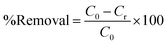Open Access Article
Open Access ArticleActivation of peroxymonosulfate for rhodamine-B removal from water: enhanced efficiency with cobalt-enriched, magnetically recoverable CNTs
M. O. Abdel-Salam†
 abc,
Hebatullah H. Farghal†
abc,
Hebatullah H. Farghal† a,
Ehab El Sawy
a,
Ehab El Sawy a,
Taeho Yoon
a,
Taeho Yoon *d and
Mayyada M. H. El-Sayed
*d and
Mayyada M. H. El-Sayed *a
*a
aDepartment of Chemistry, School of Sciences and Engineering, The American University in Cairo, Cairo, 11835, Egypt. E-mail: mayyada@aucegypt.edu
bAnalysis and Evaluation Department, Egyptian Petroleum Research Institute (EPRI), 1 Ahmed El Zomor St., Nasr City, Cairo, 11727, Egypt
cCentral Analytical Laboratories, Egyptian Petroleum Research Institute (EPRI), Nanotechnology Research, 1 Ahmed El Zomor St., Nasr City, Cairo, 11727, Egypt
dDepartment of Chemical Engineering, Kyung Hee University, Yongin-si, Gyeonggi-do, 17104, Republic of Korea. E-mail: tyoon@khu.ac.kr
First published on 26th February 2025
Abstract
Dyes are known to pose environmental threats due to their mutagenic and persistent effects. To address this concern, researchers have explored various unconventional dye degradation materials, such as metal oxides with carbon materials. However, challenges related to degradation efficiency and regeneration have been significant obstacles. Consequently, there has been a surge in interest in recent years toward using nanomaterials with carbon materials activated by peroxymonosulfate for organic pollutant degradation. In this study, we present a novel approach to prepare a hybrid nanocomposite catalyst, CoS/CoFe2O4-CNTs (CS/CF-CNTs), using a carbon nanotube decorated with cobalt ferrite and further enhanced by embedding with cobalt sulfide nanoflowers. This catalyst aims at enhancing Rhodamine-B degradation through advanced oxidation processes. The carbon nanotubes provide a stable substrate for the cobalt materials, with cobalt ferrite (CF) serving as a magnetic component, facilitating catalyst removal and regeneration for multiple uses. Due to the oxidation involved in the degradation process, high electronic conductivity of the carbon nanotubes and the active cobalt sites of the composite play a crucial role in activating peroxymonosulfate to generate reactive oxygen radicals. Notably, the CS/CF-CNTs catalyst showed a remarkable Rhodamine-B degradation rate of 98% in less than 10 min. The catalyst also exhibited excellent stability even after four cycles of regeneration. The operating reaction conditions were optimized by investigating the effects of pH, dye concentration, salinity with different salts, catalyst dose, and peroxymonosulfate dose, and the results demonstrated the superior effectiveness of CS/CF-CNTs compared to CS and to CF-CNTs, emphasizing the synergistic interaction between the carbon nanotubes and the two cobalt materials. Quenching experiments revealed the involvement of sulfate and hydroxyl radicals in the degradation reaction mechanism.
1. Introduction
Water is an instrumental natural resource for the existence of humans and living organisms. Despite its importance, all living organisms are facing the serious problems of water shortage and pollution. Water pollution arises from domestic, sewage, agricultural, and industrial wastewater.1 Among the contaminants of great concern nowadays are the emerging contaminants. These are found in water at minute concentrations that can only be detected using sophisticated analytical instruments and could pose health hazards with prolonged exposure. Examples of emerging contaminants are pharmaceuticals and personal care products, pesticides, disinfectants, and others.2 This study is concerned with the emerging contaminant Rhodamine-B, which is a basic dye that is used in the textile industry. It is carcinogenic and neurotoxic through inhalation and ingestion.3 The value of the Rhodamine-B market in 2023 was 182.37 million USD, and it is expected to rise to 252.89 million USD in 2033 (Spherical insights, https://www.sphericalinsights.com/reports/rhodamine-b-market, accessed May 6th, 2024). It is persistent in the environment, with high stability and minimal degradation tendency.4 The concentration of Rhodamine-B in textile wastewater in Hayatabad Peshawar, Khyber Pakhtunkhwa, Pakistan was found to be in the range of 20–250 mg L−1.5 Different methods have been utilized for the removal of Rhodamine-B from wastewater, such as adsorption,6 membrane filtration7 and advanced oxidation processes.8 The method applied herein is an advanced oxidation process; these processes are characterized by the generation of strong hydroxyl or sulfate radicals that can degrade even the most recalcitrant contaminants in various types of wastewater.9 Various methods have been applied for the generation of radicals, including the conventional chemical method, along with other modern sonochemical, photochemical, and electrochemical techniques. Chemical methods deploy ozone, hydrogen peroxide, and persulfate, which are not effective on their own, but require activation.10 Herein, the chemical method is applied using the oxidant peroxymonosulfate or oxone (PMS). PMS can be activated via UV light or heat treatment. Alternatively, less-energy-intensive transition-metal-induced activation can be conducted using homogeneous or heterogeneous catalysts. Heterogeneous catalysts are preferable to homogeneous catalysts, as the former have less toxicity and are more recyclable.11 Rhodamine-B was efficiently and almost completely degraded by Fe/MCM-41 catalysts in the presence of H2O2 at an initial dye concentration of 100 ppm, 1 g L−1 catalyst dose, H2O2 dose of 20 mmol L−1, pH 4, and temperature of 80 °C.12In this work, cobalt sulfide/cobalt ferrite-carbon nanotubes (CS/CF-CNTs) catalysts are combined with PMS to potentially enhance the degradation of Rhodamine-B. Cobalt-based heterogeneous catalysts are known for their superiority over the Fenton system and higher standard potential compared to other transition metals.13 The presence of cobalt ferrite should impart magnetic properties to the composite, facilitating its collection from wastewater via a magnetic field after the degradation process. Carbon nanotubes are deployed due to their excellent surface area, catalytic properties, and electron transfer ability, which makes them good candidates for water treatment.14 Numerous reports have shown the superior performance of cobalt-based heterogeneous catalysts combined with PMS. For example, a cobalt/MOF with PMS degraded about 98% of 20 ppm Rhodamine-B in 30 min.15 Additionally, CoCN–NaBH4 (Co(OH)2–g-C3N4) was able to degrade almost 100% of 100 ppm orange G at a catalyst dose of 0.1 g L−1 and a PMS dose of 0.23 mM.16
This is the first report, to the best of our knowledge, on the degradation of Rhodamine-B using cobalt sulfide/cobalt ferrite-carbon nanotubes. This work investigates the surface morphologies and physicochemical properties of the CS/CF-CNTs hybrid nanocomposite catalyst using various techniques, such as X-ray diffraction, scanning electron microscopy, transmission electron microscopy, X-ray photoelectron spectroscopy, and nitrogen adsorption–desorption isotherms. A comprehensive study is conducted on the effect of the different operating parameters on the removal, including pH, contact time, initial concentration, catalyst dose, PMS dose, and salinity with different salts. The selectivity of the catalyst and PMS toward Rhodamine-B is tested in tap water and compared to that in distilled water. In addition, the reusability of the catalyst is assessed, and the removal mechanism is explained in view of the findings of the quenching experiments.
2. Materials and methods
2.1. Materials
Oxone (PMS; 2KHSO5), ferric nitrate nonahydrate (Fe(NO3)3·9H2O), ethanol (C2H5OH), cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O), acetic acid sodium salt (CH3COONa), sulfuric acid (H2SO4), nitric acid (HNO3), ethylene glycol (C2H6O2), and methanol (CH3OH) were purchased from Sigma-Aldrich (99% analytical grade). Thiourea (H2NCSNH2), sodium chloride (NaCl), and potassium sulfate (K2SO4) were purchased from Duksan Pure Chemicals Co., Ltd (95% analytical grade). CNTs were obtained from Korea Nano Company. Rhodamine-B was obtained from Loba Chemie, PVT, LTD. All the reagents were of analytical grade and were used as obtained. All the solutions were prepared using ultrapure water.2.2. Preparation of the CS/CF-CNTs hybrid nanocomposite
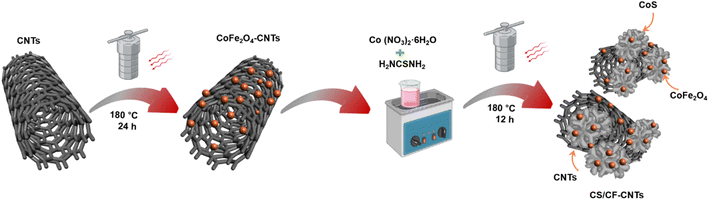
A CS/CF-CNTs hybrid nanocomposite was synthesized using a solvothermal technique. Initially, 357 mg of Co (NO3)2·6H2O were dissolved in 75 mL of C2H6O2 and ultrasonicated for 10 min to achieve complete dispersion. Subsequently, 252 mg of H2NCSNH2 were added to the mixture while ultrasonication continued. A solution of magnetic-CoFe2O4-CNTs, prepared using the procedure outlined in our previous report,11 was then added dropwise to the sonicated mixture. After 1 h of agitation, the resulting solution was transferred to a 100 mL polytetrafluoroethylene-lined autoclave. The reaction mixture underwent heating at 180 °C for 12 h in an oven, followed by cooling to room temperature as depicted in Fig. 1. The resultant black precipitate, identified as the CS/CF-CNTs composite, was washed three times with distilled water and subsequently dried in a vacuum oven at 70 °C for 6 h. CoS was synthesized using a similar procedure, excluding the addition of magnetic CoFe2O4-CNTs.2.3. Characterization of the as-synthesized (CS/CF-CNTs) nanocomposites
Various analytical techniques were employed to examine the hybrid nanocomposite prepared in this study. The crystalline structures of the samples were analyzed using X-ray diffraction (XRD) with Cu Kα radiation at 40 kV and 30 mA, scanning angles within the range of 10–70°, and a scan step of 0.02° (PANalytical, X'Pert-PRO MPD). High-resolution transmission electron microscopy (HR-TEM) at 200 kV (FEI Tecnai G2F20) and field emission scanning electron microscopy (FE-SEM) (Hitachi-Japan, S-4800) were used to investigate surface morphologies. X-ray photoelectron spectroscopy (XPS) with Al Kα monochromatized radiation (AXIS Nova) was employed to analyze the surface configuration and components of the samples. Infrared (IR) spectra were collected using a total reflectance infrared (ATR-IR) spectrometer (Bruker Instruments) in the range of 4000–400 cm−1 at a resolution of 4 cm−1. The degradation of Rhodamine-B was monitored using a Cary UV-vis Agilent G51911AA spectrophotometer. Furthermore, the hybrid nanocomposite sample underwent examination using a nitrogen adsorption–desorption apparatus (Micromeritics 3FlexSurface Characterization Analyzer) at −196 °C. Additionally, the Brunauer–Emmett–Teller (BET) surface area and pore size distribution (PSD) were estimated. The sample was degassed at 180 °C for 12 h before conducting the adsorption–desorption experiments. The magnetic properties of the samples were measured using a Lake Shore VSM 7307 vibration magnetometer. The PSD was determined using the Barrett–Joyner–Halenda (BJH) methodology, and the BET-specific surface area was calculated using the BET equation. Zeta potential measurements were performed at pHs from 3.1 to 11.1 to determine the charge of the catalyst at various pH values.2.4. Evaluating the catalytic degradation of CS/CF-CNTs with PMS activation
Catalytic degradation was studied at different catalyst and PMS doses in 100 mL of a 50 ppm Rhodamine-B solution without adjusting the pH (pH was about 3.3). The study was extended using various initial concentrations (10–50 ppm) of the dye in the presence of 10 mg catalyst and 60 mg PMS without pH adjustment. Similarly, the degradation kinetics were examined under the same conditions. The effect of pH was investigated in the range of 3.3–9.4 using the same catalyst and PMS doses indicated earlier. After degradation, 5 mL of the contaminant solution was quenched with 5 mL of sodium thiosulfate (0.5 M) and measured on a UV/VIS spectrophotometer at 554 nm. The obtained absorbance was substituted in a calibration curve to determine the remaining concentration, and the percent removal was determined as follows:where C0 is the initial concentration of Rhodamine-B, while Cr is the concentration of Rhodamine-B remaining after catalytic degradation.
2.5. Testing the regenerability, selectivity, and stability of the nanocomposite
A regeneration study was conducted over four successive cycles. In each cycle, 100 mL of a 10 ppm solution of Rhodamine-B was used at 0.1 g L–1 catalyst dose and 0.6 g L–1 PMS dose. Upon the completion of each catalytic degradation cycle, the CS/CF-CNTs hybrid nanocomposite catalyst was separated by applying an external magnetic field and then thoroughly washed with 10 mL of deionized water and dried overnight at 70 °C before commencing the subsequent recyclability experiment. The degradation efficiency of the catalyst in the presence of PMS was tested on 10 ppm Rhodamine-B in tap water and compared to that in distilled water. Additionally, the selectivity of the catalyst in the presence of PMS was tested on 50 ppm Rhodamine-B in the presence of salts such as NaCl and K2SO4 at 5 mM concentration.Quenching experiments were used to explore the underlying mechanism of pollutant degradation by identifying the reactive species responsible for the process. In this study, tert-butyl alcohol (TBA, 1 M) and ethanol (1 M) were utilized as quenching agents to differentiate between the hydroxyl radicals (˙OH) and sulfate radicals (SO4˙−) that are potentially involved in the degradation of Rhodamine-B. TBA selectively scavenges hydroxyl radicals due to its higher reactivity with ˙OH (rate constant ≈3.8–7.6 × 108 M−1 s−1) compared to its relatively slow interaction with sulfate radicals (rate constant ≈4–9.1 × 105 M−1 s−1). Conversely, ethanol acts as a dual scavenger, reacting with hydroxyl and sulfate radicals, but with different rate constants. This difference in bahavior enables a clearer understanding of which reactive species dominate under the applied experimental conditions. The quenching experiments were performed using a catalyst dosage of 10 mg, 60 mg of PMS, and a pollutant concentration of 50 mg L–1 Rhodamine-B in a reaction volume of 100 mL. To examine the catalyst's stability during Rhodamine-B degradation, leaching tests for cobalt and iron ions were conducted. The concentrations of leached cobalt and iron ions were measured using Inductively Coupled Plasma Optical Emission Spectroscopy (ICAP™ 7400 Duo, Bremen, Germany, ICP). Before analysis, calibration curves were constructed to quantify the metal ions precisely, with the detection wavelengths set at 371.993 nm for iron and 350.228 nm for cobalt.
3. Results and discussion
3.1. Characterization of the nanocatalysts
XRD analysis was conducted to examine the crystalline structure of the hybrid nanocomposite and identify the specific phases (Fig. 2). In the XRD pattern of the CNTs, notable peaks were observed at 26° and 43°, corresponding to the (0 0 2) and (1 0 0) planes, respectively. These peaks indicate the presence of concentric cylindrical features in the CNTs and suggest adequate graphitization with sp2-type atoms, devoid of carbon-based impurities or catalytic metal particles on their surfaces.17 For the CF-CNTs, distinct diffraction peaks were observed at 31.3°, 36.1°, 43.3°, 53°, 57.4°, and 63.5°, corresponding to the (2 2 0), (3 1 1), (4 0 0), (4 2 2), (5 1 1), and (4 4 0) diffraction planes, respectively. These peaks are characteristic of the spherical spinel single-phase CF.11,18 For the CS sample, the diffraction peaks appear at 31.05°, 35.68°, 47.13°, and 54.91° with low intensity, indicating the amorphous or nanocrystalline structure of CS, and these peaks correspond to the (204), (220), (306) and (330) planes, respectively.19 In the XRD pattern of the CS/CF-CNTs, the intensity slightly increased with a small shift at 47.13°, and the graphitic carbon peak could not be seen as a well-defined peak. This could be attributed to the characteristic (0 0 2) peak of graphitic carbon being weak, indicating that the carbon structures in CNTs are disordered. This observation aligns with previous reported findings.20To scrutinize its morphological structure, FE-SEM and HR-TEM analyses were conducted on the synthesized CS/CF-CNTs hybrid nanocomposite. As depicted in Fig. 3a, the morphology of the pristine CS exhibited a micro-flower-like structure,21 with spherical CF firmly anchored on the cylindrical structure of the CNTs and CS. The hydrothermal method seems to have facilitated the effective loading of CF onto the surfaces of the CNTs and CS. Fig. 3b presents the HR-TEM image of the CS/CF-CNTs hybrid nanocomposites, revealing the intertwining of CS with the CNT networks. Similar observations were demonstrated in the FE-SEM images. The successful fabrication of the CS/CF-CNTs hybrid nanocomposite is evident from the absence of free CF in the HR-TEM images and FE-SEM micrograph.
XPS analysis was employed to determine the valence state and elemental composition of the CS/CF-CNTs hybrid nanocomposites. The survey spectrum in Fig. 4a indicates the presence of Co, Fe, S, O, and C in the energy region, which is consistent with the XRD results. Further analysis of the Co 2p spectrum (Fig. 4b) based on the Gaussian fitting approach reveals two peaks at 785 and 802.8 eV, which correspond to the satellite peaks of Co2+,22 along with peaks at 778.5 and 793.7 eV corresponding to the Co 2p3/2 and 2p1/2 of cobalt sulfide.23 The peaks at 780.6 and 796.3 eV suggest the coexistence of Co3+ and Co2+, with Co3+ potentially providing additional active sites for degradation. The Fe 2p3/2 spectrum (Fig. 4c) shows peaks at 711.94, 716.8, 721.2, and 737 eV, indicating the Fe3+ and Fe2+ states. The S 2p spectrum (Fig. 4d) displays peaks at 168.5 eV (oxidized S), 164.5 and 162.5 eV (S–C bond), and 161.8 eV (CoS).24,25 In addition, Fig. 4e presents the high-resolution C 1s spectrum of the nanocomposites. The high-resolution C 1s spectrum (Fig. 4e) reveals peaks at 284.4 eV (C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds), 288.5 eV (O–C–O), and 285.31 eV (C–O/C–S),26 confirming tight bonding between C atoms and S atoms, indicating the attachment of CoS particles to the surface of the CNTs. Fig. 4f presents the deconvolution of the O 1s energy region. The two evident components have been attributed to the contribution of the crystal lattice oxygen (lower binding energy) and chemisorbed oxygen species (higher binding energy). The appearance of the latter in the shoulder peak might originate from oxygen uptake from the environment, water vapor or carbonaceous species.27
C bonds), 288.5 eV (O–C–O), and 285.31 eV (C–O/C–S),26 confirming tight bonding between C atoms and S atoms, indicating the attachment of CoS particles to the surface of the CNTs. Fig. 4f presents the deconvolution of the O 1s energy region. The two evident components have been attributed to the contribution of the crystal lattice oxygen (lower binding energy) and chemisorbed oxygen species (higher binding energy). The appearance of the latter in the shoulder peak might originate from oxygen uptake from the environment, water vapor or carbonaceous species.27
 | ||
| Fig. 4 XPS spectra of CS/CF-CNTs: (a) survey spectrum, (b) Co 2p, (c) Fe 2p, (d) S 2p, (e) C 1s, and (f) O 1s. | ||
To evaluate the magnetic properties of the prepared CS/CF-CNTs and CF-CNTs nanocomposites, the magnetization curve was measured at room temperature. As shown in Fig. 5, the saturation magnetization values of CS/CF-CNTs and CF-CNTs are 12.78 emu g−1 and 32.6 emu g−1, respectively. These values indicate that the materials are superparamagnetic.28 By comparing the magnetization curves, it can be observed that the magnetization ability of CS/CF-CNTs is lower than that of pure CF-CNTs. The decreased magnetic intensity of CS/CF-CNTs may be attributed to the CS coating. Nevertheless, the good magnetic responsiveness of the as-prepared nanocomposite enabled it to be separated conveniently with an external magnetic field, facilitating its collection and reuse.
The nitrogen adsorption–desorption isotherms of the CS/CF-CNTs hybrid nanocomposite were also obtained to determine its specific surface area (Fig. 6a) and its PSD (pore size distribution) (Fig. 6b). Following the classification scheme of the International Union of Pure and Applied Chemistry (IUPAC), the synthesized nanocomposite exhibits a type IV isotherm with a minor hysteresis loop, indicating the prevalence of mesopores along with some macropores, likely attributable to unoccupied spaces within the hybrid nanocomposite.29 This is supported by the PSD shown in Fig. 6b. As a result, the BET surface area was determined to be 34.7 m2 g−1, with the average pore size estimated at 22.4 nm and the pore volume at 0.175 cm3 g−1 using the BJH equation. The above characterization findings indicate that the CS/CF-CNTs hybrid nanocomposite possesses a mesoporous crystalline structure.
Fig. 7 shows a comparison between the different examined catalysts in their percent removal and time needed for the degradation of Rhodamine-B. As shown in the figure, CF-CNTs gave the lowest percent removal of about 83% in 20 min, whilst both CS and CS/CF-CNTs gave almost the same percent removal (about 99.5%) after 12 and 10 min, respectively. This indicates that the inclusion of magnetic cobalt ferrite did not affect the removal efficiency of the nanocomposite, but did affect the degradation rate. In this work, we focused on the catalytic degradation of Rhodamine-B using the CS/CF-CNTs nanocomposite as it provides a faster degradation rate than the other tested catalysts, and in addition it can be easily removed from wastewater by applying a magnetic field.
 | ||
| Fig. 7 Comparison between the degradation performance of several catalysts at 10 mg/100 mL catalyst dose and 60 mg/100 mL PMS at normal pH conditions for a Rhodamine-B concentration of 50 ppm. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 1
2 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250; the molar ratio used herein is 1
250; the molar ratio used herein is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51, which is in accordance with previously published literature.36
51, which is in accordance with previously published literature.36The removal efficiency of Rhodamine-B by the catalyst with PMS was investigated in the presence of the salts NaCl and K2SO4 at a concentration of 5 mM (as shown in Fig. 9b). The results indicated that the percentage removal and degradation times remained almost constant or slightly improved, suggesting that the presence of salts did not influence the degradation performance of the catalyst. This finding suggests that there was no competition between the salts and Rhodamine-B. Previous studies have stated that Cl− reacts with HSO5− to produce more active species, thereby enhancing removal efficiencies and reducing degradation times.39 Additionally, Cl− might generate HOCl/Cl2, which could accelerate dye degradation or react with sulfate radicals to generate the less-reactive Cl˙.40 Although sulfate salts have been reported to decrease reaction rates and deactivate the catalyst surface, this effect was not observed in this study, possibly because the salt concentration used was lower than in previous reports.41
| (Catalyst)III + HSO5− → (Catalyst)II + SO5˙− + H+ | (1) |
| (Catalyst)II + HSO5− → (Catalyst)III + SO4˙− + OH− | (2) |
| SO4˙− + H2O → OH˙ + SO42− + H+ | (3) |
| SO4˙− + OH− → OH˙ + SO42− | (4) |
| SO4˙−/OH˙ + Dye → H2O + CO2 (degradation product) | (5) |
This reaction mechanism was confirmed by quenching experiments in which 1 M ethanol and 1 M TBA were applied as quenching agents. It is well known that TBA quenches hydroxyl radicals (k1 = (3.8–7.6) × 108) while ethanol quenches both hydroxyl (k1 (OH˙, ethanol) = 1.2–2.8 × 109 M−1 s−1) and sulfate radicals (k1 (SO4˙−, ethanol) = 1.6–7.8 × 107 M−1 s−1).11 As can be seen from Fig. 10, TBA decreased the percent removal of Rhodamine-B by about 6.8%, while ethanol decreased the percent removal by 9%. This could imply that both sulfate radicals and hydroxyl radicals were involved in the reaction mechanism, with sulfate radicals being slightly more dominant. Notably, the reduction in percent removal by ethanol or TBA quenching was not substantial despite applying a 1 M concentration of each reagent. It could be that the degradation reaction rate is faster than the ethanol or TBA quenching rate, leading to minimal effects of quenching on the percent removal, or that the amount of the quenching agent was not sufficient to produce a prominent effect.10
4. Comparison with literature
Table 1 lists some examples of cobalt-based catalysts that were coupled with PMS for the degradation of Rhodamine-B. The results show that the activity of our catalyst exceeded that of Co-doped hydroxyapatite, FeCo-LDH, CoFe/SBA-15 and CoS-rGO in terms of catalyst/dye weight ratio. All listed catalysts showed degradation within an 8 to 15 min time frame except for CoFe/SBA-15, which required 60 min.| Catalyst | Initial concentration of dye | Conc. of catalyst | Conc. of PMS | pH | %R | Ref. |
|---|---|---|---|---|---|---|
| CoS-rGO | 14.37 mg L−1 | 0.25 g L−1 | 0.0056 g L−1 | 3–10 | 100% in 8 min | 47 |
| Co-doped hydroxyapatite | 40 mg L−1 | 0.2 g L−1 | 0.045 g L−1 | 5.5 | 93.3% in 12 min | 48 |
| Co/nitrogen-doped CNTs annealed at 700 °C | 20 mg L−1 | 0.02 g L−1 | 0.06 g L−1 | 5.67 | 99.05% in 7 min | 49 |
| CoFe/SBA-15 calcined at 700 °C | 5 mg L−1 | 0.1 g L−1 | — | No adjustment | >98% in 60 min | 50 |
| FeCo-LDH | 20 mg L−1 | 0.2 g L−1 | 0.15 g L−1 | No adjustment | >98% in 10 min | 51 |
| CS/CF-CNTs | 50 mg L−1 | 0.1 g L−1 | 0.6 g L−1 | No adjustment | >98% in 10 min | This work |
5. Conclusions
In this study, a magnetic CS/CF-CNTs catalyst was successfully synthesized via a simple hydrothermal process and exhibited excellent performance in activating PMS to degrade Rhodamine-B, a representative organic dye. The catalyst was fully characterized by various techniques to evaluate its physicochemical properties. Compared to CF-CNTs and CNTs, the CS/CF-CNTs nanocomposite displayed notably enhanced catalytic activity in removing Rhodamine-B with a degradation efficiency of >95% in only 10 min. The influence of different parameters on the degradation of Rhodamine-B was investigated, revealing pH, PMS concentration, and initial Rhodamine-B concentration as critical factors affecting the degradation rate. For example, by increasing the concentration of PMS from 20 to 60 mg/100 mL, the degradation time was decreased from 30 min to 10 min while the degradation efficiency was enhanced from 86.9% to 99.7%. Moreover, owing to its exceptional recyclability, this catalyst could be easily separated using an external magnet and reused multiple times, indicating its practical application value. This work provides a new strategy for developing an efficient reusable nanocatalyst for pollutant degradation via an environmentally safe process in which the magnetic catalyst is easily collected from water, thus mitigating its environmental impact.Data availability
The data supporting the findings of this study, including experimental results and application outputs, are available in the manuscript. Additional data are available upon reasonable request.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This study was supported by the American University in Cairo, and by the Priority Research Centers Program (NRF-2014R1A6A1031189) through the National Research Foundation of Korea (NRF), funded by the Korean Ministry of Education.References
- Z. Chen, G. Wu, Y. Wu, Q. Wu, Q. Shi, H. H. Ngo, O. A. V. Saucedo and H.-Y. Hu, Water Eco-Nexus Cycle System (WaterEcoNet) as a key solution for water shortage and water environment problems in urban areas, Water Cycle, 2020, 1, 71–77 CrossRef.
- S. Khan, M. Naushad, M. Govarthanan, J. Iqbal and S. M. Alfadul, Emerging contaminants of high concern for the environment: Current trends and future research, Environ. Res., 2022, 207, 112609 Search PubMed.
- A. A. Al-Gheethi, Q. M. Azhar, P. S. Kumar, A. A. Yusuf, A. K. Al-Buriahi, R. M. S. R. Mohamed and M. M. Al-Shaibani, Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review, Chemosphere, 2022, 287, 132080 CrossRef CAS PubMed.
- K. Mahdavi, S. Zinatloo-Ajabshir, Q. A. Yousif and M. Salavati-Niasari, Enhanced photocatalytic degradation of toxic contaminants using Dy2O3-SiO2 ceramic nanostructured materials fabricated by a new, simple and rapid sonochemical approach, Ultrason. Sonochem., 2022, 82, 105892 CrossRef CAS PubMed.
- J. Shah, M. Rasul Jan, A. Haq and Y. Khan, Removal of Rhodamine B from aqueous solutions and wastewater by walnut shells: kinetics, equilibrium and thermodynamics studies, Front. Chem. Sci. Eng., 2013, 7, 428–436 CrossRef CAS.
- W. Xiao, Z. N. Garba, S. Sun, I. Lawan, L. Wang, M. Lin and Z. Yuan, Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye, J. Cleaner Prod., 2020, 253, 119989 Search PubMed.
- R. A. Nasr and E. A. Ali, Polyethersulfone/gelatin nano-membranes for the Rhodamine B dye removal and textile industry effluents treatment under cost effective condition, J. Environ. Chem. Eng., 2022, 10, 107250 CrossRef CAS.
- P. Zawadzki and M. Deska, Degradation Efficiency and Kinetics Analysis of an Advanced Oxidation Process Utilizing Ozone, Hydrogen Peroxide and Persulfate to Degrade the Dye Rhodamine B, Catalysts, 2021, 11, 974 CrossRef CAS.
- Y. Deng and R. Zhao, Advanced oxidation processes (AOPs) in wastewater treatment, Curr. Pollut. Rep., 2015, 1, 167–176 Search PubMed.
- F. Ghanbari and M. Moradi, Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants, Chem. Eng. J., 2017, 310, 41–62 CrossRef CAS.
- M. Abdel-Salam and T. Yoon, Cobalt-ferrite/Ag-fMWCNT hybrid nanocomposite catalyst for efficient degradation of synthetic organic dyes via peroxymonosulfate activation, Environ. Res., 2022, 205, 112424 CrossRef CAS PubMed.
- D. Xu, X. Sun, X. Zhao, L. Huang, Y. Qian, X. Tao and Q. Guo, Heterogeneous Fenton degradation of rhodamine B in aqueous solution using Fe-loaded mesoporous MCM-41 as catalyst, Water, Air, Soil Pollut., 2018, 229, 1–9 Search PubMed.
- Q. Gao, G. Wang, Y. Chen, B. Han, K. Xia and C. Zhou, Utilizing cobalt-doped materials as heterogeneous catalysts to activate peroxymonosulfate for organic pollutant degradation: a critical review, Environ. Sci. Water Res. Technol., 2021, 7, 1197–1211 Search PubMed.
- S. Mishra and B. Sundaram, Efficacy and challenges of carbon nanotube in wastewater and water treatment, Environ. Nanotechnol. Monit. Manage., 2023, 19, 100764 CAS.
- T. Li, A. O. Omoniyi, Y. Wang, X. Hu and Z. Su, Enhancing dye degradation using a novel cobalt metal–organic framework as a peroxymonosulfate activator, Dalton Trans., 2024, 53, 3523–3533 RSC.
- A. B. Hamou, M. Enneiymy, S. Farsad, A. Amjlef, A. Chaoui, N. Nouj, A. Majdoub, A. Jada, M. Ez-zahery and N. El Alem, Novel chemically reduced cobalt-doped gC3N4 (CoCN-x) as a highly heterogeneous catalyst for the super-degradation of organic dyes via peroxymonosulfate activation, Mater. Adv., 2024, 5, 1960–1976 Search PubMed.
- Y. M. Moustafa, A. M. Al-Sabagh, S. A. Younis, M. M. Khalil and M. O. Abdel-Salam, Preparation of magnetic carbon nanotube nanocomposite for enhancing the separation of dissolved hydrocarbon from petroleum wastewater, J. Environ. Chem. Eng., 2017, 5, 2240–2250 Search PubMed.
- B. Babić-Stojić, V. Jokanović, D. Milivojević, Z. Jagličić, D. Makovec, N. Jović and M. Marinović-Cincović, Magnetic and structural studies of CoFe2O4 nanoparticles suspended in an organic liquid, J. Nanomater., 2013, 2013, 11 Search PubMed.
- L.-L. Ren, L.-H. Wang, Y.-F. Qin and Q. Li, One-Pot Synthesized Amorphous Cobalt Sulfide With Enhanced Electrochemical Performance as Anodes for Lithium-Ion Batteries, Front. Chem., 2022, 9, 818255 CrossRef PubMed.
- H. Wang, J. Ma, S. Liu, L. Nie, Y. Chai, X. Yang and R. Yuan, CoS/CNTs hybrid structure for improved performance lithium ion battery, J. Alloys Compd., 2016, 676, 551–556 CrossRef CAS.
- Q. Wang, L. Jiao, H. Du, W. Peng, Y. Han, D. Song, Y. Si, Y. Wang and H. Yuan, Novel flower-like CoS hierarchitectures: one-pot synthesis and electrochemical properties, J. Mater. Chem., 2011, 21, 327–329 RSC.
- X. Wang, Y. Xiao, D. Su, L. Zhou, S. Wu, L. Han, S. Fang and S. Cao, High-quality porous cobalt monoxide nanowires@ ultrathin manganese dioxide sheets core-shell nanowire arrays on Ni foam for high-performance supercapacitor, Electrochim. Acta, 2016, 194, 377–384 CrossRef CAS.
- Y. Wang, T. Zhu, Y. Zhang, X. Kong, S. Liang, G. Cao and A. Pan, Rational design of multi-shelled CoO/Co 9 S 8 hollow microspheres for high-performance hybrid supercapacitors, J. Mater. Chem. A, 2017, 5, 18448–18456 Search PubMed.
- S. J. Patil, J. H. Kim and D. W. Lee, Graphene-nanosheet wrapped cobalt sulphide as a binder free hybrid electrode for asymmetric solid-state supercapacitor, J. Power Sources, 2017, 342, 652–665 Search PubMed.
- J. Wang, Y. Zhang, J. Wang, L. Gao, Z. Jiang, H. Ren and J. Huang, Preparation of cobalt sulfide@ reduced graphene oxide nanocomposites with outstanding electrochemical behavior for lithium-ion batteries, RSC Adv., 2020, 10, 13543–13551 RSC.
- J. Huang, W. Wang, X. Lin, C. Gu and J. Liu, Three-dimensional sandwich-structured NiMn2O4@ reduced graphene oxide nanocomposites for highly reversible Li-ion battery anodes, J. Power Sources, 2018, 378, 677–684 Search PubMed.
- W. P. Wang, H. Yang, T. Xian and L. J. Jiang, XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route, Mater. Trans., 2012, 53, 1586–1589 CrossRef CAS.
- T. George, A. Sunny and T. Varghese, IOP Conf. Ser.:Mater. Sci. Eng., 2015, 73, 012050 Search PubMed.
- M. M. Rahman, A. Z. Shafiullah, A. Pal, M. A. Islam, I. Jahan and B. B. Saha, Study on Optimum IUPAC Adsorption Isotherm Models Employing Sensitivity of Parameters for Rigorous Adsorption System Performance Evaluation, Energies, 2021, 14, 7478 CrossRef CAS.
- X. Zhao, C. Niu, L. Zhang, H. Guo, X. Wen, C. Liang and G. Zeng, Co-Mn layered double hydroxide as an effective heterogeneous catalyst for degradation of organic dyes by activation of peroxymonosulfate, Chemosphere, 2018, 204, 11–21 CrossRef CAS PubMed.
- S. Luo, L. Duan, B. Sun, M. Wei, X. Li and A. Xu, Manganese oxide octahedral molecular sieve (OMS-2) as an effective catalyst for degradation of organic dyes in aqueous solutions in the presence of peroxymonosulfate, Appl. Catal., B, 2015, 164, 92–99 CrossRef CAS.
- M. Moazeni, S. M. Hashemian, M. Sillanpää, A. Ebrahimi and K.-H. Kim, A heterogeneous peroxymonosulfate catalyst built by Fe-based metal-organic framework for the dye degradation, J. Environ. Manage., 2022, 303, 113897 CrossRef CAS PubMed.
- D. A. Yaseen and M. Scholz, Impact of pH on the treatment of artificial textile wastewater containing azo dyes using pond systems, Int. J. Environ. Res., 2019, 13, 367–385 Search PubMed.
- M. A. Zazouli, F. Ghanbari, M. Yousefi and S. Madihi-Bidgoli, Photocatalytic degradation of food dye by Fe3O4–TiO2 nanoparticles in presence of peroxymonosulfate: The effect of UV sources, J. Environ. Chem. Eng., 2017, 5, 2459–2468 CrossRef CAS.
- N. T. Dung, T. V. Thu, T. Van Nguyen, B. M. Thuy, M. Hatsukano, K. Higashimine, S. Maenosono and Z. Zhong, Catalytic activation of peroxymonosulfate with manganese cobaltite nanoparticles for the degradation of organic dyes, RSC Adv., 2020, 10, 3775–3788 RSC.
- L. W. Matzek and K. E. Carter, Activated persulfate for organic chemical degradation: a review, Chemosphere, 2016, 151, 178–188 Search PubMed.
- M. Jimenez-Salcedo, M. Monge and M. T. Tena, The photocatalytic degradation of naproxen with g-C3N4 and visible light: Identification of primary by-products and mechanism in tap water and ultrapure water, J. Environ. Chem. Eng., 2022, 10, 106964 Search PubMed.
- I. Khan, N. Zada, I. Khan, M. Sadiq and K. Saeed, Enhancement of photocatalytic potential and recoverability of Fe 3 O 4 nanoparticles by decorating over monoclinic zirconia, J. Environ. Health Sci. Eng., 2020, 18, 1473–1489 Search PubMed.
- L. Zeng, L. Xiao, X. Shi, M. Wei, J. Cao and Y. Long, Core-shell Prussian blue analogues@ poly (m-phenylenediamine) as efficient peroxymonosulfate activators for degradation of Rhodamine B with reduced metal leaching, J. Colloid Interface Sci., 2019, 534, 586–594 CrossRef CAS PubMed.
- J. Di, R. Jamakanga, Q. Chen, J. Li, X. Gai, Y. Li, R. Yang and Q. Ma, Degradation of Rhodamine B by activation of peroxymonosulfate using Co3O4-rice husk ash composites, Sci. Total Environ., 2021, 784, 147258 CrossRef CAS PubMed.
- R. Jiang, D. Zhong, Y. Xu, H. Chang, P. Liao, Y. He and J. Zhang, Efficient degradation of rhodamine B by MoS2 modified Co-MOF derived nitrogen-doped carbon activated peroxymonosulfate, Colloids Surf., A, 2024, 685, 133184 CrossRef CAS.
- C. Li, J. Wu, W. Peng, Z. Fang and J. Liu, Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/β-FeOOH nanocomposites: Coexistence of radical and non-radical reactions, Chem. Eng. J., 2019, 356, 904–914 CrossRef CAS.
- C. Tan, N. Gao, Y. Deng, J. Deng, S. Zhou, J. Li and X. Xin, Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate, J. Hazard. Mater., 2014, 276, 452–460 CrossRef CAS PubMed.
- P. K. Klu, M. A. N. Khan, C. Wang, J. Qi, X. Sun and J. Li, Mechanism of peroxymonosulfate activation and the utilization efficiency using hollow (Co, Mn) 3O4 nanoreactor as an efficient catalyst for degradation of organic pollutants, Environ. Res., 2022, 207, 112148 CrossRef CAS PubMed.
- Iron in Well Water, 2025, https://www.health.state.mn.us/communities/environment/water/wells/waterquality/iron.html#:%7E:text=PointofreferenceAWaterwith,10mgFLinwater.
- N. K. Nagpal, Technical Report – Water Quality Guidelines For Cobalt, 2025, https://www2.gov.bc.ca/assets/gov/environment/air-land-water/water/waterquality/water-quality-guidelines/approved-wqgs/cobalt_tech.pdf Search PubMed.
- L. Amirache, F. Barka-Bouaifel, P. Borthakur, M. R. Das, H. Ahouari, H. Vezin, A. Barras, B. Ouddane, S. Szunerits and R. Boukherroub, Cobalt sulfide-reduced graphene oxide: An efficient catalyst for the degradation of rhodamine B and pentachlorophenol using peroxymonosulfate, J. Environ. Chem. Eng., 2021, 9, 106018 CrossRef CAS.
- Y. Pang, L. Kong, D. Chen, G. Yuvaraja and S. Mehmood, Facilely synthesized cobalt doped hydroxyapatite as hydroxyl promoted peroxymonosulfate activator for degradation of Rhodamine B, J. Hazard. Mater., 2020, 384, 121447 CrossRef CAS PubMed.
- J. Cheng, N. Wei, Y. Wang, Y. Long and G. Fan, Direct transformation of bulk cobalt foam into cobalt nanoparticles encapsulated in nitrogen-doped carbon nanotubes for peroxymonosulfate activation toward rhodamine B degradation, Sep. Purif. Technol., 2021, 277, 119441 CrossRef CAS.
- L. Hu, F. Yang, L. Zou, H. Yuan and X. Hu, CoFe/SBA-15 catalyst coupled with peroxymonosulfate for heterogeneous catalytic degradation of rhodamine B in water, Chin. J. Catal., 2015, 36, 1785–1797 CrossRef CAS.
- C. Gong, F. Chen, Q. Yang, K. Luo, F. Yao, S. Wang, X. Wang, J. Wu, X. Li and D. Wang, Heterogeneous activation of peroxymonosulfate by Fe–Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B, Chem. Eng. J., 2017, 321, 222–232 CrossRef CAS.
Footnote |
| † Authors contribute equally to the work. |
| This journal is © The Royal Society of Chemistry 2025 |