Open Access Article
Open Access ArticlePalladium-catalyzed cross-coupling of gem-difluorocyclopropanes with gem-diborylalkanes: facile synthesis of a diverse array of gem-diboryl-substituted fluorinated alkenes†
Ebrahim-Alkhalil M. A. Ahmedab,
Hongchen Zhangc,
Wen-Gen Caob and
Tian-Jun Gong *a
*a
aSchool of Chemistry and Materials Science, CAS Key Laboratory of Urban Pollutant Conversion, Anhui Provincial Key Laboratory of Biomass Chemistry, University of Science and Technology of China, Hefei 230026, China. E-mail: gongtj@ustc.edu.cn
bCollege of Chemistry and Materials Engineering, Wenzhou University, Wenzhou 325035, China
cCollege of Pharmacy, Wenzhou Medical University, Wenzhou 325035, China
First published on 9th April 2025
Abstract
This study introduces an efficacious palladium-catalyzed method for the regioselective and stereoselective cross-coupling of gem-difluorinated cyclopropanes with an array of gem-diborylalkanes under mild reaction conditions. The innovative methodology facilitates the synthesis of 2-fluoroallylic gem-diboronic esters with exceptional Z-stereo- and chemo-selectivity. Notably, this protocol extended to the ligand-modulated regio- and stereoselectivity divergence cross-coupling of 1,1-difluoro-2-vinylcyclopropane as a reaction partner. Furthermore, we explore further transformations of the fluorinated gem-diboronates, encompassing the oxidation to form ketone and hydrogenation to generate mono-fluorinated alkylated gem-diboronate.
Introduction
Organoboronates play a critical role in organic synthesis.1 Among them, gem-diborylalkanes, as a new class of organoboron compounds possess multiple transformable sp3-hybridized carbon units,2 have gained prominence in organic synthesis due to their stability and ready availability, serving as versatile intermediates in organic synthesis with valuable applications in materials science and medicinal chemistry. Over the past two decades, significant efforts have been dedicated towards construction and transformation of gem-diboron compounds,3 this includes transition metal-catalyzed or transition metal-free multi-borylation of readily available gem-dihalides,4 alkynes,5 alkenes,6 and so on.7 Additionally, the classic “lithiation-substitution” strategy provided a modular and straightforward route to gem-bis(boronates), leveraging readily available 1,1-diborylalkanes.8 Given the important value of gem-diborylalkanes in organic synthesis, the synthesis of multi-functional substituted gem-diboron compounds remains an important research topic.On the other hand, fluorinated compounds have found widespread applications across diverse fields, primarily due to the introduction of fluorine moieties that significantly enhance hydrophobicity and metabolic stability.9 Recently, gem-difluorinated cyclopropanes (gem-F2CPs)10 an easily accessible fluorinated building block,11 has undergone diverse metal-catalyzed transformations to fluorinated functional molecules. In 2015, Fu and co-workers pioneered a Pd-catalyzed C–C/C–F activation ring-opening reaction of gem-F2CPs, achieving C–N, C–O, and C–C cross-coupling reactions to produce monofluorinated alkenes with high linear selectivity.12 Since this groundbreaking work, this reaction mode has been extended to a variety of transition metal catalysts (Pd, Ni, Rh, and Co) and nucleophiles afford linear/branched monofluoroalkenes.13 For example, Li, Lv and co-workers reported the regioselective Pd/NHC-catalyzed ring-opening hydrodefluorination/defluorinative functionalization of gem-F2CPs.10b,e,13h,j Xia and co-workers reported the Rh-catalyzed regio-switchable cross-coupling of gem-F2CPs to afford different types of fluorinated compounds.10d,13g,13i,k,m,r Recently, our group reported Pd-catalysed cross-coupling of gem-F2CPs with gem-diborylalkanes and Cu/Pd bimetallic-catalyzed three-component reaction for synthesizing of boryl-substituted fluorinated alkenes.14 Despite these advancements, no effective method for the synthesizing of gem-diboryl-substituted fluorinated alkenes has been reported until now. Herein, we demonstrate an example of palladium-catalyzed cross-coupling of gem-difluorinated cyclopropanes with gem-diborylalkanes using LDA as a base to produce gem-diboryl-substituted fluorinated alkenes under mild reaction conditions with high stereoselectivity. When 1,1-difluoro-2-vinylcyclopropane is used as the substrate, ligand-modulated regio- and stereoselectivity cross-coupling can be achieved. Incorporating these versatile scaffolds with fluoroallyls, particularly considering the unique properties of fluorine atoms, would enrich the building blocks of gem-diborylalkanes (Scheme 1).
Results and discussion
We set out to investigate the cross-coupling reaction using 2-(2,2-difluorocyclopropyl)naphthalene (1a) and 2,2′-(3-phenylpropane-1,1-diyl)bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane) (2a) as model substrates (Table 1). Based on our prior studies, we initially investigated the impact of phosphorus ligands and Pd catalysts. In the presence of PdII and LDA, the reaction produced the target product 3a in low to moderate yields with monodentate ligands (Table 1, entries 1–8). When, switching to Pd0 catalysts, such as Pd2(dba)3 and [{Pd(μ-Br)(PtBu3)}2] along with phosphorus ligands, yields remained moderate but regioselectivity decreased (entries 9, 10). Delightedly, when employing [{Pd(μ-Br)(PtBu3)}2] in the presence of LDA as the base in THF, 3a could be obtained in good yield with perfect Z-selectivity (28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z/E ratio, entry 11). Subsequently, Buchwald's palladacycle precatalyst controlled the ring-coupling reaction effectively, providing reasonable yield and regioselectivity (entry 15). We also screened a variety of organic and inorganic bases and found that LDA was the most suitable, as other bases were incompatible in the process (entries 12–14, see ESI† for further details). The transformation did not conduct in the absence of LDA or palladium catalyst (entries 16, 17).
1 Z/E ratio, entry 11). Subsequently, Buchwald's palladacycle precatalyst controlled the ring-coupling reaction effectively, providing reasonable yield and regioselectivity (entry 15). We also screened a variety of organic and inorganic bases and found that LDA was the most suitable, as other bases were incompatible in the process (entries 12–14, see ESI† for further details). The transformation did not conduct in the absence of LDA or palladium catalyst (entries 16, 17).
| Entry | [Pd] | L | Base | Yieldb (%) | Z/Ec |
|---|---|---|---|---|---|
| a Standard reaction conditions: 1a (0.1 mmol), 2a (1.5 equiv.), [Pd] (10 mol%), ligand (12 mol%), base (2 equiv.), in 1.0 mL of THF at 60 °C for 24 h under Ar atmosphere.b The yield was determined by 19F NMR using trifluoromethylbenzene as internal standard. For [{Pd(μ-Br)(PtBu3)}2] and PtBu3PdG-3 catalysts, 10 mol% was used.c The ratio of Z/E isomer was determined by 19F NMR spectroscopy.d Yield represents isolated yield after purification by silica gel chromatography. dba = dibenzylideneacetone, OTFA = trifluoroacetate. LDA = lithium diisopropylamide. LiHMDS = Lithium bis(trimethylsilyl)amide. LTMP = Lithium tetramethylpiperiddide. Nap = 2-naphthyl. | |||||
| 1 | Pd(OTFA)2 | L1 | LDA | 25 | Z |
| 2 | Pd(OTFA)2 | L2 | LDA | 30 | Z |
| 3 | Pd(OTFA)2 | L3 | LDA | 35 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | Pd(OTFA)2 | L4 | LDA | 46 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | Pd(OTFA)2 | L5 | LDA | 65 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | Pd(OTFA)2 | L6 | LDA | 61 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | Pd(OTFA)2 | L7 | LDA | 13 | — |
| 8 | Pd(OTFA)2 | L8 | LDA | 10 | — |
| 9 | Pd2(dba)3 | L5 | LDA | 56 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | [{Pd(μ-Br)(PtBu3)}2] | L5 | LDA | 72 | 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | [{Pd(μ-Br)(PtBu3)}2] | — | LDA | 70 (65)d | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | [{Pd(μ-Br)(PtBu3)}2] | — | LiOtBu | n.r. | — |
| 13 | [{Pd(μ-Br)(PtBu3)}2] | — | LiHMDS | Trace | — |
| 14 | [{Pd(μ-Br)(PtBu3)}2] | — | LTMP | 10 | — |
| 15 | PtBu3PdG-3 | — | LDA | 55 | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16 | [{Pd(μ-Br)(PtBu3)}2] | — | — | n.r. | — |
| 17 | — | — | LDA | n.r. | — |
Having established the optimal reaction conditions (as described in Table 1, entry 11), a series of gem-F2CPs and gem-diborylalkanes were employed to confirm the generality of the reaction, as depicted in Scheme 2. The ring-coupling proceeded smoothly with neutrally substituted compounds (3b–3d) and electron-donating groups at various positions on aromatic rings (3e, 3f), yielding products in the range of 50–85% with reasonable to good regioselectivity. Nitrogen-containing functional groups, such as pyridine, pyrrole, morpholine, dimethylamine substituents, also participated in the reaction with various gem-diborylalkanes, furnishing mono-fluorinated gem-diborylalkenes (3g–3o) in good yields and regioselectivities. Other functional groups, such as acetal (3p), pyrrolidinone (3r, 3s), were formed smoothly when gem-F2CPs reacted with gem-diboryl derivatives, respectively. Conjugated monofluorinated gem-diboronates (3w–3y) were synthesized using the corresponding alkenyl gem-F2CPs. To our delight, alkyl-substituted gem-F2CPs were well-tolerated and converted into 3t–3v. Additionally, the simplest gem-diborylmethane also participated in the reaction, yielding 3z in moderate yield when using Buchwald's precatalysts (e.g. BrettPdG3 and tBuXPhosPdG3) instead of [{Pd(μ-Br)(PtBu3)}2]. Finally, late-stage modification of complex molecules, such as δ-tocopherol-derived gem-F2CP, was compatible and resulted in yields of 3aa (68%) and 3ab (75%). Furthermore, a gem-F2CP-derived canagliflozin participated under optimized conditions, and the corresponding ketone 3ac was formed via a further oxidation step. This approach provides valuable methods for modifying biologically active compounds.
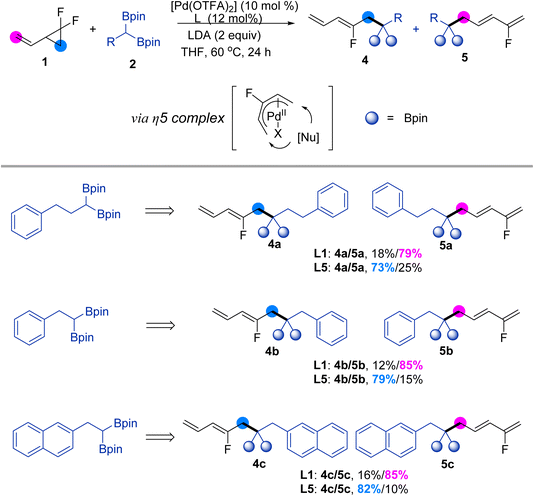
Subsequently, we investigated the scope of gem-F2CPs by performing cross-coupling reactions between 1,1-difluoro-2-vinylcyclopropane and gem-diborylalkanes, as illustrated in Scheme 3. By adjusting the optimal conditions, we found that the utilization of a PdII catalyst, such as Pd(OTFA)2, in combination with difference mono-dentate phosphor ligands, enabled successful regio- and stereoselectivity divergence cross-coupling. We examined the coupling of 1,1-difluoro-2-vinylcyclopropane with phenylpropane gem-diboronates (see ESI† for optimal conditions). For instance, using ligand L5 led to smooth ring-opening and the formation of 4a with high stereoselectivity. Interestingly, a flipped ratio products (4a/5a) was observed when PtBu3·HBF4 (L1) was used as the ligand. This trend also proved compatible with other gem-diborylalkanes, yielding the corresponding compounds (4b, 4c, 5b, 5c) under mild conditions. This represents the first regionally selectively controlled cross-coupling reaction involving 1,1-difluoro-2-vinylcyclopropane, thereby broadening the type of difluorocyclopropane ring-opening coupling reaction.
Gem-diborylalkanes and gem-difluorocyclopropanes are well-known compounds that can be easily prepared in the laboratory using readily available substrates. Phenylpropanal and cinnamaldehyde, which are biomass-derived feedstocks, can be utilized to synthesize the corresponding gem-F2CPs and gem-diborylalkanes. Utilizing our developed method, we achieved the straightforward synthesis of 6a and 6b under optimized conditions, yielding the target products in 80% yield and 57% yield, respectively, as illustrated in Scheme 4.
 | ||
| Scheme 4 Easy access C–C coupling reaction toward gem-diborylfluorinated alkenes.a Isolated yield for 0.2 mmol scale reaction. | ||
To demonstrate the utility of our cross-coupling method, we performed a gram-scale synthesis of 3c under standard conditions, resulting in a high yield of 3c (4.30 g, 85% yield). Furthermore, we explored the potential applications of mono-fluoroalkene diboronates as a versatile building block. Notably, 3c was successfully oxidized by NaBO3·4H2O to yield the corresponding ketone 7 in moderate yield. Additionally, the hydrogenation reaction was carried out to produce mono-fluoroalkane gem-diboronate 8 (Scheme 5).
The proposed reaction pathway for this strategy is elucidated based on prior studies, as depicted in Scheme 6. Initially, the Pd(0) catalyst interacts readily with gem-difluorinated cyclopropanes, leading to the formation of the four-membered-ring palladacycle intermediate (I). Subsequently, a β-F elimination step facilitates the generation of the 2-fluorinated Pd π-allyl complex (II). This complex then undergoes transmetalation with the in situ generated gem-diborylalkyl-lithium intermediate, leading to the formation of intermediate (III). Finally, a C–C bond elimination assembles the target products, while the Pd catalyst is released for further transformation.
Conclusions
In conclusion, the study demonstrates an instance of Pd-catalyzed ring-opening cross-coupling between gem-F2CPs and gem-diborylalkanes to produce gem-diboryl-substituted fluorinated alkenes. This reaction proceeds under mild conditions with LDA as the base and exhibits exceptional compatibility with diverse functional groups. Ligand-controlled regio- and stereoselective cross-coupling of 1,1-difluoro-2-vinylcyclopropane can be achieved. This achievement broadens the scope of C–C coupling reactions involving gem-F2CPs and gem-diborylalkanes, providing an efficient synthetic route to diverse gem-diboronate analogs. Ongoing research focuses on addressing remaining challenges in C–C coupling reactions to further advance this area of work.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors acknowledge support from the National Natural Science Foundation of China (22071228, 22478375), the National Natural Science Foundation of China of Research Fund for International Young Scientists (RFIS-I) (22250410263), Anhui Provincial Major Science and Technology Project, award/grant number 202305a12020034 and the Fundamental Research Funds for the Central Universities, award/grant number WK2060000096.References
- (a) D. G. Hal, Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine, Wiley-VCH, Weinheim, 2005 CrossRef; (b) S. G. Aiken, J. M. Bateman, V. K. Aggarwal and E. Fernández, Science of Synthesis: Advances in Organoboron Chemistry towards Organic Synthesis, 2019, pp 393–458 Search PubMed; (c) R. Smoum, A. Rubinstein, V. M. Dembitsky and M. Srebnik, Boron Containing Compounds as Protease Inhibitors, Chem. Rev., 2012, 112, 4156–4220 CrossRef CAS PubMed; (d) W. L. A. Brooks and B. S. Sumerlin, Synthesis and Applications of Boronic Acid-Containing Polymers: From Materials to Medicine, Chem. Rev., 2016, 116, 1375–1397 CrossRef CAS PubMed; (e) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Diboron(4) Compounds: From Structural Curiosity to Synthetic Workhorse, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed; (f) J. Hu, M. Ferger, Z. Shi and T. B. Marder, Recent advances in asymmetric borylation by transition metal catalysis, Chem. Soc. Rev., 2021, 50, 13129–13188 RSC.
- (a) A. B. Cuenca and E. Fernández, Boron-Wittig olefination with gem-bis(boryl)alkanes, Chem.Soc. Rev., 2021, 50, 72–86 RSC; (b) Y. Lee, S. Han and S. H. Cho, Catalytic Chemo- and Enantioselective Transformations of gem-Diborylalkanes and (Diborylmethyl)metallic Species, Acc. Chem. Res., 2021, 54, 3917–3929 CrossRef CAS PubMed; (c) A. B. Cuenca and E. Fernández, Boron-Wittig olefination with gem-bis(boryl)alkanes, Chem. Soc. Rev., 2021, 50, 72–86 RSC.
- (a) k. Hong, X. Liu and J. P. Morken, Simple Access to Elusive α-Boryl Carbanions and Their Alkylation: An Umpolung Construction for Organic Synthesis, Chem. Soc., 2014, 136, 10581–10584 CrossRef CAS PubMed; (b) J. Kim, S. Park, J. Park and S. H. Cho, Synthesis of Branched Alkylboronates by Copper-Catalyzed Allylic Substitution Reactions of Allylic Chlorides with 1,1-Diborylalkanes, Angew. Chem., Int. Ed., 2016, 55, 1498–1501 CrossRef CAS PubMed; (c) Y. Shi and A. H. Hoveyda, Catalytic SN2′- and Enantioselective Allylic Substitution with a Diborylmethane Reagent and Application in Synthesis, Chem. Int. Ed., 2016, 55, 3455–3458 CrossRef CAS PubMed; (d) S. A. Murray, J. C. Green, S. B. Tailor and S. J. Meek, Enantio- and Diastereoselective 1,2-Additions to α-Ketoesters with Diborylmethane and Substituted 1,1-Diborylalkanes, Angew. Chem., Int. Ed., 2016, 55, 9065–9069 CrossRef CAS PubMed; (e) X. Liu, T. M. Deaton, F. Haeffner and J. P. Morken, A Boron Alkylidene-Alkene Cycloaddition Reaction: Application to the Synthesis of Aphanamal, Angew. Chem., Int. Ed., 2017, 56, 11485–11489 CrossRef CAS PubMed; (f) M. Z. Liang and S. J. Meek, Synthesis of Quaternary Carbon Stereogenic Centers by Diastereoselective Conjugate Addition of Boron-Stabilized Allylic Nucleophiles to Enones, J. Am. Chem. Soc., 2020, 142(22), 9925–9931 CrossRef CAS PubMed; (g) J. C. Green, J. M. Zanghi and S. J. Meek, Diastereo- and Enantioselective Synthesis of Homoallylic Amines Bearing Quaternary Carbon Centers, J. Am. Chem. Soc., 2020, 142(4), 1704–1709 CrossRef CAS PubMed; (h) K. K. Das, S. Paul and S. Panda, Transition metal-free synthesis of alkyl pinacol boronates, Org. Biomol. Chem., 2020, 18, 8939–8974 Search PubMed; (i) N. Eghbarieh, N. Hanania, A. Zamir, M. Nassir, T. Stein and A. Masarwa, Stereoselective Diels-Alder Reactions of gem-Diborylalkenes: Toward the Synthesis of gem- Diboron-Based Polymers, J. Am. Chem. Soc., 2021, 143(16), 6211–6220 CrossRef CAS PubMed; (j) F. Zhang, S. Liao, L. Zhou, K. Yang, C. Wang, Y. Lou, C. Wang and Q. Song, An Olefinic 1,2-α-Boryl Migration Enables 1,2-Bis(boronic esters) via Radical-Polar Crossover Reaction, Chin. J. Chem., 2022, 40, 582–588 CrossRef CAS; (k) W. Sun, L. Xu, Y. Qin and C. Liu, Alkyne synthesis through coupling of gem-diborylalkanes with carboxylic acid esters, Nat. Synth., 2023, 2, 413–422 Search PubMed.
- (a) C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Alkylboronic Esters from Copper-Catalyzed Borylation of Primary and Secondary Alkyl Halides and Pseudohalides, Angew. Chem., Int. Ed., 2012, 51, 528–532 CrossRef CAS PubMed; (b) T. C. Atack and S. P. Cook, Manganese-Catalyzed Borylation of Unactivated Alkyl Chlorides, J. Am. Chem. Soc., 2016, 138, 6139–6142 Search PubMed; (c) B. Wang, X. Zhang, Y. Cao, L. Zou, X. Qi and Q. Lu, Electrooxidative Activation of B–B Bond in B2cat2: Access to gem-Diborylalkanes via Paired Electrolysis, Angew. Chem., Int. Ed., 2023, 62, e202218179 CrossRef CAS PubMed.
- (a) L. Zhang, X. Si, F. Rominger and A. S. K. Hashmi, Visible-Light-Induced Radical Carbo-Cyclization/gem-Diborylation through Triplet Energy Transfer between a Gold Catalyst and Aryl Iodides, J. Am. Chem. Soc., 2020, 142, 10485–10493 CrossRef CAS PubMed; (b) J. H. Docherty, K. Nicholson, A. P. Dominey and S. P. A Thomas, Boron-Boron Double Transborylation Strategy for the Synthesis of gem-Diborylalkanes, ACS Catal., 2020, 10, 4686–4691 CrossRef CAS; (c) J. Li and S. Ge, Copper-Catalyzed Quadruple Borylation of Terminal Alkynes to Access sp3-Tetra-Organometallic Reagents, Angew. Chem., Int. Ed., 2022, 61, e202213057 CrossRef CAS PubMed; (d) Y. Wang, Y. Li, L. Wang, S. Ding, L. Song, X. Zhang, Y.-D. Wu and J. Sun, Ir-Catalyzed Regioselective Dihydroboration of Thioalkynes toward gem-Diboryl Thioethers, J. Am. Chem. Soc., 2023, 145, 2305–2314 CrossRef CAS PubMed.
- (a) L. Zhang and Z. Huang, Synthesis of 1,1,1-Tris(boronates) from Vinylarenes by Co-Catalyzed Dehydrogenative Borylations-Hydroboration, J. Am. Chem. Soc., 2015, 137, 15600–15603 CrossRef CAS PubMed; (b) L. Li, T. Gong, X. Lu, B. Xiao and Y. Fu, Nickel-catalyzed synthesis of 1,1-diborylalkanes from terminal alkenes, Nat. Commun., 2017, 8, 345 CrossRef PubMed; (c) W. J. Teo and S. Ge, Cobalt-Catalyzed Diborylation of 1,1-disubstituted Vinylarenes: A Practical Route to Branched gem-Bis(boryl)alkanes, Angew. Chem., Int. Ed., 2018, 57, 1654–1658 CrossRef CAS PubMed; (d) M. Hu and S. Ge, Versatile cobalt-catalyzed regioselective chain-walking double hydroboration of 1,n-dienes to access gem-bis(boryl)alkanes, Nat. Commun., 2020, 11, 765 CrossRef CAS PubMed; (e) X. Wang, X. Cui, S. Li, Y. Wang, C. Xia, H. Jiao and L. Wu, Zirconium-Catalyzed Atom-Economical Synthesis of 1,1-Diborylalkanes from Terminal and Internal Alkenes, Angew. Chem., Int. Ed., 2020, 59, 13608–13612 CrossRef CAS PubMed; (f) Y. Zhao and S. Ge, Synergistic Hydrocobaltation and Borylcobaltation Enable Regioselective Migratory Triborylation of Unactivated Alkenes, Angew. Chem., Int. Ed., 2022, 61, e202116133 CrossRef CAS PubMed.
- (a) J. C. H. Lee, R. Mcdonald and D. G. Hall, Enantioselective preparation and chemoselective cross-coupling of 1,1-diboron compounds, Nat. Chem., 2011, 3, 894–899 CrossRef CAS PubMed; (b) X. Feng, H. Jeon and J. Yun, Regio- and Enantioselective Copper(I)-Catalyzed Hydroboration of Borylalkenes: Asymmetric Synthesis of 1,1-Diborylalkanes, Angew. Chem., Int. Ed., 2013, 52, 3989–3992 CrossRef CAS PubMed; (c) M. A. Larsen, S. H. Cho and J. Hartwig, Iridium-Catalyzed, Hydrosilyl-Directed Borylation of Unactivated Alkyl C-H Bonds, J. Am. Chem. Soc., 2016, 138, 762–765 CrossRef CAS PubMed; (d) W. N. Palmer, J. V. Obligacion, I. Pappas and P. J. Chirik, Cobalt-Catalyzed Benzylic Borylation: Enabling Polyborylation and Functionalization of Remote, Unactivated C(sp3)-H Bonds, J. Am. Chem. Soc., 2016, 138, 766–769 CrossRef CAS PubMed; (e) L. Wang, T. Zhang, W. Sun, Z. He, C. Xia, Y. Lan and C. Liu, C-O Functionalization of α-Oxyboronates: A Deoxygenative gem-Diborylation and gem-Silylborylation of Aldehydes and Ketones, J. Am. Chem. Soc., 2017, 139, 5257–5264 CrossRef CAS PubMed; (f) B. Zhao, Z. Li, Y. Wu, Y. Wang, J. Qian, Y. Yuan and Z. Shi, An Olefinic 1,2-Boryl-Migration Enabled by Radical Addition: Construction of gem-Bis(boryl)alkanes, Angew. Chem., Int. Ed., 2019, 58, 9448–9452 CrossRef CAS PubMed; (g) J. Li, H. Wang, Z. Qiu, C.-Y. Huang and C.-J. Li, Metal-Free Direct Deoxygenative Borylation of Aldehydes and Ketones, J. Am. Chem. Soc., 2020, 142, 13011–13020 CrossRef CAS PubMed; (h) J.-F. Ge, X.-Z. Zou, X.-R. Liu, C.-L. Ji, X.-Y. Zhu and D.-W. Gao, Ir-Catalyzed Enantioselective Synthesis of gem-Diborylalkenes Enabled by 1,2-Boron Shift, Angew. Chem., Int. Ed., 2023, 62, e202307447 CrossRef CAS PubMed; (i) L. Liu, B. Zhang, Y. Liu, J. Zhao, T. Li and W. Zhao, Cobalt-catalyzed deoxygenative borylation of diaryl ketones, Chin. Chem. Lett., 2023, 35, 108631 CrossRef; (j) P.-F. Ning, Y. Wei, X.-Y. Chen, Y.-F. Yang, F.-C. Gao and K. Hong, A General Method to Access Sterically Encumbered geminal Bis(boronates) via Formal Umpolung Transformation of Terminal Diboron Compounds, Angew. Chem., Int. Ed., 2024, 63, e202315232 CrossRef CAS PubMed.
- (a) D. S. Matteson and R. J. Moody, Deprotonation of 1,1-diboronic esters and reactions of the carbanions with alkyl halides and carbonyl compounds, Organometallics, 1982, 1, 20–28 CrossRef CAS; (b) M. Huang, H. Sun, F. Seufert, A. Friedrich, T. B. Marder and J. Hu, Photoredox/Cu-Catalyzed Decarboxylative C(sp3)−C(sp3) Coupling to Access C(sp3)-Rich gem-Diborylalkanes, Angew. Chem., Int. Ed., 2024, 63, e202401782 CrossRef CAS PubMed; (c) Y. Jin, J. Lee, W. Jo, J. Yu and S. H. Cho, Axially chiral α-boryl-homoallenyl boronic esters as versatile toolbox for accessing centrally and axially chiral molecules, Nat. Commun., 2024, 15, 9239 CrossRef CAS PubMed.
- (a) K. Müller, C. Faeh and F. Diederich, Fluorine in Pharmaceuticals: Looking Beyond Intuition, Science, 2007, 317, 1881 CrossRef PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Fluorine in medicinal chemistry, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (c) W. K. Hagmann, The many roles for fluorine in medicinal chemistry, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed; (d) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001-2011), Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (e) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, Applications of Fluorine in Medicinal Chemistry, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed; (f) D. O'Hagan and H. Deng, Enzymatic Fluorination and Biotechnological Developments of the Fluorinase, Chem. Rev., 2015, 115, 634–649 CrossRef PubMed; (g) C. Ni and J. Hu, The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations, Chem. Soc. Rev., 2016, 45, 5441–5454 RSC; (h) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed; (i) Q. Liu, C. Ni and J. Hu, China's flourishing synthetic organofluorine chemistry: innovations in the new millennium, Natl. Sci. Rev., 2017, 4, 303–325 CrossRef CAS; (j) J. Moschner, V. Stulberg, R. Fernandes, S. Huhmann, J. Leppkes and B. Koksch, Approaches to Obtaining Fluorinated α-Amino Acids, Chem. Rev., 2019, 119, 10718–10801 CrossRef CAS PubMed.
- (a) W. R. Dolbier and M. A. Battiste, Structure, Synthesis, and Chemical Reactions of Fluorinated Cyclopropanes and Cyclopropenes, Chem. Rev., 2003, 103, 1071–1098 CrossRef CAS PubMed; (b) L. Lv, H. Qian and Z. Li, Catalytic Diversification of gem-Difluorocyclopropanes: Recent Advances and Challenges, ChemCatChem, 2022, 14, e202200890 CrossRef CAS; (c) T. Nanda, M. Fastheem, A. Linda, B. V. Pati, S. K. Banjare, P. Biswal and P. C. Ravikumar, Recent Advancement in Palladium-Catalyzed C-C Bond Activation of Strained Ring Systems: Three- and Four-Membered Carbocycles as Prominent C3/C4 Building Blocks, ACS Catal., 2022, 12, 13247–13281 CrossRef CAS; (d) Y. Zhu, Y. Zeng, Z.-T. Jiang and Y. Xia, Recent Advances in Transition-Metal-Catalyzed Cross-Coupling Reactions of gem-Difluorinated Cyclopropanes, Synlett, 2023, 34, 1–13 CrossRef CAS; (e) L. Lv, J. Su and Z. Li, Recent developments in the ring-opening transformations of gem-difluorocyclopropanes, Org. Chem. Front., 2024, 11, 6518–6533 RSC.
- (a) I. Nowak, J. F. Cannon and M. J. Robins, Synthesis and Properties of gem-(Difluorocyclopropyl)amine Derivatives of Bicyclo[n.1.0]alkanes, Org. Lett., 2004, 6, 4767–4770 CrossRef CAS PubMed; (b) Y. Fujioka and H. Amii, Boron-Substituted Difluorocyclopropanes: New Building Blocks of gem-Difluorocyclopropanes, Org. Lett., 2008, 10, 769–772 CrossRef CAS PubMed; (c) F. Wang, T. Luo, J. Hu, Y. Wang, H. S. Krishnan, P. V. Jog, S. K. Ganesh, G. K. S. Prakash and G. A. Olah, Synthesis of gem-Difluorinated Cyclopropanes and Cyclopropenes: Trifluoromethyltrimethylsilane as a Difluorocarbene Source, Angew. Chem., Int. Ed., 2011, 50, 7153–7157 CrossRef CAS PubMed; (d) F. Wang, W. Zhang, J. Zhu, H. Li, K.-W. Huang and J. Hu, Chloride ion-catalyzed generation of difluorocarbene for efficient preparation of gem-difluorinated cyclopropenes and cyclopropanes, Chem. Commun., 2011, 47, 2411–2413 RSC; (e) S. Eusterwiemann, H. Martinez and W. R. Dolbier Jr, Methyl 2,2-Difluoro-2-(fluorosulfonyl)acetate, a Difluorocarbene Reagent with Reactivity Comparable to That of Trimethylsilyl 2,2-Difluoro-2-(fluorosulfonyl)acetate (TFDA), J. Org. Chem., 2012, 77, 5461–5464 CrossRef CAS PubMed; (f) M. Goswami, B. de Bruin and W. I. Dzik, Difluorocarbene transfer from a cobalt complex to an electron-deficient alkene, Chem. Commun., 2017, 53, 4382–4385 RSC; (g) G. Ernouf, J.-L. Brayer, B. Folleas, J.-P. Demoute, C. Meyer and J. Cossy, Synthesis of Alkylidene(gem-Difluorocyclopropanes) from Propargyl Glycolates by a One-Pot Difluorocyclopropenation/Ireland-Claisen Rearrangement Sequence, J. Org. Chem., 2017, 82, 3965–3975 CrossRef CAS PubMed; (h) P. S. Nosik, A. O. Gerasov, R. O. Boiko, E. Rusanov, S. V. Ryabukhin, O. O. Grygorenko and D. M. Volochnyuk, Gram-Scale Synthesis of Amines Bearing a gem-Difluorocyclopropane Moiety, Adv. Synth. Catal., 2017, 359, 3126–3136 CrossRef CAS; (i) H. Xu, X.-J. Fang, W.-S. Huang, Z. Xu, L. Li, F. Ye, J. Cao and L.-W. Xu, Catalytic regio- and stereoselective silicon-carbon bond formations on unsymmetric gem-difluorocyclopropenes by capture of silyl metal species, Org. Chem. Front., 2022, 9, 5272–5280 RSC; (j) J. Li, Z. Liang, Y. Ren, J. Gao and D. Du, Facile access to gem-difluorocyclopropanes via an N-heterocyclic carbene-catalyzed radical relay/cyclization strategy, Org. Chem. Front., 2023, 10, 1669–1674 RSC.
- J. Xu, E.-A. M. A. Ahmed, B. Xiao, Q.-Q. Lu, Y.-L. Wang, C.-G. Yu and Y. Fu, Pd-Catalyzed Regioselective Activation of gem-Difluorinated Cyclopropanes: A Highly Efficient Approach to 2-Fluorinated Allylic Scaffolds, Angew. Chem., Int. Ed., 2015, 54, 8231–8235 CrossRef CAS PubMed.
- (a) E.-A. M. A. Ahmed, A. M. Y. Suliman, T.-J. Gong and Y. Fu, Palladium-Catalyzed Stereoselective Defluorination Arylation/Alkenylation/Alkylation of gem-Difluorinated Cyclopropanes, Org. Lett., 2019, 21, 5645 CrossRef CAS PubMed; (b) E.-A. M. A. Ahmed, A. M. Y. Suliman, T.-J. Gong and Y. Fu, Access to Divergent Fluorinated Enynes and Arenes via Palladium-Catalyzed Ring-Opening Alkynylation of gem-Difluorinated Cyclopropanes, Org. Lett., 2020, 22, 1414–1419 CrossRef CAS PubMed; (c) H. Liu, Y. Li, D.-X. Wang, M.-M. Sun and C. Feng, Visible-Light-Promoted Regioselective 1,3-Fluoroallylation of gem-Difluorocyclopropanes, Org. Lett., 2020, 22, 8681–8686 CrossRef CAS PubMed; (d) Z. T. Jiang, J. Huang, Y. Zeng, F. Hu and Y. Xia, Rhodium Catalyzed Regioselective C−H Allylation of Simple Arenes via C−C Bond Activation of gem-difluorinated Cyclopropanes, Angew. Chem., Int. Ed., 2021, 60, 10626–10631 CrossRef CAS PubMed; (e) L. Lv and C.-J. Li, Palladium-Catalyzed Defluorinative Alkylation of gem-Difluorocyclopropanes: Switching Regioselectivity via Simple Hydrazones, Angew. Chem., Int. Ed., 2021, 60, 13098–13104 CrossRef CAS PubMed; (f) Z.-T. Jiang, Y. Zeng and Y. Xia, Rhodium-Catalyzed Direct Allylation of Simple Arenes by Using gem-Difluorinated Cyclopropanes as Allyl Surrogates, Synlett, 2021, 32, 1675–1682 CrossRef CAS; (g) B. Xiong, X. Chen, J. Liu, X. Zhang, Y. Xia and Z. Lian, Stereoselective gem-Difluorovinylation of gem-Difluorinated Cyclopropanes Enabled by Ni/Pd Cooperative Catalysis, ACS Catal., 2021, 11, 11960–11965 CrossRef CAS; (h) Y. Ai, H. Yang, C. Duan, X. Li and S. Yu, Cobalt-Catalyzed Fluoroallyllation of Carbonyls via C-C Activation of gem-Difluorocyclopropanes, Org. Lett., 2022, 24, 5051–5055 CrossRef CAS PubMed; (i) L. Wu, M. Wang, Y. Liang and Z. Shi, Ligand-Controlled Palladium-Catalyzed Regiodivergent Defluorinative Allylation of gem-Difluorocyclopropanes via σ-Bond Activation, Chin. J. Chem., 2022, 40, 2345–2355 CrossRef CAS; (j) L. Lv, H. Qian, A. B. Crowell, S. Chen and Z. Li, Pd/NHC-Controlled Regiodivergent Defluorinative Allylation of gem-Difluorocyclopropanes with Allylboronates, ACS Catal., 2022, 12, 6495–6505 CrossRef CAS; (k) X. Wang and F. W. Patureau, Pd-catalyzed access to mono- and di-fluoroallylic amines from primary anilines, Chem. Commun., 2023, 59, 486–489 RSC; (l) Y. Zeng and Y. Xia, Rhodium-Catalyzed Regio- and Diastereoselective [3+2] Cycloaddition of gem-Difluorinated Cyclopropanes with Internal Olefins, Angew. Chem., Int. Ed., 2023, 62, e202307129 CrossRef CAS PubMed; (m) D. Li, C. Shen, Z. Si and L. Liu, Palladium-Catalyzed Fluorinative Bifunctionalization of Aziridines and Azetidines with gem-Difluorocyclopropanes, Angew. Chem., Int. Ed., 2023, 62, e202310283 CrossRef CAS PubMed; (n) H. Qian, H. D. Nguyen, L. Lv, S. Chen and Z. Li, Chemo-, Stereo- and Regioselective Fluoroallylation/Annulation of Hydrazones with gem-Difluorocyclopropanes via Tunable Palladium/NHC Catalysis, Angew. Chem., Int. Ed., 2023, 62, e202303271 CrossRef CAS PubMed; (o) J. Sun, H. Ye, F. Sun, Y.-Y. Pan, X.-W. Zhu and X.-X. Wu, Palladium-Catalyzed Allylation of P(O)H Compounds: Access to 2-Fluoroallylic Phosphorus Compounds, Org. Lett., 2023, 25, 5220–5225 CrossRef CAS PubMed; (p) T.-S. Wu, Y.-J. Hao, Z.-J. Cai and S.-J. Ji, Ligand-controlled regioselective cascade C-C/C-F cleavage/annulation of gem-DFCPs: a divergent synthesis of pyrroles, Org. Chem. Front., 2023, 11, 1057–1061 RSC; (q) H. Qian, Z. P. Cheng, Y. Luo, L. Lv, S. Chen and Z. Li, Pd/IPrBIDEA-Catalyzed Hydrodefluorination of gem-Difluorocyclopropanes: Regioselective Synthesis of Terminal Fluoroalkenes, J. Am. Chem. Soc., 2024, 146, 24–32 CrossRef CAS PubMed; (r) Y. Zhu, J. Jia, X. Song, C. Gong and Y. Xia, Double strain-release enables formal C-O/C-F and C-N/C-F ring-opening metathesis, Chem. Sci., 2024, 15, 13800–13806 RSC; (s) H. Yang, Y. Zeng, X. Song, L. Che, Z.-T. Jiang, G. Lu and Y. Xia, Rhodium-Catalyzed Enantio- and Regioselective Allylation of Indoles with gem-Difluorinated Cyclopropanes, Angew. Chem., Int. Ed., 2024, 63, e202403602 CrossRef CAS PubMed.
- (a) A. M. Y. Suliman, E.-A. M. A. Ahmed, T.-J. Gong and Y. Fu, Three-component reaction of gem-difluorinated cyclopropanes with alkenes and B2pin2 for the synthesis of monofluoroalkenes, Chem. Commun., 2021, 57, 6400–6403 Search PubMed; (b) A. M. Y. Suliman, E.-A. M. A. Ahmed, T.-J. Gong and Y. Fu, Cu/Pd-Catalyzed cis-Borylfluoroallylation of Alkynes for the Synthesis of Boryl-Substituted Monofluoroalkenes, Org. Lett., 2021, 23, 3259–3263 CrossRef CAS PubMed; (c) E.-M. A. Ahmed, H.-C. Zhang, W.-G. Cao and T.-J. Gong, Palladium-Catalyzed Cross-Coupling of gem-Difluorocyclopropanes with gem-Diborylalkanes for the Synthesis of Boryl-Substituted Fluorinated Alkenes, Org. Lett., 2023, 25, 9020–9024 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and spectra data for all compounds. See DOI: https://doi.org/10.1039/d5ra00581g |
| This journal is © The Royal Society of Chemistry 2025 |