Open Access Article
Open Access ArticleStable synthesis mechanism and photocatalytic properties of TiO2 nanocrystals with different morphologies derived from potassium titanate nanowires†
Meng Chaiab,
Bo Yuanc,
Yao Band,
Jing Guo *ab,
Huifang Louab,
Hongyuan Kangab,
Qiaoling Zhangab,
Zhiwei Liuab and
Dongming Zhang
*ab,
Huifang Louab,
Hongyuan Kangab,
Qiaoling Zhangab,
Zhiwei Liuab and
Dongming Zhang ab
ab
aShanxi Province Key Laboratory of Chemical Process Intensification, School of Chemistry and Chemical Engineering, North University of China, Taiyuan 030051, China. E-mail: guojing0519@nuc.edu.cn
bState Key Laboratory of Coal and CBM Co-Mining, North University of China, Taiyuan 030051, China
cOrdos Carbon Neutral Research and Application Co., Ltd, Ordos 017010, China
dSinopec Maoming Company, Maoming 525099, China
First published on 26th February 2025
Abstract
This study explored key factors influencing TiO2 morphology using potassium titanate nanowires (KTNWs) as precursors. The pH of the hydrothermal solution was identified as critical in controlling TiO2 morphology. Different washing methods for precursors synthesized under alkaline conditions lead to varying pH environments in the subsequent hydrothermal solutions, thereby influencing the growth direction of TiO2 nuclei. Even a very slight pH change could cause a huge difference in the morphology of TiO2, by adjusting precursor washing methods, rod-like, cuboidal, and octahedral bipyramidal TiO2 nanocrystals were synthesized, the octahedral bipyramidal TiO2 exhibiting the smallest particle size. Acid treatment could stabilize the octahedral bipyramidal morphology of the TiO2 nanocrystal, and reduced particle size by nearly 86% than that of rod-like TiO2-R nanocrystal. Acid treated sample TiO2-R-H7 achieved the best photocatalytic activity, which was nearly 3 times than that of original TiO2. Adjusting (NH4)2CO3 morphology-controlling agent concentrations further regulated {001} facet exposure ratio to improve the photocatalytic activity, the TiO2-R-0.14 synthesized at 0.14 mmol per L (NH4)2CO3 showing about 2.6 times than that of original TiO2. However, excessive (NH4)2CO3 concentrations would reduce the photocatalytic activity due to increased particle size and fewer oxygen vacancies. This study provides insights into the growth mechanisms of TiO2 morphologies and highlights acid treatment as a strategy to reduce particle size and enhance photocatalytic activity, offering guidance for designing high-performance TiO2 based photocatalyst.
1. Introduction
Photocatalytic technology is extensively utilized in air purification, water treatment, and sterilization, offering an effective approach to mitigating environmental pollution. TiO2 has become one of the most widely used photocatalysts, due to its non-toxic, chemical stability, low cost, and strong photocatalytic activity. However, the wide bandgap of TiO2 and the rapid recombination of photogenerated electron–hole pairs restrict it to UV light excitation and result in low quantum efficiency, limiting its industrial applications.1 To address these limitations, TiO2 is commonly modified by methods such as ion doping, noble metal loading, semiconductor coupling, and facet engineering to enhance its photocatalytic efficiency.2,3 Among them, facet engineering normally using morphology-controlling agents to expose the high-energy crystal face of TiO2 to improve its photocatalytic activity. Furthermore, the heterojunction between the {001} and {101} facets can promote the migration and separation of photogenerated electron–hole pairs, further improving the photocatalytic activity of TiO2.4 However, following the principle of the lowest surface energy, TiO2 crystals will spontaneously expose the low-energy {101} crystal face with an energy of 0.44 J m−2 under natural conditions.5 To expose the high-energy {001} crystal face (0.90 J m−2), facet-controlling agents are needed to reduce its surface energy.6,7 Due to the slow hydrolysis rate of titanate nanowires, which is beneficial to the selective adsorption of morphology-controlling agents onto the {001} crystal face, many researchers choose titanate nanowires (K2Ti3O7 or Na2Ti3O7) as the precursor for morphology-controlled TiO2 synthesis.8,9 (NH4)2CO3 is inexpensive and readily available, can reduce the surface energy of the {001} facet, and the NH4+ can act as a pH buffer, providing a stable environment for TiO2 crystal growth.10 Therefore, this study employs (NH4)2CO3 as the morphology-controlling agent.The photocatalytic activity of the photocatalyst is not only related to the exposure of the crystal facet, but also related to its specific surface area. Previous studies have shown that acid treatment of titanates can reduce the particle size of hydrothermally synthesized TiO2 nanocrystals, increase their specific surface area, and thereby enhance their photocatalytic activity. Chacko et al. employed an alkaline hydrothermal method to convert TiO2 into potassium titanate nanowires, which was then subjected to acid treatment and ion exchange to form a titanate precursor (H2Ti3O7). Subsequent hydrothermal treatment yielded ultrafine TiO2 nanofibers. BET analysis revealed that the modified TiO2 had a specific surface area 2.5 times that of the pristine TiO2.11 Viet et al. conducted sodium titanate to water washing and acid treatment for 30 minutes, followed by hydrothermal treatment at 135 °C for 24 hours. The results showed that water washing produced irregular TiO2 nanoblocks with a particle size of approximately 38 nm, while acid treatment yielded TiO2 nanotubes with a diameter of about 10 nm and a specific surface area 2.03 times that of the original TiO2.12 Furthermore, acid treatment can reduce alkali metal ions in titanates and thereby modifying the morphology and particle size of hydrothermally synthesized TiO2. Xiong et al. treated sodium titanate nanowires with HNO3 at various concentrations for 6 hours. After acid treatment, the precursor was subjected to hydrothermal processing at 180 °C for 24 hours, the specific surface area of the produced TiO2 was 1.9 times that of the TiO2 synthesized without precursor acid treatment. The photocatalytic degradation efficiency of this TiO2 for MO was twice that of commercial P25. This acid treatment reduced the Na+ content in the precursor from 7.37% to zero, resulting in a morphological transition of the synthesized TiO2 from rod-shaped to spherical, indicating that its morphology is significantly influenced by alkali metal ions of the precursor.13 Wei et al. utilized potassium titanate nanowires as a precursor, conducting microwave-assisted hydrothermal treatment at 160 °C for 6 hours, resulting in octahedral bipyramidal TiO2 nanocrystals with a particle size of 17.2 nm. When using acid-treated potassium titanate nanowires as the precursor, hydrothermal synthesis similarly yielded octahedral bipyramidal TiO2 with a particle size of approximately 16.24 nm.14,15 It has been observed that, after acid treatment of titanate nanowires, the morphology of TiO2 obtained through hydrothermal synthesis varies among different studies. Some researchers claimed that alkali metal ion content influences TiO2 morphology, while other experimental results show no significant effect. Therefore, it is necessary to investigate whether the alkali metal ion content in the precursor is a primary factor affecting the morphology of synthesized TiO2.
Studies have also indicated that the pH of the hydrothermal solution can influence the morphology of TiO2. Du et al. treated potassium titanate nanowires with HCl, subsequently adjusting the pH of the hydrothermal solution with HCl and tetramethylammonium hydroxide at a hydrothermal temperature of 180 °C. When pH ≤ 3, rod-like TiO2 crystals with {111} facets, approximately 90 nm in length and 20 nm in width, were synthesized. At pH 5, octahedral bipyramidal TiO2 crystals with {010} facets and a particle size of 50 nm were obtained. When pH was between 7 and 14, spindle-shaped TiO2 nanocrystals, approximately 200 nm in length and 30 nm in width, with exposed {001} facets were produced.16 Wei et al. used titanates as precursors, performing microwave-assisted hydrothermal synthesis of TiO2 at 160 °C for 6 hours under pH values of 3, 6, and 11. The results showed that in both acidic and neutral environments, bipyramidal TiO2 nanocrystals of approximately 30 nm were synthesized, the particle size in neutral condition was slightly larger than acidic condition. When KOH was added to adjust the pH to 11, rod-like TiO2 nanocrystals approximately 80 nm in length were obtained.14 Chen et al. performed hydrothermal synthesis of TiO2 nanocrystals at 175 °C after acid treatment of titanates. At pH 3.5, they obtained tetragonal TiO2 nanocrystals with {101} facets and a particle size of 20 nm; at pH 11.5, they synthesized prismatic TiO2 nanocrystals with {100} facets, 150 nm in length and 30 nm in width; when the pH was raised to 13.5, irregular sheet-like TiO2 nanocrystals with {101} facets were obtained.17 The above studies indicate that the pH of the solution significantly impacts the morphology of TiO2 nanocrystals synthesized hydrothermally from titanate precursors. However, TiO2 nanocrystals synthesized by different researchers under similar hydrothermal temperatures and comparable pH conditions exhibit notable differences in morphology and particle size. Our previous work also revealed significant morphological differences in TiO2 nanocrystals synthesized in different batches under identical conditions. Therefore, when synthesizing TiO2 nanocrystals from K2Ti3O7 nanowires as a precursor, it is evident that factors such as hydrothermal pH, temperature and time, acid treatment of the precursor or not play crucial roles. Thus, it is essential to investigate the key factors affecting the morphology of TiO2 nanocrystals under controlled reactant concentration, hydrothermal temperature, and reaction time. This approach will enable the controlled synthesis of TiO2 nanocrystals, ensure batch-to-batch stability of photocatalysts, and allow facet engineering to further enhance the photocatalytic activity of TiO2 nanocrystals.
In summary, this study investigates the effect of the pH of the hydrothermal solution on the morphology of TiO2 nanocrystals by controlling the washing method of K2Ti3O7 nanowire precursors. The K+ content in K2Ti3O7 is regulated by controlling the acid treatment duration of K2Ti3O7 nanowires to explore the primary factors influencing the morphology and particle size of the synthesized TiO2. Based on stable TiO2 morphology synthesis, K2Ti3O7 nanowires treated under optimal acid conditions are used as precursors, and a series of TiO2 nanocrystals with varied {001} facet exposure ratios are synthesized by altering the concentration of (NH4)2CO3 as a morphology-controlling agent. The photocatalytic degradation performance of these nanocrystals is evaluated using methylene blue as the target pollutant, aiming to produce TiO2 nanocrystals with controlled morphology and enhanced photocatalytic activity and to provide insights for the preparation of high-activity photocatalysts.
2. Experimental
2.1 Preparation of potassium titanate nanowires
A total of 1.0 g of anatase TiO2 nanoparticles with a particle size of 20 nm was weighed and dispersed in 70 mL of 10 mol per L KOH solution. The mixture was subjected to ultrasonic treatment for 30 minutes to form a homogeneous white suspension. Subsequently, the suspension was transferred to a Teflon-lined stainless-steel autoclave for hydrothermal treatment at 180 °C for 16 hours. After the reaction, the autoclave was allowed to cool naturally to room temperature inside the oven. The reaction products were washed thoroughly using deionized water or a combination of acid washing followed by water washing to remove impurities. The washed products were separated via centrifugation and then dried to obtain the potassium titanate nanowire precursor (KTNWs). The synthesized potassium titanate nanowires were designated as R-KTNWs.2.2 Potassium titanate nanowires acid treatment
1.0 g R-KTNWs precursor was added to 60 mL of 1 mol per L HCl solution. The mixture was stirred for varying durations, followed by centrifugation to separate the solid product. The separated product was washed repeatedly with deionized water until the washing solution reached neutral pH. After the final centrifugation, the product was dried and collected for further use.2.3 Preparation of TiO2 nanocrystals
A total of 0.1 g of R-KTNWs was dispersed in 70 mL of deionized water and subjected to ultrasonic treatment to ensure uniform dispersion. The resulting suspension was transferred into a Teflon-lined stainless-steel autoclave and subjected to hydrothermal treatment at 180 °C for 16 hours. After the reaction, the autoclave was allowed to cool naturally to room temperature in the oven. The products were washed repeatedly with deionized water until the supernatant reached a neutral pH. Finally, the products were dried to obtain the series of TiO2 nanocrystals.The preparation conditions are summarized in Table 1. Specifically, the sample labeled as TiO2-R refers to TiO2 obtained from R-KTNWs without acid treatment, while TiO2-R-H0 denotes TiO2 nanocrystals prepared via hydrothermal treatment following HCl solution washing. For samples labeled as TiO2-R-HX, the value of X represents the duration of acid treatment in hours.
| R-KTNWs/g | H2O/mL | Acid treatment time/h | Named |
|---|---|---|---|
| 0.1 | 70 | — | TiO2-R |
| 0.1 | 70 | Washing | TiO2-R-H0 |
| 0.1 | 70 | 7 | TiO2-R-H7 |
| 0.1 | 70 | 24 | TiO2-R-H24 |
| 0.1 | 70 | 48 | TiO2-R-H48 |
2.4 Preparation of TiO2 nanocrystals with exposed {001} facets
0.1 g R-KTNWs precursor was added to (NH4)2CO3 solutions of varying concentrations. The mixture was ultrasonically dispersed to form a homogeneous suspension, which was then transferred to a Teflon-lined stainless-steel autoclave for hydrothermal treatment at 180 °C for 16 hours. After the reaction, the product was separated via centrifugation and washed thoroughly with distilled water until the supernatant became neutral. The washed product was subsequently dried for further analysis. The detailed preparation conditions are summarized in Table 2. In the sample notation TiO2-R-H7-Y, Y denotes the concentration of (NH4)2CO3 in the solution.| R-KTNWs/g | H2O/L | (NH4)2CO3/mmol L−1 | Acid treatment time/h | Named |
|---|---|---|---|---|
| 0.1 | 70 | 0 | 0 | TiO2-R |
| 0.1 | 70 | 0.07 | 0 | TiO2-R-0.07 |
| 0.1 | 70 | 0.14 | 0 | TiO2-R-0.14 |
| 0.1 | 70 | 0.28 | 0 | TiO2-R-0.28 |
| 0.1 | 70 | 0 | 7 | TiO2-R-H7 |
| 0.1 | 70 | 0.07 | 7 | TiO2-R-H7-0.07 |
| 0.1 | 70 | 0.14 | 7 | TiO2-R-H7-0.14 |
2.5 Characterization
Scanning electron microscopy (SEM, SIGMA500, Germany) and transmission electron microscopy (TEM, Talos F200S, G2, America) were used to observe the microscopic morphology of the samples. The light absorption capacity of the samples was analyzed and measured using a UV-visible spectrometer (Agilent Carry5000, America) with BaSO4 as the reference and a scanning wavelength range of 200–800 nm. The steady-state photoluminescence (PL) spectra were recorded using an FL fluorescence spectrophotometer (F-7000, Japan) with a stimulating wavelength range of 260 nm. The paramagnetic resonance spectrometer (EPR, Bruker MS 5000X, Germany) was used to test the active substances of the samples. The crystal structure of TiO2 catalyst was determined by X-ray diffractometer (XRD, D8 Advance). The scanning range was 2θ = 20–80°, the scanning speed was 10° min−1, the step size was 0.02°, the X-ray source was Cu Kα, the tube voltage was 40 kV, and the tube current was 100 mA.2.6 Photocatalytic evaluation
The photocatalytic degradation of methylene blue (MB) dye was investigated under a 15 W, 365 nm UV lamp to evaluate the photocatalytic activity of the series of photocatalysts. A total of 30 mg of TiO2 catalyst was added to 30 mL of 10 mg per L MB solution. The mixture was stirred in the dark for 30 min to achieve adsorption–desorption equilibrium. After centrifugation, the supernatant was collected, and its absorbance at 664 nm was measured and recorded as A0. Subsequently, samples were taken at regular intervals during UV irradiation, and the corresponding absorbance values were measured and recorded as Ai. The degradation rate of the MB dye under UV irradiation was calculated using eqn (1).
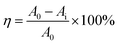 | (1) |
At low dye concentrations, the TiO2 photocatalytic reaction follows pseudo-first-order kinetics. The degradation kinetics were fitted using eqn (2), expressed as:
| ln(ct/c0) = −kappt | (2) |
3. Results and discussion
3.1 Effect of pH on the morphology of TiO2 nanocrystals
 | ||
| Fig. 1 SEM images of KTNWs precursor and TiO2 nanocrystals with different morphologies (a) R-KTNWs, (b) TiO2-R-H0, (c) TiO2-R, (d) TiO2-R-alk. | ||
Using the R-KTNWs as precursors, further hydrothermal treatment can produce a series of TiO2 nanocrystals. Analysis of experimental results from different batches indicates that, under constant reactant concentration, hydrothermal temperature, and reaction time, the surface properties of the potassium titanate precursor are likely the critical factors determining the final morphology of the TiO2 nanocrystals. The transformation of commercial TiO2 to R-KTNWs occurs in a strongly alkaline environment. Achieving a completely neutral product through simple deionized water washing is challenging. Although washing steps were standardized, variations in water volume and washing duration affected the thoroughness of precursor washing. Differences in the washing degree of R-KTNWs result in varying pH of the subsequent hydrothermal solution, potentially influencing the morphology of the synthesized TiO2 nanocrystals.
To validate this hypothesis, a series of experiments were conducted. R-KTNWs were prepared hydrothermally using anatase TiO2 nanoparticles as the raw material. The nanowires were then subjected to different washing treatments, including acid washing followed by deionized water washing to subacidity, repeated deionized water washing to slight alkalinity, and deionized water washing resulting in weakly alkaline conditions. The treated precursors were hydrothermally processed at 180 °C for 16 h to produce TiO2 nanocrystals, with their morphologies characterized via SEM, as shown in Fig. 1(b–d). Fig. 1(b) demonstrates that TiO2-R-H0 nanocrystals with a bipyramidal morphology and an average particle size of approximately 52 nm were obtained when the R-KTNWs were acid-washed and then washed with water to subacidity. In contrast, washing the nanowires only with deionized water to slight alkalinity yielded rod-like TiO2 nanocrystals under identical conditions, named TiO2-R (Fig. 1(c)), with an average width of ∼103 nm and length of ∼303 nm. When the R-KTNWs were insufficiently washed and remained weakly alkaline, the hydrothermal process resulted in cuboidal TiO2 nanocrystals, named TiO2-R-alk, as shown in Fig. 1(d), with dimensions of ∼384 nm in length and ∼228 nm in width. As show in Fig. 2, the high-resolution transmission electron microscopy (HRTEM) revealed that the lattice fringes of the TiO2 with the three different morphologies exhibited a spacing of 0.35 nm, corresponding to the {101} crystal plane, which is the most stable facet under natural conditions.
 | ||
| Fig. 2 TEM images of TiO2 nanocrystals with different morphologies (a) TiO2-R-H0, (b) TiO2-R, (c) TiO2-R-alk. | ||
The results demonstrate that the washing method of R-KTNWs precursors indirectly regulates the pH environment of the subsequent hydrothermal solution. Acid washing followed by water rinsing produces a slightly acidic solution, extensive repeated water washing results in a slightly alkaline solution, and insufficient washing leads to a more alkaline solution. These variations in the hydrothermal pH environment ultimately result in TiO2 nanocrystals with different morphologies. Although the pH of the final solution through the above three washing methods is close to 7, subtle changes in the pH of the hydrothermal solution played a critical role in controlling the morphology of TiO2 nanocrystals. Additionally, as the pH of the solution increases, the particle size of TiO2 nanocrystals also increases. TiO2 nanocrystals obtained from acid-washed R-KTNWs precursors exhibit smaller particle sizes and larger specific surface areas, which are more advantageous for enhancing photocatalytic activity.
| K2−xHxTi3O7 + (7 − 0.5x)H2O → 3Ti(OH)4 + (2 − x)KOH | (3) |
| Ti(OH)4 → TiO2 + 2H2O | (4) |
 | ||
| Fig. 3 The growth mechanism of TiO2 nanocrystals with different morphologies synthesis during different hydrothermal condition. | ||
Because of the anisotropy in adsorption stability of the capping agents, the adsorbates adsorbed onto specific crystallographic faces more strongly than others. This reduces the surface energy of the bound facet and hinders the growth of crystals or some crystal facets. Barnard et al. investigated the surface structures and energies of selected low-index surfaces for both rutile and anatase forms of TiO2. They discovered that under basic conditions, the oxygen-terminated {100} facets exhibit lower surface energy compared to the oxygen-terminated {101} and {001} facets.19,20 Yu et al. also believed that the OH−, which are gradually released from Na-titanate nanotubes, would preferentially attach to the anatase {100} facets. This selective adsorption lowers the surface energy of the {100} facets, thereby restricting the crystal growth along the a- and b-axes, the concentration of OH− would gradually decrease with the reaction, resulting in spindle or round-rod shapes.18
As illustrated in Fig. 3, in the present system, R-KTNWs with deionized water washing resulting in weakly alkaline conditions, we consider that the following hydrothermal reaction at a fixed basic condition, the selective adsorption of OH− would lower the surface energy of the {100} facets, thereby restricting the crystal growth along the a- and b-axes, the octahedral Ti(OH)4 fragments connect edge-sharing both longitudinally and laterally, with surface-edge adjustments leading to the formation of cuboidal TiO2 nanocrystals. With repeated deionized water washing to slight alkalinity close to neutral hydrothermal environment, the OH− gradually released from R-KTNWs, would preferentially attach to the anatase {100} facets and lowers the surface energy of the {100} facets, thereby restricting the crystal growth along the a- and b-axes. In this case no extra OH−, the concentration of OH− would gradually decrease with the reaction, the octahedral Ti(OH)4 fragments connect edge-sharing along the equatorial plane and point-sharing longitudinally, eventually forming rod-like TiO2 nanocrystals. R-KTNWs with acid washing followed by deionized water washing to subacidity close to neutral, part of K2Ti3O7 nanowires were transformed into H2Ti3O7 nanowires. The splitting of the nanowires maybe along the {101} and {−101} facets and the growth of nanocrystals are accompanied by Ostwald ripening process, ultimately forming octahedral bipyramidal TiO2 nanocrystals.21
Studies have shown that the morphology of TiO2 nanocrystals is primarily governed by the growth rates along the [001] and [101] directions.22 Under natural conditions, the surface energy of the {001} facet is higher (0.90 J m−2), and the growth rate along the [001] direction is faster. According to the principle of minimum surface energy, the {101} crystal plane with lower surface energy (0.44 J m−2) will eventually be exposed to minimize the surface energy.23 By adjusting the pH of the solution through different washing methods, we obtained TiO2 nanocrystals with varied morphologies, all contain {101} crystal plane, as shown in Fig. 2. This observation indicated that, the change of the pH of the hydrothermal solution will change the crystal growth mechanism to obtain TiO2 with different morphologies, but will not change the characteristics of tending to expose low-energy {101} crystal facet to minimize the total surface energy of TiO2 and make the system the most stable.
3.2 Effect of acid treatment of R-KTNWs on the morphology of TiO2 nanocrystals
Fig. 4(a–c) show SEM images of R-KTNWs precursors subjected to different acid treatment durations. Fig. 4(a) and (b) represent the SEM images of untreated R-KTNWs and those treated with acid for 7 hours, respectively. In both images, the R-KTNWs exhibit a uniform size distribution and clear, well-defined structures, with the nanowires intertwined. There are no significant morphological changes before and after acid treatment, indicating that a certain duration of acid treatment does not alter the macrostructure of the R-KTNWs. Liu et al. treated sodium titanate nanowires with 0.6 mol per L HCl for 1 h, HRTEM also showed that the morphology of the nanowires did not change before and after acid treatment, but the lattice fringe spacing changed from 0.81 nm to 0.78 nm after Na+ were replaced by H+.25 As shown in Fig. 4(c), after further extending the acid treatment time to 48 h, although the appearance morphology still maintains the nanowire structure, the nanowires are significantly shortened and messy compared with the non-acid treatment, and some nanowires also appear to adhere to each other. If the acid treatment is prolonged to 72 h, the R-KTNWs nanowires are almost completely dissolved in the acid solution, indicating that the acid treatment duration should not be excessively long. Nakahira et al. also claimed that the symmetry of the Ti environment in TiO2-derived titanate nanotubes has hardly changed with the post HCl treatment, local structures around Ti such as the coordination geometry in TiO2-derived titanate nanotubes have changed with the acid treatment using HCl, and the excess acid treatment led to the disordering, and resulted in the change of the morphology.26
 | ||
| Fig. 4 SEM images of R-KTNWs nanowires treated with acid for different time, (a) 0 h, (b) 7 h, (c) 48 h, (d) EDS spectra. | ||
Therefore, it is essential to strictly control the preparation and washing processes of R-KTNWs in experiments to ensure that the surface properties of the nanowires remain consistent across batches and to minimize their impact on subsequent experiments. Fig. 4(d) shows the EDS spectra of R-KTNWs before and after 7 hours of acid treatment. The spectra reveal a significant reduction in the relative content of K+ after acid treatment, with most K+ ions being replaced by H+ ions. The proportion of K+ decreases from approximately 10% to about 3% after 7 hours of acid treatment. As discussed previously, after K+ were replaced by H+, both of the interlayer spacing and the thermal stability of the nanowires decreased.26 The influence of the acid treatment duration of R-KTNWs precursors on the particle size, morphology, and photocatalytic activity of the TiO2 nanocrystals were further investigated by TEM and photocatalytic activity test.
It can be concluded that the variation in K+ content within potassium titanate nanowires is not the primary factor driving changes in TiO2 morphology. Instead, the pH of the hydrothermal solution is the main determinant of the formation of bipyramidal TiO2 nanocrystals. In acidic environments, TiO2 nuclei grow into bipyramidal structures through point-sharing and edge-sharing mechanisms.27 The reduction in particle size is attributed to the decrease in K+ content caused by acid treatment, which reduces the interlayer spacing of potassium titanate and ultimately results in smaller TiO2 nanocrystals.28 Smaller particle sizes correspond to larger specific surface areas, which are beneficial for enhancing photocatalytic activity.29 However, excessively long acid treatment durations can overly reduce K+ content, compromise the stability of potassium titanate nanowires, and decrease the crystallinity of TiO2 nanocrystals, leading to the formation of irregular morphologies.30
The mechanism of morphology and particle size changes in TiO2 nanocrystal after acid treatment of R-KTNWs was illustrated in Fig. 6. During acid treatment, K+ ions in R-KTNWs are gradually replaced by H+ ions. With prolonged acid treatment, the structurally stable R-KTNWs undergoes protonation to form H2Ti3O7. H2Ti3O7 exhibits weak acidity and a negative zeta potential. During hydrothermal conversion, its negatively charged surface attracts H+ ions from the reaction medium, resulting in the protonation of Ti–OH groups to form Ti–OH2+.31 Two Ti(OH)4 fragments then undergo rearrangement and dehydration to polymerize into TiO2 crystal nucleus, which grow along the {101} plane.
This transition from H2Ti3O7 to anatase TiO2 nanocrystal involves the in situ dehydration and rearrangement of Ti(OH)4 structural units and is thus considered a topochemical reaction, ultimately yielding octahedral bipyramidal TiO2 nanocrystals.31 The replacement of K+ ions in R-KTNWs during this process reduces the interlayer spacing, which in turn decreases the particle size of the TiO2 nanocrystals.
3.3 Photocatalytic activity of acid-treated TiO2 nanocrystals
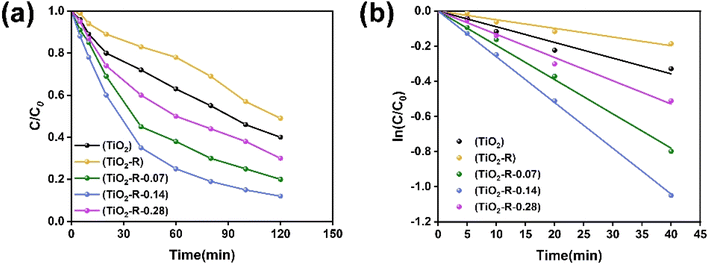
To explore the relationship between precursor acid treatment conditions and the photocatalytic activity of the synthesized TiO2 nanocrystals, a series of TiO2 nanocrystals were subjected to methylene blue (MB) photocatalytic degradation experiments. As illustrated in Fig. 7, TiO2 nanocrystals prepared from acid-treated R-KTNWs precursors exhibited better photocatalytic activity than those prepared without acid treatment (TiO2-R). However, as the acid treatment duration increased, the photocatalytic activity of the TiO2 nanocrystals initially increased and then decreased. Among the samples, TiO2-R-H7, synthesized from a precursor acid-treated for 7 hours, showed the fastest degradation rate of MB, with photocatalytic activity nearly 3 times and 5 times higher than that of the original anatase TiO2 and TiO2-R, respectively. | ||
| Fig. 7 Photocatalytic activity diagram of TiO2 nanocrystals synthesized after precursor acid treatment (a) photocatalytic degradation diagram of MB, (b) photodegradation rate diagram. | ||
The enhanced photocatalytic activity can be attributed to the morphological and particle size changes of the TiO2 nanocrystals after acid treatment. As previously analyzed, the acid treatment of R-KTNWs precursors transforms the morphology of the synthesized TiO2 from nanorods to bipyramidal structures, with particle size decreasing from 303 nm to 42 nm or smaller. The improvement in TiO2 crystallinity and the increase in specific surface area significantly enhance the photocatalytic activity of the TiO2 nanocrystals. As show in Fig. 5, with the extension of acid treatment time to 24 h or even 48 h, although the particle size was further reduced to 37.2 nm, the proportion of octahedral bipyramidal TiO2 structures decreases, and irregular morphologies emerge due to the excess acid treatment, resulting in a decline in photocatalytic activity. Additionally, acid treatment converts most of the Ti–O–K bonds on the surface of R-KTNWs into Ti–OH bonds, as H+ replaces the larger K+ ions. This substitution increases the number of surface oxygen vacancies, the hydroxyl group content, thereby enhancing the photocatalytic activity. However, with the excess acid treatment, the content of hydroxyl groups and oxygen vacancies decreases due to the continued action of the acid, leading to a reduction in photocatalytic activity.32,33
The oxygen vacancies of acid-treated TiO2 nanocrystals were analyzed using EPR, and the results are shown in Fig. 8. Theoretically, within a certain range, a higher content of oxygen vacancies is beneficial for enhancing photocatalytic activity. However, excessive oxygen vacancies can act as recombination centers for photogenerated charge carriers, leading to a decline in photocatalytic activity of TiO2.34
 | ||
| Fig. 8 Oxygen vacancy diagram of TiO2 nanocrystals synthesized by acid treatment for different time. | ||
As shown in Fig. 8, no oxygen vacancies were detected in the original anatase TiO2. The rod-like TiO2-R, synthesized from untreated precursors, exhibited the highest oxygen vacancy content at g = 2.003. The bipyramidal TiO2-R-H7, obtained after 7 hours of acid treatment, displayed a slightly lower oxygen vacancy peak intensity. The oxygen vacancy content further decreased in the TiO2-R-H24 sample synthesized after 24 hours of acid treatment. These findings indicate that the synthesis method employed in this study effectively promotes the formation of oxygen vacancies in TiO2. However, prolonged acid treatment of KTNWs will reduce the oxygen vacancy content in TiO2.
From the photocatalytic activity results in Fig. 7, it can be observed that, despite the high oxygen vacancy content, the rod-like TiO2-R nanocrystals exhibit relatively low photocatalytic activity due to their larger particle size. As the precursor acid treatment time increases, the oxygen vacancy content on the TiO2 surface gradually decreases, and the crystallinity deteriorates, ultimately resulting in reduced photocatalytic activity. This demonstrates that the photocatalytic activity of TiO2 is influenced by multiple factors, including particle size, morphology, and oxygen vacancy content. Moderate acid treatment of KTNWs precursors provides a stable approach for synthesizing bipyramidal TiO2 nanocrystals with reduced particle size and increased surface oxygen vacancy content. This is one of the effective methods to enhance the photocatalytic activity of TiO2.
3.4 Morphology of TiO2 nanocrystals controlled by (NH4)2CO3
Based on the previous work, this study employed (NH4)2CO3 as a morphology-controlling agent to investigate whether exposing highly active {001} crystal facets while maintaining small particle sizes can further enhance the photocatalytic activity of TiO2. Fully washed and acid-treated 7 h R-KTNWs precursors were selected for subsequent crystal facet control experiments. By varying the concentration of the (NH4)2CO3 solution during the hydrothermal process, the exposure ratio of the {001} crystal facets was controlled. The morphologies of the two groups of TiO2 samples are shown in Fig. 9.As shown in Fig. 9(a), using fully washed R-KTNWs as precursors, TiO2-R samples without (NH4)2CO3 were hydrothermally synthesized into rod-like TiO2 nanocrystals with a length of 303 nm and a width of 103 nm. When varying concentrations of (NH4)2CO3 morphology-controlling agents were introduced during hydrothermal synthesis using the same R-KTNWs precursors, truncated decahedral TiO2 nanocrystals were obtained. Fig. 9(b–d) illustrate the morphologies of TiO2-R-0.07, TiO2-R-0.14, and TiO2-R-0.28, corresponding to (NH4)2CO3 concentrations of 0.07 mmol L−1, 0.14 mmol L−1, and 0.28 mmol L−1, respectively. The average particle sizes of these samples were 68 nm, 98 nm, and 105 nm, respectively. Calculations showed that the proportion of exposed {001} facets was 7%, 11%, and 15%, respectively. As the proportion of {001} facets increased, the particle size of the TiO2 nanocrystals also gradually increased. The study of Han et al. also shown the phenomenon that the particle size of TiO2 nanocrystals increases with the increase of urea concentration.35 In this work, the adsorption of NH4+ on the {001} facet reduced the surface energy, changed the crystal growth direction and finally exposed the {001} facet. It is speculated that the higher the concentration of (NH4)2CO3, the more NH4+ adsorbed on the {001} plane, and the proportion of {001} plane also increased, which ultimately increased the particle size.
As shown in Fig. 9(e), when R-KTNWs acid-treated for 7 hours were used as precursors without the addition of (NH4)2CO3, bipyramidal TiO2 nanocrystals with an average particle size of approximately 42 nm were obtained. Compared to the rod-like TiO2 shown in Fig. 9(a), the particle size was reduced by approximately 86%. When the acid-treated 7 h R-KTNWs were hydrothermally synthesized in (NH4)2CO3 solutions with concentrations of 0.07 mmol L−1, 0.14 mmol L−1, and 0.28 mmol L−1, truncated decahedral TiO2 nanocrystals exposing {001} facets were similarly obtained, as shown in Fig. 9(f–h). The proportions of exposed {001} facets were approximately 6%, 10%, and 12%, respectively, and the average particle sizes of the TiO2 nanocrystals were about 71 nm, 85 nm, and 93 nm. The small particles observed on the TiO2 particle surfaces are gold clusters for the SEM test.
These results demonstrate that the (NH4)2CO3 morphology-controlling agent promotes the formation of {001} crystal facets, while the acid treatment of nanowire precursors significantly reduces the particle size of TiO2 nanocrystals and increases their specific surface area.
To further confirm the presence of {001} facets in TiO2, HRTEM characterization was performed on the TiO2-R-0.14 sample. The results, shown in Fig. 10, reveal lattice fringes with a spacing of 0.35 nm, corresponding to the {101} crystal plane of TiO2 nanocrystals, and a spacing of 0.24 nm, corresponding to the {001} crystal plane. These findings indicate that (NH4)2CO3 effectively reduces the surface energy of the TiO2 {001} facets, facilitating their exposure.10
 | ||
| Fig. 10 TEM and HRTEM images of TiO2-R-0.14 nanocrystals, (a) TEM image of TiO2-R-0.14 nanocrystals, (b) {001} crystal plane partial enlarged detail, (c) {101} crystal plane partial enlarged detail. | ||
We selected several typical samples of original TiO2, TiO2-R-0.14, TiO2-R-H-0.07 for XRD detection, the results are shown in Fig. S2.† It can be seen from the figure that the diffraction peaks of TiO2-R-0.14 and TiO2-R-H-0.07 correspond to those of anatase TiO2. It can be seen that pure anatase TiO2 nanocrystals were prepared by crystal plane control in this work.
3.5 Photocatalytic activity of TiO2 nanocrystals with exposed {001} facets
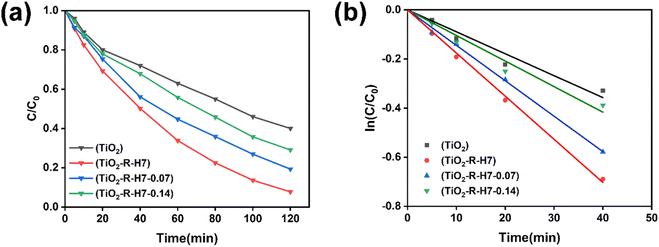
The low activity of the rod-like TiO2-R sample can be attributed to its large particle size, with a length of 303 nm, 15.2 times larger than the particle size of the original anatase TiO2. The improvement in photocatalytic activity upon introducing the (NH4)2CO3 morphology-controlling agent is primarily due to the exposure of the high-energy {001} facets. Additionally, the formation of heterojunctions between the {101} and {001} facets also enhanced the separation efficiency of electron–hole pairs. This is because of preferential flow of photogenerated holes and electrons to the oxidative {001} facets and reductive {101} facets, on which oxidation and reduction occur respectively.36 In particular, Maitani et al. demonstrated that the magnitude of electron transfer from anthracene derivatives to the {001} facet is 10 times larger than that to the {101} facets of TiO2 nanocrystals.37 This finding unambiguously suggests that the {001} facets are significantly more enriched with positively charged holes compared to the {101} facets, rendering the {001} facets more reactive for photooxidation processes. Thus, the coexistence of both the {001} facets (oxidation site) and {101} facets (reduction site) facilitate the prolonged separation of photogenerated electron–hole pairs. This spatial separation enhances the efficiency of TiO2 nanocrystals in the photocatalytic degradation of MB. However, as shown in Fig. 9(d), when the concentration of (NH4)2CO3 reached 0.28 mmol L−1, the average particle size of TiO2-R-0.14 sample increased to 105 nm and significant defects appeared on the surfaces of the TiO2 nanocrystals, along with the emergence of many irregularly shaped particles. The increase of the particle size would decrease the specific surface area of the TiO2 crystals, and the defects would act as recombination centers for electron–hole pairs, ultimately reducing the photocatalytic activity of the TiO2 nanocrystals. This indicates that although the (NH4)2CO3 morphology-controlling agent can promote the formation of high-energy {001} crystal facet of TiO2, considering the comprehensive factors affecting the photocatalytic activity, the concentration of (NH4)2CO3 in the synthesis process should not be excessively high.
The photocatalytic activity of samples with crystal facet engineering was higher than that of the original TiO2 nanoparticles. Although the photocatalytic activity of the TiO2-R-0.14 nanocrystals was about 2.6 times that of the original TiO2 nanoparticles, their particle size was approximately 98 nm, which is about 4.9 times larger than the particle size of the original anatase TiO2. This indicates that crystal facet engineering effectively enhances the photocatalytic activity of TiO2, but excessively large particle size remains a limiting factor for further improvement. Therefore, it is necessary to further reduce the particle size while maintaining the benefits of crystal facet engineering.
However, different from the series of samples without acid treatment, introducing (NH4)2CO3 to synthesize TiO2 nanocrystals from 7 h acid-treated R-KTNWs did not further enhance the photocatalytic activity. Instead, as the (NH4)2CO3 concentration increased, the photocatalytic activity of the synthesized TiO2 nanocrystals gradually decreased. Based on Fig. 9 SEM morphology and particle size analysis, this reduction in photocatalytic activity may be attributed to the lower proportion of {001} facets in the acid-treated samples, which limits the formation of crystal facet heterojunctions. Additionally, the increased particle size negatively impacts photocatalytic activity. The particle sizes of TiO2-R-H7-0.07 and TiO2-R-H7-0.14 were approximately 71 nm and 85 nm, respectively, which are about 1.7 and 2 times larger than that of the TiO2-R-H7 sample.
 | ||
| Fig. 13 The relationship between the concentration of (NH4)2CO3 and the photocatalytic degradation rate constant of a series of TiO2 nanocrystals. | ||
For samples synthesized from R-KTNWs acid-treated for 7 hours, the rate constants of TiO2-R-H7, TiO2-R-H7-0.07, and TiO2-R-H7-0.14 were 0.0213, 0.0137, and 0.0105, respectively, all of which were higher than those of the non-acid-treated series. Among these, TiO2-R-H7 had the highest rate constant, approximately 2.9 times that of the original anatase TiO2. These results indicate that TiO2-R-H7, synthesized from acid-treated potassium titanate without the addition of (NH4)2CO3, exhibited the highest photocatalytic activity. Adding (NH4)2CO3 did not further enhance the activity.
From the SEM morphology images, it can be observed that the acid-treated samples exhibit a relatively low proportion of exposed {001} facets than no acid-treated samples at the same (NH4)2CO3 concentration. Therefore, at this scale, changes in particle size have a greater impact on their photocatalytic activity. This highlights that acid treatment of potassium titanate precursors could reduce the particle size of TiO2 during crystal facet engineering, effectively enhancing the photocatalytic activity of TiO2 nanocrystals. The acid-treated precursors exhibit surface acidity, which may influence subsequent crystal facet engineering. To further improve the photocatalytic activity of TiO2 through crystal facet control, it is necessary to explore the optimal concentration of (NH4)2CO3 as a morphology-controlling agent and investigate the structure–activity relationship between acid treatment and crystal facet engineering.
3.6 Spectral analysis of the synthesized TiO2 nanocrystals
 | ||
| Fig. 14 Photoluminescence spectra of facet-controlled TiO2 nanocrystals (a) synthesized TiO2 without acid treatment, (b) synthesized TiO2 with acid treatment. | ||
As shown in Fig. 14(a), the PL peak intensity of fully washed, non-acid-treated TiO2 nanocrystals after crystal facet control initially decreases and then increases with increasing (NH4)2CO3 concentration. The TiO2-R-0.14 sample, synthesized with 0.14 mmol per L (NH4)2CO3, exhibited the lowest PL peak intensity, indicating the lowest electron–hole recombination rate and the highest photocatalytic activity. This trend is consistent with the photocatalytic activity trend shown in Fig. 11(a).
Fig. 14(b) shows that when R-KTNWs acid-treated for 7 hours are subjected to crystal facet control, the PL intensities of TiO2-R-H7, TiO2-R-H7-0.07, and TiO2-R-H7-0.14 are all lower than those of the original TiO2 nanoparticles. However, as the (NH4)2CO3 concentration increases, the PL peak intensity gradually increases, corresponding to higher electron–hole recombination rates and weaker photocatalytic activity.
A comparison of the results for the 7 h acid-treated and non-acid-treated series reveals that all acid-treated samples exhibit lower PL peak intensities than their non-acid-treated counterparts, indicating that acid treatment effectively promotes carrier separation. Among these, the bipyramidal TiO2-R-H7 sample, synthesized from 7 h acid-treated potassium titanate without (NH4)2CO3, demonstrated the highest electron–hole separation efficiency and the strongest photocatalytic activity. This PL result aligns with the photocatalytic activity tests.
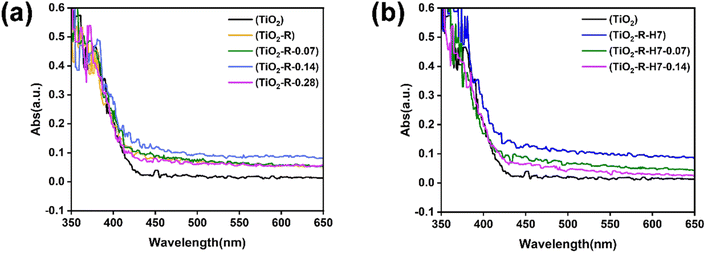 | ||
| Fig. 15 UV-vis diffuse reflectance spectra of TiO2 nanocrystals synthesized under different conditions, (a) TiO2 synthesized without acid treatment, (b) TiO2 synthesized after 7 h acid treatment. | ||
The bandgap values were determined by drawing tangents at the steepest descent points of the absorption curves,40 as shown in Fig. S3.† The original TiO2 had a bandgap of 2.8 eV, while the bandgap values for the fully washed facet-controlled TiO2-R series (TiO2-R, TiO2-R-0.07, TiO2-R-0.14, TiO2-R-0.28) were 2.75 eV, 2.68 eV, 2.6 eV, and 2.7 eV, respectively. After 7 hours of acid treatment, the bandgap values for the crystal-facet-engineered TiO2–H7 series (TiO2-R-H7, TiO2-R-H7-0.07, TiO2-R-H7-0.14) were 2.52 eV, 2.77 eV, and 2.74 eV, respectively.
For the fully washed R-KTNWs precursor, the bandgap of the crystal-facet-engineered TiO2 decreased and then increased with the rise in (NH4)2CO3 concentration, with TiO2-R-0.14 exhibiting the smallest bandgap of 2.6 eV. Similarly, for the 7 h acid-treated R-KTNWs precursor, the bandgap of the TiO2–H7 series also decreased and then increased with (NH4)2CO3 concentration. The TiO2-R-H7 sample, synthesized without (NH4)2CO3, had the smallest bandgap of 2.52 eV.
The UV-visible diffuse reflectance trends align with the photocatalytic activity trends shown in Fig. 11 and 12. Both acid treatment and crystal facet engineering caused a redshift in the light absorption edge of TiO2, with the TiO2-R-H7 sample exhibiting the largest redshift. This sample also demonstrated the highest electron–hole separation efficiency and the strongest corresponding photocatalytic activity.
 | ||
| Fig. 16 Oxygen vacancy detection of facet-controlled TiO2 nanocrystals synthesized under different conditions, (a) no acid treatment, (b) acid treatment for 7 h. | ||
The oxygen vacancy intensities for the acid-treated precursor series are shown in Fig. 16(b). The TiO2-R-H7 sample, synthesized without (NH4)2CO3, displayed relatively high oxygen vacancy peak intensity. In contrast, the TiO2-R-H7-0.07 and TiO2-R-H7-0.14 samples, synthesized with the addition of (NH4)2CO3, showed significantly reduced oxygen vacancy intensities. It is speculated that the decline in photocatalytic activity for this series with increasing (NH4)2CO3 concentration is not only related to the increase in TiO2 nanocrystal particle size but also potentially to the decrease in oxygen vacancy content.
Based on the oxygen vacancy analysis, it can be concluded that moderate acid treatment of potassium titanate nanowire precursors enables the stable synthesis of small-sized bipyramidal TiO2 nanocrystals while maintaining a sufficient level of oxygen vacancies. This effectively enhances the photocatalytic activity of TiO2.
3.7 Stable synthesis route of TiO2 nanocrystal photocatalysts with different morphologies
Highly active TiO2 nanocrystal photocatalyst can be synthesized by controlling the crystal facet derived from KTNWs nanowire precursor. But discrepancies in the size and morphology of TiO2 nanocrystals synthesized using similar methods by different researchers highlight the need to explore the key factors influencing the morphology of TiO2 nanocrystal. As show in Fig. 17, this study explored key factors influencing TiO2 nanocrystal morphology using KTNWs as precursors.We found that subtle changes in the pH of the hydrothermal solution played a critical role in controlling the morphology of TiO2 nanocrystals. The transformation of commercial TiO2 to R-KTNWs occurs in a strongly alkaline hydrothermal environment. It is difficult to achieve a completely neutral product through simple deionized water washing. Although washing steps were standardized, the difference in washing time and water dosage would also cause the difference in the surface acidity and alkalinity of the R-KTNWs precursor, which would affect the pH environment of the subsequent hydrothermal process, thereby influencing the growth direction of TiO2 nuclei. We have realized the controllable synthesis of TiO2 nanocrystals by simply adjusting the R-KTNWs precursor washing method, including deionized water washing resulting in weakly alkaline conditions, repeated deionized water washing to slight alkalinity close to neutral, and acid washing followed by deionized water washing to subacidity close to neutral, and cuboidal, rod-like, and octahedral bipyramidal TiO2 nanocrystals could be obtained, respectively. In the above several synthesis methods, acid treatment could stabilize the octahedral bipyramidal morphology of the TiO2 nanocrystal, and with the extension of acid treatment time, the particle size can be reduced. In this work, the TiO2-R-H7 sample synthesized after acid treatment of R-KTNWs for 7 h can stably obtain a uniform octahedral bipyramid structure with an average particle size of 42 nm, showing the best photocatalytic degradation of MB activity. By varying the concentration of the (NH4)2CO3 morphology control agent during the hydrothermal process, the exposure ratio of the TiO2 {001} crystal facets could be controlled. Exposing the highly active {001} facets of TiO2 could form a heterojunction of {001} and {101} facets. The electrons tend to transfer to the {101} facets, and the holes tend to transfer to the {001} facets, which promotes the spatial separation of electrons and holes, thereby improving the photocatalytic activity of TiO2 nanocrystals.42 However, in this work, after the combination of acid treatment and crystal facet regulation, the exposure of {001} facet did not further improve the photocatalytic activity of TiO2 due to the increase of particle size and insufficient exposure of {001} facet. The synergistic effect between acid treatment and crystal facet regulation needs to be further explored.
4. Conclusions
This study demonstrates that the morphology and photocatalytic activity of TiO2 nanocrystals can be effectively controlled by adjusting the washing and acid treatment of R-KTNWs. Different washing methods for precursors synthesized under alkaline conditions lead to varying pH environments in the subsequent hydrothermal solutions, thereby influencing the growth direction of TiO2 nuclei. A weakly alkaline hydrothermal solution produced cuboidal TiO2 nanocrystals, a slight alkalinity near-neutral solution yielded rod-like TiO2 nanocrystals, and a subacidity near-neutral solution resulted in octahedral bipyramidal TiO2 nanocrystals. Acid treatment could reduce particle size and stabilize octahedral bipyramidal TiO2 synthesis was considered as the best synthesis method in this work. By controlling the acid treatment duration, it was found that the reduction in K+ content would decrease the interlayer spacing of R-KTNWs, leading to smaller TiO2 particle sizes. However, prolonged acid treatment would reduce structural stability, crystallinity, and photocatalytic performance.TiO2-R-H7, synthesized from precursors acid-treated for 7 hours shown the best photocatalytic activity, exhibited a particle size reduction of 86% that of TiO2-R and a photocatalytic activity nearly5 times and 3 times that of TiO2-R and original TiO2, respectively. Introducing (NH4)2CO3 morphology-controlling agent to synthesis {001} facet exposed TiO2, but did not surpass the photocatalytic activity of TiO2-R-H7 due to increased particle size, reduced oxygen vacancies, and insufficient {001} facet exposure. Further improvement of the photocatalytic activity of TiO2 nanocrystals requires in-depth exploration of the structure–activity relationship between acid treatment and crystal facet engineering. This study provides insights into the growth mechanisms of TiO2 morphologies and highlights acid treatment as a strategy to reduce particle size and enhance photocatalytic activity, offering guidance for designing high-performance photocatalytic materials.
Data availability
The data supporting the findings of this study are available within the article and its ESI.† Additional data are available from the corresponding author and the data have not been submitted to any public repository.Author contributions
Meng Chai: writing – original draft, writing – review & editing, methodology, investigation, data curation. Bo Yuan: writing – review & editing, conceptualization. Yao Ban: investigation, methodology, data curation. Jing Guo: writing – original draft, writing – review & editing, resources, project administration, funding acquisition, conceptualization. Huifang Lou: investigation. Hongyuan Kang: investigation. Qiaoling Zhang: supervision. Zhiwei Liu: funding acquisition, writing – review & editing. Dongming Zhang: funding acquisition, writing – review & editing.Conflicts of interest
All the authors have approved the manuscript and agree with submission to your esteemed journal and there are no conflict of interest exits in the submission of this manuscript.Acknowledgements
This research was supported by the “National Natural Science Foundation of China (21808214)”, “Research Project Supported by Shanxi Scholarship Council of China (2023-126)”, “Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20220013)”, “Fundamental Research Program of Shanxi Province (202203021211085)”, “Fundamental Research Program of Shanxi Province (202403021211196)”.References
- Y. Du, X. Niu, J. He, L. Liu, Y. Liu, C. Chen, X. Yang and Q. Feng, ACS Omega, 2020, 5, 14147–14156 CrossRef CAS PubMed
.
- N. Parsafard, R. Abedi and H. Moodi, RSC Adv., 2024, 14, 19984–19995 Search PubMed
.
- L.-Y. Zhang, Y.-L. Han, M. Liu and S.-L. Deng, RSC Adv., 2023, 13, 16797–16814 RSC
.
- R. Katal, S. Masudy-Panah, M. Tanhaei, M. H. D. A. Farahani and H. Jiangyong, Chem. Eng. J., 2020, 384, 123384 Search PubMed
.
- L. Liu, Y. Du, X. Niu, W. Li, J. Li, X. Yang and Q. Feng, ChemistrySelect, 2018, 3, 9953–9959 CrossRef CAS
.
- J. Wang, B. Liu and K. Nakata, Chin. J. Catal., 2019, 40, 403–412 CrossRef CAS
.
- C. Yao, X. Wang, W. Zhao, T. Li, Y. He, X. Ran and L. Guo, J. Alloys Compd., 2020, 846, 156335 CrossRef CAS
.
- K. Zhu, H. Gao, G. Hu and Z. Shi, J. Supercrit. Fluids, 2013, 83, 28–34 CrossRef CAS
.
- X. Han, X. Wang, S. Xie, Q. Kuang, J. Ouyang, Z. Xie and L. Zheng, RSC Adv., 2012, 2, 3251 RSC
.
- Y. Du, X. Niu, K. Hou, X. He and C. Zhang, Catalysts, 2022, 12, 232 Search PubMed
.
- D. K. Chacko, A. A. Madhavan, T. A. Arun, S. Thomas, G. S. Anjusree, T. G. Deepak, A. Balakrishnan, K. R. V. Subramanian, N. Sivakumar, S. V. Nair and A. S. Nair, RSC Adv., 2013, 3, 24858 RSC
.
- P. V. Viet, B. T. Phan, L. V. Hieu and C. M. Thi, J. Nanosci. Nanotechnol., 2015, 15, 5202–5206 CrossRef CAS PubMed
.
- J. Xiong and L. He, J. Exp. Nanosci., 2017, 12, 384–393 CrossRef CAS
.
- Z. Wei, E. Kowalska, K. Wang, C. Colbeau-Justin and B. Ohtani, Catal. Today, 2017, 280, 29–36 CrossRef CAS
.
- Z. Wei, E. Kowalska, J. Verrett, C. Colbeau-Justin, H. Remita and B. Ohtani, Nanoscale, 2015, 7, 12392–12404 RSC
.
- Y. Du, Q. Feng, C. Chen, Y. Tanaka and X. Yang, ACS Appl. Mater. Interfaces, 2014, 6, 16007–16019 CrossRef CAS PubMed
.
- C. Chen, L. Xu, G. A. Sewvandi, T. Kusunose, Y. Tanaka, S. Nakanishi and Q. Feng, Cryst. Growth Des., 2014, 14, 5801–5811 CrossRef CAS
.
- J. Li and D. Xu, Chem. Commun., 2010, 46, 2301 RSC
.
- A. S. Barnard and L. A. Curtiss, Nano Lett., 2005, 5, 1261–1266 CrossRef CAS PubMed
.
- A. S. Barnard, P. Zapol and L. A. Curtiss, Surf. Sci., 2005, 582, 173–188 CrossRef CAS
.
- L. Liu, Y. Du, X. Niu, W. Li, J. Li, X. Yang and Q. Feng, ChemistrySelect, 2018, 3, 9953–9959 CrossRef CAS
.
- C. H. Cho, M. H. Han, D. H. Kim and D. K. Kim, Mater. Chem. Phys., 2005, 92, 104–111 CrossRef CAS
.
- S. Li, L. Ruan, S. Wang, Z. Wang, Z. Ren and G. Han, J. Mater. Sci. Technol., 2020, 46, 139–144 CrossRef CAS
.
- J. Wang, H. Li, H. Wang, K. Huang, G. Sun, S. Yin and T. Sato, Res. Chem. Intermed., 2011, 37, 165–175 CrossRef CAS
.
- B. Liu, J. E. Boercker and E. S. Aydil, Nanotechnology, 2008, 19, 505604 CrossRef CAS PubMed
.
- A. Nakahira, T. Kubo and C. Numako, ACS Appl. Mater. Interfaces, 2010, 2, 2611–2616 CrossRef CAS PubMed
.
- Y. Yu and D. Xu, Appl. Catal. B Environ., 2007, 73, 166–171 CrossRef CAS
.
- T. Gupta, Samriti, J. Cho and J. Prakash, Mater. Today Chem., 2021, 20, 100428 CrossRef CAS
.
- M. Humayun, F. Raziq, A. Khan and W. Luo, Green Chem. Lett. Rev., 2018, 11, 86–102 CrossRef CAS
.
- J. Xiong and L. He, J. Exp. Nanosci., 2017, 12, 384–393 CrossRef CAS
.
- M. Sluban and P. Umek, J. Phys. Chem. C, 2019, 123, 23747–23757 CrossRef CAS
.
- L. K. Dhandole, M. A. Mahadik, S.-G. Kim, H.-S. Chung, Y.-S. Seo, M. Cho, J. H. Ryu and J. S. Jang, ACS Appl. Mater. Interfaces, 2017, 9, 23602–23613 CrossRef CAS PubMed
.
- C. Lv, X. Lan, F. Li, L. Wang, L. Xiao, C. Wang, J. Shi and S. Yu, Catal. Sci. Technol., 2020, 10(3), 690–699 RSC
.
- W. Wang, C.-H. Lu, Y.-R. Ni, J.-B. Song, M.-X. Su and Z.-Z. Xu, Catal. Commun., 2012, 22, 19–23 CrossRef CAS
.
- X. Han, X. Wang, S. Xie, Q. Kuang, J. Ouyang, Z. Xie and L. Zheng, RSC Adv., 2012, 2, 3251 RSC
.
- N. Roy, Y. Sohn and D. Pradhan, ACS Nano, 2013, 7, 2532–2540 CrossRef CAS PubMed
.
- M. M. Maitani, K. Tanaka, D. Mochizuki and Y. Wada, J. Phys. Chem. Lett., 2011, 2, 2655–2659 CrossRef CAS
.
- E. P. Estévez Ruiz, J. L. Lago and S. P. Thirumuruganandham, Materials, 2023, 16, 3076 CrossRef PubMed
.
- J. Zhu, S. Zhu, X. Kong, Y. Liang, Z. Li, S. Wu, S. Luo, C. Chang and Z. Cui, ACS Appl. Nano Mater., 2020, 3, 10349–10359 CrossRef CAS
.
- X. Zhang, H. Zhang, H. Zhang, L. Peng and S. Huang, New J. Chem., 2024, 48, 17458–17464 RSC
.
- F. Li, G. Liu, F. Liu, J. Wu and S. Yang, J. Hazard. Mater., 2023, 452, 131237 CrossRef CAS PubMed
.
- A. K. R. Police, S. V. P. Vattikuti, Y.-J. Baik and B. Chan, Ceram. Int., 2019, 45, 2178–2184 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08805k |
| This journal is © The Royal Society of Chemistry 2025 |