Open Access Article
Open Access ArticleVinasse photoreforming for hydrogen production using Pt/TiO2 as catalyst under UV irradiation
Patrícia Ferreira Silvaino ,
João Coutinho Ferreira
,
João Coutinho Ferreira ,
Saulo Amaral Carminati
,
Saulo Amaral Carminati *,
Jorge Moreira Vaz and
Estevam Vitorio Spinacé
*,
Jorge Moreira Vaz and
Estevam Vitorio Spinacé *
*
Instituto de Pesquisas Energéticas e Nucleares, IPEN/CNEN, Av. Prof. Lineu Prestes, 2242-Cidade Universitária, São Paulo, SP 05508-000, Brazil. E-mail: saulocarminati89@gmail.com; espinace@ipen.br
First published on 20th February 2025
Abstract
Vinasse, a dark-colored aqueous byproduct of bioethanol production, contains a variety of organic compounds and inorganic salt ions. In this study, the photoreforming of vinasse was investigated using Pt/TiO2 as a catalyst under UV irradiation. The gaseous products generated were analyzed, revealing the formation of hydrogen (H2) along with other gases, including CO2, CH4, CO, C2H6, C2H4, C3H8, and C4H10. When using filtered vinasse, H2 and other gaseous products were produced solely through photolysis, even in the presence of the Pt/TiO2 photocatalyst. Notably, photocatalytic H2 production was observed when inorganic salt ions were removed from the vinasse, and a lower concentration of vinasse was employed in the reaction medium.
1. Introduction
The environmental impacts have stimulated the use of renewable sources in the chemical industry, however, more than 90% of hydrogen (H2) continues to be produced by reforming fossil fuel feedstocks.1The photocatalytic H2 production over nanostructured semiconductors has the advantage of renewable energy utilization and a common strategy involves the use of substrates such as methanol, ethanol and others to overcome the large thermodynamic barrier of the oxygen evolution reaction and the recombination of photogenerated charge carriers.2 On the other hand, the high cost of these substrates is an obstacle to achieving practical applications. In this sense, the low cost and easy accessibility of agricultural biomass and waste have attracted significant interest as potential substrates.3
Brazil is the second largest producer of bioethanol, which is primarily through sugarcane fermentation, generating vinasse as the main byproduct. Vinasse is an aqueous dark-colored waste containing organic compounds (residual sugars, glycerol, mannitol, organic acids and phenolic compounds) and inorganic salt ions (sodium, potassium, calcium, and magnesium cations, and chloride, nitrate, nitrite, phosphate, and sulfate anions). Due to the large volumes of vinasse resulting from bioethanol production, at least 10–15 liters for every 1 liter of ethanol, its disposal and/or use has become a major challenge. In Brazil, during the 1980s, fertigation became a widespread practice, leading to soil salinization and the leaching of metals into groundwater.4,5
More recently, other processes, such as anaerobic digestion of vinasse to produce biogas are being evaluated and developed.6,7 Additionally, the vinasse photocatalytic degradation for decolorization and total organic carbon removal is another process that has been extensively investigated.8,9
The photocatalytic water splitting over nanostructured semiconductors for H2 production offers the advantage of renewable energy utilization. A common strategy involves the use of sacrificial agents (SAs) such as methanol, ethanol and others to overcome the large thermodynamic barrier of oxygen the evolution reaction and mitigate the recombination of photogenerated charge carriers.2 On the other hand, the high cost of SAs presents a significant obstacle to achieve practical applications, making low-cost and easily accessible SAs, such as biomass and organic wastes, increasingly attractive.10
While biomass-derived substrates like glucose, polysaccharides, glycerol, lignin and cellulose have been investigated as SAs for photocatalytic H2 production,11–16 the use of raw waste materials has been less explored.17 Additionally, the photoreforming of vinasse over semiconductor-based photocatalysts for H2 production has not yet been explored.8
TiO2 has been considered one of the most promising light-harvesting materials for H2 production from water due to its low cost and stability. However, it is only activated by UV light, which corresponds to about 5% of the sunlight spectrum. Furthermore, the main limitation of sunlight-driven photoreactors is their low efficiency on cloudy days and at night. An alternative would be to use artificial light, such as a UV-LED photoreactor powered by renewable energy, to overcome these challenges and improve the efficiency of TiO2 photocatalysts.18
In this work, we explore the photoreforming of vinasse for H2 production using Pt/TiO2, the most common reference photocatalyst using UV irradiation.19 This approach could not only mitigate waste by improving resource efficiency but also contribute to more sustainable energy systems.
2. Experimental
2.1. Materials and methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water solution (3
water solution (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) and the TiO2 P25 was dispersed in this solution. The resulting mixture was stirred and heated under reflux at 180 °C for 2 h. Afterwards, the mixture was centrifuged (9000 rpm), and a light gray solid was obtained. The solid was washed with excess deionized water and dried at 90 °C for 24 h.
1 v/v) and the TiO2 P25 was dispersed in this solution. The resulting mixture was stirred and heated under reflux at 180 °C for 2 h. Afterwards, the mixture was centrifuged (9000 rpm), and a light gray solid was obtained. The solid was washed with excess deionized water and dried at 90 °C for 24 h.The elemental composition of the Pt/TiO2 was determined by WD-XRF (Wavelength-Dispersive X-ray Fluorescence) in a Rigaku Supermini200 spectrometer (Pd source, 50 kV, 200 W, zirconium filter) using a calibration curve. The shape, size and dispersion of the Pt nanoparticles on the TiO2 support were analyzed by TEM (Transmission Electron Microscopy) using a JEOL microscope, model JEM-2100 (200 kV). Photoluminescence (PL) spectra were performed in an Ocean Optics 2000 luminescence spectrometer + USB spectrometer with a CCD camera, from 200 to 1000 nm. The excitation wavelength was 265 nm, and the spectra were recorded at room temperature in the range of 200–1000 nm, with the scanning speed at 1000 nm min−1, and the PMT voltage was 650 V. Raman spectroscopy measurements were obtained with a Horiba Scientific MacroRam Raman spectrometer using a 785 nm wavelength laser. UV-Vis spectra of the vinasse solution before and after UV irradiation were recorded by using a Varian UV-Vis spectrometer model Cary 50 from 200 to 800 nm.
3. Results and discussion
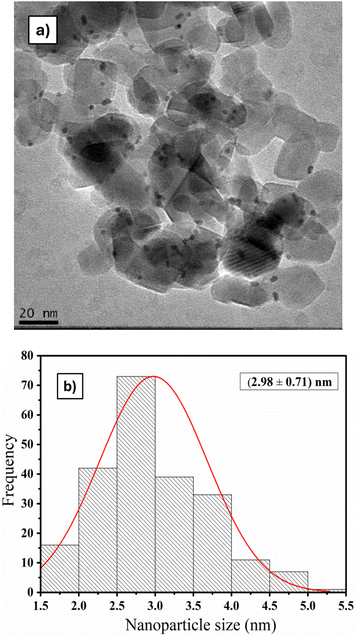
For Pt/TiO2 photocatalyst, the Pt amount determined by WDXRF was 0.52 wt%, which is very close to the nominal value. Fig. 1 shows the TEM micrograph and histogram of the Pt/TiO2 photocatalyst, where the Pt nanoparticles (small black dots) are clearly visible, exhibiting good dispersion on the TiO2 support, with sizes ranging from 2 to 5 nm.The PL spectra of the Pt/TiO2 photocatalyst and TiO2 P25 support are shown in Fig. 2, and they exhibit similar profiles, with emission peaks centered at 431 and 436 nm for Pt/TiO2 and TiO2 P25, respectively. The emission peak of the Pt/TiO2 photocatalyst has lower intensity compared to the peak of the TiO2 P25 support, indicating a decrease in the recombination rate of the electron–hole pairs.22,23
The Raman spectra of the Pt/TiO2 photocatalyst and TiO2 P25 support are shown in Fig. 3. The spectrum of TiO2 P25 presented peaks centered at 140, 196, 394, 514 and 636 cm−1, which are attributed to the vibrational modes of the anatase phase, with a small peak at 445 cm−1 corresponding to the rutile phase. These peaks are characteristic of TiO2 P25 support, which contains a mixture of both anatase and rutile phases.24,25 The Pt/TiO2 photocatalyst presented a similar profile, but with a small positive shift of 3 cm−1 in the most intense peak, which is associated with the interactions between Pt nanoparticles the TiO2 support.26,27
The photoreforming experiments are shown in Table 1. Initially, vinasse F (with the liquid phase separated from the solid phase by filtration) was tested using a vinasse F![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 50/50 (v/v) under UV irradiation. The products identified in the gas phase followed this order of formation rates: CO2 > H2 > CO > C2H6 > CH4 > C3H8 > C4H10 > C2H4. These products were formed through photochemical reactions (photolysis) since no Pt/TiO2 photocatalyst was added to the reaction medium. On the other hand, the addition of Pt/TiO2, the most common reference photocatalyst under UV irradiation,28 practically did not alter the products formed or their formation rates. Even when the amount of Pt/TiO2 was doubled (150 mg), the results remained unchanged. UV-Vis absorption spectra of vinasse (F) solution (50/50), after UV irradiation, and after UV irradiation in the presence of Pt/TiO2 as photocatalyst, are shown in Fig. 4.
H2O ratio of 50/50 (v/v) under UV irradiation. The products identified in the gas phase followed this order of formation rates: CO2 > H2 > CO > C2H6 > CH4 > C3H8 > C4H10 > C2H4. These products were formed through photochemical reactions (photolysis) since no Pt/TiO2 photocatalyst was added to the reaction medium. On the other hand, the addition of Pt/TiO2, the most common reference photocatalyst under UV irradiation,28 practically did not alter the products formed or their formation rates. Even when the amount of Pt/TiO2 was doubled (150 mg), the results remained unchanged. UV-Vis absorption spectra of vinasse (F) solution (50/50), after UV irradiation, and after UV irradiation in the presence of Pt/TiO2 as photocatalyst, are shown in Fig. 4.
| Vinasse/photocatalyst | Vinasse/H2O (v/v) | Products formation rates (μmol gcat−1 h−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C2H6 | C2H4 | C3H8 | C4H10 | CH4 | CO | CO2 | H2 | ||
| a For comparative purposes. | |||||||||
| Vinasse F/without | 50/50 | 407 | 12 | 123 | 21 | 157 | 909 | 4148 | 2004 |
| Vinasse F/(Pt/TiO2) | 50/50 | 512 | 23 | 163 | 24 | 178 | 994 | 5248 | 2200 |
| Vinasse IE/without | 50/50 | 2113 | 159 | 552 | 257 | 1792 | 973 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254 254 |
2827 |
| Vinasse IE/(Pt/TiO2) | 50/50 | 2089 | 224 | 591 | 280 | 1665 | 1096 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 759 759 |
4619 |
| Vinasse IE/without | 10/90 | 572 | 9 | 166 | 56 | 1068 | 527 | 4449 | 1787 |
| Vinasse IE/(Pt/TiO2) | 10/90 | 422 | 20 | 147 | 46 | 963 | 530 | 5685 | 5252 |
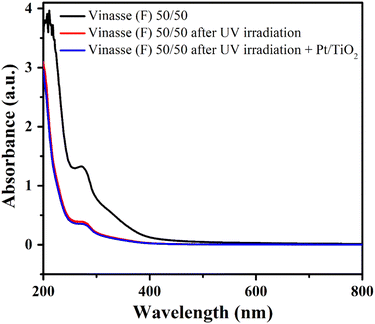 | ||
| Fig. 4 UV-Vis absorption spectra of vinasse (F) SA/H2O of 50/50, after UV irradiation and after UV irradiation in the presence of Pt/TiO2. | ||
The UV-Vis spectrum of vinasse (F) showed absorption bands in the UV region (200–400 nm) and their intensities were reduced after the photoreaction under UV irradiation indicating the degradation of vinasse by photolysis. However, when the photoreaction was carried out in the presence of Pt/TiO2 as photocatalyst, the intensities and the shape of the absorption bands were very similar to those observed for the reaction conducted only with UV light, showing that photochemical reactions (photolysis) predominated even in the presence of the photocatalyst. Therefore, under these conditions, the H2 production primarily results from the photolysis of vinasse rather than from photocatalytic process. It is likely that some organic or inorganic compounds in vinasse inhibit UV light absorption by the photocatalyst and/or block the surface catalytic sites.29
Vinasse contains appreciable amounts of inorganic salt ions,5 and some of these ionic species could absorb UV light in aqueous solution,30 as well as potentially lead to the deactivation of the photocatalysts.31,32
To eliminate the influence of these species, vinasse F was treated by an ion-exchange process (IE) and tested using vinasse![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 50/50 under UV irradiation. As a result, an increase in the formation rates of all products was observed compared to vinasse F. Interestingly, when this photoreaction took place in the presence of Pt/TiO2 photocatalyst and under UV irradiation, an increase in CO2 and H2 (4619 μmol gcat−1 h−1) formation rates was observed, while the formation of other products remained practically unchanged. Thus, two simultaneous processes may be occurring, a photochemical and a photocatalytic process, both of which contribute to the increased CO2 and H2 production.
H2O ratio of 50/50 under UV irradiation. As a result, an increase in the formation rates of all products was observed compared to vinasse F. Interestingly, when this photoreaction took place in the presence of Pt/TiO2 photocatalyst and under UV irradiation, an increase in CO2 and H2 (4619 μmol gcat−1 h−1) formation rates was observed, while the formation of other products remained practically unchanged. Thus, two simultaneous processes may be occurring, a photochemical and a photocatalytic process, both of which contribute to the increased CO2 and H2 production.
Recently, Wongyongnoi et al.17 described the green synthesis of H2 and the decolorization of distillery effluent using Au/TiO2 as photocatalyst. However, neither H2 production nor decolorization was achieved using fresh distillery effluent, even in the presence of the photocatalyst and UV irradiation. Under the studied conditions, the maximum H2 production (52.5 μmol gcat−1 h−1) with 64.4% decolorization was achieved only with 100-fold diluted distillery effluent and the addition of 15 vol% of ethanol, which also served as a sacrificial agent, to the reaction medium. In a similar study, Iervolino et al.33 described the simultaneous valorization and treatment of olive mill wastewater (OMW) through a photocatalytic process to produce hydrogen and reduce the polluting load of this waste, using a home-made sol–gel TiO2 as photocatalyst. The best results were obtained using a dilution of OMW at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 with deionized water, yielding a H2 production of about 4200 μmol gcat−1 h−1.
70 with deionized water, yielding a H2 production of about 4200 μmol gcat−1 h−1.
In this way, the volume of vinasse IE (vinasse![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 10
H2O ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90) was reduced in the reaction medium (Table 1). Under UV irradiation, a decrease in the formation rates of all products was observed compared to the vinasse
90) was reduced in the reaction medium (Table 1). Under UV irradiation, a decrease in the formation rates of all products was observed compared to the vinasse![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O ratio of 50
H2O ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. On the other hand, when the photoreaction was performed in the presence of Pt/TiO2 photocatalyst, a more pronounced increase in the CO2 and H2 formation was observed.
50. On the other hand, when the photoreaction was performed in the presence of Pt/TiO2 photocatalyst, a more pronounced increase in the CO2 and H2 formation was observed.
UV-Vis spectra of vinasse (IE) (10/90) after UV irradiation and after UV irradiation in the presence of Pt/TiO2 as photocatalyst are shown in Fig. 5. The intensities of the absorption bands of the photoreaction performed in the presence of Pt/TiO2 photocatalyst were reduced compared to the reaction performed only under UV irradiation, indicating more effective vinasse degradation. Under these conditions, the use of a lower concentration of vinasse appears to facilitate UV light absorption by the photocatalyst, making the H2 production through the photocatalytic process more effective. Despite this, there is also a significant increase in CO2 production. Therefore, the enhanced production of H2 and CO2 observed in the presence of the Pt/TiO2 photocatalyst may be associated with the activation of H2O molecules by holes (H2O + h+ → ·OH + H+), leading to the formation of highly reactive and non-selective ·OH radical, which favor the complete oxidation of vinasse to CO2. Meanwhile, the photogenerated electrons are attracted to the Pt sites, where the reduction of H+ to H2 occurs.34,35
 | ||
| Fig. 5 UV-Vis absorption spectra of vinasse (IE) 10/90 before and after UV irradiation, and after UV irradiation in the presence of Pt/TiO2. | ||
4. Conclusion
The photoreforming of the liquid phase of raw vinasse (vinasse F/H2O ratio of 50/50) under UV irradiation resulted in the production of H2, CO2, CO, and C2–C4 hydrocarbons through a photochemical process. On the other hand, the addition of Pt/TiO2 photocatalyst did not alter the products or their formation rates, suggesting that no photocatalytic activity occurred under these conditions. In contrast, using ion-exchanged vinasse (vinasse IE) under UV irradiation in conjunction with the Pt/TiO2 photocatalyst led to an increase in H2 and CO2 production, indicating that photocatalytic processes were indeed occurring. However, the photochemical process still remained dominant. Under the studied conditions, the photocatalytic H2 production was enhanced using vinasse IE and by reducing its concentration in the reaction medium (vinasse IE/H2O volume ratio of 10/90). Therefore, further investigation into the photoreforming of vinasse is warranted, and ongoing studies aim to optimize process conditions and identify more effective photocatalysts.Data availability
The data that support the findings of this study are available from the authors.Author contributions
Patricia F. Silvaino: photocatalysts preparation and characterization, photochemical experiments, data curation, editing, review. João C. Ferreira: vinasse treatments, editing, review. Saulo A. Carminati: data curation, writing-original draft, editing, review, proofreading manuscript; Jorge M. Vaz: conceptualization, investigation, data curation, supervision, editing, review; Estevam V. Spinacé: conceptualization, investigation, data curation, supervision, writing-original draft, editing, review, proofreading. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.Conflicts of interest
The authors declare that no competing financial interests or personal relationships influenced the work reported in this paper.Acknowledgements
The authors gratefully acknowledge support from São Paulo Research Foundation (FAPESP – Grant number 2021/01896-4) and from Brazilian National Council for Scientific Development (CNPq – Grant number 407967/2022-2 and 384264/2023-9). We also thank the support given by Arthur P. Machado from “Laboratório de Nanotecnologia e Energia Solar”, Chemistry Institute, UNICAMP for providing the facilities of photoluminescence experiments.References
- B. Parkinson, P. Balcombe, J. F. Speirs, A. D. Hawkes and K. Hellgardt, Energy Environ. Sci., 2019, 12, 19–40 RSC.
- C. Shi, F. Kang, Y. Zhu, M. Teng, J. Shi, H. Qi, Z. Huang, C. Si, F. Jiang and J. Hu, Chem. Eng. J., 2023, 452, 138980 Search PubMed.
- T. Zhang and S. Lu, Chem Catal., 2022, 2, 1502–1505 CrossRef CAS.
- G. C. Rego, T. B. Ferreira, L. R. Ramos, C. A. de Menezes, L. A. Soares, I. K. Sakamoto, M. B. A. Varesche and E. L. Silva, Biomass Convers. Biorefin., 2022, 12, 5527–5541 CrossRef CAS.
- M. S. Silverio, R. P. Calegari, G. M. F. L. Leite, L. M. L. M. Prado, B. C. Martins, E. A. da Silva, J. P. Neto, A. Gomig and A. S. Baptista, Braz. J. Biol. Sci., 2021, 15, 42–68 Search PubMed.
- A. Montiel-Rosales, N. Montalvo-Romero, L. E. García-Santamaría, L. C. Sandoval-Herazo, H. Bautista-Santos and G. Fernández-Lambert, Sustainability, 2022, 14, 11635 CrossRef CAS.
- J. C. de Carvalho, L. P. de Souza Vandenberghe, E. B. Sydney, S. G. Karp, A. I. Magalhães, W. J. Martinez-Burgos, A. B. P. Medeiros, V. Thomaz-Soccol, S. Vieira, L. A. J. Letti, C. Rodrigues, A. L. Woiciechowski and C. R. Soccol, Fermentation, 2023, 9, 349 CrossRef CAS.
- L. L. Nascimento, R. A. Carvalho Souza, J. Zacour Marinho, C. Wang and A. O. T. Patrocinio, J. Cleaner Prod., 2024, 449, 141709 CrossRef CAS.
- A. Montiel-Rosales, N. Montalvo-Romero, L. E. García-Santamaría, L. C. Sandoval-Herazo, H. Bautista-Santos and G. Fernández-Lambert, Sustainability, 2022, 14, 11635 CrossRef CAS.
- T. Zhang and S. Lu, Chem Catal., 2022, 2, 1502–1505 CrossRef CAS.
- A. Speltini, M. Sturini, D. Dondi, E. Annovazzi, F. Maraschi, V. Caratto, A. Profumo and A. Buttafava, Photochem. Photobiol. Sci., 2014, 13, 1410–1419 CrossRef CAS PubMed.
- K. A. Davis, S. Yoo, E. W. Shuler, B. D. Sherman, S. Lee and G. Leem, Nano Convergence, 2021, 8, 6 CrossRef CAS PubMed.
- G. Zhang, C. Ni, X. Huang, A. Welgamage, L. A. Lawton, P. K. J. Robertson and J. T. S. Irvine, Chem. Commun., 2016, 52, 1673–1676 RSC.
- H. Zhao, J. Liu, N. Zhong, S. Larter, Y. Li, M. G. Kibria, B.-L. Su, Z. Chen and J. Hu, Adv. Energy Mater., 2023, 13, 2300257 CrossRef CAS.
- J. Ma, K. Liu, X. Yang, D. Jin, Y. Li, G. Jiao, J. Zhou and R. Sun, ChemSusChem, 2021, 14, 4903–4922 CrossRef CAS PubMed.
- X. Xu, L. Shi, S. Zhang, Z. Ao, J. Zhang, S. Wang and H. Sun, Chem. Eng. J., 2023, 469, 143972 CrossRef CAS.
- P. Wongyongnoi, K. Serivalsatit, M. Hunsom and K. Pruksathorn, Int. J. Hydrogen Energy, 2024, 80, 646–658 CrossRef CAS.
- M. Rumayor, J. Corredor, M. J. Rivero and I. Ortiz, J. Cleaner Prod., 2022, 336, 130430 CrossRef CAS.
- G. M. Haselmann and D. Eder, ACS Catal., 2017, 7, 4668–4675 Search PubMed.
- E. V. Spinacé, A. O. Neto, T. R. R. Vasconcelos and M. Linardi, J. Power Sources, 2004, 137, 17–23 CrossRef.
- E. V. Spinacé, A. O. Neto, E. G. Franco, M. Linardi and E. R. Gonzalez, Quim. Nova, 2004, 27, 648–654 CrossRef.
- B. Dong, T. Liu, C. Li and F. Zhang, Chin. Chem. Lett., 2018, 29, 671–680 CrossRef CAS.
- J. Shi, J. Chen, Z. Feng, T. Chen, Y. Lian, X. Wang and C. Li, J. Phys. Chem. C, 2007, 111, 693–699 Search PubMed.
- R. S. Das and Y. K. Agrawal, Vib. Spectrosc., 2011, 57, 163–176 CrossRef CAS.
- N. Lagopati, E. P. Tsilibary, P. Falaras, P. Papazafiri, E. A. Pavlatou, E. Kotsopoulou and P. Kitsiou, Int. J. Nanomed., 2014, 9, 3219–3230 CAS.
- Y. Lou, J. Xu, Y. Zhang, C. Pan, Y. Dong and Y. Zhu, Mater. Today Nano, 2020, 12, 100093 CrossRef.
- X. Wang, S. C. Huang, T. X. Huang, H. S. Su, J. H. Zhong, Z. C. Zeng, M. H. Li and B. Ren, Chem. Soc. Rev., 2017, 46, 4020–4041 RSC.
- G. M. Haselmann and D. Eder, ACS Catal., 2017, 7, 4668–4675 CrossRef CAS.
- E. Skliri, I. Vamvasakis, I. T. Papadas, S. A. Choulis and G. S. Armatas, Catalysts, 2021, 11, 1–16 Search PubMed.
- E. Wolthuis, S. Kolk and L. Schaap, Anal. Chem., 1954, 26, 1238–1240 Search PubMed.
- A. Gopakumar, P. Ren, J. Chen, B. V. Manzolli Rodrigues, H. Y. Vincent Ching, A. Jaworski, S. Van Doorslaer, A. Rokicińska, P. Kuśtrowski, G. Barcaro, S. Monti, A. Slabon and S. Das, J. Am. Chem. Soc., 2022, 144, 2603–2613 Search PubMed.
- H. Li, B. Zhu, J. Sun, H. Gong, J. Yu and L. Zhang, J. Colloid Interface Sci., 2024, 654, 1010–1019 CrossRef CAS PubMed.
- G. Iervolino, D. Sannino, G. Pepe, M. Giovanna Basilicata, P. Campiglia and V. Vaiano, Chem. Eng. J., 2023, 468, 143725 CrossRef CAS.
- L. Yu, Y. Shao and D. Li, Appl. Catal., B, 2017, 204, 216–223 CrossRef CAS.
- L. Yu and D. Li, Catal. Sci. Technol., 2017, 9, 635–640 RSC.
| This journal is © The Royal Society of Chemistry 2025 |