Open Access Article
Open Access ArticleAdsorption of Cd2+ ions onto zeolites synthesized from a mixture of coal fly ash and oil shale ash in aqueous media†
Shuxia Baia,
Zhibing Changb,
Zhengchun Rena,
Yongqin Zhao *c and
Laixue Pang*d
*c and
Laixue Pang*d
aSchool of Construction Machinery, Shandong Jiaotong University, 250357, Jinan, Shandong, China
bSchool of Chemical & Environmental Engineering, China University of Mining and Technology (Beijing), 100083, Beijing, China
cJinan Preschool Education College, 250307, Jinan, Shandong, China. E-mail: yongqin-zhao@outlook.com
dSchool of Traffic and Civil Engineering, Shandong Jiaotong University, 250357, Jinan, Shandong, China. E-mail: lxpang@sdjtu.edu.cn
First published on 10th April 2025
Abstract
Contaminated water, especially water containing high concentrations of toxic metal ions, is frequently discharged into the environment. Thus, developing advanced adsorbents with high adsorption capacities is essential for efficient pollutant removal. In this study, X- and A-type zeolites were synthesized using industrial waste materials, specifically oil shale ash and coal fly ash. Their physicochemical properties were characterized using XRD, SEM, and nitrogen adsorption–desorption analyses. The adsorption behaviors of Cd2+ ions onto the synthesized zeolites from aqueous solutions were thoroughly investigated, including kinetic and isotherm analyses. The results showed that the adsorption process adhered to pseudo-second-order kinetics and followed the Langmuir isotherm model for all zeolites. Maximum adsorption capacities were reached within 60 min, with a dosage of 1 g L−1 and at pH 7. Among the zeolites, OSA75 demonstrated the highest Cd2+ adsorption capacity, reaching 236.41 mg g−1, indicating that the incorporation of a small amount of coal fly ash significantly enhanced the adsorption performance. The dominant mechanism governing Cd2+ adsorption was identified as cation exchange.
1. Introduction
Contaminated water, particularly water containing high concentrations of toxic metal ions, is often discharged into the environment. The persistence, accumulation, and toxicity of heavy metals in wastewater necessitate the development of effective removal strategies. Among these pollutants, cadmium is particularly detrimental to aquatic ecosystems and human health, as it is classified as a carcinogen.1 In recent years, various treatment technologies, such as chemical precipitation, flocculation, and electrochemical processes, have been proposed to mitigate the presence of heavy metals in wastewater.2,3 However, many of these approaches are hindered by limitations such as high operational costs and suboptimal efficiency. In contrast, adsorption has gained prominence as a preferred approach for removing toxic metals, owing to its straightforward operation, cost-efficiency, and remarkable effectiveness.4Various adsorbents, such as clay materials,5–7 activated carbon,2 and geopolymers8,9 have been tested for Cd2+ uptake from wastewater.10,11 Despite these efforts, the adsorption capacities of these materials remain insufficient for treating Cd2+-contaminated wastewater. Therefore, there is an urgent need to develop advanced adsorbents with high adsorption capacity. Zeolites, in particular, have garnered significant interest as promising adsorbents, attributed to their extensive surface area and highly porous structure.12–16
Zeolites can be derived from a range of sources, including both natural and synthetic materials, as well as pure chemical reagents. However, natural zeolites often exhibit low adsorption capacity and are limited by their reserves.13,17 On the other hand, synthetic zeolites are costly to produce due to the use of chemical reagents.18 To tackle these challenges, numerous researchers have explored the synthesis of zeolites from cost-effective solid waste materials, including red mud, construction waste, coal fly ash (CFA), and oil shale ash (OSA), among others.19,20 These waste materials share mineralogical composition and structural similarities with the precursors of natural zeolites, making them viable sources of alumina and silica for zeolite synthesis. Various techniques, such as the hydrothermal method, alkaline fusion method, alkaline fusion-hydrothermal method, and microwave-assisted method, have been employed to convert these materials into zeolites. This approach not only provides a sustainable waste utilization strategy but also offers an efficient solution for the removal of heavy metals from wastewater.21 This approach not only reduces the need for solid waste disposal but also helps mitigate heavy metal pollution. Several studies have investigated the potential of zeolites synthesized from CFA and OSA for heavy metal removal.11,13,22–26 However, research on synthesizing zeolites from solid wastes with varying silicon and aluminum contents remains limited.
The high silicon content in OSA and the elevated aluminum levels in CFA offer a promising opportunity for their combined use in zeolite synthesis. This study aims to synthesize zeolites from these materials and evaluate their effectiveness in removing Cd2+ from aqueous solutions. The effects of pH, contact time, and initial Cd2+ concentration on adsorption were systematically examined. Kinetic models were employed to determine adsorption parameters, while isotherm models were applied to characterize equilibrium behavior. Additionally, the mechanisms underlying Cd2+ adsorption onto the synthesized zeolites were investigated. This research provides a promising approach to mitigating environmental challenges related to both solid waste disposal and heavy metal-contaminated wastewater, aligning with the “waste-for-waste” treatment concept.
2. Experimental
2.1. Materials
This study utilized CFA and OSA as the primary raw materials, sourced from an oil-shale-fired power plant in Shandong and a coal-fired station in Shanxi, China. The chemical compositions of OSA and CFA were determined using XRF, and the results are presented in Table S1.† The SiO2/Al2O3 weight ratio for OSA was found to be 3.59, in contrast to 1.17 for CFA, indicating a higher silicon content in OSA and a higher aluminum content in CFA. X-ray diffraction (XRD) analysis (Fig. 1) revealed that OSA primarily contained quartz, while CFA exhibited a glassy amorphous phase, characterized by a broad peak between 15° and 30°. Scanning electron microscopy (SEM) images (Fig. 2) showed that OSA consisted mainly of dense phases with occasional glass beads, whereas CFA particles were granular with smooth surfaces.Analytical-grade sodium hydroxide (NaOH) and cadmium chloride (CdCl2·2.5H2O) were procured from Beijing Chemical Works. Deionized water was employed consistently as the solvent. A 1000 mg L−1 Cd2+ stock solution was prepared by accurately dissolving CdCl2·2.5H2O in deionized water, and working solutions were subsequently prepared by appropriate dilution. The pH levels of the working solutions were adjusted using HNO3 and NaOH solutions at concentrations of 0.01, 0.1, and 1 mol L−1.27
2.2. Methods
Other zeolites were synthesized following the same procedure. In addition to the reference zeolite (OSA100), blended zeolites were also produced, in which OSA was partially replaced by 25%, 50%, 75%, and 100% CFA, resulting in the zeolites labeled as OSA75, OSA50, OSA25, and CFA100, respectively.
3. Results and discussion
3.1. Characteristics of adsorbents
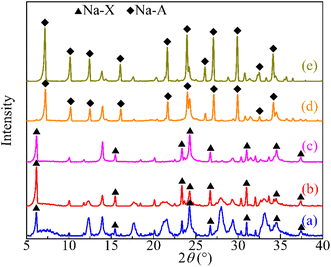
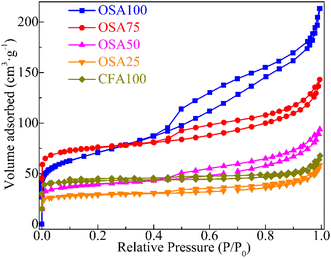
Fig. 3 presents the XRD patterns of the synthesized zeolites (OSA100, OSA75, OSA50, OSA25, and CFA100). The XRD pattern of OSA100 corresponds to X zeolite, which aligns closely with PDF#12-0246 (Na2Al2Si2.4O8.8·6.7H2O). Similar diffraction patterns were observed for OSA75 and OSA50 (Fig. 3). In contrast, the XRD profiles of CFA100 and OSA25 closely match those of Na-A zeolite (PDF#38-0241, Na2Al2Si1.85O7.7·5.1H2O). Furthermore, the synthetic zeolites exhibited well-defined crystallinity, as evidenced by the sharp and intense diffraction peaks. The Si/Al ratio of precursor materials plays a crucial role in determining the specific types of zeolites formed during hydrothermal synthesis. As the Si/Al ratio increases, the aluminum content in the synthesis system decreases, promoting the formation of X-type zeolite. Conversely, a lower Si/Al ratio creates conditions more favorable for the formation of A-type zeolite.30,31 Consequently, when synthesizing composite zeolites by blending oil shale ash and coal fly ash, an increase in the proportion of coal fly ash reduces the overall Si/Al ratio of the raw materials. This gradual decrease shifts the synthesis conditions from favoring X-type zeolite formation to those more conducive to A-type zeolite formation. In this study, the higher aluminum content in CFA compared to OSA led to the formation of Na–A zeolite when the CFA content exceeded 50%, rather than Na–X zeolite.Fig. 4 presents the N2 adsorption–desorption isotherms of the synthesized zeolites, displaying type IV profiles accompanied by H3 hysteresis loops, indicative of mesoporous structures.32 The summarized specific surface areas and pore properties of the zeolites are presented in Table 1. The BET surface area (SBET) values, as listed, decrease in the following order: OSA75 > OSA100 > OSA50 > OSA25 > CFA100. Additionally, both micropores (<2 nm) and mesopores (2–50 nm) were identified in the zeolite samples. However, significant differences were observed in the micropore-to-mesopore volume ratio and the average pore diameter among the various zeolites. The ratio of mesopore volume was higher in X-type zeolite (OSA100, OSA75 and OSA50), whereas the ratio of micropore volume was higher in A-type zeolite (OSA25 and CFA100). Furthermore, the pore size of X zeolite (OSA100, OSA75 and OSA50) were larger than that of A zeolite (OSA25 and CFA100). The variation in the specific surface area of oil shale ash/coal fly ash composite zeolites is primarily attributed to differences in pore type and pore volume. Compared to A-type zeolite, X-type zeolite has a higher density of micropores, leading to a larger pore volume and, consequently, a greater specific surface area. The differences in pore size within these composite zeolites are influenced not only by the type of pores but also by the structural characteristics of the zeolite framework. A-type zeolite belongs to the cubic crystal system, where SOD cages are arranged in a simple cubic configuration. This structure forms a three-dimensional 8-membered ring pore system with a pore diameter of 0.41 nm. In contrast, X-type zeolite falls under the hexagonal crystal system, characterized by a larger pore volume and a three-dimensional 12-membered ring pore system with a pore diameter of 0.72 nm. As a result, X-type zeolite generally exhibits a larger specific surface area, higher pore volume, and greater average pore size, whereas A-type zeolite possesses a lower specific surface area, smaller pore volume, and reduced average pore size.
| Samples | SBET (m2 g−1) | Micropore volume (cm3 g−1) | Mesopore volume (cm3 g−1) | Pore volume (cm3 g−1) | Average pore diameter (nm) |
|---|---|---|---|---|---|
| OSA100 | 252.22 | 0.109 | 0.110 | 0.229 | 2.914 |
| OSA75 | 288.55 | 0.086 | 0.132 | 0.221 | 3.068 |
| OSA50 | 215.89 | 0.038 | 0.104 | 0.145 | 3.975 |
| OSA25 | 199.04 | 0.073 | 0.033 | 0.111 | 2.330 |
| CFA100 | 170.83 | 0.055 | 0.031 | 0.104 | 2.444 |
3.2. Adsorption tests
 | ||
| Fig. 6 Impact of contact time on Cd2+ adsorption onto zeolites (m = 1 g L−1, pH 7, C0 = 100 mg L−1, T = 30 °C). | ||
To investigate the adsorption kinetics of Cd2+ on synthetic zeolites, three models were evaluated: pseudo-first-order, pseudo-second-order, and intraparticle diffusion models.10,34,35 The relevant parameters and correlation coefficients are provided in Table S2.† Among these, the pseudo-second-order model demonstrated the best agreement with the experimental data, as illustrated in Fig. 7. For all synthetic zeolites, the correlation coefficients (R2) for the pseudo-second-order model were greater than 0.999, substantially outperforming those of the other models. Furthermore, the experimentally measured adsorption capacities (qe,exp) closely matched the theoretical values (qe,cal) predicted by this model. These findings confirm that the adsorption process of Cd2+ onto synthetic zeolites is most accurately described by the pseudo-second-order model, indicating a mechanism predominantly governed by chemisorption.36
 | ||
| Fig. 8 Influence of initial Cd2+ concentration on adsorption behavior of zeolites (m = 1 g L−1, pH 7, t = 60 min, T = 30 °C). | ||
Adsorption isotherms are crucial for understanding adsorption mechanisms, as they reveal the interactions between adsorbate molecules and the active sites of the adsorbent surface. In this study, the adsorption behavior of Cd2+ on synthetic zeolites was assessed using the Langmuir, Freundlich, and Dubinin–Radushkevich (D–R) models.38–40 Fig. 9 presents the Langmuir adsorption isotherm curves for Cd2+ on synthetic zeolites along with their linear fits. The corresponding isotherm parameters and correlation coefficients are summarized in Table S3.† The Langmuir model showed the highest correlation coefficients (R2) for all zeolites, followed by the Freundlich and D–R models, indicating that the Cd2+ adsorption is most accurately described by the Langmuir isotherm. This suggests that adsorption occurs in a monolayer on adsorbents with a uniform surface energy distribution. According to the Langmuir model, the maximum Cd2+ adsorption capacities ranked as follows: OSA75 > OSA50 > OSA100 > OSA25 > CFA100, aligning closely with experimental data in Table S3.† The highest monolayer adsorption capacity was found for OSA75 at 236.41 mg g−1, followed by 211.86 mg g−1 for OSA50. These results underscore the superior adsorption performance of OSA75, which cannot be replicated by the individual raw materials. This enhanced performance can be attributed to the incorporation of a small amount of CFA, which improved the crystallinity and pore structure of the synthesized zeolite, thereby increasing its surface area and the number of exchangeable cations available for adsorption.
To evaluate the adsorption efficiency of zeolites for Cd2+ removal from aqueous systems, their maximum adsorption capacities were compared with those of other reported materials, as summarized in Table 2. It is evident from the data that the zeolites synthesized in this study exhibit significantly higher adsorption capacities than other adsorbents. This finding highlights the superior performance of zeolites derived from a mixture of coal fly ash and oil shale ash, making them a promising candidate for the treatment of cadmium-contaminated wastewater.
| Adsorption materials | Adsorption capacity (mg g−1) |
|---|---|
| Iranian natural zeolite | 4.01 (ref. 12) |
| Activated carbon | 111.78 (ref. 2) |
| Modification of nano-clays | 94.6 (ref. 5) |
| Magnetic nano-zeolite | 159.39 (ref. 17) |
| OSA100 | 187.18 (this study) |
| OSA75 | 227.24 (this study) |
| OSA50 | 205.87 (this study) |
| OSA25 | 179.54 (this study) |
| CFA100 | 156.21 (this study) |
3.3. Adsorption mechanisms
The results indicate that Cd2+ uptake was primarily governed by ion-exchange processes on the synthetic zeolites, as supported by both the adsorption kinetics and isotherms. Despite the identical adsorption mechanism, significant variations in the adsorption capacities of the synthetic zeolites were observed. This suggests that the maximum Cd2+ adsorption capacity was not solely determined by specific surface area and exchangeable cations, but also influenced by additional factors, such as the structural characteristics of the zeolites. Metal ions in aqueous solutions typically exist in a hydrated form, with Cd2+ possessing an ionic radius of 0.97 Å and a hydrated ionic radius of 4.29 Å. When comparing the crystal radii of X-type zeolite (7.4 Å) and A-type zeolite (4.5 Å) channels, it becomes evident that both hydrated and unhydrated Cd2+ ions can easily traverse the channels of X-type zeolite. In contrast, the hydrated Cd2+ ions are similar in size to the A-type zeolite channels, which may hinder their exchange unless some of the hydration water is removed. Consequently, OSA75 exhibited the highest adsorption capacity, whereas CFA100 displayed the lowest.4. Conclusions
The X- and A-type zeolites were synthesized by recycling industrial solid wastes, specifically OSA and CFA, through the alkaline fusion hydrothermal method. These zeolites exhibited significant adsorption affinity for Cd2+. Maximum adsorption capacities for all zeolites were achieved within 60 min at a concentration of 1 g L−1 and pH 7. The adsorption kinetics of Cd2+ were most accurately described by the pseudo-second-order model. Additionally, adsorption isotherm analysis revealed that the Langmuir isotherm model provided the best fit, indicating monolayer adsorption behavior. Among the zeolites investigated, OSA75 exhibited the highest Cd2+ adsorption capacity, reaching 236.41 mg g−1, with adsorption capacities following the order: OSA75 > OSA50 > OSA100 > OSA25 > CFA100. These results suggest that incorporating a small proportion of CFA during zeolite synthesis enhanced adsorption performance. The Cd2+ uptake by the synthetic zeolites was primarily attributed to ion-exchange processes, suggesting a chemical adsorption mechanism. Additionally, the structural and surface properties of the zeolites significantly contributed to their enhanced adsorption capacities. These findings suggest that synthesizing zeolites from a combination of solid wastes offers a promising approach for wastewater treatment applications.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Shuxia Bai: writing – original draft, visualization, validation, methodology, investigation, formal analysis, data curation, conceptualization. Zhibing Chang: writing – review & editing, supervision, validation, methodology. Zhengchun Ren: visualization, investigation, data curation. Yongqin Zhao: visualization, validation, methodology, investigation, formal analysis, data curation, conceptualization, supervision. Laixue Pang: writing – review & editing, validation, methodology, funding acquisition.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
The authors are grateful to the financial support of the Natural Science Foundation of Shandong Province (ZR2020ME231).References
- S. Iyer, S. M. Deshmukh and R. W. Tapre, Review on removal of heavy metals from industrial effluents by adsorption, Rev. Inorg. Chem., 2024, 22, 1–18 Search PubMed.
- S. Pap, T. Šolević Knudsen, J. Radonić, S. Maletić and S. M. Igić, et al., Utilization of fruit processing industry waste as green activated carbon for the treatment of heavy metals and chlorophenols contaminated water, J. Cleaner Prod., 2017, 162, 958–972 CrossRef CAS.
- S. Taihong, Y. Di, Y. Huizhu, Y. Jinpeng and C. Qiankun, Preparation of chitosan crosslinked modified silicon material and its adsorption capability for chromium(VI), Appl. Clay Sci., 2017, 142, 100–108 CrossRef.
- F. Fu and Q. Wang, Removal of heavy metal ions from wastewaters: a review, J. Environ. Manage., 2011, 92(3), 407–418 CrossRef CAS PubMed.
- A. Naderi, M. A. Delavar, Y. Ghorbani, B. Kaboudin and M. Hosseini, Modification of nano-clays with ionic liquids for the removal of Cd (II) ion from aqueous phase, Appl. Clay Sci., 2018, 158, 236–245 CrossRef CAS.
- P. W. Chen, M. N. Hsiao and L. W. Xiao, et al., Adsorption behavior of heavy metals onto microplastics derived from conventional and biodegradable commercial plastic products, Sci. Total Environ., 2024, 951, 175537 CrossRef.
- H. S. Ibrahim, T. S. Jamil and E. Z. Hegazy, Application of zeolite prepared from Egyptian kaolin for the removal of heavy metals: II. Isotherm models, J. Hazard. Mater., 2010, 182(1–3), 842–847 Search PubMed.
- A. Sudagar, S. Andrejkovičová, C. Patinha, A. Velosa, A. McAdam and E. F. da Silva, et al., A novel study on the influence of cork waste residue on metakaolin-zeolite based geopolymers, Appl. Clay Sci., 2018, 152, 196–210 CrossRef.
- S. Andrejkovičová, A. Sudagar, J. Rocha, C. Patinha, W. Hajjaji and E. F. da Silva, et al., The effect of natural zeolite on microstructure, mechanical and heavy metals adsorption properties of metakaolin based geopolymers, Appl. Clay Sci., 2016, 126, 141–152 CrossRef.
- S. Dash, H. Chaudhuri, R. Gupta, U. G. Nair and A. Sarkar, Fabrication and Application of Low-Cost Thiol Functionalized Coal Fly Ash for Selective Adsorption of Heavy Toxic Metal Ions from Water, Ind. Eng. Chem. Res., 2017, 56(6), 1461–1470 CrossRef.
- M. Visa, Synthesis and characterization of new zeolite materials obtained from fly ash for heavy metals removal in advanced wastewater treatment, Powder Technol., 2016, 294, 338–347 CrossRef.
- H. Merrikhpour and M. Jalali, Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by Iranian natural zeolite, Clean Technol. Environ. Policy, 2012, 15(2), 303–316 CrossRef.
- S. Bai, M. Chu, L. Zhou, Z. Chang, C. Zhang and B. Liu, Removal of heavy metals from aqueous solutions by X-type zeolite prepared from combination of oil shale ash and coal fly ash, Energy Sources, Part A Recovery, Util. Environ. Eff., 2022, 44(2), 5113–5123 Search PubMed.
- M. Z. Ahmed, Q. S. Ali, A. M. Essam, A. W. Mahmoud and K. S. Moaaz, et al., Adsorption characteristics of Na-A zeolites synthesized from Egyptian kaolinite for manganese in aqueous solutions response surface modeling and optimization, Appl. Clay Sci., 2017, 140, 17–24 CrossRef.
- M. R. Zaeri and F. Esmaeilzadeh, Performance Evaluation of Static and Dynamic CO2 Adsorption from Synthetic Gas Condensates Using Zinc Oxide, Silicon Dioxide and Zeolite 13X, Korean J. Chem. Eng., 2024, 41(3), 879–892 Search PubMed.
- T. Sirinakorn, K. Imwiset, S. Bureekaew and M. Ogawa, Inorganic modification of layered silicates toward functional inorganic-inorganic hybrids, Appl. Clay Sci., 2018, 153, 187–197 Search PubMed.
- H. A. Shamkhi, M. J. Abdulhasan, S. A. Raheem, H. A. M. Al-Zubaidi and A. S. K. Janbi, Optimization of heavy metals removal from wastewater by magnetic nano-zeolite using response surface methodology, Desalin. Water Treat., 2023, 306, 3–74 Search PubMed.
- X. D. Liu, Y. P. W, X. M. Cui, Y. He and J. Mao, Influence of synthesis parameters on NaA zeolite crystals, Powder Technol., 2013, 243, 184–193 CrossRef CAS.
- W. M. Xie, F. P. Zhou, X. L. Bi, D. D. Chen, J. Li, S. Y. Sun, J. Y. Liu and X. Q. Chen, Accelerated crystallization of magnetic 4A-zeolite synthesized from red mud for application in removal of mixed heavy metal ions, J. Hazard. Mater., 2018, 358, 441–449 Search PubMed.
- Q. L. Qiu, X. G. Jiang, G. J. Lv, Z. L. Chen, S. Y. Lu, M. J. Ni, J. H. Yan and X. B. Deng, Adsorption of heavymetal ions using zeolite materials ofmunicipal solid waste incineration fly ash modified by microwave-assisted hydrothermal treatment, Powder Technol., 2018, 335, 156–163 CrossRef CAS.
- J. L. X. Hong, T. Maneerung, S. N. Koh, S. Kawi and C.-H. Wang, Conversion of Coal Fly Ash into Zeolite Materials: Synthesis and Characterizations, Process Design, and Its Cost-Benefit Analysis, Ind. Eng. Chem. Res., 2017, 56(40), 11565–11574 CrossRef CAS.
- A. E. Burakov, E. V. Galunin, I. V. Burakova, A. E. Kucherova, S. Agarwal and A. G. Tkachev, et al., Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: a review, Ecotoxicol. Environ. Saf., 2018, 148, 702–712 Search PubMed.
- W. Bao, L. Liu, H. Zou, S. Gan, X. Xu and G. Ji, et al., Removal of Cu2+ from Aqueous Solutions Using Na-A Zeolite from Oil Shale Ash, Chin. J. Chem. Eng., 2013, 21(9), 974–982 CrossRef CAS.
- X. H. Xu, K. G. Chen, Y. Dai, X. P. Xing and L. Sun, Synthesis of Zeolite A-X from Coal Fly Ash via Ultrasonic-Alkali Fusion Hydrothermal Method for the Efficient Removal of Cr (VI) From Wastewater, Water, Air, Soil Pollut., 2025, 236(2), 137 CrossRef CAS.
- R. Shawabkeh, A. Al-Harahsheh, M. Hami and A. Khlaifat, Conversion of oil shale ash into zeolite for cadmium and lead removal from wastewater, Fuel, 2004, 83(7–8), 981–985 CrossRef CAS.
- H. Zhang, J. Wang, B. Zhang, Q. Liu, S. Li and H. Yan, et al., Synthesis of a hydrotalcite-like compound from oil shale ash and its application in uranium removal, Colloids Surf., A, 2014, 444, 129–137 Search PubMed.
- S. Bai, M. Chu, L. Zhou, Z. Chang, C. Zhang and H. Guo, et al., Modified oil shale ash and oil shale ash zeolite for the removal of Cd2+ ion from aqueous solutions, Environ. Technol., 2019, 40(11), 1485–1493 CrossRef CAS PubMed.
- S.-x. Bai, L.-m. Zhou, Z.-b. Chang, C. Zhang and M. Chu, Synthesis of Na-X zeolite from Longkou oil shale ash by alkaline fusion hydrothermal method, Carbon Resour. Convers., 2018, 1, 245–250 CrossRef CAS.
- S. Ghorai, A. Sinhamahpatra, A. Sarkar, A. B. Panda and S. Pal, Novel biodegradable nanocomposite based on XG-g-PAM/SiO2: application of an efficient adsorbent for Pb2+ ions from aqueous solution, Bioresour. Technol., 2012, 119, 181–190 CrossRef CAS PubMed.
- L. C. Hsiao and H. S. Wei, A General Method for the Conversion of Fly Ash into Zeolites as Ion Exchangers for Cesium, Ind. Eng. Res., 1998, 37, 71–78 CrossRef.
- N. Shigemoto and H. Hayashi, Selective formation of Na-X zeolite from coal fly ash by fusion with sodium hydroxide prior to hydrothermal reaction, J. Mater. Sci., 1993, 28, 4781–4786 CrossRef CAS.
- V. B. Yadav, R. Gadi and S. Kalra, Synthesis and characterization of novel nanocomposite by using kaolinite and carbon nanotubes, Appl. Clay Sci., 2018, 155, 30–36 Search PubMed.
- J. He, Y. Li, C. Wang, K. Zhang, D. Lin and L. Kong, et al., Rapid adsorption of Pb, Cu and Cd from aqueous solutions by β-cyclodextrin polymers, Appl. Clay Sci., 2017, 426, 29–39 CAS.
- F. J. López, S. Sugita, M. Tagaya and T. Kobayashi, Metakaolin-Based Geopolymers for Targeted Adsorbents to Heavy Metal Ion Separation, J. Mater. Sci. Chem. Eng., 2014, 02(07), 16–27 Search PubMed.
- Q. Zhen, Z. Xintong, H. Hui and Z. Qiuzhuo, Properties of Spartina alterniflora Loisel. derived-biochar and its effect on cadmium adsorption, J. Agro-Environ. Sci., 2018, 37(1), 172–178 Search PubMed.
- Y. Y. Wang, Y. X. Liu, H. H. Lu, R. Q. Yang and S. M. Yang, Competitive adsorption of Pb(II), Cu(II), and Zn(II) ions onto hydroxyapatite-biochar nanocomposite in aqueous solutions, J. Solid State Chem., 2018, 261, 53–61 CrossRef CAS.
- A. Alshameri, H. He, J. Zhu, Y. Xi, R. Zhu and L. Ma, et al., Adsorption of ammonium by different natural clay minerals: Characterization, kinetics and adsorption isotherms, Appl. Clay Sci., 2018, 159, 83–93 CrossRef CAS.
- V. J. Inglezakis, M. M. Fyrillas and M. A. Stylianou, Two-phase homogeneous diffusion model for the fixed bed sorption of heavy metals on natural zeolites, Microporous Mesoporous Mater., 2018, 266, 164–176 CrossRef CAS.
- Y. F. Wang, S. Liu, J. Y. Pan, H. Y. Zhang, B. Y. Wang and W. F. Yan, MER Zeolite with Remarkable Pb2+ and Cd2+ Removal Capability Cost-Effectively Synthesized from Postprocessed Natural Stellerite, Inorg. Chem., 2024, 64(1), 393–403 CrossRef PubMed.
- X. Chen, K. Wendell, J. Zhu, J. Li, X. Yu and Z. Zhang, Synthesis of nano-zeolite from coal fly ash and its potential for nutrient sequestration from anaerobically digested swine wastewater, Bioresour. Technol., 2012, 110, 79–85 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00752f |
| This journal is © The Royal Society of Chemistry 2025 |