Open Access Article
Open Access ArticleFacile fabrication of robust superhydrophobic tapered needles for collection and transportation of underwater bubbles
Huicong Liu a,
Huaxia Wang
a,
Huaxia Wang a,
Liqun Zhua,
Weiping Li
a,
Liqun Zhua,
Weiping Li a,
Haining Chen
a,
Haining Chen a and
Weitao Liang
a and
Weitao Liang *b
*b
aSchool of Materials Science and Engineering, Beihang University, No. 37 Xueyuan Road, Haidian District, Beijing 100191, China
bb, School of Aeronautic Science and Engineering, Beihang University, No. 37 Xueyuan Road, Haidian District, Beijing 100191, China. E-mail: lwt@buaa.edu.cn
First published on 20th March 2025
Abstract
The collection and transportation of underwater bubbles has attracted significant attention due to their wide range of applications in the mining, petroleum, and chemical industries. In this study, robust superhydrophobic tapered needles were successfully fabricated by spraying a superhydrophobic coating prepared by an organic–inorganic hybrid method. The prepared tapered needles present excellent surface stability and good superhydrophobicity with a contact angle (CA) of about 156°. The fabricated tapered needles demonstrate excellent performance in collection and transportation of underwater bubbles and the working mechanism was also thoroughly studied. The prepared robust superhydrophobic tapered needles provide a simple, efficient and economical way for collection and transportation of underwater bubbles.
1 Introduction
Micro-bubbles, characterized by their small diameter ranging from 10 to 50 μm, have garnered significant interest for their diverse applications,1–3 including heavy medium coal preparation,4 pipeline transportation,5–9 drag reduction,5,10–14 and more. In heavy-medium coal preparation, micro-bubbles alter the surface properties of target minerals, facilitating efficient fine coal separation. In pipeline transport, they increase the mass transfer area, effectively mitigating clogging issues. Additionally, when applied to the underwater surfaces of ships, micro-bubbles reduce skin friction between the hull and water, leading to a significant decrease in hydrodynamic resistance. On the other hand, micro-bubbles may also be harmful. For example, bubbles in crude oil will seriously affect the measurement accuracy of crude oil storage and transportation, resulting in reduced transportation and separation efficiency.15–17 At the same time, micro-bubbles in crude oil also tend to absorb small solid impurities, which can cause blockages in pipelines and damage the transport and separation equipment. The presence of micro-bubbles in a water-based coating will produce micro pores, causing cavitation erosion and seriously affecting the protective effect of coatings.15,18 Above all, it is meaningful to explore effective methods for collection and transportation of underwater bubbles. Traditional methods are mainly divided into physical methods and chemical methods,19,20 such as the defoaming method, mechanical method, heating method, ultrasonic method, and more. However, these methods are either energy-intensive or necessitate the installation of additional costly specialized equipment. Furthermore, they exhibit low efficiency, particularly when dealing with small-diameter micro-bubbles. Consequently, there is an urgent need to develop a cost-effective and highly efficient method for the collection and transportation of underwater bubbles.In recent years, super-wetting surfaces have garnered significant attention from researchers due to their broad applicability and ease of fabrication. Surfaces with tailored super-wetting properties have been engineered for a variety of applications, including corrosion protection,21,22 water vapor collection,23 and droplet directional movement,24,25 among others. Some researchers have begun to explore the potential of super-wetting surfaces as a solution for manipulating underwater bubbles.26–33 Ma Rui et al. reported a superhydrophobic sponge, which achieves rapid collection and stable storage of underwater bubbles. However, the superhydrophobic sponge did not achieve efficient control over the dynamic behavior of underwater bubbles, such as their movement or size distribution. Pei et al. reported a Janus membrane with superhydrophobic and superhydrophilic surfaces on opposite sides. It shows excellent unidirectional bubble permeability, and the results prove its effectiveness in underwater bubble manipulation. Yin et al. reported a trapezoidal platform featuring a micro/nano – structure through femtosecond laser direct cutting. The surface of this gradient platform exhibits superhydrophobicity in the air. Notably, this platform can gather bubbles in water and achieve the directional transport of bubbles without external forces. Cao et al. reported a superhydrophobic copper helix capable of achieving controlled, directional bubble transport in water. The bubble velocity can be adjusted based on the helix distance. Yu et al. reported a lubricant-infused slippery (LIS) surface with exceptional water repellency, enabling efficient manipulation of bubbles in aqueous environments. However, its poor surface strength and high consumption significantly limit applications.
Our group has been paying much attention to the preparation and application of super-wetting films for a long time.34–36 Ye et al. prepared a superhydrophobic coating with excellent mechanical durability, self-cleaning performance, and corrosion resistance through a novel organic synthesis and organic–inorganic composite method. Liang et al. prepared a composite coating for wax prevention in crude oil transportation. Yang et al. used the hydrolysis and condensation of tetraethoxysilane (TEOS) to form a silica sol–gel film while achieving controllable film material wettability. The thiol synthesis method based on click reaction has become a research hotspot in recent years due to its advantages such as low oxygen resistance, commercially accessible raw materials, few side reactions, simple operating conditions, and no use of metal catalysts.37–45
In this study, a facile click reaction was utilized to fabricate robust superhydrophobic coatings on tapered copper needles. Organic–inorganic composite coatings were fabricated via thiol-ene click reaction, leveraging its rapid kinetics and robust bonding characteristics. The influence of the organic-to-inorganic ratio on hydrophobicity was investigated, revealing that a composition of 0.4 g resin and 0.2 g SiO2 achieved a water contact angle of 156°, indicating superior hydrophobicity. The durability of coatings was assessed via cyclic abrasion tests. A water contact angle of 147° persisted after 20 friction cycles, confirming mechanical robustness. The superhydrophobic tapered copper needles demonstrated efficient underwater bubble manipulation, attributed to synergistic effects of hierarchical microstructures and surface chemistry. The superhydrophobic surface stabilizes an air plastron (Cassie–Baxter state) on the tapered copper needle submerged underwater, creating interfacial pathways for sustained directional transport of microbubbles. Due to the tapered shape, the needles will produce a gradient of Laplace pressure, which can derive a directional driving force and transport droplets directionally.46–48 The collection and transportation of bubbles by superhydrophobic copper needles under reverse gravity was also studied. This method will provide a simple and effective method to collect and transport micro-bubbles.
2 Materials and methods
2.1 Materials
All reagents were obtained at the highest purity available and used without further purification unless otherwise specified. Pentaerythritol tetra-3-mercaptopropionate (PETMP, Shanghai Aladdin Biochemical Technology Co., Ltd), benzoin dimethyl ether (Adamas Reagent Co., Ltd), acetone (Beijing Chemical Plant), dodecafluoroheptyl methacrylate, 3-(trimethoxysilyl)propyl methacrylate (Adamas Reagent Co., Ltd), tetraethoxysilane (TEOS, Guangzhou Xilong Chemical Co., Ltd), dibutyltin silicate (Tianjin Jinke Fine Chemical Research Institute), hydrophobic SiO2 nanoparticles (7–40 nm, Shanghai Aladdin Biochemical Technology Co., Ltd), CuSO4 (West long chemical).2.2 Preparation of organic–inorganic composite coating
According to the free radical mechanism, the synthesize process mainly includes the following steps. At first, a certain amount of PETMP, benzoin dimethyl ether and acetone were added to the bottle in N2 atmosphere and synthesized at 25 °C ± 2 °C. After stirring uniformly, dodecafluoroheptyl methacrylate was slowly added into the bottle through a constant pressure dropping funnel and reacted under UV light for 1 h. When completed, 3-(trimethoxysilyl)propyl methacrylate was also slowly added into the bottle and reacted under UV light for 1 h. (PETMP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dodecafluoroheptyl methacrylate
dodecafluoroheptyl methacrylate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3-(trimethoxysilyl)propyl methacrylate = 1
3-(trimethoxysilyl)propyl methacrylate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After the reaction was completed, a light-yellow liquid of fluorosilicone resin is obtained. The above fluorosilicone resin was added to the solution containing hydrophobic nano-silica, and ethyl orthosilicate, dibutyltin dilaurate and acetone were added to obtain a dispersed and uniform mixed solution. Finally, the superhydrophobic coating of organic–inorganic composite was obtained by spraying the prepared solution on the glass sheet and placing it at room temperature 25 °C for 24 h or heating it to 80 °C for 0.5 h.
2). After the reaction was completed, a light-yellow liquid of fluorosilicone resin is obtained. The above fluorosilicone resin was added to the solution containing hydrophobic nano-silica, and ethyl orthosilicate, dibutyltin dilaurate and acetone were added to obtain a dispersed and uniform mixed solution. Finally, the superhydrophobic coating of organic–inorganic composite was obtained by spraying the prepared solution on the glass sheet and placing it at room temperature 25 °C for 24 h or heating it to 80 °C for 0.5 h.
2.3 Preparation of tapered needles
A uniform-diameter copper wire (0.4 mm diameter, 8 cm length) underwent sequential ultrasonic cleaning in 1.0 mol L−1 hydrochloric acid, acetone, and ethanol, each for 5 minutes. Following deionized water rinsing, the wire was nitrogen-dried to complete pretreatment. The prepared wire was vertically mounted in an electrolytic cell (11 cm × 6 cm × 7 cm) using a copper–graphite electrode. The electrolyte consisted of CuSO4 in 0.2 mol L−1 H2SO4 solution. During electrodeposition, continuous electrolyte circulation was maintained through a syringe pump system extracting solution at 0.5 mL s−1 for 10 extraction cycles. This dynamic flow regime facilitated the formation of a tapered copper needle structure. Post-processing included surface cleaning and nitrogen-protected ambient drying. For subsequent coating application, the tapered needle was vertically fixed on a rotation stage programmed for controlled clockwise rotation. A spray gun maintained at 30 kPa pressure was positioned 20 cm from the rotating substrate to ensure uniform deposition.2.4 Characterization
The surface morphology of the fabricated coatings was characterized using field emission scanning electron microscopy (FESEM, JSM-7500F, JEOL, Japan). Chemical composition analysis was performed through Fourier transform infrared spectroscopy (FT-IR) recorded on a NEXUS-470 spectrometer (Nicolet, USA) employing the KBr-pellet method, with spectral acquisition ranging from 4000 to 500 cm−1 at 8 cm−1 resolution. Surface topography evaluation was conducted via atomic force microscopy (AFM, Veeco DI) in tapping mode under ambient conditions, utilizing a scan rate of 256 Hz over a 5 μm × 5 μm imaging area. Wettability characteristics were determined using a KRÜSS DSA20 optical contact angle analyzer (Germany) via the sessile-drop method at ambient temperature. Static contact angles were measured with 5 μL droplets, while sliding angles were determined by incrementally tilting the stage until 10 μL droplets initiated movement. Mechanical durability assessments included knife-scratch resistance and sandpaper abrasion tests, following established protocols from previous studies. For standardized testing, coatings were deposited on glass substrates. The scratch resistance evaluation involved creating grid patterns on coated surfaces using a standardized cutting tool. Abrasion resistance was quantified by subjecting specimens (100 g loading) to linear abrasion against 800-grit sandpaper (0.5 kPa pressure) along orthogonal directions. Each test cycle consisted of a 10 cm linear abrasion followed by 90° specimen rotation. Post-abrasion hydrophobicity was monitored through sequential contact angle measurements after predetermined test cycles.3 Results and discussion
3.1 Reaction mechanism and surface morphology analysis of prepared organic–inorganic composite coating
The thiol-ene click reaction mechanism and its kinetic behavior have garnered significant attention from the global materials science community since their discovery. This surface modification strategy enables simultaneous introduction of functional groups and enhancement of critical material properties including adhesion and hydrophobicity. As illustrated in Fig. 1(a), the reaction mechanism bifurcates based on olefin configuration types. The thiol-alkene system operates through a radical chain-transfer mechanism, where thiyl radicals (–S·) initiate nucleophilic attack on the carbon–carbon double bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), subsequently propagating the active site through chain transfer processes. The radical-mediated thiol-ene addition proceeds through iterative chain propagation, while thiol-methacrylate systems with conjugated olefins follow a Michael addition pathway. In the latter mechanism, the nucleophilic thiolate anion (S−) attacks the β-carbon of the electron-deficient C
C), subsequently propagating the active site through chain transfer processes. The radical-mediated thiol-ene addition proceeds through iterative chain propagation, while thiol-methacrylate systems with conjugated olefins follow a Michael addition pathway. In the latter mechanism, the nucleophilic thiolate anion (S−) attacks the β-carbon of the electron-deficient C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, forming a carbanion intermediate that regenerates active species via proton abstraction from either –SH groups or alkali cations. This chain-propagation mechanism significantly enhances reaction kinetics. Subsequent integration of –Si(OCH3)3 low-surface-energy groups into the resin matrix, coupled with TEOS as a curing agent and dibutyltin dilaurate catalysis, promotes siloxane (Si–O–Si) network formation through hydrolytic condensation. As illustrated in Fig. 1(b), this cross-linked architecture synergistically optimizes both hydrophobicity and mechanical strength in the final coating.
C bond, forming a carbanion intermediate that regenerates active species via proton abstraction from either –SH groups or alkali cations. This chain-propagation mechanism significantly enhances reaction kinetics. Subsequent integration of –Si(OCH3)3 low-surface-energy groups into the resin matrix, coupled with TEOS as a curing agent and dibutyltin dilaurate catalysis, promotes siloxane (Si–O–Si) network formation through hydrolytic condensation. As illustrated in Fig. 1(b), this cross-linked architecture synergistically optimizes both hydrophobicity and mechanical strength in the final coating.
Fig. 2 characterizes the surface morphology and chemical composition of modified SiO2 particles prepared via a photoinitiated fluorosilicone resin synthesis. FT-IR analysis in Fig. 2(a) reveals significant spectral changes post-modification: the broadened and attenuated –OH stretching vibration at 3433 cm−1 confirms successful silanol group consumption through chemical grafting. Characteristic perfluoroalkyl absorptions at 1204 cm−1 and 1149 cm−1, combined with the absence of residual C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1636 cm−1) and –SH (2564 cm−1) signals, demonstrate complete thiol-ene conversion and successful integration of fluorinated moieties into the resin matrix. SEM imaging at multiple magnifications in Fig. 2(b and c) reveals inherent micro/nanoscale hierarchical structures formed during spray deposition. High-resolution imaging confirms the coexistence of micron-sized aggregates and nanoscale surface asperities from fumed SiO2, collectively establishing a porous multilevel morphology. EDS elemental mapping in Fig. 2(d) verifies uniform surface distribution of F (17.58 ± 0.13 wt%), S, and Si – with fluorine enrichment (vs. bulk 11%) indicating surface-segregation of low-surface-energy fluoropolymer chains. The detected sulfur and silicon derive respectively from PETMP crosslinkers and SiO2 nanoparticles, consistent with the designed formulation.
C (1636 cm−1) and –SH (2564 cm−1) signals, demonstrate complete thiol-ene conversion and successful integration of fluorinated moieties into the resin matrix. SEM imaging at multiple magnifications in Fig. 2(b and c) reveals inherent micro/nanoscale hierarchical structures formed during spray deposition. High-resolution imaging confirms the coexistence of micron-sized aggregates and nanoscale surface asperities from fumed SiO2, collectively establishing a porous multilevel morphology. EDS elemental mapping in Fig. 2(d) verifies uniform surface distribution of F (17.58 ± 0.13 wt%), S, and Si – with fluorine enrichment (vs. bulk 11%) indicating surface-segregation of low-surface-energy fluoropolymer chains. The detected sulfur and silicon derive respectively from PETMP crosslinkers and SiO2 nanoparticles, consistent with the designed formulation.
3.2 Influence of surface composition on hydrophobicity
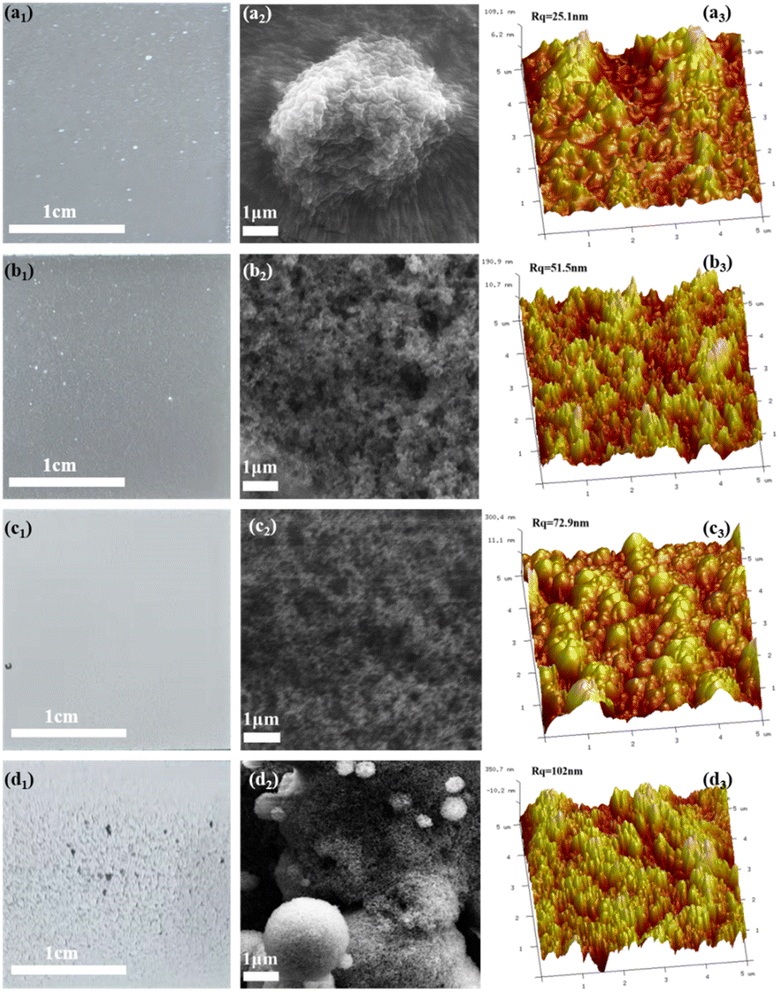
Coatings featuring micro-nano-scale roughness structures often exhibit mechanical fragility and susceptibility to abrasion, particularly on rigid substrates. Organic–inorganic nanocomposites have emerged as a promising strategy to address this limitation, achieved either through polymer-grafted nanoparticles or dispersion of surface-modified nanoparticles within polymeric matrices. Crucially, the organic/inorganic mass ratio critically determines the hydrophobic performance during composite fabrication, necessitating systematic optimization. Table 1 shows 9 formulations for follow-up research.| Group | Fluor silicone/g | SiO2/g | Acetone/g |
|---|---|---|---|
| A | 0.0 | 0.2 | 5.0 |
| A | 0.2 | 0.2 | 5.0 |
| A | 0.4 | 0.2 | 5.0 |
| A | 0.6 | 0.2 | 5.0 |
| B | 0.4 | 0.0 | 5.0 |
| B | 0.4 | 0.05 | 5.0 |
| B | 0.4 | 0.1 | 5.0 |
| B | 0.4 | 0.2 | 5.0 |
| B | 0.4 | 0.4 | 5.0 |
During the preparation process, the ratio of SiO2 particles to resin plays a great role in the hydrophobic effect. In order to determine the most suitable ratio, two sets of samples were prepared: the SiO2 particles of group A were fixed at 0.2 g, and the resin was 0 g, 0.2 g, 0.4 g, and 0.6 g. The resin of group B was fixed at 0.4 g, and the SiO2 particles were 0 g, 0.05 g, 0.1 g, 0.2 g, and 0.4 g. The solvent of the two groups is 5 g of acetone, and the curing agent is 0.2 g.
3.3 The superhydrophobic property of the prepared coating
The fluorosilicone resin–SiO2 superhydrophobic composite coating exhibits excellent superhydrophobicity, with a static water contact angle of up to 156° on the glass substrate. To evaluate its self-cleaning potential, dynamic tests were conducted. As shown in Fig. 5(a), a 10 μL water droplet rapidly slides off the inclined surface (tilt angle: 3°) within 273 ms, indicating the coating's low adhesion properties, which facilitate bubble transportation. Fig. 5(b) further characterizes the dynamic adhesion behavior: when a 5 μL water droplet on a needle contacts the coating surface, a defined pressure is applied to deform the droplet. Subsequently, the droplet detaches without leaving any residue, confirming the coating's exceptional superhydrophobicity.3.4 Collect transport bubbles
Numerous studies have demonstrated that a structurally modified conical surface generates gradients of Laplace pressure and surface free energy, creating a directional driving force for droplet collection and transport. Inspired by this mechanism, we aim to introduce analogous gradients onto conical surfaces to enable continuous collection and directional transport of microbubbles in aqueous media. In this work, a superhydrophobic coating was integrated onto copper needles to achieve efficient microbubble manipulation in deionized water. Upon immersion, the superhydrophobicity of the coated needle induces a stable air layer underwater, providing a low-resistance pathway for microbubble transportation. The Laplace pressure gradient arising from the conical geometry serves as the primary driving force, directing bubbles along the needle surface. This approach offers a simple yet effective strategy for microbubble elimination in liquid systems.As shown in Fig. 6, on the surface of the horizontally placed superhydrophobic copper needle, there are four forces acting on the moving bubbles, namely Laplace pressure (FL), synthetic resistance (FR) generated by the liquid and lubricating layer, buoyancy (FB), and the adhesion of the superhydrophobic copper needle surface to the air bubbles (FA). FL is the driving force for the directional movement of bubbles induced by the cone shape of a superhydrophobic copper cone.49–52
 | (1) |
 | (2) |
| FB = ρgV | (3) |
FA can be derived as follows:56,57
FA = γl1l2LTCL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β β
| (4) |
The collection and transport processes of microbubbles in water by superhydrophobic copper needles were studied. To prevent carbonic acid interference from dissolved CO2, a closed pipe flow system with a flow rate of 0.5 μL s−1 was utilized. The copper needle was positioned horizontally in deionized water to suppress buoyancy effects on bubble dynamics. As shown in Fig. 7, microbubbles contacting the needle surface spread rapidly, confirming its superaerophilic behavior in water. These bubbles migrated from the needle's tip to its tail at 20.5 ± 0.5 mm s−1, merging into a single large bubble. At 97 s, buoyancy exceeded the adhesive force, causing detachment of the 48 μL bubble. This cycle repeated continuously, enabling sustained microbubble collection and transport. The superhydrophobic copper needle was taken out, stood at room temperature for 24 h, and then immersed in water again, it still had the ability to collect and transport bubbles. The application of superhydrophobic copper needles in crude oil exploitation can reduce the bubble content and improve the efficiency of crude oil exploitation; when applied to waterborne coatings, it can improve the film-forming property and make the coating more uniform.
Fig. 8 investigates bubble collection and transport by the superhydrophobic copper needle under reverse gravity conditions. At a tilt angle of 20°, the average transport velocity is 17.4 ± 0.5 mm s−1 with a transport distance of 0.6 cm. When the tilt angle increases to 90°, the velocity decreases to 6.5 ± 0.5 mm s−1 and the distance shortens to 0.5 cm. These results confirm that the needle can collect and transport bubbles against gravity. Comparing these data with the 0° tilt angle case (horizontal placement, Fig. 7), we observe a clear trend: as the tilt angle increases, buoyancy increasingly opposes the adhesive force, leading to slower transport speeds and shorter distances. Nevertheless, the needle maintains functionality even at 90°, demonstrating its robustness in overcoming gravitational effects.
 | ||
| Fig. 8 Superhydrophobic copper needles collect and transport air bubbles against gravity. (a) 20° (b) 90°. | ||
3.5 Stability
The limited mechanical durability of superhydrophobic surfaces poses a significant challenge for their real-world applications. To address this, we systematically evaluated the robustness of an organic–inorganic composite coating through standardized sandpaper abrasion tests and knife scratch experiments.58,59 As illustrated in Fig. 9(a), the abrasion protocol involved cyclically moving the coating (under a 100 g load) across 800# SiC sandpaper—10 cm laterally, followed by a 90° rotation and another 10 cm longitudinal movement—to simulate severe mechanical wear. Remarkably, after 20 such cycles, the coating retained a water contact angle of 147° in Fig. 9(b), indicating robust mechanical resistance. This durability stems from the coating's “bulk superhydrophobicity”, a structural design where hydrophobic SiO2 nanoparticles and interconnected cavities are distributed not only on the surface but also throughout the interior. Consequently, even if the top layer is abraded, the underlying hydrophobic network maintains non-wettability. Comparative analysis with the DMS/ODA-PDA@PI nanofibrous membrane reported by Wenjing Ma et al.,58 revealed that our coating achieved a higher post-abrasion water contact angle (147° vs. 135° after 20 cycles), underscoring its superior mechanical resilience. Furthermore, the coating's ability to repel impacting droplets—causing them to retract or rebound—significantly reduces ice nucleation sites, suggesting strong potential for anti-icing applications in harsh environments. These results collectively demonstrate that the synergy of structural design and material composition can effectively mitigate the mechanical fragility of superhydrophobic coatings.As shown in Fig. 9(c), in the blade scratch test,60 after the blade is scratched according to the preset route, the coating still maintains superhydrophobic properties. After the test, when the dyed water droplets were dropped onto the surface of the scribed coating, they rolled off quickly without any adhesion, indicating the excellent anti-scratch stability of the composite coating.
In the actual application process, the coating may come into contact with robust acid or alkaline substances. In order to evaluate the chemical stability of the composite coating in an acid-base environment, this section adopts two methods, using aqueous solutions of different pH values to test the static contact angle of the coating surface and immersing the coating in aqueous solutions of different pH values. After a certain period of time, take out the coating and measure the static contact angle for evaluation. Fig. 9(d) shows the static contact angles of aqueous solutions with different pH values on the coating surface. It can be seen from the figure that solutions with different acidity and alkalinity all exhibit excellent superhydrophobicity on the surface of the composite coating. The contact angle of acidic droplets is generally higher and more stable than that of alkaline droplets. By comparing with the DMS/ODA-PDA@PI nanofibrous membrane prepared by Wenjing Ma et al.,58 the performance of this coating is better in acidic environment, and the hydrophobic angle is more than 154°. However, in alkaline environment, it is slightly insufficient and fluctuates greatly. This may be due to the high affinity of the nanoparticles on the surface of the coating with the sodium hydroxide solution, which allows the alkaline aqueous solution to diffuse into the porous micro–nano structure with air hidden on the surface of the coating. Further, the composite coating is immersed in a solution of hydrochloric acid and sodium hydroxide with pH of 1 and 14. The experimental results show that after immersing in a hydrochloric acid solution with a pH of 1 for 10 hours, there is no change on the surface of the coating, and the surface remains dry. After removal from the solution, the surface of the coating remains dry and does not stick to the solution. These results indicate that the superhydrophobic composite coating has outstanding stability in a robust acid environment. However, for a robust alkaline sodium hydroxide solution with a pH of 14, the surface of the coating was partially wetted and lost its mirror effect after 1 hour of soaking. After soaking for 10 hours, the surface of the composite coating was completely wetted. This indicates that the composite coating has poor resistance to robust alkaline environments.
4 Conclusions
In this article, a fast and simple method for preparing superhydrophobic coating by spraying perfluoro thiol/acrylate resin containing hydrophilic silica nanoparticles was introduced. The hydrophobic angle reaches 156°, and it has excellent mechanical properties and acid resistance. The spray deposition method of the nanoparticle-loaded resin provides the coating with a layered rough morphology on a wide range of substrates. Combined with copper cones, it can be used for gas collection and transportation in gas–water–solid three-phase systems. The superhydrophobic copper cone continuously collects microbubbles from the carbonated water, and then directionally transport them to the bottom surface driven by the gradient of Laplace pressure and surface free energy. The transport direction of the microbubbles can be controlled by the superhydrophobic copper cone.Data availability
The data supporting this study are available on request from the corresponding author.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are very grateful to the support from National Key Research and Development Program (2023YFB3408200).References
- P. Khan, W. Zhu, F. Huang, W. Gao and N. A. Khan, Micro–nanobubble technology and water-related application, Water Supply, 2020, 20, 2021–2035 CAS.
- T. Temesgen, T. T. Bui, M. Han, T.-I. Kim and H. Park, Micro and nanobubble technologies as a new horizon for water-treatment techniques: a review, Adv. Colloid Interface Sci., 2017, 246, 40–51 CrossRef CAS PubMed.
- A. Agarwal, W. J. Ng and Y. Liu, Principle and applications of microbubble and nanobubble technology for water treatment, Chemosphere, 2011, 84, 1175–1180 CAS.
- L. Dong, Y. Zhao, L. Peng, J. Zhao, Z. Luo, Q. Liu and C. Duan, Characteristics of pressure fluctuations and fine coal preparation in gas-vibro fluidized bed, Particuology, 2015, 21, 146–153 CrossRef.
- J. H. Moon, D. Fadda, D. H. Shin, J. S. Kim, J. Lee and S. M. You, Boiling-driven, wickless, and orientation-independent thermal ground plane, Int. J. Heat Mass Transfer, 2021, 167, 120817 CrossRef CAS.
- G. E. O. Celis, C. M. P. Rosero, J. B. R. Loureiro and A. P. Silva Freire, Breakup and coalescence of large and small bubbles in sudden expansions and contractions in vertical pipes, Int. J. Multiphase Flow, 2021, 137, 103548 CrossRef CAS.
- H. Setoodeh, W. Ding, D. Lucas and U. Hampel, Modelling and simulation of flow boiling with an Eulerian-Eulerian approach and integrated models for bubble dynamics and temperature-dependent heat partitioning, Int. J. Therm. Sci., 2021, 161, 106709 CrossRef.
- Z.-K. Gao, M.-X. Liu, W.-D. Dang, C. Ma, L.-H. Hou and X.-L. Hong, Multilayer limited penetrable visibility graph for characterizing the gas-liquid flow behavior, Chem. Eng. J., 2021, 407, 127229 CrossRef CAS.
- J. Cheng, S. Liu, W. Guo, Y. Song, S. Kumar, A. A. Kubar, Y. Su and Y. Li, Developing staggered woven mesh aerator with three variable-micropore layers in recycling water pipeline to enhance CO2 conversion for improving Arthrospira growth, Sci. Total Environ., 2021, 760, 143941 CrossRef CAS PubMed.
- L. Musango, S. John and M. Lloyd, CFD-DEM simulation of Small-Scale Challenge Problem 1 with EMMS bubble-based structure-dependent drag coefficient, Particuology, 2021, 55, 48–61 CrossRef.
- D. Gurera and B. Bhushan, Bioinspired movement of gas bubbles: composition, applications, generation, contact angle, and movement – an overview, Mol. Syst. Des. Eng., 2020, 5, 1555–1577 RSC.
- F. Picella, J. C. Robinet and S. Cherubini, On the influence of the modelling of superhydrophobic surfaces on laminar–turbulent transition, J. Fluid Mech., 2020, 901, A15 CrossRef CAS.
- Y. Gao, M. Wu, Y. Lin and J. Xu, Trapping and control of bubbles in various microfluidic applications, Lab Chip, 2020, 20, 4512–4527 RSC.
- L. Jiao and J. M. Floryan, Use of transpiration for reduction of resistance to relative movement of parallel plates, Phys. Rev. Fluid., 2021, 6, 014101 CrossRef.
- Z. Liu, X. Gao, L. Du, J. Li, Y. Kuang and B. Wu, Corrosion behavior of low-alloy steel with martensite/ferrite microstructure at vapor-saturated CO2 and CO2-saturated brine conditions, Appl. Surf. Sci., 2015, 351, 610–623 CrossRef CAS.
- E. Aljallis, M. A. Sarshar, R. Datla, V. Sikka, A. Jones and C.-H. Choi, Experimental study of skin friction drag reduction on superhydrophobic flat plates in high Reynolds number boundary layer flow, Phys. Fluids, 2013, 25, 025103 CrossRef.
- D. A. López, T. Pérez and S. N. Simison, The influence of microstructure and chemical composition of carbon and low alloy steels in CO2 corrosion. A state-of-the-art appraisal, Mater. Des., 2003, 24, 561–575 CrossRef.
- S. S. Kolesov, I. Kostitsyna, A. Shakhmatov, A. Davydov, R. V. Kizitov, A. B. Laptev, V. A. Polyansky, I. A. Golubev, A. A. Markov, A. Groysman, S. Webster, A. V. Shakhmatov, A. A. Alkhimenko, R. Badrak, V. V. Burlov, S. Y. Mushnikova, O. V. Shvetsov, M. Klapper, A. Dam, B. Ghai and Y. I. Kuznetsov, Study of corrosion behavior of carbon and low-alloy steels in CO2-containing environments, E3S Web Conf., 2019, 121, 04006 CrossRef.
- P. G. Kougias, P. Tsapekos, K. Boe and I. Angelidaki, Antifoaming effect of chemical compounds in manure biogas reactors, Water Res., 2013, 47, 6280–6288 CrossRef CAS PubMed.
- P. G. Kougias, K. Boe, P. Tsapekos and I. Angelidaki, Foam suppression in overloaded manure-based biogas reactors using antifoaming agents, Bioresour. Technol., 2014, 153, 198–205 CrossRef CAS PubMed.
- S. Wang, Y. Wang, Y. Zou, G. Chen, J. Ouyang, D. Jia and Y. Zhou, Biologically Inspired Scalable-Manufactured Dual-layer Coating with a Hierarchical Micropattern for Highly Efficient Passive Radiative Cooling and Robust Superhydrophobicity, ACS Appl. Mater. Interfaces, 2021, 13, 21888–21897 CrossRef CAS PubMed.
- R. Jain and R. Pitchumani, Facile Fabrication of Durable Copper-Based Superhydrophobic Surfaces via Electrodeposition, Langmuir, 2017, 34, 3159–3169 CrossRef PubMed.
- V. Sharma, H. Ali-Löytty, A. Koivikko, K. Yiannacou, K. Lahtonen and V. Sariola, Copper Oxide Microtufts on Natural Fractals for Efficient Water Harvesting, Langmuir, 2021, 37, 3370–3381 CrossRef CAS PubMed.
- M. R. Hassan, J. Zhang and C. Wang, Digital Microfluidics: Magnetic Transportation and Coalescence of Sessile Droplets on Hydrophobic Surfaces, Langmuir, 2021, 37, 5823–5837 CrossRef CAS PubMed.
- Y. Si and Z. Dong, Bioinspired Smart Liquid Directional Transport Control, Langmuir, 2020, 36, 667–681 CrossRef CAS PubMed.
- B. Chen, T. Wada and H. Yabu, Underwater Bubble and Oil Repellency of Biomimetic Pincushion and Plastron-Like Honeycomb Films, Langmuir, 2020, 36, 6365–6369 CrossRef CAS PubMed.
- L. Xie, C. Shi, X. Cui and H. Zeng, Surface Forces and Interaction Mechanisms of Emulsion Drops and Gas Bubbles in Complex Fluids, Langmuir, 2017, 33, 3911–3925 CrossRef CAS PubMed.
- R. Ma, J. Wang, Z. Yang, M. Liu, J. Zhang and L. Jiang, Bioinspired Gas Bubble Spontaneous and Directional Transportation Effects in an Aqueous Medium, Adv. Mater., 2015, 27, 2384–2389 CrossRef CAS PubMed.
- C. Pei, Y. Peng, Y. Zhang, D. Tian, K. Liu and L. Jiang, An Integrated Janus Mesh: Underwater Bubble Antibuoyancy Unidirectional Penetration, ACS Nano, 2018, 12, 5489–5494 CrossRef CAS PubMed.
- K. Yin, S. Yang, X. Dong, D. Chu, X. Gong and J.-A. Duan, Femtosecond laser fabrication of shape-gradient platform: Underwater bubbles continuous self-driven and unidirectional transportation, Appl. Surf. Sci., 2019, 471, 999–1004 CrossRef CAS.
- X. Xue, C. Yu, J. Wang and L. Jiang, Superhydrophobic Cones for Continuous Collection and Directional Transportation of CO2 Microbubbles in CO2 Supersaturated Solutions, ACS Nano, 2016, 10, 10887–10893 CrossRef CAS PubMed.
- H. Ma, M. Cao, C. Zhang, Z. Bei, K. Li, C. Yu and L. Jiang, Directional and Continuous Transport of Gas Bubbles on Superaerophilic Geometry-Gradient Surfaces in Aqueous Environments, Adv. Funct. Mater., 2017, 28, 1705091 CrossRef.
- C. Yu, X. Zhu, K. Li, M. Cao and L. Jiang, Manipulating Bubbles in Aqueous Environment via a Lubricant-Infused Slippery Surface, Adv. Funct. Mater., 2017, 27, 1701605 CrossRef.
- H. Ye, L. Zhu, W. Li, H. Liu and H. Chen, Constructing Fluorine-Free and Cost-Effective Superhydrophobic Surface with Normal-Alcohol-Modified Hydrophobic SiO2 Nanoparticles, ACS Appl. Mater. Interfaces, 2016, 9, 858–867 CrossRef PubMed.
- W. Liang, L. Zhu, W. Li, X. Yang, C. Xu and H. Liu, Bioinspired Composite Coating with Extreme Underwater Superoleophobicity and Good Stability for Wax Prevention in the Petroleum Industry, Langmuir, 2015, 31, 11058–11066 CrossRef CAS PubMed.
- X. Yang, L. Zhu, Y. Chen, B. Bao, J. Xu and W. Zhou, Controlled hydrophilic/hydrophobic property of silica films by manipulating the hydrolysis and condensation of tetraethoxysilane, Appl. Surf. Sci., 2016, 376, 1–9 CrossRef CAS.
- A. Lamoot, A. Uvyn, S. Kasmi and B. G. De Geest, Covalent Cell Surface Conjugation of Nanoparticles by a Combination of Metabolic Labeling and Click Chemistry, Angew. Chem., Int. Ed., 2021, 60, 6320–6325 CrossRef CAS PubMed.
- X. Chen, R. Chu, T. Xing and G. Chen, One-step preparation of superhydrophobic cotton fabric based on thiol-ene click chemistry, Colloids Surf., A, 2021, 611, 125803 CrossRef CAS.
- X. Dai, P. Li, Y. Sui and C. Zhang, Synthesis and performance of flexible epoxy resin with long alkyl side chains via click reaction, J. Polym. Sci., 2021, 59, 627–637 CrossRef CAS.
- X.-M. Fan, J.-J. Shen, Y.-Y. Xu, J. Gao and Y.-W. Zhang, Metabolic integration of azide functionalized glycan on Escherichia coli cell surface for specific covalent immobilization onto magnetic nanoparticles with click chemistry, Bioresour. Technol., 2021, 324, 124689 CrossRef CAS PubMed.
- X. Liu, F. Wu, K. Cai, Z. Zhao, Z. Zhang, Y. Chen, Y. Liu, J. Cheng and L. Yin, Cancer cell-targeted cisplatin prodrug delivery in vivo via metabolic labeling and bioorthogonal click reaction, Biomater. Sci., 2021, 9, 1301–1312 RSC.
- M. Yu, M. Liu, L. Zhang, M. Li, Y. Hou, D. Wang and S. Fu, Liquid-repellent and self-repairing lubricant-grafted surfaces constructed by thiol-ene click chemistry using activated hollow silica as the lubricant reservoir, J. Colloid Interface Sci., 2021, 586, 279–291 CrossRef CAS PubMed.
- P. Tang and G. Sun, Daylight-activated fumigant detoxifying nanofibrous membrane based on thiol-ene click chemistry, J. Hazard. Mater., 2021, 406, 124723 CrossRef CAS PubMed.
- I. Šarić, M. Kolympadi Markovic, R. Peter, P. Linić, K. Wittine, I. Kavre Piltaver, I. Jelovica Badovinac, D. Marković, M. Knez and G. Ambrožić, In-situ multi-step pulsed vapor phase surface functionalization of zirconia nanoparticles via copper-free click chemistry, Appl. Surf. Sci., 2021, 539, 148254 CrossRef.
- L. Xiong, W. Guo, B. M. Alameda, R. K. Sloan, W. D. Walker and D. L. Patton, Rational Design of Superhydrophilic/Superoleophobic Surfaces for Oil–Water Separation via Thiol–Acrylate Photopolymerization, ACS Omega, 2018, 3, 10278–10285 CrossRef CAS PubMed.
- Q. Wang, Q. Meng, M. Chen, H. Liu and L. Jiang, Bio-Inspired Multistructured Conical Copper Wires for Highly Efficient Liquid Manipulation, ACS Nano, 2014, 8, 8757–8764 CrossRef CAS PubMed.
- J. Ju, K. Xiao, X. Yao, H. Bai and L. Jiang, Bioinspired Conical Copper Wire with Gradient Wettability for Continuous and Efficient Fog Collection, Adv. Mater., 2013, 25, 5937–5942 CrossRef CAS PubMed.
- K. Li, J. Ju, Z. Xue, J. Ma, L. Feng, S. Gao and L. Jiang, Structured cone arrays for continuous and effective collection of micron-sized oil droplets from water, Nat. Commun., 2013, 4, 2276 CrossRef PubMed.
- Y. Zheng, H. Bai, Z. Huang, X. Tian, F.-Q. Nie, Y. Zhao, J. Zhai and L. Jiang, Directional water collection on wetted spider silk, Nature, 2010, 463, 640–643 CrossRef CAS PubMed.
- M. Cao, J. Ju, K. Li, S. Dou, K. Liu and L. Jiang, Facile and Large-Scale Fabrication of a Cactus-Inspired Continuous Fog Collector, Adv. Funct. Mater., 2014, 24, 3235–3240 CrossRef CAS.
- C. Yu, M. Cao, Z. Dong, J. Wang, K. Li and L. Jiang, Spontaneous and Directional Transportation of Gas Bubbles on Superhydrophobic Cones, Adv. Funct. Mater., 2016, 26, 3236–3243 CrossRef CAS.
- L. Lorenceau and D. Qur, Drops on a conical wire, J. Fluid Mech., 2004, 510, 29–45 CrossRef.
- J. Masliyah, R. Jauhari and M. Gray, Drag coefficients for air bubbles rising along an inclined surface, Chem. Eng. Sci., 1994, 49, 1905–1911 CrossRef CAS.
- J. C. Loudet, P. Hanusse and P. Poulin, Stokes Drag on a Sphere in a Nematic Liquid Crystal, Science, 2004, 306, 1525 CrossRef CAS PubMed.
- C. Zhang, B. Zhang, H. Ma, Z. Li, X. Xiao, Y. Zhang, X. Cui, C. Yu, M. Cao and L. Jiang, Bioinspired Pressure-Tolerant Asymmetric Slippery Surface for Continuous Self-Transport of Gas Bubbles in Aqueous Environment, ACS Nano, 2018, 12, 2048–2055 CrossRef CAS PubMed.
- T. Tate, XXX. On the magnitude of a drop of liquid formed under different circumstances, London, Edinburgh Dublin Philos. Mag. J. Sci., 2009, 27, 176–180 CrossRef.
- S. F. Jones, G. M. Evans and K. P. Galvin, Bubble nucleation from gas cavities — a review, Adv. Colloid Interface Sci., 1999, 80, 27–50 CrossRef CAS.
- W. Ma, Y. Ding, Y. Li, S. Gao, Z. Jiang, J. Cui, C. Huang and G. Fu, Durable, self-healing superhydrophobic nanofibrous membrane with self-cleaning ability for highly-efficient oily wastewater purification, J. Membr. Sci., 2021, 634, 119402 CrossRef CAS.
- W. Liang, L. Zhu, W. Li, C. Xu and H. Liu, Facile Fabrication of Binary Nanoscale Interface for No-Loss Microdroplet Transportation, Langmuir, 2016, 32, 5519–5525 CrossRef CAS PubMed.
- C. Long, Y. Qing, K. An, X. Long, C. Liu, S. Shang, C. Yang and C. Liu, Functional fluorination agents for opposite extreme wettability coatings with robustness, water splash inhibition, and controllable oil transport, Chem. Eng. J., 2021, 415, 128895 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |