Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
High-efficiency acid–base catalysts: ZrO2, TiO2, amine, and Br functionalized porous polymers for CO2 and epoxide to cyclic carbonate conversion†
Abbas A. Jawad *ab,
Sura A. Ahmedc and
Hasan J. Al-Abedib
*ab,
Sura A. Ahmedc and
Hasan J. Al-Abedib
aMidland Refineries Company MRC/AL Daura Refinery Company/Project Management Division, Baghdad, Iraq. E-mail: abbasajd5d@gmail.com; abbasajd5d@outlook.com
bDepartment of Chemical and Biochemical Engineering, Missouri University of Science and Technology, Rolla, MO 65409-1230, USA
cMidland Refineries Company MRC/AL Daura Refinery Company/Maintenance Board, Baghdad, Iraq
First published on 10th March 2025
Abstract
The development of new materials capable of converting carbon dioxide (CO2) into value-added products has emerged as a crucial strategy in addressing global climate change and promoting sustainable industrial practices. As CO2 emissions continue to rise, innovative catalytic systems that facilitate its utilization as a C1 carbon source are gaining significant attention. Such advancements not only contribute to carbon capture and utilization (CCU) efforts but also support the transition toward greener chemical processes by reducing dependence on fossil-derived feedstocks. The design of high-performance heterogeneous catalysts with synergistic acid–base sites is particularly important for improving catalytic efficiency in CO2 conversion. In this study, bifunctional catalysts were synthesized by embedding ZrO2 and TiO2 (ZT) nanoparticles into a polyamide-imide polymer dope, followed by phase inversion using a “dry-jet, wet-quench spinning” process to form porous hollow fibers (PF). The fibers were then post-grafted with 3-aminopropyltrimethoxysilane (APF) to introduce amine functional groups and further modified with 1,2-dibromopropane at 110 °C to immobilize covalent hydrogen-bond donor groups (–OH and –NH) and nucleophilic (Br−) species. These heterogeneous bifunctional catalysts were then evaluated for the synthesis of cyclic carbonates from CO2 and epoxides. The catalytic performance was systematically investigated under various reaction conditions, including temperature, reaction time, solvent selection, and CO2 pressure. The optimized catalyst system achieved 100% styrene oxide (SO) conversion and >99% selectivity for styrene carbonate (SC) via the cycloaddition reaction using the Br@ZT–APF catalyst in the presence of solvents. Beyond achieving high conversion rates, the catalyst demonstrated excellent recovery, thermal stability, and recyclability for at least five consecutive cycles without significant loss of activity.
1. Introduction
CO2 capture and catalytic conversion are critical challenges that need to be addressed, as CO2 is the primary greenhouse gas. The development of advanced catalysts is highly desirable to enable the conversion of CO2 into high-value-added compounds under moderate conditions, given its thermodynamic stability and inert nature.1 In this regard, porous organic polymers provide an excellent platform for developing heterogeneous CO2 conversion catalysts due to their large surface areas, high thermal stability, diverse building blocks, and customizable porous structures. By modifying the frameworks of porous organic polymers, active sites can be incorporated to facilitate CO2 activation and conversion. This review highlights recent advancements in the design and synthesis of porous organic polymer-based heterogeneous catalysts for CO2 conversion. We primarily focus on the synthetic strategies employed to introduce active sites into porous organic polymer frameworks, including N-doping, metalation, and ionic functionalization. One of the most important CO2 fixation processes is the cycloaddition reaction, which converts epoxides and CO2 into five-membered cyclic carbonates. This process eliminates the need for hazardous phosgene while achieving 100% atom economy.2,3 Various catalysts have been reported for this reaction, including metal oxides,4,5 porphyrins,6 salen metal complexes,7 alkali metal salts,8 transition-metal complexes,9 quaternary ammonium or quaternary phosphonium salts,10 ionic liquids,11 organocatalysts,12 and MOFs.13,14 Among these, porous heterogeneous catalysts are in high demand and have gained significant attention. Cyclic carbonate derivatives are widely used due to their low toxicity, minimal odor, and high boiling points.15 Furthermore, they have applications in fuel additives, lithium-ion battery electrolytes,16 and polycyclic carbonate synthesis.17 Because CO2 is a kinetically inert molecule, its activation is crucial for efficient conversion. In this context, the direct coordination of a metal-doped hollow fiber CO2 complex is one of the most effective strategies for inducing chemical reactions with CO2. Numerous studies have explored different catalytic systems, as the production of cyclic carbonates from CO2 and epoxides aligns with the principles of sustainable development. To move toward practical applications, recent research has focused on an innovative class of efficient and porous heterogeneous systems – hollow fiber catalysts – for CO2 conversion and fixation. These systems offer advantages such as easy separation, recyclability, and non-leaching properties.18 Bifunctional catalysts, which contain two distinct catalytic sites, can enhance reaction rates through cooperative catalysis. Recent studies have demonstrated that organic amine molecules effectively catalyze the direct reaction between CO2 and epoxides to form cyclic carbonates.19,20 Various supported amines have been produced and studied for this reaction.21,22 Metal oxides, commonly used in heterogeneous catalysis, play a crucial role as both active phases and supports due to their acid–base and redox properties. Among these, the titania–zirconia combination has attracted considerable attention. Notably, zirconium dioxide (ZrO2) and titanium dioxide (TiO2) each exhibit excellent catalytic properties and have been employed as supports for various noble and transition metals in different catalytic applications. Combining two distinct oxides can introduce new physicochemical and catalytic properties by forming stable mixed oxide compounds. To the best of our knowledge, this is the first review to focus on the relevance and significance of mixed oxides in catalysis. Basing upon our previous work23 this study investigates the incorporation of ZrO2 and TiO2 (ZT) to porous hollow fibers (PF) crosslinked with 3-aminopropyltrimethoxysilane (A) and then alkylated with 1,2-dibromopropane to investigate cooperative Lewis acid/Brønsted acid sites (LAS/BAS) and Lewis base functionality and nucleophilic species suitable for use as heterogeneous catalysts in the production of styrene carbonate (SC) from CO2 and SO. Highly porous catalysts are created and extensively characterized using XRD, BET, FTIR spectrum, TGA, and pyridine IR analysis. Additionally, the effects of various experimental parameters such as reaction temperature, reaction time, CO2 pressure, and solvent selection are systematically investigated.2. Experimental section
2.1. Chemicals
For the manufacture of polymer dope and the production of composite hollow fiber catalysts, the following compounds were used: zirconia (average particle size 100 nm, surface area of 600 m2 g−1, and pore size of 5 nm), titania (average particle size 100 nm, surface area of 41 m2 g−1, and pore size of 15 nm, and pore size of 4 nm) were purchased from Sigma-Aldrich. Torlon polyamide-imide, which is a commercially available (Solvay Advanced Polymers) (Alpharetta, GA), methanol (ACS grade, VWR), polyvinylpyrrolidone (with molecular weight = 300 from Sigma-Aldrich), N-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF) (99%) (ACS Reagent, >99.8%, VWR), (Sigma-Aldrich, Reagent Plus, 99%, Milwaukee), and hexane (ACS Reagent, VWR, >98.5%). 3-Aminopropyltrimethoxysilane (assay = 95%), styrene oxide (SO) (assay = 97%), acetonitrile (ACN) (99.8%), DMF (assay = 99.8%), and 1,2-dibromopropane (97%) were obtained from Sigma Aldrich. The ultra-high pressure (UHP) N2 and CO2 gases were provided by Airgas.2.2. Bromide-immobilized ZT–PF formation
The “dry-jet, wet-quench spinning” process employed in this study to create ZrO2–TiO2 (ZT) with poly(amide-imide) hollow fibers (PFs) has been discussed in depth in earlier publications.23 Porous hollow fibers dissolve easily in polar aprotic solvents, hence they should be post-treated before being used as heterogeneous catalysts. To enhance the ability and compatibility of the hollow fibers in polar aprotic solvents (e.g., ACN and DMF) that are commonly used in chemical transformation reactions in flow, the porous poly(amide-imide) hollow fibers were grafted with 3-aminopropyltrimethoxysilane (A) in organic network structures by further condensation of 3-aminopropyltrimethoxysilane at 80 °C for 2 h in a mixture of toluene and water. Ammonium bromide compounds were obtained by post-synthetically enhancing the ZT–APF with 1,2-dibromopropane in dry toluene at 80 °C for 4–6 h, denoted herein as Br@ZT–APF. The excess alkyl halide was then removed via solvent exchange with toluene, and the hood was left out overnight to dry. Finally, the fibers were vacuumed (30 mTorr) at 85 °C to release excess solvent from the pores as depicts in Scheme 1. | ||
| Scheme 1 Illustrates Br@ZT–APFs crosslinking and alkylation of 3-aminopropyltrimethoxysilane-grafted PFs with 1,2-dibromopropane in dry toluene. | ||
2.3. Catalyst characterization
Nitrogen physisorption isotherms were determined at 77 K using Micromeritics 3Flex surface characterization analyzer equipment. Prior to analysis, the hollow fibers were degassed for 2 h at 110 °C under vacuum. The Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) techniques were used to determine surface area and pore size distribution.Using a Bruker Tenser spectrophotometer, all post-treated fibers were evaluated via FTIR at room temperature in the range of 400–4000 cm−1 with a resolution of 4.0 cm−1.
The Br@ZT–APF composition was determined by bulk elemental analysis (ICP-MS) and CHN analyses (PerkinElmer Series II, 2400) were carried out to evaluated the concentration of TiO2 and ZrO2, nanoparticles, as well as amine and bromine loadings of the hollow fibers.
GC-MS (Hewlett Packard 5890E Series II Plus Gas Chromatograph) with a mass selective detector (Hewlett Packard/HP 5972 GC-MS System) was used to evaluate the reaction mixture as displayed in (see Fig. S2, ESI†).
The results were examined via the proton nuclear magnetic resonance (1H NMR) recorded on a 400 MHz Bruker Avance III employing CDCl3 as the solvent. Furthermore, the chemical shifts (δ), reported in parts per million (ppm), are relative to the residual CHCl3 peak (7.87 ppm for 1H NMR) (see Fig. S3, ESI†). The coupling constants (J) are reported in Hertz (Hz). 13C Magic Angle Spinning Nuclear Magnetic Resonance (MAS NMR) was recorded using solid-state NMR instrument (400 MHz Bruker Avance III) (see Fig. S4, ESI†).
Fourier-transform infrared spectroscopy (FTIR) of pyridine (Py-IR) was used with a Bruker Tensor spectrophotometer to identify the types of acid sites that were present in the samples in order to ascertain the nature of surface acid sites. For the purpose of pyridine adsorption, all samples were activated at 450 °C for four hours in order to remove moisture, and then they were cooled to 60 °C till saturation. Recalculated to a 10 mg “normalized” wafer, all observed spectra were obtained. Lewis acid site (LAS) and Brønsted acid site (BAS) were identified as the bands at 1450 and 1550 cm−1, respectively, for the purpose of quantitatively characterizing acid sites.
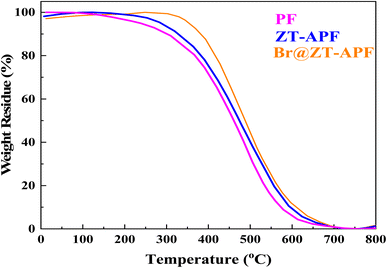
Thermogravimetric analysis (TGA) of the catalysts was carried out from 25 to 800 °C using TGA (Model Q500, TA Instruments), at a heating rate of 20 °C min−1 under nitrogen atmosphere. The flow rate was 60 mL min−1 under nitrogen atmospheres.
The surface topographies were assessed by high-performance field emission using scanning electron microscopy (SEM) on a Zeiss Merlin Gemini microscope.
2.4. Cycloaddition reactions
To carry out an example of an active catalyst catalytic process, 3.5 mL of styrene oxide, 10 mL of N,N-dimethylformamide (DMF) or acetonitrile (ACN) (used as the solvent), and a high-pressure autoclave (Parr, USA) with a magnetic stirrer and a jacket heater, 200 mg of the active catalyst was added to the reaction mix. After the reactor was sealed, it was carefully flushed three times with CO2 to remove O2, then pressurized to 5–40 bar with CO2 and heated with subsequent stirring at an operating temperature of 80–160 °C for 1–8 h. After completing the reaction, the temperature of the autoclave was slowly decreased to room temperature. Furthermore, the decompression was performed slowly for 30 minutes to reduce reaction mixture loss, after which the liquid was degassed. After depressurization, the autoclave was gently opened, and the reaction mixture was separated by filtration (Whatman, diameter 55 mm). The mixture was evaluated using gas chromatography (Varian 3800) with an Elite Wax capillary column (DB-5) and a flame ionization detector (FID).3. Results and discussion
3.1. Catalyst characterization
| Materials | SBETa [m2 g−1] | Vporeb [cm3 g−1] | TiO2 loadingc [wt%] | ZrO2 loadingc [wt%] | SiO2 loadingc [wt%] | Br loadingc [mmol gfiber−1] | N loadingc [mmol gfiber−1] |
|---|---|---|---|---|---|---|---|
| a Determined by N2 physisorption experiments at 77 K.b Determined by BJH method.c Determined by bulk elemental analysis (ICP-MS) and CHN analyses. | |||||||
| Bare-ZT–PF | 57 | 0.25 | 3.0 | 10.0 | 10.0 | — | — |
| ZT–APF | 28 | 0.12 | 2.70 | 9.55 | 9.32 | — | 4.00 |
| Br@ZT–APF (fresh 1st run) | 24 | 0.068 | 2.55 | 9.32 | 9.26 | 0.25 | 3.56 |
| Br@ZT–APF (used 5th run) | 22 | 0.058 | 2.38 | 9.15 | 9.09 | 0.21 | 3.47 |
 | ||
| Fig. 2 N2 isotherms of bare ZT–PF, ZT–APF, fresh (1st run) Br@ZT–APF, and used (5th run) Br@ZT–APF at 77 K. | ||
![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1124 cm−1; therefore, the intense absorption band at approximately
= 1124 cm−1; therefore, the intense absorption band at approximately ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1055 cm−1 is due to the Si–O–Si bond asymmetric stretching.23 Furthermore, the peaks at ≈ 760–775 cm−1 and approximately
= 1055 cm−1 is due to the Si–O–Si bond asymmetric stretching.23 Furthermore, the peaks at ≈ 760–775 cm−1 and approximately ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 505 cm−1 resulted from the existence of both tetragonal and monoclinic zirconia (Zr–O–Zr and Zr–O).37–39 Furthermore, the peak at approximately
= 505 cm−1 resulted from the existence of both tetragonal and monoclinic zirconia (Zr–O–Zr and Zr–O).37–39 Furthermore, the peak at approximately ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 450–800 cm−1 is ascribed to the vibration mode of the (Ti–O–Ti and Ti–O bonds) and the peaks at
= 450–800 cm−1 is ascribed to the vibration mode of the (Ti–O–Ti and Ti–O bonds) and the peaks at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1242, 1114, 1032, and 862 cm−1 correspond to the vibration mode of Ti–OH.40 This confirms that zirconia and titania were well dispersed in bare ZT–PF.40 The FTIR spectra of bare and amine-functionalized fibers were compared, and it was found that aminosilane grafting increased the intensity of vibrational bands vicinity ∼1000–1126 cm−1, 1618 cm−1, and 1489 cm−1 (which were attributed to Si–O–Si, O–Si–O, and N–H bending vibrations, respectively). This suggests that the condensation of 3-aminopropyltrimethoxysilane completed to form the Si–O–Si bond framework. The bare ZT–PF, ZT/APF, and Br@ZT–APF sorbents exhibit a strong imide asymmetrical carbonyl (C
= 1242, 1114, 1032, and 862 cm−1 correspond to the vibration mode of Ti–OH.40 This confirms that zirconia and titania were well dispersed in bare ZT–PF.40 The FTIR spectra of bare and amine-functionalized fibers were compared, and it was found that aminosilane grafting increased the intensity of vibrational bands vicinity ∼1000–1126 cm−1, 1618 cm−1, and 1489 cm−1 (which were attributed to Si–O–Si, O–Si–O, and N–H bending vibrations, respectively). This suggests that the condensation of 3-aminopropyltrimethoxysilane completed to form the Si–O–Si bond framework. The bare ZT–PF, ZT/APF, and Br@ZT–APF sorbents exhibit a strong imide asymmetrical carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) absorbance at
O) absorbance at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1780 cm−1, imide symmetrical carbonyl (C
= 1780 cm−1, imide symmetrical carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) absorbance at
O) absorbance at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1718 cm−1, amide carbonyl (C
= 1718 cm−1, amide carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) absorbance at
O) absorbance at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1670 cm−1, amide N–C
= 1670 cm−1, amide N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at
O stretch at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1500 cm−1, and imide C–N stretch at
= 1500 cm−1, and imide C–N stretch at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1366 cm−1. The spectra of bare ZT–PF, ZT–APF, and Br@ZT–APF sorbents exhibit absorbance peaks for amide functional groups, including a C
= 1366 cm−1. The spectra of bare ZT–PF, ZT–APF, and Br@ZT–APF sorbents exhibit absorbance peaks for amide functional groups, including a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at
O stretch at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1650 cm−1, a N–H stretch at
= 1650 cm−1, a N–H stretch at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1515–1570 cm−1, and a C–O–C stretch at
= 1515–1570 cm−1, and a C–O–C stretch at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1243 cm−1. Furthermore, the peaks at
= 1243 cm−1. Furthermore, the peaks at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 850 and 1270 cm−1 may be assigned to the Si–O and Si–CH3 stretching vibrations, respectively. The presence of a N–H bending vibration at
= 850 and 1270 cm−1 may be assigned to the Si–O and Si–CH3 stretching vibrations, respectively. The presence of a N–H bending vibration at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 688 cm−1 and a NH2 symmetric bending vibration at
= 688 cm−1 and a NH2 symmetric bending vibration at ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 1489 cm−1 of the amine-grafted PF sorbents, absent in bare PF, indicates successful grafting of the aminosilane onto the surface (FP).39 There is a slight shift in the band position of the N–H bending vibration and of the NH2 symmetric bending vibration towards lower wavenumber in the case of the amine-grafted Zr–Ti/PF sorbents, and this may be due to bonding of zirconia and titania nanoparticles with the amine groups.39 As a result, the presence of 3-aminopropyltrimethoxysilane on the surface of ZrO2 and TiO2 was verified by the vibrational band at ≈ 3410–3230 cm−1 is related to N–H groups (from 3-aminopropyltrimethoxysilane and polymer backbone),41 indicating the presence of the imidazole ring. Alkyl C–H stretching bands were identified as the bands seen in the 2871–2926 cm−1 range. The band at ≈1616 cm−1 has been previously reported in the literature42 and is attributed to a conjugated C
= 1489 cm−1 of the amine-grafted PF sorbents, absent in bare PF, indicates successful grafting of the aminosilane onto the surface (FP).39 There is a slight shift in the band position of the N–H bending vibration and of the NH2 symmetric bending vibration towards lower wavenumber in the case of the amine-grafted Zr–Ti/PF sorbents, and this may be due to bonding of zirconia and titania nanoparticles with the amine groups.39 As a result, the presence of 3-aminopropyltrimethoxysilane on the surface of ZrO2 and TiO2 was verified by the vibrational band at ≈ 3410–3230 cm−1 is related to N–H groups (from 3-aminopropyltrimethoxysilane and polymer backbone),41 indicating the presence of the imidazole ring. Alkyl C–H stretching bands were identified as the bands seen in the 2871–2926 cm−1 range. The band at ≈1616 cm−1 has been previously reported in the literature42 and is attributed to a conjugated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration of the cyclic system (imidazole) which is clearly seen in the cut-out of the IR spectra.43 The presence of the vibrational band at 596 cm−1 illustrated the functionalization of ZT–APF with 1,2-dibromoethane. The FTIR spectrum of the Br@ZT–APF confirmed the successfully 3-aminopropyltrimethoxysilane and bromide anchored on the surface of bare ZT–PF.
N vibration of the cyclic system (imidazole) which is clearly seen in the cut-out of the IR spectra.43 The presence of the vibrational band at 596 cm−1 illustrated the functionalization of ZT–APF with 1,2-dibromoethane. The FTIR spectrum of the Br@ZT–APF confirmed the successfully 3-aminopropyltrimethoxysilane and bromide anchored on the surface of bare ZT–PF.
| LOI = 17.5 + 0.4 × CR | (1) |
| Catalysts | Tonseta (°C) | Tendsetb (°C) | CRc (%) | LOId (%) |
|---|---|---|---|---|
| a Degradation temperature.b End of decomposition.c Percentage weight of material left undecomposed after TGA analysis at maximum temperature 800 °C under nitrogen atmosphere.d Limiting oxygen index (LOI) evaluating from char yield at 800 °C. | ||||
| Polyamide-imide | 300 | 581 | 55.6 | 39.74 |
| ZT–APF | 326 | 608 | 56.3 | 40.02 |
| Br@ZT–APF | 366 | 619 | 57.1 | 40.34 |
The LOI values for pure polyamide-imide, ZT–APF, and Br@ZT–APF nanocomposites are 39.74, 40.04, and 40.43 wt%, respectively, as determined from their char yield. Based on the LOI values, these materials can be categorized as self-extinguishing materials.45
3.2. Catalytic activity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 h−1.
172 h−1.
| Catalyst | Reaction temperature (°C) | Conversion (%) SO | Selectivity (%) | TOFf (h−1) | ||||
|---|---|---|---|---|---|---|---|---|
| SC | PAb | BAc | ACPd | BZAe | ||||
| a Reaction conditions: catalyst = 200 mg; styrene oxide = 3.5 mL; ACN (solvent) = 10 mL; CO2 pressure = 10 bar; reaction time = 4 h; temperature = 80, 100, 120, 140, and 160 °C.b Phenylacetaldehyde.c Benzaldehyde.d Acetophenone.e Benzoic acid.f Turnover frequency: moles of quaternary ammonium ion per mol of SC product per h. | ||||||||
| Br@ZT–APF | 80 | 90 | 23 | 45 | 17 | 8 | 7 | 4284.0 |
| 100 | 94 | 94 | 5 | 1 | — | — | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
|
| 120 | 100 | 99 | 1 | 0 | — | — | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 172 |
|
| 140 | 100 | 98 | 2 | 0 | 0 | 0 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
|
| 160 | 100 | 98 | 2 | 0 | 0 | 0 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
|
| Catalyst | Solvents | Reaction time (h) | Conversion (SO) (%) | Selectivity (%) | ||
|---|---|---|---|---|---|---|
| SC | BA | PA | ||||
| a Reaction conditions: catalyst = 200 mg; styrene oxide = 3.5 mL; acetonitrile (solvent) = 10 mL; CO2 pressure = 10 bar; temperature = 120 °C; reaction time = 1, 2, 4, 6, and 8 h. | ||||||
| Br@ZT–APF | ACN | 1 | 88 | 30 | 20 | 50 |
| 2 | 95 | 50 | 33 | 17 | ||
| 4 | 100 | 99 | 0 | 1 | ||
| 6 | 100 | 99 | 0 | 1 | ||
| 8 | 99 | 98 | 1 | 1 | ||
| Catalysts | Solvents | Reaction time (h) | Conversion SO (%) | Selectivity (%) | ||
|---|---|---|---|---|---|---|
| SC | BA | PA | ||||
| a Reaction conditions: catalyst = 200 mg; styrene oxide = 3.5 mL; solvents (ACN, and DMF) = 10 mL; CO2 pressure = 10 bar; temperature = 120 °C; reaction time = 1, 2, 4, 6, and 8 h. | ||||||
| Br@ZT–APF | ACN | 1 | 88 | 30 | 20 | 50 |
| 2 | 95 | 50 | 33 | 17 | ||
| 4 | 100 | 99 | 0 | 1 | ||
| 6 | 100 | 99 | 0 | 1 | ||
| 8 | 99 | 98 | 1 | 1 | ||
| Br@ZT–APF | DMF | 1 | 83 | 11 | 20 | 80 |
| 2 | 88 | 27 | 16 | 65 | ||
| 4 | 94 | 89 | 3 | 8 | ||
| 6 | 92 | 91 | 4 | 5 | ||
| 8 | 92 | 91 | 4 | 5 | ||
3.3. Catalyst reusability and product identification
Recycling experiments were conducted to assess the reusability of the Br@ZT–APF catalyst. After each catalytic cycle, the catalyst was separated from the reaction mixture through simple filtration, followed by multiple washes with chloroform and acetone. To ensure complete removal of adsorbed reactants and products from the pores, the catalyst was reactivated under high vacuum at 110 °C before the subsequent cycle. Reusability studies indicated that Br@ZT–APF retained its catalytic activity over five consecutive cycles, with no significant decline in performance (see Fig. 8). A slight decrease in conversion efficiency was attributed to the partial loss of bromide ions and restricted accessibility to some active sites within the porous hollow fibers, as well as catalyst leaching due to fiber swelling.56 Nevertheless, the selectivity toward styrene carbonate (SC) remained stable at approximately 98.0%. The bromide loading in the reused catalyst was measured at 0.25 mmol g−1, which closely matched that of the fresh catalyst (0.21 mmol g−1), as confirmed by elemental analysis (ICP-MS) and CHN analysis (Table 1). To obtain a clear solution, the reaction mixture was diluted with acetonitrile and filtered. Upon solidification into a yellow crystalline substance, the mixture was subjected to column chromatography using silica gel. The isolated product was characterized using FT-IR, GC-MS, and NMR spectroscopy, which confirmed the formation of a colorless crystalline solid. The FT-IR spectrum (Nujol, cm−1) displayed characteristic absorption bands at 1802, 1380, 1344, 1166, 1090, 1155, 1015, 963, 937, 748, and 670 cm−1, corresponding to SC synthesis (Fig. S1, ESI†). The absorption peak at 1802 cm−1 was attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which corresponds to carbonyl stretch of SC (Fig. S1†). Similarly, increased intensities at 1344 and 1380 cm−1 confirmed asymmetric ν(C–O) stretching vibrations of the cyclic carbonate. The rate of epoxy group consumption correlated directly with cyclic carbonate formation,46 as evidenced by the diminishing intensities of epoxide-related peaks (C–O) at 815 and 876 cm−1 for styrene oxide (SO).57 Throughout the recycling experiments, the structural integrity of the catalyst remained intact, as demonstrated by the consistency in peak characteristics between the fresh and 5th run catalysts. This demonstrates the synthetic catalyst's stability, reusability, and efficiency. These bands were consistent with those of SC, as validated by an analysis of its GC-MS spectra. The GC-MS spectrum showed the molecular ion peak at m/z 164 (M)+ which corresponds to C9H8O3 (see Fig. S2, ESI†). Other fragments were at m/z 119, 105, 91, 90 (base peak), 78 and 65. 1H NMR (400 MHz, CDCl3), δ (pmm): 8.15–7.87 (m, 5H, AR), 6.392 (t, 1H,
O, which corresponds to carbonyl stretch of SC (Fig. S1†). Similarly, increased intensities at 1344 and 1380 cm−1 confirmed asymmetric ν(C–O) stretching vibrations of the cyclic carbonate. The rate of epoxy group consumption correlated directly with cyclic carbonate formation,46 as evidenced by the diminishing intensities of epoxide-related peaks (C–O) at 815 and 876 cm−1 for styrene oxide (SO).57 Throughout the recycling experiments, the structural integrity of the catalyst remained intact, as demonstrated by the consistency in peak characteristics between the fresh and 5th run catalysts. This demonstrates the synthetic catalyst's stability, reusability, and efficiency. These bands were consistent with those of SC, as validated by an analysis of its GC-MS spectra. The GC-MS spectrum showed the molecular ion peak at m/z 164 (M)+ which corresponds to C9H8O3 (see Fig. S2, ESI†). Other fragments were at m/z 119, 105, 91, 90 (base peak), 78 and 65. 1H NMR (400 MHz, CDCl3), δ (pmm): 8.15–7.87 (m, 5H, AR), 6.392 (t, 1H,  ), 5.466 (t, 1H, CH2,
), 5.466 (t, 1H, CH2,  ), 4.995 (t, lH,
), 4.995 (t, lH,  ,
,  ) as shown in Fig. S3, ESI.† This finding is consistent with MS-GC profile (see Fig. S2, ESI†). 13C NMR (400 MHz, CDCL3), δ (ppm):155.98 (OCOO), 136.08 (ARipso), 129.58 (ARmeta), 127.08 (ARpara), 126.13 (ARortho), 79.61 (CH), 72.04 (CH2), respectively (see Fig. S4, ESI†). Spectroscopic data were identical to those found in previous literature reports for this particular chemical species.46,58 Also, the characterization results of 1H NMR and 13C NMR confirmed the structure of SC.
) as shown in Fig. S3, ESI.† This finding is consistent with MS-GC profile (see Fig. S2, ESI†). 13C NMR (400 MHz, CDCL3), δ (ppm):155.98 (OCOO), 136.08 (ARipso), 129.58 (ARmeta), 127.08 (ARpara), 126.13 (ARortho), 79.61 (CH), 72.04 (CH2), respectively (see Fig. S4, ESI†). Spectroscopic data were identical to those found in previous literature reports for this particular chemical species.46,58 Also, the characterization results of 1H NMR and 13C NMR confirmed the structure of SC.
3.4. Characterization analysis of reused catalyst
| Catalyst | Brønsted aciditya (μmol g−1) | Lewis aciditya (μmol g−1) | Catalytic cycle |
|---|---|---|---|
| a The amount of acidity for BAS and LAS was determined by Emeis.68 C(pyridine on Brønsted acid sites) = 1.88IA(B)R2/W, C(pyridine on Lewis acid sites) = 1.42IA(L)R2/W (R = 13 mm, W = 30 mg). | |||
| Br@ZT–APF | 12.0 | 30.0 | Fresh (after 1st run) |
| 11.0 | 27.84 | Used (after 3rd run) | |
| 9.54 | 24.21 | Used (after 5th run) | |
3.5. Proposed mechanism of cyclic carbonate synthesis from styrene oxide and CO2
In this study, the polyamide imide hollow fibers are functionalized using aminosilanes and a bromine source to immobilize nucleophilic [Br−] species and covalently hydrogen-bond donor groups (–OH and –NH) on the porous hollow fiber surface. Highly efficient acid–base-nucleophilic producing trifunctional catalysts as shown in Scheme 2. The cycloaddition reaction is initiated by coordination of the oxygen atom of the epoxide with unsaturated Lewis acidic (ZrO and TiO) centers present in the larger channel, which activates the epoxy ring, the Lewis acid plays a crucial role in activating the epoxide by interacting an electrophilic attack on one of the oxygen atoms (SO). At the same time, the Br− anion, generated from ammonium salt (polyamide-imide backbone structure) nucleophilically attacks the sterically less hindered carbon atom in the epoxy ring and opens the epoxide ring, which forms the metal coordinated bromo-alkoxide, and considered as rate-limiting step.2,69 In the following step, the primary amine groups of 3-aminopropyltrimethoxysilane-grafted ZT–PFs polarize the CO2 molecule to create a carbamate, and the electrophilic carbon of the incoming CO2 forms a tetrel bond with the negatively charged oxygen of metal-bound bromo-alkoxide. The insertion of a C–O bond of CO2 between the (Zr and Ti)–O(bromo-alkoxide) link results in the production of a metal carbonate intermediate, which has been claimed to be a more thermodynamically preferred phase.70 The –NH2 groups that are present in the crosslinked ZT–APFs structure may be rotated, which would further enhance this interaction. Finally, the metal carbonate species undergoes ring-closing, resulting in the elimination of bromide ions. At the same time, the cyclic carbonate is removed from the metal center, and the active catalyst is regenerated. The presence of both the Lewis acidic (Zr and Ti) centers and the N basic moiety in 3-aminopropyltrimethoxysilane-grafted ZT–PFs would make Br@ZT–APF an attractive candidate for the CO2 cycloaddition process. As a result, the oxygen anion from SO attacks the carbamate carbon, which converts into the corresponding cyclic carbonate (SC), as proved by cycloaddition with regeneration of the catalyst. According to the proposed mechanism illustrated in Scheme 2, the inclusion of tertiary amine results in a beneficial synergistic effect that improves catalyst performance. Additionally, the presence of halide anion increased nucleophilicity and leaving ability, with better leaving groups giving the most activity. This demonstrated the significance of nucleophilic attack on the epoxide C atom in the progression of the reaction.23,71 | ||
| Scheme 2 The proposed reaction mechanism for cycloaddition of epoxide and CO2, as catalyzed by Br@ZT–APF. | ||
To prove the reaction mechanism, the sites for CO2 activation by the catalyst were studied using the FT-IR spectrum (Fig. 10). After the catalyst was exposed to CO2 under optimum reaction conditions, a number of additional transmission bands emerged. The band at 1802 and 1783 cm−1 are due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O bonds respectively.42 Comparing the FTIR of the used catalyst with that of the fresh catalyst allowed for additional confirmation that the carbamate anion had formed. The peaks at 1624 and 1225 cm−1 are assigned to bidentate bicarbonate species, b-HCO3−, whereas monodentate bicarbonate, m-HCO3−, was present at 1478 and 1422 cm−1 as well as the peaks at 1450 and 1430 cm−1 are assigned to polydentate carbonate species, p-CO32−.72,73 The peaks at 1595 and 1314 cm−1 can be assigned to bidentate carbonate species, b-CO32−, and the peaks at 1375 and 1355 cm−1 can be assigned to monodentate carbonate species, m-CO32−.74 The peaks at 1565 and 1466 cm−1 was assigned to asymmetric and symmetric stretching vibration of the RCOO−.42,75–77 Sun et al.78 investigated the effect anchorage of CO2 with 15 wt% monoethanolamine (MEA). Several new bands at 1568, 1486, and 1322 cm−1 were tethered to COO− asymmetric and symmetric stretching, and N–COO− stretching vibration of the carbamate species, respectively.79–81
O and C–O bonds respectively.42 Comparing the FTIR of the used catalyst with that of the fresh catalyst allowed for additional confirmation that the carbamate anion had formed. The peaks at 1624 and 1225 cm−1 are assigned to bidentate bicarbonate species, b-HCO3−, whereas monodentate bicarbonate, m-HCO3−, was present at 1478 and 1422 cm−1 as well as the peaks at 1450 and 1430 cm−1 are assigned to polydentate carbonate species, p-CO32−.72,73 The peaks at 1595 and 1314 cm−1 can be assigned to bidentate carbonate species, b-CO32−, and the peaks at 1375 and 1355 cm−1 can be assigned to monodentate carbonate species, m-CO32−.74 The peaks at 1565 and 1466 cm−1 was assigned to asymmetric and symmetric stretching vibration of the RCOO−.42,75–77 Sun et al.78 investigated the effect anchorage of CO2 with 15 wt% monoethanolamine (MEA). Several new bands at 1568, 1486, and 1322 cm−1 were tethered to COO− asymmetric and symmetric stretching, and N–COO− stretching vibration of the carbamate species, respectively.79–81
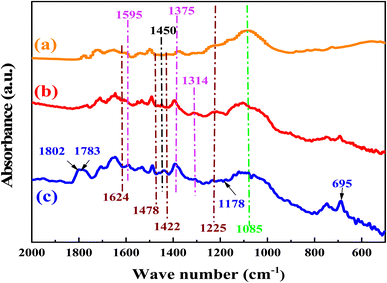 | ||
| Fig. 10 The FTIR spectra of the Br@ZT–APF. (a) Before reaction, (b) after reaction, (c) adsorption of catalyst with CO2. | ||
4. Conclusions
In this study, we developed and investigated Br@ZT–APF as a bifunctional acid–base heterogeneous catalyst for the production of cyclic carbonates from epoxides and CO2. The synergistic interaction between amine groups and transition metal oxides (ZrO2 and TiO2) played a crucial role in activating CO2 and epoxides, facilitating an efficient cycloaddition reaction. Additionally, the incorporation of catalytically active Br− ions within the porous ZT–APF framework significantly enhanced catalytic performance. This technology offers a sustainable and efficient pathway for CO2 utilization, operating under moderate reaction conditions, including low solvent use, reduced reaction temperature, shorter reaction times, and lower pressure (CO2) compared to previously our work.23 The Br@ZT–APF catalyst demonstrated outstanding activity, achieving 100% styrene oxide (SO) conversion and >99% selectivity for styrene carbonate (SC) under optimized reaction conditions. Furthermore, excellent catalyst stability and recyclability were confirmed, with consistent performance maintained over at least five consecutive cycles. Beyond its catalytic efficiency, this study contributes to the broader goal of carbon capture and utilization (CCU) by providing a viable approach for converting CO2 into valuable chemical products. The ability to transform CO2 into cyclic carbonates aligns with global sustainability initiatives, reducing greenhouse gas emissions while supporting the production of environmentally friendly materials. Cyclic carbonates have widespread industrial applications, including their use in polymer production, battery electrolytes, and pharmaceutical intermediates, making this catalytic approach highly relevant to green chemistry and industrial sustainability. Overall, this work lays the foundation for further advancements in the design of porous heterogeneous catalysts for CO2 valorization. Future research could focus on scaling up the process, optimizing catalyst design, and exploring other functionalization strategies to enhance performance and expand the applicability of this technology in various CO2 conversion processes.Data availability
All data generated or analyzed during this study are included in this published article in the main manuscript and ESI.†Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The authors would like to thank the Higher Committee for Educational Development in Iraq (HCED) for their financial support. The authors would also like to acknowledge the Midland Refineries Company MRC/AL-Daura Refinery Company for financial their support. The authors thank the Missouri University of Science and Technology for supporting this work.References
- K. Huang, J. Y. Zhang, F. Liu and S. Dai, ACS Catal., 2018, 8, 9079–9102 Search PubMed.
- V. B. Saptal, B. Nanda, K. M. Parida and B. M. Bhanage, ChemCatChem, 2017, 9, 4105–4111 Search PubMed.
- K. Zhu, Y. Li, Z. Li, Y. Liu, H. Wu and H. Li, Chem. Commun., 2022, 58, 12712–12715 Search PubMed.
- J. Liang, R. P. Chen, X. Y. Wang, T. T. Liu, X. S. Wang, Y. B. Huang and R. Cao, Chem. Sci., 2017, 8, 1570–1575 Search PubMed.
- A. N. Ahmed, A. T. Jarulah, B. A. A. Altabakh, A. M. Ahmed and H. J. Mohammed, Journal of Petroleum Research and Studies, 2023, 13, 115–130 Search PubMed.
- T. Ema, Y. Miyazaki, J. Shimonishi, C. Maeda and J. Y. Hasegawa, J. Am. Chem. Soc., 2014, 136, 15270–15279 Search PubMed.
- M. V. Escárcega-Bobadilla, M. Martínez Belmonte, E. Martin, E. C. Escudero-Adán and A. W. Kleij, Chem.–Eur. J., 2013, 19, 2641–2648 CrossRef PubMed.
- Z. Wu, H. Xie, X. Yu and E. Liu, ChemCatChem, 2013, 5, 1328–1333 CrossRef CAS.
- R. Luo, X. Zhou, S. Chen, Y. Li, L. Zhou and H. Ji, Green Chem., 2014, 16, 1496–1506 Search PubMed.
- S. Liu, Y. Kumatabara and S. Shirakawa, Green Chem., 2016, 18, 331–341 RSC.
- T. Wang, W. Wang, Y. Lyu, X. Chen, C. Li, Y. Zhang, X. Song and Y. Ding, RSC Adv., 2017, 7, 2836–2841 RSC.
- M. Alves, B. Grignard, S. Gennen, C. Detrembleur, C. Jerome and T. Tassaing, RSC Adv., 2015, 5, 53629–53636 RSC.
- F. Guo and X. Zhang, Dalton Trans., 2020, 49, 9935–9947 Search PubMed.
- H. J. Kadhim1 and B. M. A. Dabbagh, Journal of Petroleum Research and Studies, 2023, 13, 118–131 Search PubMed.
- Q. Wen, X. Yuan, Q. Zhou, H. J. Yang, Q. Jiang, J. Hu and C. Y. Guo, Materials, 2023, 16, 1–13 Search PubMed.
- C. Zhao, X. Luo, C. Chen and H. Wu, Nanoscale, 2016, 8, 9511–9516 Search PubMed.
- H. Matsukizono and T. Endo, J. Am. Chem. Soc., 2018, 140, 884–887 Search PubMed.
- A. Jawad, RSC Adv., 2023, 13, 33129–33145 RSC.
- R. Srivastava, D. Srinivas and P. Ratnasamy, Microporous Mesoporous Mater., 2006, 90, 314–326 CrossRef CAS.
- A. Jawad and S. Ahmed, RSC Adv., 2023, 13, 11081–11095 Search PubMed.
- S. R. Jagtap, V. P. Raje, S. D. Samant and B. M. Bhanage, J. Mol. Catal. A:Chem., 2007, 266, 69–74 Search PubMed.
- X. Zhang, N. Zhao, W. Wei and Y. Sun, Catal. Today, 2006, 115, 102–106 Search PubMed.
- A. Jawad, F. Rezaei and A. A. Rownaghi, J. CO2 Util., 2017, 21, 589–596 Search PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquero and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603–619 CrossRef CAS.
- D. Tan, P. Yuan, F. Annabi-bergaya, D. Liu, L. Wang, H. Liu and H. He, Appl. Clay Sci., 2014, 96, 50–55 CrossRef CAS.
- G. Liu, S. Zheng, D. Yin, Z. Xu, J. Fan and F. Jiang, J. Colloid Interface Sci., 2006, 302, 47–53 CrossRef CAS PubMed.
- A. Jawad, F. Rezaei and A. A. Rownaghi, Catal. Today, 2020, 350, 80–90 Search PubMed.
- L. A. Solovyov, A. N. Shmakov, V. I. Zaikovskii, S. H. Joo and R. Ryoo, Carbon, 2002, 40, 2477–2481 CrossRef CAS.
- F. Adam, S. Balakrishnan and P.-L. Wong, J. Phys. Sci., 2006, 17, 1–13 CAS.
- R. B. Pujari, A. C. Lokhande, J. H. Kim and C. D. Lokhande, RSC Adv., 2016, 6, 40593–40601 RSC.
- A. Jawad, F. Rezaei and A. A. Rownaghi, in 17th International Congress on Catalysis Meeting, 2020 Search PubMed.
- N. M. Ahmed, M. H. Flayyih and H. A. Abdul Hussein, Journal of Petroleum Research and Studies, 2021, 11, 17–35 CrossRef.
- G. Zhu, R. Graver, L. Emdadi, B. Liu, K. Y. Choi and D. Liu, RSC Adv., 2014, 4, 30673 RSC.
- A. Jawad and S. Ahmed, J. Thermoplast. Compos. Mater., 2023, 1–31 Search PubMed.
- G. N. Bondarenko, E. G. Dvurechenskaya, O. G. Ganina, F. Alonso and I. P. Beletskaya, Appl. Catal., B, 2019, 254, 380–390 Search PubMed.
- A. Jawad, Eng. Technol. J., 2014, 32, 1618–1639 CrossRef.
- W. Aperador, A. Delgado and M. D. Lagos, Int. J. Electrochem. Sci., 2014, 9, 4144–4157 CrossRef.
- A. Kant, Y. He, A. Jawad, X. Li, F. Rezaei, J. D. Smith and A. A. Rownaghi, Chem. Eng. J., 2017, 317, 1–8 CrossRef CAS.
- S. Mallick, S. Rana and K. Parida, Dalton Trans., 2011, 40, 9169–9175 RSC.
- O. Harizanov, A. Harizanova and T. Ivanova, Solid State Ionics, 2000, 128, 261–265 Search PubMed.
- E. F. Da Silva and H. F. Svendsen, Ind. Eng. Chem. Res., 2004, 43, 3413–3418 CrossRef CAS.
- J. N. Appaturi and F. Adam, Appl. Catal., B, 2013, 136–137, 150–159 CrossRef CAS.
- F. Adam, K. M. Hello and H. Osman, Appl. Catal., A, 2010, 382, 115–121 CrossRef CAS.
- S. Mallakpour and A. Zadehnazari, J. Chem. Sci., 2013, 125, 203–211 Search PubMed.
- S. Mallakpour and A. Zadehnazari, J. Thermoplast. Compos. Mater., 2015, 28, 1644–1661 CrossRef CAS.
- J. N. Appaturi and F. Adam, Appl. Catal., B, 2013, 136–137, 150–159 CrossRef CAS.
- Y. He, A. Jawad, X. Li, M. Atanga, F. Rezaei and A. A. Rownaghi, J. Catal., 2016, 341, 149–159 CrossRef CAS.
- A. Siewniak, A. Forajter and K. Szymańska, Catalysts, 2020, 10, 1–12 CrossRef.
- A. Jawad and N. Salah, Eng. Technol. J., 2013, 31, 976–990 CrossRef.
- S. N. Shatub, Journal of Petroleum Research and Studies, 2022, 12, 104–120 CrossRef.
- R. H. Saihod, Z. M. Shakoor and A. Jawad, Al-Khwarizmi Engineering Journal, 2014, 10, 47–61 Search PubMed.
- J. Kegere, S. S. Alneyadi, A. P. Paz, L. A. Siddig, A. Alblooshi, M. A. Alnaqbi, A. Alzamly and Y. E. Greish, Nanoscale Adv., 2024, 6, 4804–4813 RSC.
- J. A. Castro-Osma, J. W. Comerford, R. H. Heyn, M. North and E. Tangstad, Faraday Discuss., 2015, 183, 19–30 RSC.
- M. W. C. Robinson, A. M. Davies, R. Buckle, I. Mabbett, S. H. Taylor and A. E. Graham, Org. Biomol. Chem., 2009, 7, 2559–2564 Search PubMed.
- A. Jawad and N. J. Saleh, Eng. Technol. J., 2013, 31, 2039–2054 CrossRef.
- A. K. Gupta, N. Guha, S. Krishnan, P. Mathur and D. K. Rai, J. CO2 Util., 2020, 39, 101173 CrossRef CAS.
- W. Clegg, R. W. Harrington, M. North and R. Pasquale, Chem.–Eur. J., 2010, 16, 6828–6843 CrossRef CAS PubMed.
- D. Urbani, C. Rovegno, A. Massi, M. E. Leblebici, E. Kayahan, E. Polo and P. Dambruoso, J. CO2 Util., 2023, 67, 102328 CrossRef CAS.
- W. Dong, Z. Shen, B. Peng, M. Gu, X. Zhou, B. Xiang and Y. Zhang, Sci. Rep., 2016, 6, 1–8 CrossRef PubMed.
- J. De, O. N. Ribeiro, D. G. Da Silva, D. C. L. Vasconcelos and W. L. Vasconcelos, Adsorption, 2021, 27, 1207–1220 CrossRef PubMed.
- H. Song, J. Wang, Z. Wang, H. Song, F. Li and Z. Jin, J. Catal., 2014, 311, 257–265 Search PubMed.
- A. Jouve, S. Cattaneo, D. Delgado, N. Scotti, C. Evangelisti, J. M. L. Nieto and L. Prati, Appl. Sci., 2019, 9, 2287 Search PubMed.
- M. A. Atanga, F. Rezaei, A. Jawad, M. Fitch and A. A. Rownaghi, Appl. Catal., B, 2017, 220, 429–445 CrossRef.
- A. Jawad, Doctoral dissertations, Missouri University of Science and Technology, 2020, p. 2958. https://scholarsmine.mst.edu/doctoral_dissertations/2958. Search PubMed.
- M. North and R. Pasquale, Angew. Chem., Int. Ed., 2009, 48, 2946–2948 CrossRef CAS PubMed.
- F. Paquin, J. Rivnay, A. Salleo, N. Stingelin and C. Silva, J. Mater. Chem. C, 2015, 3, 10715–10722 RSC.
- A. Jawad and A. Rownaghi, in 2019 AIChE Annual Meeting, AlChE, 2019 Search PubMed.
- C. A. Emeis, J. Catal., 1993, 141, 347–354 CrossRef CAS.
- X. Y. Li, L. N. Ma, Y. Liu, L. Hou, Y. Y. Wang and Z. Zhu, ACS Appl. Mater. Interfaces, 2018, 10, 10965–10973 CrossRef CAS PubMed.
- K. Xu, A. M. P. Moeljadi, B. K. Mai and H. Hirao, J. Phys. Chem. C, 2018, 122, 503–514 CrossRef CAS.
- A. C. Kathalikkattil, R. Babu, R. K. Roshan, H. Lee, H. Kim, J. Tharun, E. Suresh and D. W. Park, J. Mater. Chem. A, 2015, 3, 22636–22647 RSC.
- C. Morterra and L. Orio, Mater. Chem. Phys., 1990, 24, 247–268 Search PubMed.
- K. Pokrovski, K. T. Jung and A. T. Bell, Langmuir, 2001, 17, 4297–4303 CrossRef CAS.
- K. Pokrovski, K. T. Jung and A. T. Bell, Langmuir, 2001, 4297–4303 Search PubMed.
- C. Nyambo, P. Songtipya, E. Manias, M. M. Jimenez-Gasco and C. a. Wilkie, J. Mater. Chem., 2008, 18, 4827–4838 RSC.
- C. Xu, Z. Bacsik and N. Hedin, J. Mater. Chem. A, 2015, 3, 16229–16234 RSC.
- A. Danon, P. C. Stair and E. Weitz, J. Phys. Chem. C, 2011, 115, 11540–11549 CrossRef CAS.
- C. Sun and P. K. Dutta, Ind. Eng. Chem. Res., 2016, 55, 6276–6283 Search PubMed.
- P. Jackson, K. Robinson, G. Puxty and M. Attalla, Energy Procedia, 2009, 1, 985–994 CrossRef CAS.
- K. Robinson, A. McCluskey and M. I. Attalla, Chemphyschem, 2011, 12, 1088–1099 CrossRef CAS PubMed.
- G. H. Gunasekar, K. Park, K.-D. Jung and S. Yoon, Inorg. Chem. Front., 2016, 3, 882–895 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Including the mass spectrum of the product styrene carbonate and 1H NMR spectra for styrene carbonate in CDCl3. See DOI: https://doi.org/10.1039/d5ra00392j |
| This journal is © The Royal Society of Chemistry 2025 |