Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Co-cultivation effects of Lactobacillus plantarum and Pichia pastoris on the key aroma components and non-volatile metabolites in fermented jujube juice†
Tao Feng a,
Weitong Caia,
Wei Sunb,
Shixing Yub,
Jianhua Caob,
Min Suna,
Huatian Wanga,
Chuang Yu
a,
Weitong Caia,
Wei Sunb,
Shixing Yub,
Jianhua Caob,
Min Suna,
Huatian Wanga,
Chuang Yu a,
Wencui Kang*a and
Lingyun Yao*a
a,
Wencui Kang*a and
Lingyun Yao*a
aSchool of Perfume and Aroma Technology, Shanghai Institute of Technology, Shanghai 201418, China. E-mail: kangcsong@sit.edu.cn; Lyyao@sit.edu.cn
bHunan Wuzizui Industrial Group Co., Ltd, Xiangtan 411228, China
First published on 7th April 2025
Abstract
Fermented jujube products are gradually becoming popular. However, few studies have focused on the relationship between the metabolites and aroma compounds in jujube during the fermentation process. Hence, in this study, jujube was fermented with the co-culture of Lactobacillus plantarum and Pichia pastoris, and the key volatile organic components (VOCs) and non-volatile organic components (nVOCs) in the fermented jujube juice (FJJ) were studied to determine the possible aromatic production pathway during microbial metabolism and propose the possibility of regulating flavor during fermentation. Headspace solid-phase microextraction-gas chromatography-mass spectrometry (HS-SPME-GC-MS) was employed to analyze and compare the VOCs in the jujube juice before and after fermentation, which showed that the fermented aroma had increased floral, winy and sour notes. Specifically, 13 key aroma compounds were found using the aroma extract dilution analysis (AEDA) and aroma recombination/omission model. Additionally, 32 differential nVOC metabolites, mainly involved in amino acid and nucleotide metabolism pathways, were screened in FJJ using liquid chromatography-tandem mass spectrometry (LC-MS/MS) combined with multivariate statistical analysis. After correlation analysis, 14 nVOCs were significantly correlated with 8 key aroma compounds. This study indicates that the combination of Lactobacillus plantarum and Pichia pastoris may supply a new mixed fermentation agent towards fermented jujube products and provides reference values for flavor regulation in the co-fermentation of jujube juice.
1 Introduction
With the great demand for flavor choice and functional attributes of food products, fermented jujube juice (FJJ) has become more and more popular in beverage market and is gaining popularity among consumers.1 FJJ can be fermented using mono-culture or mixed strains, resulting in different nutrient and quality features under various inoculum fermentation.2,3 Prior to jujube fermentation, some pretreatments, such as enzymatic hydrolysis,4 hot water soaking,5 and various sterilization methods,6 can cause changes in the quality characteristics of FJJ products. During the jujube fermentation process, the microbial strain and environmental factors play important roles in the metabolic profiles and quality features of the FJJ.7,8 For example, different lactic acid bacteria (LAB) strains have been found to reveal a clear distinction in both aroma and taste sensations, as reported previously, where Lactobacillus plantarum fermented jujube generated more aroma VOCs and exhibited lower bitterness, astringency, and aftertaste.9 Additionally, the use of LAB in co-fermentation with acetic acid bacteria (AAB) or yeast has been extensively employed for the preparation of fermented fruit drinks in recent years, which improves the flavor complexity, nutritional value, and shelf-life of the final product.10,11 Consequently, the co-fermentation strategy (LAB and AAB) has been successfully adopted for FJJ production owing to its superior capacity in enhancing the functionality and sensory quality of the product.3,7Numerous works have been conducted to screen the potential active components in FJJ in the past few years,12,13 whereas the flavor characteristics and key aroma active compounds of the fermented fruit juice have been less investigated.14 Flavor is an important factor affecting the sensory perception of food. Under co-cultivation conditions, the synergic interactions between various strains may form higher levels of aroma VOCs in the fermented fruit substrates.11 For FJJ, a similar phenomenon was observed during the co-cultivation of Lactiplantibacillus and Streptococcus. Non-targeted metabolomic analysis and sensory evaluation results revealed that the acids and aroma volatiles were enriched and the characteristics were improved during co-cultivation compared with monoculture fermentation.14 Besides LAB and AAB, non-Saccharomyces yeasts are well known microbes for the biosynthesis of aroma compounds, which can be employed for fruit juice fermentation.15 In recent years, the co-fermentation of LAB with non-Saccharomyces yeast has been revealed to be a more efficient method for improving the bioactive capacity and flavor characteristic of fruit-derived non-alcoholic beverages.16,17 For example, co-fermentation of L. plantarum with Rhodotorula glutinis would improve the total phenolic content and antioxidant capacity and increase the norisoprenoid aroma compounds of fermented mango juice.18 However, information on the co-cultivation of LAB with non-Saccharomyces yeast in terms of metabolite profile and sensory quality of FJJ is still limited.
During the fermentation process, the Pichia yeast has considerable advantages among non-Saccharomyces yeasts such as lower production of alcohol and accumulation of more aroma substances.15 In the present work, the non-Saccharomyces yeast Pichia pastoris (CGMCC 2.4869) was employed for co-cultivation with two L. plantarum strains for FJJ production due to the improved flavor characteristic and acceptability based on preliminary selection. The volatile profile and key aroma compounds of FJJ were characterized by the SPME-GC-MS-O method. Meanwhile, the non-volatile metabolites were identified using LC-MS/MS, and the correlation between metabolites and key aroma compounds was evaluated by Pearson's correlation analysis. The obtained results will provide useful information for understanding and regulating the changes in aroma profile and non-volatile metabolites of FJJ during the co-fermentation process.
2 Experiments
2.1 Materials and reagents
Dried jujube, from Ruoqiang, Xinjiang, was purchased from the Ecological and Healthy Jujube Garden in Milan Town, Ruoqiang County, Xinjiang. Cellulase (enzyme activity 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) and pectinase (enzyme activity 30
000 U g−1) and pectinase (enzyme activity 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U g−1) were purchased from Jiangsu Yinong Biotechnology Co., Ltd, China. The detailed information for the other standard chemicals is shown in Table S1.†
000 U g−1) were purchased from Jiangsu Yinong Biotechnology Co., Ltd, China. The detailed information for the other standard chemicals is shown in Table S1.†
2.2 Preparation of bacterial strains and FJJ
L. plantarum (CICC 21825, LP1) was purchased from China Center of Industrial Culture Collection (CICC), while L. plantarum (CGMCC 1.12394, LP2) and Pichia pastoris (CGMCC 2.4869, PP) were purchased from China General Microbiological Culture Collection Center (CGMCC), and each strain was cultured according to the product manual. Also, the strains were activated to approximately 107 CFU mL−1 for later use.Jujube was cored with boiling water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, g mL−1) using a cooking machine and extracted for 30 min to obtain jujube juice (JJ). Then, 0.2% pectinase and cellulase (2
6, g mL−1) using a cooking machine and extracted for 30 min to obtain jujube juice (JJ). Then, 0.2% pectinase and cellulase (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) were enzymatically hydrolyzed for 120 min at 49.9 °C and the JJ was pasteurized at 90 °C for 20 min. Based on a pre-experiment, LP1, LP2 and PP (1
1, w/w) were enzymatically hydrolyzed for 120 min at 49.9 °C and the JJ was pasteurized at 90 °C for 20 min. Based on a pre-experiment, LP1, LP2 and PP (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1:1 v/v/v) at a 3% (v/v) inoculation volume were added simultaneously after cooling and fermented under the conditions of stirring at 120 rpm min−1 for 18 h at 34 °C.
1:1 v/v/v) at a 3% (v/v) inoculation volume were added simultaneously after cooling and fermented under the conditions of stirring at 120 rpm min−1 for 18 h at 34 °C.
The total plate count during the fermentation period (0 h, 6 h, 12 h, and 18 h) was determined using the plate counting agar culture method, following the national food safety standard GB 4789.2-2022. The final counting result was in log CFU mL−1 (per mL of FJJ). A desktop pH meter was used to directly measure the pH of FJJ during fermentation, taking the average of three measurements each time.
2.3 Sensory evaluation
The sensory evaluation test was performed at the Shanghai Institute of Technology. The sensory evaluation method was performed according to a previous study with slight modification.19 Before the formal sensory evaluation, the sensory team members received 7 consecutive days of training (1–2 hours per day), mainly to be familiar with the sensory characteristics of JJ before and after fermentation. A consensus was reached on 6 descriptive words to describe the flavor of JJ before and after fermentation, including jujube-like, winy, sour, floral, fruity, and woody. The corresponding compounds were employed to refer to the sensory attributes of these sensory descriptors, namely, methyl dodecanoate, pentan-1-ol, hexanoic acid, β-damascenone, ethyl tetradecanoate, and cedrol, respectively. Each sensory descriptor was scored on an intensity scale of 0–9, with 0 indicating the lowest intensity and no feeling, 9 indicating the strongest intensity and obvious feeling.20 This study was reviewed and approved by the School's Institutional Review Board (IRB) and informed consent was obtained from each subject prior to their participation in the study, and the work did not involve experiments on living animals.2.4 Determination of VOCs in FJJ
The GC-O (GC 8860, Agilent, Palo Alto, USA; Olfactory Detection Port ODP4, Gerstel, Mülheim, Germany) programmed temperature conditions were the same as GC-MS, and the aroma description, peak time and aroma intensity of the smelt compounds were recorded during the olfactory detection. The intensity of the aroma was scored on a scale of 0–4, where 0 meant that the aroma was not felt, and 4 meant the aroma was strongly felt.22 A sample was sniffed three times.
Quantitative analysis was performed using two methods, i.e., semi-quantitative internal standard method and external standard method. The internal standard method referred to Sun et al.25 The external standard method was described by Zhu & Xiao with modification.26 Firstly, dichloromethane was used to extract the fermented juice to gain the tasteless fermented juice matrix. The compound standard with different concentrations (0.023–6.448 mg kg−1) was mixed with the internal standard and the volume was fixed with ethanol. Then, the samples were diluted in an odorless matrix at 7 different concentration ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, 1
5, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 1
10, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1
50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, 1
100, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, 1
200, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 and 1
400 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000). The peak area of the detected compounds was calculated according to the concentration formula (Ax/Ai) = a(Cx/Ci) + b, where A represents the peak area, C represents the concentration of each compound, x represents the compound standard, i represents the internal standard, and a and b represent the slope of the standard curve of each compound and the intercept on the Y-axis, respectively.24,26
1000). The peak area of the detected compounds was calculated according to the concentration formula (Ax/Ai) = a(Cx/Ci) + b, where A represents the peak area, C represents the concentration of each compound, x represents the compound standard, i represents the internal standard, and a and b represent the slope of the standard curve of each compound and the intercept on the Y-axis, respectively.24,26
The OAV value was used to evaluate the contribution of an aroma compound to the overall aroma of the sample, where generally, the larger the OAV value, the stronger the contribution.29 The method for its calculation was the content of a compound obtained after quantification by the external standard method divided by its detection threshold.24
To assess the critical importance of an aroma compound to its aroma or overall aroma composition, the aroma omission model was used to make a judgment. In simple terms, multiple aroma models were prepared, and each time an entire note or just one aroma compound in one note left out. According to the triangle test (ISO 4120:2021), the combined aroma model and two odorless substrates were formed into one group, and 10 members of the sensory team were selected for testing. The aroma included in the omission/recombination model was similar to the sensory attributes including jujube-like, winy, sour, floral, fruity and woody.
2.5 Determination of nVOCs in FJJ
The nVOCs of FJJ were extracted according to the method reported by Chen et al.30 Firstly, the freeze-dried FJJ was dissolved with 500 μL 70% methanol and centrifuged (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g, 5 min) at 4 °C to obtain the supernatant sample for LC-MS/MS analysis. The analysis for all samples was the same under positive and negative ion conditions. Briefly, in the elution method, 0.1% formic acid was used in water and acetonitrile as solvent A and B, respectively, in the following gradient: 5% A in 2 min, increased to 60% A in the following 3 min, then increased to 99% A in 1 min and held for 1.5 min, and back to 5% B within 0.1 min, and held for 2.4 min through a T3 column (Waters ACQUITY Premier HSS T3 Column 1.8 μm, 2.1 mm × 100 mm). The ion spray voltage floating of MS (Triple TOF 6600+, SCIEX, Foster City, CA, USA) was 5 kV and 4 kV in positive and negative modes, and the source gas ion spray was 50 psi. Also, the data were collected in the ion scan range of 50–1000 m/z. The original data of non-targeted metabolomics were extracted by XCMS software and corrected for retention time, and then metabolites were identified by the self-built database in the Metware Metabolic platform. Finally, there were 2445 metabolites in positive ion mode and 3830 metabolites in negative ion mode.
000 g, 5 min) at 4 °C to obtain the supernatant sample for LC-MS/MS analysis. The analysis for all samples was the same under positive and negative ion conditions. Briefly, in the elution method, 0.1% formic acid was used in water and acetonitrile as solvent A and B, respectively, in the following gradient: 5% A in 2 min, increased to 60% A in the following 3 min, then increased to 99% A in 1 min and held for 1.5 min, and back to 5% B within 0.1 min, and held for 2.4 min through a T3 column (Waters ACQUITY Premier HSS T3 Column 1.8 μm, 2.1 mm × 100 mm). The ion spray voltage floating of MS (Triple TOF 6600+, SCIEX, Foster City, CA, USA) was 5 kV and 4 kV in positive and negative modes, and the source gas ion spray was 50 psi. Also, the data were collected in the ion scan range of 50–1000 m/z. The original data of non-targeted metabolomics were extracted by XCMS software and corrected for retention time, and then metabolites were identified by the self-built database in the Metware Metabolic platform. Finally, there were 2445 metabolites in positive ion mode and 3830 metabolites in negative ion mode.
2.6 Data analysis
The results for all the test samples was expressed in the form of mean ± standard deviation using Office Excel 2011 (Redmond, Washington, USA). ANOVA (analysis of variance) and significance analysis were completed using SPSS 20 (Chicago, USA), while line, bar, radar charts and heatmaps were generated using Origin 2024b (Northampton, Massachusetts, USA).3 Results and discussion
3.1 Microbial growth and sensory evaluation of FJJ
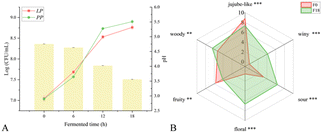
Viable count is a more intuitive method to observe the growth process of microorganisms. As shown Fig. 1A, LAB grew rapidly in the early stage of fermentation (0–6 h), which was similar to the reported observation.31 The rich content of fructose and glucose in jujube were high quality carbon sources for the growth and cultivation of LAB, and thus LAB had a higher utilization rate in the early stage of fermentation. In addition, acidification is an essential part of the fermentation process of LAB.32 Organic substances in the substrate will be converted by LAB into organic acids (especially lactic acid) and other by-products, thereby reducing the overall pH value of the environment.33 Meanwhile, the pH value of the mixed bacteria FJJ showed a slow downward trend in the early stage of fermentation (from 4.75 to 4.65), and then dropped rapidly to about 3.60 in the middle and late stage of fermentation (6–18 h) (Fig. 1A). This may be due to the acidic condition caused by the acid production of LAB, which easily inhibited the growth of LAB. The two kinds of bacteria would compete for the saccharides in JJ,34,35 and thus the yeast grew more rapidly in the middle and late stage of fermentation, and the final total colony was close to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log (CFU mL−1) (Fig. 1A).
log (CFU mL−1) (Fig. 1A).
Sensory evaluation was conducted on the aroma properties of FJJ for 0 h and 18 h, resulting in a sensory attribute radar chart (Fig. 1B). The results showed that there was a significant change in aroma before and after fermentation. Besides the unique sweet and fruity aroma of jujube itself, which were more pronounced at 0 h of fermentation, all other sensory attributes scored higher after 18 h fermentation (F18) including floral (6.64), sour (6.39), and wine (4.17). Studies have shown that non-Saccharomyces have higher glucosidase activity, which can hydrolyze more aroma precursor substances to produce higher alcohols, complex esters, and volatile fatty acid compounds, thereby enriching the aroma characteristics such as floral and winy aroma.36
3.2 Analysis of key aroma VOCs in FJJ
A total of 35 compounds was detected in JJ after 0 h and 18 h fermentation, including 13 acids, 9 esters, 4 aldehydes, 5 alcohols and 4 ketones (Table S2†). The number and content of the compound types before and after fermentation showed significant changes (Fig. 2A and Table S2†). It can be clearly seen that JJ itself was rich in a variety of acid and aroma components, given that 9 acid compounds were detected before fermentation (F0), while the content of acids after fermentation (F18) was significantly different from that of F0 (for example, the content of octanoic acid (A7) increased from 0.09 mg mL−1 to 0.12 mg mL−1, and the content of dodecanoic acid (A11) increased from 0.60 mg mL−1 to 6.25 mg mL−1). This is because with the fermentation process, the metabolism of LAB continued to produce organic acids and small molecular acids with flavor by consuming sugar-containing substrates.31 At the same time, the aroma intensity and AEDA results of F18 are shown in Table 1. Hexanoic acid (A4) and heptanoic acid (A5) had a more pronounced sour aroma and a cheese-like sour odor, while A11 had a strong coconut oil aroma and a soapy feeling. | ||
| Fig. 2 (A) Number of compound types between F0 and F18 JJ and (B) sensory radar score plot compared with F18 jujube juice and recombination model (RM), “JJ”: jujube juice. | ||
| No. | Compound | CAS no. | RI | Odor description | Aroma intensity | FD value | Identification method |
|---|---|---|---|---|---|---|---|
| a Odor description was detected at the sniffing port of ODP4; FD value was flavor dilution value; MS, compounds were identified using MS spectra: NIST 20; RI, compounds were identified through calculated value and searched value; O, compounds were identified by comparison of their odor with that of authentic compounds using GC-O; and S, compounds were identified by comparison with standards. | |||||||
| A | Acid | ||||||
| A4 | Hexanoic acid | 142-62-1 | 1831 | Sour cheese | 2 | 8 | MS, RI, O, Std |
| A5 | Heptanoic acid | 111-14-8 | 1950 | Rancid sour | 2 | 4 | MS, RI, O, Std |
| A8 | Decanoic acid | 334-48-5 | 2265 | Sour rancid | 2 | 2 | MS, RI, O, Std |
| A11 | Dodecanoic acid | 143-07-7 | 2503 | Oily coconut soapy | 2 | 8 | MS, RI, O, Std |
| A13 | Tetradecanoic acid | 544-63-8 | 2713 | Waxy fatty | 1 | 4 | MS, RI, O, Std |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| B | Ester | ||||||
| B1 | Methyl decanoate | 110-42-9 | 1604 | Sweet fruity winy | 2 | 2 | MS, RI, O, Std |
| B3 | Methyl dodecanoate | 111-82-0 | 1815 | Waxy fatty oily | 1 | 8 | MS, RI, O, Std |
| B5 | Methyl tetradecanoate | 124-10-7 | 2020 | Warm waxy | 1 | 1 | MS, RI, O, Std |
| B7 | Ethyl tetradecanoate | 124-06-1 | 2057 | Sweet waxy floral | 3 | 8 | MS, RI, O, Std |
| B8 | Methyl hexadecanoate | 112-39-0 | 2226 | Oily sweet floral | 2 | 1 | MS, RI, O, Std |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| C | Alcohol | ||||||
| C1 | Pentan-1-ol | 71-41-0 | 1244 | Fermented alcohol | 2 | 2 | MS, RI, O, Std |
| C4 | 2-Phenylethanol | 60-12-8 | 1872 | Like sweet rose | 3 | 32 | MS, RI, O, Std |
| C5 | (1R,2R,5R,7S,8R)-2,6,6,8-Tetramethyltricyclo[5.3.1.01,5]undecan-8-ol | 77-53-2 | 2149 | Woody sweet | 1 | 4 | MS, RI, O, Std |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| D | Aldehyde | ||||||
| D3 | 2-Phenylacetaldehyde | 122-78-1 | 1663 | Strong green floral | 4 | 16 | MS, RI, O, Std |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||
| E | Ketone | ||||||
| E2 | (E)-1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one | 23726-93-4 | 1831 | Rose honey floral | 3 | 64 | MS, RI, O, Std |
| E3 | Pentadecan-2-one | 2345-28-0 | 2025 | Fresh green floral | 1 | 1 | MS, RI, O, Std |
| E4 | 6,10,14-Trimethylpentadecan-2-one | 502-69-2 | 2131 | Oily | 1 | 1 | MS, RI, O, Std |
The formation of ester compounds was mainly due to the non-enzymatic esterification reaction of alcohols produced by yeast metabolism and organic acids produced by LAB,37 which were considered to be the main class of compounds with a fruity aroma. F0 contained methyl dodecanoate (B3), methyl tetradecanoate (B5) and methyl hexadecanoate (B8). Based on fruity, they also have the special fat wax fragrance and oil fragrance of jujube, which are the representative compounds in Ruoqiang jujube,38 and their content increased in F18. The olfaction results in Table 1 show that the FD value of B3 is higher, which is described as having the aroma of fatty, waxy and sweet. In addition, methyl decanoate (B1) and ethyl tetradecanoate (B7) were newly produced after fermentation, and although their content was low, they could still be detected many times in the olfactory results (FD = 2 and 8). B1 was fruity and winy, while B7 had a more floral note.
Alcohols are mainly metabolites of yeast bacteria. They are produced by amino acids through decarboxylation and dehydrogenation pathways.39 For example, phenylmethanol (C3) and 2-phenylethanol (C4) are produced by the phenylalanine pathway. However, C4 (FD = 32) could be clearly perceived in the olfactory results, which had a rose sweet aroma, while C3 could not be perceived due to its low content. In addition, pentan-1-ol (C1) and its isomers are important fermentation flavor compounds in the food industry. They are often found in the flavor compounds of liquor, which endow it with a full-bodied aroma and mainly generated through the biosynthetic pathway of leucine or valine.40 C1 was considered to have ethanol-like translucency and fermented aroma in smell, but due to its certain volatility, its FD value was only 2.
Aldehyde compounds are easily oxidized or reduced, mainly through the oxidation of amino acids or fatty acids. Nonanal (D1), 2-phenylacetaldehyde (D3) and 2-benzylideneoctanal (D4) were newly generated aldehydes with floral fragrance in F18. D1 had a thick sense of rose fragrance and lipid-like fragrance, and D3 had a floral fragrance like hyacinth.41 However, only the aroma characteristics of D3 (FD = 16) were felt, as shown in Table 1. Ketones and aldehydes are formed in a similar way and obtained by the degradation of their precursor amino acids or the transformation of alcohols. 6,10,14-Trimethylpentadecan-2-one (E4) was the original of F0 and its content increased slightly with the fermentation process. It had an oily fragrance and a slightly sweet floral fragrance,39 where (E)-1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)but-2-en-1-one (known as β-damascenone) (E2) was the ketone compound with the highest content in F18 (0.3367 mg mL−1), which is produced by yeast metabolism and considered to be an important aroma component of wine beverages. It had a strong rose-like sweet and honey-like sweet fragrance.39 Because of its low threshold value and high aroma vitality value, it had a high value of aroma activity, and also its FD factor was as high as 64 in the diluted smell, which was the compound with the highest FD value in F18, and this it was initially considered to be an important aroma compound in sweet floral fragrance. Moreover, 17 VOCs with FD factors were subjected to the use of an external standard and threshold detection analysis, as shown in Table S3.†
To further determine the key aroma compounds in mixed bacteria FJJ, the aroma characteristics of F18 were simulated by aroma recombination and omission experiments. Compounds with FD ≥ 2 and OAV ≥ 1 were selected and mixed in the odorless matrix according to the actual concentration to obtain the recombination model of F18. The sensory group scored and evaluated the attributes of each group, and the results are shown in Fig. 2B. Compared with F18, the recombination model had significantly lower scores for jujube and woody, slightly lower scores for fruity, and higher scores for winy. Only the scores of floral and sour notes were similar to F18. Jujube-like and woody note were considered to be close to the original dried jujube and the incense similar to jujube kernel when the sensory attributes were first proposed. These aroma features were derived from nature and cannot be obtained simply by mixing several monomer aroma compounds. This may require synergistic effects between a variety of aroma compounds. Overall, the aroma recombination model showed good similarity compared to F18.
The compounds in the omission model were classified by aroma note, and the aroma omission test was carried out by omitting a group of notes or a single compound in one note, and the results are shown in Table 2. Similar to the aroma recombination model, the group members were more sensitive to floral and sour notes, especially hexanoic acid (3-1), octanoic acid (3-2), β-damascenone (4-1) and 2-phenylethanol (4-2). In the jujube-like note, the absence of dodecanoic acid (1-1) and tetradecanoic acid (1-3) was perceived by most members. The description of aroma loss mainly mentioned the absence of fatty or lipid waxy note. Some studies concluded that the main characteristic aroma of jujube is fatty and fruity, and its aroma is not only provided acid compounds with more than 10 carbon atoms, but also conferred by aldehydes such as nonan-2-al and decan-2-al.42,43 However, although the OAV values of phenylacetaldehyde (4-3) and cedrol (6) were >10, they were not obviously felt in the omission model, which was related to the lower threshold of 4-1 and 4-2 and their high OAV values (OAV = 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 828 and 3302) in the floral fragrance omission model, respectively, while the loss of woody note was not easily felt by members. This may be due to the fact that the content of cedrol (6), represented by woody aroma, was relatively low in the overall composition, which was easily masked by the winy compound pentan-1-ol (2-1). At the same time, methyl decanoate (4-1) in the fruit aroma also had the aroma of fruity and winy, which was also the reason why the winy aroma score, as shown in Fig. 2B, was higher than the actual value. In conclusion, as further confirmed by the aroma recombination/omission model, dodecanoic acid, tetradecanoic acid, methyl dodecanoate, pentan-1-ol, hexanoic acid, heptanoic acid, β-damascenone, 2-phenylethanol, methyl decanoate, and cedrol were the most important aroma compounds in FJJ, and the compounds with FD ≥ 2 and OAV ≥ 1 were phenylacetaldehyde, decanoic acid and methyl tetradecanoate, which also contributed to the overall aroma.
828 and 3302) in the floral fragrance omission model, respectively, while the loss of woody note was not easily felt by members. This may be due to the fact that the content of cedrol (6), represented by woody aroma, was relatively low in the overall composition, which was easily masked by the winy compound pentan-1-ol (2-1). At the same time, methyl decanoate (4-1) in the fruit aroma also had the aroma of fruity and winy, which was also the reason why the winy aroma score, as shown in Fig. 2B, was higher than the actual value. In conclusion, as further confirmed by the aroma recombination/omission model, dodecanoic acid, tetradecanoic acid, methyl dodecanoate, pentan-1-ol, hexanoic acid, heptanoic acid, β-damascenone, 2-phenylethanol, methyl decanoate, and cedrol were the most important aroma compounds in FJJ, and the compounds with FD ≥ 2 and OAV ≥ 1 were phenylacetaldehyde, decanoic acid and methyl tetradecanoate, which also contributed to the overall aroma.
| Constructed flavor note | Model no. | Omitted odorants | Difference in odor | Number of correct answers |
|---|---|---|---|---|
| a **Highly significant (p ≤ 0.01); *significant (p ≤ 0.05); and not significant (p > 0.05). | ||||
| Jujube-like note | 1 | Jujube-like waxy | Less waxy and soapy | 6* |
| 1-1 | Dodecanoic acid | Lack of soap and crayon fragrance | 9** | |
| 1-2 | Methyl dodecanoate | Less fatty | 5* | |
| 1-3 | Tetradecanoic acid | Lack of oily smell | 8** | |
| Fermented note | 2 | Pentan-1-ol | Lacking the aroma of wine and ethanol | 5* |
| Sour note | 3 | Sour | Lacking the sour aroma of nasal stimulation | 9** |
| 3-1 | Hexanoic acid | The lack of sharp thorns in the acid, the grassy aroma is heavy | 7** | |
| 3-2 | Heptanoic acid | Sharp acid deficiency is more pronounced | 9** | |
| 3-3 | Decanoic acid | Slightly weak sour aroma | 2 | |
| Floral note | 4 | Floral | Without floral and sweet | 7** |
| 4-1 | (E)-1-(2,6,6-Trimethyl-1,3-cyclohexadien-1-yl)-2-buten-1-one (β-damascenone) | Without the sweet fragrance of flowers | 9** | |
| 4-2 | 2-Phenylethanol | Less floral fragrance with a hint of green leaves | 6* | |
| 4-3 | 2-Phenylacetaldehyde | The sweet fragrance of flowers still lingers, and one cannot feel the lack | 2 | |
| Fruity note | 5 | Fruity | Less sweet but different with floral | 4* |
| 5-1 | Methyl decanoate | Less winy | 4* | |
| 5-2 | Ethyl tetradecanoate | Less some sweet | 1 | |
| Woody note | 6 | (1R,2R,5R,7S,8R)-2,6,6,8-Tetramethyltricyclo[5.3.1.01,5]undecan-8-ol (cedrol) | Less smoked woody | 3 |
3.3 Differential analysis of characteristic nVOCs in FJJ
To study the difference and key metabolites during the fermentation process, the fermented juice was sampled every six hours, centrifuged and frozen. The grouped samples were detected by LC-MS/MS, and the data were sorted by multivariate statistical analysis to finally obtain the key differential metabolites before and after fermentation. Before the difference analysis, principal component analysis (PCA) was performed on the grouped samples to observe the overall variation between and within groups of the grouped samples.44 As shown in Fig. 3A and B, the PCA analysis results of the two modes are ideal. The QC sample points were used to monitor the stability of the instrument, and the three parallel and close overlaps indicate that the instrument had good stability and repeatability.44 The principal component contribution rate of the positive ion mode (Fig. 3A) was close to 60%. In the negative ion mode (Fig. 3B), F0 and F6 overlapped, indicating that the metabolite content slightly changed. Similarly, F12 and F18 were close to each other, indicating that the juice fermented for four different periods was clustered into two groups.To further separate the samples obtained at different periods, the supervised multivariate statistical analysis method OPLS-DA was selected to distinguish samples from different groups, and 200 permutation tests were used to verify the feasibility of the fitting model and the VIP value was calculated to identify the key variables affecting the classification of groups.45 As shown in Fig. S1,† the four groups of samples were grouped in pairs and had good separation. In the permutation test (Fig. S2†), the Q2 and R2Y values were close to 1, and no over-fitting phenomenon was shown, indicating that the OPLS-DA model was effective and had good predictive ability, which could be used to predict the differential metabolites of FJJ before and after fermentation. Therefore, in the subsequent difference analysis, we mainly focused on the difference between F0 and F18.
The variable importance in the projection (VIP) value reflects the importance of a variable in the projection model, and its size is used to measure the contribution of each metabolite to the classification ability of the model, which is an important parameter in OPLS-DA. Usually, a VIP value of >1 is considered a differential metabolite. In differential analysis, VIP > 1 and p < 0.05 are commonly used to determine the differential metabolites between groups.44,46 The metabolic substances with VIP > 1 and p < 0.05 were screened in the OPLS-DA model before and after fermentation (F0 vs. F18), and 1264 substances were detected, among which 138 were up-regulated and 1126 were down-regulated. The hierarchical clustering heat map and volcano map (Fig. 3C and D, respectively) were used to more intuitively observe the relative content changes in the metabolites before and after fermentation. As shown in Fig. 3C, darker red represents a greater content of this substance, while darker green represents less of this substance, and as shown in Fig. 3D, red represents the up-regulated substance, and green represents the down-regulated substance. Both showed that most of the metabolites were consumed after fermentation, with only nearly one-tenth of the metabolites showing an increase. In addition, the fold change (FC) refers to the ratio of the expression level of metabolites in F0 and F18, which can reflect the change fold in the expression level of a metabolite before and after fermentation. The bar chart in Fig. 3E shows the top 20 metabolites with the largest log2 FC absolute values among the 1264 metabolites. Most of the down-regulated metabolites were amino acids and their derivatives. Jujube is rich in a variety of free amino acids, including eight amino acids necessary in the human body. Men et al. studied the changes in the nutritional composition of JJ before and after fermentation by enzymatic hydrolysis and L. plantarum mixed with Pediococcus pentose.47 The results showed that this fermentation method could increase the production of GABA and increase the content of branched chain amino acids and free amino acids, such as aspartate, glutamic acid, and proline, which provides a theoretical basis for LAB fermentation of JJ to improve the content of nutrients. Liu et al. pointed out that amino acids were the energy source for the growth of LAB, and the content of free amino acids in FJJ decreased significantly.48 In addition, in the study on the single yeast fermentation of fruit wine, it was found that the concentration of amino acids or ammonium ions would affect the growth and metabolism rate of yeast, and eventually reduce the possibility of ethanol metabolism and affect the flavor and taste of fruit wine.49
3.4 KEGG pathway enrichment analysis of the differential metabolites
In the KEGG enrichment pathway analysis (Fig. 4A), the main pathways with significant differences (p < 0.05) were ABC transporter, amino acid metabolism and nucleotide metabolism, among which the metabolic pathways were similar to some previous studies.42,47,49 ABC transporters are an important class of membrane transporters, which mainly drive the transport of various substances across the membrane by hydrolyzing ATP and considered enzyme or transporter.50 Through the search of the KEGG enrichment pathways, we sorted and plotted the main metabolic pathways of 32 metabolites together (Fig. 4B), and combined the KEGG metabolic pathways with OPLS-DA and analyzed the findings together (Table S4†). Finally, 14 metabolites out of 32 involved in the major metabolic pathways were identified as key differential metabolites (VIP ≥ 1, p ≤ 0.05). | ||
| Fig. 4 (A) KEGG enrichment analysis of differential metabolite pathways in 0 h vs. 18 h and (B) metabolic pathways of major nVOCs during the fermentation of JJ from 0 h to 18 h. “JJ”: jujube juice. | ||
3.5 Correlation analysis between key metabolites and key aroma components
As shown in the cluster heat map in Fig. 5A, 14 differential metabolites had obvious content changes in different fermentation periods. In the early fermentation period (F0 and F6), these metabolites were rapidly used and metabolized by LAB and yeast, while in the late fermentation period (F12 and F18), due to the decline in the growth rate of LAB, most of the metabolites had a similar content distribution. Only α-ketoglutarate acid continued to accumulate during the later stages of fermentation. Previous studies have shown that α-oxaloacetic acid (α-ketoglutarate acid) is produced through the glycolysis or phosphoketolase pathway to pyruvate, and then enters the tricarboxylic acid cycle to be transformed by citrate.51,52 At the same time, in the tricarboxylic acid cycle, many amino acids are metabolites of citrate and α-ketoglutarate acid under the action of transaminases. For example, valine and leucine with bitter taste were converted to α-ketoglutarate acid during the fermentation process of LAB, and then more aldehydes, acids and alcohols were generated under the action of some dehydrogenases, thus enriching the aroma after fermentation.53 Aspartic acid, an amino acid with a sour and umami taste, may change the metabolism of D-glucose and lead to the excessive production of acetic acid, which in turn can combine with alcohols produced by yeast to produce esters with fruity taste.54 Therefore, the tricarboxylic acid cycle is an important metabolic process in microbial fermentation. In addition, the key nucleotides involved in the KEGG metabolic pathway are mainly uridine, adenosine and deoxyinosine. The reason for the reduction in these nucleotides may be the salvage pathway occurring in the fermentation process. Due to the continuous decomposition of nutrients in the substrate, a series of specific enzymes could be used to recycle nucleoside substances, with the purpose of reducing the high energy consumption of microorganisms. Simply, under certain conditions, nucleoside compounds can be used as alternative energy sources to support cell growth in an environment where glucose is completely lacking, and microbial metabolic pathways support ATP energy production.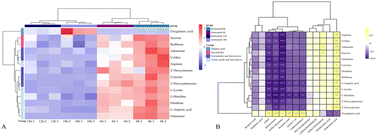 | ||
| Fig. 5 (A) Relative content heatmap of 14 selected key nVOCs of FJJ and (B) heatmap representation of Pearson's correlation analysis of selected differential nVOCs and key VOCs. | ||
To better illustrate the influence of fermentation of LAB and yeast on the aroma profile of FJJ, a correlation analysis between key selected metabolites and key aroma components was also performed. As shown in Fig. 5B, a total of 8 key aroma components was significantly correlated with 14 key selected metabolites. It is not difficult to find that α-ketoglutarate acid was positively correlated with most aroma components. α-Ketoglutarate acid is produced by glutamate dehydrogenase acting on glutamate, and it is also a key node connecting carbon-nitrogen metabolism in cells (Fig. 5B).52 As an intermediate metabolite, α-ketoglutarate acid can indirectly participate in the formation of volatile aroma substances. It can also be found in Fig. 5B that nucleotide and amino acid compounds were negatively correlated with most of the aroma components, which also verified that the metabolic decomposition of amino acids and nucleotides during fermentation was the main metabolic pathway and may indirectly produce VOCs with aroma.
As shown in Fig. 5B, histidine, leucine, and aspartic acid showed relatively significant negative correlations with most aroma components, while decanoic acid, heptanoic acid, ethyl tetradecanoate, and phenylacetaldehyde may not be significantly correlated due to their low content. Previous studies have shown that LAB such as L. plantarum and L. acidophilus, which contain more genomic fragments, can participate in different metabolic pathways to produce flavor compounds through the action of enzymes and other functional proteins encoded by the genome on amino acids.7,53 Research on the metabolic pathways of yeast used in alcohol fermentation shows that amino acids are metabolized in yeast by the Ehrlich pathway and produce higher alcohols and other aromatic substances.55 In the process of nucleotide metabolism, LAB and yeast could obtain the corresponding nucleoside through the dephosphorylation of nucleotidases contained in microorganisms.56 As shown in Fig. 5B, the main nucleosides negatively correlated with methyl decanoate, cedrol, β-damascenone, hexanoic acid and pentan-1-ol were uridine, cytidine, inosine and cytosine, respectively. Overall, these metabolites are complex and the provided conditions produced volatile aroma components during the fermentation of JJ.
4 Conclusions
In summary, the mixed fermentation of L. plantarum and P. pastoris could not only increase the total plate count of FJJ, but also enrich its aroma and flavor. Through GC-MS external standard quantification and sensory analysis, the key aroma components of FJJ were determined to be dodecanoic acid, tetradecanoic acid, methyl dodecanoate, pentan-1-ol, hexanoic acid, heptanoic acid, β-damascenone, 2-phenylethanol, methyl decanoate, cedrol, phenylacetaldehyde, decanoic acid and methyl tetradecanoate. These aroma components endowed FJJ with jujube-like, winy, sour, floral, woody, and fruity aromas. In addition, through untargeted metabolomics analysis, it was found that the main metabolic pathways of co-fermentation of JJ were amino acid and nucleotide metabolism. Ultimately, 14 key non-volatile metabolites were identified in FJJ at four fermentation periods, among which, cedrol, β-damascenone, methyl decanoate, pentan-1-ol, 2-phenylethanol, and dodecanoic acid were significantly correlated with the key metabolites. This study demonstrates the possibility of regulating fermentation flavor, but the metabolite pathways are complex, and the enzymes involved in the reaction are diverse. Thus, further bioengineering research is needed to confirm the relationship between aroma components and metabolites.Data availability
All data included in this study are available from the corresponding author upon request.Author contributions
Tao Feng: conceptualization, funding acquisition, project administration, supervision, writing-review & editing. Weitong Cai: data curation, formal analysis, methodology, software, writing-original draft. Wei Sun: conceptualization, project administration. Shixing Yu: conceptualization, project administration. Jianhua Cao: conceptualization, project administration. Min Sun: supervision, validation. Huatian Wang: supervision, validation. Chuang Yu: methodology, visualization. Wencui Kang: visualization, writing-review & editing. Lingyun Yao: conceptualization, visualization, writing-review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships with other people or organizations that could inappropriately influence the work reported in this paper.Acknowledgements
This project named ‘Solution for Evaluation and Optimization of Betel Leaf Extract Liquor’ was funded by Hunan Wuzizui Industrial Co., Ltd, which was numbered J2024-041.References
- K. Rashwan, N. Karim, M. R. L. Shishir, T. Bao, Y. Lu and W. Chen, J. Funct. Foods, 2020, 75, 104205 CrossRef.
- W. Cai, F. Tang, C. Shan, Q. Hou, Z. Zhang, Y. Dong and Z. Guo, Food Sci. Nutr., 2020, 8(9), 4965–4975 CrossRef CAS PubMed.
- T. A. Boasiako, A. A. Yinka, X. Yuqing, I. D. Boateng and Y. Ma, Food Biosci., 2024, 57, 103534 CrossRef CAS.
- L. Zhang, M. Zha, S. Li and W. Zong, J. Food Meas. Char., 2022, 16(4), 3196–3207 CrossRef.
- G. Liu, M. Wu, Y. Li, N. Qayyum, X. Li, J. Zhang and C. Wang, LWT--Food Sci. Technol., 2023, 181, 114692 CrossRef CAS.
- L. Zhang, M. Zha, S. Li and W. Zong, J. Food Sci. Technol., 2022, 59(10), 3765–3774 CrossRef CAS PubMed.
- X. Pan, S. Zhang, X. Xu, F. Lao and J. Wu, LWT--Food Sci. Technol., 2022, 154, 112772 CrossRef CAS.
- H. Li, L. Fan, S. Yang, W. Lei, P. Tan, J. Liang and Z. Gao, Food Biosci., 2024, 57, 103496 CrossRef CAS.
- W. Cai, F. Tang, X. Zhao, Z. Guo, Z. Zhang, Y. Dong and C. Shan, J. Food Process. Preserv., 2019, 43(9), e14095 Search PubMed.
- J. Y. H. Toy, Y. Lu, D. Huang, K. Matsumura and S. Liu, Crit. Rev. Food Sci. Nutr., 2022, 62(7), 1890–1911 CrossRef CAS PubMed.
- S. Rajendran, P. Silcock and P. Bremer, Molecules, 2023, 28(7), 3236 CrossRef CAS PubMed.
- T. Li, T. Jiang, N. Liu, C. Wu, H. Xu and H. Lei, Food Chem., 2021, 339, 127859 CrossRef CAS PubMed.
- W. Ren, Y. Ma, D. Liu, P. Liang, J. Du, S. Yang, L. Tang and Y. Wu, J. Food Sci., 2022, 87(2), 664–685 CrossRef CAS PubMed.
- J. Li, H. Xu, H. Li, Y. Xie, K. Ding, S. Xu, Z. Wang, R. Wang, C. Yi and S. Ding, Food Res. Int., 2024, 196, 115093 CrossRef CAS.
- Y. Bao, M. Zhang, W. Chen, H. Chen, W. Chen and Q. Zhong, Food Biosci., 2021, 44, 101414 CrossRef CAS.
- Y. Li, T. T. H. Nguyen, J. Jin, J. Lim, J. Lee, M. Piao, I. Mok and D. Kim, Food Sci. Biotechnol., 2021, 30, 555–564 CrossRef CAS PubMed.
- L. Nyhan, A. W. Sahin and E. K. Arendt, Eur. Food Res. Technol., 2023, 249(1), 167–181 CrossRef CAS PubMed.
- Q. Zhong, R. Chen, M. Zhang, W. Chen, H. Chen and W. Chen, Fermentation, 2023, 9(6), 563 CrossRef CAS.
- Z. Wang, Q. Xiao, J. Zhuang, T. Feng, C. T. Ho and S. Song, J. Agric. Food Chem., 2019, 68(1), 267–278 Search PubMed.
- Y. Chen, Y. Huang, Y. Bai, C. Fu, M. Zhou, B. Gao, C. Wang, D. Li, Y. Hu and N. Xu, LWT--Food Sci. Technol., 2017, 84, 753–763 CAS.
- Y. Liu, Y. Sang, J. Guo, W. Zhang, T. Zhang, H. Wang, S. Cheng and G. Chen, Food Sci. Nutr., 2021, 9(12), 6617–6626 CAS.
- L. Wang, P. Wang, W. Deng, J. Cai and J. Chen, LWT--Food Sci. Technol., 2019, 108, 400–406 CrossRef CAS.
- Y. Feng, Y. Cai, D. Sun-Waterhouse, C. Cui, G. Su, L. Lin and M. Zhao, Food Chem., 2015, 187, 44–52 CAS.
- W. Song, M. Sun, H. Lu, S. Wang, R. Wang, X. Shang and T. Feng, Foods, 2024, 13(5), 684 CAS.
- Y. Sun, Y. Zhang and H. Song, J. Food Process. Preserv., 2020, 45, 15036 Search PubMed.
- J. Zhu and Z. Xiao, J. Agric. Food Chem., 2018, 66(29), 7722–7734 CAS.
- G. Lytra, S. Tempere, A. Le Floch, G. de Revel and J. C. Barbe, J. Agric. Food Chem., 2013, 61(36), 8504–8513 CrossRef PubMed.
- Y. Yang, P. Yu, J. Sun, Y. Jia, C. Wan, Q. Zhou and F. Huang, Food Chem., 2022, 373, 131607 CrossRef CAS PubMed.
- X. Du, A. Plotto, E. Baldwin and R. Rouseff, J. Agric. Food Chem., 2011, 59(23), 12569–12577 CrossRef CAS PubMed.
- S. Chen, H. Liu, X. Zhao, X. Li, W. Shan, X. Wang and X. Yu, Food Res. Int., 2020, 128, 108778 CrossRef CAS PubMed.
- M. Zhao, F. Zhang, L. Zhang, B. Liu and X. Meng, Int. J. Food Sci. Technol., 2019, 54(8), 2624–2631 CrossRef CAS.
- Q. Gao, C. Wu and M. Wang, J. Agric. Food Chem., 2013, 61, 3351–3363 CrossRef CAS PubMed.
- W. Fu and A. Mathews, Biochem. Eng. J., 1999, 3(3), 163–170 CrossRef CAS.
- M. Gobbetti, A. Corsetti and J. Rossi, Appl. Microbiol. Biotechnol., 1994, 41, 456–460 CrossRef CAS.
- Y. Liao, X. Ao, H. Kang, T. He and L. Yang, Food Ferment. Ind., 2023, 49(03), 340–346 Search PubMed.
- X. Wang, G. Fan, Y. Peng, N. Xu, Y. Xie, H. Zhou, H. Liang, J. Zhan, W. Huang and Y. You, J. Food Compos. Anal., 2023, 84, 105660 Search PubMed.
- X. Jiang, D. Peng, W. Zhang, M. Duan, Z. Ruan, S. Huang, S. Zhou and Q. Fang, Food Chem., 2021, 344, 128681 CAS.
- M. Gou, Q. Chen, Y. Qiao, X. Jin, J. Zhang, H. Yang, M. L. Fauconnier and J. Bi, J. Food Compos. Anal., 2023, 116, 105072 CrossRef CAS.
- Q. Chen, J. Song, J. Bi, X. Meng and X. Wu, Food Res. Int., 2018, 105, 605–615 CAS.
- L. Wang, L. Zhu, F. Zheng, F. Zhang, C. Shen, X. Gao, B. Sun, M. Huang, H. Li and F. Chen, J. Food Sci., 2021, 86(5), 2061–2074 CAS.
- W. Fu, J. Ren, S. Li, D. Ren, X. Li, C. Ren, X. Zhao, J. Li and F. Li, Foods, 2023, 12(17), 3184 CAS.
- Y. Jia, C. Wang, Y. Zhang, W. Deng, Y. Ma, J. Ma and G. Han, Foods, 2024, 13(8), 1193 CAS.
- R. Liu, L. Ma, X. Meng, S. Zhang, M. Cao, D. Kong, X. Chen, Z. Li, X. Pang and W. Bo, Plants, 2024, 13(11), 1517 CAS.
- J. Ai, Q. Wu, M. Battino, W. Bai and L. Tian, Food Chem., 2021, 364, 130425 CAS.
- B. Huang, Q. Zha, T. Chen, S. Xiao, Y. Xie, P. Luo, Y. Wang, L. Liu and H. Zhou, Phytomedicine, 2018, 45, 8–17 CrossRef CAS PubMed.
- C. Kang, Y. Zhang, M. Zhang, J. Qi, W. Zhao, J. Gu, W. Guo and Y. Li, Food Chem., 2022, 387, 132932 CrossRef CAS PubMed.
- Y. Men, P. Zhu, Y. Zhu, Y. Zeng, J. Yang and Y. Sun, Food Sci. Nutr., 2019, 7(4), 1302–1310 CrossRef CAS PubMed.
- W. Liu, X. Luo, S. Qiu, W. Huang, Y. Su and L. Li, BMC Microbiol., 2023, 23(1), 67 CrossRef CAS PubMed.
- A. Mas, J. M. Guillamon, M. J. Torija, G. Beltran, A. B. Cerezo, A. M. Troncoso and M. C. Garcia-Parrilla, BioMed Res. Int., 2014, 1, 898045 Search PubMed.
- C. F. Higgins, Annu. Rev. Cell Biol., 1992, 8(1), 67–113 CrossRef CAS.
- L. De Vuyst and F. Leroy, FEMS Microbiol. Rev., 2019, 44(4), 432–453 CrossRef.
- T. Xiao, A. Khan, Y. Shen, L. Chen and J. D. Rabinowitz, Nat. Chem. Biol., 2022, 18(12), 1380–1387 CrossRef CAS PubMed.
- Y. Ardö, Biotechnol. Adv., 2006, 24(2), 238–242 CrossRef.
- Y. Vasserot, C. Dion, E. Bonnet, A. Maujean and P. Jeandet, J. Appl. Microbiol., 2001, 90(3), 380–387 CrossRef CAS PubMed.
- B. Kim, B. R. Cho and J. S. Hahn, Biotechnol. Bioeng., 2014, 111(1), 115–124 CAS.
- M. Kilstrup, K. Hammer, P. Ruhdal Jensen and J. Martinussen, FEMS Microbiol. Rev., 2005, 29(3), 555–590 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00193e |
| This journal is © The Royal Society of Chemistry 2025 |