Open Access Article
Open Access ArticleSurface catalytic effect of Ag nanoparticles and influence of dissolved oxygen on photocatalytic reduction of nitrate†
Bin Huangab,
Wei Liubc,
Kun Chaobd,
Dongran Wangbe,
Pengxiang Wangbe,
Tengfeng Xie f,
Fangming Han
f,
Fangming Han *be,
Yan Mi*a,
Haibin Tang
*be,
Yan Mi*a,
Haibin Tang *be and
Guowen Meng
*be and
Guowen Meng be
be
aGuangxi Key Laboratory of Chemistry and Engineering of Forest Products, Guangxi Minzu University, Nanning, 530006, P. R. China. E-mail: miyan@gxmzu.edu.cn
bKey Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanotechnology, Institute of Solid State Physics, HFIPS, Chinese Academy of Sciences, 350 Shushanhu Road, Hefei, Anhui 230031, P. R. China. E-mail: hbtang@issp.ac.cn; fmhan@issp.ac.cn
cInstitutes of Physical Science and Information Technology, School of Materials Science and Engineering, Anhui University, Hefei 230601, P. R. China
dSchool of Advanced Materials and Nanotechnology, Xidian University, Xi'an, 710126, P. R. China
eUniversity of Science and Technology of China, 96 Jinzhai Road, Hefei, Anhui 230026, P. R. China
fInstitute of Physical Chemistry, College of Chemistry, Jilin University, Changchun 130012, P. R. China
First published on 20th March 2025
Abstract
Photocatalytic reduction of nitrate is a promising green strategy to remove nitrogen pollutants from water bodies. However, it is a considerable challenge to directly reduce nitrate using photogenerated electrons. In this work, a g-C3N4/Ag-nanoparticle composite demonstrating high catalytic activity toward nitrate is constructed using an in situ photodeposition method. Based on photoelectrochemical, ion chromatography and nuclear magnetic resonance characterization, Ag nanoparticles were found to exhibit strong surface catalytic activity for reducing NO3− to NO2−, while g-C3N4/Au-nanoparticles exhibited no photocatalytic reduction activity toward NO3−. Additionally, the effects of dissolved oxygen on the photocatalysis system of nitrate reduction are analyzed for the first time.
1. Introduction
The massive use of nitrogenous fertilizers in agricultural production and the rapid development of the nitrogen industry have resulted in serious nitrate (NO3−) contamination of underground water and natural water bodies.1–3 NO3− can be converted into toxic nitrosamines when ingested by the body, which can induce cellular cancer, malformations and embryotoxicity.4–7 Thus, removal of NO3− from water is critical for ensuring environment safety and human health. Compared with traditional methods such as adsorption,8,9 ion exchange,10,11 electrodialysis,12–14 and biological denitrification,15,16 photocatalytic reduction of NO3− to N2 gas is a promising technology.17,18 However, NO3− is chemically stable because of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and two N–O bonds with bond energies of 607 and 230 kJ mol−1, respectively. The reduction potential is extremely high for typical semiconductor photocatalysts; e.g., for the first reaction of NO3−/˙NO32−, it is −0.89 V (vs. standard hydrogen electrode, SHE). Moreover, most reported photocatalytic systems are TiO2-based photocatalysts requiring various hole sacrificial agents, especially formic acid, which results in high production of ˙CO2− radicals after oxidation by photogenerated holes and has strong reducibility toward nitrate (E(CO2−/˙CO2−) = −1.81 V vs. SHE) and high selectivity for N2.19–23 To replace reducing additives for potential application, high conduction band semiconductors with a higher driving force of photogenerated electrons have been studied to reduce NO3−. For example, ZnS,24 LiNbO3,25 Pd/GdCrO3,26 and ZnSe/BiVO4 (ref. 27) showed photocatalytic efficiency and considerable N2 selectivity with added sacrificial agents. However, they all require strong UV light illumination owing to their wide bandgaps. Moreover, UV light can be strongly absorbed by NO3− and NO2−, which will be excited to excitation states, thus facilitating their reduction.28
O and two N–O bonds with bond energies of 607 and 230 kJ mol−1, respectively. The reduction potential is extremely high for typical semiconductor photocatalysts; e.g., for the first reaction of NO3−/˙NO32−, it is −0.89 V (vs. standard hydrogen electrode, SHE). Moreover, most reported photocatalytic systems are TiO2-based photocatalysts requiring various hole sacrificial agents, especially formic acid, which results in high production of ˙CO2− radicals after oxidation by photogenerated holes and has strong reducibility toward nitrate (E(CO2−/˙CO2−) = −1.81 V vs. SHE) and high selectivity for N2.19–23 To replace reducing additives for potential application, high conduction band semiconductors with a higher driving force of photogenerated electrons have been studied to reduce NO3−. For example, ZnS,24 LiNbO3,25 Pd/GdCrO3,26 and ZnSe/BiVO4 (ref. 27) showed photocatalytic efficiency and considerable N2 selectivity with added sacrificial agents. However, they all require strong UV light illumination owing to their wide bandgaps. Moreover, UV light can be strongly absorbed by NO3− and NO2−, which will be excited to excitation states, thus facilitating their reduction.28
Development of photocatalysts with a narrow bandgap, i.e. improving the utilization of visible light, is a developing research direction for promoting practical application.29,30 At present, only few materials, such as oxygen vacancy-enriched P25 decorated with Ag/Cu nanoparticles31 and graphitic carbon nitride (g-C3N4) decorated with Ag nanoparticles,32 have been reported to exhibit photocatalytic reduction of nitrate performance under visible light, although the selectivity for N2 is low with NO2− as the primary product. Furthermore, revealing their working mechanisms is of great significance for the future design of visible light-sensitive photocatalysts. However, the mechanism is not clear to date. Additionally, all previously reported experimental systems worked in an aqueous solution in the absence of dissolved O2. However, for practical application, the presence of dissolved O2 in natural water bodies cannot be neglected. Nevertheless, the effects of dissolved O2 on the photocatalytic reduction of nitrate have not been studied.
Herein, we prepared composite photocatalysts of g-C3N4 decorated with Ag nanoparticles (Ag-NPs) and Au-NPs (denoted as CNAg and CNAu, respectively). The surface catalysis activity of Ag-NPs was found to be the dominant mechanism for efficient photocatalytic reduction of nitrate. Moreover, the negative influence of dissolved O2 in inhibiting nitrate reduction and positive influence in improving oxidation capacity were revealed, providing further guidance for practical applications.
2. Experimental details
2.1 Materials
Urea, silver nitrate, methanol, anhydrous ethanol were purchased from Sinopharm Group Chemical Reagent Co., Ltd. All reagents were of analytical grade and used without further purification. Deionized water (18.2 MΩ) used in all experiments was produced using a Millipore Direct-Q system.2.2 Synthesis of g-C3N4 and g-C3N4 decorated with Ag, Au, and Pt nanoparticles
2.3 Characterization
The samples were characterized through X-ray diffraction (XRD, Bruker D8 Advance) using CuKα radiation (λ = 1.54178 Å). Transmission electron microscopy (TEM, JEOLJEM-2010) characterizations were performed at 200 kV. UV-vis diffuse reflectance spectra were performed on a UV-vis spectrophotometer (UV3600-MPC3100). Photoluminescence spectra were recorded on a spectrometer (OmniFluo990LSP) under an excitation wavelength of 310 nm at room temperature.2.4 Photocatalytic reduction and analysis
Photocatalysis experiment was carried out in a container with a glass sleeve (0.2 L, Quartz). The light source was placed on top of the quartz reactor, and the outer layer was cooled with water. Potassium nitrate was used as a nitrate source for degradation. The initial concentration of nitrate (100 mg L−1) was 10 times the maximum permissible concentration limit in drinking water. The catalyst concentration in the solution was 1.2 g L−1, and methanol was used as a hole sacrificial agent. A 300 W xenon lamp (Microsolar 300) was used as the light source for the photocatalysis experiment. The solution was kept under constant stirring conditions during the whole experiment. After every 60 min, 1 mL of solution was extracted and filtered for ion chromatography measurements (Dionex Aquion and Dionex ICS-600).The NO3− conversion rate (CNO3−, %) and N2 selectivity (SN2, %) were evaluated using eqn (1) and (2), respectively.
 | (1) |
 | (2) |
2.5 Electrochemical measurements
Firstly, the catalyst ink was obtained by dispersing 3 mg of catalyst in 90 μL of anhydrous ethanol and 10 μL of Nafion, followed by sonication for 10 min. Subsequently, 5 μL of the catalyst ink was coated on an L-shaped glassy carbon electrode and dried to obtain the working electrode. The Ag/AgCl electrode was used as the reference electrode and the Pt electrode as the counter electrode. The electrochemical impedance spectroscopy (EIS) results and Mott–Schottky curves of the catalysts were measured using an electrochemical workstation (CHENHUA, CHI660E, China) in 0.25 M K2SO4 solution. Cyclic voltammetry (CV) and linear scanning voltammetry (LSV) were performed in 0.25 M K2SO4 and 0.2 g per L KNO3 solutions. For photoelectrochemical tests, a 300 W xenon lamp was used as the light source.3. Results and discussion
The morphology of the achieved CNAg composite was determined using TEM (Fig. 1 and S1†). Under a longer photodeposition duration, the size of Ag-NPs gradually increased (Fig. S2†). Fig. 1a shows the morphology of CNAg-30 under a photodeposition of 30 min; Ag-NPs of about 10 nm were loaded onto lamellar-structured g-C3N4. The lattice spacing of the exposed crystal faces of Ag-NPs was 0.21 nm, which was attributed to the (200) crystal faces, in accordance with the Fourier-transform image (Fig. 1b). Energy-dispersive X-ray maps (Fig. 1c–f) show the uniform distribution of C and N in g-C3N4 and the successful loading of Ag-NPs. | ||
| Fig. 1 (a) TEM image, (b) high-resolution image, and (c–f) EDS mapping of C, N and Ag elements of CNAg-30. | ||
Fig. 2a shows the X-ray diffraction pattern of the as-prepared samples. The strong diffraction peak signal near 27.6° corresponds to the interlayer stacking planes of the conjugated heptazine frameworks.34 After photodeposition of Ag for more than 30 min, the reduced Ag-NPs crystallized (PDF# 89-3722). Similar crystallization of Au-NPs is shown in Fig. S3.† Fig. 2b shows the light absorption of the as-prepared samples. The absorption peaks at 470 and 550 nm after loading Ag and Au nanoparticles were attributed to the localized surface plasmon resonance (LSPR) absorption of Ag- and Au-NPs, respectively. The forbidden bandgap of g-C3N4 was determined to be 2.8 eV from the Tauc plot (Fig. 2c) using the formula (αhν)1/n = B(hν – Eg), where n = 0.5 since g-C3N4 is a direct bandgap semiconductor. The flat band potential of g-C3N4 was calculated to be −1.0 V (vs. SHE) based on the Mott–Schottky curve (Fig. 2d). Next, the conduction band edge of g-C3N4 was deduced to be −1.2 V (vs. SHE), according to the empirical formula that the semiconductor's conduction band value and flat-band potential differ by 0.2 eV,35 which was consistent with previously reported results.36
 | ||
| Fig. 2 (a) XRD and (b) UV-vis spectra of different samples. (c) Mott–Schottky and (d) Tauc plot of g-C3N4. | ||
Nitrate photocatalytic reduction performances were evaluated (Fig. 3a). Pure g-C3N4 showed no reduction effect. The CNAu composites showed no considerable photocatalytic reduction performance either. However, after loading Ag-NPs, the CNAg composites showed significant photocatalytic reduction efficiency toward nitrate. Especially, CNAg-30 exhibited the optimal nitrate reduction rate, and total nitrates were degraded within 1 h. Nevertheless, N2 selectivity was only about 38% (Fig. 3b) and the predominant reduction product was NO2− (Fig. S4†), indicating that the reduction driving force was limited under xenon lamp irradiation, which contains limited UV light. The XRD patterns of the samples before and after the cyclic photocatalytic reaction were basically unchanged (Fig. S5†), indicating the relative stability of the as-prepared photocatalyst.
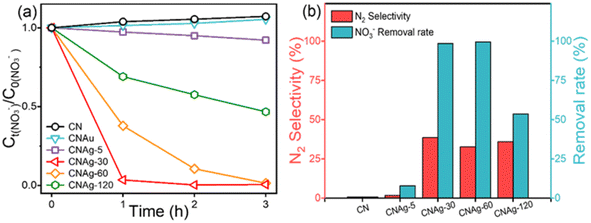 | ||
| Fig. 3 (a) Nitrate removal dynamics of different samples and (b) removal rates and N2 selectivity after 3 h of photocatalysis. | ||
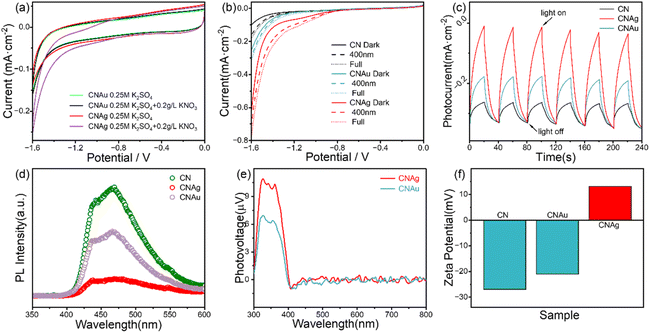
The photocatalytic mechanism of CNAg toward NO3− was revealed by comparing the photoelectrochemical response. A previous work claimed that the adsorbed hydrogen (H*) was the reason for the photocatalytic activity of CNAg.37 However, if H* plays a role in NO3− reduction, the CNAu composite could reduce NO3− as it can produce H* too.38 To reveal the function of H*, g-C3N4 decorated with Pt-NPs was synthesized (Fig. S6†). However, no nitrate reduction was observed in the CN–Pt system. Thus, H* should not be the real reason for the photocatalytic reduction of NO3−. Other possible mechanisms include specific surface catalytic activity, surface plasmonic hot electrons, or enhanced separation efficiency of photogenerated carriers. The CV curves of CNAg and CNAu in the dark with/without NO3− illustrate that CNAu has no catalytic effect on NO3− (Fig. 4a); however, CNAg shows considerably increased catalytic activity with an onset potential of about −0.9 V (vs. Ag/AgCl). LSV curves for different light illumination conditions demonstrate that all the photocatalysts showed a light response (Fig. 4b), with the CNAg composite demonstrating the highest increase in current after light illumination, which is more clearly depicted in Fig. 4c. Under chopped light illumination, the photocurrent of CNAg was significantly higher than that of CNAu or bare g-C3N4. The slow response speed of the photocurrent demonstrated that the photocurrent is induced by the reduction of nitrate owing to the slow reaction dynamics. Additionally, the recombination probability of photogenerated carriers decreased after decoration with the metal nanoparticle, especially Ag-NPs, which was demonstrated by photoluminescence (PL) spectroscopy (Fig. 4d). Moreover, the surface photovoltage reveals that the CNAg composite can induce higher separation efficiency of photogenerated charge carriers (Fig. 4e), which can generate higher photocatalytic performance. Electrochemical impedance spectroscopy (Fig. S7†) reveals the CNAg composite has a lower impedance value, which is favourable for the separation and transport of charge carriers. Moreover, the zeta potentials (Fig. 4f) of CN and CNAu are negative, whereas they are positive for CNAg. As NO3− is negatively charged, electrostatic repulsion will inhibit the adsorption of NO3− for CN and CNAu, while CNAg can benefit adsorption, which can accelerate the photocatalytic reduction. Thus, based on the significant positive shift of the onset potential of NO3− reduction without light illumination (Fig. 4a) and no surface photovoltage at the wavelength of LSPR of the Ag- and Au-NPs (Fig. 4e), the surface catalytic activity of Ag-NPs, but not the plasmonic hot electrons, is believed to be the predominant reason for the outstanding photocatalytic NO3− reduction by CNAg. Furthermore, the Ag-NPs enhanced the separation efficiency of photogenerated electrons and holes, resulting in improved photocatalytic performance. Thus, the small size and large number of Ag nanoparticles should benefit the performance. Characterization results (Fig. 1, S1 and S2†) demonstrate that Ag nanoparticles with photodeposition of 30 min exhibited the optimal balance of size and density (i.e., small size and large density), which can provide more reaction sites that act as electron traps to accelerate the reduction reaction.
Dissolved O2 has always existed in the real application case; however, the influence on photocatalytic nitrate reduction has not been studied. Fig. 5a depicts the ion chromatography measurement results recorded for a photocatalytic reaction of 6 h, in which the dissolved oxygen in the solution is not excluded in the initial 3 h and removed via argon bubbling in the next 3 h with CNAg-30 as a photocatalyst and methanol as a hole scavenger. When dissolved oxygen is present, a chromatography peak attributed to formic acid gradually increases with the photocatalytic duration, which is further confirmed by NMR hydrogen spectroscopy with a peak located near 8.15 ppm (Fig. 5b).39–41 Although it has been widely reported that the oxidation of formic acid by holes can generate ˙CO2− radicals, which can efficiently reduce NO3−,42–45 the degradation of NO3− is not observed with dissolved O2 based on the chromatography peak intensity of NO3− (Fig. 5a). This is probably attributed to the consumption of photogenerated electrons by reacting with dissolved O2, producing superoxide radicals (˙O2−), and the inverse oxidation of NO2− by the resultant hydroxyl radicals (˙OH) or single-linear oxygen (1O2).46,47 Argon bubbling was used to rapidly remove dissolved O2. Thereafter, electrons and ˙CO2− could efficiently reduce NO3−, resulting in a rapid reduction in concentration. If the photocatalysis is started in the absence of dissolved O2, no considerable formic acid and the consequent ˙CO2− can be produced, and thus, NO3− is reduced only by photogenerated electrons with limited driving force, which is determined by the conduction band of the photocatalyst. As a result, a large amount of NO2− (Fig. 5c, NO2− concentration is represented in blue and NO3− concentration in red) and low N2 selectivity (Fig. 5d) are obtained. However, if the photocatalysis is carried out with dissolved O2 for 3 h followed by subsequent removal of O2, the reactivated photocatalytic reduction of NO3− produces no NO2− and extremely high N2 selectivity (Fig. 5d) as a result of the supplementary reduction capacity of ˙CO2− radicals generated from formic acid. Thus, the dissolved O2 demonstrates a critical inhibition of photocatalytic reduction of NO3−, although it can help produce ˙CO2− to improve the catalytic activity and N2 selectivity. As a result, in future research, the design of a photocatalyst or device without influence of dissolved O2 would be the biggest bottleneck in practical applications.
4. Conclusions
In summary, Ag- and Au-NPs were in situ grown on g-C3N4 for nitrate reduction. Ag-NPs showed improved surface catalytic activity and carrier separation efficiency, which significantly enhanced photocatalytic NO3− reduction. Additionally, CNAg could oxidize methanol to formic acid and, consequently, to ˙CO2− radicals in the dissolved O2 atmosphere, which could improve the reduction dynamics and N2 selectivity. However, the dissolved O2 in practical cases would be a critical bottleneck owing to the consumption of photogenerated electrons. The work provides new perspectives for subsequent development of solar-driven removal of nitrate from contaminated water.Data availability
All the data related to this work are available in the main manuscript or ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants No. 52472309, 52232007, 51972308) and HFIPS Director's Fund (Grant No. BJPY2022B03).References
- J. Wang, X. Liu, A. H. W. Beusen and J. J. Middelburg, Environ. Sci. Technol., 2023, 57, 19395–19406 CAS.
- S. Qu, L. Duan, Z. Shi, H. Mao, G. Wang, T. Liu, R. Yu and X. Peng, J. Cleaner Prod., 2022, 376, 134289 CAS.
- J. Yan, J. Chen and W. Zhang, Water Res., 2023, 243, 120379 CAS.
- P. J. Clemmensen, J. Schullehner, N. Brix, T. Sigsgaard, L. T. Stayner, H. A. Kolstad and C. H. Ramlau-Hansen, Curr. Environ. Health Rep., 2023, 10, 250–263 CAS.
- N. G. Hord, Curr. Atheroscler. Rep., 2011, 13, 484–492 CrossRef CAS PubMed.
- N. Medgyesi Danielle, B. Trabert, J. Sampson, J. Weyer Peter, A. Prizment, A. Fisher Jared, E. Beane Freeman Laura, H. Ward Mary and R. Jones Rena, Environ. Health Perspect., 2022, 130, 057012 CrossRef CAS PubMed.
- J. A. Alcolea, C. Donat-Vargas, A. C. Chatziioannou, P. Keski-Rahkonen, N. Robinot, A. J. Molina, P. Amiano, I. Gómez-Acebo, G. Castaño-Vinyals, L. Maitre, M. Chadeau-Hyam, S. Dagnino, S. L. Cheng, A. Scalbert, P. Vineis, M. Kogevinas and C. M. Villanueva, Environ. Sci. Technol., 2023, 57, 19316–19329 CrossRef CAS PubMed.
- S. Lu, Q. Zhu and R. Li, J. Colloid Interface Sci., 2023, 652, 1481–1493 CrossRef CAS PubMed.
- P. Loganathan, S. Vigneswaran and J. Kandasamy, J. Environ. Manage., 2013, 131, 363–374 CrossRef CAS.
- B. Yan, K. Kato, S. Shimoda, R. Otomo and Y. Kamiya, Chem. Eng. J., 2024, 498, 155721 CrossRef CAS.
- T. Nur, W. G. Shim, P. Loganathan, S. Vigneswaran and J. Kandasamy, Int. J. Environ. Sci. Technol., 2014, 12, 1311–1320 CrossRef.
- J. Ma, W. Wei, G. Qin, L. Jiang, N. Hing Wong, J. Sunarso and S. Liu, Sep. Purif. Technol., 2022, 292, 121010 CrossRef CAS.
- Y. Zhou, L. Zhang, Z. Zhu, M. Wang, N. Li, T. Qian, C. Yan and J. Lu, Angew. Chem., Int. Ed., 2024, 63, e202319029 CrossRef CAS PubMed.
- J. Bi, C. Peng, H. Xu and A.-S. Ahmed, Desalin. Water Treat., 2011, 34, 394–401 CrossRef CAS.
- H. Duan, L. Zhang, H. Wang, S. Li, X. Li and Y. Zhuang, Sci. Total Environ., 2024, 951, 175745 CrossRef CAS PubMed.
- Z. S. Wei, J. B. Wang, Z. S. Huang, X. L. Xiao, M. R. Tang, B. L. Li and X. Zhang, Biomass Bioenergy, 2019, 126, 34–40 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS PubMed.
- F. Yang, P. Hu, F. Yang, X.-J. Hua, B. Chen, L. Gao and K.-S. Wang, Tungsten, 2023, 6, 77–113 Search PubMed.
- H. O. Tugaoen, S. Garcia-Segura, K. Hristovski and P. Westerhoff, Sci. Total Environ., 2017, 599–600, 1524–1551 CrossRef PubMed.
- J. M. A. Freire, M. A. F. Matos, D. S. Abreu, H. Becker, I. C. N. Diógenes, A. Valentini and E. Longhinotti, J. Environ. Chem. Eng., 2020, 8, 103844 CAS.
- W. Yang, J. Wang, R. Chen, L. Xiao, S. Shen, J. Li and F. Dong, J. Mater. Chem. A, 2022, 10, 17357–17376 CAS.
- S. Yu, S. Shen, C. Zhang, R. Chen, H. Dan, J. Li and F. Dong, Appl. Catal., B, 2024, 358, 124419 CAS.
- W.-M. Wang, S.-J. Tseng, Y.-S. Huang, Q.-Y. Wu, W.-L. Wang and J. J. Wu, J. Ind. Eng. Chem., 2023, 119, 386–394 CAS.
- K. T. Ranjit, R. Krishnamoorthy and B. Viswanathan, J. Photochem. Photobiol., A, 1994, 81, 55–58 CAS.
- G. Liu, S. You, M. Ma, H. Huang and N. Ren, Environ. Sci. Technol., 2016, 50, 11218–11225 CAS.
- Z. Hou, F. Chen, J. Wang, C. P. François-Xavier and T. Wintgens, Appl. Catal., B, 2018, 232, 124–134 CAS.
- H. Shi, C. Li, L. Wang, W. Wang, J. Bian and X. Meng, Sep. Purif. Technol., 2022, 300, 121854 CAS.
- R. Yin, P. U. Dao, J. Zhao, K. Wang, S. Lu, C. Shang and H. Ren, Environ. Sci. Technol., 2024, 58, 20676–20686 CAS.
- K. Yin, Z. Yan, N. Fang, W. Yu, Y. Chu, S. Shu and M. Xu, Sep. Purif. Technol., 2023, 325, 124636 CAS.
- H. Wang, X. Li, X. Zhao, C. Li, X. Song, P. Zhang, P. Huo and X. Li, Chin. J. Catal., 2022, 43, 178–214 CAS.
- C. Wang, S. Dong, Y. Wang, T. Guo, G. Gao, Z. Lu and B. Pan, Chem. Eng. J., 2020, 397, 125435 CAS.
- Y. Kim, E. B. Creel, E. R. Corson, B. D. McCloskey, J. J. Urban and R. Kostecki, Adv. Energy Mater., 2018, 8, 1800363 Search PubMed.
- P. M. Schaber, J. Colson, S. Higgins, D. Thielen, B. Anspach and J. Brauer, Thermochim. Acta, 2004, 424, 131–142 CAS.
- A. Zambon, J. M. Mouesca, C. Gheorghiu, P. A. Bayle, J. Pecaut, M. Claeys-Bruno, S. Gambarelli and L. Dubois, Chem. Sci., 2016, 7, 945–950 CAS.
- F. Li, X. Li, S. Tong, J. Wu, T. Zhou, Y. Liu and J. Zhang, Nano Energy, 2023, 117, 108849 CAS.
- N. Shehzad, M. Tahir, K. Johari, T. Murugesan and M. Hussain, J. CO2 Util., 2018, 26, 98–122 CrossRef CAS.
- S. J. P. Varapragasam, J. M. Andriolo, J. L. Skinner and E. M. Grumstrup, ACS Omega, 2021, 6, 34850–34856 CrossRef CAS PubMed.
- Z. Mo, H. Xu, Z. Chen, X. She, Y. Song, P. Yan, Y. Xu, Y. Lei, S. Yuan and H. Li, Chin. J. Catal., 2018, 39, 760–770 CrossRef CAS.
- W.-M. Zhang, K.-W. Feng, R.-G. Hu, Y.-J. Guo and Y. Li, Chem, 2023, 9, 430–442 CAS.
- J. Shi, H. He, S. Zhou, J. Li and W. Cai, Green Chem., 2024, 26, 2638–2644 RSC.
- J. Wu, J. Wen and Y.-X. Yu, Appl. Surf. Sci., 2021, 565, 150567 CrossRef CAS.
- L. Wang, W. Fu, Y. Zhuge, J. Wang, F. Yao, W. Zhong and X. Ge, Chemosphere, 2021, 278, 130298 CrossRef CAS PubMed.
- H. Shi, C. Li, R. Zheng, L. Wang, W. Wang, J. Bian and X. Meng, J. Cleaner Prod., 2023, 407, 137088 CrossRef CAS.
- H. Shi, C. Li, L. Wang, W. Wang and X. Meng, J. Hazard. Mater., 2022, 424, 127711 CrossRef CAS PubMed.
- I. Hong, H. S. Moon, B. J. Park, Y.-A. Chen, Y.-P. Chang, B. Song, D. Lee, Y. Yun, Y.-J. Hsu, J. W. Han and K. Yong, Chem. Eng. J., 2024, 484, 149506 CrossRef CAS.
- K. Tennakone, S. Punchihewa and R. U. Tantrigoda, Environ. Pollut., 1989, 57, 299–305 CrossRef CAS PubMed.
- L. Yang, Z. Chen, Q. Cao, H. Liao, J. Gao, L. Zhang, W. Wei, H. Li and J. Lu, Adv. Mater., 2024, 36, 2306758 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00782h |
| This journal is © The Royal Society of Chemistry 2025 |