma atomic emission spectrometry, Metall. Anal., 2014, 34(11), 42–45 CAS.
- M. P. Q. Argaaraz, K. Jori, J. M. Ramallo-López, A. Visintin, F. G. Requejo and M. G. Ortiz, Enhancement of LiFePO4 cathodic material through incorporation of reduced graphene oxide via a simple two-step procedure, J. Phys. Chem. Solids, 2025, 196, 112353 Search PubMed.
- J. Duan, Y. Zheng, W. Luo, W. Wu, T. Wang and Y. Xie, et al., Is graphite lithiophobic or lithiophilic?, Natl. Sci. Rev., 2020, 7(7), 1208–1217 CAS.
- B. Gangaja, S. V. Nair and D. Santhanagopalan, Reuse, Recycle, and Regeneration of LiFePO4 Cathode from Spent Lithium-Ion Batteries for Rechargeable Lithium- and Sodium-Ion Batteries, ACS Sustain. Chem. Eng., 2021, 9(13), 4711–4721 CAS.
- X. Lu, L. Zhao, X. He, R. Xiao, L. Gu and Y. S. Hu, et al., Lithium Storage in Li4Ti5O12 Spinel: The Full Static Picture from Electron Microscopy, Adv. Mater., 2012, 24(24), 3233–3238 CAS.
- S. Qiu, W. N. Yan, Li Wang, L. S. Zhang, C. Chen and Li. J. Mu, et al., Facile Synthesis of Si@LiAlO2 Nanocomposites as Anode for Lithium-Ion Battery, Chin. J. Inorg. Chem., 2022, 38(8), 1655–1662 CAS.
- Y. Lu, L. Yu and X. W. Lou, Nanostructured Conversion-type Anode Materials for Advanced Lithium-Ion Batteries, Chem, 2018, 4(5), 972–996 CAS.
- T. Wang, Z. Wang, H. Li, L. Cheng, Y. Wu and X. Liu, et al., Recent status, key strategies, and challenging prospects for fast charging silicon-based anodes for lithium-ion batteries, Carbon, 2024, 230(11), 119615 CrossRef CAS.
- G. N. Lewis and F. G. Keyes, The potential of the lithium electrode, J. Am. Chem. Soc., 1913, 35(4), 340–344 CrossRef CAS.
- C. Dong, W. Dong, X. Lin, Y. Zhao, R. Li and F. Huang, Recent progress and perspectives of defective oxide anode materials for advanced lithium ion battery, EnergyChem, 2020, 2(6), 100045 Search PubMed.
- L. Y. Beaulieu, K. W. Eberman, R. L. Turner, L. J. Krause and J. R. Dahn, Colossal Reversible Volume Changes in Lithium Alloys, Electrochem. Solid-State Lett., 2001, 4(9), A137 CAS.
- J. Wang, B. Ge, H. Li, M. Yang and Q. Peng, Challenges and Progresses of Lithium-Metal Batteries, Chem. Eng. J., 2021, 420(8), 129739 CAS.
- L. Porcarelli, A. S. Shaplov, F. Bella, J. R. Nair, D. Mecerreyes and C. Gerbaldi, Single-Ion Conducting Polymer Electrolytes for Lithium Metal Polymer Batteries that Operate at Ambient Temperature, ACS Energy Lett., 2016, 1(4), 678–682 CAS.
- D. Zhou, D. Shanmukaraj, A. Tkacheva, M. Armand and G. Wang, Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects, Chem, 2019, 5(9), 2326–2352 CAS.
- R. Wang, L. Wang, R. Liu, X. Li, Y. Wu and F. Ran, “Fast-Charging” Anode Materials for Lithium-Ion Batteries from Perspective of Ion Diffusion in Crystal Structure, ACS Nano, 2024, 18(4), 2611–2648 CAS.
- L. Zhang, X. Wu, W. Qian, K. Pan, X. Zhang and L. Li, et al., Exploring More Functions in Binders for Lithium Batteries, Electrochem. Energy Rev., 2023, 6(1), 36 CAS.
- Y. Zhang, S. Yang, Y. Xin, B. Cai, P. Hu and H. Dai, et al., Designing Symmetric Gradient Honeycomb Structures with Carbon-Coated Iron-Based Composites for High-Efficiency Microwave Absorption, Nano–Micro Lett., 2024, 16(1), 234 Search PubMed.
- W. Yan, Z. Mu, Z. Wang, Y. Huang, D. Wu and P. Lu, et al., Hard-carbon-stabilized Li-Si anodes for high-performance all-solid-state Li-ion batteries, Nat. Energy, 2023, 8(8), 800–813 CAS.
- D. Deng, M. G. Kim, J. Y. Lee and J. Cho, Green energy storage materials: Nanostructured TiO2 and Sn-based anodes for lithium-ion batteries, Energy Environ. Sci., 2009, 2(8), 818–837 CAS.
- E. M. H. White, L. M. Rueschhoff, S. W. Martin and I. E. Anderson, Novel One-Step Production of Carbon-Coated Sn Nanoparticles for High-Capacity Anodes in Lithium-Ion Batteries, Batteries, 2024, 10(11), 386 CAS.
- G. Derrien, J. Hassoun, S. Panero and B. Scrosati, Nanostructured Sn-C Composite as an Advanced Anode Material in High-Performance Lithium-Ion Batteries, Adv. Mater., 2010, 19(17), 2336–2340 Search PubMed.
- J. Liu, W. Li and A. Manthiram, Dense core-shell structured SnO2/C composites as high performance anodes for lithium ion batteries, Chem. Commun., 2010, 46(9), 1437–1439 CAS.
- M. S. Park, G. Wang, Y. Kang, D. Wexler and H. Liu, Preparation and electrochemical properties of SnO2 nanowires for application in lithium-ion batteries, Angew. Chem., Int. Ed., 2010, 46(5), 750–753 Search PubMed.
- H. U. Renzong, H. Liu, M. Zeng, J. Liu and M. Zhu, Progress on Sn-based thin-film anode materials for lithium-ion batteries, Chin. Sci. Bull., 2012, 57(32), 4119–4130 Search PubMed.
- W. Hui and C. Yi, Designing nanostructured Si anodes for high energy lithium ion batteries, Nano today, 2012, 7(5), 414–429 CrossRef.
- H. Chen, Z. Wu, Z. Su, S. Chen, C. Yan and M. Al-Mamun, et al., A mechanically robust self-healing binder for silicon anode in lithium ion batteries, Nano Energy, 2021, 81(1), 105654 CrossRef CAS.
- W. Zhao, J. Yi, P. He and H. Zhou, Solid-State Electrolytes for Lithium-Ion Batteries: Fundamentals, Challenges and Perspectives, Electrochem. Energy Rev., 2019, 2(4), 574–605 CrossRef CAS.
- J. Deng, H. Ji, C. Yan, J. Zhang, W. Si and S. Baunack, et al., Naturally Rolled-Up C/Si/C Trilayer Nanomembranes as Stable Anodes for Lithium-Ion Batteries with Remarkable Cycling Performance, Angew. Chem., Int. Ed., 2013, 52(8), 2326–2330 CrossRef CAS PubMed.
- M. W. Verbrugge, D. R. Baker, X. Xiao, Q. Zhang and Y. Cheng, Experimental and Theoretical Characterization of Electrode Materials that Undergo Large Volume Changes and Application to the Lithium-Silicon System, J. Phys. Chem. C, 2015, 119(10), 5341–5349 CrossRef CAS.
- W. Li, Y. Tang, W. Kang, Z. Zhang, X. Yang and Y. Zhu, et al., Core-Shell Si/C Nanospheres Embedded in Bubble Sheet-like Carbon Film with Enhanced Performance as Lithium Ion Battery Anodes, Mater. Lett., 2015, 199(15), 139–142 Search PubMed.
- W. Yu, C. Liu, P. Hou, L. Zhang, X. Shan and F. Li, et al., Lithiation of Silicon Nanoparticles Confined in Carbon Nanotubes, ACS Nano, 2015, 9(5), 5063–5071 CAS.
- J. Huang, X. Liu, Y. Liu, A. Kushima, J. Li and T. Zhu, In-Situ TEM Experiments of Electrochemical Lithiation and Delithiation of Individual Nanostructures, Adv. Energy Mater., 2012, 2(7), 722–741 Search PubMed.
- F. Dou, L. Shi, G. Chen and D. Zhang, Silicon/Carbon Composite Anode Materials for Lithium-Ion Batteries, Electrochem. Energy Rev., 2019, 2(1), 149–198 CAS.
- H. Kim, M. Seo, M.-H. Park and J. Cho, A Critical Size of Silicon Nano-Anodes for Lithium Rechargeable Batteries, Angew. Chem., Int. Ed., 2010, 49(12), 2146–2149 CAS.
- J. Graetz, C. C. Ahn, R. Yazami and B. Fultz, Highly Reversible Lithium Storage in Nanostructured Silicon, Electrochem. Solid-State Lett., 2003, 6(9), A194–A197 CAS.
- Z. Chen, A. Soltani, Y. Chen, Q. Zhang, A. Davoodi and S. Hosseinpour, et al., Emerging Organic Surface Chemistry for Si Anodes in Lithium-Ion Batteries: Advances, Prospects, and Beyond, Adv. Energy Mater., 2022, 12(32), 2200924 CrossRef CAS.
- Y. Oumellal, N. Delpuech, D. Mazouzi, N. Dupré, J. Gaubicher and P. Moreau, et al., The failure mechanism of nano-sized Si-based negative electrodes for lithium ion batteries, J. Mater. Chem., 2011, 21(17), 6201–6208 RSC.
- C. K. Chan, R. Ruffo, S. S. Hong and Y. Cui, Surface chemistry and morphology of the solid electrolyte interphase on silicon nanowire lithium-ion battery anodes, J. Power Sources, 2009, 189(2), 1132–1140 CrossRef CAS.
- I. A. Shkrob, J. F. Wishart and D. P. Abraham, What Makes Fluoroethylene Carbonate Different?, J. Phys. Chem. C, 2015, 119(27), 14954 CrossRef CAS.
- G. Hou, B. Cheng, Y. Cao, M. Yao, B. Li and C. Zhang, et al., Scalable production of 3D plum-pudding-like Si/C spheres: Towards practical application in Li-ion batteries, Nano Energy, 2016, 24, 111–120 CrossRef CAS.
- S. Goriparti, E. Miele, F. D. angelis, E. M. D. Fabrizio, R. P. Zaccaria and C. Capiglia, Review on Recent Progress of Nanostructured Anode Materials for Li-Ion Batteries, J. Power Sources, 2022, 257(1), 421–443 Search PubMed.
- B. Liang, Y. Liu and Y. Xu, Silicon-based materials as high capacity anodes for next generation lithium ion batteries, J. Power Sources, 2014, 267(1), 469–490 CrossRef CAS.
- M. Ashuri, Q. He and L. L. Shaw, Silicon as a potential anode material for Li-ion batteries: where size, geometry and structure matter, Nanoscale, 2015, 8(1), 74–103 RSC.
- H. Liu, Z. Guo, J. Wang and K. Konstantinov, Si-based anode materials for lithium rechargeable batteries, J. Mater. Chem., 2010, 20(45), 10055–10057 RSC.
- F. Luo, B. Liu, J. Zheng and G. Chu, Review-Nano-Silicon/Carbon Composite Anode Materials Towards Practical Application for Next Generation Li-Ion Batteries, J. Electrochem. Soc., 2015, 162(14), A2509–A2528 CrossRef CAS.
- S. P. V. Nadimpalli, V. A. Sethuraman, S. Dalavi, B. Lucht, M. J. Chon and V. B. Shenoy, et al., Quantifying Capacity Loss due to Solid-Electrolyte-Interphase Layer Formation on Silicon Negative Electrodes in Lithium-ion Batteries, J. Power Sources, 2012, 215(5), 145–151 CrossRef CAS.
- L. Wei, C. Chen, Z. Hou and H. Wei, Poly (acrylic acid sodium) grafted carboxymethyl cellulose as a high performance polymer binder for silicon anode in lithium ion batteries, Sci. Rep., 2016, 6, 19583 CrossRef CAS PubMed.
- M. Liu, C. Ye, L. Peng and J. Weng, Influence of Binder on Impedance of Lithium Batteries: A Mini-review, J. Electr. Eng. Technol., 2022, 17(2), 1281–1291 CrossRef.
- B. I. Yoo, H. M. Kim, M. J. Choi and J. K. Yoo, Synergetic Effect of Hybrid Conductive Additives for High-Capacity and Excellent Cyclability in Si Anodes, Nanomaterials, 2022, 12(19), 3354 CrossRef CAS PubMed.
- X. Liu, L. Zhong, S. Huang, S. X. Mao, T. Zhu and J. Y. Huang, Size-Dependent Fracture of Silicon Nanoparticles During Lithiation, ACS Nano, 2012, 6(2), 1522–1531 CrossRef CAS PubMed.
- J. M. Park, J. H. Cho, J. H. Ha, H. S. Kim, S. W. Kim and J. Lee, et al., Reversible crystalline-amorphous phase transformation in Si nanosheets with lithi-/delithiation, Nanotechnology, 2017, 28(25), 255401 CrossRef PubMed.
- S. W. Lee, M. T. McDowell, L. A. Berla, W. D. Nix and Y. Cui, Fracture of crystalline silicon nanopillars during electrochemical lithium insertion, Proc. Natl. Acad. Sci. U.S.A., 2012, 109(11), 4080–4085 CrossRef CAS PubMed.
- C. Li, C. Yuan, J. Zhu, X. Ni, K. Li and L. Wang, et al., Fabrication of silicon nanoparticles/porous carbon@porous carbon nanofibers core-shell structured composites as high-performance anodes for lithium-ion batteries, Colloids Surf., A, 2022, 655, 129721 CrossRef CAS.
- Z. Yu, S. Fang, N. Wang, B. Shi, Y. Hu and Z. Shi, et al., In-situ growth of silicon nanowires on graphite by molten salt electrolysis for high performance lithium-ion batteries, Mater. Lett., 2020, 273, 127946 CrossRef CAS.
- F. Sun, Z. Tan, Z. Hu, J. Chen and R. Zheng, Ultrathin Silicon Nanowires Produced by a Bi-Metal-Assisted Chemical Etching Method for Highly Stable Lithium-Ion Battery Anodes, Nano-Brief Rep. Rev., 2020, 15, 2050076 CAS.
- C. Keller, A. Desrues, S. Karuppiah, E. Martin, J. P. Alper and F. Boismain, et al., Effect of Size and Shape on Electrochemical Performance of Nano-Silicon-Based Lithium Battery, Nanomaterials, 2021, 11(2), 307 CrossRef CAS PubMed.
- C. Hwang, T. H. Kim, Y. G. Cho, J. Kim and H. K. Song, All-in-one assembly based on 3D-intertangled and cross-jointed architectures of Si/Cu 1D-nanowires for lithium ion batteries, Sci. Rep., 2015, 5, 8623 CrossRef PubMed.
- T. Song, J. Xia, J. H. Lee, D. H. Lee, M. S. Kwon and J. M. Choi, et al., Arrays of Sealed Silicon Nanotubes As Anodes for Lithium Ion Batteries, Nano Lett., 2010, 10(5), 1710–1716 CrossRef CAS PubMed.
- M. R. Zamfir, H. T. Nguyen, E. Moyen, Y. H. Lee and D. Pribat, Silicon nanowires for Li-based battery anodes: a review, J. Mater. Chem. A, 2013, 1(34), 9566–9586 RSC.
- N. S. Choi, Y. Yao, Y. Cui and J. Cho, One dimensional Si/Sn-based nanowires and nanotubes for lithium-ion energy storage materials, J. Mater. Chem., 2011, 21(27), 9825 RSC.
- P. P. Prosini, C. Cento, A. Rufoloni, F. Rondino and A. Santoni, A lithium-ion battery based on LiFePO4 and silicon nanowires, Solid State Ionics, 2015, 269, 93–97 CrossRef CAS.
- Z. Wen, G. Lu, S. Mao, H. Kim, S. Cui and K. Yu, et al., Silicon nanotube anode for lithium-ion batteries, Electrochem. Commun., 2013, 29, 67–70 CrossRef CAS.
- M. H. Park, M. G. Kim, J. Joo, K. Kim, J. Kim and S. Ahn, et al., Silicon Nanotube Battery Anodes, Nano Lett., 2009, 9(11), 3844–3847 CrossRef CAS PubMed.
- T. Song, L. Hu and U. Paik, One-Dimensional Silicon Nanostructures for Li Ion Batteries, J. Phys. Chem. Lett., 2014, 5(4), 720–731 CAS.
- W. Yao, J. Chen, L. Zhan, Y. Wang and S. Yang, Two-dimensional Porous Sandwich-like C/Si-Graphene-Si/C Nanosheets for Superior Lithium Storage, ACS Appl. Mater. Interfaces, 2017, 9(45), 39371–39379 CrossRef CAS PubMed.
- B. Shi, Y. Zou, G. Xu, C. Tu, C. Jin and F. Sun, et al., Preparation of pure hydrogenated amorphous silicon film via PECVD method as anode materials for high-performance lithium-ion batteries, Solid State Ionics, 2023, 402, 116366 CAS.
- J. P. Maranchi, A. F. Hepp, A. G. Evans, N. T. Nuhfer and P. N. Kumta, Interfacial Properties of the a-Si/Cu:Active–Inactive Thin-Film Anode System for Lithium-Ion Batteries, J. Electrochem. Soc., 2006, 153(6), A1246 CAS.
- Q. Wu, B. Shi, J. Bareño, Y. Liu, V. Maroni and D. Zhai, et al., Investigations of Si Thin Films as Anode of Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2018, 10(4), 3487–3494 CAS.
- N. Bensalah, F. Z. Kamand, M. Zaghou, H. D. Dawoud and T. A. Tahtamouni, Silicon nanofilms as anode materials for flexible lithium ion batteries, Thin Solid Films, 2019, 690(30), 137516 CAS.
- S. Suresh, Z. Wu, S. F. Bartolucci, S. Basu, R. Mukherjee and T. Gupta, et al., Protecting Silicon Film Anodes in Lithium-Ion Batteries Using an Atomically Thin Graphene Drape, ACS Nano, 2017, 11(5), 5051–5061 CAS.
- Y. Ren, L. Xiang, X. Yin, R. Xiao, P. Zuo and Y. Gao, et al., Ultrathin Si Nanosheets Dispersed in Graphene Matrix Enable Stable Interface and High Rate Capability of Anode for Lithium-ion Batteries, Adv. Funct. Mater., 2022, 32(16), 2110046 CAS.
- G. Lv, B. Zhu, X. Li, C. Chen, J. Li and Y. Jin, et al., Simultaneous Perforation and Doping of Si Nanoparticles for Lithium-Ion Battery Anode, ACS Appl. Mater. Interfaces, 2017, 9(51), 44452–44457 CrossRef CAS PubMed.
- T. Yanagishita, M. Imaizumi, K. Nishio and H. Masuda, Fabrication of Porous Si Particles by Electrochemical Etching, ECS Solid State Lett., 2013, 2(12), P117–P119 CrossRef CAS.
- O. Kuntyi, G. Zozulya and M. Shepida, Porous Silicon Formation by Electrochemical Etching, Adv. Mater. Sci. Eng., 2022, 2022(4), 1–15 Search PubMed.
- A. Santos and T. Kumeria, Electrochemical Etching Methods for Producing Porous Silicon, Electrochem. Eng. Nanoporous Mater., 2015, 198, 110695 Search PubMed.
- M. Deguchi, K. Shinohara, H. Kobayashi, K. Kuratani, H. Takahara and H. Shiomi, et al., Preparation of porous silicon using magnesiothermic reduction of porous silica glass and electrode characteristics for lithium-ion batteries, J. Mater. Res., 2024, 39(12), 1758–1769 CrossRef CAS.
- X. Ma, W. Fei, X. Zhang, J. Ji and X. Zhou, Preparation of Mesoporous Si Nanoparticles by Magnesiothermic Reduction for the Enhanced Reactivity, Molecules, 2023, 28(7), 3274 CrossRef CAS PubMed.
- B. Wang, W. Li, T. Wu, J. Guo and Z. Wen, Self-template construction of mesoporous silicon submicrocube anode for advanced lithium ion batteries, Energy Storage Mater., 2018, 15, 139–147 CrossRef.
- A. T. Tesfaye, R. Gonzalez, J. L. Coffer and T. Djenizian, Porous Silicon Nanotube Arrays as Anode Material for Li-Ion Batteries, ACS Appl. Mater. Interfaces, 2015, 7(37), 20495–20498 CrossRef CAS PubMed.
- Y. Yao, M. T. Mcdowell, I. Ryu, H. Wu, N. Liu and L. Hu, et al., Interconnected Silicon Hollow Nanospheres for Lithium-Ion Battery Anodes with Long Cycle Life, Nano Lett., 2011, 11(7), 2949–2954 CrossRef CAS PubMed.
- J. Xie, L. Tong, L. Su, Y. Xu, L. Wang and Y. Wang, Core-shell yolk-shell Si@C@Void@C nanohybrids as advanced lithium ion battery anodes with good electronic conductivity and corrosion resistance, J. Power Sources, 2017, 342(28), 529–536 CrossRef CAS.
- Y. Mei, Y. He, H. Zhu, Z. Ma, Y. Pu and Z. Chen, et al., Recent Advances in the Structural Design of Silicon/Carbon Anodes for Lithium Ion Batteries: A Review, Coatings, 2023, 13(2), 436 CrossRef CAS.
- S. Zhu, Q. Sun, C. Ma, Z. Yu, Y. Zhang and J. Wang, et al., Graphene-Encapsulated Si@C with Dual Carbon Layer Structure as High-Performance Anode Materials for Lithium-Ion Batteries, ChemNanoMat, 2024, 10(4), e202300518 CAS.
- C. Du, M. Chen, L. Wang and G. Yin, Covalently-functionalizing synthesis of Si@C core–shell nanocomposites as high-capacity anode materials for lithium-ion batteries, J. Mater. Chem., 2011, 21, 15692–15697 CAS.
- Q. Xu, J. Y. Li, J. K. Sun, Y. X. Yin, L. J. Wan and Y. G. Guo, Watermelon-Inspired Si/C Microspheres with Hierarchical Buffer Structures for Densely Compacted Lithium-Ion Battery Anodes, Adv. Energy Mater., 2017, 7(3), 1601481 CrossRef.
- J. Sung, N. Kim, J. Ma, J. H. Lee, S. H. Joo and T. Lee, et al., Subnano-sized silicon anode via crystal growth inhibition mechanism and its application in a prototype battery pack, Nat. Energy, 2021, 6(12), 1164–1175 Search PubMed.
- H. Juan, C. Kun, G. Xu, M. Stapelberg, K. Yuan and P. Sun, et al., Novel silicon/copper nanowires as high-performance anodes for lithium ion batteries, J. Alloys Compd., 2021, 875, 159927 Search PubMed.
- H. Zeng, Y. He and M. Chamas, Si/Cu composite as anode material for lithium-ion batteries, Front. Energy Res., 2022, 10, 968259 Search PubMed.
- E. M. Lotfabad, P. Kalisvaart, K. Cui, A. Kohandehghan and D. Mitlin, ALD TiO2 coated silicon nanowires for lithium ion battery anodes with enhanced cycling stability and coulombic efficiency, Phys. Chem. Chem. Phys., 2013, 15(32), 13646–13657 Search PubMed.
- P. Zhang, Q. Ru, H. Yan, X. Hou, F. Chen and S.-j. Hu, et al., Hierarchically 3D structured milled lamellar MoS2/nano-silicon@carbon hybrid with medium capacity and long cycling sustainability as anodes for lithium-ion batteries, J. Mater. Sci. Technol., 2019, 35(9), 1840–1850 CAS.
- I. Marriam, M. Tebyetekerwa, H. A. Memon, H. Chathuranga, J. Yang and K. Sun, et al., 1D Textile Yarn Battery with MoS2@Si Anode and NCM Cathode, Adv. Mater. Technol., 2024, 10(3), 2400753 Search PubMed.
- U. V. Kawade, A. A. Ambalkar, R. P. Panmand, R. S. Kalubarme, S. R. Kadam and S. D. Naik, et al., Silicon nanoparticle-sandwiched ultrathin MoS2-graphene layers as an anode material for Li-ion batteries, Mater. Chem. Front., 2019, 3, 587–596 CAS.
- Y. Su, Y. Lv, M. R. Habibipour, K. Liu, A. Esfandiar and Z. Wang, et al., Dynamic stable interface between CNT and nanosilicon for robust anode with large capacity and high rate performance, Energy Storage Mater., 2023, 61, 102892 Search PubMed.
- H. Chen, M. Zheng, Y. Chen, Y. Xu and H. Zhang, PANI-based conductive polymer composites as water-soluble binders for nano-silicon anodes in lithium-ion batteries, Ionics, 2021, 27(2), 587–597 CAS.
- G. Liu, S. Xun, N. Vukmirović, X. Song, P. Olalde-Velasco and H. Zheng, et al., Polymers with Tailored Electronic Structure for High Capacity Lithium Battery Electrodes, Adv. Mater., 2011, 23(40), 4679–4683 CAS.
- X. Fan, Z. Wang, T. T. Cai, Y. Yang, H. Wu and S. Cao, et al., An integrated highly stable anode enabled by carbon nanotube-reinforced all-carbon binder for enhanced performance in lithium-ion battery, Carbon, 2021, 182, 749–757 CAS.
- Y. Xiao, X. Hao, T. Li, Y. Mao, T. Zhu and J. Zang, et al., Multifunctional Cross-Linking Composite Binder Enables the Stable Performance of Si-Based Anodes for High-Energy-Density Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2024, 16(31), 41036–41047 CAS.
- M.-Y. Park, C.-G. Kim and J.-H. Kim, CNT-Coated Quartz Woven Fabric Electrodes for Robust Lithium-ion Structural Batteries, Appl. Sci., 2020, 10(23), 8622 CAS.
- A. M. Leonova, O. A. Bashirov, N. M. Leonova, A. S. Lebedev, A. A. Trofimov and A. V. Suzdaltsev, Synthesis of C/SiC Mixtures for Composite Anodes of Lithium-Ion Power Sources, Appl. Sci., 2023, 13(2), 901 CAS.
- Y. Chen, B. Jiang, Y. Zhao, H. Liu and T. Ma, Diatomite and Glucose Bioresources Jointly Synthesizing Anode/Cathode Materials for Lithium-Ion Batteries, Coatings, 2023, 13(1), 146 CAS.
- X. Tang, G. Wen and Y. Song, Novel scalable synthesis of porous silicon/carbon composite as anode material for superior lithium-ion batteries, J. Alloys Compd., 2017, 739, 510–517 CrossRef.
- Y. Charles-Blin, H. Todoki, N. Zettsu and K. Teshima, Molecular gate effects observed in fluoroalkylsilane self-assembled monolayers grafted on LiNi0.5Mn1.5O4 cathodes: an application to efficient ion-exchange reactions, Mater. Adv., 2021, 2(16), 5406–5414 RSC.
- H. Wang, X. Zhang, Y. Li and L. Xu, Siloxane-based Organosilicon Materials in Electrochemical Energy Storage Devices, Angew. Chem., 2022, 61(49), e202210851 CrossRef CAS PubMed.
- C. Zhang, F. Wang, J. Han, S. Bai, J. Tan and J. Liu, et al., Challenges and Recent Progress on Silicon-Based Anode Materials for Next-Generation Lithium-Ion Batteries, Small Struct., 2021, 2(6), 2100009 CAS.
- R. Nölle, K. Beltrop, F. Holtstiege, J. Kasnatscheew, T. Placke and M. Winter, A reality check and tutorial on electrochemical characterization of battery cell materials: How to choose the appropriate cell setup, Mater. Today, 2020, 32, 131–146 CrossRef.
- G. M. Overhoff, R. Nölle, V. Siozios, M. Winter and T. Placke, A Thorough Analysis of Two Different Pre-Lithiation Techniques for Silicon/Carbon Negative Electrodes in Lithium Ion Batteries, Batteries Supercaps, 2021, 4(7), 1163–1174 CrossRef CAS.
- Q. Meng, G. Li, p. Jun, Q. Xu, Y. Yin and Y. Guo, High-Performance Lithiated SiOx Anode Obtained by a Controllable and Efficient Prelithiation Strategy, ACS Appl. Mater. Interfaces, 2019, 11(35), 32062–32068 CrossRef CAS PubMed.
- T. Watanabe, T. Tsuda, N. Ando, S. Nakamura and F. Matsumoto, An improved pre-lithiation of graphite anodes using through-holed cathode and anode electrodes in a laminated lithium ion battery, Electrochim. Acta, 2019, 324(3), 134848 CrossRef CAS.
- A. Jamaluddin, B. Umesh, K.-H. Tseng, C.-W. Huang, F. Chen and J.-K. Chang, et al., Control of Graphene Heteroatoms in a Microball Si@Graphene Composite Anode for High-Energy-Density Lithium-Ion Full Cells, ACS Sustainable Chem. Eng., 2020, 8(51), 18936–18946 CrossRef CAS.
- Y. Shen, J. Zhang, Y. Pu, H. Wang, B. Wang and J. Qian, et al., Effective Chemical Prelithiation Strategy for Building a Silicon/Sulfur Li-Ion Battery, ACS Energy Lett., 2019, 4, 1717–1724 CrossRef CAS.
- Y. Chen, Y. Hu, Z. Shen, R. Chen, X. He and X. Zhang, et al., Hollow core–shell structured silicon@carbon nanoparticles embed in carbon nanofibers as binder-free anodes for lithium-ion batteries, J. Power Sources, 2017, 342, 467–475 CrossRef CAS.
- Y. Chen, Y. Hu, J. Shao, Z. Shen, R. Chen and X. Zhang, et al., Pyrolytic carbon-coated silicon/carbon nanofiber composite anodes for high-performance lithium-ion batteries, J. Power Sources, 2015, 298, 130–137 CrossRef CAS.
- F. Sun, Z. Tan, Z. Hu, J. Chen, J. Luo and X. Wu, et al., Ultrathin Silicon Nanowires Produced by a Bi-Metal-Assisted Chemical Etching Method for Highly Stable Lithium-Ion Battery Anodes, Nano, 2020, 15(06), 2050076 CrossRef CAS.
- J. Ha and U. Paik, Hydrogen treated, cap-opened Si nanotubes array anode for high power lithium ion battery, J. Power Sources, 2013, 244, 463–468 CrossRef CAS.
- M.-H. Park, M. G. Kim, J. Joo, K. Kim, J. Kim and S. Ahn, et al., Silicon Nanotube Battery Anodes, Nano Lett., 2009, 9(11), 3844–3847 CAS.
- Y. Ren, L. Xiang, X. Yin, R. Xiao, P. Zuo and Y. Gao, et al., Ultrathin Si Nanosheets Dispersed in Graphene Matrix Enable Stable Interface and High Rate Capability of Anode for Lithium-ion Batteries, Adv. Funct. Mater., 2022, 32(16), 2110046 CrossRef CAS.
- Y. Yang, D. Chen, B. Liu and J. Zhao, Binder-Free Si Nanoparticle Electrode with 3D Porous Structure Prepared by Electrophoretic Deposition for Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2015, 7(14), 7497–7504 CAS.
- F. Wang, L. Sun, W. Zi, B. Zhao and H. Du, Solution Synthesis of Porous Silicon Particles as an Anode Material for Lithium Ion Batteries, Chemistry, 2019, 25(38), 9071–9077 CAS.
- Q. Ma, Y. Dai, H. Wang, G. Ma, H. Guo and X. Zeng, et al., Directly conversion the biomass-waste to Si/C composite anode materials for advanced lithium ion batteries, Chin. Chem. Lett., 2021, 32(1), 5–8 CAS.
- D. Liang, X. Zhang, J. Qi, L. Jiang, L. Yue and L. Zhang, et al., Mesoporous silicon–carbon composite anodes for lithium-ion battery prepared with the P123-templated SBA-15, J. Energy Storage, 2025, 113, 115654 Search PubMed.
- J. Xie, L. Tong, L. Su, Y. Xu, L. Wang and Y. Wang, Core–shell yolk-shell Si@C@Void@C nanohybrids as advanced lithium ion battery anodes with good electronic conductivity and corrosion resistance, J. Power Sources, 2017, 342, 529–536 CAS.
- C.-Z. Ke, F. Liu, Z.-M. Zheng, H.-H. Zhang, M.-T. Cai and M. Li, et al., Boosting lithium storage performance of Si nanoparticles via thin carbon and nitrogen/phosphorus co-doped two-dimensional carbon sheet dual encapsulation, Rare Met., 2021, 40(6), 1347–1356 CAS.
- G. P. Pandey, S. A. Klankowski, Y. Li, X. S. Sun, J. Wu and R. A. Rojeski, et al., Effective Infiltration of Gel Polymer Electrolyte into Silicon-Coated Vertically Aligned Carbon Nanofibers as Anodes for Solid-State Lithium-Ion Batteries, ACS Appl. Mater. Interfaces, 2015, 7(37), 20909–20918 CAS.
- M. Ko, S. Chae, J. Ma, N. Kim, H.-W. Lee and Y. Cui, et al., Scalable synthesis of silicon-nanolayer-embedded graphite for high-energy lithium-ion batteries, Nat. Energy, 2016, 1(9), 16113 CAS.
- X. Li, C. Bian, J. Zhang, J. Hong, R. Fu and X. Zhou, et al., Chemical Pre-lithiation of SiOx Anodes with a Weakly Solvating Solution of Polycyclic Aromatic Hydrocarbons for Lithium-Ion Batteries, ACS Appl. Energy Mater., 2023, 6(17), 8919–8928 CAS.
| This journal is © The Royal Society of Chemistry 2025 |
