Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Chemical CO2 fixation by a heterogenised Zn(II)-hydrazone complex†
Neda Heydari a,
Rahman Bikas
a,
Rahman Bikas *a and
Tadeusz Lisb
*a and
Tadeusz Lisb
aDepartment of Chemistry, Faculty of Science, Imam Khomeini International University, Qazvin, 34148-96818, Iran. E-mail: bikas@sci.ikiu.ac.ir; bikas_r@yahoo.com
bFaculty of Chemistry, University of Wroclaw, Joliot-Curie 14, Wroclaw 50-383, Poland
First published on 26th February 2025
Abstract
A new Zn(II) coordination compound, [Zn(HL)(OAc)2] (1), with ONN-donor hydrazone ligand (HL = (E)-4-amino-N′-(1-(pyridin-2-yl)ethylidene)benzohydrazide) was synthesized and structurally characterized by using spectroscopic techniques and X-ray analysis. These analyses indicated that the Zn(II) ion in the resulting coordination compound is five coordinated and the compound has a free amine functionality on the phenyl ring. Thus, [Zn(HL)(OAc)2] (1) was immobilized on the surface of propionylchloride functionalized silica gel through an amidification process via the reaction of the aniline part of compound 1 and the acyl chloride group of the support. The synthesized heterogeneous catalyst, Si-[Zn(HL)(OAc)2], was characterized by several analytical methods and the results confirmed the successful support of 1 on the surface of the support. Si-[Zn(HL)(OAc)2] was used in a chemical CO2 fixation reaction and styrene epoxide was used as a model substrate to investigate the catalytic performance of the supported Zn(II) catalyst. Si-[Zn(HL)(OAc)2] can efficiently catalyze the formation of cyclic carbonate from the reaction of epoxide and CO2 in the presence of TBAB as a co-catalyst.
1. Introduction
Human activities on the planet have caused carbon dioxide to accumulate significantly in the earth's atmosphere and caused increased concerns about climate change.1 These concerns have caused research on the absorption and use of carbon dioxide to reduce greenhouse gases. One of the innovative strategies is the chemical fixation of carbon dioxide and its conversion into other valuable chemical products2 such as polymers, fuels, carboxylic acids and cyclic carbonates.3 CO2 fixation can be done in the presence or absence of catalysts. Transition metal complexes (especially zinc, ruthenium and copper) are important catalysts for chemical CO2 fixation, and they play a key role in converting CO2 to other compounds under mild conditions.4 These catalysts have high ability to activate carbon dioxide and increase the efficiency of the formation of various organic compounds.5 Carbon dioxide can be converted to methane or carbon monoxide through electrolysis,6 or it can be converted to various carbonates by reacting with hydroxides and oxides of alkali metals.7 In CO2 fixation in the absence of a catalyst, the chemical processes are not carried out under mild conditions and the use of high pressure and high temperature are required for converting CO2 to other materials.8 Thus, the use of catalysts and performing CO2 fixation reaction under mild condition has attracted much attention during recent decades. The reaction of CO2 with epoxides in the presence of a catalyst and converting them to cyclic carbonates is one of the most interesting and efficient methods in chemical CO2 fixation9 because cyclic carbonates are useful intermediates in the chemical industry, and they have several applications in various fields.10The use of zinc coordination compounds as catalyst in chemical fixation of CO2 has attracted high attention due to adjustable reactivity, low cost and abundance of zinc compounds.11 Zinc, as a good Lewis acid, can efficiently interact with carbon dioxide and activate it to involve in CO2 fixation reactions and this matter considerably facilitates efficient conversion of CO2 into other organic compounds.12 The mechanism of this process mainly includes the coordination and activation of carbon dioxide to the zinc core of coordination compounds and the formation of active intermediates that can easily react with other suitable reagents and convert to the final products.13 The coordination environment of Zn(II) ion and the structure of organic ligands can considerably influence on the rate of the catalytic reactions and selectivity of the product.14 Thus, several studies by using various Zn(II) coordination compounds should be done to increase our knowledge about the details of such transformations and to design efficient catalysts for CO2 fixation under milder and safer conditions. Both homogeneous and heterogeneous catalytic systems have been used for chemical CO2 fixation reaction15 but, the use of heterogeneous systems has some advantages like easy separation, simple recovery process and reusability of the catalyst16 which make them interesting and attractive materials for industrial applications. Therefore, developing heterogeneous catalytic systems based on coordination compounds can be considered as one of the top research fields in catalysis science and technology and also in CO2 fixation reactions.17
Hydrazone ligands are a type of famous nitrogen and oxygen donor ligands in Schiff base family which are synthesized from the reaction of hydrazides (R–CO–NH–NH2) and appropriate aldehyde/ketone derivatives.18 Hydrazone ligands are known for their versatile coordination chemistry and tunable properties, and they mostly form stable coordination compounds.19 Their coordination compounds have several applications in biological, chemical and material sciences and usually show high catalytic activity and stability in various reactions.20 Although hydrazone coordination compounds are known as homogeneous catalysts, during recent years several strategies have been used for converting them into heterogeneous systems.21 Among them, introducing a suitable functional group on the structure of the hydrazone ligands and connecting them onto the surface of a heterogeneous substrate (like silica) is the most successful way.
In this report, we describe the synthesis, crystal structure, spectroscopic properties and catalytic activity of a new Zn(II) coordination compound with a hydrazone ligand. Since there is a free aniline functionality on the structure of the synthesized compound, it was converted to a heterogeneous catalyst by supporting on the surface of functionalized silica gel. The resulting heterogeneous compound was characterized by various analytical methods and its catalytic activity was investigated in chemical fixation of CO2.
2. Experimental
2.1. Materials
2-Acetylpyridine, 4-aminobenzoic hydrazide, propionylchloride-functionalized silica gel (≈1 mmol g−1 loading), zinc(II) acetate dihydrate and other chemicals were purchased from Sigma-Aldrich. Solvents with the highest grade were prepared by Merck and used as received. The ligand (HL) was prepared according to our previous report.222.2. Characterization methods
Powder X-ray diffraction analyses were performed on a Bruker D8 Advance PXRD instrument. Thermal gravimetric analysis was recorded on a TA Q600 instrument (at 25–1000 °C). Scanning electron microscopy and energy-dispersive spectroscopy were taken with a TESCAN MIRA III instrument. Elemental analyses (carbon, hydrogen and nitrogen) were provided using a Carlo ERBA Model EA1108 analyzer and the zinc content of the compounds was measured by a Varian AA-220 atomic absorption instrument. The UV-vis spectra were obtained by using a thermos-spectronic Helios Alpha spectrophotometer (200–800 nm). Photoluminescence (PL) spectra were recorded on a Hitachi F2700 spectrophotometer. Diffuse-reflectance UV-vis spectroscopy was measured with a Sinco S4100 instrument (200–1000 nm). NMR spectra were recorded on a Bruker DRX-300 spectrometer (at 250 MHz). FT-IR spectra were carried out as KBr disks with a Bruker Tensor27 spectrophotometer (4000–400 cm−1). An Agilent 7890B gas chromatography (GC) instrument equipped with FID detector (temperature 300 °C) and a HP-5 capillary column (phenyl methyl siloxane 30 m × 320 μm × 0.25 μm) was used to check the progress of the catalytic reactions.2.3. Synthesis of [Zn(HL)(OAc)2] (1)
For the synthesis of zinc complex, Zn(OAc)2·2H2O (0.44 g, 2.0 mmol) was added to the solution of HL (0.508 g, 2.0 mmol) in 25 ml methanol (see Scheme 1). After a short time, the color of the solution turned to yellow and the mixture was stirred for 2 hours at RT. Then, the resulting mixture was heated and stirred under reflux condition for 4 hours. The volume of the solvent was reduced by vacuum and the resulting yellow precipitate was isolated by filtration and washed with methanol. The obtained powder was dissolved in hot methanol and the air stable yellow block crystals, suitable for X-ray analysis, were obtained by evaporating of the solvent. Yield: 79.4% (0.695 g). Anal. calc. For C18H20N4O5Zn (437.75 g mol−1): C, 49.39; H, 4.61; N, 12.80; Zn, 14.94%. Found: C, 49.45; H, 4.65; N, 12.74; Zn, 14.80%. FT-IR (KBr, cm−1): 3555 (m, br), 3432 (s, br), 3333 (s, br), 3205 (s, br), 2996 (w), 2970 (w), 2926 (w), 1635 (s), 1617 (s), 1560 (s), 1513 (s), 1473 (s), 1397 (vs.), 1335 (s), 1287 (vs.), 1182 (vs.), 1166 (vs.), 1123 (s), 1017 (s), 975 (w), 935 (w), 902 (w), 845 (vs.), 785 (s), 767 (s), 745 (w), 672 (s), 637 (m), 626 (s), 567 (s), 551 (m), 507 (s), 477 (w), 430 (w), 417 (w). 1H NMR (250 MHz, DMSO-d6, TMS, ppm): δ = 1.83 (s, 6H); 2.66 (s, 3H), 5.53 (s, 2H); 6.52 (m, 2H), 7.27, (s, 1H), 7.51 (s, 1H), 7.85 (m, 2H), 7.04 (m, 1H) and 8.45 ppm (s, 1H). 13C NMR (62.9 MHz, DMSO-d6, 25 °C, ppm): δ = 12.4, 22.3, 113.0, 122.0, 123.8, 125.0, 130.1, 140.6, 146.9, 148.6, 150.8, 151.8, 160.4, 174.0 and 174.9 ppm. UV-vis (2.5 × 10−5 M, CH3OH): λmax (ε, M−1 cm−1): 222 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600), 285 (27
600), 285 (27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300), 360 nm (60
300), 360 nm (60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500).
500).
 | ||
| Scheme 1 Synthesis pathway of (a) HL; (b) [Zn(HL)(OAc)2] (1); and (c) heterogenised Zn catalyst (Si-[Zn(HL)(OAc)2]). | ||
2.4. Synthesis of heterogeneous catalyst (Si-[Zn(HL)(OAc)2])
Functionalized silica gel with propionylchloride was used to prepare silica supported Zn catalyst via amidification reaction (see Scheme 1). For this matter, 0.175 g of 1 (0.40 mmol) and 0.40 g of silica gel (≈1 mmol g−1 loading) were reacted in 10 ml of dried acetonitrile. The reaction mixture was stirred and heated at 80 °C for one day. The resulting yellow solid was filtered, washed several times with distilled water and dried at air. Yield: 94.58% (0.530 g). FT-IR (KBr, cm−1): 3546 (m, br), 3447 (m, br), 3416 (m, br), 3232 (m, br), 1725 (m), 1637 (s), 1616 (s), 1601 (s), 1561 (w), 1517 (w), 1494 (m), 1461 (m), 1424 (w), 1400 (w), 1359 (s), 1317 (m), 1288 (m), 1062 (br), 945 (m), 909 (m), 805 (s), 771 (s), 745 (w), 708 (w), 684 (w), 631 (m), 468 (w, br).2.5. Catalytic studies
For investigating the catalytic activity of Si-[Zn(HL)(OAc)2] in chemical CO2 fixation, the reactions were done in two necked round bottom flasks connected to a condenser. The flask was charged with styrene epoxide (5.0 mmol; as substrate), solvent (10.0 ml; CH3CN, EtOH, THF or CHCl3), and tetrabutylammonium bromide as a co-catalyst (0.1–2.0 mmol). As a catalyst, Si-[Zn(HL)(OAc)2] (15.0 mg, 9.20 μmol Zn(II) ion) was added to the reaction mixture and CO2 was bubbled from the solution at atmospheric pressure to have a CO2 saturated solution. Then, a CO2 balloon was connected to the top of the condenser. The flask was inserted into an oil bath at the desired temperature and the mixture was continuously stirred. The progress of the reactions was monitored by TLC technique and GC instrument. The carbonate product was isolated from the reaction mixture by solvent extraction method and after purification processes, it was characterized by spectroscopic methods. The heterogeneous catalyst was simply recovered by filtration (or decantation) and reused in the next rounds.2.6. X-ray crystallographic study and Hirshfeld surface analysis
Single crystal of [Zn(HL)(OAc)2] was selected for X-ray analysis and its data were collected at 100 K using a Kuma KM-4 diffractometer with monochromatic Mo Kα radiation (λ = 0.71073 Å). Unit cell refinement, data collection, data reduction and absorption correction were performed with the CrysAlisPro software package.23 The data were obtained by ω-scan mode. The structure of the compound was refined by full matrix least squares using SHELX-2013 software.24 The crystallographic data and important refinement parameters are collected in Table 1. Crystal-Explorer17 program25 was used to investigate the intermolecular interactions in the structure of [Zn(HL)(OAc)2].| Formula | C18H20N4O5Zn |
|---|---|
| MW/g mol−1 | 437.75 |
| Crystal shape, color | Block, yellow |
| Crystal size/mm | 0.36 × 0.27 × 0.15 |
| T/K | 100 |
| Crystal system | Triclinic |
| Space group | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 9.037(2) |
| b/Å | 10.049(3) |
| c/Å | 10.795(3) |
| α/° | 80.44(4) |
| β/° | 78.53(4) |
| γ/° | 79.74(3) |
| V/Å−3 | 936.7(5) |
| Z | 2 |
| Dcalc/g cm−3 | 1.552 |
| μ/mm−1 | 1.35 |
| Tmin, Tmax | 0.672, 0.853 |
| F(000) | 452 |
| θ range/° | 2.8–25 |
| Rint | 0.042 |
| R[F2 > 2σ(F2)] | 0.051 |
| wR(F2) | 0.142 |
| S | 1.04 |
| Abs. correction | Analytical |
| Hydrogen refinement | Mixed |
| Measured reflections | 7344 |
| Independent reflections | 3855 |
| Reflections with I > 2σ(I) | 3579 |
| Parameters | 268 |
| Restraints | 0 |
| Δρmax/Δρmin/e Å−3 | 0.50/−0.85 |
3. Results and discussion
3.1. Synthesis of HL, [Zn(HL)(OAc)2] (1) and supported catalyst (Si-[Zn(HL)(OAc)2])
[Zn(HL)(OAc)2] was obtained as yellow block single crystals by slow evaporating of the methanolic solution. The structure of the resulting crystals was determined by X-ray analysis and the product was also characterized by NMR, FT-IR, PL, UV-vis, TGA and elemental analyses. By having a free NH2 group in the structure of this compound, [Zn(HL)(OAc)2] (1), we supported it on the surface of silica gel. The reaction between the amine group of [Zn(HL)(OAc)2] and propionylchloride group of the functionalized silica gave the supported catalyst (Si-[Zn(HL)(OAc)2]) as a yellowish powder which was characterized by atomic absorption, FT-IR, UV-DRS, TGA, SEM, EDX and XRD techniques.3.2. X-ray structure of [Zn(HL)(OAc)2] (1) and Hirshfeld surface analysis
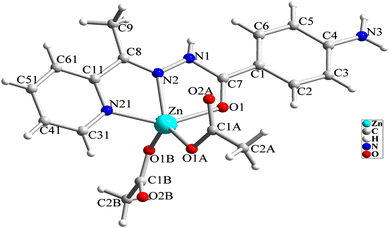
The structure of [Zn(HL)(OAc)2] is shown in Fig. 1 and Table 2 shows the selected bond lengths and angles around the Zn(II) in this structure. According to X-ray diffraction studies, the product is a neutral mononuclear Zn(II) coordination compound with a general formula of [Zn(HL)(OAc)2] and crystallizes in triclinic system with P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The Zn(II) atom is five-coordinated and pursues a distorted square pyramidal [ZnN2O3] geometry that was inferred by calculating the values of Addison parameter (τ = 0.179). In the structure of this compound, the ligand acts as a NNO-donor ligand and is coordinated to the Zn(II) center as a neutral ligand in amidic form (HL). The imine and pyridine nitrogen atoms (N2 and N21) together with the amidic oxygen atom (O1) of HL are coordinated to the Zn(II) center and fill three corners of the square plane. The remaining corner is filled by the oxygen atom of the acetate ligand (O1A). In the equatorial plane, the distances of Zn–N2, Zn–N21, Zn–O1 and Zn–O1A are 2.118(2), 2.142(3), 2.177(2) and 1.954(2) Å, respectively. These bond lengths are in the normal range observed in the similar Zn(II) complexes.26 The axial position is filled by the oxygen atom of the second acetate ligand (O1B) with the Zn–O1B distance of 1.963(2) Å. In the crystal of [Zn(HL)(OAc)2], there are several intermolecular hydrogen bonds and π⋯π stacking interactions (see Fig. 2 and 3) which stabilize the crystal. Hydrogen bonding data are given in Table 3.
space group. The Zn(II) atom is five-coordinated and pursues a distorted square pyramidal [ZnN2O3] geometry that was inferred by calculating the values of Addison parameter (τ = 0.179). In the structure of this compound, the ligand acts as a NNO-donor ligand and is coordinated to the Zn(II) center as a neutral ligand in amidic form (HL). The imine and pyridine nitrogen atoms (N2 and N21) together with the amidic oxygen atom (O1) of HL are coordinated to the Zn(II) center and fill three corners of the square plane. The remaining corner is filled by the oxygen atom of the acetate ligand (O1A). In the equatorial plane, the distances of Zn–N2, Zn–N21, Zn–O1 and Zn–O1A are 2.118(2), 2.142(3), 2.177(2) and 1.954(2) Å, respectively. These bond lengths are in the normal range observed in the similar Zn(II) complexes.26 The axial position is filled by the oxygen atom of the second acetate ligand (O1B) with the Zn–O1B distance of 1.963(2) Å. In the crystal of [Zn(HL)(OAc)2], there are several intermolecular hydrogen bonds and π⋯π stacking interactions (see Fig. 2 and 3) which stabilize the crystal. Hydrogen bonding data are given in Table 3.
| Bond | Length/Å | Bond | Angle/° |
|---|---|---|---|
| Zn–O1A | 1.954(2) | O1A–Zn–O1B | 116.97(9) |
| Zn–O1B | 1.963(2) | O1A–Zn–N2 | 136.36(9) |
| Zn–N2 | 2.118(3) | O1B–Zn–N2 | 105.63(9) |
| Zn–N21 | 2.142(3) | O1A–Zn–N21 | 106.11(9) |
| Zn–O1 | 2.177(2) | O1B–Zn–N21 | 99.12(9) |
| C7–O1 | 1.244(4) | N2–Zn–N21 | 74.51(10) |
| C7–N1 | 1.380(4) | O1A–Zn–O1 | 93.43(9) |
| N1–N2 | 1.365(4) | O1B–Zn–O1 | 94.93(9) |
| N2–C8 | 1.290(4) | N2–Zn–O1 | 73.15(9) |
| C4–N3 | 1.355(4) | N21–Zn–O1 | 147.15(9) |
 | ||
| Fig. 2 Hydrogen bond interactions (N–H⋯O = green dashed line) and π⋯π stacking (pink dashed line) in the crystal of [Zn(HL)(OAc)2] (1). | ||
 | ||
| Fig. 3 Hydrogen bond interactions (N–H⋯O = green and C–H⋯O = pink) in the structure of [Zn(HL)(OAc)2] (1). | ||
Due to the presence of several intermolecular interactions in the crystal of [Zn(HL)(OAc)2], Hirshfeld surface (HS) analysis was used for investigating these interactions in its structure. The Hirshfeld surfaces of [Zn(HL)(OAc)2] mapped with (a) dnorm, (b) curvedness and (c) shape index are shown in Fig. 4. The dnorm Hirshfeld surface is shown in Fig. 4a and indicates the interaction between the closer atoms outside the surface (de) and inside the surface (di) such as red (close proximity), white (medium proximity) and blue colors (little proximity of the outside atoms).27 As shown in Fig. 4a, the dnorm Hirshfeld surface of [Zn(HL)(OAc)2] shows four main red spots which are related to the close proximity such as N–H⋯O and C–H⋯O contacts. The curvedness Hirshfeld of [Zn(HL)(OAc)2] is shown in Fig. 4b which includes two flat areas on the curvedness surface showing the presence of π⋯π stacking interactions. The shape index Hirshfeld of [Zn(HL)(OAc)2] is shown in Fig. 4c. The presence of blue (bulging) and orange (hollow) regions indicates the presence of intermolecular C–H⋯π interactions. For this crystal, the fingerprint plots and the contribution of some main contacts are shown in Fig. 4d and 5, respectively. The fingerprint plots of [Zn(HL)(OAc)2] indicate that H⋯H contacts have the most contribution (45.1%) and the contribution of O⋯H, C⋯H and N⋯H contacts are 12.6%, 7.9% and 2.4%, respectively.
 | ||
| Fig. 4 Hirshfeld surface of [Zn(HL)(OAc)2] mapped with (a) dnorm, (b) curvedness and (c) shape index (d) fingerprint plots. | ||
3.3. Spectroscopic characterization of the synthesized compounds
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching frequency that is shifted to 1617 cm−1 in [Zn(HL)(OAc)2] indicating the coordination of HL to Zn(II) ion through imine nitrogen atom.28 The amidic C
N stretching frequency that is shifted to 1617 cm−1 in [Zn(HL)(OAc)2] indicating the coordination of HL to Zn(II) ion through imine nitrogen atom.28 The amidic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band of HL is appeared as a strong band at 1655 cm−1, and it is shifted to 1635 cm−1 in the spectrum of [Zn(HL)(OAc)2]. It should be noted that the C
O band of HL is appeared as a strong band at 1655 cm−1, and it is shifted to 1635 cm−1 in the spectrum of [Zn(HL)(OAc)2]. It should be noted that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band of acetate groups is overlapped with the amidic C
O band of acetate groups is overlapped with the amidic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which confirms the coordination of C
O, which confirms the coordination of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O oxygen to the Zn(II) ion. The NH stretching band in the spectra of HL and [Zn(HL)(OAc)2] is observed at 3229 and 3205 cm−1, respectively.29 The bands related to the NH2 group in the spectrum of [Zn(HL)(OAc)2] are observed at 3333 and 3432 cm−1 and these bands are located at 3367 and 3338 cm−1 in the spectrum of HL. In the FT-IR spectrum of Si-[Zn(HL)(OAc)2], there are three strong and broad bands at 1062, 805 and 468 cm−1 which are related to the vibration of the silica support.30 The broad band at 3416 cm−1 is due to the OH group in the structure of supported catalyst. Two absorption bands at 1637 and 1601 cm−1 are respectively assigned to the amide C
O oxygen to the Zn(II) ion. The NH stretching band in the spectra of HL and [Zn(HL)(OAc)2] is observed at 3229 and 3205 cm−1, respectively.29 The bands related to the NH2 group in the spectrum of [Zn(HL)(OAc)2] are observed at 3333 and 3432 cm−1 and these bands are located at 3367 and 3338 cm−1 in the spectrum of HL. In the FT-IR spectrum of Si-[Zn(HL)(OAc)2], there are three strong and broad bands at 1062, 805 and 468 cm−1 which are related to the vibration of the silica support.30 The broad band at 3416 cm−1 is due to the OH group in the structure of supported catalyst. Two absorption bands at 1637 and 1601 cm−1 are respectively assigned to the amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and imine C
O and imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups of the supported [Zn(HL)(OAc)2]. The FT-IR spectra of these compounds confirm the successful support of [Zn(HL)(OAc)2] on silica support.
N groups of the supported [Zn(HL)(OAc)2]. The FT-IR spectra of these compounds confirm the successful support of [Zn(HL)(OAc)2] on silica support.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C–NH2 and amide C
N, C–NH2 and amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively. The peaks observed at δ = 174.0 and 174.9 ppm are related to the carboxyl group of the acetate ligands31 which indicate two acetate groups have slightly different conditions and one of the carbon peaks is observed at a slightly different position.
O groups, respectively. The peaks observed at δ = 174.0 and 174.9 ppm are related to the carboxyl group of the acetate ligands31 which indicate two acetate groups have slightly different conditions and one of the carbon peaks is observed at a slightly different position.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
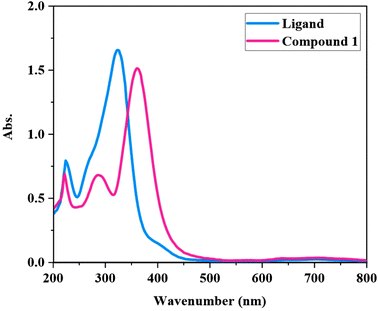
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups to the metal ion and due to this, the intraligand n → π* transitions are eliminated in the spectrum of complex. In the spectrum of [Zn(HL)(OAc)2], a new strong and relatively broad band is observed in the range of 330–450 nm with λmax = 360 nm, which is devoted to the charge transfer transitions. The slight shift of π → π* transitions, the elimination of intraligand n → π* transitions and the observation of charge transfer transitions in the spectrum of [Zn(HL)(OAc)2] clearly confirm the coordination of HL to the Zn(II) ion.
O groups to the metal ion and due to this, the intraligand n → π* transitions are eliminated in the spectrum of complex. In the spectrum of [Zn(HL)(OAc)2], a new strong and relatively broad band is observed in the range of 330–450 nm with λmax = 360 nm, which is devoted to the charge transfer transitions. The slight shift of π → π* transitions, the elimination of intraligand n → π* transitions and the observation of charge transfer transitions in the spectrum of [Zn(HL)(OAc)2] clearly confirm the coordination of HL to the Zn(II) ion.
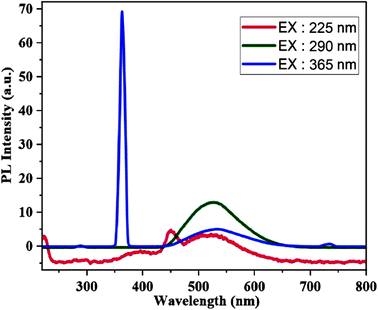
The emission spectra of [Zn(HL)(OAc)2] in the range of 220–800 nm were recorded at the excitation wavelengths (225, 290 and 365 nm), which are shown in Fig. 7. The spectra display emission bands and in all the excitation wavelengths, broad emission bands appear in the range of 450–600 nm with maximum intensity at 522, 525 and 530 nm. There are two weak emissions at about 450 and 730 nm by excitations at 225 and 365 nm, respectively, which are the second order Rayleigh scattering of these excitations.32 Zn(II) compounds do not exhibit d–d transitions because of their d10 configuration. As a result, the observed peaks are the result of electron relaxation from higher to lower energy levels.
The UV-DRS spectrum of Si-[Zn(HL)(OAc)2] was recorded in the range of 300–1000 nm (see Fig. S7†). There are two obvious absorption bands at 361 and 411 nm in this spectrum which are in agreement with the UV-vis spectrum of [Zn(HL)(OAc)2] in methanol solution. These bands are related to intraligand and charge transfer transitions, respectively. The slight differences between solid state UV-DRS and solution UV-vis spectra are related to the phase and environmental differences and also to the changes in the intermolecular interactions in these two different conditions.
3.4. Catalytic CO2 fixation
The results of various analyses confirmed the successful supporting of [Zn(HL)(OAc)2] on the silica support. The loading of Zn(II) ion by atomic absorption analysis was obtained as 4.01 wt% which is equal to 0.613 mmol g−1 of the catalyst. Due to the high activity of Zn(II) compounds in the chemical CO2 fixation reactions, the catalytic activity of Si-[Zn(HL)(OAc)2] was investigated in the formation of cyclic carbonates from the reaction of CO2 and epoxide. The catalytic reactions were done in a round bottom flask containing Si-[Zn(HL)(OAc)2] (as catalyst), styrene epoxide (as substrate), solvent (CH3CN, EtOH, THF or CHCl3), and tetrabutylammonium bromide (TBAB, as co-catalyst) under CO2 rich atmosphere (see Scheme 2). The control experiments in the absence of the catalyst or co-catalyst indicated there are low amounts of the cyclic product in the absence of one of them. When both the catalyst and co-catalyst were simultaneously employed, the rate of the reaction was considerably increased, and styrene epoxide was converted to phenylethylene carbonate. Thus, the effect of the ratio of co-catalyst was studied by using various amounts of TBAB in the presence of constant amounts of the other reagents. The results of these studies are collected in Table 4 and indicate 1.0 mmol of TBAB has better performance than others. Temperature of the reaction was also investigated, and the best result was obtained at 70 °C in CH3CN solvent. Although the catalyst in this reaction is heterogeneous, we interested to investigate the effect of the solvent on its performance by considering the fact that solvent can considerably increase the interaction between substrate, co-catalyst and catalyst. Moreover, CO2 can considerably dissolve in the solvent and, due to this; solvent also has a considerable effect on the interaction between CO2 and epoxide. By changing the solvent of the reaction, there was no considerable change in the conversion of epoxide to cyclic carbonate but, it was found that the rate of the reaction is higher in CH3CN which maybe is related to the better solubility of the reagents in this solvent. Higher dielectric constant of CH3CN (37.5) than other used solvents (THF = 7.58, CHCl3 = 4.81 and EtOH = 24.55) can be also influential in this process. The low boiling point of THF (66 °C) and CHCl3 (61.2 °C) in comparison to CH3CN (82 °C) and its limiting effect is also effective on the reaction rate in these solvents. It should be noted that 1-phenyl-1,2-ethanediol was obtained as a byproduct in ethanol solvent, which can be attributed to the epoxide ring opening reaction by the attack of the water molecules in this solvent. Therefore, due to the formation of byproduct and lower yield of the cyclic carbonate, ethanol was the worst solvent among the investigated ones. | ||
| Scheme 2 The reaction pathway of CO2 coupling by styrene epoxide in the presence of Si-[Zn(HL)(OAc)2]. | ||
| Entry | TBAB (mmol) | Solvent | Temp. (°C) | Time (h) | Yieldb (%) | TON | TOF (h−1) |
|---|---|---|---|---|---|---|---|
| a Reaction condition: Si-[Zn(HL)(OAc)2] (15.0 mg, 9.20 μmol Zn(II) ion), styrene epoxide (5.0 mmol), solvent (10 ml).b Isolated yield of cyclic carbonate.c Without catalyst. | |||||||
| 1 | — | CH3CN | 50 | 18 | 10 | 54 | 3.0 |
| 2 | 0.10 | CH3CN | 50 | 18 | 61 | 332 | 18.4 |
| 3 | 0.50 | CH3CN | 50 | 12 | 70 | 380 | 31.7 |
| 4 | 1.00 | CH3CN | 50 | 12 | 82 | 446 | 37.2 |
| 5 | 1.50 | CH3CN | 50 | 12 | 84 | 456 | 38.0 |
| 6 | 2.00 | CH3CN | 50 | 12 | 87 | 473 | 39.4 |
| 7 | 1.00 | CH3CN | 70 | 12 | 94 | 511 | 42.6 |
| 8 | 1.00 | CH3CN | 80 | 12 | 95 | 516 | 43.0 |
| 9 | 1.00 | CH3CN | RT | 12 | 46 | 250 | 20.8 |
| 10 | 1.00 | THF | 70 | 12 | 68 | 370 | 30.8 |
| 11 | 1.00 | CHCl3 | 70 | 12 | 72 | 391 | 32.6 |
| 12 | 1.00 | EtOH | 70 | 12 | 66 | 359 | 29.9 |
| 13c | 1.00 | CH3CN | 70 | 12 | 19 | — | — |
When the catalytic reaction was finished, the product was extracted by solvent and Si-[Zn(HL)(OAc)2] was recovered from the reaction mixture by filtration. The remaining compound was characterized by FT-IR spectroscopy to investigate the stability of Si-[Zn(HL)(OAc)2] during the catalytic reaction. Considering the FT-IR spectrum of the recovered Si-[Zn(HL)(OAc)2] (Fig. S12†) indicated that it is comparable with the spectrum of the fresh one. This matter confirms that there is no considerable change in the structure and composition of Si-[Zn(HL)(OAc)2] during and after catalytic reaction and it has acceptable stability under the investigated catalytic reaction. The recovered catalyst showed acceptable activity in the next runs and it could efficiently catalyze the CO2 fixation reaction without meaningful decrease in its activity. Only 6% decrease in the conversion of substrate was observed after five recovering and reusing cycles. The catalytic activity of [Zn(HL)(OAc)2] with same loading of Zn(II) ion (9.20 μmol, 4.0 mg) was also investigated in the optimum condition obtained for Si-[Zn(HL)(OAc)2] (Temp. = 70 °C, TBAB = 1.0 mmol, CH3CN = 10 ml). In this case the reaction was completed after about 8 hours which indicates [Zn(HL)(OAc)2] in homogeneous condition has higher catalytic activity than heterogenised Si-[Zn(HL)(OAc)2]. This matter is mainly related to higher interaction of catalyst with reagents in the homogeneous condition.
The catalytic activity of Si-[Zn(HL)(OAc)2] was compared with the activity of the other homogeneous and heterogeneous Zn(II) coordination compounds (see Table 5) and the results indicated that this compound has moderate activity in comparison with the similar catalytic systems. The mechanism of the chemical CO2 fixation in the presence of Zn(II) catalysts has been investigated in the literature.34 The proposed mechanism for chemical CO2 fixation reaction in the presence of Si-[Zn(HL)(OAc)2] is illustrated in Scheme S1.† By considering the structure and composition of Si-[Zn(HL)(OAc)2] and also the results of the catalytic reactions, it is predictable that the mechanism of this system is similar to the previous reports and the cyclic carbonate produces by the cyclization reaction of CO2 with activated epoxide by TBAB co-catalyst in the presence of Zn(II) ion.
| Catalyst | Co-catalyst | Pressure | Temp. (°C) | Time (h) | Yield (%) | TOF (h−1) | Ref. |
|---|---|---|---|---|---|---|---|
| Si-[Zn(HL)(OAc)2] | TBAB | 1 atm | 70 | 12 | 94 | 42.6 | This study |
| La–Zn complex with diglycolamine-bis(phenolate) ligand | TBAB | 1 atm | 25 | 24 | 93 | 7.8 | 35 |
| Zn porphyrin complex | TBAB | 1 atm | 70 | 4 | 99> | 13.88 | 36 |
| ZnL2 (L = 4,5-diphenyl-1H-imidazole-2-yl)phenol derivatives) | TBAI | 2 MPa | 110 | 5 | 93.1 | 186.2 | 37 |
| [Zn(Me6Tren)ClO4]ClO4 (Me6Tren = tris[2-(dimethylamino)ethyl]amine | TBAI | 1 bar | 80 | 6 | 85 | 14.2 | 38 |
| [Zn(Me6Tren)NO3]NO3 | TBAI | 1 bar | 80 | 6 | 77 | 12.8 | 38 |
| ZnTCPP (TCPP = 5,10,15,20-tetrakis(4-carboxyphenyl)-porphyrinato) | TBAB | 1 bar | 40 | 14 | 52.8 | 3.97 | 39 |
| Zn(OH-salC2NH2Am) (OH-salC2NH2Am = N,N-diethyl-2-((2,3,4-trihydroxybenzylidene)amino)ethanaminium bromide) | TBAB | 0.1 MPa | 120 | 12 | 90 | 15 | 40 |
| [PS-Zn(II)L] (polystyrene supported Zn(II) complex with bis-phenolate ligand) | TBAB | 0.1 MPa | RT | 8 | 98 | 21.9 | 41 |
| ZnCl2 | TBAI | 1 bar | RT | 24 | 27 | 11.3 | 42 |
| ZnLAPIPOAc (LAPIP = 2,4-di-tert-butyl-6-(((E)-2-(((E)-pyridin-2-lmethylene)amino) benzylidene)-amino)phenol) | TBAB | 0.1 MPa | 70 | 4 | 40 | 100 | 43 |
| ZnMOF-1 | TBAB | 1 atm | 40 | 8 | 68 | 4.7 | 44 |
| {[(CH3)2NH2][Zn2(L)(H2O)PO4]·2DMF}n (H2L = 5-(1H-imidazole-1-yl)isophthalic acid) | TBAB | 1 atm | 70 | 12 | 99 | 4.5 | 45 |
4. Conclusion
In summary, a new Zn(II) coordination compound containing a free amine functionality on the ligand, [Zn(HL)(OAc)2] (1), was synthesized and characterized by several methods. The Zn(II) ion in this compound is five coordinated and its coordination environment is created by coordination of ONN-donor atom of organic ligand and two oxygen atoms of acetate anions. The compound was successfully supported on the functionalized silica gel by using amidification reaction and the resulting heterogeneous catalyst, Si-[Zn(HL)(OAc)2], was characterized by atomic absorption, TGA, FT-IR, XRD, SEM, EDX and DRS analyses. The results confirmed that compound 1 is successfully supported on the silica, and it has high stability during and after catalytic reactions. The catalytic activity of Si-[Zn(HL)(OAc)2] was investigated in chemical CO2 fixation reaction and production of cyclic carbonate by using styrene epoxide as a model substrate. Si-[Zn(HL)(OAc)2] showed catalytic properties in the formation of cyclic carbonate and the results indicated that temperature, solvent and co-catalyst can improve the catalytic activity of this compound.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for the reported compound have been deposited at the Cambridge Crystallographic Data Center (CCDC No. 2409956) and can be obtained free of charge from https://www.ccdc.cam.ac.uk/structures/ and also from the online version of this article at https://doi.org/10.1039/D4RA09026H.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the Imam Khomeini International University for supporting this study.References
- (a) Y. Abdullatif, A. Sodiq, N. Mir, Y. Bicer, T. Al-Ansari, M. H. El-Naas and A. I. Amhamed, RSC Adv., 2023, 13, 5687–5722 RSC; (b) J. Fang, J. Zhu, S. P. Wang, C. Yue and H. H. Shen, Sci. China Earth Sci., 2011, 54, 1458–1468 CrossRef; (c) S. R. Weart, Wiley Interdiscip. Rev. Clim., 2010, 1, 67–81 CrossRef.
- (a) G. A. Olah, A. Goeppert and G. K. S. Prakash, J. Org. Chem., 2011, 74, 487 CrossRef PubMed; (b) A. M. Appel, J. E. Bercaw, A. B. Bocarsly, H. Dobbek, D. L. DuBois, M. Dupuis, J. G. Ferry, E. Fujita, R. Hille, P. J. A. Kenis, C. A. Kerfeld, R. H. Morris, C. H. F. Peden, A. R. Portis, S. W. Ragsdale, T. B. Rauchfuss, J. N. H. Reek, L. C. Seefeldt, R. K. Thauer and G. L. Waldrop, Chem. Rev., 2013, 113, 6621–6658 CrossRef CAS PubMed.
- P. H. Phua, S. Y. Chew and L. Zhao, Chem. Soc. Rev., 2016, 45, 954–985 RSC.
- (a) T. K. Pal, D. De and P. K. Bharadwaj, Coord. Chem. Rev., 2020, 408, 213173 CrossRef CAS; (b) S. Muthuramalingam, M. Velusamy and R. Mayilmurugan, J. Chem. Sci., 2018, 130, 78 CrossRef; (c) K. Huang, C.-L. Sun and Z.-J. Shi, Chem. Soc. Rev., 2011, 40, 2435–2452 RSC; (d) A. Rawat, S. Dhakla, P. Lama and T. K. Pal, J. CO2 Util., 2022, 59, 101939 CrossRef CAS; (e) D. Yu, S. P. Teong and Y. Zhang, Coord. Chem. Rev., 2015, 293–294, 279–291 CrossRef CAS.
- (a) Y. Wang, H. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2019, 58, 1701–1705 Search PubMed; (b) K. Koike, M. Ueno, H. Inoue and N. Morita, Angew. Chem., Int. Ed., 2014, 53, 4220–4223 Search PubMed.
- (a) K. C. Poon, W. Y. Wan, H. Su and H. Sato, RSC Adv., 2022, 12, 22703–22721 RSC; (b) L. Chen, F. Chenb and C. Xia, Energy Environ. Sci., 2014, 7, 4018–4022 RSC; (c) D. Monzer and C. Bouallou, Int. J. Hydrogen Energy, 2024, 52, 152–166 CrossRef CAS; (d) T. A. Saleh, RSC Adv., 2022, 12, 23869–23888 RSC; (e) J.-H. Zhou, C.-Y. Yuan, Y.-L. i Zheng, H.-J. Yin, K. Yuan, X.-C. Sun and Y.-W. Zhang, RSC Adv., 2021, 11, 38486–38494 RSC.
- X. B. Lu, D. J. Darensbourg and D. J. Darensbourg, Acc. Chem. Res., 2017, 50, 592–595 CrossRef PubMed.
- J. Klankermayer, S. Wesselbaum, K. Beydoun and W. Leitner, Angew. Chem., Int. Ed., 2016, 55, 7296–7343 CrossRef CAS PubMed.
- (a) Z. Li, B. Yan, J. Sun, Q. Ma and H. Jiang, Green Chem., 2020, 22, 1457–1479 Search PubMed; (b) C. Claver, M. B. Yeamin, M. Reguero and A. M. Masdeu-Bultó, Green Chem., 2020, 22, 7665–7706 RSC; (c) M. Usman, A. Rehman, F. Saleem, A. Abbas, V. C. Eze and A. Harvey, RSC Adv., 2023, 13, 22717–22743 RSC.
- (a) P. Rollin, L. K. Soares, A. M. Barcellos, D. R. Araujo, E. J. Lenardão, R. G. Jacob and G. Perin, Appl. Sci., 2021, 11, 5024 CrossRef CAS; (b) Sahil and N. Gupta, Renew. Sustain. Energy Rev., 2024, 193, 114297 CrossRef CAS.
- (a) N. J. English, M. M. El-Hendawy, D. A. Mooney and J. M. D. MacElroy, Coord. Chem. Rev., 2014, 269, 85–95 CrossRef CAS; (b) J. Chen, X. Wu, H. Ding, N. Liu, B. Liu, L. He and A. C. S. Sus, Chem. Eng., 2021, 9, 16210–16219 CAS.
- (a) J. Tapiador, P. Leo, A. Rodríguez-Diéguez, D. Choquesillo-Lazarte, G. Calleja and G. Orcajo, Catal. Today, 2022, 390–391, 230–236 CrossRef CAS; (b) P. Dadmehr, R. Bikas and T. Lis, Dalton Trans., 2024, 53, 15246–15257 RSC; (c) A. Eskemech, H. Chand, A. Karmakar, V. Krishnan and R. R. Koner, Inorg. Chem., 2024, 63, 3757–3768 CrossRef CAS PubMed.
- W. H. Wang and Y. G. Wang, Green Chem., 2020, 22, 1038–1061 RSC.
- (a) R. Sanyal, A. Guha, T. Ghosh, T. K. Mondal, E. Zangrando and D. Das, Inorg. Chem., 2014, 53, 85–96 CrossRef CAS PubMed; (b) A. Erxleben, Coord. Chem. Rev., 2003, 246, 203–228 CrossRef CAS; (c) Z. Fang, Y.-J. Zhu, D. Wang, Z. Wang, C. Lian, K.-L. Huang, J.-F. Qian, M.-Y. He, S.-C. Chen and Q. Chen, Mol. Catal., 2024, 569, 114507 CrossRef CAS; (d) Y. Zhang, J. Yang, R. Ge, J. Zhang, J. M. Cairney, Y. Li, M. Zhu, S. Li and W. Li, Coord. Chem. Rev., 2022, 461, 214493 CrossRef CAS.
- (a) Z.-J. Li, J.-F. Sun, Q.-Q. Xu and J.-Z. Yin, ChemCatChem, 2021, 13, 1848–1866 CrossRef CAS; (b) S.-T. Bai, G. D. Smet, Y. Liao, R. Sun, C. Zhou, M. Beller, B. U. W. Maes and B. F. Sels, Chem. Soc. Rev., 2021, 50, 4259–4298 RSC; (c) H. Chen, Z. Yang, C.-L. Do-Thanh and S. Dai, ChemSusChem, 2020, 13, 6182–6200 CrossRef CAS PubMed.
- (a) F. Zaera, Chem. Rev., 2022, 122, 8594–8757 CrossRef CAS PubMed; (b) I. Fechete, Y. Wang and J. C. Védrine, Catal. Today, 2012, 189, 2–27 CrossRef CAS.
- (a) H. Chen, Z. Guo, G. Liu, Y. Zhang and W. Dai, Adv. Sus. Sys., 2021, 5, 2000134 Search PubMed; (b) P. Bhanja, A. Modak and A. Bhaumik, Chem.–Eur. J., 2018, 24, 7278–7297 CrossRef CAS PubMed; (c) I. H. Chowdhury, A. H. Chowdhury, P. Sarkar and M. Islam, ChemNanoMat, 2021, 7, 580–591 CrossRef; (d) Y. Song and H. Tüysüz, Acc. Chem. Res., 2024, 57, 2038–2047 CrossRef CAS PubMed.
- (a) H.-W. Zheng, D.-D. Yang, Y.-S. Shi, T. Xiao, H.-W. Tan and X.-J. Zheng, Inorg. Chem., 2023, 62, 6323–6331 CrossRef CAS PubMed; (b) R. Bikas, M. Darvishvand, V. Kuncser, G. Schinteie, M. Siczek and T. Lis, Polyhedron, 2020, 190, 114751 CrossRef CAS.
- (a) T. Gebretsadik, Q. Yang, J. Wu and J. Tang, Coord. Chem. Rev., 2021, 431, 213666 CrossRef CAS; (b) N. N. Kuranova, O. A. Pimenov, M. N. Zavalishin and G. A. Gamov, Int. J. Mol. Sci., 2024, 25, 5046 CrossRef CAS PubMed; (c) G. Sahu, K. Sahu, S. A. Patra, D. Mohapatra, R. Khangar, S. Sengupta and R. Dinda, ACS Appl. Bio Mater., 2023, 6, 5360–5371 CrossRef CAS PubMed.
- (a) R. Liu, J. Cui, T. Ding, Y. Liu and H. Liang, Molecules, 2022, 27, 8393 CrossRef CAS PubMed; (b) M. Fallah-Mehrjardi, H. Kargar and K. S. Munawar, Inorg. Chim. Acta, 2024, 560, 121835 CrossRef CAS; (c) J. O. C. Brum, T. C. C. França, S. R. LaPlante and J. D. F. Villar, Mini Rev. Med. Chem., 2020, 20, 342–368 CrossRef PubMed; (d) S. N. Mali, B. R. Thorat, D. R. Gupta and A. Pandey, Eng. Proc., 2021, 11, 21 Search PubMed.
- (a) M. R. Maurya, A. Patter, A. Chauhan and N. Kumar, Top. Catal., 2024, 67, 466–482 CrossRef CAS; (b) M. Sarkheil and M. Lashanizadegan, Appl. Organomet. Chem., 2018, 32, e4459 CrossRef; (c) A. H. Ahmed and M. S. Thabet, J. Mol. Strucut., 2011, 1006, 527–535 CrossRef CAS; (d) M. Emami, R. Bikas, N. Noshiranzadeh, A. Kozakiewicz and T. Lis, ACS omega, 2020, 5, 13344–13357 CrossRef CAS PubMed.
- N. Heydari, R. Bikas, M. Shaterian, M. S. Krawczyk and T. Lis, Appl. Organomet. Chem., 2023, 37, e6976 CrossRef CAS.
- CrysAlis PRO, Oxford Diffraction/Agilent Technologies Ltd, Yarnton, Oxfordshire, England, 2014.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- (a) M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, P. R. Spackman, D. Jayatilaka and M. A. Spackman, CrystalExplorer17, University of Western Australia, 2017 Search PubMed; (b) P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, J. Appl. Cryst., 2021, 54, 1006–1011 CrossRef CAS PubMed.
- (a) S. Dasgupta, S. Karim, S. Banerjee, M. Saha, K. D. Saha and D. Das, Dalton Trans., 2020, 49, 1232–1240 RSC; (b) F. Marchetti, R. Pettinari, F. Verdicchio, A. Tombesi, S. Scuri, S. Xhafa, L. Olivieri, C. Pettinari, D. Choquesillo-Lazarte, A. García-García, A. Rodríguez-Diéguez and A. Galindo, Dalton Trans., 2022, 51, 14165–14181 RSC; (c) Z. Zabihollahi, R. Bikas, M. Hossaini-Sadr, A. Kozakiewicz-Piekarz and B. Soltani, J. Mol. Struct., 2022, 1265, 133356 CrossRef CAS.
- (a) Z. Keleşoğlu, C. Ersanlı and G. K. Kantar, J. Mol. Struct., 2019, 1181, 25–37 CrossRef; (b) A. H. Bakheit and H. M. Alkahtani, Molecules, 2023, 28, 6859 CrossRef CAS PubMed; (c) A. H. Bakheit, H. A. Abuelizz and R. Al-Salahi, Crystals, 2023, 13, 1410 CrossRef CAS.
- (a) F. Soltani, R. Bikas, N. Heydari and A. Kozakiewicz-Piekarz, New J. Chem., 2023, 47, 6102–6113 RSC; (b) R. Bikas, M. Emami, K. Ślepokura and N. Noshiranzadeh, New J. Chem., 2017, 41, 9710–9717 RSC; (c) M. Aligholivand, Z. Shaghaghi, R. Bikas and A. Kozakiewicz, RSC Adv., 2019, 9, 40424–40436 RSC.
- (a) N. Heydari, R. Bikas, D. A. Kalofolias and T. Lis, Polyhedron, 2024, 264, 117207 CrossRef CAS; (b) R. Bikas, Z. Shaghaghi, Y. Heshmati-Sharabiani, N. Heydari and T. Lis, Photosynth. Res., 2022, 154, 383–395 CrossRef CAS PubMed; (c) F. Ajormal, R. Bikas, N. Noshiranzadeh, A. Kozakiewicz-Piekarz and T. Lis, New J. Chem., 2022, 46, 19468–19481 RSC.
- (a) N. Heydari, R. Bikas, M. Siczek and T. Lis, Dalton Trans., 2023, 52, 421–433 RSC; (b) R. Bikas, N. Heydari and T. Lis, J. Mol. Struct., 2023, 1281, 135120 CrossRef CAS; (c) R. Bikas, N. Heydari, P. Asadollahi and T. Lis, Catal. Lett., 2024, 154, 116–131 CrossRef CAS; (d) N. Heydari, R. Bikas, M. Shaterian and T. Lis, Appl. Organomet. Chem., 2023, 37, e6939 CrossRef CAS.
- (a) S. J. Mohammed, K. M. Omer and F. E. Hawaiz, RSC Adv., 2023, 13, 14340–14349 RSC; (b) H. Li, Y. Ma, L. Yu, H. Xue, Y. Wang, J. Chen and S. Zhang, RSC Adv., 2020, 10, 8480–8489 RSC.
- (a) R. J. Clarke and A. Oprysa, J. Chem. Educ., 2004, 81, 705–707 CrossRef CAS; (b) M. A. Morozova, V. N. Tumasov, I. V. Kazimova, T. V. Maksimova, E. V. Uspenskaya and A. V. Syroeshkin, Pharmaceutics, 2022, 14, 767 CrossRef CAS PubMed; (c) D. M. Jameson, Introduction to Fluorescence, CRC Press, 1st edn, 2014 CrossRef; (d) N. Wu, H. Guo, M. Wang, L. Peng, Y. Chen, B. Liu, Z. Pan, Y. Liu and W. Yang, Spectrochim. Acta, Part A, 2022, 270, 120858 CrossRef CAS PubMed; (e) J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 2006 CrossRef.
- N. Heydari, R. Bikas, M. Shaterian and T. Lis, RSC Adv., 2022, 12, 4813–4827 RSC.
- (a) T. T. Wang, Y. Xie and W. Q. Deng, J. Phys. Chem. A, 2014, 118, 9239–9243 CrossRef CAS PubMed; (b) S. K. Kim, J. J. Kim, S. D. Lee, M. S. Lah, D. Moon and H. G. Jang, Chem.–Eur. J., 2003, 9, 678–686 CrossRef PubMed; (c) Y. Wu, S. Shi, X. Su, Z. Zhang, P. Liu, O. Oderinde, G. Yi, G. Xiao and Y. Zhang, Mol. Catal., 2021, 515, 111894 CrossRef CAS; (d) F. Castro-Gómez, G. Salassa, A. W. Kleij and C. Bo, Chem.–Eur. J., 2013, 19, 6289–6298 CrossRef PubMed; (e) T. Yan, H. Liu, Z. X. Zeng and W. G. Pan, J. CO2 Util., 2023, 68, 102355 CrossRef CAS; (f) A. K. Ghosh, U. Saha, S. Biswas, Z. A. Alothman, M. A. A. Islam and M. Dolai, Ind. Eng. Chem. Res., 2022, 61, 175–186 CrossRef CAS; (g) J. B. Cruz and C. H. Hung, Catal. Sci. Technol., 2021, 11, 2144–2154 RSC; (h) R. Ma, L.-N. He and Y.-B. Zhou, Green Chem., 2016, 18, 226–231 RSC; (i) Y. Wu, X. Song, S. Xu, Y. Chen, O. Oderinde, L. Gao, R. Wei and G. Xiao, Dalton Trans., 2020, 49, 312–321 RSC; (j) Y. Wu, X. Song, S. Xu, T. Yu, J. Zhang, Q. Qi, L. Gao, J. Zhang and G. Xiao, Mol. Catal., 2019, 475, 110485 CrossRef CAS; (k) Y. Wu, Y. Xiao, H. Yuan, Z. Zhang, S. Shi, R. Wei, L. Gao and G. Xiao, Micropor. Mesopor. Mat., 2021, 310, 110578 CrossRef CAS.
- K. Yin, L. Hua, L. Qu, Q. Yao, Ya. Wang, D. Yuan, H. You and Y. Yao, Dalton Trans., 2021, 50, 1453–1464 RSC.
- A. Farhadian, M. B. G. Afshani, A. B. Miyardan, M. R. Nabid and N. Safari, ChemistrySelect, 2017, 2, 1431–1435 CrossRef CAS.
- J. Peng, H.-J. Yang, Z. Wei and C.-Y. Guo, RSC Adv., 2015, 5, 53063–53072 RSC.
- K. Naveen, H. Ji, T. S. Kim, D. Kim and D.-H. Cho, Appl. Catal., B, 2021, 280, 119395 CrossRef CAS.
- J. Liang, Y.-Q. Xie, Q. Wu, X.-Y. Wang, T.-T. Liu, H.-F. Li, Y.-B. Huang and R. Cao, Inorg. Chem., 2018, 57, 2584–2593 CrossRef CAS PubMed.
- X.-D. Lang, Y.-C. Yu and L.-N. He, J. Mol. Catal. A: Chem., 2016, 420, 208–215 CrossRef CAS.
- S. Biswas, R. Khatun, M. Sengupta and S. M. Islam, Mol. Catal., 2018, 452, 129–137 CrossRef CAS.
- H. Kisch, R. Millini and I. J. Wang, Chem. Ber., 1986, 119, 1090–1094 CrossRef CAS.
- Z. Alaji, E. Safaei and A. Wojtczak, New J. Chem., 2017, 41, 10121–10131 RSC.
- P. Patel, B. Parmar, R. I. Kureshy and N. H. Khan, ChemCatChem, 2018, 10, 2401–2408 CrossRef CAS.
- M. Gupta, D. De, K. Tomar and P. K. Bharadwaj, Inorg. Chem., 2017, 56, 14605–14611 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: FT-IR, NMR and DRS spectra of the compounds, TGA curves, XRD pattern of the supported catalyst and suggested mechanism for the CO2 fixation reaction. CCDC no. 2409956 contains the supplementary crystallographic data for compound 1. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra09026h |
| This journal is © The Royal Society of Chemistry 2025 |