Open Access Article
Open Access ArticleManganese-based nanoparticles plus gambogic acid targeted hypoxic tumor microenvironment by enhancing ROS generation and provided antitumor treatment and improved immunotherapy†
Zixin Liang‡
 a,
Xinyi Xua,
Ning Wanga,
Xintao Heb,
Xingzhi Hanc,
Liuqi Sangd,
Jing Hu*a and
Xiaoping Qian*a
a,
Xinyi Xua,
Ning Wanga,
Xintao Heb,
Xingzhi Hanc,
Liuqi Sangd,
Jing Hu*a and
Xiaoping Qian*a
aDepartment of Oncology, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, China. E-mail: xiaopingqian@nju.edu.cn; doctorhujing@163.com
bDepartment of Pathology, Nanjing Drum Tower Hospital Clinical College of Nanjing University of Chinese Medicine, Nanjing, China
cNanjing Drum Tower Hospital, Affiliated Hospital of Medical School, Nanjing University, Nanjing, China
dNanjing Drum Tower Hospital Clinical College of Xuzhou Medical University, Nanjing, China
First published on 11th April 2025
Abstract
Colorectal cancer (CRC) remains a major global health challenge, particularly in advanced stages where drug resistance leads to high recurrence rates and poor survival outcomes. This study investigates a novel therapeutic approach combining gambogic acid (GA) with manganese dioxide (MnO2) nanoparticles (MBG NPs) to enhance anti-tumor efficacy in the acidic and hypoxic tumor microenvironment (TME). The development of MBG NPs involved conjugating MnO2 nanosheets with bovine serum albumin (BSA) for effective GA encapsulation, optimizing the delivery of both components. We explored the potential of Mn2+ ions released from MnO2 to synergize with GA to alleviate tumor hypoxia and modulate the TME, thereby improving immune response. In vitro assays demonstrated significant cytotoxicity of MBG NPs against mouse colon cancer cells (CT26 cells), with enhanced apoptosis and elevated reactive oxygen species (ROS) levels. In vivo studies using BALB/c mice showed that treatment with MBG NPs significantly reduced tumor volumes and improved survival rates compared to controls. Additionally, MBG NPs combined with programmed death-1 inhibitor (aPD-1) further augmented therapeutic effects. Histological analyses confirmed tumor necrosis and changes in TME composition, indicating the potential of this synergistic strategy to overcome drug resistance in microsatellite stable (MSS) CRC, inhibit tumor growth and benefit patient survival. These findings highlight the promising application of nanoparticle-based platforms in enhancing immunotherapy outcomes for advanced colorectal cancer.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, with high incidence and mortality rates. Although the prevalence of early cancer screening and improved treatment options have increased the five-year survival rate for patients, the issue of drug resistance often leads to tumor recurrence, with a mere 14% five-year survival rate in advanced CRC patients.1 Thus, it is crucial to explore new treatment strategies for advanced CRC and to improve drug resistance. Immune-checkpoint inhibitors (ICIs) constitute a major research direction in immunotherapy, offering hope for patients with advanced CRC. However, most patients with proficient DNA mismatch repair (pMMR) or microsatellite stable (MSS) CRC show limited responses to ICIs.2 In-depth studies of molecular mechanisms indicate that the tumor microenvironment (TME), characterized by low pH, hypoxia, and high levels of oxidative stress, is a major reason for poor responses to ICIs therapy. The acidic and hypoxic TME hinders the infiltration and activity of immune cells, resulting in T cell exhaustion and tumor immune evasion.3 Therefore, the urgent challenge is to combine immunotherapy with other drugs, to overcome immune resistance induced by the TME and reverse ICIs resistance.Gambogic acid (GA), derived from the traditional Chinese medicine gamboge, has been found to curb the progression of various malignancies through multiple mechanisms, including the induction of autophagy, inhibition of tumor metastasis, and anti-angiogenesis.4 Additionally, recent studies suggest that GA can induce apoptosis in cancer cells through reactive oxygen species (ROS) signaling, with elevated ROS levels associated with GA-mediated cell death.5 Our team has successfully developed nanoparticle-based drug delivery systems to effectively encapsulate GA which overcome the disadvantages of GA such as poor water solubility,6 and we found that GA can promote dendritic cells (DC) maturation.7 But the mechanism behind its potential enhancement of programmed death-1 inhibitor (aPD-1) is still unclear. In this study, we also aim to investigate this further.
In recent years, gold nanostructures and two-dimensional nanomaterials have garnered significant attention as drug carriers for combined cancer therapies.8 Among reported nanoparticle-based drug delivery systems, MnO2 nanostructures serve as a unique TME-responsive carrier, offering several distinct advantages: first, MnO2 can decompose H2O2 present in the TME into O2.9 Second, MnO2 can react with H+ or glutathione (GSH) in the TME to produce Mn2+ ions, targeting the tumor's acidic microenvironment.10,11 Furthermore, Mn2+ is a valuable T1 contrast agent, and many studies have utilized this property for magnetic resonance imaging (MRI) to continuously monitor drug kinetics in vivo.12,13 In conclusion, MnO2 nanostructures have been found to enhance various cancer therapies.14 Bovine serum albumin (BSA) possesses high drug loading capacity and effectively extends drug half-lives,15 making it a beneficial candidate for developing nanoparticle-based drugs. Nab-paclitaxel (Abraxane®), a formulation for delivering paclitaxel, has been widely used and approved by the Food and Drug Administration (FDA).16
During the sonication process, the collapse of microbubbles generates high temperature and pressure, which facilitates the incorporation of BSA into the layered MnO2.17 Compared to traditional MnO2 nanoparticles (MnO2 NPs), protein-templated MnO2 NPs exhibited better biocompatibility.18 So, we synthesized MnO2-BSA (MB NPs) by conjugating MnO2 nanostructures with BSA. Combining Mn2+ ions with GA can synergistically improve the hypoxic and acidic TME. It will enhance tumor killing and augmenting anti-tumor immune responses. Thus, we further load GA into MB NPs to generate MnO2-BSA-GA (MBG NPs). The MnO2 in the material can react in an acidic environment to release Mn2+ ions. This material can simultaneously increase the concentration of Mn2+ ions and GA at the tumor site for optimal anti-tumor effects. They both against and regular the acidic hypoxic TME of MSS CRC. Compared with other existing GA NPs, we successfully developed simple to fabricate MBG NPs that enable cells to produce ROS and improve hypoxic acidic TEM, providing a new possibility for clinical translation of GA, which will provide a new strategy for MSS CRC.13,19
So, comprehensive in vitro and in vivo evaluations were performed. Our research focuses on determining how to target the tumor site, increase the concentration of Mn2+ ions and GA within the tumor and maintain biosafety and explore the reasons why GA enhances the efficacy of aPD-1.
2. Results and discussion
2.1 Preparation and characterization of MBG NPs
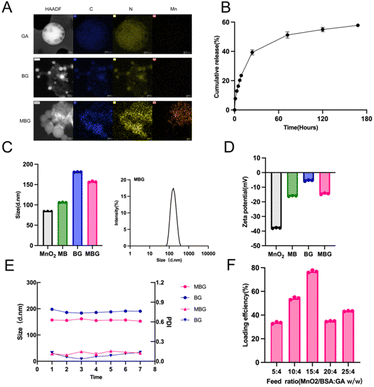
We prepared MBG NPs in two steps (Fig. 1A): first, MnO2 nanostructures were obtained by ultrasonication of BSA solution. Subsequently, GA was loaded onto the carrier using Nab™ technology. Compared to a simple BSA formulation, the addition of MnO2 transformed the drug from a yellow clear liquid to a brownish-yellow clear liquid. MnO2 nanostructures can degrade H2O2 present in TME into O2, alleviating tumor hypoxia.9 Additionally, they can react with H+ or GSH in the TME, targeting the acidic microenvironment and acting as a strong adjuvant.10 So, we hypothesized that combining the three components would produce a “1 + 1 + 1 > 3” effect. The morphology of GA, BG NPs and MBG NPs were observed by TEM, the observed images were shown in Fig. 2A. It was observed that GA samples appeared in spherical form and adsorbed GA through BSA and MnO2. Elemental analysis was then performed by using EDX mappings. The analysis results showed that, compared with GA and BG NPs, there was significant Mn enrichment in MBG NPs, confirming the structural characteristics observed by TEM and the successful construction of MBG NPs. DLS measurement indicated that the average size of MnO2, MB, BG and MBG NPs were approximately 84.86 ± 0.15 nm, 106.1 ± 0.35 nm, 181.1 ± 0.26 nm and 157 ± 0.92 nm(Fig. 2C). The zeta potential of MnO2 was −37.8 ± 0.14 mV, after modification with BSA, the zeta potential of MB NPs changed to −16 ± 0.15 mV, and the potential of the final product MBG NPs was −14.47 ± 0.29 mV (Fig. 2D). These changes in particle size reconfirm the successful loading of GA.20–22 Additionally, we evaluated the long-term stability of the NPs; dispersing them in PBS for one week showed no significant changes in particle size or polymer dispersity index (PDI), indicating excellent stability and ensuring the feasibility of subsequent experiments (Fig. 2E). To enhance GA encapsulation efficiency, we investigated the encapsulation efficiency under different feeding ratios. As shown in Fig. 2F, we found that as the MB ratio increased, the encapsulation efficiency improved, reaching a maximum of 76.86 ± 0.61% when the ratio of MB to GA was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, indicating good loading capacity. This specific drug loading condition was selected for subsequent experiments.
4, indicating good loading capacity. This specific drug loading condition was selected for subsequent experiments.
2.2 NPs demonstrated initial burst GA release with dose-dependent cytotoxicity in CT26 cells and good biocompatibility
We studied the release profile of GA from the NPs over seven days (Fig. 2B). The results showed an initial burst release of GA, with nearly 50% of the loaded GA released within 72 hours. The release rate slowed in the following days, and approximately 60% of the loaded GA was released from the NPs within one week. Overall, the NPs supported a sustained release of GA for at least one week. Cell viability was measured using the CCK-8 assay. As shown in Fig. 3A, MBG NPs exhibited strong dose-dependent cytotoxicity against CT26 cells, with effective killing observed at concentrations above 0.4 μg mL−1. Additionally, the MBG NPs demonstrated significantly greater cytotoxicity compared to the BG NPs. However, for NCM460 cells, neither the BG nor the MBG NPs exhibited noticeable cytotoxicity in the concentration, with cell viability remaining above 60% at 0.6 μg mL−1. All these indicated that the NPs have good biocompatibility. Using an apoptosis kit, we assessed drug-induced late-stage apoptosis. FCM results (Fig. 3B) indicated no significant cytotoxicity in the NS, MB NPs, whereas both the BG and MBG NPs showed cell killing effects, with the MBG NPs being more pronounced, further confirming the enhanced in vitro anti-tumor activity of our NPs.2.3 MBG NPs preferentially targeted tumor cells, increasing ROS and 1O2 levels and enhancing anti-tumor effects
To evaluate drug cellular uptake, we incubated various cell lines with DIO-labeled NPs. As shown in Fig. 4A and C, CT26, 4T1, and HGC27 cells showed green fluorescence (MB/DIO) clearly surrounding the blue nuclei (nuclei labeled with DAPI dye) and even overlapping each other. However, NCM460 cells showed significantly lower fluorescence intensity than other cells during the same incubation time. Fig. 4C showed the quantitative results of relative fluorescence density, and found that the relative fluorescence densities of CT26 and 4T1 were not statistically significant, while the fluorescence intensity of NCM460 was was indeed low. This observation showed that the drugs encapsulated within the NPs can preferentially target tumor cells, enhancing their anti-tumor efficacy.The biodistribution of MBG NPs was detected by Near-infrared (NIR) fluorescence imaging (Fig. 4B and D), it was found that 24 hours after intravenous injection of the nanoparticles, red fluorescence began to appear at the tumor site. At 48 hours, a strong red fluorescence was detected and continued for 72 hours. This represents targeted enrichment of drugs in tumor and tumor-draining lymph nodes (TDLN), and recommends 24–48 hours after injection as the optimal treatment period.
Studies have shown that ROS can induce oxidative stress, which may lead to lipid peroxidation, protein damage, and DNA fragmentation.23 We utilized DCFH-DA probes to indicate ROS levels in CT26 cells, observed through confocal microscopy and FCM. As expected, MBG NPs greatly increased the ROS levels in CT26 cells, reversed the hypoxic acidic TME. Confocal microscopy showed the fluorescence intensity in the MBG NPs was significantly stronger than that of the MnO2 or GA monotherapy groups (Fig. 3C). FCM (Fig. 3D) reflected a gradual increase in the percentage of ROS-positive cells, peaking after treatment with MBG NPs. Singlet oxygen(1O2)is one of the radicals that can participate in the non-specific reactions that lead to the destruction of cells.24 It was shown by Fig. 3E, MBG NPs also induced CT26 cells to produce a higher concentration of 1O2.
2.4 MBG NPs inhibited tumor growth in vivo, increased CD8+ T cell infiltration, reduced M2-type macrophages, and promoted anti-tumor immunity
To evaluate the anti-tumor effects of MBG NPs in vivo, we established a subcutaneous CT26 colon cancer model in male BALB/c mice and administered treatment via peritumoral injection at specified time points (Fig. 5A). Three days after the last administration, mice were euthanized. As shown in Fig. 5B and C, tumors in the MBG NPs showed significant growth inhibition, with tumor weights and volumes consistent. Tumors, spleens, and TDLNs were then excised for FCM analysis. Fig. 5D and E showed that the percentage of CD3+CD8+ T cells in the spleens of the MBG NPs (36.2%) was significantly higher than that in the NS (25%) and BG groups (27.8%), with increased programmed death-1 (PD-1) expression on CD8+ T cells. In tumors, the MBG NPs also showed nearly double the percentage of CD3+CD8+ T cells (44.8%) compared to the NS group (23.2%) (Fig. 5F). In this study, we found that MBG NPs could reverse hypoxia and increase the expression of CD8+ T cells. Previous studies have shown that improving hypoxia can promote M2-to-M1 polarization of TAMs.25 So, we investigated the expression of tumor-associated macrophages (TAMs) in tumor tissues, type macrophages (CD11b+CD206+) in the MBG NPs compared to controls (Fig. 5G). Furthermore, the MBG NPs increased mature DCs in TDLNs (Fig. 5H). In conclusion, MBG NPs release high ROS levels, regulate the TME, reduce hypoxia, enhance anti-tumor immunity, and promote immune-mediated tumor killing by transforming “cold tumors” into “hot tumors”.2.5 MBG NPs showed optimal tumor accumulation 24–48 hours post-injection, with no significant systemic toxicity or organ damage
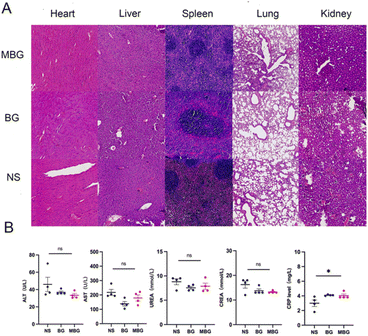
We assessed the biodistribution of MB/DIR NPs using IVIS Lumina III system (Fig. 4B), as high accumulation of NPs in tumors is crucial for achieving favorable therapeutic outcomes. Forty-eight hours post intravenous injection, we detected strong red fluorescence in the tumor region of CT26 tumor-bearing mice, which persisted up to 72 hours, while NPs in the liver were gradually eliminated after 48 hours. These results indicate the fluorescence detectability of NPs and suggest that 24–48 hours post-injection is the optimal treatment window.In mice treated with different NPs, we histologically evaluated the systemic toxicity of BG NPs and MBG NPs by assessing major organs (heart, liver, spleen, lung, and kidney) with H&E staining (Fig. 6A). No significant histological differences were observed between the NS and BG, MBG NPs, and no obvious signs of inflammatory response were detected in these critical organs. Additionally, serum biochemical assays (Fig. 6B) showed no significant differences in serum biochemical markers among groups, the C-reactive protein (CRP) level in the MBG NPs was slightly elevated but remained within the normal range. These findings indicate that MBG NPs possess good biocompatibility and safety in vivo.
2.6 MBG NPs enhanced aPD-1 immunotherapy
The hypoxic TME,26 insufficient T cell activation and infiltration27 and other factors, limit the benefit that patients can gain from ICIs. Our previous studies confirmed that GA synergizes with aPD-1 to achieve better anti-tumor effects,7 but the exact reason remains unclear. Prior reports have suggested that acidic tumor microenvironment with hypoxia can induce high programmed cell death ligand-1 (PD-L1) expression, leading to immune escape in tumors.28–30 Mn2+ can improve hypoxic and acidic environment, disrupt the PD-1/PD-L1 axis, and reverse the immunosuppressive microenvironment.9 Mn2+ combined with ICIs can enhance antitumor efficacy while reducing the required dose of aPD-1 in mice.31 So, we established a mouse xenograft tumor model using CT26 cells to verify this. Tumor-bearing mice were randomly divided into five groups (n = 5) and treated with NS, BG, MBG, BG + aPD-1, and MBG + aPD-1, monitoring tumor volume and body weight every 2–3 days until the endpoint (Fig. 7A). As shown in Fig. 7B, the tumors in mice injected with NS or BG NPs grew rapidly. However, the addition of MnO2 enhanced the anti-tumor efficacy, and the MBG + aPD-1 group demonstrated the most remarkable tumor inhibitory effect. Fig. 7C displayed tumor growth over 30 days, with nearly two-thirds of mice in the MBG + aPD-1 group showing slow tumor growth, maintaining volumes below 500 mm3. Body weight monitoring revealed no significant differences among groups (Fig. 7D). The mice in the NS group survived for an average of 20 days. Those in the BG, MBG, and BG + aPD-1 groups survived for 30–35 days. Meanwhile, the MBG + aPD-1 group survived for a significantly longer period (Fig. 7E). Our results suggest that the combination of MBG NPs and aPD-1 provided the best overall antitumor effect, significantly prolonged mouse survival.2.7 Immunohistochemistry and immunofluorescence staining showed MBG NPs change TME
CA9 is a membrane-associated zinc metalloenzyme that enables tumor cells to survive in hypoxic acidic environments.30 It also has been reported that hypoxia and acidity contribute to tumor resistance.29 In the H&E-stained sections (Fig. 8A), we observed a general trend of decreased cellular density in tumors treated with MBG NPs. In staining for CA9, the levels significantly decreased in tumors treated with Mn2+ NPs, supporting our earlier investigation into the targeted effects of this material on tumors. We also noted significantly ki67 lower expression levels in the MBG NPs, alongside an increase in TUNEL expression (Fig. 8B), which was more pronounced in the MBG NPs combined with aPD-1. The quantitative results of positive area in Fig. 8C also prove this conclusion. These results suggested that MBG NPs treatment recruited more T cells to activate systemic antitumor immunity and reduced the expression of CA9 and the resistance of drug. That may explain why it has a better synergistic effect with aPD-1. Collectively, the experiments confirm that MBG NPs, by promoting ROS generation, have the potential to remodel the TME, potentially enhancing the efficacy of immunotherapy by alleviating hypoxia-induced acid environment and aPD-1 resistance.3. Materials and methods
3.1 Materials
The reagents used in this study were as follows: gambogic acid (purity ≥98%) purchased from Source Leaf Biotechnology (Shanghai, China); manganese chloride (MnCl2) purchased from Mcklin (Shanghai, China); tetramethylammonium hydroxide (TMA·OH, 25% wt) purchased from Aladdin (Shanghai, China); bovine serum albumin (BSA, purity ≥97%) purchased from Pusitang Biotechnology (Beijing, China); hydrogen peroxide (H2O2, 30% wt) purchased from Sigma (USA); annexin V-FITC/PI apoptosis kit purchased from LinkBio (Hangzhou, China); reactive oxygen species assay kit (including 2,7-dichlorofluorescein diacetate (DCFH-DA)) purchased from Biyuntian Biotechnology (Shanghai, China); 1,3-diphenylbenzofuran (DPBF) purchased from MedChemExpress (USA)and mouse antibodies including CD3, CD4, CD8a, programmed death-1 antibodies (PD-1), CD11c, CD80, CD86, CD11b, and CD206, all purchased from Biolegend (USA).3.2 Preparation and characterization of MBG NPs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes. The precipitate was collected and washed three times with methanol and ultrapure water. Subsequently, the precipitate was freeze-dried using a vacuum freeze dryer. Under ultrasonic agitation(950 W, 25 kHz for 8 h in an ice-bath),21 15 mg of BSA was added to 1 mg of the dispersed MnO2 nanosheet system and reacted for 8 hours to obtain the MnO2-BSA (MB) carrier, which was stored at 4 °C. In this study, the Nab™ technique was used to prepare GA NPs: the aqueous phase was an BSA solution, and the oil phase was a mixed solution of dichloromethane and ethanol dissolving GA. The oil phase was transferred to the aqueous phase, uniformly emulsified by ultrasound, and then the organic solvent was removed by rotary evaporation. The solution was filtered through a 0.45 μm filter membrane to remove potential Garcinia precipitate, yielding MBG NPs for later use.
000 rpm for 10 minutes. The precipitate was collected and washed three times with methanol and ultrapure water. Subsequently, the precipitate was freeze-dried using a vacuum freeze dryer. Under ultrasonic agitation(950 W, 25 kHz for 8 h in an ice-bath),21 15 mg of BSA was added to 1 mg of the dispersed MnO2 nanosheet system and reacted for 8 hours to obtain the MnO2-BSA (MB) carrier, which was stored at 4 °C. In this study, the Nab™ technique was used to prepare GA NPs: the aqueous phase was an BSA solution, and the oil phase was a mixed solution of dichloromethane and ethanol dissolving GA. The oil phase was transferred to the aqueous phase, uniformly emulsified by ultrasound, and then the organic solvent was removed by rotary evaporation. The solution was filtered through a 0.45 μm filter membrane to remove potential Garcinia precipitate, yielding MBG NPs for later use.3.3 In vitro cell studies
In a separate experiment, CT26 cells that have been treated differently were then resuspended in 1 mL of serum-free medium and stained with 0.1 mM DCFH-DA at 37 °C in the dark for 30 minutes. Fluorescence was measured using FCM, and ROS levels were determined from the fluorescence intensity.
3.4 Biodistribution study
CT26 cells (1 × 106) were subcutaneously injected into the right flank of BALB/c mice. One week later, MB/DIR was intravenously injected into the mice. The mice were anesthetized with isoflurane and imaged at 3, 24, 48, and 72 hours using near-infrared fluorescence imaging system (IVIS Lumina III system) (PerkinElmer, USA). The average fluorescence intensity was analyzed using region of interest (ROI) selection. MB/DIR NPs were prepared by loading MB with DIR dye.3.5 In vivo experiments
3.6 Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 9.0). Fluorescence and immunohistochemistry density was performed using Image J software. All experiments were repeated at least three times. Data are expressed as mean ± standard error of the mean (SEM). An unpaired one-tailed Student's t-test was used to compare two groups, and a one-way analysis of variance (ANOVA) with Tukey's post-hoc test was employed to compare more than two groups. Survival analysis was conducted using Kaplan–Meier estimates with log-rank tests. Statistical significance was defined as: *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Graphs were generated using Adobe Photoshop 2021.4. Conclusions
In summary, we first combined MnO2 and GA, both of which target acid environment and improve hypoxia, using BSA as a compatible delivery method for GA, achieving a synergistic effect in enhancing the killing of tumor cells and improving tumor cell resistance. This particle enhances T cell expression in tumor regions and effectively improves the efficacy of aPD-1 treatment for MSS CRC. Moreover, the particle exhibits excellent biocompatibility, ensuring precise targeting without causing severe toxic side effects in the host. However, due to laboratory limitations, we currently cannot explore molecular pathways in depth, nor have we fully investigated optimal concentration ratios. In future research, we will delve into the specific molecular regulatory mechanisms in CRC and the optimal concentration ratios of Mn2+, GA, and BSA to achieve more precise targeting and treatment. Overall, this study offers a combined therapeutic strategy that enhances tumor killing while alleviating hypoxia and improving the efficacy of ICIs. We hope this research will have a positive impact in the era of precision cancer treatment.Ethical statement
All animal experiments were approved by the Laboratory Animal Care and Use Committee of the Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School (Checking number: 2024AE01034), and were carried out in compliance with all relevant ethical regulations.Data availability
All the data supporting this article have been included in the research article. If the raw data is required, it will be made available on request.Author contributions
Zixin Liang: writing – original draft, software, resources, project administration, methodology, investigation, formal analysis, data curation. Xinyi Xu: investigation, formal analysis. Ning wang: investigation. Xintao He: investigation. Xingzhi Han: investigation. Liuqi Sang: investigation. Jing Hu: writing – review & editing, conceptualization. Xiaoping Qian: writing – review & editing, funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was supported by grants from Nanjing health science and technology development key program (no ZKX21028), Jiangsu scientific and technological development of traditional Chinese medicine Key projects (no ZD202227), provincial natural science foundation of Jiangsu (no BK20211007). Figure support was provided by Figdraw.References
- R. L. Siegel, A. N. Giaquinto and A. Jemal, CA Cancer J. Clin., 2024, 74, 12–49 CrossRef PubMed.
- M. Fakih, J. Sandhu, D. Lim, X. Li, S. Li and C. Wang, JAMA Oncol., 2023, 9, 627–634 CrossRef PubMed.
- S. Jiao, S. K. Subudhi, A. Aparicio, Z. Ge, B. Guan, Y. Miura and P. Sharma, Cell, 2019, 179, 1177–1190 CrossRef CAS PubMed.
- E. Hatami, M. Jaggi, S. C. Chauhan and M. M. Yallapu, Biochim. Biophys. Acta, Rev. Cancer, 2020, 1874, 188381 CrossRef CAS PubMed.
- Q. Zhang, Y. Zhang, C. Wang, H. Tang, A. Ma, P. Gao, Q. Shi, G. Wang, S. Shen, J. Zhang, F. Xia, Y. Zhu and J. Wang, Phytomedicine, 2024, 129, 155657 CrossRef CAS PubMed.
- Z. Zhang, H. Qian, M. Yang, R. Li, J. Hu, L. Li, L. Yu, B. Liu and X. Qian, Int. J. Nanomed., 2017, 12, 1593–1605 CrossRef CAS PubMed.
- F. Huang, Q. Zhang, J. Xiao, X. Zhang, X. Han, X. Shi, J. Hu, L. Li and X. Qian, Int. J. Nanomed., 2023, 18, 2261–2273 CrossRef CAS PubMed.
- Z. Z. Lim, J. E. Li, C. T. Ng, L. Y. Yung and B. H. Bay, Acta Pharmacol. Sin., 2011, 32, 983–990 CrossRef CAS PubMed.
- B. Ding, J. Yue, P. Zheng, P. Ma and J. Lin, J. Mater. Chem. B, 2021, 9, 7117–7131 RSC.
- L. Hou, C. Tian, Y. Yan, L. Zhang, H. Zhang and Z. Zhang, ACS Nano, 2020, 14, 3927–3940 CrossRef CAS PubMed.
- T. Song, Y. Liao, Q. Zuo, N. Liu and Z. Liu, J. Mater. Chem. B, 2022, 10, 3474–3490 RSC.
- J. Y. Hong, Y. G. Lim, Y. J. Song and K. Park, Int. J. Biol. Macromol., 2023, 226, 121–131 CrossRef CAS PubMed.
- Y. Jing, C. Wang, C. Li, Z. Wei, D. Lei, A. Chen, X. Li, X. He, L. Cen, M. Sun, B. Liu, B. Xue and R. Li, Int. J. Biol. Macromol., 2024, 270, 132348 CrossRef CAS PubMed.
- Z. Geng, F. Chen, X. Wang, L. Wang, Y. Pang and J. Liu, Biomaterials, 2021, 275, 120897 CrossRef CAS PubMed.
- S. S. Ahmed, M. Z. Baba, U. Wahedi, J. Koppula, M. V. Reddy, D. Selvaraj, S. Venkatachalam, J. Selvaraj, V. Sankar and J. Natarajan, Int. J. Biol. Macromol., 2024, 279, 135487 CrossRef CAS PubMed.
- C. Weekes and V. Narayanan, Gastrointest. Cancer: Targets Ther., 2015, 11 Search PubMed.
- Y. Gan, N. Hu, C. He, S. Zhou, J. Tu, T. Liang, Y. Pan, D. Kirsanov, A. Legin, H. Wan and P. Wang, Biosens. Bioelectron., 2019, 130, 254–261 CrossRef CAS PubMed.
- B. Xiao, X. Zhou, H. Xu, B. Wu, D. Hu, H. Hu, K. Pu, Z. Zhou, X. Liu, J. Tang and Y. Shen, ACS Nano, 2018, 12, 12682–12691 CrossRef CAS PubMed.
- L. Deng, T. Wei, Y. Zhang, A. Shen, X. He, S. Gao, X. Li, W. He, A. Haleem, R. Hu, H. Cheng and S. Chen, Int. J. Pharm., 2024, 660, 124303 CrossRef CAS PubMed.
- L. Han, Y. Wang, X. Huang, F. Liu, C. Ma, F. Feng, J. Zhang, W. Liu, W. Qu, H. Pang and J. Xue, Biomaterials, 2020, 257, 120228 CrossRef CAS PubMed.
- W. Xiu, S. Gan, Q. Wen, Q. Qiu, S. Dai, H. Dong, Q. Li, L. Yuwen, L. Weng, Z. Teng, Y. Mou and L. Wang, Research, 2020, 2020, 9426453 CrossRef CAS PubMed.
- G. Yang, L. Xu, Y. Chao, J. Xu, X. Sun, Y. Wu, R. Peng and Z. Liu, Nat. Commun., 2017, 8, 902 CrossRef PubMed.
- D. Wang, W. Ma, Y. Zhang, Y. Wang, L. Sun, J. Jiang, L. Jiao, R. Li, Y. Zhang, M. Zhang and Q. Zhou, Biomaterials, 2025, 313, 122778 CrossRef CAS PubMed.
- K. Malarz, W. Borzęcka, P. Ziola, A. Domiński, P. Rawicka, K. Bialik-Wąs, P. Kurcok, T. Torres and A. Mrozek-Wilczkiewicz, Bioorg. Chem., 2025, 155, 108127 CrossRef CAS PubMed.
- Y. Gong, W. Gao, J. Zhang, X. Dong, D. Zhu and G. Ma, J. Nanobiotechnol., 2024, 22, 341 CrossRef CAS PubMed.
- G. Luo, X. Li, J. Lin, G. Ge, J. Fang, W. Song, G. G. Xiao, B. Zhang, X. Peng, Y. Duo and B. Z. Tang, ACS Nano, 2023, 17, 15449–15465 CrossRef CAS PubMed.
- J. M. Zaretsky, A. Garcia-Diaz, D. S. Shin, H. Escuin-Ordinas, W. Hugo, S. Hu-Lieskovan, D. Y. Torrejon, G. Abril-Rodriguez, S. Sandoval, L. Barthly, J. Saco, B. Homet Moreno, R. Mezzadra, B. Chmielowski, K. Ruchalski, I. P. Shintaku, P. J. Sanchez, C. Puig-Saus, G. Cherry, E. Seja, X. Kong, J. Pang, B. Berent-Maoz, B. Comin-Anduix, T. G. Graeber, P. C. Tumeh, T. N. Schumacher, R. S. Lo and A. Ribas, N. Engl. J. Med., 2016, 375, 819–829 CrossRef CAS PubMed.
- A. Akinleye and Z. Rasool, J. Hematol. Oncol., 2019, 12, 92 CrossRef PubMed.
- C. B. Yang, H. X. Feng and C. L. Dai, Cancer Med., 2022, 11, 2329–2341 CrossRef CAS PubMed.
- A. W. Eckert, S. Horter, D. Bethmann, J. Kotrba, T. Kaune, S. Rot, M. Bache, U. Bilkenroth, W. Reich, T. Greither, C. Wickenhauser, D. Vordermark, H. Taubert and M. Kappler, Int. J. Mol. Sci., 2019, 20, 375 CrossRef PubMed.
- M. Lv, M. Chen, R. Zhang, W. Zhang, C. Wang, Y. Zhang, X. Wei, Y. Guan, J. Liu, K. Feng, M. Jing, X. Wang, Y. C. Liu, Q. Mei, W. Han and Z. Jiang, Cell Res., 2020, 30, 966–979 CrossRef CAS PubMed.
- C. Chu, H. Lin, H. Liu, X. Wang, J. Wang, P. Zhang, H. Gao, C. Huang, Y. Zeng, Y. Tan, G. Liu and X. Chen, Adv. Mater., 2017, 29(23), 1–13 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08547g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |