DOI:
10.1039/D5RA00650C
(Paper)
RSC Adv., 2025,
15, 8506-8522
Reusing the expired Ceftazidime drug as an inhibiting agent for zinc metal corrosion in HCl medium†
Received
27th January 2025
, Accepted 10th March 2025
First published on 19th March 2025
Abstract
Drug disposal costs and drug-related environmental pollution could be reduced by reusing expired drugs. A well-known cephalosporin antibiotic that was listed as an essential medication by the World Health Organization is Ceftazidime. In this study, the corrosion mitigation properties of the expired Ceftazidime (CDZ) drug for zinc corrosion in 1 mol per L HCl solution were examined by weight loss (WL), frequency modulation (EFM), potentiodynamic polarization (PDP), and impedance spectroscopy (EIS) techniques. Theoretical calculations were carried out by Density Functional Tight Binding (DFTB) and Monte Carlo (MC) simulations. Furthermore, scanning electron microscopy (SEM) and energy dispersion X-rays (EDX) were used for examining the appearance and structure of the corroded zinc surface, respectively. For a CDZ concentration of 300 ppm, EFM techniques have shown an inhibitive efficiency (IE) of 70.3% at 298 K. The IE increased as the drug concentration increased from 50 to 300 ppm, whereas it reduced as the temperature increased. The inhibition effect ceased to improve at concentrations greater than 300 ppm. A mixed form of adsorption (physisorption and chemisorption) was suggested for inhibition. The adsorption process followed the Temkin model. The spontaneous character and exothermicity of the adsorption process were demonstrated by the  and Kads magnitudes. The PDP study demonstrated that CDZ was a mixed-type inhibitor as it retards both the cathodic and anodic reactions. According to EFM results, increasing the concentration of CDZ from 50 to 300 ppm reduces the corrosion current density from 163.5 to 78.7 μA cm−2. SEM and EDX examinations revealed the adsorption of CDZ drug at the zinc surface. The DFTB and MC simulations revealed that the CDZ drug bonds to the zinc surface via the heteroatoms' lone pair of electrons and the pyridinium ring's π-electrons. The adsorption energy of the drug on the Zn surface was found to be −77.41 kJ mol−1.
and Kads magnitudes. The PDP study demonstrated that CDZ was a mixed-type inhibitor as it retards both the cathodic and anodic reactions. According to EFM results, increasing the concentration of CDZ from 50 to 300 ppm reduces the corrosion current density from 163.5 to 78.7 μA cm−2. SEM and EDX examinations revealed the adsorption of CDZ drug at the zinc surface. The DFTB and MC simulations revealed that the CDZ drug bonds to the zinc surface via the heteroatoms' lone pair of electrons and the pyridinium ring's π-electrons. The adsorption energy of the drug on the Zn surface was found to be −77.41 kJ mol−1.
1. Introduction
Metal corrosion is an internationally significant problem affecting both industrial and natural environments. It has a negative impact on the quality of the environment, industrial equipment, and infrastructure asset durability. Therefore, it is essential to design and implement corrosion protection techniques to minimize economic damage to materials, machinery, and buildings.1,2
Zinc is a valuable metal, having different uses in various fields. It is used to make a wide variety of alloys, coatings, and chemicals. Another typical use for it is to galvanize steel to prevent corrosion. Galvanized steel is used in the construction, automotive, and other industries. Because of its exceptional casting characteristics, Zn alloys are employed in die casting applications.3–5 Both acidic and basic solutions can cause zinc to corrode. Within the pH range of 6.0 to 12.5 solutions, corrosion is relatively slow. Beyond this range, it is more prevalent.6,7 Zinc metal corrodes in harmful conditions, resulting in white rust.8 Zinc is susceptible to corrosion when subjected to industrial procedures, including scale removal and acidic solution cleaning. This makes zinc material inappropriate for use in industrial applications.9–12 It has been established that using inhibitors is one of the best ways to prevent corrosion in metals.13,14 These are chemicals that, by prohibiting the dissolution of metal, inhibit the corrosion of metallic substances.15 The primary factor determining an inhibitor's efficacy is its capacity to absorb on a metal surface, resulting in substituting a water molecule at a corroding surface.16
Corrosion damage of zinc is commonly prevented through cathodic protection, utilizing galvanic or impressed current methods, often combined with paint or organic coatings. While polymer coatings offer a simple application, they provide less durable protection against environmental corrosion compared to cathodic protection techniques. Studies by Yasakau et al.17 have shown that Layered Double Hydroxide (LDH) conversion coatings significantly enhance zinc's corrosion resistance. Specifically, LDH coatings incorporating vanadate resulted in a twenty-fold increase in impedance and a reduction in local ionic currents compared to uncoated zinc. This improved protection is attributed to the controlled release of vanadate anions from the LDH structure. The new study by Ouyang et al.18 was explored on-site fabrication of biomimetic Slippery Liquid-Infused Porous Surfaces (SLIPS) coatings for zinc corrosion protection. A simple displacement reaction creates a dendritic zinc structure, which is then made superhydrophobic and infused with lubricant. This SLIPS coating significantly reduces corrosion current and enhances icing resistance, offering a practical, facile approach for field application.
To inhibit the deterioration of metals and alloys, several kinds of potent organic inhibitors are employed. These organic compounds have molecules with heteroatoms of N, O, and S, or structures with π-electrons. By covering the metal surfaces with a protective layer, these organic compounds inhibit the ionization of metals by blocking the diffusion of chemical species involved in the process.19,20 Nevertheless, most of these organic inhibitors are pricey, hazardous, and harmful to the environment. This has encouraged researchers worldwide to investigate the potential of pharmaceuticals as corrosion inhibitors. Many studies have shown that using drugs as corrosion inhibitors is safe and has no effect on the surrounding environment. These findings have encouraged the application of medicines as corrosion inhibitors, which has led to the replacement of old, harmful corrosion inhibitors.21
When compared to industry-standard organic inhibitors, most drugs are more costly. Therefore, using a fresh drug as an anti-corrosion agent is not cost-effective. Thus, using the properties of expired medications to suppress corrosion is reasonable. Pharmaceuticals retain at least 90% of their original efficacy even after they have expired; nonetheless, their usage has been limited for therapeutic purposes due to liability issues and professional restrictions.22 Utilizing expired medications could solve two more important problems, such as lowering drug-related environmental pollution and drug disposal expenses.23,24
Most of the inhibitors used to inhibit zinc corrosion were harmful, and an inhibitor's solubility in corrosive media is an important factor. To protect zinc from corroding, non-toxic pharmaceutical drugs are being used. Several drugs, including Guaifenesin,25 Seroquel,26 Clotrimazole,27 Ketosulfone,28 Floctafenine,29 Hemorheologic,30 Furosemide,31 Telmisartan,32 Paromomycin, Streptomycin, Spectinomycin,33 Atenolol,34 N-arylpyrroles,35 Anisidines,36 Gabapentin,37 Erythromycin,38 and Hexamine,6 were used to prohibit zinc corrosion. The drugs, including Guaifenesin, Seroquel, Clotrimazole, and others, were tested in corrosive media such as HCl at varying concentrations and temperatures, as well as H2SO4. The inhibition efficiency, measured as a percentage, varied significantly depending on the drug, its concentration, and the specific corrosive environment. Clotrimazole exhibited the highest inhibition efficiency, reaching 90% at a concentration of 500 ppm in 0.1 mol per L HCl at 298 K, indicating its strong protective capabilities. In contrast, Ketosulfone displayed a significantly lower efficiency of 30% at 20 ppm in the same corrosive medium and temperature, highlighting its weaker inhibitory effect. Guaifenesin and expired Hemorheologic achieved 81% and 84% inhibition at 300 ppm in 2 mol per L HCl at 298 K and 0.1 mol per L HCl at 303 K, respectively, demonstrating comparable performance under different conditions. Seroquel reached 84% at 1000 ppm in 1 mol per L HCl at 333 K, while expired Gabapentin achieved 85% at 400 ppm in 0.1 mol per L HCl at 298 K. Floctafenine showed a 72% efficiency at 25 ppm in 0.1 mol per L HCl at 333 K. The efficiency of the other drugs ranged from 57% to 77% at varying concentrations and corrosive conditions, showing that concentration, temperature, and the composition of the corrosive medium play significant roles in the effectiveness of the drugs as corrosion inhibitors.
Because pharmaceutical compounds contain heteroatoms such as oxygen, sulfur, and nitrogen, they provide promising potential for inhibiting corrosion. Their large molecular sizes and great water solubility make this particularly intriguing.39
A third-generation cephalosporin antibiotic, Ceftazidime (CDZ) drug, is free of poisonous metallic components.40,41 The CDZ drug is one of the most significant drugs that are crucial to treating various infections, including joint and vibrio infections, in the healthcare system. It contains many binding centers, such as atoms of N, S, and O. The inhibitive impact of the CDZ drug is usually attributed to the way it interacts with the metal surface via the adsorption process.42 Table 1 shows the CDZ drug's structural formula, IUPAC identification, molecular mass, and molecular structure. Additionally, our earlier study on the effective inhibition of corrosion of copper metal using expired Ceftazidime42 led us to employ Ceftazidime to suppress the corrosion of zinc, another essential industrial metal. Additionally, most of the characteristics needed to be employed as a corrosion inhibitor are present in CDZ, such as electron-rich heteroatoms (N, S, and O), electron-rich efficient groups (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, OH, NH, NH2, C
N, OH, NH, NH2, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, COO−, π-electrons of aromatic ring), good structure to cover the metal surface, and dissolved in water. Therefore, expired CDZ was used as an inhibitor in the current investigation to study zinc corrosion mitigation in a 1 mol per L HCl medium. The experiments were carried out using WL, PDP, EIS, and EFM methodologies. The composition and morphology of the zinc metal surface were investigated using EDX and SEM analyses. The drug's type of adsorption on zinc metal was evaluated using several adsorption isotherms. Additionally, the thermodynamic characteristics pertaining to the adsorption process were established. The mechanism of inhibition was investigated. The interaction between the CDZ molecules and the zinc surface was described by DFTB, MC, and computational studies.
O, COO−, π-electrons of aromatic ring), good structure to cover the metal surface, and dissolved in water. Therefore, expired CDZ was used as an inhibitor in the current investigation to study zinc corrosion mitigation in a 1 mol per L HCl medium. The experiments were carried out using WL, PDP, EIS, and EFM methodologies. The composition and morphology of the zinc metal surface were investigated using EDX and SEM analyses. The drug's type of adsorption on zinc metal was evaluated using several adsorption isotherms. Additionally, the thermodynamic characteristics pertaining to the adsorption process were established. The mechanism of inhibition was investigated. The interaction between the CDZ molecules and the zinc surface was described by DFTB, MC, and computational studies.
Table 1 The chemical structure, IUPAC name, molecular mass, and molecular formula of the expired Ceftazidime (CDZ) drug
| Drug |
Structural formula |
Characteristics |
| Ceftazidime (CDZ) |
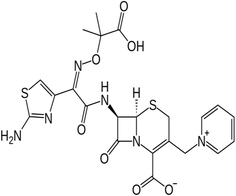 |
(6R,7R,Z)-7-(2-(2-Aminothiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetamido)-8-oxo-3-(pyridinium-1-ylmethyl)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate |
| Molecular formula: C22H22N6O7S2 |
| Molecular mass: 546.58 g mol−1 |
2. Materials and methods
2.1 Zn strips
The corrosion rate was determined using high-purity 99.2% zinc metal strips (Cd 0.52%, Sn 0.07%, Pd 0.18%, Fe 0.035%, and Zn remaining). After the metal strips were abraded using 400, 800, 1500, and 2000 grade silicon carbide emery papers, they were cleaned with distilled water, degreased in acetone, and thoroughly dried.43 Zinc strips measuring 2 × 2 × 0.1 cm3 were used for WL experiments in a 1 mol per L HCl solution. Electrochemical investigations were conducted using zinc strips with a 1 cm2 exposure area. AR-grade HCl (32%) and double-distilled water were used to prepare the 1 mol per L HCl solution (blank). This concentration was chosen for its balance between inducing significant corrosion and maintaining a reasonable level of safety during experimentation.
2.2 Inhibitor
A stock solution containing 1000 mg L−1 (ppm) was prepared by dissolving 1 gram of expired CDZ medication (expired 1 year ago) in 1 Liter of bi-distilled water. The stock solution was then diluted with bi-distilled water to achieve concentrations ranging from 50 to 300 ppm. Different concentrations of CDZ were added to 1 mol per L HCl to create the corrosive medium. To determine if the Ceftazidime molecule degraded after expiration, FTIR spectroscopy was performed. The resulting spectra of both fresh and expired samples showed nearly identical patterns, indicating that the functional groups and overall chemical structure were preserved (Fig. S1, ESI†). No notable structural alterations or degradation products were observed.
2.3 WL measurements
According to ASTM-G1,44 WL measurements were established. To conduct WL tests, cleaned zinc strips were immersed in 100 cm3 blank solution having various CDZ concentrations for 6 hours. The studies were conducted between 298 and 318 K. The rusted Zn metal strips were removed after 6 hours, cleaned with distilled water, allowed to dry in acetone, and then weighed. Three sets of measurements were made, and the average value was recorded ± standard deviation.
2.4 Electrochemical studies
ASTM-G102 was followed for conducting the electrochemical tests.24 The Gamry Instrument PCI4300 Potentiostat/Galvanostat ZRA was utilized to evaluate the potentiodynamic polarization (PDP), the impedance spectroscopy (EIS), and the frequency modulation (EFM) at 298 K. Experiments were conducted using a three-electrode system that included a working electrode (Zn strip with 1 cm2 of exposure), an auxiliary electrode (Pt), and a reference electrode (saturated calomel). The zinc electrode was submerged in the corrosive medium for 30 minutes before each electrochemical measurement to allow the open circuit potential to achieve equilibrium. Polarization curves were obtained within the potential window of −0.3 to +0.2 V regarding the open circuit potential value (OCP) with a scan rate of 1 mV s−1.
EIS studies were accomplished utilizing AC signals in the frequency range of 100 kHz to 0.5 Hz, with an amplitude of 10 mV s−1. The ZSimp Win 3.21 program was used to fit all impedance values to the relevant equivalent circuits. Nyquist and Bode's plot provided the impedance parameters. The reproducibility of the EIS measurements was confirmed by performing triplicate experiments under identical conditions. The consistency of the obtained spectra and fitted parameters demonstrates the reliability of the data. The EFM investigation employed two frequencies, 2 and 5 Hz. Every second, the waveform renews itself at the shortest frequency of 100 mHz. The spectrum includes current actions caused by inter-modification and harmonic current peaks. To compute the corrosion current, Tafel slopes (βc and βa), and causality factors (CF-2 and CF-3), the bigger peaks were used.
2.5 SEM studies
A scanning electron microscope (model: JOEL JSM-6510LV) was utilized to examine the surface morphologies of the zinc specimens. To conduct the studies, zinc specimens were submerged in a blank solution for 6 hours in the absence and presence of 300 ppm of the CDZ inhibitor. The metal samples were removed, washed with bi-distilled water, dried, and subjected to SEM analysis. The accelerating beam used had a potential of 35 kV.
2.6 EDX analysis
The EDX device (model: EDX-FEI-QUANTA FEG 250) was used to investigate the chemical analysis of the zinc surface.
2.7 Modeling and computations
Chemical simulations were used to describe the adsorption mechanism. The inhibitory efficiency of the corrosion inhibitor was connected to the quantum chemical characteristics. In the quantum computing package, DFTB +1.3 is utilized. Slater–Koster file libraries provided the empirical parameters required for the DFTB integral evaluation computation. For the current system, Auorg parameters were employed.45–47 Energy of the lowest unoccupied molecular orbital (ELUMO), energy of the highest occupied molecular orbital (EHOMO), adsorption energy (Eads), and energy gap (ΔE) were among the thermodynamic and electronic characteristics that were computed to evaluate the effectiveness of the CDZ drug for inhibiting corrosion.
The preferred adsorption arrangements of the CDZ molecule on the adsorption centers of zinc's periodic structure were examined via MC simulation. Utilizing a simulated annealing algorithm, MC checks of possible substrate-adsorbate arrangements were conducted to find possible adsorption sites.45 A periodic slab model box of a super unit cell with a 25 Å vacuum layer was used for MC to avoid interference between periodic unit cells. A single inhibitor molecule and a 20 Å vacuum layer in the Z-direction cover a zinc (002) supercell surface with 8 atomic layers. The mechanism of interactions between the drug and the zinc surface and the behavior of the CDZ molecule during adsorption were clarified by the compass field of force.
3. Results and discussion
3.1 WL tests
The WL studies were carried out with and without different concentrations of CDZ ranging from 50 to 300 ppm in a blank acid solution. Fig. 1 illustrates how the inhibition efficiency (IEw) changes with the CDZ concentration at various temperatures. Zinc weight loss and corrosion rate (CR) decrease, and inhibition efficiency increases when CDZ is added to a 1 mol per L HCl solution. The results revealed that zinc corroded rapidly when exposed to a 1 mol per L HCl solution, while the rate of breakdown was slowed down when CDZ expired drug was present. Furthermore, an increase in inhibitory efficacy with concentration indicates a strong interaction between the drug and the zinc surface.48 The results also revealed that CDZ drug might effectively block the Zn surface in a 1 mol per L HCl solution. Additionally, as demonstrated by Fig. 1, the efficiency of inhibition drops as the temperature gets higher, suggesting that some of the drug that was previously adsorbed may desorb at higher temperatures. This indicates that the CDZ molecules were physically coated on the zinc surface, and physisorption is the term for the adsorption process.49
 |
| | Fig. 1 Effect of temperature on IEw at different concentrations of expired Ceftazidime drug for zinc corrosion in 1 mol per L HCl. | |
The corrosion rate (CR) was computed by eqn (1).50
| |
 | (1) |
where Δ
W is the WL of the Zn strips in mg,
A is the area of the Zn strip in cm
2, and
t is the submersion period in minutes. The inhibition efficacy (IE
w), and the surface coverage (
θ) were determined by
eqn (2).
51| |
 | (2) |
where
Wcorr and
Winh stand for the WL of zinc strips free of and with the drug, respectively.
θ is the surface coverage of zinc. Table 2 presents data for the corrosion inhibition of zinc in a blank solution by CDZ. The WL measurements were conducted 240 minutes at varying temperatures and CDZ concentrations. The data suggests that CDZ is an effective inhibitor for zinc corrosion in an acidic environment. The inhibition efficacy is influenced by both the quantity of CDZ and the temperature of the solution. The addition of CDZ to the solution significantly inhibits zinc corrosion. The WL and CR decrease with increasing CDZ concentration. The CR decreases to 10.62 × 10−3 mg cm−2 min−1 at 298 K when the medication exists with a concentration of 300 ppm. This indicates the drug's capability to develop a shielding film on the zinc surface. As temperature increases, the corrosion rate of zinc generally increases in both the absence and presence of CDZ. This is expected behavior as higher temperatures provide more energy for the corrosion process. As the inhibition efficiency did not improve beyond 300 ppm (Fig. 1), the electrochemical experiments were performed exclusively within the 50–300 ppm concentration range. The surface coverage and IEw increase with increasing CDZ concentration. At 298 K, with 300 ppm of CDZ present, the IEw reaches 64.1%. This suggests that the drug molecules are effectively adsorbing onto the zinc surface, blocking corrosive sites.
Table 2 Data of WL measurements at 240 min for zinc in a 1 mol per L HCl, in the absence and presence of different concentrations of the expired Ceftazidime drug at different temperatures
| Temp. (K) |
Dose of drug (ppm) |
Weight loss (mg cm−2) |
CR × 10−3 (mg cm−2 min−1) |
Surface coverage (θ) |
IEw (%) |
| 298 |
0.00 |
7.11 |
25.45 |
— |
— |
| |
50 |
4.35 |
18.12 |
0.388 |
38.8 ± 0.1 |
| |
100 |
3.75 |
15.62 |
0.472 |
47.2 ± 0.3 |
| |
150 |
3.22 |
13.41 |
0.547 |
54.7 ± 0.2 |
| |
200 |
2.98 |
12.48 |
0.580 |
58.0 ± 0.3 |
| |
250 |
2.82 |
11.75 |
0.603 |
60.3 ± 0.2 |
| |
300 |
2.55 |
10.62 |
0.641 |
64.1 ± 0.1 |
| 303 |
0.00 |
8.22 |
34.25 |
— |
— |
| |
50 |
5.32 |
21.16 |
0.352 |
35.2 ± 0.2 |
| |
100 |
4.82 |
20.08 |
0.413 |
41.3 ± 0.1 |
| |
150 |
4.56 |
19.37 |
0.445 |
44.5 ± 0.3 |
| |
200 |
3.98 |
16.58 |
0.515 |
51.5 ± 0.2 |
| |
250 |
3.58 |
14.16 |
0.564 |
56.4 ± 0.3 |
| |
300 |
3.11 |
12.95 |
0.621 |
62.1 ± 0.1 |
| 308 |
0.00 |
9.70 |
38.29 |
— |
— |
| |
50 |
6.82 |
29.60 |
0.301 |
30.1 ± 0.2 |
| |
100 |
6.56 |
27.33 |
0.359 |
35.9 ± 0.3 |
| |
150 |
6.21 |
25.87 |
0.413 |
41.3 ± 0.1 |
| |
200 |
5.38 |
22.41 |
0.445 |
44.5 ± 0.2 |
| |
250 |
5.11 |
21.23 |
0.473 |
47.3 ± 0.4 |
| |
300 |
4.98 |
20.75 |
0.486 |
48.6 ± 0.3 |
| 318 |
0.00 |
10.65 |
49.79 |
— |
— |
| |
50 |
7.60 |
36.41 |
0.286 |
28 2 ± 0 28.6 ± 0.2 |
| |
100 |
7.24 |
35.08 |
0.320 |
30.2 ± 0.3 |
| |
150 |
6.38 |
33.41 |
0.409 |
40.9 ± 0.1 |
| |
200 |
6.14 |
32.55 |
0.423 |
42.3 ± 0.3 |
| |
250 |
5.98 |
36.81 |
0.438 |
43.8 ± 0.2 |
| |
300 |
5.78 |
29.50 |
0.467 |
46.7 ± 0.4 |
A comparison of Ceftazidime's corrosion inhibition with varying expiration dates revealed that two, and three-year expired samples performed poorly (Fig. S2 and S3, Tables S1 and S2 in ESI†). This led us to prioritize the one-year expired drug, which demonstrated the most effective inhibition.
Acid dissolves metal by an electrochemical reaction. Zinc dissolves at the anode, producing corresponding ions.52
In an aerated solution of acidic chloride, the reaction occurring in the cathodic area is:
or
| | |
4H+ + O2 + 4e− → 2H2O
| (6) |
Therefore, the overall corrosion reaction in an aerated solution will be:
| | |
2Zn + 4H+ + O2 → 2H2O + 2Zn2+
| (7) |
3.2 Adsorption behavior and thermodynamic parameters
The adsorption phenomenon, in which inhibitor molecules prefer to adsorb on the corroding surface layer, has been proposed as the origin of corrosion inhibition. In this work, the extent of surface coverage occupied by CDZ molecules (θ) was computed by applying the traditional WL method to investigate the most appropriate adsorption mode. The Temkin model with high fitting (R2 ranging between 0.990 and 0.997) was proposed (Fig. 2) to shed further light on the inhibitory effect of CDZ on the Zn surface. Eqn (8) was used to assess the Temkin model's applicability.42| |
 | (8) |
 |
| | Fig. 2 Temkin adsorption curves for zinc in 1 mol per L HCl with different concentrations of CDZ at different temperatures (298–318 K). | |
For this equation, C represents the CDZ drug concentration, a is a molecular interaction parameter, Kads is the equilibrium constant, and θ is the fraction of surface covering (θ = IEw/100). A straight line having a slope of (2.303/a) with an intercept of [(2.303/a) log Kads] is established by plotting logC against θ (Fig. 2). Table 3 displays the values of Kads. High values of Kads indicate a strong and stable adsorbed film.53 Rising temperatures cause the value of Kads to drop. The physical nature of adsorption causes CDZ molecules to desorb from zinc surfaces more quickly at higher temperatures.
Table 3 Adsorption parameters for zinc at various concentrations of the expired Ceftazidime drug at different temperatures in 1 mol per L HCl solutions
| Temperature (K) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kads Kads |
Kads |

(kJ mol−1) |
| 298 |
3.60 |
3981.07 |
30.49 |
| 303 |
3.00 |
1000.00 |
27.07 |
| 308 |
2.88 |
758.58 |
26.38 |
| 313 |
2.41 |
257.04 |
23.70 |
| 318 |
2.25 |
127.83 |
21.97 |
Eqn (9) was employed to obtain the standard free energy of adsorption,  , which was linked to Kads as previously established.54,55
, which was linked to Kads as previously established.54,55
| |
 | (9) |
where
T is the Kelvin temperature and
R is the gas constant. The calculated

of the CDZ medication at various temperatures (298–318 K) are shown in
Table 3. The negative sign of

refers to the adsorption process of the CDZ drug on the Zn surface being stable and spontaneous.
56 Moreover, some studies have discriminated against the chemisorption and physisorption processes using

values.
57,58 The magnitudes of

below 20 kJ mol
−1 were linked to physisorption, or the interaction of electrostatic charges, whereas values above 40 kJ mol
−1 were related to chemisorption, or the transfer of electrons between the drug molecules and the Zn surface. The CDZ medication has

values ranging between −21.97 and −30.49 kJ mol
−1 in the current investigation. Hence, rather than being a simple case of physisorption or chemisorption, the adsorption of the CDZ medication on the Zn surface is a complex combination of chemical and physical adsorption.
56
3.3 PDP study
The PDP curves of the zinc electrode in a blank solution were displayed in Fig. 3 at a voltage scan rate of 1 mV s−1 and 298 K, without and with different CDZ drug concentrations. Electrochemical parameters, including corrosion current density (icorr), corrosion potential (Ecorr), and the cathodic and anodic Tafel slopes (βc and βa), were determined (Table 4) by applying the Tafel extrapolation method to the polarization curves shown in Fig. 3. The Tafel extrapolation method is a fundamental electrochemical technique used to determine corrosion parameters by analyzing a Tafel plot. This plot, which graphs the logarithm of current density against electrode potential, reveals distinct linear regions corresponding to anodic and cathodic reactions. By extrapolating these linear regions, their intersection point provides the corrosion potential (Ecorr) and corrosion current (icorr). Ecorr signifies the potential where anodic and cathodic reaction rates equalize, while icorr directly correlates with the corrosion rate.59 Furthermore, the slopes of these linear regions, known as Tafel slopes (βc and βa), offer insights into the kinetics of the electrochemical reactions. Essentially, Tafel extrapolation harnesses the linear behavior of electrochemical polarization curves to quantify corrosion rates and understand the underlying reaction mechanisms. The following equation was used to determine the percentage inhibition efficiency (IEp).60| |
 | (10) |
 and icorr stand for the corrosion current density in the absence and presence of the CDZ drug, respectively. Significant corrosion developed when the blank solution came into direct contact with zinc metal. Zinc dissolves more quickly in corrosive solutions when aggressive ions like Cl− and H3O+ are present. The insertion of CDZ into the corrosive solution causes notable drops in current densities, which slowed the zinc's rate of corrosion. The anodic and cathodic sections of the measured polarization curves (Fig. 3) progressively shift toward lower current density as the concentration of CDZ increases, indicating a decline in the overall corrosion rate.61 Anodic and cathodic branches' shapes were altered, demonstrating that the existence of CDZ controlled both anodic and cathodic processes. The cathodic polarization curves depict the evolution of hydrogen, but the dissolution of zinc is represented by anodic polarization curves.
and icorr stand for the corrosion current density in the absence and presence of the CDZ drug, respectively. Significant corrosion developed when the blank solution came into direct contact with zinc metal. Zinc dissolves more quickly in corrosive solutions when aggressive ions like Cl− and H3O+ are present. The insertion of CDZ into the corrosive solution causes notable drops in current densities, which slowed the zinc's rate of corrosion. The anodic and cathodic sections of the measured polarization curves (Fig. 3) progressively shift toward lower current density as the concentration of CDZ increases, indicating a decline in the overall corrosion rate.61 Anodic and cathodic branches' shapes were altered, demonstrating that the existence of CDZ controlled both anodic and cathodic processes. The cathodic polarization curves depict the evolution of hydrogen, but the dissolution of zinc is represented by anodic polarization curves.
 |
| | Fig. 3 Potentiodynamic polarization curves of zinc in 1 mol per L HCl without and with different concentrations of the Ceftazidime drug at 298 K. | |
Table 4 Potentiodynamic polarization parameters of zinc in 1 mol per L HCl without and with different concentrations of the Ceftazidime drug at 298 K
| [CDZ] (ppm) |
−Ecorr (mV vs. SCE) |
βa (mV dec−1) |
−βc (mV dec−1) |
icorr (μA cm−2) |
θ |
IEp (%) |
| Blank |
117 |
119 |
199 |
235 |
— |
— |
| 50 |
113 |
112 |
146 |
150 |
0.362 |
36.2 ± 0.2 |
| 100 |
111 |
107 |
127 |
123 |
0.476 |
47.6 ± 0.1 |
| 150 |
108 |
102 |
116 |
105 |
0.553 |
55.3 ± 0.1 |
| 200 |
106 |
99 |
112 |
93 |
0.604 |
60.4 ± 0.2 |
| 250 |
104 |
91 |
105 |
84 |
0.642 |
64.2 ± 0.4 |
| 300 |
100 |
78 |
87 |
73 |
0.689 |
68.9 ± 0.3 |
In relation to the blank solution, the inhibitor acts as a mixed-type inhibitor if the Ecorr is smaller than 85 mV and as a cathodic or anodic type if it is bigger than 85 mV. According to Table 4, there is a 17 mV difference between Ecorr in the drug-containing solution (300 ppm) and the blank solution in this study, suggesting that the CDZ medication is a mixed inhibitor.57 Tafel plots in Fig. 3 illustrate a positive shift in the corrosion potential (Ecorr) relative to the drug-free blank solution. The polarization curves and collected data demonstrate that the drug influences both the anodic and cathodic half-reactions. Notably, both the anodic and cathodic current densities significantly drop in comparison to the blank solution, confirming that the CDZ drug functions as a mixed inhibitor.62 Data shown in Table 4 demonstrates a substantial reduction in current density with the insertion of the CDZ medication. When the drug's concentration reaches 300 ppm, the efficiency of inhibition rises to 68.9%. As the quantity of CDZ drug in the blank solution increases, the βa and βc show a decrease in value. The possible reason for these effects could be the adsorption of CDZ molecules onto the zinc surface.63
3.4 EIS study
The Nyquist diagrams of zinc in blank solution free of and with varying CDZ medication doses were shown in Fig. 4A. For both the blank and CDZ drug solutions, the Nyquist diagrams display only one semicircle of depressed capacitance. Electrodes that show frequency dispersion because of surface defects and other inhomogeneities of the metal were characterized by depressed semicircles, which correlate to Nyquist diagrams.64 The Zn corrosion reaction was thought to be controlled by a single charge transfer process, as indicated by the singular capacitive loop.64 In accordance with the polarization study, this shape remained unchanged with the presence of CDZ at various dosages, indicating that the process of corrosion is under activation control and does not alter.65 It is worth observing that the centers of the depressed semicircles were laid under the X-axis. The frequency dispersion can cause this phenomenon. The influence of the inhomogeneous Zn surface and the interfacial impedance on frequency dispersion could be the reason for this behavior.66 Usually, the presence of inhibitor adsorption and coarseness/roughness is the cause.67,68 Furthermore, compared to a free HCl solution, the existence of CDZ medication increased the diameter of the capacitive loops, indicating that the drug inhibits zinc metal from corroding by covering the zinc metal's surface with a barrier coating.69
 |
| | Fig. 4 Nyquist (A) and Bode (B) plots of zinc in 1 mol per L HCl without and with different concentrations of the expired Ceftazidime drug at 298 K, and (C) the equivalent circuit used to fit the EIS data. | |
Because of the inhomogeneities of the metal surface, the Bode plot shown in Fig. 4B generally produces a depressed semicircle for the metal-solution interface. Zinc corrosion in acidic conditions increases the metal's surface roughness, which lowers the phase angle.26 The phase angle increased as drug concentration increased, as shown by the Bode's plot. The data also supports the inhibitor's progressive adsorption on the zinc surface. This blocks the sites that are active and lowers the extent of corrosion.26 The Bode graphs (Fig. 4B) demonstrate that the impedance module |Z| shifts towards larger values in comparison to the blank when CDZ is present. This displacement was caused by the adsorption of the CDZ drug on the zinc surface.56 Consequently, the assessed CDZ slowed down the kinetics of the zinc corrosion in HCl medium.70 Fig. 4B shows that for the Bode plots at the mid-frequency range, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z and log
Z and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f have a direct proportionality. Also, the phase angle value is near −60°, and the slope approaches −1. This verifies the inadequate capacitive performance at intermediate frequencies.58 Perfect capacitive performance was attained at moderate frequencies when the slope is −1 and the angle of phase is −90°. Compared to uninhibited solutions, inhibited solutions have larger slope and phase angle magnitudes. This makes clear how efficiently the drug under investigation inhibits zinc degradation.57
f have a direct proportionality. Also, the phase angle value is near −60°, and the slope approaches −1. This verifies the inadequate capacitive performance at intermediate frequencies.58 Perfect capacitive performance was attained at moderate frequencies when the slope is −1 and the angle of phase is −90°. Compared to uninhibited solutions, inhibited solutions have larger slope and phase angle magnitudes. This makes clear how efficiently the drug under investigation inhibits zinc degradation.57
Adapting the results of EIS studies with the described circuit in Fig. 4C allows one to examine the impedance spectra. Table 5 shows the impedance parameters, which include the double layer capacitance (Cdl), inhibition efficiency (IEEIS), and charge transfer resistance (Rct). When CDZ concentration increases, Rct and IEEIS values increase, indicating that a shielding film has developed on the metal/solution interface.68,70 The Cdl, was inversely proportional to Rct based on the following equation.
| |
 | (11) |
where
fmax represents the frequency at which the imaginary part of the impedance is at its highest value. In the presence of CDZ medication, the
Cdl values decreased (
Table 5). This results from an increase in the thickness of the double layer surrounding the shielding film and/or a decrease in the local dielectric constant caused by the deposited CDZ molecules permuting the H
2O molecules at the Zn surface.
68 The effective adsorption mechanism may be the cause of the decreased
Cdl values with increasing CDZ medication additions. This finding implies that the drug molecules may initially cover the active centers on the zinc surface by adsorption.
71 Using the following equation,
72 the inhibition efficiency values (
Table 4) can be calculated from the R
ct values.
| |
 | (12) |
where
Rct and

stand for Zn's electric charge transfer resistance in blank solution with and free of CDZ, respectively. As the concentration of CDZ increases, the values of IE
EIS increase, reaching an efficiency of 68.6% at 300 ppm and 298 K. The findings suggest that a more protective coating was formed on the zinc surface with the addition of CDZ. These findings validate the consistency between the outcomes from EIS and alternative methods.
Table 5 EIS data of zinc in 1 mol per L HCl without and with different concentrations of the Ceftazidime drug at 298 K
| [CDZ] (ppm) |
Rct (Ω cm2) |
Cdl ×10−6 (F cm−2) |
θ |
IEEIS (%) |
| Blank |
110.6 |
348 |
— |
— |
| 50 |
179.2 |
100.2 |
0.383 |
38.3 ± 0.2 |
| 100 |
211.3 |
83.6 |
0.477 |
47.7 ± 0.1 |
| 150 |
255.2 |
65.8 |
0.567 |
56.7 ± 0.1 |
| 200 |
288.5 |
56.2 |
0.617 |
61.7 ± 0.3 |
| 250 |
311.8 |
48.5 |
0.645 |
64.5 ± 0.1 |
| 300 |
352.0 |
42.1 |
0.686 |
68.6 ± 0.4 |
3.5 EFM study
Several advantages of the EFM technique, including direct, nondestructive, quick corrosion rate measurement and data validation, make it a perfect choice for online corrosion monitoring applications.51 The EFM approach is a small signal AC technique that resamples the EIS. On the other hand, two distinct sine waves are applied simultaneously in this technique. Since there can be no direct relationship (non-linear) between I and V, the examined system responds to the excitation potential non-linearly.42 As seen in Fig. 5, the input frequencies as well as their frequency components were considered in the current response. The frequency constituents are the sum of the two input frequencies, as well as their multiples and differences. The EFM diagrams of the zinc metal, under study, in a blank solution having varying dosages of the CDZ medication at 298 K are shown in Fig. 5. Because of the excitation frequencies, there are two prominent peaks at 0.2 and 0.5 Hz. The larger peaks were used to compute the slopes of Tafel, the causality factors (CF2 and CF3), and the corrosion current (icorr). The kinetic features of the corrosion process, such as inhibition efficiency (IEEFM), icorr, Tafel parameters, and causality factors, are shown in Table 6. The insertion of the expired CDZ drug into the blank solution resulted in a reduction in the icorr of zinc metal. Increasing the concentration of CDZ from 50 to 300 ppm reduces the corrosion current density from 163.5 to 78.7 μA cm−2. This is likely due to the adsorption of the drug molecules onto the zinc surface. The CDZ acts as a barrier and inhibits the corrosion process.42
 |
| | Fig. 5 EFM spectra for the corrosion of zinc in the blank solution without and with different concentrations of Ceftazidime drug at 298 K. | |
Table 6 Electrochemical kinetic parameters obtained by the EFM technique for zinc metal in 1 mol per L HCl in the absence and presence of different concentrations of the expired Ceftazidime drug at 298 K
| [CDZ] (ppm) |
icorr ×10−6 (A cm−2) |
βa × 10−3 (V dec−1) |
−βc × 10−3 (V dec−1) |
CF-2 |
CF-3 |
IEEFM (%) |
| Blank |
265 |
91 |
323 |
1.92 |
2.93 |
— |
| 50 |
163.5 |
73.2 |
188.8 |
1.82 |
2.87 |
38.3 ± 0.1 |
| 100 |
135.1 |
71.4 |
129.2 |
1.89 |
3.12 |
49.0 ± 0.2 |
| 150 |
110.2 |
55.1 |
110.5 |
1.95 |
3.10 |
58.4 ± 0.3 |
| 200 |
99.8 |
52.2 |
87.5 |
1.87 |
3.09 |
62.3 ± 0.1 |
| 250 |
85.2 |
45.9 |
59.2 |
2.10 |
2.89 |
67.8 ± 0.2 |
| 300 |
78.7 |
41.2 |
57.4 |
2.03 |
2.85 |
70.3 ± 0.3 |
The efficiency of the inhibition (IEEFM) increases with Ceftazidime concentration. According to Table 6, the causality factors closely resemble the ones that were predicted. This might ensure that the different parameters and corrosion current densities are accurate, according to EFM theory.68 With the EFM data as a basis, eqn (13) computes IEEFM.
| |
 | (13) |
where

and
idrug stand for the corrosion current densities without and with CDZ drug, respectively.
3.6 SEM and EDX examinations
Using the SEM technique, it was possible to determine whether the zinc surface had a protective film from the expired CDZ. Moreover, EDX analyses were conducted to ascertain whether the medication is adsorbed on the zinc surface or not. Fig. 6 displays SEM photographs of the cleaned zinc surface and the zinc surface after submerging in a blank solution free of and with 300 ppm of the medication CDZ. The polished zinc surface is smooth and compact without any defects (Fig. 6A). The SEM micrographs for the zinc surface after 8 hours submersion in a blank solution free of and with 300 ppm of CDZ medication are displayed in Fig. 6B and C, respectively. The SEM image (Fig. 6B) of the corroded zinc sample in 1 mol per L HCl solution reveals a badly damaged zinc surface covered by corrosion products. Smaller pits evenly spread laterally and internally may have led to the extended corrosion region. On the other hand, zinc metal corrosion is significantly suppressed when the CDZ medication is present (Fig. 6C). Consequently, the presence of the CDZ medication on the zinc surface lowers the rate at which it dissolves in the HCl medium.
 |
| | Fig. 6 SEM micrographs of polished zinc (A), zinc after 8 h immersion in a 1 mol per L Cl without (B), and with (C) 300 ppm of expired Ceftazidime drug at 298 K. | |
To confirm that the CDZ medication molecules are present on the zinc surface, EDX analysis is used. Fig. 7 shows the corresponding EDX spectra. Zinc peaks were the only ones found for the polished zinc sample (Fig. 7A). Because ZnCl2 forms at the zinc surface in the presence of the blank solution, there were peaks for both zinc and chloride elements (Fig. 7B).53 As seen in Fig. 7C, there were peaks for C, S, N, and O elements in the EDX spectrum for zinc coupons subjected to the CDZ drug. This result demonstrates that the medication was adsorbed onto the zinc surface, significantly impeding the zinc's ability to dissolve in the HCl solution.42
 |
| | Fig. 7 EDX spectra of polished zinc (A), zinc after 8 h immersion in a 1 mol per L HCl without (B), and with (C) 300 ppm of expired Ceftazidime drug at 298 K. | |
3.7 Computations involving quantum chemistry
3.7.1 DFTB simulations. Significant details concerning the electronic and thermodynamic characteristics of corrosion-inhibitory compounds can be obtained by Density Functional Tight-Binding (DFTB) simulations. The DFTB method was used to assess the frontier molecular orbitals (FMOs) of CDZ (Fig. 8), which comprise the highest occupied (HOMO) and lowest unoccupied (LUMO) molecular orbitals. The pyridinium-1-ylmethyl group that lies at the 3-position of the antibiotic's cephem ring contains the HOMOs of CDZ. On the other hand, LUMOs are positioned at the two oxygens of the carboxylic group.
 |
| | Fig. 8 FMO diagram of Ceftazidime (a) LUMO and (b) HOMO. | |
According to the results, the HOMO and LUMO energies were −5.833 and −3.381, respectively. With a computed energy gap (ΔE) of 2.452, CDZ exhibited good stability and the ability to successfully reduce corrosion (Table 7). The metal's surface binding capability can be increased by decreasing the inhibitor's ΔE value, which will increase the reactivity between the metal and the inhibitor molecule. This increase in binding capacity may lead to an improvement in the inhibition efficiency of the compound because less energy is required to liberate the electron from the HOMO orbital.73
Table 7 The calculated quantum chemical parameters, obtained from DFTB data
| Parameter (au) |
Value |
| Energy of highest occupied molecular orbital (EHOMO) |
−5.833 |
| Energy of lowest unoccupied molecular orbital (ELUMO) |
−3.381 |
| Energy gap (ΔE) |
2.452 |
| Chemical hardness (η) |
1.226 |
| Chemical softness (σ) |
0.816 |
| Chemical potential (μ) |
−4.607 |
| Electronegativity (χ) |
4.607 |
| Electron affinity (A) |
3.381 |
| Ionization potential (I) |
5.833 |
| Electrophilicity index (ω) |
8.656 |
| Maximum charge transfer index (ΔNmax) |
3.758 |
| Nucleophilicity (ε) |
0.116 |
Electrostatic potential (ESP) maps assist in offering a better understanding of the electron-rich and electron-deficient regions of molecules.74 The red areas (negative portions) of the map indicate places that support an electrophilic attack, whereas the blue regions (positive portions) of the map indicate sites that support a nucleophilic attack (Fig. 9). A light blue area refers to a slightly electron-deficient location; the yellow area indicates a slightly electron-rich position, and the green area indicates a neutral location. The ESP map of the CDZ compound showed that the pyridinium-1-ylmethyl group acts as a nucleophile (red to orange areas); on the other hand, the carboxylic group functions as an electrophile (blue area). The hydrophobic carbon chain repelled serious pitting chloride ions away from the zinc surface, facilitating the adhesion of adsorbed molecules.75
 |
| | Fig. 9 Electrostatic potential map of Ceftazidime molecule. | |
DFTB data can be used to compute a few descriptors that evaluate the CDZ drug's inhibitory performance. The energies of the highest occupied (EHOMO) and the lowest unoccupied (ELUMO) molecular orbitals can be used to calculate them. The energy gap (ΔE), ionization potential (I), electron affinity (A), chemical potential (μ), electronegativity (χ), hardness (η), softness (σ), electrophilicity index (ω), nucleophilicity (ε), and a proportion of electron transfer (ΔNmax) are reported in Table 7, which is based on the literature.76 According to the following relations, the parameters are computed.77
| |
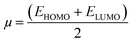 | (18) |
| |
 | (19) |
| |
 | (21) |
| |
 | (22) |
The chemical reactivity of molecules is indicated by their ionization potentials. Compared to high ionization potentials that indicate the high stability of the molecules, smaller values represent more activity for the molecules.78 Due to its small ionization potential (5.833), CDZ has good inhibitory efficiency, as seen in Table 7. An important consideration when assessing the CDZ drug's responsiveness is its electronegativity (χ) value. The value of 4.607 refers to the degree to which it can hold electrons.79 A substantial interaction between CDZ and the zinc metal was confirmed by a low value of softness and a big value of hardness.80 The reactivity of the CDZ molecule is demonstrated by its electrophilicity (ω) value of 8.656. ε is nucleophilicity, which is the reciprocal of electrophilicity. With an estimated ΔNmax value of 3.758, CDZ is capable of releasing electrons and supports the development of a shielding layer on the zinc surface.81 The computation studies show that the present drug has efficient adsorption centers that can readily adsorb on the zinc surface and create a protective layer.
3.7.2 Monte Carlo simulation studies. While electronic parameters are effective in describing the drug's mechanism of action, they are insufficient to figure out the trend of the drug's inhibitive performance.82 Therefore, it is crucial to carry out detailed modeling of the drug's direct interactions with the Zn surface. Adsorption is thought to be the main way by which corrosion is inhibited. The nature of interactions between the investigated drug and the Zn (002) crystal surface in 1 mol per L HCl was thus established by simulating the adsorption of the CDZ compound on the Zn surface. Because it possesses the most stable surface, the Zn (002) surface is utilized for the MC simulation study.Using an MC simulation, Fig. 10 displays the drug's most stable low-energy adsorption configuration on Zn (002). Through the process of adsorption, the drug forms a protective film on the zinc surface. The film protects the zinc surface and keeps the corrosive solution away from it. Table 8 shows the values of the adsorption, the deformation, and the rigid adsorption energies for the adsorption of the CDZ drug on the Zn (002) surface. The adsorption energy of −77.41 kJ mol−1 indicates a significant interaction between the CDZ molecule and the zinc surface. This suggests that the drug is likely to bind effectively to the metal.24 The deformation energy of 1.88 kJ mol−1 suggests that the CDZ molecule undergoes a slight conformational change upon adsorption. This is expected as the molecule needs to adjust its shape to optimize its interaction with the surface. The rigid adsorption energy of −79.29 kJ mol−1 provides an estimate of the adsorption energy without considering the conformational changes of the molecule. Comparing this value to the adsorption energy suggests that the deformation energy has a minor impact on the overall adsorption strength.
 |
| | Fig. 10 The side view of the adsorption of the Ceftazidime drug on the zinc surface. | |
Table 8 The adsorption, the deformation, and the rigid adsorption energies for the adsorption of the Ceftazidime drug on the Zn (002) surface
| Total energy (kJ mol−1) |
Adsorption energy (kJ mol−1) |
Deformation energy (kJ mol−1) |
Rigid adsorption energy (kJ mol−1) |
| 680.25 |
−77.41 |
1.88 |
−79.29 |
Eqn (24) is used to compute the adsorption energy, or Eads.
| | |
Eads = EZn-inh − (Einh − EZn)
| (24) |
where
EZn is the zinc surface's total energy, and
Einh is the inhibitor's total energy. The surface energy of zinc (002) is zero.
83 Given the strong adsorption energy (−77.41 kJ mol
−1) and the relatively small deformation energy (1.88 kJ mol
−1); it is likely that the CDZ molecule adopts a relatively flat configuration on the zinc surface. This would maximize the contact area between the molecule and the metal, leading to stronger interactions. This enables the transfer of the lone pair of electrons of the heteroatoms and π-electrons from the pyridinium ring of the drug to the zinc metal.
84 The side view orientation could be influenced by specific interactions between functional groups on the CDZ molecule and the zinc surface. For example, the carboxylate group (–COO–) in CDZ might interact favorably with the zinc atoms due to electrostatic interactions. The CDZ molecule has two sulfur atoms. Sulfur atoms have relatively lower electronegativities, enabling relatively stronger coordinate bonding with surface metallic atoms. Many investigators reported the high inhibition efficiency of sulfur compounds.
85 Heteroatoms' inhibitory effect followed their inverse electronegativity order, which is S > N > O. Higher inhibition efficiency and more charge transfer are associated with lower electronegativity. Because these heteroatoms are protonated and exist in their cationic forms in acidic solutions,
85 they were adsorbed by means of the electrostatic force of attraction during the initial stages of the interaction.
3.8 Mechanism of inhibition
The dissolution and dissociation of Zn metal in the anodic region and the reduction of O2 gas in the cathodic region are both impacted by the introduction of the CDZ medication to the acidic medium, as indicated by the values of Ecorr, βa, and βc in Table 4. The values of the Tafel cathodic and anodic constants decrease when the dosage of the CDZ drug in the acidic blank solution increases. Since the expired medication slows down both the cathodic and anodic reactions, it is confirmed to function as a mixed inhibitor.24 Such impacts might result from CDZ molecules adhering to the zinc surface.63
Table 3 shows that the computed values of  are −21.97 to −30.49 kJ mol−1, which indicates that the drug under study binds to zinc metal by chemisorption and physisorption.56–58 The drug's interaction with the zinc surface could be due to the existence of COO−, N, O, S, and π-electrons in its structure. At the cathode, O2 and H+ ions in the solution combine to make H2O, while at the anode, Zn metal is ionized to Zn2+ (en (3)). While the absence of vacant d-orbitals in zinc might seem to limit its ability to chemisorb, other mechanisms like s–p hybridization,86 electrostatic interactions,87 and charge transfer88 can still lead to strong adsorption. Furthermore, the CDZ molecule retro-donates electrons from the zinc metal via the carboxylic group's two oxygens (LUMO orbital).24 In addition, a protective coating is established on the Zn surface via physisorption between the protonated CDZ molecules and the Cl− ions in the corrosive environment.40 The film inhibits further zinc oxidation and decreases the metal's surface area in contact with the corrosive solution.24 Fig. 11 shows the suggested mechanism of inhibition.
are −21.97 to −30.49 kJ mol−1, which indicates that the drug under study binds to zinc metal by chemisorption and physisorption.56–58 The drug's interaction with the zinc surface could be due to the existence of COO−, N, O, S, and π-electrons in its structure. At the cathode, O2 and H+ ions in the solution combine to make H2O, while at the anode, Zn metal is ionized to Zn2+ (en (3)). While the absence of vacant d-orbitals in zinc might seem to limit its ability to chemisorb, other mechanisms like s–p hybridization,86 electrostatic interactions,87 and charge transfer88 can still lead to strong adsorption. Furthermore, the CDZ molecule retro-donates electrons from the zinc metal via the carboxylic group's two oxygens (LUMO orbital).24 In addition, a protective coating is established on the Zn surface via physisorption between the protonated CDZ molecules and the Cl− ions in the corrosive environment.40 The film inhibits further zinc oxidation and decreases the metal's surface area in contact with the corrosive solution.24 Fig. 11 shows the suggested mechanism of inhibition.
 |
| | Fig. 11 Proposed mechanism of the Ceftazidime drug as a corrosion inhibitor for zinc metal. | |
3.9 A comparison of the previously mentioned drugs with the expired Ceftazidime drug
In Table 9, the performance of the expired Ceftazidime drug under examination towards zinc protection is compared with other corrosion-inhibiting drugs published in the literature. According to this comparison, the expired CDZ is a good inhibitor and can be used to protect zinc metal in a 1 mol per L HCl medium.
Table 9 The comparison between other drugs and the tested expired Ceftazidime
| The drug |
The corrosive medium |
Inhibition efficiency |
Reference |
| Pentoxifylline |
0.1 M HCl at 298 K |
84.3% at 300 ppm |
89 |
| Expired cefazolin |
0.5 M HCl at 298 K |
87.3% at 300 ppm |
24 |
| Expired glycyrrhizin |
0.5 M H2SO4 at 298 K |
93.6% at 500 ppm |
90 |
| Expired gatifloxacin |
3.5% NaCl at 298 K |
87.0% at 500 ppm |
91 |
| Expired levofloxacin |
3.5% NaCl at 298 K |
89.0% at 500 ppm |
91 |
| Expired amiodarone |
1.0 M HCl at 298 K |
88.8% at 0.001 M |
92 |
| Expired hemorheologic |
0.1 M HCl at 303 K |
84% at 300 ppm |
30 |
| Telmisartan |
0.1 M HCl at 298 K |
77.0% at 20 ppm |
32 |
| Expired gabapentin |
0.1 M HCl at 298 K |
85.0% at 400 ppm |
37 |
| Hexamine |
0.15 M HCl at 301 K |
62.0% at 0.01M |
6 |
| Expired Ceftazidime |
1.0 M HCl at 298 K |
70.3% at 300 ppm |
This work |
4. Conclusions
In this study, Ceftazidime, an expired drug, was successfully reused to reduce zinc metal corrosion in a 1 mol per L hydrochloric acid solution. FTIR spectroscopy showed no significant change in chemical structure between fresh and expired drugs. The performance of the CDZ drug was examined using PDP, EFM, WL, and EIS methods. The WL method, the EFM, PDP, and EIS techniques showed good agreement. In a 1 mol per L HCl medium, CDZ inhibited zinc corrosion well. The inhibition performance improved with increasing CDZ drug concentration, reaching 70.3% efficacy at 300 ppm, while it was reduced at higher temperatures. Concentrations above 300 ppm failed to enhance the inhibition effect. The inhibition is caused by expired drug adsorption on the zinc surface. The adsorption behavior was consistent with the Temkin model.  have negative values below 40 kJ mol−1. The thermodynamic data indicated that the drug was absorbed both physically and chemically, establishing a protective film on the zinc surface. Ceftazidime is classified as a mixed-type inhibitor. The adsorption of CDZ medication on the zinc surface reduced the corrosion rate, as demonstrated by SEM and EDX analyses. The DFTB and MC simulation findings showed that the expired medication attached to the zinc surface by the heteroatoms' lone pair of electrons. In addition to the pyridinium ring's π-electrons. The drug's adsorption energy with the zinc surface was −77.41 kJ mol−1. Zinc metal in HCl medium can be protected by using an expired Ceftazidime drug, which is an effective inhibitor.
have negative values below 40 kJ mol−1. The thermodynamic data indicated that the drug was absorbed both physically and chemically, establishing a protective film on the zinc surface. Ceftazidime is classified as a mixed-type inhibitor. The adsorption of CDZ medication on the zinc surface reduced the corrosion rate, as demonstrated by SEM and EDX analyses. The DFTB and MC simulation findings showed that the expired medication attached to the zinc surface by the heteroatoms' lone pair of electrons. In addition to the pyridinium ring's π-electrons. The drug's adsorption energy with the zinc surface was −77.41 kJ mol−1. Zinc metal in HCl medium can be protected by using an expired Ceftazidime drug, which is an effective inhibitor.
Data availability
Data will be made available on request.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors extend their appreciation to Taif University, Saudi Arabia, for supporting this work through project number (TU-DSPP-2025-19).
References
- M. J. Mazumder, Glob. J. Eng. Sci., 2020, 5, 1–5 Search PubMed.
- A. Kellenberger, D. A. Duca, M. L. Dan and M. Medeleanu, Materials, 2022, 15, 2918 CrossRef CAS PubMed.
- H. Kania and M. Saternus, Appl. Sci., 2023, 13, 2003 CrossRef CAS.
- A. Pola, M. Tocci and F. E. Goodwin, Metals, 2020, 10, 253 CrossRef CAS.
- G. A. Gaber, A. Y. Hassan, M. S. Kadh, N. M. Saleh, E. S. Abou-Amra and A. M. Hyba, Chem. Pap., 2024, 78, 3189–3203 CrossRef CAS.
- R. T. Vashi, Int. J. Res. Appl. Sci. Eng. Technol., 2020, 8, 128–135 CrossRef.
- M. B. Mahida and H. G. Chaudhari, Der Pharm. Chem., 2012, 4, 2305 Search PubMed.
- A. V. Shanbhag, T. V. Venkatesha, R. A. Prabhu and B. M. Praveen, Bull. Mater. Sci., 2011, 34, 571–576 CrossRef CAS.
- M. D. Shah, A. S. Patel, G. V. Mudaliar and N. K. Shah, Port. Electrochim. Acta, 2011, 29, 101–113 CrossRef CAS.
- N. Hebbar, B. M. Praveen, B. M. Prasanna and V. T. Venkatesha, Trans. Indian Inst. Met., 2015, 68, 543–551 CrossRef CAS.
- M. M. Solomon, S. A. Umoren, I. I. Udosoro and A. P. Udoh, Corros. Sci., 2010, 52, 1317–1325 CrossRef CAS.
- S. Ade, S. Nana and S. M. Lonkar, Int. J. ChemTech Res., 2014, 6, 3642–3650 CAS.
- G. Karthik and M. Sundaravadivelu, Egypt. J. Pet., 2016, 25, 183–191 CrossRef.
- S. Karthikeyan, Int. J. ChemTech Res., 2016, 9, 251–259 CAS.
- R. S. A. Hameed, H. I. AlShafey and A. H. Abu-Nawwas, Int. J. Electrochem. Sci., 2014, 9, 6006–6019 CrossRef.
- A. S. Fouda, M. N. EL-Haddad and Y. M. Abdalla, Int. J. Innovat. Stud. Sci. Eng. Technol., 2013, 2(12), 7073 Search PubMed.
- K. A. Yasakau, A. Kuznetsova, H. M. Maltanava, S. K. Poznyak, M. G. S. Ferreira and M. L. Zheludkevich, Corros. Sci., 2024, 229, 111889 CrossRef CAS.
- Y. Ouyang, Y. Wei, R. Zhang, R. Li, Z. Lin, S. Shi and R. Qiu, Colloids Surf. A Physicochem. Eng. Asp., 2024, 681, 132779 CrossRef CAS.
- M. Abdallah, E. A. M. Gad, M. Sobhi, J. H. Al-Fahemi and M. M. Alfakeer, Egypt. J. Pet., 2019, 28, 173–181 CrossRef.
- N. Vaszilcsin, V. Ordodi and A. Borza, Int. J. Pharm., 2012, 431, 241–244 CrossRef CAS PubMed.
- S. Hooshmand Zaferani, M. Sharifi, D. Zaarei and M. R. Shishesaz, J. Environ. Chem. Eng., 2013, 1, 652–657 CrossRef CAS.
- D. Gikonyo, A. Gikonyo, D. Luvayo and P. Ponoth, Afr. Health Sci., 2019, 19, 2737–2739 CrossRef PubMed.
- N. Vaszilcsin, A. Kellenberger, M. L. Dan, D. A. Duca and V. L. Ordodi, Materials, 2023, 16, 5555 CrossRef CAS.
- R. A. Alsaiari, M. M. Kamel and M. M. Mohamed, Molecules, 2024, 29, 1157 CrossRef CAS PubMed.
- H. Adil, J. Al. Nahrain. Univ., 2015, 18(1), 60–65 CrossRef.
- A. M. Guruprasad, H. P. Sachin, G. A. Swetha and B. M. Prasanna, Int. J. Ind. Chem., 2019, 10, 17–30 CrossRef CAS.
- A. M. Guruprasad, H. P. Sachin, G. A. Swetha and B. M. Prasanna, Surf. Interfaces, 2020, 19, 100478 CrossRef CAS.
- N. Hebbar, B. M. Praveen, B. M. Prasanna and T. V. Venkatesha, J. Fund. Appl. Sci., 2015, 7, 271–289 CrossRef CAS.
- N. Hebbar, B. M. Praveen, B. M. Prasanna and T. V. Venkatesha, Int. J. Ind. Chem., 2015, 6, 221–231 CrossRef CAS.
- J. C. Abbar, G. A. Swetha and H. P. Sachin, Colloids Surf., A, 2022, 650, 129518 CrossRef CAS.
- E. Yousif, Y.-F. Win, A. H. Al-Hamadani, A. A. Al-Amiery, A. A. H. Kadhum and A. B. Mohamad, Int. J. Electrochem. Sci., 2015, 10, 1708–1715 CrossRef.
- G. A. Swetha and H. P. Sachin, Chem. Data Collect., 2021, 32, 100668 CrossRef CAS.
- M. Abdallah, I. A. Zaafarany and B. A. A. Jahdaly, J. Mater. Environ. Sci., 2016, 7(4), 1107–1118 CAS.
- A. Alwash, D. H. Fadhil, A. Ali, F. Abdul-Hameed and E. Yousif, Rasayan J. Chem., 2017, 10, 922–928 CAS.
- E. Stupnišek-Lisac, S. Podbršček and T. Sorić, J. Appl. Electrochem., 1994, 24, 779–784 CrossRef.
- R. T. Vashi and D. Naik, Asian J. Chem., 2010, 22, 7761 CAS.
- G. A. Swetha, H. P. Sachin and J. R. Choudhuri, Emergent Mater., 2023, 6, 721–740 CrossRef CAS.
- N. O. Eddy, S. A. Odoemelam, E. C. Ogoko and B. I. Ita, Port. Electrochim. Acta, 2010, 28, 15–26 CrossRef CAS.
- G. Gece, Corros. Sci., 2011, 53, 3873–3898 CrossRef CAS.
- A. K. Singh, S. K. Shukla and M. A. Quraishi, Int. J. Electrochem. Sci., 2011, 6, 5802–5814 CrossRef CAS.
- A. K. Singh, S. K. Shukla, M. Singh and M. A. Quraishi, Mater. Chem. Phys., 2011, 129, 68–76 CrossRef CAS.
- M. M. Kamel, Q. Mohsen, Z. M. Anwar and M. A. Sherif, J. Mater. Res. Technol., 2021, 11, 875–886 CrossRef CAS.
- Z. G. Luo, Y. Zhang, H. Wang, S. Wan, L. F. Song, B. K. Liao and X. P. Guo, Corros. Sci., 2024, 227, 111705, DOI:10.1016/j.corsci.2023.111705.
- Designation: G 1–03; Standard practice for preparing, cleaning, and evaluating corrosion test specimens, ASTM International, West Conshohocken, PA, USA, 1990, pp. 19428–22959 Search PubMed.
- D. Frenkel and B. Smit, Understanding Molecular Simulation: from Algorithms to Applications, Elsevier, 2023 Search PubMed.
- M. Gatchell, M. Goulart, L. Kranabetter, M. Kuhn, P. Martini, B. Rasul and P. Scheier, Phys. Chem. Chem. Phys., 2018, 20, 7739–7745 RSC.
- G. Zheng, H. A. Witek, P. Bobadova-Parvanova, S. Irle, D. G. Musaev, R. Prabhakar, K. Morokuma, M. Lundberg, M. Elstner, C. Köhler and T. Frauenheim, J. Chem. Theory Comput., 2007, 3, 1349–1367 CrossRef PubMed.
- S. L. Muhammad and mad B. Ibrahim, Bayero J. Pure Appl. Sci., 2022, 13, 149–162 Search PubMed.
- O. Abakedi and J. Asuquo, J. Basic Appl. Res. Biomed., 2016, 2, 556–560 Search PubMed.
- M. A. Hegazy, J. Mol. Liq., 2015, 208, 227–236 CrossRef CAS.
- M. Bobina, A. Kellenberger, J.-P. Millet, C. Muntean and N. Vaszilcsin, Corros. Sci., 2013, 69, 389–395 CrossRef CAS.
- M. Pais and P. Rao, J. Bio Tribo Corros., 2021, 7, 117 CrossRef.
- I. Abdelfattah, W. Abdelwahab and A. El-Shamy, Egypt. J. Chem., 2022, 65, 687–694 Search PubMed.
- H. M. Abd El-Lateef, V. M. Abbasov, L. I. Aliyeva, E. E. Qasimov and I. T. Ismayilov, Mater. Chem. Phys., 2013, 142, 502–512 CrossRef CAS.
- L. H. Abdel-Rahman and A. M. Abu-Dief, Mater. Chem. Phys., 2013, 142, 502–512 CrossRef.
- M. M. Kamel, M. A. Ghanem, S. M. Rashwan, M. A. Mahmoud, S. A. El-Mekawy, K. M. H. Mohammed and H. E. Ibrahim, RSC Adv., 2024, 14, 19428–19445 RSC.
- M. Kamel, M. Hegazy, S. Rashwan and M. El Kotb, Chin. J. Chem. Eng., 2021, 34, 125–133 CrossRef CAS.
- M. A. Hegazy, S. M. Rashwan, S. Meleek and M. M. Kamel, Mater. Chem. Phys., 2021, 267, 124697 CrossRef CAS.
- L. B. Furtado, R. C Nascimento, M. J. O. C. Guimarães, F. J. F. S. Henrique, J. C. Rocha, P. R. Seidl and J. A. C. P. Gomes, Sustainable Chem. Pharm., 2021, 19, 100353 CrossRef CAS.
- T. S. Chu, W. J. Mai, H. Z. Li, B. X. Wei, Y. Q. Xu and B. K. Liao, Int. J. Mol. Sci., 2025, 26, 561, DOI:10.3390/ijms26020561.
- F. Bentiss, M. Lebrini and M. Lagrenée, Corros. Sci., 2005, 47, 2915–2931 CrossRef CAS.
- M. A. Migahed, S. M. Rashwan, M. M. Kamel and R. E. Habib, Cogent Eng., 2017, 4, 1366255 CrossRef.
- M. M. Kamel, M. A. Ghanem, A. S. Fouda, S. M. Rashwan, O. M. Abdelkader, N. Y. Mostafa and K. M. H. Mohammed, Egypt. J. Chem., 2024, 67, 413–427 Search PubMed.
- S. B. Aoun, RSC Adv., 2017, 7, 36688–36696 RSC.
- A. D. Becke, in Book of Abstracts, 212th ACS National Meeting, Orlando, FL, August 25-29, 1996 Search PubMed.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS.
- J. A. Syed, S. Tang, H. Lu and X. Meng, Ind. Eng. Chem. Res., 2015, 54, 2950–2959 CrossRef CAS.
- I. Sekine, M. Sanbongi, H. Hagiuda, T. Oshibe, M. Yuasa, T. Imahama, Y. Shibata and T. Wake, J. Electrochem. Soc., 1992, 139, 3167 CrossRef CAS.
- P. Arellanes-Lozada, V. Díaz-Jiménez, H. Hernández-Cocoletzi, N. Nava, O. Olivares-Xometl and N. V. Likhanova, Corros. Sci., 2020, 175, 108888 Search PubMed.
- B. S. Sanatkumar, J. Nayak and A. Nityananda Shetty, Int. J. Hydrogen Energy, 2012, 37, 9431–9442 CrossRef CAS.
- S. A. El Wanees, M. I. Alahmdi, M. A. Alsharif and Y. Atef, Egypt. J. Chem., 2019, 62, 811–825 Search PubMed.
- M. A. Amin, S. S. Abd El-Rehim, E. E. F. El-Sherbini and R. S. Bayoumi, Electrochim. Acta, 2007, 52, 3588–3600 CrossRef CAS.
- U. T. Timothy, J. Med. Nanomater. Chem., 2024, 6, 81–94, DOI:10.48309/jmnc.2024.1.7.
- A. Thakur and A. Kumar, Eur. J. Chem., 2023, 14, 246–253 CrossRef CAS.
- A. Nasser, N. M. El Basiony, M. A. Migahed, H. M. Abd-El-Bary and T. A. Mohamed, Egypt. J. Chem., 2022, 65, 845–867 Search PubMed.
- M. Pais and P. Rao, Chem. Pap., 2021, 75, 1387–1399 CrossRef CAS.
- M. M. Kamel, S. M. Rashwan, M. A. A. Mahmoud, S. A. A. El-Mekawy, M. K. Awad and H. E. Ibrahim, ACS Omega, 2022, 7, 17609–17619 CrossRef CAS.
- T. Chakraborty, K. Gazi and D. C. Ghosh, Mol. Phys., 2010, 108, 2081–2092 CrossRef CAS.
- A. Singh, K. R. Ansari, A. Kumar, W. Liu, C. Songsong and Y. Lin, J. Alloys Compd., 2017, 712, 121–133 CrossRef CAS.
- S. S. Al-Najjar and A. Y. Al-Baitai, Phys. Chem. Res., 2022, 10, 179–194 CAS.
- N. Khalil, Electrochim. Acta, 2003, 48, 2635–2640 CrossRef CAS.
- A. Bello, A. Uzairu and G. A. Shallangwa, JOTCSA, 2019, 6, 451–462 CrossRef.
- L. Guo, X. Ren, Y. Zhou, S. Xu and Y. Gong, Monte Carlo simulations of corrosion inhibition of copper by two Schiff bases, Proceedings of the 2015 International Conference on Materials, Environmental and Biological Engineering, Atlantis Press, 2015, pp. 622–625 Search PubMed.
- N. M. El Basiony, A. Elgendy, A. E. El-Tabey, A. M. Al-Sabagh, Gh. M. Abd El-Hafez, M. A. El-raouf and M. A. Migahed, J. Mol. Liq., 2020, 297, 111940 CrossRef CAS.
- Y. Qiang, H. Zhi, L. Guo, A. Fu, T. Xiang and Y. Jin, J. Mol. Liq., 2022, 351, 118638 CrossRef CAS.
- G. L. Miessler and D. A. Tarr, Solutions Manual, Inorganic Chemistry, Prentice Hall, 1999 Search PubMed.
- P. W. Atkins, J. D. Paula and J. Keeler, Atkins' Physical Chemistry, Oxford University Press, 2023 Search PubMed.
- D. P. Woodruff and T. A. Delchar, Modern Techniques of Surface Science, Cambridge University Press, Cambridge, 2nd edn, 1994. ISBN 0-521-42498-4 Search PubMed.
- J. C. Abbar, G. A. Swetha and H. P. Sachin, Colloids Surf. A Physicochem. Eng. Asp., 2022, 650, 129518 CrossRef CAS.
- Q. H. Chen, Z. Y. Zhou, M. T Feng, J. H. He, Y. Q. Xu and B. K. Liao, J. Taiwan Inst. Chem. Eng., 2025, 168, 105913 CrossRef CAS.
- A. Toghan, O. K. Alduaij, N. Alqarni, E. M. Masoud, H. Alhussain, A. M. Mostafa, A. A. Farag and A. Fawzy, Results Chem., 2025, 13, 102006 CrossRef CAS.
- H. M. K. Sheit, S. M. Kani, M. A. Sathiq, S. S. S. Abuthahir, P. Subhapriya, K. S. Nivedhitha, M. A. Umarfarooq, I. A. Badruddin, S. Kamangar and A. S. Shaik, Materials, 2024, 17, 751, DOI:10.3390/ma17030751.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Safaa N. Abdoub,
Zainab M. Anwara,
Magdy A. Sherifa and
Nasser Y. Mostafa
*a,
Safaa N. Abdoub,
Zainab M. Anwara,
Magdy A. Sherifa and
Nasser Y. Mostafa a
a
 and Kads magnitudes. The PDP study demonstrated that CDZ was a mixed-type inhibitor as it retards both the cathodic and anodic reactions. According to EFM results, increasing the concentration of CDZ from 50 to 300 ppm reduces the corrosion current density from 163.5 to 78.7 μA cm−2. SEM and EDX examinations revealed the adsorption of CDZ drug at the zinc surface. The DFTB and MC simulations revealed that the CDZ drug bonds to the zinc surface via the heteroatoms' lone pair of electrons and the pyridinium ring's π-electrons. The adsorption energy of the drug on the Zn surface was found to be −77.41 kJ mol−1.
and Kads magnitudes. The PDP study demonstrated that CDZ was a mixed-type inhibitor as it retards both the cathodic and anodic reactions. According to EFM results, increasing the concentration of CDZ from 50 to 300 ppm reduces the corrosion current density from 163.5 to 78.7 μA cm−2. SEM and EDX examinations revealed the adsorption of CDZ drug at the zinc surface. The DFTB and MC simulations revealed that the CDZ drug bonds to the zinc surface via the heteroatoms' lone pair of electrons and the pyridinium ring's π-electrons. The adsorption energy of the drug on the Zn surface was found to be −77.41 kJ mol−1.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, OH, NH, NH2, C
N, OH, NH, NH2, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, COO−, π-electrons of aromatic ring), good structure to cover the metal surface, and dissolved in water. Therefore, expired CDZ was used as an inhibitor in the current investigation to study zinc corrosion mitigation in a 1 mol per L HCl medium. The experiments were carried out using WL, PDP, EIS, and EFM methodologies. The composition and morphology of the zinc metal surface were investigated using EDX and SEM analyses. The drug's type of adsorption on zinc metal was evaluated using several adsorption isotherms. Additionally, the thermodynamic characteristics pertaining to the adsorption process were established. The mechanism of inhibition was investigated. The interaction between the CDZ molecules and the zinc surface was described by DFTB, MC, and computational studies.
O, COO−, π-electrons of aromatic ring), good structure to cover the metal surface, and dissolved in water. Therefore, expired CDZ was used as an inhibitor in the current investigation to study zinc corrosion mitigation in a 1 mol per L HCl medium. The experiments were carried out using WL, PDP, EIS, and EFM methodologies. The composition and morphology of the zinc metal surface were investigated using EDX and SEM analyses. The drug's type of adsorption on zinc metal was evaluated using several adsorption isotherms. Additionally, the thermodynamic characteristics pertaining to the adsorption process were established. The mechanism of inhibition was investigated. The interaction between the CDZ molecules and the zinc surface was described by DFTB, MC, and computational studies.




 , which was linked to Kads as previously established.54,55
, which was linked to Kads as previously established.54,55
 of the CDZ medication at various temperatures (298–318 K) are shown in Table 3. The negative sign of
of the CDZ medication at various temperatures (298–318 K) are shown in Table 3. The negative sign of  refers to the adsorption process of the CDZ drug on the Zn surface being stable and spontaneous.56 Moreover, some studies have discriminated against the chemisorption and physisorption processes using
refers to the adsorption process of the CDZ drug on the Zn surface being stable and spontaneous.56 Moreover, some studies have discriminated against the chemisorption and physisorption processes using  values.57,58 The magnitudes of
values.57,58 The magnitudes of  below 20 kJ mol−1 were linked to physisorption, or the interaction of electrostatic charges, whereas values above 40 kJ mol−1 were related to chemisorption, or the transfer of electrons between the drug molecules and the Zn surface. The CDZ medication has
below 20 kJ mol−1 were linked to physisorption, or the interaction of electrostatic charges, whereas values above 40 kJ mol−1 were related to chemisorption, or the transfer of electrons between the drug molecules and the Zn surface. The CDZ medication has  values ranging between −21.97 and −30.49 kJ mol−1 in the current investigation. Hence, rather than being a simple case of physisorption or chemisorption, the adsorption of the CDZ medication on the Zn surface is a complex combination of chemical and physical adsorption.56
values ranging between −21.97 and −30.49 kJ mol−1 in the current investigation. Hence, rather than being a simple case of physisorption or chemisorption, the adsorption of the CDZ medication on the Zn surface is a complex combination of chemical and physical adsorption.56

 and icorr stand for the corrosion current density in the absence and presence of the CDZ drug, respectively. Significant corrosion developed when the blank solution came into direct contact with zinc metal. Zinc dissolves more quickly in corrosive solutions when aggressive ions like Cl− and H3O+ are present. The insertion of CDZ into the corrosive solution causes notable drops in current densities, which slowed the zinc's rate of corrosion. The anodic and cathodic sections of the measured polarization curves (Fig. 3) progressively shift toward lower current density as the concentration of CDZ increases, indicating a decline in the overall corrosion rate.61 Anodic and cathodic branches' shapes were altered, demonstrating that the existence of CDZ controlled both anodic and cathodic processes. The cathodic polarization curves depict the evolution of hydrogen, but the dissolution of zinc is represented by anodic polarization curves.
and icorr stand for the corrosion current density in the absence and presence of the CDZ drug, respectively. Significant corrosion developed when the blank solution came into direct contact with zinc metal. Zinc dissolves more quickly in corrosive solutions when aggressive ions like Cl− and H3O+ are present. The insertion of CDZ into the corrosive solution causes notable drops in current densities, which slowed the zinc's rate of corrosion. The anodic and cathodic sections of the measured polarization curves (Fig. 3) progressively shift toward lower current density as the concentration of CDZ increases, indicating a decline in the overall corrosion rate.61 Anodic and cathodic branches' shapes were altered, demonstrating that the existence of CDZ controlled both anodic and cathodic processes. The cathodic polarization curves depict the evolution of hydrogen, but the dissolution of zinc is represented by anodic polarization curves.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z and log
Z and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f have a direct proportionality. Also, the phase angle value is near −60°, and the slope approaches −1. This verifies the inadequate capacitive performance at intermediate frequencies.58 Perfect capacitive performance was attained at moderate frequencies when the slope is −1 and the angle of phase is −90°. Compared to uninhibited solutions, inhibited solutions have larger slope and phase angle magnitudes. This makes clear how efficiently the drug under investigation inhibits zinc degradation.57
f have a direct proportionality. Also, the phase angle value is near −60°, and the slope approaches −1. This verifies the inadequate capacitive performance at intermediate frequencies.58 Perfect capacitive performance was attained at moderate frequencies when the slope is −1 and the angle of phase is −90°. Compared to uninhibited solutions, inhibited solutions have larger slope and phase angle magnitudes. This makes clear how efficiently the drug under investigation inhibits zinc degradation.57

 stand for Zn's electric charge transfer resistance in blank solution with and free of CDZ, respectively. As the concentration of CDZ increases, the values of IEEIS increase, reaching an efficiency of 68.6% at 300 ppm and 298 K. The findings suggest that a more protective coating was formed on the zinc surface with the addition of CDZ. These findings validate the consistency between the outcomes from EIS and alternative methods.
stand for Zn's electric charge transfer resistance in blank solution with and free of CDZ, respectively. As the concentration of CDZ increases, the values of IEEIS increase, reaching an efficiency of 68.6% at 300 ppm and 298 K. The findings suggest that a more protective coating was formed on the zinc surface with the addition of CDZ. These findings validate the consistency between the outcomes from EIS and alternative methods.


 and idrug stand for the corrosion current densities without and with CDZ drug, respectively.
and idrug stand for the corrosion current densities without and with CDZ drug, respectively.


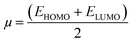



 are −21.97 to −30.49 kJ mol−1, which indicates that the drug under study binds to zinc metal by chemisorption and physisorption.56–58 The drug's interaction with the zinc surface could be due to the existence of COO−, N, O, S, and π-electrons in its structure. At the cathode, O2 and H+ ions in the solution combine to make H2O, while at the anode, Zn metal is ionized to Zn2+ (en (3)). While the absence of vacant d-orbitals in zinc might seem to limit its ability to chemisorb, other mechanisms like s–p hybridization,86 electrostatic interactions,87 and charge transfer88 can still lead to strong adsorption. Furthermore, the CDZ molecule retro-donates electrons from the zinc metal via the carboxylic group's two oxygens (LUMO orbital).24 In addition, a protective coating is established on the Zn surface via physisorption between the protonated CDZ molecules and the Cl− ions in the corrosive environment.40 The film inhibits further zinc oxidation and decreases the metal's surface area in contact with the corrosive solution.24 Fig. 11 shows the suggested mechanism of inhibition.
are −21.97 to −30.49 kJ mol−1, which indicates that the drug under study binds to zinc metal by chemisorption and physisorption.56–58 The drug's interaction with the zinc surface could be due to the existence of COO−, N, O, S, and π-electrons in its structure. At the cathode, O2 and H+ ions in the solution combine to make H2O, while at the anode, Zn metal is ionized to Zn2+ (en (3)). While the absence of vacant d-orbitals in zinc might seem to limit its ability to chemisorb, other mechanisms like s–p hybridization,86 electrostatic interactions,87 and charge transfer88 can still lead to strong adsorption. Furthermore, the CDZ molecule retro-donates electrons from the zinc metal via the carboxylic group's two oxygens (LUMO orbital).24 In addition, a protective coating is established on the Zn surface via physisorption between the protonated CDZ molecules and the Cl− ions in the corrosive environment.40 The film inhibits further zinc oxidation and decreases the metal's surface area in contact with the corrosive solution.24 Fig. 11 shows the suggested mechanism of inhibition. have negative values below 40 kJ mol−1. The thermodynamic data indicated that the drug was absorbed both physically and chemically, establishing a protective film on the zinc surface. Ceftazidime is classified as a mixed-type inhibitor. The adsorption of CDZ medication on the zinc surface reduced the corrosion rate, as demonstrated by SEM and EDX analyses. The DFTB and MC simulation findings showed that the expired medication attached to the zinc surface by the heteroatoms' lone pair of electrons. In addition to the pyridinium ring's π-electrons. The drug's adsorption energy with the zinc surface was −77.41 kJ mol−1. Zinc metal in HCl medium can be protected by using an expired Ceftazidime drug, which is an effective inhibitor.
have negative values below 40 kJ mol−1. The thermodynamic data indicated that the drug was absorbed both physically and chemically, establishing a protective film on the zinc surface. Ceftazidime is classified as a mixed-type inhibitor. The adsorption of CDZ medication on the zinc surface reduced the corrosion rate, as demonstrated by SEM and EDX analyses. The DFTB and MC simulation findings showed that the expired medication attached to the zinc surface by the heteroatoms' lone pair of electrons. In addition to the pyridinium ring's π-electrons. The drug's adsorption energy with the zinc surface was −77.41 kJ mol−1. Zinc metal in HCl medium can be protected by using an expired Ceftazidime drug, which is an effective inhibitor.







