Open Access Article
Open Access ArticleDifferent conjugates of Fe3O4 nanoparticles, drug, and dye: optical and magnetic properties for in vivo bimodal imaging
Thi Thu Huong Le a,
Ke Son Phanb,
Bich Thuy Doan
a,
Ke Son Phanb,
Bich Thuy Doan c,
Thi Thu Trang Maib,
Ha Bao Hung Buib,
Hong Nam Phamb,
Thi Dieu Thuy Ungb,
Yiqian Wangc,
Ngoc Quyen Tran
c,
Thi Thu Trang Maib,
Ha Bao Hung Buib,
Hong Nam Phamb,
Thi Dieu Thuy Ungb,
Yiqian Wangc,
Ngoc Quyen Tran d,
Le Hang Dangd and
Phuong Thu Ha
d,
Le Hang Dangd and
Phuong Thu Ha *b
*b
aFaculty of Natural Resources and Environment, Vietnam National University of Agriculture, Hanoi, Vietnam
bInstitute of Materials Science, Vietnam Academy of Science and Technology, Hanoi, Vietnam. E-mail: thuhp@ims.vast.ac.vn
cThe Institute I-CLeHS Institute of Chemistry for Life and Health Sciences, ENSCP Chimie ParisTech, PSL Université, CNRS UMR 8060, Paris, France
dInstitute of Advanced Technology, Vietnam Academy of Science and Technology, Ho Chi Minh City, Vietnam
First published on 31st March 2025
Abstract
Bioimaging is very important in medicine, especially in cancer diagnosis and treatment. In this study, we determined the impact of different components on the optical and magnetic properties of various conjugates. The three components, i.e., Fe3O4 nanoparticles (NPs), drug (doxorubicin – Dox), and dye (Cyanine 5.5 – Cy 5.5), were incorporated to form the four conjugates of A1 (Fe3O4–Cy 5.5), A2 (Fe3O4–Dox), A3 (Fe3O4–Cy 5.5–Dox), and A4 (Cy 5.5–Dox). The conjugates were characterized by DLS, UV-Vis spectra, fluorescence spectra, VSM, XRD, and TEM methods. After that, in vitro near-infrared (NIR) fluorescence imaging and magnetic resonance imaging (MRI) experiments were carried out to determine the conjugate suitable for bimodal imaging. The results show that A3 exhibits the highest quantum yield and radiance ratio and also has the highest ratio of r2/r1. The in vivo MRI and NIR fluorescent imaging results of CT26-bearing mice injected with A3 conjugates prove that the conjugate has potential in bimodal cancer imaging applications.
1 Introduction
Cancer has become one of the leading threats to human health in today's society, leading to high morbidity and mortality rates worldwide. According to a report from the International Agency for Research on Cancer (IARC), there were nearly 20 million new cancer cases and 9.7 million cancer deaths in 2022. The report also estimates that about 1 in 5 men or women will develop cancer during their lifetime, while about 1 in 9 men or 1 in 12 women will die from cancer.1 Therefore, developing new methods for diagnosing and treating cancer, which directly target cancer cells without affecting healthy cells, is of great interest to many research groups.Tumor imaging plays an important role in the advancement of cancer diagnosis because it allows non-invasive measurement of the biodistribution and pharmacokinetics of drugs, allowing us to determine where the drugs have gone, whether they have reached the tumor, how long they stay in the body, and how they are cleared.2 Magnetic resonance imaging (MRI) and fluorescence imaging attract much attention among various imaging techniques. Compared to other techniques, MRI can provide the best spatial resolution. However, its specificity is not high (which can cause a false positive rate of 10% for breast cancer). Therefore, it is necessary to use MRI contrast-enhancing agents such as superparamagnetic Fe3O4 nanoparticles (NPs).2 Besides, fluorescence imaging provides non-ionizing, portable, low-cost radiation with some advantages, including real-time imaging, higher resolution, and high sensitivity. Recently, near-infrared (NIR) fluorescence probes have been rapidly developed due to their important advantages over conventional optical imaging, including deeper tissue penetration, lower tissue absorption, and higher signal-to-background ratio.3 However, although NIR imaging is highly sensitive for diagnosing tumor tissue, it cannot differentiate between anatomical structures and has a limited penetration depth (less than 10 mm).4 Thus, no single imaging modality is perfect or sufficient to capture all the necessary information, so combining them will help to obtain more comprehensive information about the tumor and avoid the shortcomings of each individual technique.
Fe3O4 nanoparticles (MNPs) have shown great promise for diagnostic and therapeutic applications. Fe3O4 nanoparticles smaller than 20 nm are typically in a superparamagnetic state at room temperature, i.e., their magnetism can reach saturation value when exposed to an external magnetic field. In the absence of an external magnetic field, their magnetic moments align randomly, and their magnetization becomes zero. Due to their unique magnetic properties and size comparable to biologically important objects, Fe3O4 nanoparticles are being studied for a variety of biomedical applications, such as drug delivery, MRI contrast enhancement, and hyperthermia.5 A small amount of these NPs can be used for MRI tumor detection because when placed in a magnetic field, they will disturb the magnetic field, causing water protons to relax faster, thus allowing for increased MRI contrast.6 Multifunctional nanosystems based on Fe3O4 NPs were also reported.7,8
Cyanine compounds have many important photophysical and photochemical properties. At the same time, they have high biocompatibility and low toxicity, which makes them used in many applications in biomedicine, diagnostic imaging, and biochemistry. Recent studies have also shown that combining these advantages of Cyanine with nanoparticles allows the creation of many conjugates with improved desired functions.9 A 2014 study fabricated a conjugate system containing Fe/Fe3O4 nanoparticles and Cyanine 5.5 (covalently bound to the surface of the particles) to measure the activity of cancer-related proteases in biological samples.10 The results of the study showed that the measurement using the nanosystem was highly sensitive (as low concentration as 1 × 10−16 mol L−1 for 12 proteases), selective, and rapid (time required: 60 min). Cyanine 5.5 (Cy5.5) was conjugated with chitosan glycol to form spherical nanoparticles (containing 5.8% Cy5.5) with an average size of about 260 nm, stable in an aqueous medium. Thanks to the near-infrared (NIR) fluorescence of Cy5.5, the study determined that the maximum accumulation time in the tumor of the nanoparticles was from 48 to 96 hours, and the main organ that helped eliminate the nanoparticles from the body was the kidney.11 Fe3O4 nanoparticles surface functionalized with branched polyethyleneimine (b-PEI) and conjugated with Cy 5.5 produced a red signal on confocal fluorescence microscopy images, allowing to determine intracellular uptake of the nanoparticles by cells and showing the potential application in in vivo fluorescence imaging.12
Doxorubicin (Dox) is the most widely used drug in the treatment of many types of cancer in different organs (lung, stomach, breast, ovary, …). However, its high toxicity to healthy cells and serious side effects limit its applications in clinical treatment.13 To overcome these disadvantages, various DOX carrier systems, especially nanostructured drug carriers, have been studied to reduce the side effects of DOX while improving its therapeutic efficacy.14–17 Although Dox can absorb at 488 nm and emit fluorescence at 591 nm, its quantum yield is only 9% compared to that of rhodamine 6G (95%).18 Therefore, in vivo imaging usually requires other fluorophores like FCR-675 amine19 or DiR.20
Alginate, a natural polysaccharide extracted from brown algae, has various applications in the pharmaceutical and biomedical fields thanks to its biocompatibility and biodegradability. In addition, its stable and viscous aqueous solution makes alginate suitable for being used in different drug delivery nanosystems.21,22 In our previous studies, a nanosystem of Cyanine 5.5, Dox, and Fe3O4 was prepared based on synthetic PLA-TPGS copolymer. The in vitro toxicity on cancer cell lines and some in vitro optical and MRI imaging experiments of the system were carried out.23 The in vivo biodistribution of the nanosystem was also determined in normal mice.24 In this work, various conjugates (A1: Fe3O4–Cy5.5; A2: Fe3O4–Dox; A3: Fe3O4–Cy5.5–Dox; A4: Cy5.5–Dox) of the three components were prepared based on a natural polymer (alginate). The conjugates were fully examined for their optical and magnetic properties including quantum yield and magnetic inductive heating performance. The optimized conjugate was chosen for in vivo bimodal MRI and NIR-fluorescence imaging in tumor-bearing mice.
2 Materials and method
2.1. Materials
FeCl3·6H2O ≥99%, FeCl2·4H2O ≥9%, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), and doxorubicin hydrochloride (Dox), NaOH ≥99%, HCl (37%), NH3 solution (26%), 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminos-tyryl)-4H-pyran (DCM), ethanol, alginate (Alg) with a molecular weight of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were provided by Sigma Aldrich. Cyanine 5.5 NHS ester (C44H46N3BF4O4) was purchased from Lumiprobe GmbH (Europe). (3-Aminopropyl)triethoxysilane (APTES), and phosphate buffered solution (PBS, pH 7.4) were purchased from Merck. All chemicals were used without further purification. Distilled water was used for all experiments.
000 were provided by Sigma Aldrich. Cyanine 5.5 NHS ester (C44H46N3BF4O4) was purchased from Lumiprobe GmbH (Europe). (3-Aminopropyl)triethoxysilane (APTES), and phosphate buffered solution (PBS, pH 7.4) were purchased from Merck. All chemicals were used without further purification. Distilled water was used for all experiments.
2.2. Methods
| No. | Sample | Concentration (mg mL−1) | |||
|---|---|---|---|---|---|
| Fe3O4 | Cy 5.5 | Dox | |||
| 1 | A1 | Fe3O4–Cy 5.5 | 2 | 0.05 | 0 |
| 2 | A2 | Fe3O4–Dox | 2 | 0 | 0.25 |
| 3 | A3 | Fe3O4–Cy 5.5–Dox | 2 | 0.05 | 0.25 |
| 4 | A4 | Cy 5.5–Dox | 0 | 0.05 | 0.25 |
To calculate their quantum yield (QY), the samples were prepared as aqueous solutions with the absorbance values at 355 nm below 0.1. DCM was also dissolved in ethanol to obtain a reference solution with a similar absorbance value. The fluorescence spectra of the samples and the reference solution were recorded in the same conditions to determine the integrated fluorescence intensity of the emitted light. QY of the samples were calculated by eqn (1):
 | (1) |
The hydrodynamic size and zeta potential of the nanosystems in water were determined by dynamic light scattering using a Malvern Nano-ZS ZEN 3600 instrument. X-ray diffraction (XRD) method (SIEMENS-D5000) was used to determine the crystal structure of the Fe3O4-containing nanosystems. The magnetic properties of the systems were measured using a homemade vibrating sample magnetometer (VSM) at room temperature. The morphology of the nanoparticles was determined by transmission electron microscopy (TEM) on a JEOL JEM-1010.
Dox entrapment efficiency (EE%) of A2, A3, and A4 was determined by UV-Vis spectra at 485 nm based on a calibration curve of standard Dox solutions. EE% was calculated by the following formula:
 | (2) |
Magnetic inductive heating curves of A1, A2, and A3 were recorded on a commercial generator (Model UHF-20A) with the magnetic field amplitude varying from 100 to 250 Oe and at the frequency of 450 kHz. An optical thermometer (GaAs sensor, Opsens) was used to measure the sample temperature in the range of 0 to 250 °C.
Specific absorption rate (SAR) and intrinsic loss power (ILP) of the samples were calculated by eqn (3) and (4):
 | (3) |
 | (4) |
 is the slope of the heating curve, H and f are the magnetic field and frequency of the applied field.
is the slope of the heating curve, H and f are the magnetic field and frequency of the applied field.
6 Tumor-bearing mice were placed in a 7 T vertical MRI instrument (300WB, Bruker, Avance II, Wissembourg, France) and anesthetized with 1.5% isoflurane in air/O2 mixture (0.5 L min−1 and 0.2 L min−1, respectively). After T2-weighted MRI imaging before injection, the mice were injected with 150 μL of A3 at the concentration of 25 mM iron through a catheter placed in the tail vein of the animal. The remaining 3 mice were used as controls. T2-weighted MRI images were acquired 24 h after injection. ParaVision software (version 5.1) was used to process the acquired MRI data. All images were acquired at the same imaging depth to compare the darkening caused by the NPs. The muscle signal was used to normalize the relative signal intensity ratio of nanoparticles in the tumors of nano-injected and control mice.
For the data processing, an MRI image of each slice was opened in the ImageJ software (National Institutes of Health, Bethesda) using the plugin BrukerOpener. The ROI corresponding to the tumor was drawn as equally sized circles (∼5 mm in diameter), and the pixel intensity distribution was obtained. The pixel intensity distributions for each slice of the tumor were compiled using MATLAB software (R2023a, Natick, Massachusetts, United States) to obtain a pixel intensity distribution for the whole tumor. The percentage of pixels under the I0.25 value was then calculated with the same software by eqn (5):
| I0.25 = 0.25 × (Imax − Imin) | (5) |
3 Results and discussion
3.1. Size and optical properties
Fig. 1 presents the DLS and Dox EE results of the nanoconjugates. Hydrodynamic sizes of A1 to A4 are from 136 to 210 nm, which belongs to the optimal size range (100–200 nm) of nanoparticles to achieve enhanced permeability and retention effect and escape the filtration of spleen or liver.29,30 The particle size of the 2-component Fe3O4–Dox (A2) is larger than those of other conjugates, even the 3-component A3. This observation can be explained by the fact that in A1 and A3, APTES tightly attachs to Fe3O4 NPs and makes the structure of the conjugates more compact. | ||
| Fig. 1 Hydrodynamic size (a), zeta potential (b), and Dox encapsulation efficiency (c) of the conjugates. | ||
All four conjugates exhibit highly negative zeta potential, ranging from −32.6 mV to −37.5 mV, implying the high colloidal stability of the conjugates. In this study, alginate was used as a surfactant for the synthesis of the nanoconjugates. Alginate possesses a variety of carboxylate groups that help to maintain the electrostatic repulsion between the NPs.22 Moreover, the carboxyl groups on the surface of the conjugates make Cyanine 5.5 or DOX combine with other components more tightly.31 As a result, DOX was efficiently entrapped in the nanoconjugates (Fig. 1c). The encapsulation efficiency (EE%) of A2, A3, and A4 is around 80%. A4 shows a slightly lower EE compared to A2 and A3. This can be due to the role of Fe3O4 NPs that could be used as an effective Dox deliverer25,32,33 in A2 and A3 conjugates.
The UV-Vis and PL spectra of the conjugates are shown in Fig. 2. For the conjugates containing Dox (A2, A3, A4), the absorption of Dox appears at around 500 and 535 nm, and the corresponding emission bands are at 551 and 593 nm. Free Cyanine 5.5 has an absorption maximum of 684 nm and an emission maximum of 710 nm. A1 and A3 show minor redshifts of the NIR peaks in both absorption and emission spectra (690 and 712 nm, respectively). The high intensity of the 712 nm emission peaks confirms that Fe3O4 NPs do not interfere with the fluorescence emission of Cyanine 5.5.12 Fig. 2a shows that at 355 nm, A1 absorbs more than A3 while Fig. 2b shows that it emits less than A3 (at the same concentration of Cyanine 5.5). This indicates a higher fluorescent efficiency of A3 compared to that of A1 at the near infra-red region (712 nm).34 On the contrary, A4 shows a broad absorption from around 600 to 750 nm while the emission at the NIR region disappears. This result is in agreement with another report, in which the emission intensity in the NIR region of indocyanine green also decreases with the increase in the concentration of Dox.35 Notably, A3, a conjugate of Cyanine 5.5 with both fluorescent quenching agent of Fe3O4 (ref. 36 and 37) and a high concentration of Dox (5 times to the concentration of Cy 5.5), shows the highest emission intensity. Further calculation of quantum yield for the samples (Fig. 2c) confirms that A3 has the highest quantum yield compared to the other samples. Although there is a small portion of Cyanine 5.5 in A3, the quantum yield of this sample is about ¾ of that of pure Cyanine 5.5 (20%, as stated by the producer Lumiprobe GmbH). This result indicates the potential of A3 in NIR fluorescent imaging.
 | ||
| Fig. 2 UV-Vis spectra (a) and PL spectra (b) of the conjugates at C0/10 concentration and quantum yield of the samples (c). | ||
Table 2 displays the radiance ratio of the conjugates and control (agar) at different excitation/emission wavelengths, and Fig. 3 shows the in vitro fluorescent imaging of the conjugates at their optimal excitation/emission wavelength. The radiance of a sample is the number of photons per second that are leaving a square centimeter of the sample tube and radiating into a solid angle of one steradian (ph s−1 cm−2 sr−1). As shown in Table 2, A2 exhibits the best fluorescent signal at λex = 537 nm and λem = 597 nm, while the optimal excitation wavelength of A1, A3, and A4 is 687 nm, and their optimal emission wavelengths vary in the NIR region from 722 to 772 nm (the bold number in the table). The difference in emission wavelengths might relate to the difference in size of the conjugates.38 The results are also in agreement with the absorption and emission spectra of the conjugates (Fig. 2). Moreover, A3 shows the highest radian ratio, and its red fluorescent signal can be observed at a concentration as low as C0/129 = 5 × 10−4 mM Cy 5.5 (Fig. 3).
| λex = 487 nm | λex = 512 nm | λex = 537 nm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λem = 547 nm | λem = 572 nm | λem = 597 nm | λem = 622 nm | λem = 547 nm | λem = 572 nm | λem = 597 nm | λem = 622 nm | λem = 572 nm | λem = 597 nm | λem = 622 nm | |
| A1 | 0.52 | 0.43 | 0.34 | 0.23 | 0.80 | 0.61 | 0.62 | 0.41 | 0.69 | 0.95 | 0.76 |
| A2 | 0.63 | 0.86 | 1.06 | 0.89 | 0.72 | 1.01 | 1.25 | 1.04 | 1.93 | 2.38 | 1.94 |
| A3 | 1.44 | 1.54 | 1.95 | 1.22 | 1.34 | 2.47 | 3.08 | 1.90 | 5.58 | 6.74 | 4.13 |
| A4 | 2.37 | 3.15 | 4.0 | 2.93 | 4.1 | 5.7 | 7.69 | 5.45 | 6.6 | 8.8 | 6.22 |
| λex = 637 nm | λex = 662 nm | λex = 687 nm | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| λem = 697 nm | λem = 722 nm | λem = 747 nm | λem = 772 nm | λem = 697 nm | λem = 722 nm | λem = 747 nm | λem = 772 nm | λem = 722 nm | λem = 747 nm | λem = 772 nm | |
| A1 | 19.2 | 30.1 | 22.3 | 21.2 | 41.6 | 68.0 | 51.5 | 50 | 75.0 | 73.3 | 84.1 |
| A2 | 0.54 | 0.59 | 0.60 | 0.59 | 0.53 | 0.56 | 0.58 | 0.58 | 0.62 | 0.57 | 0.57 |
| A3 | 73.2 | 178.8 | 99.9 | 121.6 | 62.5 | 229.8 | 124.8 | 67.3 | 236.7 | 108.4 | 49.2 |
| A4 | 1.94 | 2.31 | 2.06 | 1.97 | 7.75 | 7.77 | 5.96 | 5.62 | 8.62 | 9.63 | 8.83 |
3.2. Magnetic properties
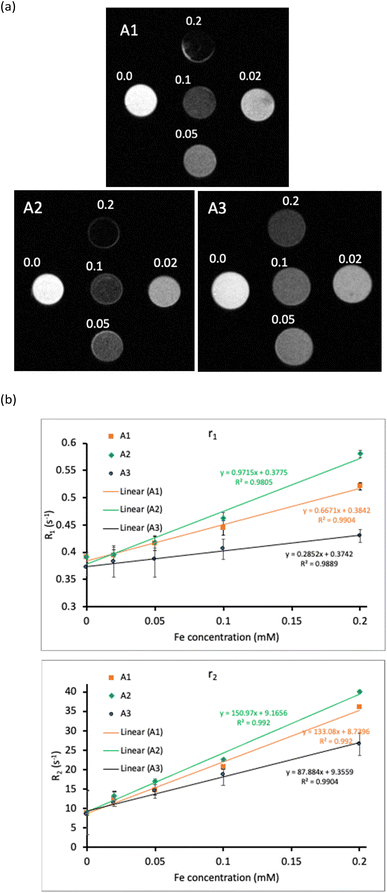
Magnetic properties of Fe3O4-containing conjugates (A1, A2, A3) are summarized in Table 3. The saturation magnetization (Ms) and the coercivity (Hc) values of the conjugates were determined by a vibrating magnetometer in the magnetic field of −11000 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Oe (Fig. 4a). Ms for A1, A2, and A3 are 63.6, 59.9, and 45.9. emu g−1, respectively. The decrease over the series is consistent with the decrease in the content of Fe3O4 present in each conjugate (see Table 1). The small Hc values of A1, A2, and A3 confirm the superparamagnetic properties of these conjugates.39 The magnetic heating curves (Fig. 4b–d) of the conjugates clearly show that increasing in magnetic field strength (from 100 to 250 Oe) makes the temperature change of the samples increase. Therefore, it is possible to achieve an expected temperature by changing the field strength. The magnetic heating experiments were carried out at room temperature (25–35 °C). In case the temperature of the sample reached the boiling point of water (100 °C), the heating experiment would stop before 1500 s. At the same field strength (100 Oe or 150 Oe), the ΔT1500s of the A1 is higher than that of A2, and the smallest ΔT1500s belongs to A3. At 100 Oe, after 1500 s of magnetic field application, A3 can change the medium's temperature by 25.3 °C. If the starting temperature is the body temperature (37 °C), the temperature can reach over 62 °C, allowing the ablation of tumors by heat.40 Calculated SAR and ILP values of the conjugates (Table 3) also have the same trend with ΔT1500s because of the decrease in Ms from A1 to A3.41 Despite having the lowest value, the ILP of A3 (5.1 nH m2 kg−1) is still higher than many reported Fe3O4 NPs.42–44 Thus, the three conjugates can serve as potential hyperthermia agents.
000 Oe (Fig. 4a). Ms for A1, A2, and A3 are 63.6, 59.9, and 45.9. emu g−1, respectively. The decrease over the series is consistent with the decrease in the content of Fe3O4 present in each conjugate (see Table 1). The small Hc values of A1, A2, and A3 confirm the superparamagnetic properties of these conjugates.39 The magnetic heating curves (Fig. 4b–d) of the conjugates clearly show that increasing in magnetic field strength (from 100 to 250 Oe) makes the temperature change of the samples increase. Therefore, it is possible to achieve an expected temperature by changing the field strength. The magnetic heating experiments were carried out at room temperature (25–35 °C). In case the temperature of the sample reached the boiling point of water (100 °C), the heating experiment would stop before 1500 s. At the same field strength (100 Oe or 150 Oe), the ΔT1500s of the A1 is higher than that of A2, and the smallest ΔT1500s belongs to A3. At 100 Oe, after 1500 s of magnetic field application, A3 can change the medium's temperature by 25.3 °C. If the starting temperature is the body temperature (37 °C), the temperature can reach over 62 °C, allowing the ablation of tumors by heat.40 Calculated SAR and ILP values of the conjugates (Table 3) also have the same trend with ΔT1500s because of the decrease in Ms from A1 to A3.41 Despite having the lowest value, the ILP of A3 (5.1 nH m2 kg−1) is still higher than many reported Fe3O4 NPs.42–44 Thus, the three conjugates can serve as potential hyperthermia agents.
| Sample | Ms (emu g−1) | Hc (Oe) | dT/dt (°C s−1) (100 Oe) | ΔT1500s (100 Oe) | SAR (W g−1) (100 Oe) | ILP (nH m2 kg−1) | r1 (s−1 mM−1) | r2 (s−1 mM−1) | r2/r1 |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 63.6 | 6.7 | 0.12 | 43.7 | 250.8 | 8.7 | 0.6671 | 133.08 | 199.49 |
| A2 | 59.9 | 5.0 | 0.11 | 38.6 | 229.9 | 8.0 | 0.9715 | 150.97 | 155.40 |
| A3 | 45.9 | 8.3 | 0.07 | 25.3 | 146.3 | 5.1 | 0.2852 | 87.884 | 308.15 |
 | ||
| Fig. 4 Magnetic hysteresis loops (a) and magnetic heating curves of A1 (b), A2 (c), and A3 (d) conjugates. | ||
The ability to enhance the MRI contrast of A1, A2, and A3 was determined by in vitro MR images at different iron concentrations taken in T1-weighted and T2-weighted modes. Fig. 5a shows the T2-weighted images of the nanoconjugates. As the Fe concentration increases, the T2-weighted MR images become darker. The protons of water molecules are affected by the dipole moment of the nanosystems, causing their spin–spin relaxation time (T2) to decrease. On the other hand, the presence of carboxylate functional groups of alginate causes more water molecules to adsorb on the surface of the nanosystems, also changing the longitudinal relaxation time (T1).45 Fig. 5b shows the linear relationship between the relaxation rate (R1 = 1/T1 and R2 = 1/T2) and Fe concentration. The slopes of the plots are longitudinal relaxivity (r1) and transverse relaxivity (r2) (Table 3). While A2 has the highest r2 value, A3 shows the highest r2/r1 value. Several previous studies also revealed that the presence of Dox24 or other drug like curcumin can improve the r2 value.46 A2 and A3, therefore, can be used as T2-contrast enhancement agents.
3.3. A3 and its in vivo bimodal imaging
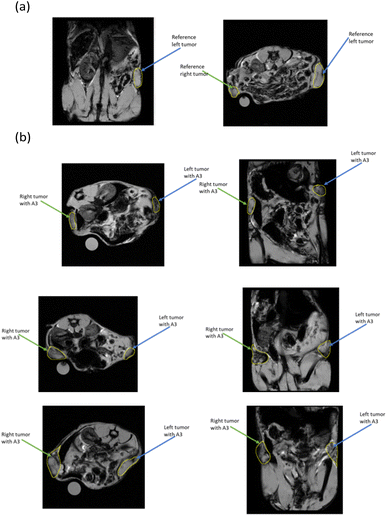
Based on the above optical and magnetic properties results, A3 was selected for in vivo imaging experiments. Fig. 6 provides some more details of A3. The XRD pattern of A3 shows characteristic peaks at 2θ of 30.1°, 35.4°, 43.1°, 53.4°, 57.0°, 62.6°, corresponding to the (220), (311), (400), (422), (511), and (440) characteristic peaks of Fe3O4 NPs.47 The TEM image shows the core size of A3 is about 10 to 15 nm. The significant difference in the size of A3 measured by TEM (Fig. 6) and by DLS method (Fig. 1a) resulted from the different states of the sample in the 2 measurements. To obtain TEM images, A3 solution was spread in a copper grid and then dried before being applied a high energy electron beam. The electron beam can burn the organic components (alginate, Dox, or Cyanine 5.5), and the image obtained records the size of the Fe3O4 core only. In DLS method, the sample was kept in an aqueous medium in which the alginate polymer easily expands and causes the overall hydrodynamic size of the A3 sample much larger than its Fe3O4 core. Similar phenomena are also reported elsewhere.25,48,49Fig. 7 displays the in vivo MRI images of Balb/C mice with CT26 colon tumors (control and A3-injected mice). The tumor images of A3-injected mice become darker than those of control mice. The dark regions also confirm the dispersion of the A3 conjugate in the tumors (pointed by the arrows) and demonstrate the ability to increase the contrast in MR images with  weighted mode. This effect allows for a more accurate assessment of the size, shape, and internal structure of the tumor, as well as an easier assessment of the presence or absence of a tumor when it appears inside the body.50
weighted mode. This effect allows for a more accurate assessment of the size, shape, and internal structure of the tumor, as well as an easier assessment of the presence or absence of a tumor when it appears inside the body.50
To quantify the MRI signal and evaluate the in vivo accumulation of the A3 conjugate in tumors, the semi-quantitative method% I0.25 was used.51 Regions of interest (ROIs) were drawn on each slice of the tumor on the MRI image, and the pixel intensity distribution for each slice was obtained. The synthesis of these pixel intensity distribution plots gave a unique pixel intensity distribution for each tumor. Fig. 8 shows that the percentage of pixels below I0.25 was significantly different (p < 0.001) between control mice and A3-injected mice (4.00 ± 0.38% and 21.72 ± 3.49%, respectively). This reflects the efficient passive accumulation of the nanoconjugate in these tumors.
 | ||
| Fig. 8 Percentage of pixels below I0.25 of control mice and A3-injected mice (***: different significantly at p < 0.001). | ||
Fig. 9 shows the in vivo distribution of A3 at 5 min, 15 min, 30 min, 1 h, 3 h, 6 h, and 24 h after injection. Immediately after 5 minutes of injection, the fluorescence signal was observed and then increased to reach the maximum at 3 h after injection. After that, the signal was reduced.
 | ||
| Fig. 9 In vivo distribution over time of A3 determined by fluorescence imaging in the representation of the rainbow signal. | ||
The fluorescence signal was then quantitatively analyzed using M3 vision software (BioSpace Lab, France) and expressed as average radiance (ph s−1 cm−2 sr−1). Regions of interest (ROIs) were drawn around the subcutaneous tumors on both flanks and adjacent tissues to determine the average radiance. The signal was then normalized relative to that of the control mouse to assess the degree of signal enhancement compared to the background (corresponding biological tissue) (Fig. 10a). After 3 hours of injection, the highest signal is obtained in the tumor, followed by the liver, kidney, and spleen. This result is also consistent with the MRI quantitative results above, showing the passive targeting effect of A3 to the tumor site. From 3 h to 24 h, the fluorescence signal at the tumor decreases sharply while the signal intensity at the liver and spleen slightly decreases. The signal at the kidney increases until 6 h after injection, indicating that after 3 h, the nanoconjugate was transferred from tumors to the kidney before clearance.
 | ||
| Fig. 10 Signal quantification over time (a and b); ex vivo fluorescent images of tumors and other organs (c) and signal quantification at 36 h after injection (d and e). | ||
In addition, the tumor/liver signal ratio, an index of tumor-specific nanoparticle distribution relative to the RES,52,53 was calculated (Fig. 10b). The tumor/liver signal ratio increases and reaches its highest point at the same time as the highest point of both tumor and liver signal (3 h after injection). This indicates that the conjugate was successful in passively targeting tumors. Further analysis of the liver/kidney signal ratio allowed us to evaluate the role of each organ in the metabolism and excretion of the nanoconjugate.54 During the first 3 hours, the liver/kidney signal ratio was >1, indicating that the metabolism in the liver was dominant. After 6 and 24 hours of injection, the liver/kidney signal ratio was <1.0, indicating the dominant role of the kidney. In another study, the in vivo distribution in a CT26 tumor mouse model of the PEG–BPSi–Cyanine 7.5 nanosystem (size 140 nm) was evaluated. It was shown that after 24 hours, the nanosystem was eliminated mainly through the liver and spleen, followed by fecal excretion.55 Lyli Mai's research group demonstrated that Cyanine 5.5-conjugated nanobubbles could persist at the tumor site for more than 24 hours in a mouse model of H22 liver tumor. In contrast, free Cyanine 5.5 is rapidly eliminated by renal filtration, followed by urinary excretion.56
After 36 hours, the mice were sacrificed, and the tumor, lung, spleen, liver, and kidney were dissected. Ex vivo fluorescence imaging (Fig. 10c) was performed to quantify the fluorescence signal in these organs (Fig. 10d and e). The highest fluorescence intensity was in the kidney, followed by the liver and tumor. This result shows that the kidney and liver are the main organs in nanoconjugate elimination. The ratio of tumor/liver signal <0.5 and liver/kidney <0.5 showed that after 36 hours of injection, the nanoconjugate was mainly distributed in the kidney (Fig. 10e) and would be eliminated from the body through the kidney.
4 Conclusion
In conclusion, various conjugates of the three components, Fe3O4 NPs, drug (Dox), and dye (Cyanine 5.5), have been prepared based on alginate polymer. Despite containing both fluorescence quenching agents of Fe3O4 NPs and Dox, A3 conjugate shows the highest fluorescent intensity in both PL spectra and fluorescence images. A2 (Fe3O4–Dox) and A3 (Fe3O4–Cy 5.5–Dox) have lower saturate magnetization values but exhibit higher r2 or r2/r1 ratio compared to A1 (Fe3O4–Cy 5.5). Selected conjugate A3 was used for in vivo MRI and NIR fluorescence imaging. The results confirm that A3 can passively target tumors and enhance MRI contrast. Based on optical images, A3 reaches the maximum concentration at tumors 3 h after injection and then transfers to the kidney for clearance. Thus, the results demonstrate that the A3 conjugate is a promising multifunctional nanoconjugate for in vivo MRI and NIR-fluorescence tumor imaging.Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the University of Paris, referral CEEA34 apafis 18.037 and approved by the Animal Ethics Committee No. 34 of the University.Data availability
All data that support the findings of this study are included in the manuscript.Author contributions
T. T. H. L.: investigation, writing original draft; review and editing; K. S. P.: investigation, data analysis, review and editing; B. T. D., H. B. H. B., H. N. P., T. D. T. U., Y. W., L. H. D.: investigation; T. T. T. M.: investigation, review and editing; N. Q. T.: investigation, funding acquisition; P. T. H.: conceptualization, funding acquisition, review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Vietnam Academy of Science and Technology (VAST) under Grant Number NCXS.01.01/23-25.References
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263 Search PubMed.
- E. Atlihan-Gundogdu, D. Ilem-Ozdemir, M. Ekinci, E. Ozgenc, E. S. Demir, B. Sánchez-Dengra and I. González-Alvárez, J. Pharm. Invest., 2020, 50, 349–361 Search PubMed.
- S. Yoon, S. Y. Cheon, S. Park, D. Lee, Y. Lee, S. Han, M. Kim and H. Koo, Biomater. Res., 2024, 26, 57 Search PubMed.
- C. Zeng, W. Shang, X. Liang, X. Lieng, Q. Chen, C. Chi, Y. Du, C. Fang and J. Tian, ACS Appl. Mater. Interfaces, 2016, 8, 29232–29241 Search PubMed.
- H. Rui, R. Xing, Z. Xu, Y. Hou, S. Goo and S. Sun, Adv. Mater., 2010, 22, 2729–2742 Search PubMed.
- H. Gu, S. Fu, Z. Cai and H. Ai, J. Polym. Sci., 2024, 62, 3103–3121 CAS.
- M. E. Sadat, M. Kaveh Baghbador, A. W. Dunn, H. P. Wagner, R. C. Ewing, J. Zhang, H. Xu, G. M. Pauletti, D. B. Mast and D. Shi, Appl. Phys. Lett., 2014, 105, 1–6 Search PubMed.
- P. Q. Thong, L. T. Thu Huong, N. D. Tu, H. T. My Nhung, L. Khanh, D. H. Manh, P. H. Nam, N. X. Phuc, J. Alonso, J. Qiao, S. Sridhar, H. P. Thu, M. H. Phan and N. T. Kim Thanh, Nanomedicine, 2022, 17, 1677–1693 CAS.
- G. A. Marcelo, J. Galhano and E. Oliveira, Dyes Pigm., 2023, 208, 110756 CrossRef.
- H. Wang, D. N. Udukala, T. N. Samarakoon, M. T. Basel, M. Kalita, G. Abayaweera, H. Manawadu, A. Malalasekera, C. Robinson, D. Villanueva, P. Maynez, L. Bossmann, E. Riedy, J. Barriga, N. Wang, P. Li, D. A. Higgins, G. Zhu, D. L. Troyer and S. H. Bossmann, Photochem. Photobiol. Sci., 2014, 13, 231–240 CrossRef CAS PubMed.
- K. C. On, J. Rho, H. Y. Yoon, H. Chang, J. Y. Yhee, J. S. Yoon, S. Y. Jeong, H. K. Kim and K. Kim, Pharmaceutics, 2020, 12, 1–14 Search PubMed.
- D. Yoo, C. Lee, B. Seo and Y. Piao, RSC Adv., 2017, 7, 12876–12885 CAS.
- K. Butowska, A. Woziwodzka, A. Borowik and J. Piosik, Materials, 2021, 14, 1–19 Search PubMed.
- G. Prabha and V. Raj, Mater. Sci. Eng., C, 2017, 79, 410–422 Search PubMed.
- P. I. P. Soares, A. I. Sousa, J. C. Silva, I. M. M. Ferreira, C. M. M. Novo and J. P. Borges, Carbohydr. Polym., 2016, 147, 304–312 CAS.
- W. Cai, M. Guo, X. Weng, W. Zhang and Z. Chen, Mater. Sci. Eng., C, 2019, 98, 65–73 CAS.
- M. Norouzi, V. Yathindranath, J. A. Thliveris, B. M. Kopec, T. J. Siahaan and D. W. Miller, Sci. Rep., 2020, 10, 1–18 Search PubMed.
- N. Sadat, H. Motlagh, P. Parvin, F. Ghasemi and F. Atyabi, Biomed. Opt. Express, 2016, 7, 2400–2406 Search PubMed.
- D. Ahn, J. Lee, S. Park, Y. Kwark and K. Y. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 22069–22077 CAS.
- S. Cao, J. Guo, Y. He, M. Alahdal, S. Tang, Y. Zhao, Z. Yang, H. Gao, W. Hu, H. Jiang, L. Qin and L. Jin, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 642–652 CrossRef CAS.
- M. A. Taemeh, A. Shiravandi, M. A. Korayem and H. Daemi, Carbohydr. Polym., 2020, 228, 115419 CAS.
- D. Kothale, U. Verma, N. Dewangan, P. Jana, A. Jain and D. Jain, Curr. Drug Delivery, 2020, 17, 755–775 CAS.
- K. Son Phan, H. Nghi Do, B. Thuy Doan, T. Thu Huong Le, T. Thu Trang Mai, Q. Bao Ngoc Nguyen, T. Nham Dong, B. Hung Bui Ha, V. Dung Dang, L. H. Dang, N. Quyen Tran and P. Thu Ha, ChemMedChem, 2024, 20(5), e202400586 Search PubMed.
- K. Son Phan, B. Thuy Doan, T. Thu Huong Le, T. Thu Trang Mai, T. Dieu Thuy Ung, T. Quang Bui, S. Boumati and P. Thu Ha, ChemistrySelect, 2023, 8(40), e202303580 CAS.
- T. T. H. Le, T. Q. Bui, T. M. T. Ha, M. H. Le, H. N. Pham and P. T. Ha, J. Mater. Sci., 2018, 53, 13826–13842 CAS.
- H. T. T. Le, K. S. Phan, D. T. T. Ung, L. A. T. Tran, N. T. Dong and T. P. Hà, Vietnam J. Sci. Technol., 2022, 60, 33–42 Search PubMed.
- K. Lawson-wood, Fluoresc. Spectrosc., 2018, 1–5 Search PubMed.
- T. T. T. Huong, N. T. Loan, L. Van Long, T. D. Phong, T. Ung Thi Dieu and N. Q. Liem, Opt. Mater., 2022, 130, 112564 CAS.
- J. Wu, J. Pers. Med., 2021, 11(8), 771 CrossRef PubMed.
- M. Longmire, P. L. Choyke and H. Kobayashi, Nanomedicine, 2008, 3, 703–717 CrossRef CAS.
- Y. Zhao, S. Tang, J. Guo, M. Alahdal and S. Cao, Nat. Publ. Gr., 2017, 7, 44758 Search PubMed.
- G. Prabha and V. Raj, Mater. Sci. Eng., C, 2017, 79, 410–422 Search PubMed.
- R. Pashaei-Asl, S. Motaali, E. Ebrahimie, M. Mohammadi-Dehcheshmeh, M. Ebrahimi and M. Pashaeiasl, Pathol., Res. Pract., 2024, 155667 Search PubMed.
- M. Vaishakh and V. P. N. Nampoori, Thermooptic techniques: a tool for interdisciplinary studies, Photoacoustic and Photothermal Spectroscopy - Principles and Application, ed. S. N. Thakur, V. N. Rai and J. P. Singh, Elsevier, 2023, ch 8, pp. 185–216 Search PubMed.
- S. Jaiswal, S. B. Dutta, D. Nayak and S. Gupta, ACS Omega, 2021, 6, 34842–34849 Search PubMed.
- Q. Zhang, R. Su, J. P. Wang and Q. Zhang, J. Nanosci. Nanotechnol., 2016, 16, 7427–7432 CAS.
- C. E. Rivera-Enríquez, M. Ojeda-Martínez, M. E. Cano, V. M. Rentería-Tapia, F. González, H. J. Ojeda Galván and C. Velásquez-Ordoñez, Ceram. Int., 2023, 49, 41133–41141 Search PubMed.
- Z. Popović, W. Liu, V. P. Chauhan, J. Lee, C. Wong, A. B. Greytak, N. Insin, D. G. Nocera, D. Fukumura, R. K. Jain and M. G. Bawendi, Angew Chem. Int. Ed. Engl., 2010, 49, 8649–8652 Search PubMed.
- A. K. Singh, O. N. Srivastava and K. Singh, Nanoscale Res. Lett., 2017, 12, 298 Search PubMed.
- S. K. Sharma, N. Shrivastava, F. Rossi, L. D. Tung and N. T. K. Thanh, Nano Today, 2019, 29, 100795 CrossRef CAS.
- A. Singh, P. Kumar, S. Pathak, K. Jain, P. Garg, M. Pant, A. K. Mahapatro, D. Rath, L. Wang, S.-K. Kim, K. K. Maurya and R. P. Pant, J. Alloys Compd., 2023, 968, 171868 CrossRef CAS.
- A. Hervault, A. E. Dunn, M. Lim, C. Boyer, D. Mott, S. Maenosono and N. T. K. Thanh, Nanoscale, 2016, 8, 12152–12161 RSC.
- B. Dutta, A. Nema, N. G. Shetake, J. Gupta, K. C. Barick, M. A. Lawande, B. N. Pandey, I. K. Priyadarsini and P. A. Hassan, Mater. Sci. Eng., C, 2020, 112, 110915 Search PubMed.
- F. Reyes-Ortega, B. L. Checa Fernández, A. V Delgado and G. R. Iglesias, Pharmaceutics, 2019, 11(10), 517 Search PubMed.
- N. Torkashvand and N. Sarlak, Eur. Polym. J., 2019, 118, 128–136 Search PubMed.
- M. Ghorbaanee, A. A. Salarian and V. Saba, J. Inorg. Organomet. Polym. Mater., 2018, 28, 2169–2178 Search PubMed.
- B. Wang, Q. Wei and S. Qu, Int. J. Electrochem. Sci., 2013, 8, 3786–3793 Search PubMed.
- Y. Ye, J. He, Y. Qiao, Y. Qi, H. Zhang, H. A. Santos, D. Zhong, W. Li, S. Hua, W. Wang, A. Grzybowski, K. Yao and M. Zhou, Theranostics, 2020, 10, 8541–8557 CrossRef CAS PubMed.
- T. M. M. Ways, S. K. Filippov, S. Maji, M. Glassner, M. Cegłowski, R. Hoogenboom, S. King, W. M. Lau and V. V Khutoryanskiy, J. Colloid Interface Sci., 2022, 626, 251–264 Search PubMed.
- A. Nomani, S. Yousefi, D. Sargsyan and A. Hatefi, J. Controlled Release, 2024, 368, 728–739 CAS.
- C. J. Thébault, G. Ramniceanu, A. Michel, C. Beauvineau, C. Girard, J. Seguin, N. Mignet, C. Ménager and B.-T. Doan, Mol. Imaging Biol., 2019, 21, 269–278 Search PubMed.
- P. Lebert, M. Adens-Fauquembergue, M. Azahaf, V. Gnemmi, H. Behal, A. Luciani and O. Ernst, Eur. Radiol., 2019, 29, 5742–5751 CAS.
- Y. Hama and E. Tate, Br. J. Radiol., 2022, 95, 20211131 Search PubMed.
- B. C. Yung and S. Sostre, Clin. Nucl. Med., 1994, 19, 228–232 Search PubMed.
- K. Tamarov, J. T. W. Wang, J. Kari, E. Happonen, I. Vesavaara, M. Niemelä, P. Perämäki, K. T. Al-Jamal, W. Xu and V. P. Lehto, ACS Appl. Mater. Interfaces, 2021, 13, 40392–40400 CrossRef CAS PubMed.
- L. Mai, A. Yao, J. Li, Q. Wei, M. Yuchi, X. He, M. Ding and Q. Zhou, PLoS One, 2013, 8, e61224 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |