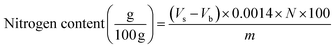Open Access Article
Open Access ArticleNutritional and chemical characterization of cow, camel, and goat meat from Kebridehar, Ethiopia: a comparative analysis and statistical approaches†
Mahamed Abdi Walia,
Yared Shewarega Lemmab,
Fikadu Siyum Mekonon b,
Mulusew Birara Yizengawb,
Kebede Mamo Aderab,
Medidi Raja Sekhar
b,
Mulusew Birara Yizengawb,
Kebede Mamo Aderab,
Medidi Raja Sekhar *b and
Sathish Mohan Botsa
*b and
Sathish Mohan Botsa *c
*c
aCollege of Dry Land and Agriculture, Department of Animal Science, University of Kabridahar, P.O. Box 250, Kebri Dehar, Somali Region, Ethiopia
bDepartment of Chemistry, College of Computational and Natural Sciences, University of Kabridahar, P.O. Box 250, Kebri Dehar, Somali Region, Ethiopia. E-mail: medidirajasekhar16@gmail.com; bsathish401@gmail.com
cBio Enviro Chemical Solutions, Visakhapatnam-530017, India
First published on 10th March 2025
Abstract
The study analyzed fresh camel, cow, and goat meat for physicochemical properties, including pH, moisture, protein, fat, ash, crude fiber, vitamins, and metal concentrations. Camel meat had the highest pH (6.01 ± 0.04) and fat content (6.48 ± 0.03%), cow meat had the highest moisture, and goat meat exhibited the highest protein (23.63 ± 0.01%) and ash content (1.03 ± 0.03%). Vitamins A, E, and D levels were consistently low across samples. Essential metals such as sodium (452.55–508.81 mg kg−1), potassium (2994.13–3503.58 mg kg−1), and calcium (282.41–594.05 mg kg−1) were within acceptable ranges. Camel meat showed elevated sodium, selenium, and copper, while goat meat had higher potassium, iron, and manganese. The study highlights species-specific differences in nutritional composition and metal content, influenced by environmental and dietary factors, with implications for public health regarding both nutritional benefits and heavy metal risks.
1. Introduction
The global population is increasing at an unprecedented rate, significantly elevating the demand for food worldwide and exacerbating food crises.1 This surge in population, coupled with advancements in technology, has heightened awareness of the nutritional value of food and increased incomes, further driving the demand for various food products2 By 2050, it is expected that annual meat production will rise to 470 million tons. The consumption of red meats, particularly from camels, cattle, sheep, and goats, plays a crucial role in human development at all stages of existence.Camels, being multipurpose animals, are integral to the socio-economic fabric of many arid and semi-arid regions worldwide. They provide milk, meat, and hides and are used for transportation, entertainment, celebrations, and competitions such as racing and beauty shows. Camel meat, a primary source of animal protein in numerous African and Asian countries, is especially vital in harsh conditions where other livestock struggle to thrive. It is preferred for its medicinal properties and affordability, offering a healthier alternative to beef with its higher content of polyunsaturated fatty acids and lower levels of fat and cholesterol, which reduce the risk of cardiovascular diseases.3 Additionally, camel meat is used to treat various ailments, including hyperacidity, hypertension, pneumonia, and respiratory diseases.4
Similarly, goat meat is recognized for its nutritional value, offering more protein and less fat than sheep meat, making it easier to digest.5 It is healthier option than red meat due to its low content of saturated fatty acids and cholesterol, reducing the risk of stroke and coronary diseases. Goat meat also contains essential amino acids such as lysine, threonine, and tryptophan, further enhancing its nutritional profile. These factors make goat meat a preferred choice for those seeking a nutritious diet with less total fat and cholesterol.
Beef on other hand, a significant source of essential nutrients, including proteins, fats, zinc, phosphorus, cholesterol and iron. These nutrients play critical roles in body functions such as growth, reproduction, metabolism, and maintaining strong bones and teeth. Beef also provides various vitamins B necessary for energy metabolism and overall health. Meat has always been a cornerstone of human diets, providing vital nutrients necessary for survival and development. As the demand for meat continues to grow, understanding the nutritional value and health implications of consuming different types of meat becomes increasingly important. This study aims to compare the nutritional values of camel, cow and goat meats to address nutritional deficiencies and improve food security in regions like Kebridehar, where food availability is a significant concern. The findings will help highlight the importance of improving meat production and consumption to ensure a balanced diet and better health outcomes.
Camel meat has gained recognition for its unique chemical composition and nutritional benefits. Studies have shown that camel meat contains higher moisture, lower ash, and lower fat compared to meats from other farm animals such as beef, lamb, goat, and chicken, while maintaining a similar protein content.6 Research conducted in various regions has provided detailed chemical composition profiles of camel meat, highlighting its nutritional value. For instance, in Pakistan, camel meat samples were reported to have 71.29% moisture, 71.29% protein, 15.37% fat, and 2.20% ash.7 Similar studies in Sudan and Iran have also provided comprehensive data on the moisture, protein, fat, and ash content of camel meat, underscoring its nutritional benefits.8
Comparative studies between camel meat and other meats such as beef or goat, further emphasize the distinct characteristics of camel meat. Fresh camel meat from young animals has been found to have higher moisture, protein, and glycogen but lower fat and ash compared to beef.6 Similar trends are observed when comparing camel meat with goat meat, with camel meat generally exhibiting higher moisture and protein but lower fat and ash content.
The pH of camel meat is another crucial factor influencing its quality, affecting attributes such as color, water-holding capacity, texture, and overall consumer acceptance. The pH value of camel meat, measured after 24 hours post-mortem, ranges from 5.7 to 6.0, reflecting the impact of pre-slaughter handling, nutrition, and stress levels on the meat's quality.9 Proper management practices can significantly influence the ultimate pH of camel meat, thereby affecting its shelf life and susceptibility to microbial growth.10
Understanding the chemical composition and quality attributes of camel meat is essential for enhancing its utilization and marketability. This study provides a comparative analysis of camel, cow, and goat meat (area of Kebridehar), focusing on their proximate composition, which is rarely explored in a single framework. The findings highlight significant differences in moisture, protein, fat, and fiber content, offering valuable insights into their nutritional and culinary properties. By combining detailed proximate data with standardized methods, this research sets the stage for optimizing meat selection based on dietary needs and processing requirements.
2. Materials and methods
2.1. Description of the study area and samples collection
Kebrideharchem (Somali: Qabri-Dahare) is a city in the eastern part of Ethiopia known as the Somali Region. Located in the Korahey Zone of the Somali Region, this town has a latitude and longitude of 6°44′N 44°16′E coordinates, and an elevation of 393 meters above sea level (Fig. S1†). The three animals of camel, goat and cow fresh meat samples were collected in three study areas Kebri-Dehar city (Korahey market) in amber bottle and was marked according to the type of animal.2.2. Experimental site
Experimental works were done at Ethiopian Conformity Assessment Enterprise (ECAE), Addis Ababa, Ethiopia.2.3. Instruments, apparatus and chemicals
The instruments used for this study were Inductively Coupled Plasma-Mass Spectroscopy (ICP-MS system Model 7900, Agilent, Palo Alto, CA, USA) and Inductively Coupled Plasma-Optical emission Spectroscopy (ICP-OES) for selected metal determination; High Performance Liquid Chromatography (HPLC) for Vitamin (AED) determination, High Performance Microwave Digestion System (for selected-metal), Bochi heating bath B-490, a Macroprossecer based PH-EC-TDS Meter for the determination of pH, Fat extractor (E-500), Kjel-Digester (K-449) for protein determination, kjelflex (k-360) for distillation (protein), Kjel-Flex (K-300) and de-ionizer were used.Common laboratory apparatus used during the study were refrigerator, Amber-bottles, Stomacher, hot plate, different sized beakers, sonicator, syringe filter (diameter: 25 mm & pore size: 0.45 μm), crucibles, glass rod, moisture dish, separatory funnel, vortex mixer, conical flasks, funnels, Petridish, micro pipette, thimble, cotton, aluminum foil, volumetric flasks, steam bath, water bath, oil bath, cooler, thin glass rod, fume hood, glass pipettes, micro-pipettes, singer glass, spatula, measuring cylinders, ice-box, filter paper, vial, test tubes, stop watch, sample bottles, desiccators, burette, wash bottle, vinyl gloves, analytical balance, drying oven, furnace and a mercury thermometer (°C) were used during the laboratory work. All glassware used throughout the experiments were washed and then dried in oven at 75 °C.
All the chemicals and reagents used during this work were analytical reagent grade. Sodium (Na), potassium (K), cadmium (Cd), copper (Cu), chromium (Cr), selenium (Se), iron (Fe), manganese (Mn), nickel (Ni), lead (Pb), zinc (Zn), molybdenum (Mo), calcium (Ca), cobalt (Co) and vitamin (AED) standards were used. Acetone (CH3COCH3), a mixture of 4.98 g potassium sulfate (K2SO4), 0.02 g copper sulfate (CuSO4·5H2O), 98% sulfuric acid (H2SO4), 30% Hydrogen peroxide (H2O2), 0.5 N hydrochloric acid (HCl), petroleum ether (C6H14), Celite-545, 0.313 N sodium hydroxide (NaOH), ethanol (C2H5OH), boric acid (H3BO3), 50% potassium hydroxide (KOH), pyrogallol, petroleum ether, n-hexane (C6H14), methanol (CH3OH) and concentrated nitric acid (HNO3) were used during the analysis.
2.4. Sample preparation
The meat sampling and preparation for experimental analysis were conducted using standardized methods to ensure accuracy and reliability. These methods included protocols such as ES ISO 1442:2005 for moisture content, ES ISO 1443:2005 for fat analysis, and BCTL/SOP guidelines for other parameters like protein, pH, vitamins, and metal content. In brief, 1 kg sample of each fresh meat sample species (camel, cow and goat) were purchased from randomly selected commercial markets (Kabridahar) after being butchered in a slaughterhouse. The samples were carefully packed in sterile polyethylene and in an insulated box filled with ice during transportation to laboratory. Upon arrival in the lab, the meat was washed with chilled sterilized and deionized water, deport fat and connective tissues were then removed. The proximate analysis was done for the raw meat samples which include pH, moisture content, ash content, crude protein content, crude fat content, crude fiber content and vitamin (AED) content. The samples were prepared and examined according to the technique recommended by ECAE: the samples were grounded using Stomacher then the chopped materials were transferred to an amber bottle with an airtight cover, identified, and were stored in the refrigerator till used.2.5. pH (BCTL/SOP/M00.01)
The pH-meter (HI 255 Combined Meter) was calibrated at acidic (pH-4), neutral (pH-7) and basic level (pH-9), then the pH of each meat sample was measured using the calibrated pH meter and the readings were recorded.2.6. Moisture (ESISO14421, 2005)
Five grams from each homogenized meat sample was transferred to the prepared dish and weighed the dish with its content and the glass rod to the nearest 0.001 g (m1). Then the contents were mixed with the glass rod. The dishes with their content and the glass rod were heated for 2 h in the oven seat at 103 °C. Then the dishes with their contents and the glass rods were removed from the oven and were placed them in a desiccators. The dishes with their contents and the glass rods were allowed to cool at room temperature and weighed to the nearest 0.001 g (m2). Then the moisture content (w), as a percentage by mass calculated as;where m0: the mass in gram of the moisture dish and the rod m1: the mass in grams of the moisture dish containing the test portion and the rod before drying. m2: the mass in grams of the moisture dish containing the test portion and the rod after drying.
2.7. Crude protein determination (ESISO 1871:2013)
Crude protein was determined by the micro-Kjeldhal method: 0.6 grams of the grounded meat sample, a mixed catalyst (4.98 g K2SO4 and 0.02 g CuSO4·5H2O) and 1 glass bead was added in to the digestion tube. The tubes were put into a fume hood; 15 ml of 98% sulfuric acid followed by 3 ml of 30% hydrogen peroxide was added to each digestion tubes and shaken slowly using a universal shaker. Then the digestion tubes were placed in to the Kjel-Digester (K-449) at low temperature (to prevent frothing and briskly) until the solution is green and oxidation was completed. Then the digested samples were cooled and were made ready for distillation step. Then the digestion tubes were fitted with the distillation unit and 250 ml Erlenmeyer flask containing as a receiver was placed on the distillation unit. Then the samples were run sequentially and the resultant distillate was titrated with a 0.5 N hydrochloric acid solution until the first appearance of the pink color and the volume of the acid consumed was recorded. Then the titre value obtained was used to calculate the percent crude protein content using the following formula;where Vs: volume of titrant HCl consumed by the sample in ml Vb: volume of titrant HCl consumed by the blank in ml N: normality of 0.5 N HCl solution used m: mass of the test portion C: conversion factor (6.25 for meat) Ash Determination (ESISO official method 936:2005)
Ash content was determined using ESISO official method 936:2005. Crucibles were heated for 20 min in the muffle furnace set at 550 °C and were allowed to cool in the desiccators to room temperature: and weighed with analytical balance (m0). Five gram (5 g) from each grounded meat sample placed into a dried crucible and noted as (m1). Then, the crucibles with their contents were placed in the cool muffle furnace and the temperature was raised 550 °C for 6 h until the ash has a grey-white appearance. Then the crucibles were taken out from the muffle furnace and allowed to cool in the desiccators to room temperature; and weighed with analytical balance (m2). The ash percentage was calculated as;
2.8. Crude fat determination (ESISO 1443:2005)
To determine the crude fat content 5 grams from each grounded meat sample was added into 250 ml conical flask. For each sample containing conical flask 50 ml HCl was poured and covered with a small watch glass. Then boiled for one (1) h in a water-bath; and 150 ml hot-boiled water was added to each flask. Then a fluted filter paper was moistened which held in a glass funnel with distilled water and the hot contents from the flask were poured onto the filter. Then the filter papers were rolled up and inserted in to the extraction thimble. Then fat contents from the Petridish were removed using cotton wool and moistened with petroleum ether, and the cotton wools transferred to the thimble. Then the thimbles were placed in the fat extractor (E-500). Then after extraction the flask containing the liquid were taken from the extraction apparatus; distilled off the solvent and transferred to the previous conical flask. Then the conical flasks were dried for 1 h in the drying oven at 103 °C and were allowed to cool at room temperature in the desiccators. Then the total fat content of the sample were expressed as a percentage by mass was calculated as;where: m0: the mass of the test portion m1: the mass of the empty conical flask m2: the mass of the test portion with conical flask.
2.9. Determination of fiber content (BCTL/SOP/M017.01)
For the determination of fiber content 1 grams of the grounded fresh meat sample was weighed in a pre-dried singer glass to 525 °C for 30 min which was cooled at room temperature. To simplify the filtration 1 g of Celite-545 was added to the singer glass before the sample. Then the singer glass and its content were connected to the cold extraction unit; and 25 ml di-water was poured to each. Then it was stand for 5 min in a vacuum to draw-off the di-water and the extracted fat. The residue was washed with di-water in a similar manner to ensure drying. Then the filter was placed in the fiber Tec Hot extraction unit and 150 ml of boiled 0.255 N of H2SO4 (aq.) was poured in to the assembled cylinder. Then the liquid was brought to the boil vigorously for 30 min. Then the tap was opened to the discharge pipe heating unite for digestion with H2SO4, and, was under vacuum. Then the sulfuric acid was filtered through the filter crucible and washed the residue with three consecutive 30 ml portion of boiling water.Then the outlet tap was closed and 150 ml of boiled 0.313 N NaOH (aq.) solutions was poured. In addition 3 drops of n-octanol was added to prevent foaming and heat to boiling, and the liquid was boiled vigorously for 30 minutes. Then the filtration and the washing procedure were repeated used for the sulphuric acid step.
The singer glasses were dried to a constant mass in the oven at 130 °C. After each drying, the singer glasses were cooled in the desiccators and weighed (M2). Then the singer glasses were placed in a muffle furnace and ashed the residue to constant mass at 550 °C. Then cooled to room temperature in desiccators and weighed (M3).
where, M0 = mass of empty filter crucible singer glass M1 = mass of sample in gram M2 = the total mass, in g, of the residue after oven drying M3 = is the loss of mass after ashing during the determination in gram c1 = mass of Celite in gram c2 = mass of Celite residue after oven drying in gram c3 = mass of Celite residue after furnace ignition in gram.
2.10. Determination of vitamin A, E and D extraction
For the determination of selected vitamins (A, E & D) 5 grams from each grounded samples were measured and added into around bottom flask. Then 40 ml ethanol, 10 ml 50% KOH and a few amount of pyrogallol (as antioxidant) were poured in to each sample containing round bottom flasks. The flasks were attached to a reflux condenser which were reflux at 95 °C for 45 min (were mixed and agitated every 10 min). Then each were cooled immediately using cold water and 40 ml di-water added for each flask and transferred in to a separatory funnel.For first extraction; added to the flask and was rinsed with 70 ml petroleum ether and transferred in to a separatory funnel. The 20 ml ethanol added into the flasks, rinsed and transferred to the separatory funnel. Then the extraction was done by shaking the separatory funnels for 5 min (by letting the air out from time to time). Then the lower layer was collected in to the previous round bottom flask and then upper layer (ether layer) in to another second separatory funnel.
The second extraction was continued; the solutions from the round bottom flasks were transferred to the first separatory funnel. The 70 ml n-hexane was poured to each round bottom flask and rinsed well then transferred to the first separatory funnel. Then the extraction was done by shaking the separatory funnels each for 5 min by letting the air out from time to time. Then the lower was collected in to the previous round bottom flask and the upper layer (n-hexane layer) was transferred in to the second separatory funnel.
Third extraction: the solution was transferred from the round bottom flask to the first separatory funnel. Then 30 ml petroleum ether, 30 ml n-hexane were added to each round bottom flask and were rinsed well and then transferred into the first separatory funnel. Then the separatory funnels were shaken for 5 min by letting the air out from time to time. Then the lower was discarded and collected the upper layer (n-hexane layer) in to the second separatory funnel.
Then the collected organic layers were washed with distilled water for 5 min and the washings were free from alkaline then the lower layer (water) was discarded. Then the organic (upper layer) was filtered with a filter paper in a second round bottom flask. Then the collected organic layer was evaporated using a Rota-vapor at 40 °C until it becomes around 5 ml. Then the rest was evaporated and dried with nitrogen. Then 10 ml methanol was added to each sample (crystal left) and sonicated using a Sonicator around 1 min. Then each sample was filtered using a syringe filter (diameter: 25 mm & pore size: 0.45 μm). The filtrates were transferred to a vial, mixed with a vortex mixer and were kept at room temperature for further determination (HPLC).
2.11. Minerals contents
0.2 g from each grounded samples were poured into a micro wave digestion vessels. Then 15 ml concentrated HNO3 and 3 ml of 30% hydrogen peroxide was added to each digestion vessels. The internal standard was added to each digestion vessel; caped the vessels securely and were placed in to the microwave digestion system according to the manufacturers instruction. Then the samples were digested at a temperature of 190 °C for 10 min. Then after the filtration step was done and were kept for further determination (ICP-MS and ICP-OES).Quantitative analysis was performed via the calibration curve method. Calibration curves were built with a minimum of five concentrations of standards per element.
3. Results and discussion
3.1. Proximate ccomposition analysis
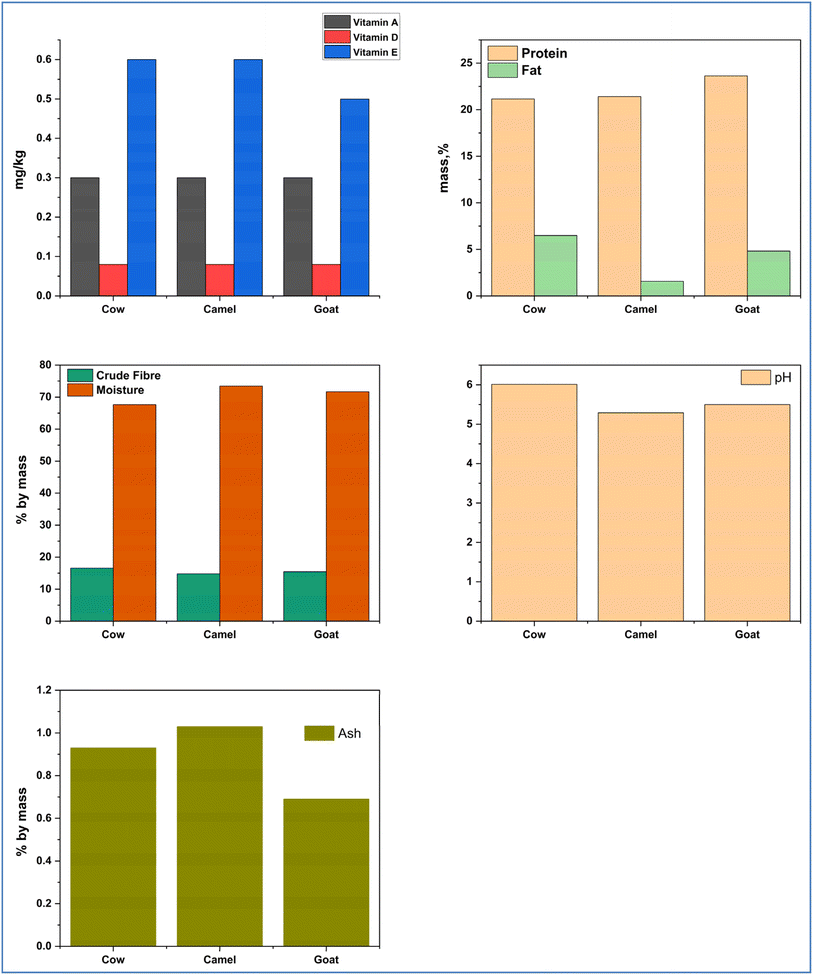
Each of the samples collected were analyzed, for pH, moisture, protein, fat, ash, crude fiber, vitamin (A, E and D) and the results were displayed in Fig. 3. Table 1 presents the laboratory results of all examined physico-chemical properties.| Parameters | Test method | Cow | Camel | Goat |
|---|---|---|---|---|
| pH | BCTL/SOP/M00.01 | 6.01 ± 0.04 | 5.29 ± 0.01 | 5.50 ± 0.00 |
| Moisture, % by mass | ES ISO 1442:2005 | 67.66 ± 0.03 | 73.46 ± 0.02 | 71.71 ± 0.04 |
| Protein (N* 6.25), % by mass | BCTL/SOP/MO14.01 | 22.16 ± 0.01 | 21.41 ± 0.04 | 23.63 ± 0.01 |
| Fat, % by mass | ES ISO 1443:2005 | 6.48 ± 0.03 | 1.56 ± 0.04 | 4.81 ± 0.04 |
| Ash, % by mass | BCTL/SOP/MO14.01 | 0.93 ± 0.02 | 1.03 ± 0.03 | 0.69 ± 0.02 |
| Crude fiber, % by mass | BCTL/SOP/M017.01 | 16.56 ± 0.03 | 14.80 ± 0.03 | 15.45 ± 0.01 |
| Vitamin A, mg kg−1 | BCTL/SOP/M006.01 | <0.50 | <0.50 | <0.50 |
| Vitamin E, mg kg−1 | BCTL/SOP/M006.01 | <0.70 | <0.70 | <0.70 |
| Vitamin D, mg kg−1 | BCTL/SOP/M006.01 | <0.09 | <0.09 | <0.09 |
| Metals | Test method | Cow | Camel | Goat |
|---|---|---|---|---|
| Essential trace elements, mean ± SD (mg kg−1) | ||||
| Na | ICP-OES | 508.81 ± 0.02 | 452.55 ± 0.01 | 505.97 ± 0.02 |
| K | ICP-OES | 2994.13 ± 0.02 | 3467.15 ± 0.04 | 3503.58 ± 0.02 |
| Ca | AOAC 2015.01 ICP-MS | 509.47 ± 0.04 | 594.05 ± 0.04 | 282.41 ± 0.03 |
| Cu | AOAC 2015.01 ICP-MS | 7.07 ± 0.04 | 2.98 ± 0.03 | 2.0 ± 0.02 |
| Cr | AOAC 2015.01 ICP-MS | <0.01 | <0.01 | <0.01 |
| Se | AOAC 2015.01 ICP-MS | 0.66 ± 0.00 | 0.25 ± 0.02 | 0.30 ± 0.01 |
| Fe | AOAC 2015.01 ICP-MS | 1.80 ± 0.02 | 5.80 ± 0.02 | 10.33 ± 0.04 |
| Mn | AOAC 2015.01 ICP-MS | 3.0 ± 0.00 | 4.38 ± 0.02 | 9.64 ± 0.03 |
| Zn | AOAC 2015.01 ICP-MS | 5.79 ± 0.02 | 6.98 ± 0.04 | 4.81 ± 0.02 |
| Mo | ICP-OES | <0.25 | <0.25 | <0.25 |
| Co | ICP-OES | <0.16 | <0.16 | <0.16 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Heavy metals, mean ± SD (mg kg−1) | ||||
| Cd | AOAC 2015.01 ICP-MS | 0.01 ± 0.01 | 0.05 ± 0.01 | 0.04 ± 0.01 |
| Ni | AOAC 2015.01 ICP-MS | 1.35 ± 0.03 | 0.65 ± 0.01 | 0.81 ± 0.00 |
| Pb | AOAC 2015.01 ICP-MS | 0.20 ± 0.00 | 0.13 ± 0.001 | 0.07 ± 0.00 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg kg−1, pregnant women as 2900 mg kg−1, for teens (boys) 14–18 years 3000 mg kg−1, teens (girls) 14–18 years 2300 mg kg−1, pregnant teens 2600 mg kg−1, pregnant women 2900 mg kg−1, breast feeding teens 2500 mg kg−1, breast feeding women 2800 mg kg−1 etc.28 The Goat meat is found to be more as 3503 mg kg−1 and the least reported for camel 2994 mg kg−1 which is permissible limits for all the three meats.
000 mg kg−1, pregnant women as 2900 mg kg−1, for teens (boys) 14–18 years 3000 mg kg−1, teens (girls) 14–18 years 2300 mg kg−1, pregnant teens 2600 mg kg−1, pregnant women 2900 mg kg−1, breast feeding teens 2500 mg kg−1, breast feeding women 2800 mg kg−1 etc.28 The Goat meat is found to be more as 3503 mg kg−1 and the least reported for camel 2994 mg kg−1 which is permissible limits for all the three meats.
Muscle weakening, which might lead to more tender flesh after slaughter, is caused by a K deficiency. According to a human investigation, the weakening of periodic paralysis may be brought on by abnormal muscle absorption of K, which raises the ions intracellular to extracellular concentration ratio. This causes the muscle membrane to become hyperpolarized, which is linked to a decrease in the muscle's reactivity to nerve stimulation, the spread of excitement, and contraction. According to studies, osmotic pressure changes continue during storage and do not cease 24 hours after death.29 In addition to being crucial for animal growth and muscular force, giving animals the right food and ensuring that they absorb and use K enough may also improve meat softness in light of the effects of K that are now recognized. Strong evidence suggests that for a significant section of the population, this imbalance that is high sodium consumption on the one hand and low intakes of potassium, calcium, and magnesium on the other causes and maintains increased blood pressure. Elevated blood pressure can be lowered by reducing sodium intake alone and increasing potassium, calcium, and magnesium intakes separately. Combining all three elements a reduction in sodium and an increase in intakes of potassium, calcium, and magnesium as part of the so-called dietary approaches to stop hypertension diets has a very good effect on lowering blood pressure. A complete approach involving the reduction of sodium intake and the increase of potassium, calcium, and magnesium intake should be implemented in communities to prevent and treat high blood pressure. It is anticipated that the so-called ‘functional food/nutraceutical/food-ceutical’ approach, which corrects the mineral-nutrient composition of widely consumed processed meals, will be especially successful in bringing about instant positive results.
The analysis of essential trace elements and heavy metals in camel, cow, and goat reveals significant variations in their mineral content, reflecting the diverse dietary and environmental conditions experienced by these animals. Notably, potassium (K) levels are highest in goats (3503.58 ± 0.02 mg kg−1), followed closely by cows (3467.15 ± 0.04 mg kg−1), whereas camels exhibit the lowest concentration (2994.13 ± 0.02 mg kg−1). Calcium (Ca) content is most abundant in cows (594.05 ± 0.04 mg kg−1), suggesting a diet rich in calcium or better absorption mechanisms, while goats have the lowest calcium levels (282.41 ± 0.03 mg kg−1). Copper (Cu) levels are significantly higher in camels (7.07 ± 0.04 mg kg−1) compared to cows (2.98 ± 0.03 mg kg−1) and goats (2.0 ± 0.02 mg kg−1), indicating a potentially greater requirement or accumulation in camels.
Regarding heavy metals, camels show a higher concentration of nickel (Ni) (1.35 ± 0.03 mg kg−1) compared to cows (0.65 ± 0.01 mg kg−1) and goats (0.81 ± 0.00 mg kg−1), which could be attributed to environmental exposure or differences in metabolic processing. Lead (Pb) levels are relatively low across all species, with camels having the highest concentration (0.20 ± 0.00 mg kg−1) and goats the lowest (0.07 ± 0.00 mg kg−1). This data underscores the importance of monitoring and managing mineral and heavy metal intake in livestock to ensure their health and the safety of animal products for human consumption. The findings also highlight the unique nutritional profiles of these animals, which can inform dietary supplementation and environmental management practices.
| Cow | Camel | Goat | ||
|---|---|---|---|---|
| N | 22 | 22 | 22 | |
| Mean | 188.75 | 211.6127 | 42.7027 | |
| Median | 2.40 | 2.3150 | 1.4050 | |
| Std. Deviation | 643.835 | 743.24256 | 119.89376 | |
| Minimum | 0 | 0.05 | 0.00 | |
| Maximum | 2994 | 3467.15 | 505.97 | |
| Percentiles | 25 | 0.28 | 0.2600 | 0.1875 |
| 50 | 2.40 | 2.3150 | 1.4050 | |
| 75 | 17.71 | 16.4525 | 11.6100 | |
The agglomeration schedule provides a hierarchical clustering process, where clusters are progressively combined based on similarity (indicated by coefficients). At each stage, two clusters (columns “Cluster 1” and “Cluster 2”) are merged, starting with small coefficients, meaning close similarities between the clusters (Table S1†). For instance, in Stage 1, clusters Cd and vitamin D are combined with a minimal coefficient of 0.007, showing a high level of similarity (Fig. 4). As the stages progress, the coefficients increase, reflecting more dissimilar or distant clusters are merged, until stage pH, where the coefficient skyrockets to over 20 million, indicating the final merging of highly disparate clusters. This hierarchical process reveals the underlying structure of the data, where clusters of similar items merge early, and larger, more disparate groups form later in the process.
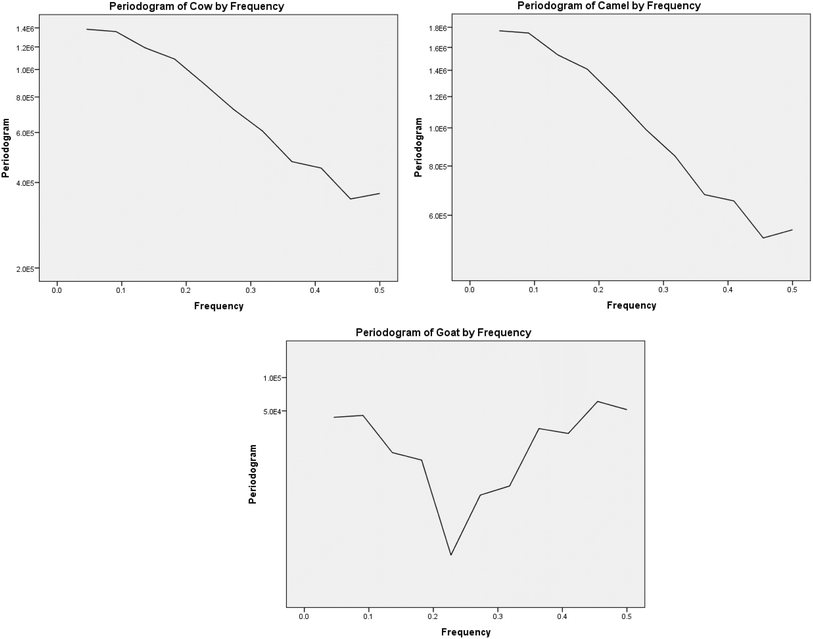
Fig. 5 shows the three periodograms display the frequency spectra for the cow, camel, and goat data. A periodogram reveals the strength of various frequency components in a time series. Strongly we concluded that the cow and camel share a similar spectral signature with dominance at low frequencies, while the goat exhibits a more complex frequency pattern. These differences could be attributed to distinct behavioral or physiological processes underlying the time series data for each animal.
4. Conclusion and recommendations
Meat and meat products are crucial components of the human diet, necessitating an understanding of their elemental content, especially those posing health risks due to toxicity, bioaccumulation, and bio-magnification. Camel meat offers distinct advantages over cow and goat meats due to its high fiber content, rich mineral profile, and elevated levels of beneficial components like vitamins D, E, K, and omega-3 fatty acids. These attributes make it particularly valuable in reducing the risks of obesity, cancer, type-II diabetes, cardiovascular diseases, and bowel disorders. Additionally, its low levels of heavy metals, combined with its superior nutritional composition, position camel meat as a healthier and safer option for regular consumption. This study compared the quality of fresh camel, cow, and goat meats by evaluating their chemical compositions, including pH, moisture, protein, fat, ash, crude fiber, vitamins (A, E, and D), and fourteen metals (Na, K, Ca, Cu, Cr, Se, Fe, Mn, Zn, Mo, Co, Cd, Ni, and Pb) using standard procedures.Key findings are high pH, fat content, Na and fiber in camel meat with enrich amount of essential vitamins D, E, K and omega 3- fatty acids. But low level of heavy metals presented except Cu even no toxic. In contrast, heavy metals are under permissible limits in both meats of cow and goat. Significant results showed at moisture, Cr, ash content, and minimal concentrations of Pb, Cd and Mo in cow meat. However in goat meat, high K, Zn, Fe, Mn and protein observed at maximum.
•Camel meat's high fiber content and rich minerals contribute to its beneficial physicochemical properties, potentially reducing risks of obesity, cancer, type-II diabetes, cardiovascular diseases, and bowel disorders.
•Cow meat's high moisture content aids muscle protein synthesis, and its balanced ash content ensures safety from toxic minerals.
•Goat meat's high potassium content supports muscle function, fluid balance, blood pressure, and bone health.
•Despite the presence of heavy metals, their concentrations in these meats are minimal, posing negligible health risks.
•Essential minerals like chromium and zinc regulate blood sugars and cholesterol, selenium aids DNA synthesis and cell protection, iron boosts immunity and oxygen transport, and manganese supports tissue and bone formation.
•Regular monitoring of copper levels in these meats is recommended to ensure safety.
•The consumption of camel, cow, and goat meats is recommended for their nutritional benefits, provided that heavy metal concentrations remain within safe limits.
•Further studies are needed to analyze additional vitamins in these meats to better understand their nutritional impact on human health.
Conflicts of interest
Authors declare that no conflicts of interest.References
- United Nations, Global Report of Food Crisis, World Food Program (WFP). Italy, Rome, 2017 Search PubMed.
- D. I. Ekine, C. O. Albert and T. A. Peregba, Expenditure pattern for beef consumption in selected households in Southern Nigeria, Dev. Ctry. Stud., 2012, 2(7), 1–5 Search PubMed.
- A. E. D. Bekhit and M. M. Farouk, Nutritive and Health Value of Camel Meat. Camel Meat and Meat Products, CBI Publishing, 2013. pp. 205–223 Search PubMed.
- M. Y. Kurtu, An assessment of the productivity for meat and the carcass yield of camels (Camelus dromedarius) and the consumption of camel meat in the eastern region of Ethiopia, Trop. Anim. Health Prod., 2004, 36(1), 65–76 CrossRef CAS PubMed.
- M. Anaeto, J. A. Adeyeye, G. O. Chioma, A. O. Olarinmoye and G. O. Tayo, Goat products: meeting the challenges of human health and nutrition, Agric. Biol. J. North Am., 2010, 1(6), 1231–1236 CrossRef.
- S. M. S. Abd-Allah, H. A. M. A. Ismail and M. A. S. Ahmed, A comparative study of some nutritional aspects of camel and cattle meats and the effect of chilling and freezing storage on meat lipid peroxidation, Int. J. Agric. Food Sci., 2018, 5(7), 1–22 Search PubMed.
- B. F. Muhammad, A. B. Mahmud and A. Mustapha, Effect of processing method on composition and consumer acceptability of camel (Camelus dromedarious) meat and beef, Niger. J. Anim. Prod., 2011, 38(1), 135–144 CrossRef.
- S. A. A. Mohammed, A study of cholesterol concentrations of camel meat and beef, Int. J. Agric. Res., 2019, 7(4), 397–401 Search PubMed.
- S. Cristofaneli, M. Antonini, D. Torres and C. Renieri, Meat and carcass quality from Peruvian llama (Lama glama) and alpaca (Lama pacos), Meat Sci. J., 2004, 66, 589–593 Search PubMed.
- K. Ronald, Influence of Ultimate pH on Meat Quality and Consumer Purchasing Decisions, 5M Publishing, 2005 Search PubMed.
- G. A. Ibrahim and I. A. Nour, Physical and chemical properties of camel meat burgers, J. Camelid Sci., 2010, 3, 39–43 Search PubMed.
- E. A. Elgasim and G. A. Elhag, Carcass characteristics of the Arabian camel, Camel News Letter, 1982, 4, 523–529 Search PubMed.
- I. T. Kadim, A. Al-Karousi, O. Mahgoub, W. Al-Marzooqi, S. K. Khalaf and R. S. Al-Maqbali, et al., Chemical composition, quality, and histochemical characteristics of individual dromedary camel (Camelus dromedarius) muscles, Meat Sci, 2013, 93(3), 564–571 CAS.
- P. G. V. Nair and S. Mathew, Biochemical Composition of Fish and Shellfish, Central Institute of Fisheries Technology, Cochin, 2001 Search PubMed.
- B. E. Emmanuel, C. Oshionebo and N. F. Aladetohun, Comparative analysis of the proximate composition of Tarpon atlanticus and Clarias gariepinus from culture systems in South-Western Nigeria, Afr. J. Food, Agric., Nutr. Dev., 2011, 11, 5344–5359 Search PubMed.
- R. B. Shirley and C. M. Parsons, Effect of ash content on protein quality of meat and bone meal, Poult. Sci., 2001, 80(5), 626–632 CAS.
- W. N. Baba, N. Rasool, M. Selvamuthukumara and S. Maqsood, A review on nutritional composition, health benefits, and technological interventions for improving consumer acceptability of camel meat: an ethnic food of Middle East, J. Ethnic Foods., 2021, 8(1), 1–13 Search PubMed.
- T. N. Rawdah, M. Zamil E1-Faer and S. A. Koreish, Fatty Acid Composition of the Meat and Fat of theOne-Humped Camel (Camelus dromedarius), Meat Sci., 1994, 37, 149–155 CAS.
- A. N. Al-Owaimer, G. M. Suliman, A. S. Sami, B. Picard and J. F. Hocquette, Chemical composition and structural characteristics of Arabian camel (Camelus dromedarius) m. longissimus thoracis, Meat Sci., 2014, 96(3), 1233–1241 CAS.
- I. T. Kadim, I. S. Al-Amri, A. Y. Alkindi and Q. M. Haq, Nutritional values and health benefits of dromedary camel meat, Anim. Front., 2022, 12(4), 61–70 Search PubMed.
- L. E. Hernández-Castellano, A. Morales-delaNuez, I. Moreno-Indias, A. Torres, D. Sánchez-Macías, J. Capote and A. Argüello, Carcass and meat quality determination as a tool to promote local meat consumption in outermost regions of Europe, J. Appl. Anim. Res., 2013, 41(3), 269–276 Search PubMed.
- K. Pearce and R. Jacob, Saltbush lifts sheep meat vitamin content, Farming Ahead, 2004, 153, 63 Search PubMed.
- O. K. Horbańczuk and A. Wierzbicka, Technological and nutritional properties of ostrich, emu, and rhea meat quality, J. Vet. Res., 2016, 60(3), 279–286 CrossRef.
- ATSDR. Agency for Toxic Substances and Diseases Registry, Division of Toxicology, Clifton Road, NE Atlanta, CA, 2004, available from: http://www.atsdr.cde.goo/toxprotiles Search PubMed.
- A. J. Garmyn, G. G. Hilton, R. G. Mateescu, J. B. Morgan, J. M. Reecy, R. G. Tait Jr, D. C. Beitz, Q. Duan, J. P. Schoonmaker, M. S. Mayes, M. E. Drewnoski, Q. Liu and D. L. VanOverbeke, Estimation of relationships between mineral concentration and fatty acid composition of longissimus muscle and beef palatability traits, J. Anim. Sci., 2011, 89(9), 2849–2858 CrossRef CAS PubMed.
- F. Fervenza, T. Tsao and R. Rabkin, Paradoxical body and kidney growth in potassium deficiency, Ren. Fail., 2001, 23(3–4), 339–346 CrossRef CAS PubMed.
- L. D. Campbell and W. K. Roberts, The requirements and role of potassium in ovine nutrition, Can. J. Anim. Sci., 1965, 45(3), 147–156 CrossRef CAS.
- J. T. Dwyer, M. F. Picciano, J. M. Betz, K. D. Fisher, L. G. Saldanha and E. A. Yetley, et al., Progress in development of an integrated dietary supplement ingredient database at the NIH Office of Dietary Supplements, J. Food Compos. Anal., 2006, 19, S108–S14 CrossRef PubMed.
- E. Veiseth, S. D. Shackelford, T. L. Wheeler and M. Koohmaraie, Factors regulating lamb longissimus tenderness are affected by age at slaughter, Meat Sci, 2004, 68(4), 635–640 CrossRef CAS PubMed.
- G. Xiang, S. Wen, X. Jiang, X. Liu and L. He, Determination of trace copper (II) in food samples by Flame Atomic Absorption Spectrometry after cloud point extraction, Iran. J. Chem. Chem. Eng., 2011, 30(3), 101–107 CAS.
- M. Malede, M. Tefera and B. Mehari, Trace metals in the leaves of selected plants used to treat Hepatitis in Dembia, Ethiopia, J. Herbs, Spices Med. Plants, 2020, 26(1), 101 CrossRef CAS.
- S. Oe, K. Miyagawa, Y. Honma and M. Harada, Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease, Exp. Cell Res., 2016, 347(1), 192–200 CrossRef CAS PubMed.
- I. C. Fuentealba and E. M. Aburto, Animal models of copper-associated liver disease, Comp. Hepatol., 2003, 2(1), 5 CrossRef PubMed.
- X. Wang, et al., Spatial analysis of heavy metals in meat products in China during 2015–2017, Food Control, 2017, 174–180 Search PubMed.
- Agency for Toxic Substances and Disease Registry (ATSDR), Toxicologic Profile for Selenium, US Department of Health and Human Services, Public Health Service, Atlanta, GA, 2003 Search PubMed.
- Institute of Medicine (IOM), Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc, National Academies Press, Washington, DC, 2001 Search PubMed.
- A. M. Fan and K. W. Kizer, Selenium. Nutritional, toxicologic, and clinical aspects, West. J. Med., 1990, 153(2), 160 CAS.
- A. J. Colter and B. G. Mahler, Iron in Drinking Water. University of Idaho, 2006 Search PubMed.
- S. Montes, H. Riojas-Rodriguez, E. Sabido-Pedraza and C. Rios, Biomarkers of manganese exposure in population living close to a mine and mineral processing plant in Mexico, Environ. Res., 2008, 106, 89–95 CrossRef CAS PubMed.
- H. K. Hudnell, Effects from environmental Mn exposures, a review of the evidence from non-occupational exposure studies, Neurotoxicology, 1999, 20, 379–397 CAS.
- M. D. Taylor, K. M. Erikson, A. W. Dobson, V. A. Fitsanakis, D. C. Dorman and M. Aschner, Effects of inhaled manganese biomarkers of oxidative stress in the rat brain, Neurotoxicology, 2006, 2, 788–797 CrossRef PubMed.
- M. Malakootian, M. Tahergorabi, M. Daneshpajooh and K. Amirtaheri, Determination of Pb, Cd, Ni, and Zn concentrations in canned fish in southern Iran, Sacha J. Environ. Stud., 2011, 1, 94–100 Search PubMed.
- A. Bernard, Cadmium and its adverse effects on human health, Indian J. Med. Res., 2008, 128, 557–564 CAS.
- A. Gregoriadou, K. Delidou, D. Dermosonoglou, P. P. Tsoum, C. Edipidi and B. Katsougiannopoulos, Heavy metals in drinking water in Thessaloniki area, Greece, in. Proceedings of the 7th International Conference on Environmental Hazards Mitigation, Cairo University, Egypt, 2001, pp. 542–556 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07096h |
| This journal is © The Royal Society of Chemistry 2025 |