 Open Access Article
Open Access ArticleFluorescent silver hydrosol for the dual fluorometric sensing of gallic acid and Cd2+
Mamta Sahu,
Mainak Ganguly * and
Priyanka Sharma
* and
Priyanka Sharma
Solar Energy Conversion and Nanomaterials Laboratory, Department of Chemistry, Manipal University Jaipur, Dehmi Kalan, Jaipur 303007, Rajasthan, India. E-mail: mainak.ganguly@jaipur.manipal.edu
First published on 8th May 2025
Abstract
Herein, silver-enhanced fluorescence was obtained using a small molecule of salicylaldehyde (SL) in an alkaline solution in the presence of silver nitrate. Ag+ was reduced to Ag0, while SL was oxidized to the quinone form OSL. OSL was a very weak fluorophore and exhibited metal-enhanced fluorescence (MEF) in the presence of proximal silver nanoparticle surfaces. MEF was selectively quenched by gallic acid and restored by Cd2+. Thus, a dual-sensing platform was obtained for gallic acid [limit of detection (LOD) = 1.77 × 10−5 M; linear detection range = 10−8 to 5 × 10−5 M)] and Cd2+ (LOD = 2.4 × 10−6 M; linear detection range = 5 × 10−8 to 10−6 M) in a one-pot. The observed lightning rod effect and higher radiative decay rate were ascribed to MEF, while replacing the capping agents was responsible for the tuning of MEF. The sensing protocol was applied to natural samples from real sample sources, and satisfactory results were obtained.
Introduction
Nowadays, the use of fluorescence spectroscopy is almost ubiquitous in life sciences, with diverse applications, such as in the identification of chemical or biological analytes or species.1,2 Intensive research has been conducted on biosensor platforms that generate amplified fluorescence signals. In these platforms, metallic nanostructures and metallic colloidal nanoparticles are effectively used for enhancing the optical properties of fluorophores. They can interact with proximal fluorophores and generate an enhanced quantum yield with improved photostability at an optimal distance of 5–90 nm. MEF is the term used to describe this phenomenon3–6Pawar et al.7 showed that when AuNP was bonded with physiologically significant Zn2+, it induced MEF from the modified bipyridine-based construct 4-(pyridine-2-yl)-3H-pyrrolo[2,3-c]quinoline (PPQ). This effect was utilized in a biocompatible bilayer vesicle platform in aquatic settings. When compared with the fluorescence intensity without AuNPs, MEF was visible in the presence of AuNPs when PPQ was bonded with Zn2+ to form a complex. Fluorescence enhancement was seen across the three AuNPs utilized in this study with an average diameter of 33 nm, while no enhancement was seen for the AuNPs sized 18 and 160 nm. An et al.8 used the “seed growth” method to synthesize AgNPs of various sizes and the wet oxidation method to create carbon dots (CDs). A straightforward hybrid glass slide-silver nanoparticle-carbon dot (GS-AgNP-CD) structure was employed to successfully detect the MEF effect. To examine the impact of altering the metal group's particle size and the distance between the particles and the fluorophore on the MEF effect, this structure was used to vary the sizes of the AgNPs and the distance between the AgNPs and CDs. The fluorescence improvements of fluorescein attached to various-sized silver colloids were investigated by Lukomska et al.9 They reported the selective binding of thiolated 23-mer oligonucleotide (ssDNA-SH) to silver colloids on quartz slides treated with 3-aminopropyltriethoxysilane (APS). The quantity of fluorescein-labeled complementary oligonucleotide (ssFl-DNA) added was much less than the quantity of unlabelled DNA attached to the colloids. Similar hybridization kinetics were seen on the tiny (30–40 nm) and large (120 nm) colloids, as evidenced by the increase in fluorescence emission. Nonetheless, the sample with large colloids had a final fluorescence intensity that was around 50% greater than the sample with small colloids. Because of its consistent fluorescence signal, the reference sample devoid of ssDNA-SH was utilized to estimate the fluorescence increases of fluorescein attached to the small colloids (E 2.7) and large colloids (E 4.1).
The plasmonic enhancement of the fluorophore 4-(pyridine-2-yl)-3H-pyrrolo[2,3-c]quinoline (PPQ) and its Zn2+ complex caused by gold nanoparticles (AuNPs) was observed in a meticulously constructed bilayer structure of a “niosome”, which is a significant class of pharmaceutical nanocarriers composed of nonionic surfactants.7 Choi et al. developed a brand-new proteolytic enzyme nanobiosensor using Au nanoparticles (AuNPs) and a particular peptide sequence (DEVD) that could recognize and cleave caspase-3. The peptide and single-stranded DNA (ssDNA) formed a double bridge between the AuNPs and fluorescein isothiocyanate (FITC), and the short peptide length caused the fluorescence signal to be extinguished. Both the distance and the ability to selectively break down the peptide were enhanced. Following the peptide cleavage procedure, ssDNA was used to keep the Au and the FITC apart. Because the ideal distance of MEF is around 7 to 8 nm, the FITC-ssDNA-AuNP produced a stronger fluorescence signal than the original fluorescence signal from FITC.10
Gallic acid (GAc), i.e., 3, 4, 5-trihydroxybenzoic acid, is one of the most frequently encountered phenolic acids in various plants. It is prevalent in multiple plant-based consumables, including leaves, fruits, seeds, tea, and wine,11,12 and also has applications in sustainable packaging development and food preservation. Food preservation is aided by its antibacterial and antifungal qualities against resistant microorganisms and biofilm formation, its capacity to stop lipid and protein oxidation, and its potential as a color indicator for monitoring food freshness. It provides a method for creating edible packaging by its inclusion into edible polymers. The addition of gallic acid inside a polymer matrix can enhance the packaging capabilities of materials, depending on the dosage and material composition.13,14
According to numerous studies, GAc can scavenge free radicals as an antioxidant. GAc may be used as an antioxidant in the human diet, with several research studies showing its anti-carcinogenic, antimutagenic, and anti-oxidative qualities.15 GAc can have an effect on oxidative stress, the body's molecular oxidation process, which may be prevented or delayed, reducing the risk of illness.16–19 Furthermore, some human diseases, including diabetes, Parkinson's disease, cancer, Alzheimer's disease, and cardiovascular problems, have been linked to oxidative stress.
One of the most hazardous substances to which humans may be exposed at work or in the environment is cadmium (Cd), a byproduct of the manufacturing of zinc. After absorption, Cd can be effectively stored in the human body, where it can accumulate over a lifetime. The kidney is the primary accumulation location for cadmium, which is particularly harmful to the proximal tubular cells. Through direct bone injury or indirectly through renal failure, cadmium can also result in bone demineralization. Excessive exposure to airborne Cd in the workplace can harm the lung function and raise the risk of lung cancer.20,21
In conjunction with zinc, cadmium is a rare but extensively distributed hazardous element that may have detrimental health effects on people involved in various activities or in contact with various materials, including mining, water, smelting, phosphate fertilizers, plating, plastics, Ni–Cd batteries, and pigments. The WHO recommends that the cadmium threshold in drinking water should be no more than 3 μg L−1. Furthermore, it is difficult to remove cadmium from ecological systems since it is not biodegradable. Cadmium buildup in human organs can cause renal problems, bone fractures, and several malignancies. Therefore, identifying this heavy metal ion in biological and environmental samples is a critical issue.22–26
In this study, we synthesized fluorescent silver nanoparticles (OSLAg), capped with quinone, for the selective sensing of gallic acid and Cd2+ in one pot based on turn-off–turn-on emission.
Experimental section
All the chemicals and reagents utilized in the experiments were analytical grade and were used as received without further purification. All the experiments were performed using triple-distilled water. Following the creation of fresh aqua regia, all the glassware was cleaned using soapy water and copious amounts of distilled water, and was thoroughly dried before use. The silver nitrate was purchased from Sigma-Aldrich. The NaOH was provided by Qualigens and Fisher Scientific, respectively. All the metal salts and salicylaldehyde were supplied by Loba Chemicals. A UV-2600 digital spectrophotometer (Shimadzu) was used to record the UV/vis absorption spectra. A Horiba FluoroMax-4 spectrophotometer was used to analyze the fluorescence at room temperature. The morphology of the particles was examined using a JEOL Make JSM-7610FPlus FESEM system, under high-resolution scanning (1 kV at 1.0 nm, 15 kV at 0.8 nm).Synthesis of OSLAg
A stock solution of 0.047 M SL was made by dissolving the appropriate amount of SL in 500 mL of 0.005 M NaOH solution. Next, 10 mL of 0.001 M AgNO3 solution, 3 mL of the SL stock solution, and 37 mL of NaOH solution (0.005 M) were mixed thoroughly and aged overnight on the bench at room temperature (30 °C) to obtain the fluorescent hydrosol OSLAg. No stirring was performed during synthesis.Results and discussion
OSLAg: quinone-capped fluorescent silver hydrosol
SL is an aromatic compound with –OH and –CHO functional groups at the ortho position. As SL is insoluble in water, it was dissolved in 0.2 M NaOH to obtain a pale-yellow alkaline SL solution. The introduction of an aqueous AgNO3 solution produced the strongly emissive zero-valent silver hydrosol OSLAg.27 The phenolates in the SL alkaline solution were transformed to the quinone form during the formation of OSLAg. It was reported that fluorophores sized in between 5–90 nm from metal nanostructure exhibit MEF with a high scattering cross-section and low absorption cross-section.5,28 Here, the Ag nanoparticles in OSLAg, being in proximity with a weakly fluorescent fluorophore, bestowed it with a higher rate of excitation (lightning rod effect) and a higher rate of emission. Thus, MEF was observed. No external energy was employed in the reaction mixture for the synthesis of OSLAg and the detection of the analytes. Therefore, the process was cost-effective, simple, and energetically convenient.The optical characteristics of metallic surfaces and particles are varied and intricate. The vibrant hues of noble metal colloids, absorption of surface plasmon resonance of thin metal films, and the quenching of excited fluorophores close to metal surfaces are some examples. Fluorophores' interactions with metal surfaces have recently been exploited to boost the fluorescence intensities of materials, create tests based on gold colloids quenching fluorescence, and to achieve directional radiation from fluorophores close to thin metal films. It is challenging to forecast whether a certain metal structure—such as a colloid, fractal, or continuous surface—will increase or quench fluorescence in the case of metal-enhanced fluorescence.24,29,30
Lakowicz proposed a straightforward theory based on radiating plasmons (RPs) to explain how metals influence fluorescence. The concept is relatively easy to grasp, even if the physics behind it is complicated. The RP model states that the optical characteristics of a metal structure, as determined by electrodynamics, Mie theory, and/or Maxwell equations, may be used to forecast the emission or quenching of a fluorophore close to the metal. For instance, Mie's theory states that the extinction of metal colloids might be caused by either absorption or scattering, depending on the particles' size and shape.29,31
Absorption dissipates incident energy, while scattering produces far-field radiation. The RP model predicts that tiny colloids would suppress fluorescence because absorption would outweigh scattering. Conversely, for larger colloids, since the scattering component would outweigh the absorption, larger colloids should improve fluorescence.
Here, OSLAg still demonstrated strong fluorescence (quantum yield = 6.5%, λex = 290 nm, λem = 452 nm) after 8 h aging with alkaline SL and Ag+. Such 8 h of aging was important for the evolution of fluorescence. SL + Ag+ without aging could not generate fluorescence. The UV-Vis spectrum of OSLAg showed the appearance of a new peak at 295 nm due to the strong interaction between the quinone and silver in OSLAg (Fig. 1).
 | ||
| Fig. 1 (a) Schematic representation of the formation of silver nanoparticles. (b) Fluorescence spectra and (c) absorption spectra before and after the formation of OSLAg. | ||
The nanoparticle formation was further gauged by zeta potential and dynamic light scattering (DLS) analyses. The zeta potential before aging (−50 mV) was increased (to −40 mV) after OSLAg formation (after overnight aging). A decrease in negative zeta potential indicated the formation of zero-valent Ag after nanoparticle formation. The negative charge density was a little decreased due to the conversion of Ag+ to Ag0. The zeta potential of −40 mV of OSLAg was high enough to stabilize the silver hydrosol.1 It is to be noted that the zeta potential was not the sole factor for the stability of the hydrosol. Steric stabilization, depletion (including kinetic interaction, specific adhesion, and specific contact), and delayed aggregation are crucial to nanoparticle stability.9,32,33 The hydrodynamic diameter of 208.6 nm (polydispersity index 5.7%, mean intensity 324.9 kcps, diffusion coefficient 2.4 μm2 s−1) (before aging) was decreased to 64.5 nm (polydispersity index 27.7%, mean intensity 301.34 kcps, diffusion coefficient 7.6 μm2 s−1) after OSLAg formation (after overnight aging). Thus, the overnight aging of the reaction mixture generated smaller silver nanoparticles with MEF. The FESEM and TEM images also indicated the size of the spherical nanoparticles of OSLAg was ∼60 nm (Fig. 2). The XRD pattern indicated that silver was in a zero-oxidation state in OSLAg (JCPDS file no. 04-0783). The noted 2θ values of 38.36°, 44.6°, 64.56°, and 77.7°, respectively, supported the zero-valent Ag nanoparticles' (111), (200), (210), and (311) planes34,35 Using Debye–Scherrer's equation D = 0.9λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ (where D is the crystalline size, λ is the X-ray wavelength, β is the full width at half maximum of the diffraction peak, and θ is the Bragg's angle), the size of the Ag nanoparticles was determined. Based on the width of the reflection planes, the size of the produced Ag nanoparticles was estimated to be around 14.8 nm.36
θ (where D is the crystalline size, λ is the X-ray wavelength, β is the full width at half maximum of the diffraction peak, and θ is the Bragg's angle), the size of the Ag nanoparticles was determined. Based on the width of the reflection planes, the size of the produced Ag nanoparticles was estimated to be around 14.8 nm.36
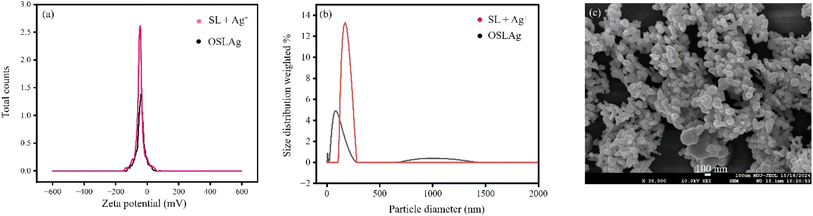 | ||
| Fig. 2 (a) Zeta potential and (b) DLS plots of OSLAg before and after the formation of OSLAg via aging. (c) FESEM image of OSLAg. | ||
The lattice fringe of 0.48 nm observed in the HRTEM image was in accord with the (111) plane of zero-valent silver (Fig. 3).37
Sensing of gallic acid based on turn-off fluorescence
The strong fluorescence of OSLAg was efficiently decreased by the inclusion of GAc in OSLAg. No other competitive molecules (Zn2+, Na+, K+, Mg2+, succinic acid, citric acid, ascorbic acid, urea, fructose, gallic acid) could quench the fluorescence efficiently, unlike GAc. Such selectivity prompted us to design a fluorometric sensing platform for GAc. The limit of detection (LOD) was 1.77 × 10−5 M while the linear detection range was 10−8 to 5 × 10−5 M (Fig. 4). Here, 2 mL of OSLAg and 1 mL of GAc of various concentrations were mixed thoroughly, aged for ∼0.5 h, and the fluorescence spectra then recorded. We calculated LOD values using the slope obtained in the linear detection range. We fitted the data using Excel software and used the LOD formula below to calculate the LOD using the experimental data:| LOD = 3σ/s |
Fluorescence quenching refers to any process that decreases the fluorescence intensity of a material. A variety of molecular interactions can result in quenching. These include excited-state reactions, molecular rearrangements, energy transfer, ground-state complex formation, and collisional quenching. Quenching can be induced by different mechanisms, which are usually classified into dynamic quenching and static quenching. Dynamic, i.e., collisional quenching, occurs for excited-state fluorophores.39
Quenching data are usually presented as plots of I0/I versus [Q]. This is because I0/I is expected to be linearly dependent on the concentration of the quencher. A plot of I0/I versus [Q] yields a slope equal to the Stern–Volmer quenching constant KSV. A linear Stern–Volmer plot is generally indicative of a single class of fluorophores, all equally accessible to the quencher. In many cases, the fluorophore can be quenched both by collision and by complex formation with the same quencher. When this is the case, the Stern–Volmer plot exhibits an upward curvature, concave toward the y-axis at high [Q].39
The Stern–Volmer plot in our present study showed an upward curvature, implying that both static and dynamic quenching mechanisms were operative. At a lower concentration range (10−8 to 5 × 10−5 M), a linear transformer plot was observed with KSV = 531 (Fig. 4).
We summarized some different methods and parameters for sensing reported in the available literature in Table 1 in comparison with the present work. Using fluorometric sensing techniques, several research teams have investigated various nanostructures for the detection of GAc. Tan et al.40 developed a fluorescent sensor for the detection of GAc based on the reversible “off–on” fluorescence change of N-acetyl-L-cysteine-capped CdTe QDs, with KMnO4 utilized to effectively quench the fluorescence intensity of the QDs. In this kind of sensing, the QDs' fluorescence is first quenched, turned off, and then recovered, or turned on, in the subsequent analyte sequence step. In the light-switching process, this offers the benefits of high selectivity and versatility. The synthesis method though for the QDs was difficult in comparison to our method. Specifically, in their method, the hydrothermal method with modification and N2 as a protective gas were used for synthesis of the QDs, whereas our synthesis involved a single step only, and no additional reducing agent was required. Zhang et al.41 also reported needing many steps for the synthesis of nanoparticles@zeolitic imidazolate frameworks as a fluorescent nanoprobe.
| Nanoparticles | Method of sensing | LOD for GAc | Detection range | |
|---|---|---|---|---|
| GAc | N-Acetyl-L-cysteine-carbon dots40 | Fluorometric | 0.56 ng mL−1 | 0.6–12.6 μg mL−1 |
| Carbon dots45 | Fluorometric | 1.2 μmol L−1 | 2.0–60.0 μmol L−1 | |
| Nanoparticles@zeolitic imidazolate frameworks41 | Fluorometric | 0.35 μM | 0–30 μM | |
| Bovine serum albumin-gold/silver nanoclusters46 | Fluorometric | 45.27 nM | 1.25–40.0 μM | |
| Present study | Fluorometric | 1.77 × 10−5 M | 10−8 to 5 × 10−5 M | |
| Octamethoxy resorcin[4]arene tetrahydrazide-silver nanoparticles43 | Fluorometric | 10−8 M | 10−8 M to 10−6 M | |
| Cd2+ | 1,13-Bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane-silver nanoparticles44 | Colorimetric | 2.0 μM | 0.5–6.0 μM |
| Sulfosalicylic acid-silver nanoparticles47 | Colorimetric | 3.0 nM | 0.05–1.1 μM | |
| Present study | Fluorometric | 2.4 × 10−6 M | 5 × 10−8 to 10−6 M |
For the detection of cadmium, a fast, dependable, and extremely sensitive sensor is required.42 According to recent developments, extremely luminous metal nanoclusters can be expertly created inside biocompatible scaffolds. Using octamethoxy resorcin[4]arene tetrahydrazide (OMRTH) as a reducing and stabilizing agent, Makwana et al.43 reported the straightforward production of fluorescent Ag nanoparticles. They also reported that Cd2+ could quench the emission of the stable silver nanoparticles sensitively and selectively.43 For the determination of Cd2+, Fallahi and Khayatian44 suggested a straightforward, quick, and sensitive colorimetric technique, using 1,13-bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane-modified AgNPs. We believe the reported nanostructures given in Table 1 as sensing platforms are too expensive for cadmium sensing. Moreover, we disclosed here turn-on fluorescence for Cd2+ involving silver nanoparticles, unlike in the existing literature.
GAc is an aromatic organic molecule with three phenolate groups. Herein, GAc was oxidized to its quinone48 form (OGAc) in the reaction mixture. OGAc replaced the capping of OSL on the surface of Ag. Fluorescence was quenched due to the lack of a fluorophore (OSL) in the proximity of the silver nanoparticles surface. Thus, GAc quenched MEF selectively due to the structural parity of OGAc and OSL (Scheme 1).
The zeta potential of OSLAg + OGAc was found to be −28.8 mV and the hydrodynamic diameter (obtained from DLS analysis) was 112.4 nm. FESEM image also indicated an organic coating on silver nanoparticles and showed that the particles were aggregated due to this coating (Fig. 5).
Sensing of Cd2+ based on the restoration of fluorescence
To investigate the effect of interference of other species for GAc sensing, we added both organic (L-leucine, glutathione, uric acid, fructose urea, citric acid, succinic acid, ascorbic acid) and inorganic (Cd2+, Pb2+, Cu2+, Zn2+, Ba2+, Fe3+, Al3+, Ca2+, Hg2+, Mg2+, Na+, and K+) materials in OSLAg + OGAc. The quenched fluorescence of OSLAg + OGAc was restored in the presence of Cd2+ selectively. No other metal ions could enhance the fluorescence of OSLAg + OGAc. Thus, the Cd2+ sensing was made possible with the formation of fluorescent OSLAg + OGAc + Cd2+. The LOD was 2.4 × 10−6 M, while the linear detection range was 10−6 to 5 × 10−8 M (Fig. 6). In the experiments, 2 mL of OSLAg, 1 mL of 0.001 M GAc, and various concentrations of Cd2+ were mixed thoroughly and aged for 12 h to record the fluorescence spectra.OSLAg + OGAc + Cd2+ had a zeta potential of −46 mV, indicating the high stability of the hydrosol, while the hydrodynamic diameter was found to be 1185 nm. Spherical particles sized ∼1 μm were also observed in the FESEM image. In the large spheres, silver particles (white color) remained similar to the eyeballs in the eye (Fig. 7).
Cd2+ removed OGAc from the surface of the silver nanoparticles and OSL-capped Ag was regenerated with the restoration of MEF (Scheme 1). The presence of divalent Cd in OSLAg + OGAc + Cd2+ was confirmed by XPS analysis and Cd(OH)2 formation was verified. According to the XPS spectra, the peaks at binding energies of 405.01 and 411.73 eV, respectively, matched the divalent cadmium 3d5/2 and 3d3/2 states. We did not find peaks for Ag in the XPS survey as Ag was buried by Cd(OH)2 (as confirmed by the FESEM image). The XRD pattern also supported the formation of Cd(OH)2 and Ag2O (Fig. 8).49,50 The fluorescence of OSLAg was 1.7 times higher than that of OSLAg + OGAc + Cd2+ owing to the surface oxidation of Ag by GAc and Cd2+ treatment.
Effect of temperature variation
To test the effects of temperature, we kept OSLAg and OSLAg + OGAc + Cd2+ in the chamber of the fluorimeter and varied the temperature from 25 °C to 65 °C. With the increase in the temperature, fluorescence decreased monotonously for both hydrosols. Increasing Brownian motion was the pivotal factor in the decrease in fluorescence. Energy loss via dynamic quenching by the solvent molecules was facilitated by their Brownian motion.51 However, in the case of OSLAg + OGAc + Cd2+, the reduction in fluorescence was more rapid than that in OSLAg due to the mobility of Cd2+ at higher temperatures. Complex formation between Cd2+ and OGAc was interrupted at high temperatures. Thus a rapid fluorescence decrement was observed for OSLAg + OGAc + Cd2+ due to the joint effect of increased Brownian motion and the increased free quencher [OGAc] at higher temperatures. We plotted IT/IRT at various experimental temperatures, as shown in Fig. 9 (where IT and IRT represent the fluorescence intensity at temperature T and room temperature, respectively).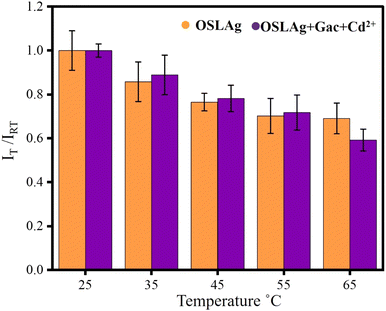 | ||
| Fig. 9 Fluorescence intensities of OSLAg and OSLAgGAcCd2+ at various temperatures with respect to the room temperature. | ||
Real sample analysis
In order to assess the viability of the system for practical application, the fluorescence technique mentioned above was used to detect GAc and Cd2+ in various natural water samples. We collected Ganga water from Prayagraj (Uttar Pradesh, India), rain water from Jaipur (Rajasthan, India), and tap water from Manipal University Jaipur (India). We filtered the real water samples using Whatman filter paper. We spiked known concentrations of both analytes in three real samples separately to obtain 3.00 × 10−5 M analytes, as mentioned in Table 2. Next, we added the real sample solutions in OSLAg and OSLAgGAc to estimate the [GAc] and [Cd2+] by recording their fluorescence spectra and matching them with their linear detection ranges (Fig. 4 and 6). The added analyte concentrations (known concentrations) and estimated analyte concentrations were found to be close. We checked the calculated % recovery values in Table 2. Though it seems unusual to represent % recovery values >100, it is the way of representation we found in different literature for case % recovery values.40| Sample | Spiked concentration (M) | Measured (M) | Recovery (%) | RSD (n = 3, %) | |
|---|---|---|---|---|---|
| GAc | Ganga water | 3.00 × 10−5 | 2.94 × 10−5 | 98 | 3.7 |
| Rain water | 3.00 × 10−5 | 3.14 × 10−5 | 105 | 4.1 | |
| Tap water | 3.00 × 10−5 | 2.75 × 10−5 | 92 | 4.5 | |
| Cd2+ | Ganga water | 3.00 × 10−5 | 3.09 × 10−5 | 103 | 1.5 |
| Rainwater | 3.00 × 10−5 | 3.12 × 10−5 | 104 | 2.9 | |
| Tap water | 3.00 × 10−5 | 2.82 × 10−5 | 94 | 2.2 |
Conclusions
A one-pot sensing platform was designed for gallic acid and Cd2+ based on turn-off–turn-on MEF from quinone-capped silver nanoparticles. The observed lightning rod effect and higher rate of radiative decay (due to the high plasmonic density) were ascribed to MEF. Replacement of the capping agents by analytes played a pivotal role in the tuning of MEF in association with nanosensing. For turbid water, the proposed technique has limited applications. Though we anticipate that given the in situ formation of orthoquinone, more research is warranted to identify its formation in the aqueous state both theoretically and experimentally. This paper provides a hint on how to tune MEF for nanosensing for many analytes. This paper is expected to be an asset for young scientists venturing into the field of sustainable water management and environmental science.Data availability
The authors are unable or have chosen not to specify which data have been used.Author contributions
Mamta Sahu: writing – original draft, Mainak Ganguly: writing – review & editing, conceptualization, Priyanka Sharma: visualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are thankful to Central Analytical Facilities (MUJ) and Sophisticated Analytical Instrument Facility (MUJ) for instrumental facilities.References
- S. Dawadi, S. Katuwal, A. Gupta, U. Lamichhane, R. Thapa, S. Jaisi, G. Lamichhane, D. P. Bhattarai and N. Parajuli, Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications, J. Nanomater., 2021, 2021, 6687290, DOI:10.1155/2021/6687290.
- C. D. Geddes, Metal-enhanced fluorescence, Phys. Chem. Chem. Phys., 2013, 15, 19537, 10.1039/c3cp90129g.
- Y. Jeong, Y. M. Kook, K. Lee and W. G. Koh, Metal enhanced fluorescence (MEF) for biosensors: general approaches and a review of recent developments, Biosens. Bioelectron., 2018, 111, 102–116, DOI:10.1016/j.bios.2018.04.007.
- M. Ganguly, J. Jana, A. Pal and T. Pal, Synergism of gold and silver invites enhanced fluorescence for practical applications, RSC Adv., 2016, 6, 17683–17703, 10.1039/c5ra26430h.
- K. Aslan, I. Gryczynski, J. Malicka, E. Matveeva, J. R. Lakowicz and C. D. Geddes, Metal-enhanced fluorescence: an emerging tool in biotechnology, Curr. Opin. Biotechnol., 2005, 16, 55–62, DOI:10.1016/j.copbio.2005.01.001.
- M. T. Yaraki and Y. N. Tan, Metal Nanoparticles-Enhanced Biosensors: Synthesis, Design and Applications in Fluorescence Enhancement and Surface-enhanced Raman Scattering, Chem.–Asian J., 2020, 15, 3180–3208, DOI:10.1002/asia.202000847.
- S. Pawar, A. Bhattacharya and A. Nag, Metal-Enhanced Fluorescence Study in Aqueous Medium by Coupling Gold Nanoparticles and Fluorophores Using a Bilayer Vesicle Platform, ACS Omega, 2019, 4, 5983–5990, DOI:10.1021/acsomega.9b00036.
- L. Y. Jia An, H. Nan and Y. Chengyue, Metal-enhanced fluorescence (MEF) effect based on silver nanoparticles with different UV spectra on a surface carbon dot-based novel dry platform, Spectrochim. Acta, Part A, 2024, 322, 124744 CrossRef PubMed.
- J. Lukomska, J. Malicka, I. Gryczynski, Z. Leonenko and J. R. Lakowicz, Fluorescence enhancement of fluorophores tethered to different sized silver colloids deposited on glass substrate, Biopolymers, 2005, 77, 31–37, DOI:10.1002/bip.20179.
- J.-H. Choi and J.-W. Choi, Metal-Enhanced Fluorescence by Bifunctional Au Nanoparticles for Highly Sensitive and Simple Detection of Proteolytic Enzyme, Nano Lett., 2020, 20, 7100–7107, DOI:10.1021/acs.nanolett.0c02343.
- M. Liu, H. Xie, Y. Ma, H. Li, C. Li, L. Chen, B. Jiang and B. Nian, High Performance Liquid Chromatography and Metabolomics Analysis of Tannase Metabolism of Gallic Acid and Gallates in Tea Leaves, J. Agric. Food Chem., 2020, 68, 4946–4954, DOI:10.1021/acs.jafc.0c00513.
- A. Septembre-malaterre, G. Stanislas, E. Douraguia and M. Gonthier, Evaluation of nutritional and antioxidant properties of the tropical fruits banana, litchi, mango, papaya, passion fruit and pineapple cultivated in Réunion French Island, Food Chem., 2016, 212, 225–233, DOI:10.1016/j.foodchem.2016.05.147.
- I. Rahmawati, A. Wedi, S. Abdi, M. Nur, A. Firmanda, F. Haidir, A. Nur, M. Ansori and H. Sucipto, Case studies in chemical and environmental engineering gallic acid: a promising bioactive agent for food preservation and sustainable packaging development, Case Stud. Chem. Environ. Eng., 2024, 10, 100776, DOI:10.1016/j.cscee.2024.100776.
- C. O. Chikere, N. H. Faisal, P. Kong, T. Lin and C. Fernandez, The Synergistic Effect between Graphene Oxide Nanocolloids and Silicon Dioxide Nanoparticles for Gallic Acid Sensing, J. Solid State Electrochem., 2019, 23, 1795–1809 CrossRef CAS.
- J. D. Hsu, S. H. Kao, T. T. Ou, Y. J. Chen, Y. J. Li and C. J. Wang, Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27Kip1 attributed to disruption of p27 Kip1/Skp2 complex, J. Agric. Food Chem., 2011, 59, 1996–2003, DOI:10.1021/jf103656v.
- I. Pinchuk, H. Shoval, Y. Dotan and D. Lichtenberg, Evaluation of antioxidants: Scope, limitations and relevance of assays, Chem. Phys. Lipids, 2012, 165, 638–647, DOI:10.1016/j.chemphyslip.2012.05.003.
- A. M. Pisoschi, C. Cimpeanu and G. Predoi, Electrochemical Methods for Total Antioxidant Capacity and its Main Contributors Determination: A review, Open Chem., 2015, 13, 824–856, DOI:10.1515/chem-2015-0099.
- H. Zhao, Q. Ran, Y. Li, B. Li, B. Liu, H. Ma, M. Zhang and S. Komarneni, Highly sensitive detection of gallic acid based on 3D interconnected porous carbon nanotubes/carbon nanosheets modified glassy carbon electrode, J. Mater. Res. Technol., 2020, 9, 9422–9433, DOI:10.1016/j.jmrt.2020.05.102.
- W. Ma, D. Han, S. Gan, N. Zhang, S. Liu, T. Wu, Q. Zhang, X. Dong and L. Niu, Rapid and specific sensing of gallic acid with a photoelectrochemical platform based on polyaniline–reduced graphene oxide–TiO2, Chem. Commun., 2013, 49, 7842–7844, 10.1039/c3cc43540g.
- A. Bernard, Cadmium & its adverse effects on human health, Indian J. Med. Res., 2008, 128, 557–564 CAS.
- J. Chmielowska-b, J. Gzyl, R. Ruci, M. Arasimowicz-jelonek and J. Deckert, The new insights into cadmium sensing, 5 ( 2014) 1–14. doi: DOI:10.3389/fpls.2014.00245.
- M. Yang, T. J. Jiang, Z. Guo, J. H. Liu, Y. F. Sun, X. Chen and X. J. Huang, Sensitivity and selectivity sensing cadmium(II) using amination functionalized porous SnO2 nanowire bundles-room temperature ionic liquid nanocomposite: combined efficient cation capture with control experimental conditions, Sens. Actuators, B, 2017, 240, 887–894, DOI:10.1016/j.snb.2016.09.060.
- C. ye Hui, Y. Guo, H. Li, C. xian Gao and J. Yi, Detection of environmental pollutant cadmium in water using a visual bacterial biosensor, Sci. Rep., 2022, 12, 1–11, DOI:10.1038/s41598-022-11051-9.
- M. Y. He, Y. J. Lin, Y. L. Kao, P. Kuo, C. Grauffel, C. Lim, Y. S. Cheng and H. H. D. Chou, Sensitive and Specific Cadmium Biosensor Developed by Reconfiguring Metal Transport and Leveraging Natural Gene Repositories, ACS Sens., 2021, 6, 995–1002, DOI:10.1021/acssensors.0c02204.
- S. L. Wang, C. Y. Hsieh, C. R. Wu, J. C. Chen and Y. L. Wang, Highly sensitive FET sensors for cadmium detection in one drop of human serum with a hand-held device and investigation of the sensing mechanism, Biomicrofluidics, 2021, 15, 024110, DOI:10.1063/5.0042977.
- M. Sahu, M. Ganguly and P. Sharma, Role of silver nanoparticles and silver nanoclusters for the detection and removal of Hg(II), RSC Adv., 2024, 14, 22374–22392, 10.1039/d4ra04182h.
- M. Ganguly, J. Pal, C. Mondal, A. Pal and T. Pal, Intriguing Manipulation of Metal-Enhanced Fluorescence for the Detection of CuIIand Cysteine, Chem.–Eur. J., 2014, 20, 12470–12476, DOI:10.1002/chem.201402505.
- M. Sahu, M. Ganguly, P. Sharma, A. Doi and Y. Negishi, Simultaneous ionic cobalt sensing and toxic Congo red dye removal: a circular economic approach involving silver-enhanced fluorescence, Nanoscale Adv., 2024, 6, 6173–6183, 10.1039/d4na00588k.
- J. R. Lakowicz, Y. Shen, S. D'Auria, J. Malicka, J. Fang, Z. Gryczynski and I. Gryczynski, Radiative decay engineering: 2. Effects of silver island films on fluorescence intensity, lifetimes, and resonance energy transfer, Anal. Biochem., 2002, 301, 261–277, DOI:10.1006/abio.2001.5503.
- M. Sahu, M. Ganguly and A. Doi, Metal-Enhanced Fluorescence due to Salicylaldehyde-Silver Nanoparticle Interactions for Fe3+ Sensing, ChemistrySelect, 2023, 8, e202301017, DOI:10.1002/slct.202301017.
- J. Jana, M. Ganguly and T. Pal, Evolution, Stabilization, and Tuning of Metal-Enhanced Fluorescence in Aqueous Solution, Surface Plasmon Enhanced, Coupled and Controlled Fluorescence, 2017,pp. 151–178, doi: DOI:10.1002/9781119325161.ch9.
- P. Sharma, M. Ganguly and M. Sahu, Na+ detection via brightening of synergistically originated noble metal nanoclusters, RSC Adv., 2024, 14, 31624–31632, 10.1039/d4ra05501b.
- P. Attard, Recent advances in the electric double layer in colloid science, Curr. Opin. Colloid Interface Sci., 2001, 6, 366–371, DOI:10.1016/S1359-0294(01)00102-9.
- M. Sahu, M. Ganguly and P. Sharma, Recent applications of coinage metal nanoparticles passivated with salicylaldehyde and salicylaldehyde-based Schiff bases, Nanoscale Adv., 2024, 6, 4545–4566, 10.1039/d4na00427b.
- M. Sahu, M. Ganguly and P. Sharma, Fluorescent Giant Clusters with Myriad Applications, NanoWorld J., 2023, 9, 496–504, DOI:10.17756/nwj.2023-s5-080.
- Y. Meng, A Sustainable Approach to Fabricating Ag Nanoparticles/PVA Hybrid Nanofiber and Its Catalytic Activity, Nanomaterials, 2015, 5, 1124–1135, DOI:10.3390/nano5021124.
- N. A. Bakar, J. G. Shapter, M. M. Salleh and A. A. Umar, Self-Assembly of high density of triangular silver nanoplate films promoted by 3-aminopropyltrimethoxysilan, Appl. Sci., 2015, 5, 209–221, DOI:10.3390/app5030209.
- J. Li, B. Zheng, Z. Zheng, Y. Li and J. Wang, Highly sensitive and selective colorimetric and SERS dual-mode detection of arsenic (III) based on glutathione functionalized gold nanoparticles, Sens. Acutators Rep., 2020, 2, 100013, DOI:10.1016/j.snr.2020.100013.
- J. Mariam, P. M. Dongre and D. C. Kothari, Study of Interaction of Silver Nanoparticles with Bovine Serum Albumin Using Fluorescence Spectroscopy, J. Fluoresc., 2011, 21, 2193–2199, DOI:10.1007/s10895-011-0922-3.
- X. Tan, Q. Li and J. Yang, CdTe QDs based fluorescent sensor for the determination of gallic acid in tea, Spectrochim. Acta, Part A, 2020, 224, 117356, DOI:10.1016/j.saa.2019.117356.
- Y. Zhang, L. Ning, D. Gao, D. Jia, W. Gu and X. Liu, A highly sensitive upconversion nanoparticles@zeolitic imidazolate frameworks fluorescent nanoprobe for gallic acid analysis, Talanta, 2021, 233, 122588, DOI:10.1016/j.talanta.2021.122588.
- S. Velanki, S. Kelly, T. Thundat, D. A. Blake and H. Ji, Detection of Cd(II) using antibody-modified microcantilever, Sensors, 2007, 107, 1123–1128, DOI:10.1016/j.ultramic.2007.01.011.
- B. A. Makwana, D. J. Vyas, K. D. Bhatt, S. Darji and V. K. Jain, Novel fluorescent silver nanoparticles: sensitive and selective turn off sensor for cadmium ions, Appl. Nanosci., 2016, 6, 555–566, DOI:10.1007/s13204-015-0459-x.
- M. R. Fallahi and G. Khayatian, Cadmium determination based on silver nanoparticles modified with 1,13-bis(8-quinolyl)-1,4,7,10,13-pentaoxatridecane, J. Iran. Chem. Soc., 2017, 14, 1469–1476, DOI:10.1007/s13738-017-1087-z.
- X. H. Qin Tan, X. An, S. Pan, S. Zhen and Y. Hu, A facile and sensitive ratiometric fluorescent sensor for determination of gallic acid, Microchem. J., 2022, 172, 106922 CrossRef.
- M. Wang, Z. Liu, M. Wu, T. Wong, X. Yu, N. Niu and L. Chen, Ratiometric luminescent sensor based on BSA-coated gold/silver nanoclusters for the selective determination and spatiotemporal imaging of gallic acid in plants, Microchim. Acta, 2024, 191, 60 CrossRef CAS.
- W. Jin, P. Huang, F. Wu and L. H. Ma, Ultrasensitive colorimetric assay of cadmium ion based on silver nanoparticles functionalized with 5-sulfosalicylic acid for wide practical applications, Analyst, 2015, 140, 3507–3513, 10.1039/c5an00230c.
- G. Ziyatdinova, E. Guss, E. Morozova, H. Budnikov, R. Davletshin, V. Vorobev and Y. Osin, Simultaneous voltammetric determination of gallic and ellagic acids in cognac and brandy using electrode modified with functionalized SWNT and poly(pyrocatechol violet), Food Anal. Methods, 2019, 12, 2250–2261, DOI:10.1007/s12161-019-01585-6.
- L. A. Saghatforoush, S. Sanati, R. Mehdizadeh and M. Hasanzadeh, Solvothermal synthesis of Cd(OH)2 and CdO nanocrystals and application as a new electrochemical sensor for simultaneous determination of norfloxacin and lomefloxacin, Superlattices Microstruct., 2012, 52, 885–893, DOI:10.1016/j.spmi.2012.07.019.
- Y. C. Zhang and G. L. Wang, Solvothermal synthesis of CdO hollow nanostructures from CdO2 nanoparticles, Mater. Lett., 2008, 62, 673–675, DOI:10.1016/j.matlet.2007.06.031.
- M. Ganguly, A. Pal, Y. Negishi and T. Pal, Diiminic Schiff bases: an intriguing class of compounds for a copper-nanoparticle-induced fluorescence study, Chem.–Eur. J., 2012, 18, 15845–15855, DOI:10.1002/chem.201201242.
| This journal is © The Royal Society of Chemistry 2025 |








