DOI:
10.1039/D5RA00749F
(Review Article)
RSC Adv., 2025,
15, 10565-10572
Five-membered heterocycles as promising platforms for molecular logic gate construction
Received
1st February 2025
, Accepted 28th March 2025
First published on 4th April 2025
Abstract
The field of molecular logic gates began thirty years ago with the early pioneers de Silva et al. (A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42) laying the foundation for modern molecular-scale switches and devices. Recent reports of lab-on-a-molecule and molecular calculators (Moleculators) demonstrate the potential of this bottom-up approach. Five-membered heterocycles were central to the first reported logic gates in 1994 and remain valuable scaffolds in the present day. This review provides an overview of logic gate design using Boolean logic, introduces the work of the first pioneers, and highlights recently reported five-membered heterocycle logic gates.
1 Introduction
The mid-20th century to modern day is often described as the information age due to technological advancement in the creation, processing, storage, and transmission of digital information.1 The semiconductor transistor is the foundation of all modern electronics2 performing various functions including switches alternating between ON and OFF voltage states. Combining multiple transistors enables complex calculations to be performed.3,4 While early transistors were centimetres in size, modern transistors are nanometres enabling billions of transistors to be combined on a single microchip.5 Society has benefited greatly from this miniaturization resulting in portable devices (smartphones and smartwatches) with processing powers unthinkable just two decades ago.6 Moore's law famously states the number of transistors in an integrated circuit doubles every two years and this has historically been correct over the past 60 years (Fig. 1A).10 In 2016 the electronic industry recognised current technological limits acknowledging new approaches were required to maintain increases in processing power (expressed as clock speed in Fig. 1A).7,7b,10 The quest for miniaturization is not limited to transistors, the field of lab-on-a-chip8 involves performing laboratory analysis on the microscale. Lab-on-a-chip devices include nuclear magnetic resonance (NMR) (Fig. 1B)8b,c and HPLC analysis (Fig. 1C).9 This concept of miniaturization can be expanded to the molecular scale in the construction of molecular switches and devices. The ability to perform laboratory analysis at the molecular level, referred to as lab-on-a-molecule11 validates this bottom-up approach. The first molecular-scale transistor12a was reported in 2009 with multiple reports of molecular-scale switches mimicking semiconductor transistors.12b,c As we reach the limits of current semiconductor transistors, advancement in the design and construction of atomic-scale devices to solve macroscale problems remains more critical than ever. While numerous chemical scaffolds have been adapted for molecular-scale switches and devices, this review will focus specifically on the five-membered heterocycles pyrazoline, pyrazole, imidazole, and pyrrole. These simple heterocycles are well suited for molecular logic gate construction due to their modular configuration, well-established fluorescence properties, and ease of synthesis from inexpensive commercially available starting materials. To provide a viable alternative to current semiconductor transistors, its essential molecular devices are manufactured on a cost-effective industrial scale. The five-membered heterocycles offer distinct advantages to achieve this research need. A general overview of molecular logic gate construction and an introduction to the early pioneers from the mid-1990s to recent developments will be discussed.
 |
| | Fig. 1 Approaching the end of Moore's law (panel A),7b a lab-on-chip NMR (panel B)8c and HPLC (panel C).9 Images reproduced from ref. 7b, 8c and 9 with permission from RSC, copyright 2019, 2005 and 2017 respectively. | |
2 Logic gates
In 1847 George Boole devised the foundation for computer science and the digital age, referred to as Boolean algebra.13 This type of binary (0 or 1) algebraic logic is expressed as logic gates wherein an OUTPUT signal is triggered with specific INPUT configurations. These logical operations are expressed graphically in a truth table (Fig. 2). Combining multiple semiconductor transistor switches to create logic gates enables complex calculations to be performed. This process can be emulated using molecules in which the OUTPUT signal, typically fluorescence emission (λem), is triggered only in the presence of certain INPUT signals. This bottom-up approach could potentially overcome the technological limits of current semiconductor transistors. It is important to distinguish between the molecule itself and the logic gate properties derived from its response to the inputs. To that end, each logic gate will have the additional (LG) to highlight the logic gate properties which are mimicked under certain inputs. An AND logic gate only results in an OUTPUT of 1 if both INPUT 1 and INPUT 2 are present. Anthracene A with a crown ether attached via an amine spacer (Fig. 3) was reported in 1993 by de Silva et al.14 Molecule A has two receptor units, the amine unit can bind to INPUT 1 (a proton) and the crown ether binds to INPUT 2 (a sodium ion). Upon excitation in the absence of both proton (H+) and sodium ion (Na+) photoelectron transfer (PET)15 from either the amine or alkylbenzene unit (red arrows in Fig. 3) quenches fluorescence. The binding of a single input (either H+ or Na+) is not sufficient to fully deactivate the PET pathway. Both INPUT 1 and 2 are required to fully deactivate PET resulting in increased fluorescence (λem) at 428 nm. A(LG) was the first reported AND molecular logic gate.
 |
| | Fig. 2 Truth tables for basic logic gates using Boolean algebra.13 | |
 |
| | Fig. 3 Anthracene molecule A and its AND logic gate A(LG).14 | |
An OR gate triggers an OUTPUT of 1 if either INPUT 1, 2, or both are 1. The first OR logic gate B(LG) (Fig. 4) was reported in 1994 by de Silva et al.16 and is based on a fluorophore-space-receptor format around pyrazoline B. In the absence of either INPUT 1 (Ca2+) or INPUT 2 (Mg2+) the PET pathway quenches fluorescence (Fig. 4). The addition of Ca2+, Mg2+, or both deactivates PET resulting in “turn on” λem at 490 nm. While AND logic gate A(LG) operates in an organic medium, OR gate B(LG) operates in water at pH 7.3 suggesting logic gates could serve a valuable function for the detection of biologically important analytes in living systems. The opposite of an AND gate is a NOR gate, an OUTPUT of 1 is only triggered when both INPUT 1 and INPUT 2 are 0. A variety of different configurations are possible (Fig. 2) but the INHIBIT (INH) logic gate is of particular interest. In the INH gate an OUTPUT of 1 is triggered when INPUT 2 is present only (Fig. 2). If INPUT 2 and INPUT 1 are 1 then INPUT 1 inhibits the gate and the OUTPUT is 0. It is noteworthy to highlight the symbol for the INH gate is related to an AND gate with the addition of a small circle on the INPUT 1 line to symbolise the inhibitory function of INPUT 1. While useful as single units, combining logic gates allows sophisticated devices to be constructed. A molecular keypad lock17 in which the INPUT signals must be entered in a particular order alongside molecular scale calculators18 has been reported. These calculators, often referred to as Moleculators, can perform simple arithmetic using Boolean algebra. While numerous scaffolds have been used for logic gate construction,19 this review will focus specifically on the five-membered heterocycles (Fig. 5). These simple scaffolds offer significant advantages in future molecular-scale switches and devices.
 |
| | Fig. 4 Pyrazoline molecule B and its OR logic gate B(LG) application reported by de Silva et al.16 in 1993. | |
 |
| | Fig. 5 Four different five-membered heterocycles used for logic gate construction. | |
3 Pyrazoline-based logic gates
Pyrazolines, a five-membered non-aromatic heterocycle with two adjacent nitrogen atoms, are privileged structures20 with a diverse range of medicinal applications.21 Pyrazolines have well-established fluorescent properties22 for logic gate construction. Pyridine-based pyrazoline 1 (Fig. 6) reported in 1999 by de Silva et al.24 was the first NOR gate (combining a NOT and OR logic functions) with two inputs, proton (H+) and mercury ions (Hg2+). Logic gate 1(LG) was designed according to the fluorophore-receptor system in which fluorescence output at λem 440 nm is present only in the absence of both INPUT 1 (H+) and INPUT 2 (Hg2+) (Fig. 6).25 The presence of either or both INPUT quenched fluorescence. Pyrazoline 1 is a useful “turn on” fluorescence sensor for Zn2+ with λem 568 nm in MeCN.25 Substitution of the N1 phenyl group in 1 for a methyl unit resulted in “turn on” sensor 1A for both Zn2+ and Cd2+ at λem 460 nm in MeCN (Fig. 6) however no logic gate properties were reported.23 Ferrocene-based pyrazolines 2 and 3 reported by Magri et al.26 are designed according to the electron donor-spacer-fluorophore-receptor system (Fig. 7) as INHIBIT logic gates. In the absence of INPUT 1 (H+) and INPUT 2 (Fe3+) both 2 and 3 undergo photoelectron transfer (PET)15 quenching fluorescence at λem 445 and 448 nm respectively. Addition of Fe3+ inhibited PET triggering fluorescence in the absence of H+. At high H+ (1 M H+) there is approx. 40-fold decrease in quantum yield therefore both 2 and 3 are functioning as INHIBIT logic gates 2(LG) and 3(LG) (Fig. 7).
 |
| | Fig. 6 Pyrazoline 1 and its NOR logic gate 1(LG) application, pyrazoline 1A with Zn2+ and Cd2+ image reproduced from ref. 23 with permission from RSC, copyright 2012.23,24 | |
 |
| | Fig. 7 Ferrocene-based pyrazoline 2 and 3 and their application as INHIBIT logic gates 2(LG) and 3(LG).26 | |
Sensors that detect pH and redox conditions are also known as Pourbaix sensors27 with 2 and 3 leading examples. N,N-Dimethylaniline- based pyrazoline 4 was designed as an off-on-off ternary logic gate (Fig. 8) which would respond to various proton concentrations.28 At low pH (<10−3 M H+) the PET15 process is active quenching fluorescence (red arrows in Fig. 8). The carboxylic acid unit on 4 enabled the attachment of 4 to silica microparticles generating pyrazoline 5 (Fig. 8) via DCC coupling and amide bond formation.28 Excitation of 5 resulted in blue fluorescence confirming attachment to microparticles was not detrimental to fluorescence (Fig. 8). This confirmed pyrazolines can be bound to solid substrates without loss of function, vital for industrial applications. Naphthalimide–pyrazoline 6 was an INHIBIT logic gate in which Fe3+ but not H+ would trigger λem at 530 nm (Fig. 9).29 In the presence of INPUT 2 (Fe3+) PET15 was inhibited triggering a “turn on” fluorescence response (Fig. 9). The addition of INPUT 1 (H+) disrupted this process, therefore, 6(LG) is an INHIBIT logic gate (Fig. 9). Pyrazoline 6 demonstrates combining the photophysical properties of naphthalimide with the rich electron ferrocene ring to fine-tune the desired logic gate properties.29
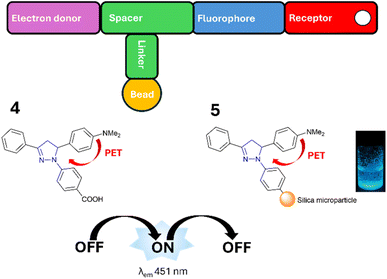 |
| | Fig. 8 Pyrazoline 4 and 5 off-on-off logic gates, image reproduced from ref. 28 with permission from RSC, copyright 2021.28 | |
 |
| | Fig. 9 Naphthalimide–pyrazoline 6 and its application as INHIBIT logic gate (6LG), image reproduced from ref. 29 with permission from RSC, copyright 2022.29 | |
A further naphthalimide–pyrazoline bearing a crown ether 7 was developed as a receptor-spacer-fluorophore-receptor system (Fig. 10).30 The addition of the crown ether enables 7 to be receptive to biologically important metal ions, for example, sodium ions (Na+) and magnesium ions (Mg2+) enabling a range of different input signals to be used. In the absence of INPUT 1–3 (H+, Na+ or Mg2+) PET is active from crown ether to pyrazoline (red arrows in Fig. 10), and no fluorescence is observed. On addition of INPUT 2 (Na+) PET is inactive and λem 597 nm is observed (Fig. 10). On addition of INPUT 3 (Mg2+) white light is observed. If INPUT 1 (H+) is also present, then protonation of the N2 on the pyrazoline ring reduces λem, and therefore 7 is acting as an INHIBIT logic gate 7(LG1). In 7 the OUTPUT signal is dependent on INPUT allowing this structure to selectively detect Na+ over Mg2+ at different wavelengths. An alternative configuration is an OR gate coupled with an INHIBIT gate to give 7(LG2) (Fig. 10). In this arrangement the presence of either INPUT 1 or 2 (or both) but in the absence of INPUT 3 (H+) triggers OUTPUT 1. This unlocks the potential of 7 in medical applications involving the detection of biological metals at low pH. In summary, pyrazolines offer several advantages for the construction of logic gates (Fig. 11). Pyrazolines are easily synthesised from chalcone precursors enabling modular construction and orientation of specific R1–R3 groups around the pyrazoline ring. The pyrazoline is non-aromatic enabling activation and deactivation of the PET pathway. The N2 on the pyrazoline has a lone pair of electrons which can act as a acceptor for H+ ions enabling the sensing of different pH values (Fig. 11). Pyrazoline 5(LG) validated the application to solid substrates28 (Fig. 8), logic gate 6(LG) demonstrated the advantages of combining different functional units29 (Fig. 9), and pyrazoline 7(LG) provided confirmation biologically relevant inputs (Na+ and Mg2+) can be utilised (Fig. 10).
 |
| | Fig. 10 Naphthalimide–pyrazoline with crown ether 7 and its application as logic gates 7(LG1) and 7(LG2), images reproduced from ref. 30 with permission from RSC, copyright 2023.30 | |
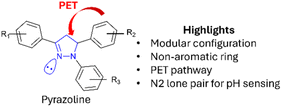 |
| | Fig. 11 Properties of pyrazoline heterocycles for logic gates. | |
4 Pyrazole-based logic gates
The pyrazole heterocycle, composed of an aromatic five-membered ring with two adjacent nitrogen atoms, is a privileged structure31 present in a diverse range of biological applications.32 Pyrazoles have unique fluorescence properties33 valuable for logic gate applications. Ferrocene-based pyrazole 8 (Fig. 12) was designed for INHIBIT and NOR logic functions based on INPUT 1 (Hg2+) and INPUT 2 (Cu2+) ions (Fig. 12).34 It is interesting to compare the structural similarity of 8 to ferrocene-pyrazoline logic gates 2, 3 (Fig. 7), and 6 (Fig. 9) designed around a Fe3+ INPUT. In the presence of INPUT 1 only (Hg2+) there is λem at 424 nm however if INPUT 2 is also present (Cu2+) this fluorescence is reduced. In the presence of INPUT 2 (Cu2+) only and not INPUT 1 (Hg2+) then λem 381 nm is present. This logic gate approach was further developed in pyrazole 9 where the phenyl unit was replaced by a pyridine unit. Logic gate 9(LG) is also designed around INPUT 1 (Hg2+) and INPUT 2 (Cu2+).35 Of note is that ferrocene-based pyrazoles 8 and 9 operate around a chelation-enhanced fluorescence effect (CHEF) whereas ferrocene-based pyrazolines 2, 3, and 6 operate via disruption of the photoinduced electron transfer (PET) effect. This highlights that while pyrazolines and pyrazole are structurally very similar, the difference in aromaticity results in profoundly separate fluorescence mechanisms. Pyrazole 10 incorporating a pyridine unit was designed to function as an Al3+ sensor in MCF3, a breast cancer, cell line (Fig. 13).36
 |
| | Fig. 12 Ferrocene-pyrazole 8 and 9 and their application as logic gates 8(LG) and 9(LG).34,35 | |
 |
| | Fig. 13 Pyridine-based pyrazole 10 and 11 and their application as logic gates 10(LG) and 11(LG).36,37 | |
In the presence of INPUT 1 (Al3+) only a fluorescence response at λem 500 nm is triggered (Fig. 13) due to the chelation-enhanced fluorescence (CHEF). If INPUT 1 (Al3+) and INPUT 2 (picric acid, PA) are present then fluorescence is inhibited (Fig. 12). Of note are the Al3+ limit of detection of 10−7 M and confirmation 10(LG) operates in vitro demonstrating real-world applications. Pyridine-based pyrazole 11 (Fig. 13) was confirmed as logic gate 11(LG) in vitro in H549, a lung cancer, cell line.37 This logic gate performed an INHIBIT function with λem at 506 nm due to CHEF in the presence of INPUT 1 (Al3+) only, when INPUT 2 (H2PO4) was present fluorescence was inhibited (Fig. 13). Rhodamine-pyrazole 12 displayed a fluorescence “turn on” response to a range of metals including Cu2+, Fe3+, Al3+ and Hg2+ in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeCN
1 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O with λem approx. 560 nm (Fig. 14).38 12(LG) functioned as an INHIBIT logic gate in the presence of INPUT 2 (Fe3+) triggering a “turn on” response at 560 nm (Fig. 14). If INPUT 1 and INPUT 2 (Cu2+) are present, then λem is disrupted. The ability to function in aqueous solution is a useful feature of 12. Rhodamine-pyrazole 13 also displayed a “turn on” response to numerous metals including Al3+, Fe3+, and Cr3+ in 7
H2O with λem approx. 560 nm (Fig. 14).38 12(LG) functioned as an INHIBIT logic gate in the presence of INPUT 2 (Fe3+) triggering a “turn on” response at 560 nm (Fig. 14). If INPUT 1 and INPUT 2 (Cu2+) are present, then λem is disrupted. The ability to function in aqueous solution is a useful feature of 12. Rhodamine-pyrazole 13 also displayed a “turn on” response to numerous metals including Al3+, Fe3+, and Cr3+ in 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOH
3 EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution with λem 558 nm due to CHEF (Fig. 14).39 The INHIBIT logic gate 13(LG) composed of NOT and AND functionality was constructed in which INPUT 1 was Al3+, Fe3+ or Cr3+ and INPUT 2 was EDTA (Fig. 14). The ability to detect metals in vitro in SiHa, cervical cancer, cell line demonstrates real-world application of 13(LG). In summary, pyrazole-based logic gates have found real-world logic gate applications in vitro. Pyrazolines and pyrazole logic gates share the ability to incorporate different functional units around a central heterocycle core. This includes the addition of electron-rich ferrocene units, well-established chelators, or fluorescence dyes for optimised photophysical properties. Of note is pyrazole logic gates often differ in their fluorescence mode of action from pyrazolines and this must be factored into future design (Fig. 15).
H2O solution with λem 558 nm due to CHEF (Fig. 14).39 The INHIBIT logic gate 13(LG) composed of NOT and AND functionality was constructed in which INPUT 1 was Al3+, Fe3+ or Cr3+ and INPUT 2 was EDTA (Fig. 14). The ability to detect metals in vitro in SiHa, cervical cancer, cell line demonstrates real-world application of 13(LG). In summary, pyrazole-based logic gates have found real-world logic gate applications in vitro. Pyrazolines and pyrazole logic gates share the ability to incorporate different functional units around a central heterocycle core. This includes the addition of electron-rich ferrocene units, well-established chelators, or fluorescence dyes for optimised photophysical properties. Of note is pyrazole logic gates often differ in their fluorescence mode of action from pyrazolines and this must be factored into future design (Fig. 15).
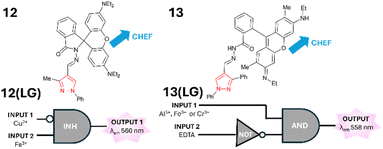 |
| | Fig. 14 Rhodamine-pyrazole 12 and 13 and their application as logic gates 12(LG) and 13(LG).38,39 | |
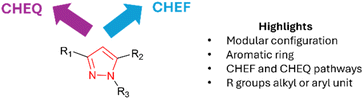 |
| | Fig. 15 Properties of the pyrazole heterocycle for logic gates. | |
5 Imidazole-based logic gates
Imidazole, a five-membered heterocycle with two nitrogen atoms separated by a carbon displays numerous biological properties.40 Imidazoles are aromatic and more alike to pyrazole than pyrazolines. Simple diphenyl substituted imidazole 14 was a “turn on” sensor for the fluoride ion (F−) with λem 490 nm in MeCN (Fig. 16).41 Upon the addition of H2O the “turn on” response is significantly reduced therefore 14 was developed as a water sensor that functions as logic gate 14(LG). Upon addition of INPUT 1 (14) and INPUT 2 (F−) but not INPUT 3 (H2O) both AND gates are satisfied, and OUTPUT is triggered at λem 490 nm (Fig. 16). If even small traces of H2O are present the NOT gate is active closing the second AND gate disrupting OUTPUT. A real-world application of applying 14 to paper test strips and the detection of H2O in a range of food products was confirmed. Brominated imidazole 15 was a “turn off” sensor for Cu2+ in DMSO (Fig. 16).42 A XNOR logic gate 15(LG) was constructed in which OUTPUT at λem 372 nm was only triggered in the absence of both INPUT 1 (Cu2+) and INPUT 2 (EDTA) or the presence of both Cu2+ and EDTA (Fig. 16). The presence of just Cu2+ or just EDTA did not trigger λem 372 nm. Schiff base imidazole 16 is a “turn off” sensor for Cu2+ and Fe2+ in DMSO.43 Logic gate 16(LG) was constructed (Fig. 17) in which OUTPUT at 320 nm is only active in the presence of INPUT 1 (EDTA) or the presence of both INPUT 1 and INPUT 2 (ergo the NOT gate is satisfied). In this situation Cu2+ or Fe2+ are disrupting the CHEF however if EDTA is present this sequesters the metal ion (Fig. 17). In summary, imidazole-based logic gates have been developed and found applications within in vitro studies. The fluorescence mode of action is primarily CHEF with a modular configuration around the heterocycle akin to pyrazoline and pyrazole logic gates (Fig. 18).
 |
| | Fig. 16 Diphenyl-imidazole 14 and pyridine-imidazole 15 and their corresponding logic gates 14(LG) and 15(LG).41,42 | |
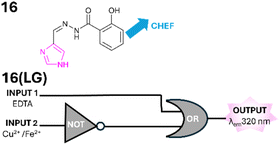 |
| | Fig. 17 Schiff base imidazole 16 and its logic gate 16(LG).43 | |
 |
| | Fig. 18 Properties of the pyrazole heterocycle for logic gates. | |
6 Pyrrole logic gates
Pyrrole is a five-membered aromatic heterocycle with a single nitrogen atom with multiple useful properties.44 Pyrrole 17 is a “turn on” sensor for Al3+ and a “turn off” sensor for Cu2+ and Cd2+ in MeOH.45 Logic gate 17(LG) composed of a NOT and OR gate was structured (Fig. 19) and demonstrated in the absence of INPUT 1 (Cu2+) there was fluorescence emission at λem 418 nm either with or without INPUT 2 (S2−) (Fig. 19). The “turn off” response is triggered only in the presence of Cu2+. Reversible binding was confirmed for 17 confirming it can be used for multiple cycles of detection (Fig. 19). Additional logic gates for Al3+ and Cd2+ were constructed and investigated.45 The application of 17 to filter paper test strips for the detection of Al3+ in real-world environmental and industrial settings was suggested. Pyrazole 18 was also developed as logic gate 18(LG) for the detection of Cu2+ using the inhibition of aggregation-induced emission (AIE) mode of action (Fig. 19).46 A “turn off” response at 554 nm was detected either in the absence of INPUT 1 (Cu2+) or the presence of both INPUT 1 and INPUT 2 (S2−). 18 was applied to filter paper and suggested as a useful tool for the detection of Cu2+ in real-world applications.
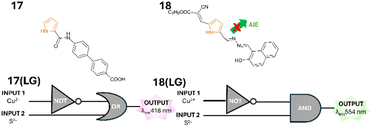 |
| | Fig. 19 Pyrrole 17 and 18 and logic gates 17(LG) and 18(LG).45,46 | |
Pyrrole 19 (Fig. 20) was initially designed as a phosphorescent probe for the fluoride ion (F−) however further investigation revealed OR logic gate functionality for the hydroxide (OH−) and acetate (AcO−) ions (Fig. 20).47 Upon addition of hydroxide and acetate ions the emission bands at 615 nm and 700 nm increase due to binding to the Eu3+ centre (Fig. 20). The limit of detections for hydroxide and acetate were calculated as 0.7 μM and 0.87 μM in MeCN. Lanthanide based logic gates are rapidly emerging as a promising platform for logic gate design.19d While the number of pyrrole-based logic gates is currently low, this scaffold has potential for a range of unexplored metals such as the lanthanides (Fig. 21).
 |
| | Fig. 20 Pyrrole-lanthanide complex 19 and its logic gate 19(LG).47 | |
 |
| | Fig. 21 Properties of the pyrrole heterocycle for logic gates. | |
7 Conclusions
Multiple logic gates have been reported over the last thirty years19 building on the pioneering work of de Silva et al. in 1993–4.14,16 Magri et al. have expanded and advanced the pyrazoline scaffold further resulting in INHIBIT logic gates 2(LG), 3(LG), 6(LG), and 7(LG) with useful applications.26,28–30 The pyrazoline “turn on” fluorescence response operates via inhibition of the PET pathway15 whereas the structurally related pyrazoles operate via CHEF or CHEQ. Numerous in vitro applications have confirmed pyrazoles can perform valuable operations within living systems for example 10(LG), 11(LG), and 13(LG).36–39 Imidazole and pyrrole heterocycles have recently emerged as useful scaffolds for the development of logic gates enabling a range of previously unexplored inputs to be utilized. While pyrazoline and pyrazole inputs were typically based around proton (H+) and metal ion (Hg2+, Fe3+, Mg2+), imidazole and pyrrole logic gates 17(LG), 18(LG) and 19(LG) have expanded the range of inputs to other ions (S2−, F−, OH− and AcO−).41–47 High throughput fluorescent screening platforms48 enable multitudes of different inputs to be screened rapidly and efficiently. Recent developments in artificial intelligence and machine learning in drug discovery49 could provide further insight into the development of heterocyclic logic gates. The algorithms developed for drug discovery could be modified for logic gate development resulting in highly unexpected logic gate designs and unusual inputs. While the last thirty years have firmly proven the concept of molecular scale devices, it's likely the next decade will focus on incorporating these cutting-edge technologies in the search for new logic gates to replace current semiconductor devices. The five-membered heterocycles provide many advantages and will be a valuable resource for the foreseeable future.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.
Author contributions
Alexander Ciupa authored the manuscript.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The Materials Innovation Factory, created as part of the UK Research Partnership Innovation Fund (Research England) and co-funded by the Sir Henry Royce Institute is acknowledged for providing funding.
References
- N. Stafford, Nature, 2010, 467, S19 CrossRef CAS PubMed.
- M. Li, H. Wu, E. M. Avery, Z. Qin, D. P. Goronzy, H. D. Nguyen, T. Liu, P. S. Weiss and Y. Hu, Science, 2023, 382, 585 CAS.
- M. S. Lundstrom and M. A. Alam, Science, 2022, 378, 722 Search PubMed.
- C. Auth and A. Shankar, IEEE Solid-St Circ. Mag., 2023, 15, 20 Search PubMed.
- B. Dyatkin, MRS Bull., 2021, 46, 16 Search PubMed.
- D. Burg and J. H. Ausubel, PLoS One, 2021, 16, e0256245 CrossRef CAS PubMed.
- Selected examples
(a) T. N. Theis and H. S. P. Wong, Comput. Sci. Eng., 2017, 19, 41 Search PubMed;
(b) S. Gao, X. Yi, J. Shang, G. Liu and R.-W. Li, Chem. Soc. Rev., 2019, 48, 1531 Search PubMed.
- Selected examples
(a) V. Narayanamurthy, Z. E. Jeroish, K. S. Bhuvaneshwari, P. Bayat, R. Premkumar, F. Samsuri and M. M. Yusoff, RSC Adv., 2020, 10, 11652 Search PubMed;
(b) Z. Li, Q. Bao, C. Liu, Y. Li, Y. Yang and M. Liu, Lab Chip, 2023, 23, 1213 Search PubMed;
(c) H. Wensink, F. Benito-Lopez, D. C. Hermes, W. Verboom, H. Gardeniers, D. N. Reinhoudt and A. van den Berg, Lab Chip, 2005, 5, 280 Search PubMed.
- J. J. Heiland, R. Warias, C. Lotter, L. Mauritz, P. J. W. Fuchs, S. Ohla, K. Zeitler and D. Belder, Lab Chip, 2017, 17, 76 Search PubMed.
- M. M. Waldrop, Nature, 2016, 530, 144 CrossRef CAS PubMed.
- Selected examples
(a) D. C. Magri, G. J. Brown, G. D. McClean and A. P. de Silva, J. Am. Chem. Soc., 2006, 128, 4950 CrossRef CAS PubMed;
(b) K. Chen, Q. H. Shu and M. Schmittel, Chem. Soc. Rev., 2015, 44, 136 CAS;
(c) G. J. Scerri, J. C. Spiteri, C. J. Mallia and D. C. Magri, Chem. Commun., 2019, 55, 4961 RSC;
(d) M. Schmittel and H. W. Lin, Angew. Chem., Int. Ed., 2007, 46, 893 CAS.
- Selected examples:
(a) H. Song, Y. Kim, Y. H. Jang, H. Jeong, M. A. Reed and T. Lee, Nature, 2009, 462, 1039 CAS;
(b) I. Gallardo, G. Guirado, J. Hernando, S. Morais and G. Prats, Chem. Sci., 2016, 7, 1819 Search PubMed;
(c) P. Remon, S. M. Li, M. Grotli, U. Pischel and J. Andreasson, Chem. Commun., 2016, 52, 4659 CAS.
- A. P. de Silva, Molecular Logic-Based Computation, Royal Society of Chemistry, Cambridge, UK, 2013 Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42 Search PubMed.
- Selected examples
(a) A. P. de Silva and R. A. D. D. Rupasinghe, J. Chem. Soc., Chem. Commun., 1985, 1669–1670 Search PubMed;
(b) B. Daly, J. Ling and A. P. de Silva, Chem. Soc. Rev., 2015, 44, 4203 Search PubMed;
(c) A. P. de Silva, T. S. Moody and G. D. Wright, Analyst, 2009, 134, 2385 Search PubMed;
(d) H. Niu, J. Liu, H. M. O'Connor, T. Gunnlaugsson, T. D. James and H. Zhang, Chem. Soc. Rev., 2023, 52, 2322 CAS;
(e) D. C. Magri, Coord. Chem. Rev., 2021, 426, 213598 Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne and G. E. M. Maguire, J. Chem. Soc., Chem. Commun., 1994, 10, 1213 RSC.
- D. Margulies, C. E. Felder, G. Melman and A. Shanzer, J. Am. Chem. Soc., 2007, 129, 347 CrossRef CAS PubMed.
- D. Margulies, G. Melman and A. Shanzer, J. Am. Chem. Soc., 2006, 128, 4865 CrossRef CAS PubMed.
- Selected examples
(a) D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105 RSC;
(b) L. Liu, P. Liu, L. Ga and J. Ai, ACS Omega, 2021, 6, 30189 CrossRef CAS PubMed;
(c) N. I. Georgiev, V. V. Bakov and V. B. Bojinov, Molecules, 2023, 28, 6327 CrossRef CAS PubMed;
(d) S. Zanella, M. A. Hernandez-Rodriguez, R. A. S. Ferreira and C. D. S. Brites, Chem. Commun., 2023, 59, 7863 RSC.
- B. Varghese, S. N. Al-Busa, F. O. Suliman and S. M. Al-Kindy, RSC Adv., 2017, 7, 46999 RSC.
- A. Tiwari, A. Bendi and A. S. Bhathiwal, ChemistrySelect, 2021, 6, 12757 CrossRef CAS.
- Selected examples
(a) A. Ciupa, RSC Adv., 2024, 14, 34918 RSC;
(b) Z. L. Gong, F. Ge and B. X. Zhao, Sens. Actuators, B, 2011, 159, 148 CrossRef CAS;
(c) Y. P. Zhang, Q. Teng, Y. S. Yang, H. C. Guo and J. J. Xue, Inorg. Chim. Acta, 2021, 525, 120469 CrossRef CAS;
(d) A. Ciupa, RSC Adv., 2024, 14, 3519 RSC.
- A. Ciupa, M. F. Mahon, P. A. De Bank and L. Caggiano, Org. Biomol. Chem., 2012, 10, 8753 RSC.
- A. P. de Silva, I. M. Dixon, H. Q. N. Gunaratne, T. Gunnlaugsson, R. Pamela, R. S. Maxwell and T. E. Rice, J. Am. Chem. Soc., 1999, 121, 1393 CrossRef CAS.
- P. Wang, N. Onozawa-Komatsuzaki, Y. Himeda, H. Sugihara, H. Arakawa and K. Kasuga, Tetrahedron Lett., 2001, 42, 9199 CrossRef CAS.
- G. J. Scerri, M. Cini, J. S. Schembri, P. Fontoura da Costa, A. D. Johnson and D. C. Magri, ChemPhysChem, 2017, 18, 1742 CrossRef CAS PubMed.
- Selected examples
(a) D. C. Magri, Supramol. Chem., 2017, 20, 741 CrossRef;
(b) M. Vella Refalo, J. C. Spiteri and D. C. Magri, New J. Chem., 2018, 42, 16474 RSC.
- N. Zerafa, M. Cini and D. C. Magri, Mol. Syst. Des. Eng., 2021, 6, 93 RSC.
- D. Sammut, N. Bugeja, K. Szaciłowski and D. C. Magri, New J. Chem., 2022, 46, 15042 RSC.
- D. C. Magri and A. A. Camilleri, Chem. Commun., 2023, 59, 4459 RSC.
- M. A. Alam, Future Med. Chem., 2023, 15, 2011 CrossRef CAS PubMed.
- O. Ebenezer, M. Shapi and J. A. Tuszynski, Biomedicines, 2022, 10, 1124 CrossRef CAS PubMed.
- Selected examples
(a) A. Tigreros and J. Portilla, RSC Adv., 2020, 10, 19693 RSC;
(b) S. Melo-Hernández, M.-C. Rios and J. Portilla, RSC Adv., 2024, 14, 39230 RSC;
(c) A. Ciupa, New J. Chem., 2024, 48, 13900 RSC.
- V. S. Elanchezhian and M. Kandaswamy, Inorg. Chem. Commun., 2010, 13, 1109 CrossRef.
- V. S. Elanchezhian, E. Kasirajan, P. Muthirulan, P. Muthukrishnan and M. Kandaswamu, J. Mol. Struct., 2024, 1313, 138687 CrossRef.
- S. Saha, A. De, A. Ghosh, A. Ghosh, K. Bera, K. S. Das, S. Akhtar, N. C. Maiti, A. K. Das, B. B. Das and R. Mondal, RSC Adv., 2021, 11, 10094 CAS.
- B. Das, M. Dolai, A. Ghosh, A. Dhara, A. D. Mahapatra, D. Chattopadhyay, S. Mabhai, A. Jana, S. Dey and A. Misra, Anal. Methods, 2021, 13, 4266 RSC.
- S. Gond, P. Yadav, A. Singh, S. Garai, A. Shekher, S. C. Gupta and V. P. Singh, Org. Biomol. Chem., 2023, 21, 4482 RSC.
- A. I. Said, N. I. Georgiev and V. B. Bojinov, Spectrochim. Acta, Part A, 2019, 223, 117304 CAS.
- A. Siwach and P. K. Verma, BMC Chem., 2021, 15, 12 CAS.
- X. Yu, Y. Li, Y. Li, Y. Liu and Y. Wang, Spectrochim. Acta A, 2025, 326, 125245 CAS.
- Z. Ekmekcia, G. Yilmaz and E. Duman, Chem. Phys., 2020, 532, 110693 CrossRef.
- J. M. Priya, H. D. Revanasiddappa, B. Jayalakshmi, A. Swamynayaka, M. Madegowda, M. Iqbal, C. Shivamallu and S. P. Kollur, Polyhedron, 2024, 260, 117110 CrossRef.
- V. Bhardwaj, D. Gumber, V. Abbot, S. Dhiman and P. Sharma, RSC Adv., 2015, 5, 15233 RSC.
- V. Kumar, P. Kumar and R. Gupta, RSC Adv., 2017, 7, 23127 RSC.
- B. Tharmalingam, O. Anitha, J. Aiswarya, T. Thiruppathiraja, S. Lakshmipathi and B. Murugesapandian, J. Photochem. Photobiol., A, 2023, 442, 114757 CrossRef CAS.
- M. Bilmez, A. Degirmenci, M. P. Algi and F. Algi, New J. Chem., 2018, 42, 450 RSC.
-
(a) X. Fang, Y. Zheng, Y. Duan, Y. Liu and W. Zhong, Anal. Chem., 2019, 91, 482 CrossRef CAS PubMed;
(b) E. Farrant, ACS Med. Chem. Lett., 2020, 11, 1506 CrossRef CAS PubMed;
(c) M. Trobe and M. D. Burke, Angew. Chem., Int. Ed., 2018, 57, 4192 CrossRef CAS PubMed.
- Selected examples
(a) J. Vamathevan, D. Clark, P. Czodrowski, I. Dunham, E. Ferran, G. Lee, B. Li, A. Madabhushi, P. Shah, M. Spitzer and S. Zhao, Nat. Rev. Drug Discovery, 2019, 18, 463 CrossRef CAS PubMed;
(b) A. Blanco-González, A. Cabezón, A. Seco-González, D. Conde-Torres, P. Antelo-Riveiro, A. Piñeiro and R. Garcia-Fandino, Pharmaceuticals, 2023, 16, 891 CrossRef PubMed;
(c) R. Gupta, D. Srivastava, M. Sahu, S. Tiwari, R. K. Ambasta and P. Kumar, Mol. Divers, 2021, 25, 1315 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *
*











![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 MeCN
1 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O with λem approx. 560 nm (Fig. 14).38 12(LG) functioned as an INHIBIT logic gate in the presence of INPUT 2 (Fe3+) triggering a “turn on” response at 560 nm (Fig. 14). If INPUT 1 and INPUT 2 (Cu2+) are present, then λem is disrupted. The ability to function in aqueous solution is a useful feature of 12. Rhodamine-pyrazole 13 also displayed a “turn on” response to numerous metals including Al3+, Fe3+, and Cr3+ in 7
H2O with λem approx. 560 nm (Fig. 14).38 12(LG) functioned as an INHIBIT logic gate in the presence of INPUT 2 (Fe3+) triggering a “turn on” response at 560 nm (Fig. 14). If INPUT 1 and INPUT 2 (Cu2+) are present, then λem is disrupted. The ability to function in aqueous solution is a useful feature of 12. Rhodamine-pyrazole 13 also displayed a “turn on” response to numerous metals including Al3+, Fe3+, and Cr3+ in 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 EtOH
3 EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O solution with λem 558 nm due to CHEF (Fig. 14).39 The INHIBIT logic gate 13(LG) composed of NOT and AND functionality was constructed in which INPUT 1 was Al3+, Fe3+ or Cr3+ and INPUT 2 was EDTA (Fig. 14). The ability to detect metals in vitro in SiHa, cervical cancer, cell line demonstrates real-world application of 13(LG). In summary, pyrazole-based logic gates have found real-world logic gate applications in vitro. Pyrazolines and pyrazole logic gates share the ability to incorporate different functional units around a central heterocycle core. This includes the addition of electron-rich ferrocene units, well-established chelators, or fluorescence dyes for optimised photophysical properties. Of note is pyrazole logic gates often differ in their fluorescence mode of action from pyrazolines and this must be factored into future design (Fig. 15).
H2O solution with λem 558 nm due to CHEF (Fig. 14).39 The INHIBIT logic gate 13(LG) composed of NOT and AND functionality was constructed in which INPUT 1 was Al3+, Fe3+ or Cr3+ and INPUT 2 was EDTA (Fig. 14). The ability to detect metals in vitro in SiHa, cervical cancer, cell line demonstrates real-world application of 13(LG). In summary, pyrazole-based logic gates have found real-world logic gate applications in vitro. Pyrazolines and pyrazole logic gates share the ability to incorporate different functional units around a central heterocycle core. This includes the addition of electron-rich ferrocene units, well-established chelators, or fluorescence dyes for optimised photophysical properties. Of note is pyrazole logic gates often differ in their fluorescence mode of action from pyrazolines and this must be factored into future design (Fig. 15).









