Open Access Article
Open Access ArticleUnlocking dual Mn3+/Mn4+ emissions in garnet phosphors for WLED and plant growth lighting applications†
Khuat Thi Thua,
Vu Dinh Huana,
Nguyen Tu *b,
Do Quang Trung
*b,
Do Quang Trung b,
Nguyen Van Dub,
Nguyen Van Quangc,
Ta Ngoc Bachd,
Le Tien Hae,
Nguyen Duy Hungf,
Dao Xuan Vietf,
Nguyen Tri Tuang,
Nguyen Minh Hieu
b,
Nguyen Van Dub,
Nguyen Van Quangc,
Ta Ngoc Bachd,
Le Tien Hae,
Nguyen Duy Hungf,
Dao Xuan Vietf,
Nguyen Tri Tuang,
Nguyen Minh Hieu a,
Manh Trung Tran
a,
Manh Trung Tran *a and
Pham Thanh Huy
*a and
Pham Thanh Huy a
a
aFaculty of Materials Science and Engineering, Phenikaa University, Yen Nghia, Ha-Dong District, Hanoi 10000, Vietnam. E-mail: trung.tranmanh@phenikaa-uni.edu.vn
bFaculty of Fundamental Science, Phenikaa University, Yen Nghia, Ha-Dong District, Hanoi 10000, Vietnam. E-mail: tu.nguyen@phenikaa-uni.edu.vn
cDepartment of Chemistry, Hanoi Pedagogical University, 2, Phuc Yen, Vinh Phuc, Vietnam
dInstitute of Materials Science, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet Street, Cau Giay District, Hanoi 10000, Vietnam
eInstitute of Science and Technology, TNU-University of Sciences, Thai Nguyen, 250000, Vietnam
fFaculty of Electronic Materials and Components, School of Materials Science and Engineering, Hanoi University of Science and Technology (HUST), 01 Dai Co Viet Street, Hanoi 10000, Vietnam
gCollege of Science, Cantho University, 3/2, Ninh Kieu, Cantho 94000, Vietnam
First published on 17th March 2025
Abstract
Garnet-based lattices (A3B5O12) have emerged as promising hosts for Mn co-doping due to their wide band gap and robust mechanical properties. However, challenges in stabilizing Mn3+, balancing Mn3+/Mn4+ emissions, and optimizing synthesis for thermal stability and efficiency have limited practical applications. In this study, Gd3Ga5O12 (GGG) garnet was synthesized with Mn3+ and Mn4+ co-doping via a simple solid-state reaction. The incorporation of Mn ions into octahedral [GaO6] sites and optimizing the synthesis condition to enhance photoluminescence (PL) properties were investigated. The phosphor achieved 100% color purity, 0.32 eV activation energy, 20.0% internal quantum efficiency, and a 0.0408 ms lifetime. A prototype pc-LED was fabricated, demonstrating that the Mn3+/Mn4+-doped Garnet-based phosphor can be used as potential WLED and plant growth LED components. These results provide insights into stabilizing Mn3+, controlling Mn3+/Mn4+ balance, and improving thermal stability for advanced lighting applications.
1. Introduction
Lighting plays a pivotal role in modern society, influencing a wide range of domains, including industry, broad-based applications, and daily human activities. Among the most notable technological advancements of the 21st century are light-emitting diodes (LEDs) utilizing phosphor materials, celebrated for their numerous advantages.1,2 Commercial white light-emitting diodes (WLEDs) based on YAG:Ce3+ phosphor coated on GaN chip LED (460 nm) exhibit a deficiency in the red region of the emission spectrum. This limitation results in a low color rendering index (CRI) and a highly correlated color temperature (CCT).3,4 To address these challenges and achieve a full-visible-spectrum emission in WLEDs, extensive research has focused on developing red-emitting phosphors.5–7 Notable examples include Li2Ca2Mg2Si2N6:Eu2+,8 which emits at 638 nm and enhances the CRI of WLEDs to as high as 91, as well as Ca2LuSbO6:Eu3+ (λem = 613 nm)9 and Sr2LiScB4O10: Eu3+ (λem = 617 nm).10 However, despite the success of Eu3+-doped materials, recent research increasingly focuses on Mn-based ions, particularly Mn3+ and Mn4+, which offer unique advantages for red-emission phosphors due to their partially filled d-orbitals. Mn3+ (with d4 configuration) exhibits red and far-red emission via 5E′ and 5E′′ transitions, induced by Jahn–Teller splitting of the 2E ground state.11–13 In contrast, Mn4+ (with a d3 configuration) emits deep red light through 2E → 4A2 and 4T2 → 4A2 transitions.14,15 Co-doping these ions is particularly promising, as their complementary emission properties enable the creation of a broader and tunable red-emission spectrum. The fact that Mn is cost-effective and environmentally friendly further enhances its appeal. However, challenges remain in controlling the emission profiles of Mn3+ and Mn4+, which are highly sensitive to fabrication conditions. A major challenge in achieving the coexistence of Mn3+ and Mn4+ in phosphor materials is the precise regulation of manganese valence states during synthesis.16,17 Mn3+ is particularly unstable, as it readily oxidizes to Mn4+ under oxidizing conditions or reduces to Mn2+ in a reducing environment.18,19 This instability complicates the control of the Mn3+/Mn4+ ratio, which is essential for fine-tuning the red emission properties of the phosphor.Garnet-based lattices (A3B5O12) are highly promising for Mn co-doping due to their wide band gap and excellent mechanical properties. Among these, Y3Al5O12 (YAG) has been extensively studied as a host for Mn4+, which can substitute for Al3+ in octahedral sites with minimal lattice distortion.20 In contrast, Gd3Ga5O12 garnet, despite its similar structural properties, has received relatively little attention.21 The Gd3Ga5O12 lattice, characterized by Ga3+ ions (r = 0.62 Å, CN = 6), is well-suited to accommodate both Mn3+ ions (r = 0.65 Å, CN = 6)22,23 and Mn4+ ions (r = 0.53 Å, CN = 6),22,24 making it an ideal candidate for co-doping.13 Incorporating both Mn3+ and Mn4+ into the Gd3Ga5O12 lattice enables the exploration of their unique emission characteristics and offers the potential to fine-tune the red-emission spectrum by controlling the Mn3+/Mn4+ ratio.25 Studies by L. Marciniak and K. Trejgis have investigated this co-doping strategy Mn3+ and Mn4+ in the Gd3Ga5O12 lattice,11 while S. Kuck analyzed the PL properties of Mn3+-doped Gd3Ga5O12.13 Despite progress, the full potential of Gd3Ga5O12:Mn for LEDs remains unrealized, with challenges in balancing Mn3+/Mn4+ emissions and optimizing synthesis for red emission, stability, and efficiency.11 Precise annealing, charge compensation, and non-radiative loss suppression are crucial to stabilizing Mn3+ and advancing Mn-doped garnet phosphors for next-generation WLEDs.
This study employs a simple solid-state reaction technique to synthesize the dual red and far-red emitting (Mn3+, Mn4+)-activated Gd3Ga5O12 phosphors. The effects of experimental conditions on crystal structure, morphology, elemental composition, bonding characteristics, and photoluminescent properties of (Mn3+, Mn4+)-activated Gd3Ga5O12 phosphors are systematically investigated. Key performance metrics are evaluated, including color purity, lifetime, thermal stability, and internal quantum efficiency of the synthesized phosphors. Additionally, a prototype-tested LED device was successfully fabricated by coating a layer of the optimized phosphor onto the surface of a NUV-chip LED (310 nm).
2. Experimental
2.1. Synthesis of the Gd3Ga5O12:Mn phosphors
A series of Gd3Ga5O12:x%Mn (x = 0.2–2.0) was fabricated by a simple solid-state reaction method. The starting materials included Gd2O3 (99.99% purity, Merck), Ga2O3 (99.99% purity, Merck), and MnCl2·4H2O (99.9% purity, Merck) were magnetically stirred in 100 ml of deionized water at room temperature. The mixture was then dried at 150 °C in the air for 10 hours, ground by a high-energy planetary ball milling at 200 rpm for 30 minutes, and annealed at 1000–1300 °C for 5 hours in the air to receive the final Gd3Ga5O12:x%Mn phosphors. The maximum annealing temperature was set at 1300 °C, as higher temperatures lead to melting phenomena and partial decomposition of the garnet phase into perovskite (REGaO3) and Ga2O3 liquidus, compromising phase purity and structural integrity.26 The experimental process is illustrated in Fig. S1.†2.2. Characterization
The crystal structure of the samples was analyzed by X-ray diffraction (XRD) patterns measured over a 2θ range of 25° to 75° with a D8 Advance diffractometer. High-resolution transmission electron microscopy (HRTEM) was performed on a JEM2500SE microscope (JEOL) operating at 200 kV to provide further structural insights. Structural visualization was created using VESTA software. The samples' surface morphology and element composition were examined using field emission scanning electron microscopy (FESEM) images and the energy dispersion spectrum (EDS) using JSM-7600F equipment. Bonding energies of the ions were characterized by X-ray photoelectron spectroscopy (XPS) using a Thermo Scientific XPS system with an Al Kα X-ray source. The optical properties of the samples were evaluated through PL and photoluminescence excitation (PLE) spectra, obtained with a NanoLog spectrophotometer (Horiba) equipped with a 450 W xenon discharge lamp as the excitation source. The PL decay times were measured using a fluorescence spectrophotometer (Agilent Cary Eclipse).2.3. LED packaging
The optimized Gd3Ga5O12:Mn phosphors were used to produce a prototype of an LED device. Initially, a polydimethylsiloxane (PDMS) solution (Dow Corning OE-7340 Optical Encapsulant) was obtained by blending an elastomer and a hardener in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Then, the PDMS and the optimized phosphor were combined at a mass ratio of 1
1 ratio. Then, the PDMS and the optimized phosphor were combined at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and thoroughly mixed using a planetary mixer (Kurabo Mazerustar KK-V300SS) for 270 seconds. The resulting mixture was then applied to the top surface of a 310 nm LED chip using an i-DRS310A Desktop Dispensing System. Finally, the phosphor-coated LED was cured in an oven (ASONE AVO-310SB-D) at 150 °C for 4 hours to remove any residual solvents. The optical properties of the fabricated LED chip were characterized using a Gamma Scientific GS-1290 spectroradiometer (RadOMA).
4 and thoroughly mixed using a planetary mixer (Kurabo Mazerustar KK-V300SS) for 270 seconds. The resulting mixture was then applied to the top surface of a 310 nm LED chip using an i-DRS310A Desktop Dispensing System. Finally, the phosphor-coated LED was cured in an oven (ASONE AVO-310SB-D) at 150 °C for 4 hours to remove any residual solvents. The optical properties of the fabricated LED chip were characterized using a Gamma Scientific GS-1290 spectroradiometer (RadOMA).
3. Results and discussion
3.1. XRD and HRTEM analysis
Fig. 1a shows the XRD patterns of the Gd3Ga5O12:0.2%Mn samples annealed at different temperatures in the air for 5 hours. At an annealing temperature of 1100 °C, the XRD pattern reveals the initial formation of the Gd3Ga5O12 phase. As the annealing temperature increases, the intensity of the diffraction peaks associated with the Gd3Ga5O12 phase becomes more pronounced, accompanied by a narrowing of the peaks. These indicate a significant improvement in crystalline with increasing temperature. At 1300 °C, all diffraction peaks corresponding to the cubic Gd3Ga5O12 phase (PDF # 13-0493) are distinctly observed at 2θ = 26.54°, 28.68°, 31.96°, 35.06°, 39.49°, 45.65°, 50.53°, 52.79°, 54.97°, 59.13°, 67.00°, 68.81°, 70.71°. The peaks correspond to the (321), (400), (420), (422), (521), (611), (444), (640), (642), (800), (840), (842), (664) crystal planes, respectively.27,28 The above result confirms that the single-phase Gd3Ga5O12 is most effectively stabilized at 1300 °C compared to lower annealing temperatures.Fig. 1b shows the XRD patterns of the Gd3Ga5O12:x%Mn (x = 0.0–2.0) samples, annealed at 1300 °C in air for 5 hours. Notably, no significant differences in the XRD patterns suggest that doping concentration does not affect the crystal structure of Gd3Ga5O12.29–31 Fig. 1c illustrates the cubic structure of the Gd3Ga5O12 lattice, visualized using VESTA software. It is well-known that the difference in radii between the dopant and substituted ions (Dr) can be calculated using the following eqn (1):32
 | (1) |
Fig. 2 shows the Rietveld refinement of the Gd3Ga5O12:x%Mn (x = 0.0–2.0) phosphors annealed at 1300 °C for 5 hours. Table 1 demonstrates a strong agreement between the theoretical and experimental data, with low χ2 values ranging from 1.14 to 1.45. As Mn doping increases, both the lattice parameter, a, and cell volume, V, increase from 12.3756 Å and 1895.3909 Å3 at 0.0% Mn to 12.3766 Å and 1895.8504 Å3 at 2.0% Mn. Furthermore, the refinement indicators Rp and Rwp decrease higher Mn doping levels, indicating improved refinement accuracy. These findings confirm the reliability of the Rietveld analysis and align with the X-ray diffraction results, validating the structural modifications in the Gd3Ga5O12 lattice caused by Mn doping.
 | ||
| Fig. 2 Rietveld refinement for the Gd3Ga5O12:x%Mn (x = 0.0–2.0) phosphors annealed at 1300 °C for 5 hours in air. | ||
| Doping concentration (mol%) | Rp (%) | Rwp (%) | χ2 | Lattice parameter a = b = c (Å) | Cell volume V (Å3) |
|---|---|---|---|---|---|
| 0.0% | 116 | 61.7 | 1.14 | 12.3756 | 1895.3909 |
| 0.2% | 62.1 | 47.9 | 1.42 | 12.3760 | 1895.5747 |
| 0.6% | 58.2 | 45.9 | 1.41 | 12.3759 | 1895.5287 |
| 1.0% | 55.1 | 46.0 | 1.35 | 12.3763 | 1895.7125 |
| 1.5% | 68.4 | 50.9 | 1.41 | 12.3765 | 1895.8045 |
| 2.0% | 68.0 | 51.4 | 1.45 | 12.3766 | 1895.8504 |
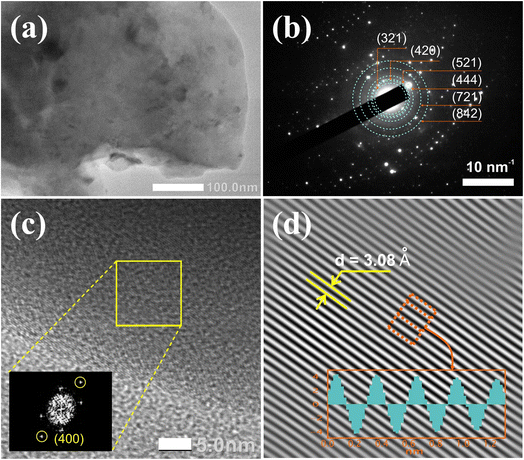
Fig. 3a–d presents the TEM image, selected area electron diffraction (SAED) pattern, and high-resolution TEM (HRTEM) image of the Gd3Ga5O12:0.2%Mn sample annealed at 1300 °C for 5 hours in the air. The SAED pattern in Fig. 3b shows diffraction rings corresponding to the crystallite planes of the Gd3Ga5O12 structure. The fast Fourier transform (FFT) image in the inset of Fig. 3c reveals symmetric point pairs that align with the Gd3Ga5O12 phase. In Fig. 3d, the inverse fast Fourier transform (IFFT) of the marked point pair indicates an orderly atomic arrangement with a d-spacing of 0.308 nm, corresponding to the (400) plane of the Gd3Ga5O12.31,39 Additionally, the XRD data demonstrates the presence of the (400) plane with an interplanar spacing of d = 3.09 Å, which aligns well with previous reports.29–31 These results confirm that the synthesized phosphor is a single-phase Gd3Ga5O12 structure.
3.2. FESEM image and EDS spectra investigation
Fig. 4a–d shows the FESEM images of Gd3Ga5O12:0.2%Mn samples annealed at various temperatures in air for 5 hours. As the annealing temperature increases, the particle size grows, reflecting enhanced crystallization of the Gd3Ga5O12 structure.1 The sample annealed at 1300 °C shows the largest particles, approximately 2 μm, making it suitable for LED chip fabrication.37 The EDS spectrum (Fig. 4e) confirms the presence of Gd (14.06 at%), Ga (23.33 at%), O (62.50 at%), and Mn (0.11 at%), with no impurities detected. Elemental mapping (Fig. 4f–k) corroborates these findings, revealing a uniform distribution of these elements across the sample. These results confirm that the sample achieved high purity and a particle size closely aligned with the standard requirements for phosphor applications.3.3. XPS spectra investigation
Fig. 5 presents the XPS spectra of the Gd3Ga5O12:Mn sample, providing a comprehensive analysis of the chemical composition and valence states of the constituent elements. The survey spectrum (Fig. 5a) confirms the presence of all expected elements without detectable impurities, consistent with the earlier elemental mapping results.30 The C 1s spectrum, peaking at 285.7 eV (Fig. 5b), serves as a reference for energy shift corrections.40,41 The Gd 4d spectrum (Fig. 5c) shows two prominent peaks at 147.6 eV and 142 eV, representing Gd 4d3/2 and Gd 4d5/2 states of Gd3+ ion.42 The Gd 4d5/2 state bonding energy can be decomposed into five peaks at 145.8 eV, 144.8 eV, 143.9 eV, 142.5 eV and 141.5 eV, attributed to the interaction between the 4d photoholes and valence electrons of the 4f7 level.42–44 The Ga 2p spectrum (Fig. 5d) reveals two peaks at 1117.9 eV and 1144.8 eV, corresponding to the Ga 2p3/2 and Ga 2p1/2, respectively.30,45 The O 1s spectrum (Fig. 5e) consists of three components with peaks at 530.3 eV, 528.7 eV, and 528.6 eV, representing oxygen bonding states associated with the measurement environment, Ga–O and Gd–O bonds, respectively.30,46 The Mn 2p spectrum (Fig. 5f) reveals two bands, deconvoluted into four peaks at 641.8 eV, 645.4 eV, 653.0, and 654.3 eV.47,48 The peaks at 641.8 eV and 653.0 eV correspond to the 2p3/2 and 2p1/2 states of Mn3+ ions, while the peaks at 645.4 eV and 654.3 eV originated from the 2p3/2 and 2p1/2 states of Mn4+ ions.47,48 These results indicate the coexistence of Mn3+ and Mn4+ oxidation states in the Gd3Ga5O12 lattice. | ||
| Fig. 5 XPS spectra of Gd3Ga5O12:0.2%Mn sample: (a) survey spectrum, (b) C 1s, (c) Gd 4d, (d) Ga 2p, (e) O 1s and (f) Mn 2p. | ||
3.4. Photoluminescence study
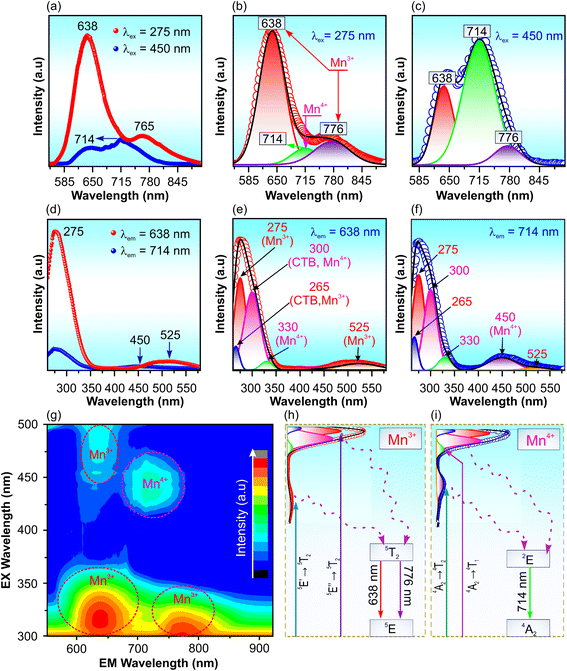
Fig. 6a shows the PL spectra of Gd3Ga5O12:0.2%Mn sample annealed at 1300 °C for 5 hours under excitation at 275 and 450 nm, revealing three deconvoluted emission peaks at 638, 714, and 776 nm, as shown in Fig. 6b and c. The peaks at 638 and 776 nm correspond to the 5T2 → 5E′ and 5T2 → 5E′′ transitions of the Mn3+ ion, respectively, while the 714 nm peak arises from the 2E → 4A2 transition of Mn4+ ions in the Gd3Ga5O12 lattice.11–13 This assignment is further substantiated by the presence of fine structure (zero-phonon lines and vibrionics) in the PL spectrum of Mn4+ ions (Fig. 6c), which is a defining characteristic of the 2E → 4A2 transition in Mn4+.33 The PLE spectra recorded at 638 and 714 nm, shown in Fig. 6d, reveal absorption bands in the NUV and blue-green regions, with distinct peaks at 265, 275, 300, 330, 450, and 525 nm (Fig. 6e and f). The observed Stokes shift from the 4A2 → E2 absorption band further confirms the Mn4+ luminescence characteristics.34 The peaks at 265 and 300 remain attributed to O2− → Mn3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 and O2− → Mn4+
13 and O2− → Mn4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33,34 charge-transfer transition, respectively. Meanwhile, the peaks at 275, 525 and 330 nm in Fig. 6e are assigned the 5E′′ → 5T2 transition, 5E′ → 5T2 transition of Mn3+ ions13,22 and 4A2 → 4T1 transition of Mn4+ ions. The PLE spectrum measured at 714 nm (Fig. 6f) exhibits six fitted peaks at 265, 275, 300, 330, 450 and 525 nm. The 275 and 525 nm peaks reaffirm the Mn3+ transitions (5E′′ → 5T2 and 5E′ → 5T2, respectively),13,22 whereas the 330 and 450 nm peaks are associated with the 4A2→ 4T1 and 4A2 → 4T2 transitions of Mn4+ ions.33,34 These findings confirm that the observed red and far-red emissions originate from the coexistence of Mn3+ and Mn4+ ions in the lattice. To further investigate the optical properties of the Gd3Ga5O12:Mn sample, a 3D spectrum was measured (Fig. 6g). The results indicate that under UV excitation, the PL intensity governed by Mn3+, while blue-light excitation predominantly enhances Mn4+ ions emissions. This suggests that the red and far-red emission regions can be tuned by varying the excitation conditions. Finally, a proposed energy-level scheme is provided in Fig. 6h and i that illustrates the absorption and emission mechanisms of Mn3+ and Mn4+ ions in the Gd3Ga5O12 lattice.
33,34 charge-transfer transition, respectively. Meanwhile, the peaks at 275, 525 and 330 nm in Fig. 6e are assigned the 5E′′ → 5T2 transition, 5E′ → 5T2 transition of Mn3+ ions13,22 and 4A2 → 4T1 transition of Mn4+ ions. The PLE spectrum measured at 714 nm (Fig. 6f) exhibits six fitted peaks at 265, 275, 300, 330, 450 and 525 nm. The 275 and 525 nm peaks reaffirm the Mn3+ transitions (5E′′ → 5T2 and 5E′ → 5T2, respectively),13,22 whereas the 330 and 450 nm peaks are associated with the 4A2→ 4T1 and 4A2 → 4T2 transitions of Mn4+ ions.33,34 These findings confirm that the observed red and far-red emissions originate from the coexistence of Mn3+ and Mn4+ ions in the lattice. To further investigate the optical properties of the Gd3Ga5O12:Mn sample, a 3D spectrum was measured (Fig. 6g). The results indicate that under UV excitation, the PL intensity governed by Mn3+, while blue-light excitation predominantly enhances Mn4+ ions emissions. This suggests that the red and far-red emission regions can be tuned by varying the excitation conditions. Finally, a proposed energy-level scheme is provided in Fig. 6h and i that illustrates the absorption and emission mechanisms of Mn3+ and Mn4+ ions in the Gd3Ga5O12 lattice.
Fig. 7 reveals key insights into the effect of annealing temperature and doping concentration on the PL properties of Gd3Ga5O12:Mn (x = 0.2–2.0) samples. Under 275 nm excitation (Fig. 7a), Mn3+ emission becomes evident only after the annealing temperatures exceed 1000 °C, with intensity increasing at higher temperatures. The spectral contributions of Mn3+ and Mn4+ ions were deconvoluted and analyzed in Fig. S2 and Table S1 of the ESI† and presented in the insert of Fig. 7a. It demonstrates a gradual increase in Mn3+ and Mn4+ emission intensities as annealing temperature rises. This is attributed to improved crystallization and enhanced diffusion of impurity ions.49 Under 450 nm excitation (Fig. 7c), Mn4+ emission intensity dominates, and both Mn3+ and Mn4+ emissions follow a similar increasing trend with annealing temperature (Fig. S3 and Table S2 of ESI† and the inset of Fig. 7c), further supporting the temperature-dependent emission enhancement.
Doping concentration also significantly influences PL intensity. As shown in Fig. 7b and d, the highest PL intensity is achieved at an Mn doping concentration of 0.2% under 275 and 450 nm excitations. Beyond this concentration, PL intensity decreases due to the concentration quenching effect.50,51 Fitting analyses for Mn3+ and Mn4+ emissions, shown in Fig. S4 and S5 of the ESI,† confirm that the maximum PL intensity occurs at 0.2% Mn doping. Tables S3 and S4 of the ESI† present the integral intensities of deconvoluted Mn3+ and Mn4+ emissions for various Gd3Ga5O12:x% (x = 0.2–2.0) Mn samples, emphasizing the effects of annealing temperature and doping concentration on their PL properties.
3.5. Lifetime, color purity, thermal stability, and internal quantum efficiency of phosphors
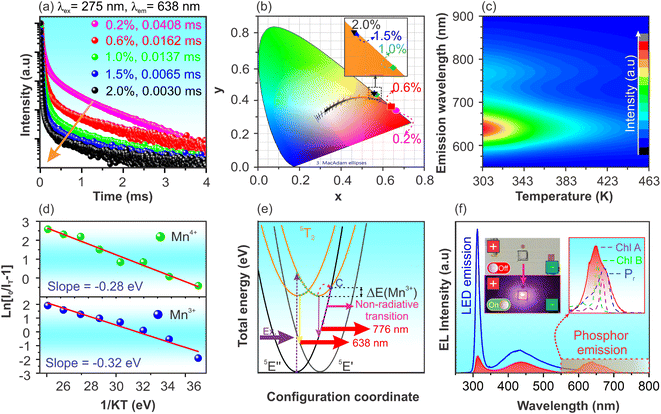
The potential application of Gd3Ga5O12:Mn phosphor was assessed by evaluating the lifetime, color purity, thermal stability, and internal quantum efficiency (IQE). Fig. 8a presents the PL decay under 275 nm excitation wavelength of Gd3Ga5O12:x%Mn (x = 0.2–2.0) phosphors. Due to the existence of two emission sources from Mn3+ and Mn4+ ions occupying different lattice sites, all data were well-fitted using a double-exponential model (see Fig. S7†), described by an eqn (2):50,51
 | (2) |
 | (3) |
Using this approach, the lifetimes of the samples were determined, with the optimized Gd3Ga5O12:0.2% Mn sample showing the highest average lifetime of 0.0408 ms. The observed decrease in lifetime with increasing Mn concentration suggests energy transfer among impurity ions, promoting phonon-assisted non-radiative decay rather than PL.52 As the dopant concentration rises, the reduced distance between Mn ions enhances non-radiative energy transfer mechanisms such as cross-relaxation and energy migration,50,51 leading to accelerated luminescence decay. Similar findings are also observed in the PL decay for the 714 nm peak under 450 nm excitation in Gd3Ga5O12:x% Mn (x = 0.2–2.0) samples (Fig. S6 and S8†).
The chromatic coordinates (x, y) of Gd3Ga5O12:x%Mn (x = 0.2–2.0) samples annealed at 1300 °C for 5 hours are shown in Fig. 8b, based on the CIE 1931 diagram. The calculated chromatic coordinates (x, y) of samples are listed in Table 2. As shown in Fig. 8b, the samples display red chromatic coordinates close to the boundary of the CIE diagram, demonstrating high color purity. To quantitatively confirm this, the color purity of the sample was further quantified using eqn (4):53
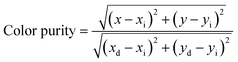 | (4) |
| Mn concentration (mol%) | Chromaticity coordinates (x; y) | Color purity (%) | Lifetime (ms) |
|---|---|---|---|
| 0.2 | (0.6617; 0.3380) | 100 | 0.0408 |
| 0.6 | (0.6391; 0.3603) | 99.9 | 0.0162 |
| 1.0 | (0.5666; 0.4322) | 99.8 | 0.0137 |
| 1.5 | (0.5579; 0.4409) | 99.8 | 0.0065 |
| 2.0 | (0.5573; 0.4415) | 99.8 | 0.0030 |
The temperature-dependent PL spectra of the optimized Gd3Ga5O12:0.2%Mn phosphor ranging from 300 to 463 K are shown in Fig. 8c. A significant decline in PL intensity occurs as the temperature rises, primarily due to thermal quenching.54,55 The PL spectra measured at various temperatures were also fitted and present in Fig. S9 and Table S5 of the ESI.† At 423 K (150 °C), the PL intensities of Mn3+ and Mn4+ emissions retain approximately 21.9% and 10.0% of their initial values at 300 K, respectively. To better understand this behavior, the activation energy (Ea) of the sample was estimated using eqn (5):2,56
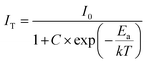 | (5) |
 | (6) |
A prototype pc-LED was obtained by applying the optimized phosphor coating onto the surface of a 310 nm LED chip. The electroluminescence (EL) spectra of the phosphor-coated and uncoated pc-LED device are shown in Fig. 8f. The EL spectrum of the uncoated LED chip displays two emission regions, with peaks at 310 and 438 nm attributed to the emission from the LED chip and the oxidation layer on its surface. In contrast, the EL spectrum of the phosphor-coated LED shows an additional red emission region resulting from the emission of the Gd3Ga5O12:Mn phosphor. The phosphor's internal quantum efficiency (IQE) could be estimated by eqn (7).61,62
 | (7) |
| Phosphors | Excitation wavelength (nm) | Emission wavelength (nm) | Quantum efficiency (QE%) | Ref. |
|---|---|---|---|---|
| Gd3Ga5O12:Mn3+ | — | — | <5 | 38 |
| Gd3Ga5O12:Mn3+, Mn4+ | 18.2 | 11 | ||
| Gd3Al5O12:Mn3+, Mn4+ | — | — | 19.0 | 11 |
| Y3Al5O12:Mn3+, Mn4+ | — | — | 22.6 | 11 |
| YAlO3:Mn3+, Mn4+ | — | — | 17.8 | 11 |
| Lu3Ga5O12:Mn3+, Mn4+ | — | — | 20.9 | 11 |
| Gd3Al3Ga2O12:Mn | 450 | 628 | 25.9 | 63 |
| Gd3GaAl4O12:Mn | 333, 468 | 630 | 22.4 | 31 |
| Gd3Al2Ga3O12:Mn | 450 | 628 | 8.4 | 63 |
| Gd3Al4Ga1O12:Mn | 450 | 628 | 29.6 | 63 |
| Gd3Ga5O12:Mn | 310 | 638 | 20.0 | This work |
4. Conclusions
In conclusion, this study successfully synthesized Mn3+/Mn4+-co-doped Gd3Ga5O12 phosphors, confirming Mn incorporation into the host lattice and demonstrating broad red-to-far-red emission. The optimized phosphor, Gd3Ga5O12:0.2%Mn annealed at 1300 °C, exhibited a long lifetime (0.0408 ms), high color purity (100%), significant activation energy (0.32 eV), and a quantum efficiency of 20.0%. The luminescence properties were strongly influenced by Mn oxidation states and synthesis conditions, highlighting the importance of precise control over the doping process. A pc-LED prototype incorporating the synthesized phosphor demonstrated its versatility for high-quality white lighting and plant growth applications. Its dual-excitation capability (275 nm and 450 nm) enables flexible integration, enhancing WLED CRI and optimizing deep-red emission for horticultural lighting. These findings support the development of next-generation Mn-doped phosphors with improved stability, efficiency, and multifunctionality.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research is supported by the Ministry of Science and Technology (Vietnam) under Grant No. ĐTĐL.CN-32/2023.References
- L. Sun, B. Devakumar, J. Liang, S. Wang, Q. Sun and X. Huang, J. Alloys Compd., 2019, 785, 312–319 CrossRef CAS.
- Y. Han, S. Wang, H. Liu, L. Shi, J. Zhang, Z. Zhang, Z. Mao, D. Wang, Z. Mu, Z. Zhang and Y. Zhao, J. Lumin., 2020, 220, 116968 CrossRef CAS.
- S. He, F. Xu, T. Han, Z. Lu, W. Wang, J. Peng, F. Du, F. Yang and X. Ye, Chem. Eng. J., 2020, 392, 123657 CrossRef CAS.
- W. Chen, Y. Cheng, L. Shen, C. Shen, X. Liang and W. Xiang, J. Alloys Compd., 2018, 762, 688–696 CrossRef CAS.
- S. Li, Y. Xia, M. Amachraa, N. T. Hung, Z. Wang, S. P. Ong and R. J. Xie, Chem. Mater., 2019, 31, 6286–6294 CrossRef CAS.
- X. Zhang, D. Zhang, Z. Zheng, B. Zheng, Y. Song, K. Zheng, Y. Sheng, Z. Shi and H. Zou, Dalton Trans., 2020, 49, 17796–17805 RSC.
- D. Wu, C. Shi, J. Zhou, Y. Gao, Y. Huang, J. Ding and Q. Wu, Chem. Eng. J., 2022, 430, 133062 CrossRef CAS.
- X. Yang, Y. Zhang, X. Zhang, J. Chen, H. Huang, D. Wang, X. Chai, G. Xie, M. S. Molokeev, H. Zhang, Y. Liu and B. Lei, J. Am. Ceram. Soc., 2020, 103, 1773–1781 CrossRef CAS.
- J. Liang, B. Devakumar, L. Sun, G. Annadurai, S. Wang, Q. Sun and X. Huang, J. Lumin., 2019, 214, 116605 CrossRef CAS.
- Q. Chen, B. Miao, P. S. Kumar and S. Xu, Opt. Mater., 2021, 116, 111093 CrossRef CAS.
- L. Marciniak and K. Trejgis, J. Mater. Chem. C, 2018, 6, 7092–7100 RSC.
- M. Czaja, R. Lisiecki, A. Chrobak, R. Sitko and Z. Mazurak, Phys. Chem. Miner., 2018, 45, 475–488 CrossRef CAS.
- S. Kück, S. Hartung, S. Hurling, K. Petermann and G. Huber, Phys. Rev. B, 1998, 57, 2203 CrossRef.
- S. V. Bulyarskiǐ, A. V. Zhukov and V. V. Prikhod’ko, Opt. Spectrosc., 2003, 94, 538–544 CrossRef.
- Y. Li, S. Qi, P. Li and Z. Wang, RSC Adv., 2017, 7, 38318–38334 RSC.
- E. Jara, R. Valiente, M. Bettinelli and F. Rodríguez, J. Phys. Chem. C, 2021, 125, 27118–27129 CrossRef CAS.
- M. Gao, Y. Pan, Y. Jin and J. Lin, RSC Adv., 2021, 11, 760–779 RSC.
- L. Wu, H. Chen, L. Wu, F. Bo, J. Jian, H. Zhang, L. Zheng, Y. Kong, Y. Zhang and J. Xu, J. Mater. Chem. C, 2019, 7, 7096–7103 RSC.
- A. Yadav, B. Tandon and A. Nag, RSC Adv., 2016, 6, 79153–79159 RSC.
- K. Trejgis and L. Marciniak, Phys. Chem. Chem. Phys., 2018, 20, 9574–9581 RSC.
- A. Suchocki, S. Biernacki, G. Boulon, A. Brenier, M. Potemski and A. Wysmołek, Chem. Phys., 2004, 298, 267–272 CrossRef CAS.
- S. Kück, S. Hartung, S. Hurling, K. Petermann and G. Huber, Spectrochim. Acta, Part A, 1998, 54, 1741–1749 CrossRef.
- S. Kück, S. Hartung, S. Hurling, K. Petermann and G. Huber, Phys. Rev. B, 1998, 57, 2203 CrossRef.
- S. V. Bulyarskiǐ, A. V. Zhukov and V. V. Prikhod’ko, J. Exp. Theor. Phys., 2001, 74, 543–546 Search PubMed.
- M. Nakayama and M. Nogami, Solid State Commun., 2010, 150, 1329–1333 CrossRef CAS.
- M. A. Digiuseppe and S. L. Soled, J. Cryst. Growth, 1980, 49, 746–748 CrossRef CAS.
- A. Luchechko, L. Kostyk, S. Varvarenko, O. Tsvetkova and O. Kravets, Nanoscale Res. Lett., 2017, 12, 1–6 CrossRef CAS PubMed.
- M. Pang and J. Lin, J. Cryst. Growth, 2005, 284, 262–269 CrossRef CAS.
- B. Zhang, Y. Feng, Y. Fu, K. Li, M. Liu, B. Zhang, Y. Zhang, J. Sun, C. Zhang, X. Qian, Z. Wang and Q. Liu, Ceram. Int., 2024, 50, 54753–54761 CrossRef CAS.
- K. Li, X. Chen, Y. Feng, Y. Fu, P. Chen, J. Lu, B. Wei, L. Zheng, J. Tao and Q. Liu, J. Alloys Compd., 2024, 979, 173582 CrossRef CAS.
- L. Dong, L. Zhang, Y. Jia, B. Shao, W. Lu, S. Zhao and H. You, ACS Appl. Mater. Interfaces, 2020, 12, 7334–7344 CrossRef CAS PubMed.
- K. Li, H. Lian, M. Shang and J. Lin, Dalton Trans., 2015, 44, 20542–20550 RSC.
- S. Adachi, ECS J. Solid State Sci. Technol., 2020, 9, 016001 CrossRef CAS.
- S. Adachi, ECS J. Solid State Sci. Technol., 2019, 8, R183 CrossRef CAS.
- H. Taguchi, K. Hirota, S. Nishihara, S. Morimoto, K. Takaoka, M. Yoshinaka and O. Yamaguchi, Phys. Rev. B:Condens. Matter Mater. Phys., 2005, 367, 188–194 CrossRef CAS.
- K. Li, H. Lian, R. Van Deun and M. G. Brik, Dyes Pigm., 2019, 162, 214–221 CrossRef CAS.
- D. Q. Trung, N. Tu, N. V. Quang, M. T. Tran, N. V. Du, P. T. Huy, N. Tu, N. V. Quang, M. T. Tran, N. V. Du and P. T. Huy, J. Alloys Compd., 2020, 845, 156326 CrossRef CAS.
- S. Kück, Appl. Phys. B:Lasers Opt., 2001, 72, 515–562 CrossRef.
- F. P. Yu, D. R. Yuan, X. L. Duan, S. Y. Guo, X. Q. Wang, X. F. Cheng and L. M. Kong, J. Alloys Compd., 2008, 465, 567–570 CrossRef CAS.
- S. T. Jackson and R. G. Nuzzo, Appl. Surf. Sci., 1995, 90, 195–203 CrossRef CAS.
- P. S. Bagus, C. Sousa and F. Illas, J. Chem. Phys., 2016, 145, 144303 CrossRef PubMed.
- N. Ullah, M. Imran, K. Liang, C. Z. Yuan, A. Zeb, N. Jiang, U. Y. Qazi, S. Sahar and A. W. Xu, Nanoscale, 2017, 9, 13800–13807 RSC.
- N. Zhang, D. Chen, F. Niu, S. Wang, L. Qin and Y. Huang, Sci. Rep., 2016, 6, 1–11 CrossRef PubMed.
- T. B. Thiede, M. Krasnopolski, A. P. Milanov, T. De Los Arcos, A. Ney, H. W. Becker, D. Rogalla, J. Winter, A. Devi and R. A. Fischer, Chem. Mater., 2011, 23, 1430–1440 CrossRef CAS.
- L. Kong, J. Ma, C. Luan, W. Mi and Y. Lv, Thin Solid Films, 2012, 520, 4270–4274 CrossRef CAS.
- P. N. Meitei, B. Moirangthem, C. Ngangbam, M. W. Alam and N. K. Singh, J. Mater. Sci.:Mater. Electron., 2022, 33, 10705–10714 CrossRef CAS.
- J. Zha, C. He, F. Chen, H. Wang, B. Dong, L. Liu, M. Xia, C. Deng, Q. Li, Y. Lu and H. Chen, J. Fluoresc., 2024, 1–12 Search PubMed.
- M. Wang, K. Chen, J. Liu, Q. He, G. Li and F. Li, Catalysts, 2018, 8, 138 CrossRef.
- M. T. Tran, D. Q. Trung, N. Tu, D. D. Anh, N. V Du, N. V Quang, N. T. Huyen, D. X. Viet, N. D. Hung and P. T. Huy, J. Alloys Compd., 2021, 884, 1–13 CrossRef.
- N. Tu, N. Van Quang, N. Van Du, D. Q. Trung, T. N. Bach, N. D. Hung, D. X. Viet, L. T. Ha, N. M. Hieu, M. T. Tran and P. T. Huy, J. Mol. Struct., 2024, 1316, 139004 CrossRef CAS.
- N. T. Huyen, N. Tu, N. Van Quang, D. Quang Trung, M. T. Tran, N. Van Du, N. D. Hung, D. X. Viet, N. D. Trung Kien and P. T. Huy, ACS Appl. Electron. Mater., 2022, 4, 4322–4331 CrossRef CAS.
- Z. Zhou, Y. Li, M. Xia, Y. Zhong, N. Zhou and H. T. (Bert) Hintzen, Dalton Trans., 2018, 47, 13713–13721 RSC.
- R. Naik, S. C. Prashantha, H. Nagabhushana, S. C. Sharma, H. P. Nagaswarupa and K. M. Girish, J. Alloys Compd., 2016, 682, 815–824 CrossRef CAS.
- Y. Tian, J. Solid State Light., 2014, 1, 1–15 CrossRef.
- Y. Wei, L. Cao, L. Lv, G. Li, J. Hao, J. Gao, C. Su, C. C. Lin, H. S. Jang, P. Dang and J. Lin, Chem. Mater., 2018, 30, 2389–2399 CrossRef CAS.
- S. Wang, J. Cai, R. Pang, H. Wu, Y. Luo, T. Tan, W. Yuan, L. Jiang, C. Li and H. Zhang, Dalton Trans., 2021, 50, 5666–5675 RSC.
- Y. Yan, C. Luo, S. Ling, J. Liang, S. Liao and Y. Huang, Opt. Mater., 2022, 132, 112818 CrossRef CAS.
- J. M. Gonçalves, M. Stefanski, R. Tomala, A. Musialek, B. Cichy, M. Bettinelli and W. Strek, J. Phys. Chem. C, 2025, 129, 2204–2210 CrossRef.
- Q. Chen, L. Shang, H. Xu, C. Ma and C. K. Duan, J. Phys. Chem. C, 2021, 125, 21780–21790 CrossRef CAS.
- J. H. You, R. C. Wang, F. Han, R. Guo and X. W. Liu, Rare Met., 2018, 37, 439–446 CrossRef CAS.
- D. Q. Trung, N. V. Quang, M. T. Tran, N. V. Du, N. Tu, N. D. Hung, D. X. Viet, D. D. Anh and P. T. Huy, Dalton Trans., 2021, 50, 9037–9050 RSC.
- I. Mackevic, J. Grigorjevaite, M. Janulevicius and A. Linkeviciute, Opt. Mater., 2019, 89, 25–33 CrossRef CAS.
- B. Sun, B. Jiang, J. Fan, L. Zhang and L. Zhang, J. Am. Ceram. Soc., 2022, 106, 513–526 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00345h |
| This journal is © The Royal Society of Chemistry 2025 |