 Open Access Article
Open Access ArticleMolecular recognition and potentiometric determination of neostigmine and pyridostigmine by a methylene-bridged naphthotube†
Jian-Fang Wu‡
ab,
Wen-Jie Chen‡b,
Li-Li Wang b,
Liu-Pan Yang
b,
Liu-Pan Yang *b,
Yan-Fang Wang
*b,
Yan-Fang Wang *cd and
Dehua Liao*a
*cd and
Dehua Liao*a
aDepartment of Pharmacy, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, Hunan 410000, China. E-mail: liaodehua1125@126.com
bSchool of Pharmaceutical Science, Hengyang Medical School, University of South China, Hengyang, Hunan 421001, China. E-mail: yanglp@usc.edu.cn
cDepartment of Chemistry, Zhejiang University, Hangzhou 310058, P. R. China. E-mail: 11930562@mail.sustech.edu.cn
dZJU-Hangzhou Global Scientific and Technological Innovation Center, ZhejiangUniversity, Hangzhou 311215, P. R. China
First published on 31st March 2025
Abstract
A macrocyclic arene featuring rigid bis-naphthalene possesses a remarkable ability to strongly bind neostigmine (Ka = 2.7 × 104 M−1) and pyridostigmine (Ka = 2.2 × 105 M−1) within its deep cavity. This facilitates the host as a potential ionophore for the potentiometric determination of neostigmine and pyridostigmine with low detection limits of 1.0 μM, highlighting its promising applications in pharmaceutical analysis.
Anticholinesterase agents, also known as indirect-acting cholinomimetics, are a class of drugs that can bind to acetylcholinesterase and inhibit its activity, leading to the accumulation of acetylcholine at nerve terminals and exerting an excitatory effect on cholinergic receptors.1 Neostigmine (NEO) and pyridostigmine (PYR) are two of the most representative reversible acetylcholinesterase inhibitors, which form complexes with the anionic site of acetylcholinesterase through their positively charged quaternary ammonium groups to inhibit the enzyme's activity. These two drugs are the most widely used acetylcholinesterase inhibitors in clinical practice, mainly for the treatment of myasthenia gravis, postoperative abdominal distension, urinary retention, paralytic ileus, glaucoma, and reversal of neuromuscular blockade.2 The development of simple analytical methods for NEO and PYR is of great significance for drug quality control and the study of drug metabolism kinetics. Several methods have been reported for the determination of drugs in different dosage forms and biological fluids, including high-performance liquid chromatography (HPLC),3 gas chromatography,4 spectrophotometry,5 potentiometric titration,6 etc. Among these methods, HPLC is the most widely studied due to its high accuracy, however, this method requires complex sample preparation and is time-consuming.7 Therefore, developing more efficient and rapid methods for the detection of NEO and PYR is of significant importance.
Since the last century, ion-selective electrodes (ISEs) have been widely used in drug analysis and clinical testing due to their simple operation, efficiency, and ease of on-site monitoring.8 The principle of ISEs relies on the partition equilibrium of analytes at the liquid–membrane interface, where the binding affinity of ionophores toward target analytes fundamentally governs both the sensitivity and selectivity of the electrode. Classical macrocyclic hosts such as cyclodextrins and calixarenes have been successfully employed for detecting quaternary ammonium pharmaceuticals. For instance, El-Rahman's research group fabricated an ion-selective electrode utilizing calixarene-based ion carriers, achieving potentiometric detection of NEO with excellent selectivity and a linear response range.9a Similarly, Khorshid's team developed a β-cyclodextrin-modified carbon paste sensor that demonstrated enhanced performance with a lower detection limit of 6.3 × 10−8 mol L−1 for NEO.9b However, these conventional macrocyclic hosts suffer from certain limitations when employed as ion carriers. Cyclodextrins and sulfonated calixarenes exhibit high water solubility, leading to ionophore leaching and subsequent signal drift during operation.10 Additionally, the inherent conformational flexibility of calixarenes compromises their binding specificity. Consequently, to achieve superior detection performance, the development of novel macrocyclic hosts featuring rigid architectures and enhanced hydrophobicity is imperative for advanced ion carrier applications.
In recent years, macrocyclic arenes with methylene bridges have attracted wide attention due to their simple synthesis, diverse structures, and ease of modification.11 The majority of macrocyclic arenes are formed by planar aromatic rings connected by methylene bridges, where the free rotation of the two single bonds around the methylene group can lead to collapse of the macrocyclic cavity and structural flexibility, limiting their molecular recognition and application performance.12 To construct macrocyclic arenes with rigid cavities, we synthesized a new type of macrocycle – methylene-bridged naphthotubes using rigidly bridged bisnaphthalenes with an arched structure as building blocks (Fig. 1a).13 The introduction of the rigid bisnaphthalene14 structure reduces the involvement of methylene groups during cyclization, decreasing the structural flexibility of the macrocycle while providing a deeper electron-rich cavity (Fig. 1b). Therefore, methylene-bridged naphthotubes exhibit strong binding affinity towards organic cations. Both NEO and PYR have a positive charge in their structures, leading us to speculate that they are suitable guests for methylene naphthotubes. Herein, in this study, we have re-synthesized the macrocyclic host (H1) to ensure its structural integrity and purity for subsequent investigations. We report the binding behavior of H1 toward NEO and PYR, and further utilize the host as an ionophore to construct ion-selective electrodes, enabling accurate detection of these two drugs in different formulations and fetal bovine serum.
Initially, we investigated the binding interactions between host H1 and guests NEO and PYR in solution (Fig. 2). The 1H NMR spectrum of an equimolar mixture of host H1 and NEO in CD2Cl2 revealed a singular set of resonances, indicating the formation of a new complex (NEO@H1). This observation suggests that the binding process occurs via fast exchange dynamics. Notably, the signals corresponding to the guest within the complex exhibited broadening and, in some cases, disappeared into the baseline. Additionally, a significant downfield shift (Δδ = 0.28 ppm) was observed for the H3 proton signal of host H1. A similar influence was noted with the aromatic cation PYR, which also induced notable chemical shifts in the aromatic protons of host H1. Importantly, the chemical shifts observed for H1 in the PYR@H1 complex were generally greater than those seen in NEO@H1, suggesting that the binding affinity between host H1 and PYR is stronger than that with NEO. Diffusion-ordered spectroscopy (DOSY) experiments (Fig. S1–S3†) reveal a single assembly with diffusion coefficients of 5.97 × 10−10, 5.56 × 10−10, and 7.13 × 10−10 m2 s−1 for H1, NEO@H1 and PYR@H1, respectively, indicating similar hydrodynamic radii before and after host–guest binding. Moreover, there are two representative conformers for the host–guest complexes for NEO@H1 and PYR@H1, and the conformation of the host–guest complexes were determined based on NOE signals between the host and guest observed in the rotating Overhauser effect spectroscopy (ROESY) experiments (Fig. S4 and S5†).
 | ||
Fig. 2 Partial 1H NMR spectra (500 MHz, CD2Cl2, 1.0 mM, 25 °C) of (a) NEO, (b) a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H1 and NEO, (c) H1, (d) a 1 1 mixture of H1 and NEO, (c) H1, (d) a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H1 and PYR, and (e) PYR. 1 mixture of H1 and PYR, and (e) PYR. | ||
Electrospray ionization mass spectrometry experiments were conducted to analyze the equimolar mixture of H1 and the cationic guests NEO·BarF or PYR·BarF. The ESI mass spectrum revealed two intense peaks: one corresponding to [NH4@H1]+ (m/z ∼1458.7160) and the other to [NEO⊂H1]+ (m/z 1663.8251, Fig. 3a) or [PYR⊂H1]+ (m/z 1621.7771, Fig. 3b). These results confirm the formation of host–guest complexes through ESI mass analysis.
NMR titration experiments were then conducted to determine the association constants for the host–guest systems, as the formation of complexes occurs in a fast exchange on the NMR timescale. The titrations were performed using solutions with fixed and varying concentrations of the host molecule H1 and the guest molecules (NEO and PYR), respectively. For example, when examining the guest NEO, it was observed that upon its addition, the chemical shifts of protons 3, 15, and 16 on the host exhibited significant changes, making them particularly amenable to monitoring (Fig. 4a). By fitting these variations in chemical shifts to corresponding guest concentrations based on a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model (Fig. 4b), we calculated three Ka values (2.83 × 104 M−1, 2.69 × 104 M−1 and 2.68 × 104 M−1), which are consistent with each other. We also analyzed the NMR titration data using the Musketeer software15 developed by Hunter group, and the fitting result is similar with above results (Fig. S6†). Similarly, we determined the binding constant of PYR using the same method (Fig. S7†), which was found to be 2.2 × 105 M−1. This value is an order of magnitude higher than that of NEO and aligns with the predictions obtained from the 1
1 binding model (Fig. 4b), we calculated three Ka values (2.83 × 104 M−1, 2.69 × 104 M−1 and 2.68 × 104 M−1), which are consistent with each other. We also analyzed the NMR titration data using the Musketeer software15 developed by Hunter group, and the fitting result is similar with above results (Fig. S6†). Similarly, we determined the binding constant of PYR using the same method (Fig. S7†), which was found to be 2.2 × 105 M−1. This value is an order of magnitude higher than that of NEO and aligns with the predictions obtained from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 NMR analysis. The strong binding affinity of methylene-bridged naphthotube towards these two drug molecules also establishes a foundation for its application as an ion carrier in electrochemical detection.16
1 NMR analysis. The strong binding affinity of methylene-bridged naphthotube towards these two drug molecules also establishes a foundation for its application as an ion carrier in electrochemical detection.16
ISEs are typically constructed from PVC-based membranes doped with plasticizers, ion exchangers, and ionophores.17 Ion exchangers and ionophores improve selectivity;18 the latter should possess a strong binding affinity for the target analyte, thus lowering the free energy required for its permeation through the aqueous membrane interface. This study prepared two membrane electrodes to assess the efficacy of methylene-bridged naphthotubes as ionophores for NEO and PYR. The fabrication method was based on established protocols, employing PVC, ortho-nitrophenyl octyl ether (o-NPOE) and potassium tetrakis[3,5-bis(trifluoromethyl)phenyl] borate as the polymer matrix, plasticizer, ion exchanger, respectively. H1 functioned as the ionophore, while a control “blank” sensor contained no ionophores. Bu4N+ served as a reference compound due to its common use in potentiometry for evaluating ionophores owing to its high hydrophobicity. The more effective NEO/PYR ionophore was determined by comparing responses across different formulations (Fig. S8†). The potential differences were measured as electromotive force (EMF) values among ISEs: one without an ionophore and one containing H1 for NEO and PYR relative to Bu4N+. The electrodes with H1 displayed smaller potential differences than the blank electrode, indicating that its incorporation significantly reduces the free energy needed for NEO and PYR permeation across the aqueous membrane interface.
After confirming the efficacy of the ionophore, we proceeded to optimize the selection of plasticizer and working pH. The results indicated that o-NPOE demonstrated markedly superior sensitivity as a plasticizer in comparison to dioctyl sebacate (Fig. S9†). This finding underscores the importance of plasticizer choice in enhancing sensor performance. During the pH optimization process, We observed that within the pH range of 2 to 11, the potential responses of the two sensors are remarkably stable (Fig. S10†). Consequently, these types of sensors exhibit an extensive operational pH range.
In subsequent experimental procedures, a specific volume of a standard solution (0.1 M) containing the analyte was introduced into the buffer at intervals of 100 seconds while undergoing magnetic stirring. Potential responses were recorded within an analyte concentration range of 10−8–10−2 M (Fig. 5a). The outcomes indicate that the potential response is swift and attains equilibrium almost immediately upon alterations in the analyte concentration. This rapid equilibration can be attributed to the fast equilibrium at the membrane/solution interface. Based on these potential responses, a calibration curve was established for the concentration range of 10−8–10−2 M (Fig. 5b). Following the recommendations of the International Union of Pure and Applied Chemistry (IUPAC), the detection limit at 1 μM coincides with the intersection point of the linear response range, adhering to both the Nernst equation and regions of non-responsiveness. Compared to a blank electrode devoid of ion carriers, the electrode employing H1 as an ionophore demonstrates a reduced detection limit and a steeper slope (Fig. S11†). This enhancement is primarily attributed to the robust host–guest interaction between H1 and the analyte's ester bonds. After completing the study on NEO, a response standard curve for PYR was constructed using a similar methodology. The measurements indicated a linear range of 10−6 to 10−2 mol L−1, with a slope of 58.52 ± 0.52 mV per decade and a detection limit of 1.0 × 10−6 mol L−1 (Fig. S12†). Although the result is higher than the reported value,9 this electrode system demonstrates rapid response characteristics and are adequate in detecting these two substances thereby facilitating the accurate determination of NEO and PYR in real-world samples.
 | ||
| Fig. 5 (a) Response of EMF to incremental changes in the NEO concentration. (b) Calibration curves of the H1-based electrode to NEO. | ||
To further investigate the performance of the membrane electrode sensor, systematic tests were conducted to evaluate its selectivity and anti-interference capabilities. The experiment selected common cations and representative amino acids as analytes, specifically Na+, K+, NH4+, Fe3+, proline, histidine and glycine. As shown in Fig. 6, the electromotive force for NEO and PYR is significantly higher than that of other interfering substances. The calculated selectivity coefficients for various interfering substances (Table S1†) indicate that the selectivity coefficient values for the target substances are notably greater than those for other interferents. This suggests that the sensor exhibits high selectivity and good anti-interference capability towards the target substances. The primary reason for this selectivity is that the electron-rich deep cavity of H1 can form specific host–guest complexes with the organic cations of NEO and PYR through weak interactions such as cation-π interactions. This mechanism implies that in a mixed system containing multiple substances, the membrane electrode preferentially interacts with NEO and PYR, thereby enhancing detection selectivity. A comparative analysis with reported sensors9 reveals that the present system demonstrates comparable or superior selectivity parameters, suggesting significant potential for real-sample detection applications.
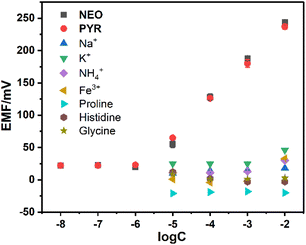 | ||
| Fig. 6 The selectivity for NEO and PYR over Na+, K+, NH4+, Fe3+, proline, histidine, and glycine of the electrodes containing H1. | ||
To investigate the detection performance of our sensor in complex samples, we first attempted to detect NEO and PYR in fetal bovine serum. Three concentrations of NEO and PYR were prepared using the spiking recovery method. The experimental results indicated that the sensor responded quickly within the fetal bovine serum matrix, and the results were accurate and reliable, with recoveries ranging from 98% to 102% (Table 1). This demonstrates that the sensor is effective for detecting NEO and PYR in complex biological samples. Furthermore, in the field of pharmaceutical analysis, precise determination of drug component content is crucial for ensuring drug quality. We subsequently examined the application performance of the sensor in measuring drug content in methanesulfonate injection of neostigmine and bromide tablets. The results showed that the measured contents were consistent with the labeled amounts on the drug packaging, with recoveries generally exceeding 95% (Table S2†). This indicates that this analytical method has significant potential for application in pharmaceutical analysis.
In conclusion, to achieve rapid and efficient detection of NEO and PYR, we have successfully engineered an advanced ion-selective electrode membrane sensor utilizing methylene-bridged naphthotube as ionophore. This sensor exhibits exceptional sensitivity and selectivity, while eliminating the need for sample preparation, thereby offering a robust technique for the rapid and accurate detection of NEO and PYR. With a minimum detection limit of 1.0 μM, it stands out as a promising analytical tool. Furthermore, its cost-effectiveness and environmentally friendly characteristics position it as a highly attractive option in the realm of pharmaceutical analysis, likely to gain significant traction in the field.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by National Natural Science Foundation of China (no. 22174059), Hunan Provincial Natural Science Foundation of China (no. 2022JJ40363), and the Young Science and Technology Innovation Program of Hunan Province (no. 2022RC1230). The APC was funded by University of South China.Notes and references
- (a) G. Pepeu and M. Giovannini, Curr. Alzheimer Res., 2009, 6, 86–96 Search PubMed; (b) Q.-S. Yu, H. W. Holloway, W. Luo, D. K. Lahiri, A. Brossi and N. H. Greig, Bioorg. Med. Chem., 2010, 18, 4687–4693 Search PubMed.
- (a) A. S. Habib and T. J. Gan, CNS Drugs, 2006, 20, 821–839 Search PubMed; (b) N. Tajaate, J.-U. Schreiber, T. Fuchs-Buder, Y. Jelting and P. Kranke, Eur. J. Anaesthesiol., 2018, 35, 184–192 Search PubMed.
- M. Takahiro, O. Masaki, N. Toshiaki, I. Yuji and A. Takao, J. Pharm. Biomed. Anal., 2006, 40, 331–337 Search PubMed.
- K. Mariitta, J. Chromatogr. A, 1993, 648, 501–506 Search PubMed.
- T. Sakai, X. Q. Liu and Y. Maeda, Talanta, 1999, 49, 997–1001 Search PubMed.
- (a) E. P. Diamandis and T. K. Christopoulos, Anal. Chim. Acta, 1983, 152, 281–284 Search PubMed; (b) K. A. Fouad and Y. M. Issa, Pharm. Biosci. J., 2015, 3, 30–39 Search PubMed.
- F. Varin, J. Couture and H. Gao, Biomed. Sci. Appl., 1999, 732, 319–323 Search PubMed.
- (a) M. K. A. El-Rahman, H. E. Zaazaa, N. B. ElDin and A. A. Moustafa, Talanta, 2015, 132, 52–58 Search PubMed; (b) G. A. E. Sayed, M. R. Abukhadra, S. M. Mostafa, M. Rabia, M. A. Koranya and M. M. Khalil, RSC Adv., 2023, 13, 34715–34723 Search PubMed; (c) A. A. Mouhamed, B. M. Eltanany, N. M. Mostafa, T. A. Elwaie and A. H. Nadim, RSC Adv., 2023, 13, 23138–23146 CAS.
- (a) A. M. El-Kosasy, M. Nebsen, M. K. A. El-Rahman, M. Y. Salem and M. G. El-Bardicy, Talanta, 2011, 85, 913–918 CAS; (b) A. F. Khorshid and Y. M. Issa, Biosens. Bioelectron., 2014, 51, 143–149 CrossRef CAS PubMed.
- M. A. El-Sayed, Sens. Actuators B: Chem., 2014, 190, 101–110 CrossRef CAS.
- (a) X.-N. Han, Y. Han and C.-F. Chen, Chem. Soc. Rev., 2023, 52, 3265–3298 RSC; (b) Z.-Y. Zhang and C. Li, Acc. Chem. Res., 2022, 55, 916–929 CAS; (c) Q. Shi, X. Wang, B. Liu, P. Qiao, J. Li and L. Wang, Chem. Commun., 2021, 57, 12379–12405 CAS; (d) S. Bleus and W. Dehaen, Coord. Chem. Rev., 2024, 509, 215762 CAS; (e) Y.-H. Tian, H. Qin, M.-H. Ding, L.-L. Tang and F. Zeng, RSC Adv., 2023, 13, 14539 CAS; (f) F. Zeng, L.-L. Tang, H. Yu, F.-P. Xu and L. Wang, Chin. Chem. Lett., 2023, 34, 108304 CAS; (g) C. Ruan, Z. Li, W. Lin, W. W. Xie, H. Li, Y. Lu, R. Wang, S. Li and L. Wang, Org. Lett., 2024, 26, 4122 CAS.
- (a) P. D. Sala, R. D. Regno, C. Talotta, A. Capobianco, N. Hickey, S. Geremia, M. D. Rosa, A. Spinella, A. Soriente, P. Neri and C. Gaeta, J. Am. Chem. Soc., 2020, 142, 1752–1756 Search PubMed; (b) L. P. Yang and W. Jiang, Angew. Chem., Int. Ed., 2020, 59, 15794–15796 CrossRef CAS.
- (a) Y. F. Wang, H. Yao, L. P. Yang, M. Quan and W. Jiang, Angew. Chem., Int. Ed., 2022, 61, e202211853 CrossRef CAS PubMed; (b) Mi. Yan and J. Zhou, Org. Chem. Front., 2023, 10, 2340 RSC.
- (a) L. P. Yang, X. Wang, H. Yao and W. Jiang, Acc. Chem. Res., 2019, 53, 198–208 Search PubMed; (b) C.-D. Zhao, H. Yao, S.-Y. Li, F. Du, L.-L. Wang and L.-P. Yang, Chin. Chem. Lett., 2024, 35, 108879 CrossRef CAS; (c) Y.-F. Wang, S.-M. Wang, X. Zhang, H. Nian, L.-S. Zheng, X. Wang, G. Schreckenbach, W. Jiang, L.-P. Yang and L.-L. Wang, Angew. Chem., Int. Ed., 2023, 62, e202310115 CAS; (d) S.-M. Wang, Y.-F. Wang, L. Huang, L.-S. Zheng, H. Nian, Y.-T. Zheng, H. Yao, W. Jiang, X. Wang and L.-P. Yang, Nat. Commun., 2023, 14, 5645 CAS.
- D. O. Soloviev and C. A. Hunter, Chem. Sci., 2024, 15, 15299 CAS.
- Y. Lu, S.-M. Wang, S.-S. He, Q. Huang, C.-D. Zhao, S. Yu, W. Jiang, H. Yao, L.-L. Wang and L.-P. Yang, Chem. Sci., 2024, 15, 14791 RSC.
- (a) S. Ogawara, J. L. Carey, X. U. Zou and P. Bühlmann, ACS Sens., 2015, 1, 95–101 CrossRef; (b) Y. Ishige, S. Klink and W. Schuhmann, Angew. Chem., Int. Ed., 2016, 55, 4831–4835 Search PubMed.
- Q. Chen, L.-P. Yang, D.-H. Li, J. Zhai, W. Jiang and X. Xie, Sens. Actuators B: Chem., 2021, 326, 128836 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra09021g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |



