Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silver-containing composites based on copolymers of β-cyclodextrin and TiO2 for enhanced photocatalytic degradation of methyl orange in environmental water†
Serhii Kobylinskyi ,
Sergii Sinelnikov,
Larysa Kobrina,
Yuliia Bardadym and
Sergii Riabov
,
Sergii Sinelnikov,
Larysa Kobrina,
Yuliia Bardadym and
Sergii Riabov *
*
Institute of Macromolecular Chemistry, National Academy of Sciences of Ukraine, 48, Kharkivske shose, Kyiv 02155, Ukraine. E-mail: sergii.riabov@gmail.com
First published on 30th May 2025
Abstract
This study aims to develop effective photocatalysts by combining the photocatalytic properties of TiO2 and Ag nanoparticles (AgNPs) with the adsorption ability of β-cyclodextrin-containing polymers (CDPs). For this purpose, TiO2/CDP/AgNP hybrid catalysts were prepared via radical and thermal polymerization of cyclodextrin derivatives with trimethylolpropane trimethacrylate on the TiO2 surface, followed by introduction of 1 wt% silver nanoparticles into their bulk via reduction of Ag+ salts under UV irradiation or using NaOH. The structure of the nanocomposite photocatalysts was investigated through X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), and thermal gravimetric analysis (TGA), and particle size distribution was determined via dynamic light scattering (DLS). The photocatalytic degradation process was monitored via UV-vis spectroscopy in an aqueous environment at pH 2.5. The best photodegradation results for methyl orange (MO) were obtained using samples prepared from β-CD methacrylate and silver methacrylate. In this case, complete decolorization of the MO solution was achieved within 10 min at pH 2.5, which is 7.5 times faster than that achieved by pure TiO2 and 4 times faster than that that achieved by the TiO2/CDP sample.
1. Introduction
Water pollution caused by organic compounds, such as dyes, is a serious environmental problem that threatens ecosystems and human health. Dyes are widely used in the textile, food, paper and cosmetic industries. Owing to their high resistance to biodegradation, they persist in the environment for long periods, which make them particularly hazardous to water systems.1 Traditional water treatment methods, such as physical (activated carbon adsorption and filtration), chemical (coagulation and chlorination) and biological (biodegradation) processes, have a number of limitations in removing dyes, especially those that are stable and toxic even at low concentrations.2Photocatalytic degradation using titanium dioxide (TiO2)-based nanomaterials is one of the most promising methods for water purification through the removal of persistent organic pollutants, including dyes. TiO2 has attracted considerable attention owing to its high activity, chemical stability, and environmental safety.3–6 However, there are a number of drawbacks that limit the widespread industrial application of this technology. One of the major disadvantages of TiO2 is that it is only activated by ultraviolet radiation, which comprises only 5% of the solar spectrum. This severely limits the use of solar energy for photocatalysis under natural conditions. Even in the presence of UV light, the efficiency of photocatalytic reactions is often reduced owing to the rapid recombination of excited electrons and holes. This shortens their lifetime and likelihood of interaction with organic contaminant molecules. For effective degradation, pollutants must be adsorbed onto the catalyst surface. However, many dyes exhibit low affinity for the TiO2 surface, reducing their contact and, consequently, reaction efficiency. The photocatalytic degradation of dyes can produce toxic intermediates that pose a threat to aquatic organisms and humans. This requires further study of the kinetics and mechanisms of degradation to prevent the formation of hazardous by-products. In addition to process efficiency, it is important to ensure the stability and long-term performance of photocatalytic materials in aqueous environments because some materials may lose activity owing to particle aggregation or chemical modification during the reaction.
This study aims to modify TiO2 to solve these problems. One of the directions is the doping of TiO2 with metals (Ag, Pt, Au) and non-metals (nitrogen, carbon, fluorine) to extend its activity in the visible light range.6 Another promising area is the creation of hybrid nanocomposites based on TiO2 with organic molecules such as cyclodextrins (CDs), which can improve the adsorption of organic compounds on the catalyst surface and reduce electron and hole recombination. These solutions significantly improve the efficiency of processes and make them suitable for practical applications.7–9
Several reports have described the preparation of TiO2/β-CD and TiO2/β-CD/Ag composites.10–23 García-Díaz et al.10 studied the use of TiO2 microspheres coated with β-CD polymer obtained from condensation polymerization with tetrafluoroterephthalonitrile, resulting in the formation of a cross-linked, covalently bonded β-CD film. The use of β-CD as a coating improves the stability of the microspheres and increases the duration of the catalytic effect to at least 1000 hours, which was studied in the photocatalytic degradation of bisphenol A (BPA) in wastewater. Cyclodextrin improves BPA adsorption, accelerating its degradation. Coated TiO2 microspheres have a more stable surface and better charge separation, which reduces the recombination rate. Sangari et al.11 investigated the effect of β-cyclodextrin on the photocatalytic degradation of Metanil Yellow dye using TiO2. β-CD was utilized to enhance the photocatalytic activity by increasing the adsorption of the dye on the TiO2 surface. β-CD forms inclusion complexes with dye molecules, which accelerates their transfer to the photocatalyst surface and improves the reaction under UV light. The study showed a significant increase in the degradation rate of methylene yellow compared to the use of TiO2 alone. In,7 a β-CD polymer/TiO2 composite was prepared by the in situ growth of TiO2 on a β-CDP matrix synthesized by epichlorohydrin crosslinking. The photodegradation efficiency of this composite toward tetracycline was attributed to its mesoporous structure, large pore volume and high surface area, which provided many active sites for photocatalytic activity and effectively improved the separation of photogenerated electrons and holes. Colpani et al.12 considered the functionalization of TiO2 with carboxymethyl-β-cyclodextrin and lanthanum doping. Such modifications improve the absorption of visible light and increase the activity of photocatalysts for the degradation of organic pollutants. This study13 investigates a floating photocatalytic membrane based on TiO2 doped with silver and β-cyclodextrin (β-CD). These membranes were designed to improve their adsorption and photocatalytic activity under visible light. Silver broadens the absorption spectrum of visible light, while β-CD enhances the adsorption of organic pollutants. The synergy between Ag and β-CD promotes more efficient photocatalytic decomposition. The membranes showed high efficiency in the dynamic adsorption of pollutants and higher activity under visible light compared to pure TiO2. In,14 a hybrid system based on TiO2 decorated with gold (Au) nanoclusters and cyclodextrin was investigated. In the developed hybrid material, the combination of the properties of SH-β-CDs, Au NCs and TiO2 NPs led to a significant improvement in the photodegradation of methyl orange (∼98%, t = 10 min) due to the increased availability of catalytic centers and inhibition of electron-hole pair recombination. In the article,15 titanium dioxide nanoparticles doped with Ag and β-cyclodextrin (β-CD) were synthesized on activated carbon (AC). The resulting Ag-β-CD/TiO2/AC composite exhibited high photocatalytic activity (98.4%) and rate constant for naphthalene under visible light irradiation. In our previous articles, we studied the effect of both the original cyclodextrin and its derivatives,19,20 as well as the cross-linked copolymers of cyclodextrin methacrylate and maleate as additives21 that are grafted onto the surface of titanium dioxide through silicon derivatives,22,23 on the photodegradation of methyl orange. It has been shown that β-CD maleates, both alone and as part of cross-linked copolymers, have a positive effect on the photodegradation rate of methyl orange in distilled water.
At the same time, there is a lack of data in the literature on the effect of modification of TiO2 by cross-linked polymers based on CD together with AgNPs on its photocatalytic activity, and the effect of CD, the chemical composition of the silver salt used, and the method of silver ion reduction on the formation of AgNPs has not been studied, but such information is important from scientific and practical points of view. Thus, in this study, we elaborated polymer composites based on TiO2, cyclodextrin derivatives: methacrylate, maleate and AgNPs. The photocatalytic activity of the obtained composite catalysts towards methyl orange as a model object was investigated in environmental water media.
2. Experimental part
2.1. Reagents
β-Cyclodextrin (β-CD) (Cavamax W7, Wacker), titanium dioxide (TiO2), silver nitrate (AgNO3), silver nanoparticles (AgNPs) (<100 nm), methacrylic anhydride (MethA), maleic anhydride (MA), acryloyl chloride (AC), trimethylolpropane trimethacrylate (TMPTA), α,α′-azoisobutyronitrile (AIBN), α,α-dimethoxy-α-phenylacetophenone (ketal), trimethylamine, trisodium citrate, ascorbic acid, acetone, dimethylformamide (DMF), butyl acetate (BA), hydrochloric acid (37%), sodium hydroxide were supplied by Sigma-Aldrich (Germany). A stock solution of 1.5 g L−1 methyl orange was prepared by dissolving 0.75 g of MO in 500 ml of double-distilled water.2.2. Synthesis of silver(I) precursors
Silver citrate (Ag3Citr) was synthesized by precipitation from the reaction between AgNO3 and sodium citrate at a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.24 This reaction resulted in the formation of a white precipitate, which was filtered, washed with distilled water, and dried at 50 °C away from light.
1.24 This reaction resulted in the formation of a white precipitate, which was filtered, washed with distilled water, and dried at 50 °C away from light.
2.3. Instrumental methods
1H NMR spectra were acquired on a Bruker DPX 300 NMR spectrometer (Germany) using DMSO-d6 as solvent. UV-vis spectra were recorded using a UV-vis spectrophotometer UV-2401 PC (Shimadzu, Japan) in the frequency range 190–800 nm. FTIR spectra were obtained using a Tensor-37 Fourier Transform Infrared Spectrometer (Bruker, Germany) in the range 400–4000 cm−1. Thermogravimetric analysis of the samples was performed using a TGA Q50 (TA Instruments, USA) in an air atmosphere in the range of 20–550 °C at a heating rate of 20 °C min−1 and using a Mettler Toledo simultaneous TGA/SDTA851 thermogravimetric analyzer in a N2 atmosphere at a gas flow rate of 100 ml min−1 at a heating rate of 10 °C min−1 in the range of 20–1000 °C. X-ray diffraction (XRD) of the photocatalysts was recorded by a PAN Analytical X'Pert Pro high-resolution diffractometer using CuKα radiation (λ = 1.54 Å). XRD profiles were fitted with the Voigt functions. The instrumental broadening correction was performed using a polycrystalline SiO2 standard and the Voigt function method. The average crystallite size (D) and interplanar spacing (d) of TiO2 and the prepared composites were calculated using the Scherer and Bragg equations, respectively:where K is a constant depending on the particle morphology of the scattering object and varies from 0.89 to 1.39 (i.e. for particles of unknown shape – 0.9, for spherical particles – 0.94); β is the full width at half maximum (FWHM) of the peak; θ is the Bragg angle; λ is the wavelength of characteristic X-ray radiation (λ = 1.54 Å for CuKα radiation).
The lattice parameters of solids can be calculated using the following formula:
The particle size of each sample was measured in triplicate at 25 °C by dynamic light scattering using a Litesizer 500 (Anton Paar, Austria) (40 mW red semiconductor laser of 658 nm) at a scattering angle of 175°. The TiO2 samples were ultrasonically dispersed in water at a concentration of 1 mg ml−1 for 30 min before testing. The size measurements of each sample (∼1 ml per sample) were presented in terms of three metrics, intensity-based size, number-based size and volume-based size. The zeta potential was determined using the Smoluchowski approximation from the electrophoretic mobility measurements based on Henry's equation. The measuring angle was automatically set to 15° by the instrument, and the measuring range was −600 to +600 mV.
For the quantitative determination of silver, the sample (0.1000 g) was suspended in 10 ml of dilute HNO3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and CO2-free distilled water (total 50 ml) and analyzed in duplicate by atomic absorption spectroscopy (spectrometer C-115M1) at 328.1 nm.
1) and CO2-free distilled water (total 50 ml) and analyzed in duplicate by atomic absorption spectroscopy (spectrometer C-115M1) at 328.1 nm.
The N2 adsorption/desorption isotherms were performed at −196 °C on a Quantachrome instrument (NOVA-2200 Gas Sorption Analyzer, USA) using the Nova Win 2.0 software. Before the measurements, the samples were outgassed at 150 °C for 20 h. The specific surface area of the materials was calculated using the Brunauer–Emmet–Teller (BET), the Barrett–Joyner–Halenda (BJH) and Dollimore–Heal (DH) methods. The pore size distribution of the samples was calculated using the quenched solid density functional theory (QSDFT) method with a slit/cylindrical pore model. The total pore volume (Vtotal) was calculated from the volume of adsorbed nitrogen converted to liquid at a pressure close to P/P0 = 1. The BJH and DH methods were used to calculate the mesopore volume (Vmeso). The average pore radius (Rav) was determined from the total pore volume (Vtotal) and its specific surface area (SBET) using the equation Rav = 2Vtotal/SBET. The micropore volume (Vmicro) was calculated from the difference between Vtotal and Vmeso using the Dubinin–Radushkevich (DR) and Saito–Foley (SF) methods.
2.4. Procedures for the reduction of silver ions in water solution
Thus, the reduction of Ag+ ions was fulfilled with NaOH in the presence of β-cyclodextrin in aqueous solution as follows: 0.4 ml of β-cyclodextrin solution (0.7 μmol) with a concentration of 2 g L−1, 0.2 ml of AgNO3 (6.31 μmol), and 28.9 ml of distilled water were placed in a glass tube and stirred for 2 min at room temperature and then 0.5 ml of NaOH (0.0105 mmol) was added. The molar ratio of β-CD to AgNO3 and NaOH was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. This solution was heated at 85 °C for 6 hours. The synthesis of TiO2/AgNPs samples was conducted under the same conditions, with the addition of the required amount of titanium dioxide. After reduction, the product was centrifuged at 4000 rpm, washed with distilled water, and dried at 70°C. A similar sample was obtained using silver methacrylate. The silver content determined by atomic absorption spectroscopy was 1 wt%.
15. This solution was heated at 85 °C for 6 hours. The synthesis of TiO2/AgNPs samples was conducted under the same conditions, with the addition of the required amount of titanium dioxide. After reduction, the product was centrifuged at 4000 rpm, washed with distilled water, and dried at 70°C. A similar sample was obtained using silver methacrylate. The silver content determined by atomic absorption spectroscopy was 1 wt%.
2.5. Synthesis of β-cyclodextrin derivatives (monomers)
Synthesis of acylated β-cyclodextrin derivatives (β-CD-(Meth)n and β-CD-(Mal)n).19,212.6. Preparation of cross-linked β-cyclodextrin-containing polymers on TiO2 surfaces
The thermo-polymerization of monomers (β-cyclodextrin methacrylate or β-cyclodextrin maleate) on the TiO2 surface was carried out in a mixture (30 ml) of i-PrOH/H2O (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 v/v), initiator AIBN, and, in the case of UV polymerization, in H2O using ketal as initiator.26 Cross-linker in both cases was trimethylolpropane trimethacrylate (TMPTA). The ratios of the starting components are given in Table 1. To perform thermo-polymerization, the mixture composition was stirred on a magnetic stirrer at a temperature of 80 °C for 6 h; in turn, UV polymerization was performed at room temperature for 90 min. The products were then centrifuged, washed several times with distilled water, and dried at 70 °C to constant weight.
50 v/v), initiator AIBN, and, in the case of UV polymerization, in H2O using ketal as initiator.26 Cross-linker in both cases was trimethylolpropane trimethacrylate (TMPTA). The ratios of the starting components are given in Table 1. To perform thermo-polymerization, the mixture composition was stirred on a magnetic stirrer at a temperature of 80 °C for 6 h; in turn, UV polymerization was performed at room temperature for 90 min. The products were then centrifuged, washed several times with distilled water, and dried at 70 °C to constant weight.
| Sample | Content of initial components, g | Solvent/initiator | Silver precursor/reduction |
|---|---|---|---|
| TiO2/TMPTA/CD(Mal)5 | |||
| TiO2-P1 | 0.5/0.0505/0.241 | i-PrOH/H2O/AIBN | — |
| TiO2-P2 | 0.5/0.087/0.412 | i-PrOH/H2O | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| TiO2/TMPTA/CD(Meth)5 | |||
| TiO2-P3 | 0.5/0.023/0.101 | H2O/ketal | — |
| TiO2-P4 | H2O/AIBN | — | |
| TiO2-P5Ag | 0.5/0.023/0.101 | H2O/UV polymerization/ketal | 0.0093 g AgNO3/UV reduction |
| TiO2-P6Ag | 0.0106 g AgMeth/UV reduction | ||
| TiO2-P7Ag | 0.094 g Ag3Citr/UV reduction | ||
| TiO2-P8Ag | 0.094 g Ag3Citr/reduction by NaOH | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| TiO2/TMPTA/CD(Ac)7 | |||
| TiO2-P9 | 0.5/0.024/0.108 | H2O/UV polymerization/ketal | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| TiO2/TMPTA/CD(Meth)7 | |||
| TiO2-P10 | 0.5/0.024/0.108 | H2O/UV polymerization/ketal | — |
| TiO2-P10Ag | 0.2 (TiO2-P10) | H2O/UV polymerization/ketal | 0.0032 g Ag3Citr/reduction by NaOH |
| TiO2-P11Ag | 0.5/0.024/0.108 | 0.01 g Ag3Citr/reduction by NaOH | |
| TiO2-P12Ag | 0.01 g Ag3Citr/UV reduction | ||
| TiO2-P13Ag | 0.0109 g AgMeth/reduction by NaOH | ||
| TiO2-P14 | 0.5/–/0.12 | DMF/BuAc | — |
| TiO2-P15Ag | 0.5/–/0.12 | 0.0108 g AgMeth/ascorbic acid/KOH | |
2.7. Preparation of silver-containing composites
Silver-containing composites were obtained by reduction of silver ions of appropriate salts-nitrate, citrate, and methacrylate, at the rate of 1% by weight in the final product (TiO2/CDP) using NaOH, ascorbic acid (AA) or UV irradiation. TiO2 samples containing the copolymer and silver nanoparticles (AgNPs) were prepared without separation of the intermediate product (copolymer), except for the TiO2P10-Ag one. After UV polymerization of CD monomers, an appropriate amount of the corresponding silver salt and the required quantity of NaOH were added. The mixture was then stirred on a magnetic stirrer at a temperature of 80 °C for 6 hours. The product was then centrifuged, washed several times with distilled water, and dried at 70 °C up to constant weight was achieved.The TiO2-P14 and TiO2-P15Ag composites were prepared without TMPTA as follows: 0.12 g CD(Meth)7 was dissolved in 1 ml DMF, and then 10 ml butyl acetate and 0.5 g TiO2 were added. The mixture was refluxed with stirring for 1.5 h, cooled, and then the product was filtered, washed with EtOH, distilled water, and dried at 70 °C. The TiO2-P15Ag composite was similarly synthesized. Silver methacrylate was added to the mixture after 30 min of refluxing TiO2 with CD(Meth)7, after 5 min of stirring solution of ascorbic acid and KOH in EtOH was added in the molar ratio AgMeth![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA
AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KOH 1
KOH 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25.
0.25.
2.8. Photocatalysis experiment
The photodestruction experiments were carried out in a 100 ml quartz test tube, to which the required volume of MO solution and 40 mg of photocatalyst were added. The total volume was 80 ml. The resultant suspension was exposed to UV irradiation under stirring. The irradiation source was a system of three 8 W UV lamps with wavelengths of 365 nm (2 lamps) and 254 nm (1 lamp) placed vertically on three sides of the test tube.20 The following reagent concentrations were used in the study: TiO2 0.5 g L−1, MO – 25 mg L−1, artesian water was acidified to pH 2.5 from pH 7.3–7.5. To study the kinetics of photodegradation, the MO concentration was measured at certain intervals (after centrifugation of the solid phase) and determined spectrophotometrically at 504 nm.3. Results and discussion
3.1. Synthesis of silver citrate and methacrylate
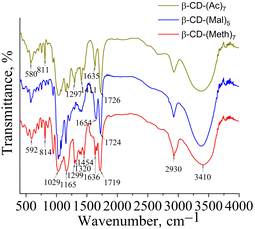
Silver methacrylate (AgMeth) was obtained by mixing aqueous solutions of silver nitrate and sodium methacrylate at an equimolar ratio. The formation of these silver salts was confirmed by FTIR spectroscopy (Fig. 1). In the spectra of silver citrate and methacrylate, the strong bands at 1590, 1556 cm−1 and 1386, 1384 cm−1 characterize the asymmetric and symmetric stretching vibrations of carboxylate groups coordinated with Ag+.3.2. Synthesis and characterization of the TiO2/CD/copolymers and TiO2/CD/copolymers/Ag composites
 | ||
| Fig. 2 1H NMR spectra of β-cyclodextrin derivatives: (1) – β-CD methacrylate (m = 5), (2) – β-CD maleate (m = 5). | ||
First, TiO2/CD copolymers' objects were synthesized by thermo- or UV polymerization of β-cyclodextrin monomers (methacrylate or maleate) on the TiO2 surface, involving trimethylolpropane trimethacrylate (TMPTA) as a cross-linker at different temperature conditions. In the second stage, composites with silver nanoparticles (1 wt%) (TiO2/CDP/AgNPs) were obtained by reduction of silver ions from the appropriate salts (nitrate, citrate, methacrylate) using NaOH, UV irradiation or ascorbic acid Scheme 1.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations of the copolymer samples were observed at 1718–1730 cm−1. The characteristic valence bands of the C(
O stretching vibrations of the copolymer samples were observed at 1718–1730 cm−1. The characteristic valence bands of the C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)C–O methacrylate groups, which were used in particular to analyze the polymerization process, were observed at 1300, 1320 cm−1 and 1635 cm−1 of the C
O)C–O methacrylate groups, which were used in particular to analyze the polymerization process, were observed at 1300, 1320 cm−1 and 1635 cm−1 of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 fragment (νC
CH2 fragment (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C). The intensity of the band at 1251–1261 cm−1, which is attributed to the C(
C). The intensity of the band at 1251–1261 cm−1, which is attributed to the C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)C–O ester groups of the copolymer, increased compared with that of the initial monomer.27 The appearance of these bands in the spectra of the modified TiO2 confirms the formation of the polymer on its surface. The absorption bands of stretching bridging vibrations of Ti–O–Ti and stretching vibrations of Ti–O bonds are in the range of 450 to 800 cm−1 and appear as two bands at 520 cm−1 and 682 cm−1, respectively.28–31 In the spectra of modified TiO2, the band at 520 cm−1 was shifted (partially or completely) to higher frequencies (for the TiO2-P1 sample, it is not seen). Such a shift as observed in TiO2/Ag samples is generally due to the strengthening of Ti–O bonds, which, in our case, may be caused by an increase in crystallinity and compression of the crystal lattice. When titanium dioxide is doped with metal ions such as iron, cobalt, or silver, a shift of the Ti–O bond position toward the low-frequency region is often observed, indicating the weakening of this bond. This weakening of the oxide bonds is associated with the formation of oxygen vacancies in the TiO2 crystal lattice.32–35 Oxygen vacancies are formed during the formation of M–O bonds by substitution of Ti4+ with Mn+. Such substitution creates a charge imbalance in the crystal lattice, and oxygen vacancies are formed to maintain charge neutrality. Instead, the formation of hydrogen bonds of the hydroxyl group on the surface of titanium dioxide with the groups of CD or copolymer, as well as the probable formation of Ti–O–C bonds, whose vibrations include the band at 943–952 cm−1,36 which appears in the spectra of samples synthesized based on β-CD methacrylates, would lead to a shift of the Ti–O bands to lower frequencies,33,37 which is associated with the weakening of the bonds due to changes in the electron density around oxygen atoms. The FTIR spectra of the silver-containing samples are shown in Fig. 5. A similar position of the bands was observed as for the starting copolymers.
O)C–O ester groups of the copolymer, increased compared with that of the initial monomer.27 The appearance of these bands in the spectra of the modified TiO2 confirms the formation of the polymer on its surface. The absorption bands of stretching bridging vibrations of Ti–O–Ti and stretching vibrations of Ti–O bonds are in the range of 450 to 800 cm−1 and appear as two bands at 520 cm−1 and 682 cm−1, respectively.28–31 In the spectra of modified TiO2, the band at 520 cm−1 was shifted (partially or completely) to higher frequencies (for the TiO2-P1 sample, it is not seen). Such a shift as observed in TiO2/Ag samples is generally due to the strengthening of Ti–O bonds, which, in our case, may be caused by an increase in crystallinity and compression of the crystal lattice. When titanium dioxide is doped with metal ions such as iron, cobalt, or silver, a shift of the Ti–O bond position toward the low-frequency region is often observed, indicating the weakening of this bond. This weakening of the oxide bonds is associated with the formation of oxygen vacancies in the TiO2 crystal lattice.32–35 Oxygen vacancies are formed during the formation of M–O bonds by substitution of Ti4+ with Mn+. Such substitution creates a charge imbalance in the crystal lattice, and oxygen vacancies are formed to maintain charge neutrality. Instead, the formation of hydrogen bonds of the hydroxyl group on the surface of titanium dioxide with the groups of CD or copolymer, as well as the probable formation of Ti–O–C bonds, whose vibrations include the band at 943–952 cm−1,36 which appears in the spectra of samples synthesized based on β-CD methacrylates, would lead to a shift of the Ti–O bands to lower frequencies,33,37 which is associated with the weakening of the bonds due to changes in the electron density around oxygen atoms. The FTIR spectra of the silver-containing samples are shown in Fig. 5. A similar position of the bands was observed as for the starting copolymers.
 | ||
| Fig. 4 FTIR spectra of TiO2 (1), TiO2-P1 (2), TiO2-P2 (3), TiO2-P3 (4), TiO2-P10 (5) and TiO2-P14 (6). | ||
 | ||
| Fig. 5 FTIR spectra of TiO2-P5Ag (7), TiO2-P8Ag (8), TiO2-P11Ag (9), TiO2-P10Ag (10), TiO2-P12Ag (11), TiO2-P13Ag (12), and TiO2-P15Ag (13). | ||
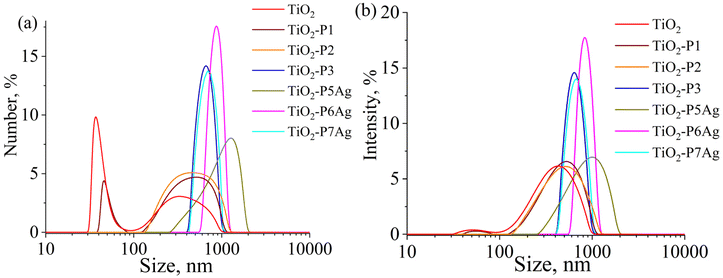
Fig. 6 illustrates the particle size distribution of neat TiO2, TiO2/CDP, and TiO2/CDP/AgNPs obtained by UV reduction of Ag ions from its nitrate (P5Ag), methacrylate (P5Ag) and citrate (P6Ag).
The range of particle sizes (Table 2) for TiO2-P1 and P2 almost coincide with that of pure TiO2, but it is narrower for TiO2-P3, which was obtained from CD(Meth)5 and TMPTA and contains a larger number of CD moieties, as well as for the silver-containing samples TiO2-P6Ag and TiO2-P7Ag. The increase in particle size for these samples compared to pure TiO2 is most likely due to the formation of a 200–300 nm layer of cross-linked, branched polymer around the TiO2 particles rather than particle aggregation. For samples TiO2-P1 and P2, whose polymeric macromolecules contain practically no CD, the layer thickness can be estimated to be 50–70 nm, based on the increase in average particle size. It is also interesting to note that the sample prepared from AgMeth exhibited the lowest polydispersity index and the highest negative zeta potential value.
| Sample | Mean particle size, nm | Hydrodynamic diameter, nm | Particle size range, nm | Polydispersity index | Mean zeta potential, mV |
|---|---|---|---|---|---|
| TiO2 | 55.4/421.4 | 379.3 | 34–953 | 0.23 | −32.0 |
| TiO2-P1 | 58.2/495.0 | 451.5 | 44–77/136–1034 | 0.25 | −0.01 |
| TiO2-P2 | 512.4 | 452.6 | 148–1216 | 0.23 | −24.4 |
| TiO2-P3 | 654.1 | 773.7 | 460–954 | 0.19 | −25.6 |
| TiO2-P5Ag | 915.5 | 1077.9 | 283–1823 | 0.25 | −25.2 |
| TiO2-P6Ag | 847.9 | 1072.2 | 636–1121 | 0.03 | −35.5 |
| TiO2-P7Ag | 684.0 | 796.3 | 460–1034 | 0.22 | −30.8 |
3.2.2.1. XRD of TiO2/copolymers and TiO2/copolymers/AgNPs.. The X-ray diffraction patterns of the TiO2, TiO2/CDP/AgNPs, TiO2/CDP, and TiO2/CDP/AgNP composites were studied (Fig. 7).
 | ||
| Fig. 7 XRD patterns of TiO2, TiO2/AgNPs (a), TiO2/CDP (b), and TiO2/CDP/AgNPs (c and d – enlarged b). | ||
The XRD pattern of pristine TiO2 presents several sharp peaks at 2θ = 25.26, 37.80, 48.00 (Fig. 7b), which are assigned to the Miller indices (101), (004), (200) planes of the anatase phase according to JCPDS no. 21-1272.
For silver nanoparticles obtained in the presence of β-cyclodextrin from silver citrate (Fig. 7a), two broad maxima were observed at 38.5° and 44.3°. The crystallite size of Ag was 5.6 nm. For TiO2/Aguv, the diffraction maxima were observed at 38.1°, 44.3°, 64.5, and 77.4 corresponding to the crystallographic planes of the silver face-centered cubic lattice with Miller indices (111), (200), (220), and (311), respectively (JCPDS card no. 04-0783).
The average crystallite size (D), interplanar spacing (d), and lattice parameters are listed in Table 3. The cyclodextrin-containing polymer AgNPs or their combination increased the crystallite size from 28.16 nm to 40.03–51.35 nm. However, their d-spacing and lattice parameters also changed. For example, for the sample containing practically no β-cyclodextrin moieties (TiO2-P1), the position of the reflexes and thus the lattice parameters do not change compared to the sample (TiO2-P4), while for the samples TiO2-P5Ag and TiO2-P7Ag the reflexes shift to smaller angles, indicating an increase in d-spacing and lattice parameters. This implies that silver atoms in these samples, in contrast to all other samples, may have replaced titanium or oxygen atoms. This assumption is consistent with the FTIR spectra data, in which changes in the Ti–O vibrational frequency range were observed, in particular the Ti–O–Ti band at 520 cm−1 toward lower frequencies, which is quite clearly seen in the example of the TiO2-P5Ag sample. For the other composites obtained by UV polymerization and reduction of silver ions, only a shoulder in the low-frequency region was found in the spectra, except for the sample obtained from silver methacrylate, for which, on the contrary, a clear shift toward 580 cm−1 occurred. That is, for samples TiO2-P5Ag and TiO2-P7Ag obtained by UV reduction of silver ions, the replacement of titanium atoms by silver atoms is more likely to occur, leading to an increase in the lattice parameters.
| Sample | 2θ, ° | FWHM, ° | D, nm | d, Å | Lattice parameters | ||
|---|---|---|---|---|---|---|---|
| a, Å | c, Å | V, Å3 | |||||
| TiO2 | 25.26 | 0.2891 | 28.16 | 3.521 | 3.783 | 9.627 | 137.77 |
| TiO2/Ag/nitr/CD/NaOH | 25.34 | 0.1622 | 50.21 | 3.510 | 3.779 | 9.476 | 135.32 |
| TiO2/Ag/meth/CD/NaOH | 25.33 | 0.1649 | 49.38 | 3.512 | 3.780 | 9.490 | 135.60 |
| TiO2-P1 | 25.25 | 0.1933 | 42.11 | 3.522 | 3.785 | 9.623 | 137.86 |
| TiO2-P3 | 25.34 | 0.2034 | 40.03 | 3.511 | 3.779 | 9.489 | 135.51 |
| TiO2-P5Ag | 25.20 | 0.1980 | 41.12 | 3.529 | 3.789 | 9.707 | 139.36 |
| TiO2-P7Ag | 25.23 | 0.1989 | 40.93 | 3.526 | 3.786 | 9.669 | 138.59 |
| TiO2-P13Ag | 25.37 | 0.1591 | 51.18 | 3.507 | 3.778 | 9.436 | 134.68 |
| TiO2-P15Ag | 25.33 | 0.1586 | 51.35 | 3.512 | 3.780 | 9.492 | 135.63 |
Fig. 8 shows the TGA curves of the pure and modified TiO2 composites. For all samples, the weight loss is insignificant in the first stage, which corresponds to moisture evaporation in the range of 0.2–1.1%, except for two samples, TiO2-P14 and TiO2-P15Ag, for which it was 2.4 and 1.6%. The degradation of the sample based on maleoyl-β-CD (TiO2-P1) proceeded in one stage with a maximum degradation rate at 459.5 °C. However, a rather slow degradation in the range of 280–390 °C without a distinguished stage can be attributed to the destruction of the CD moieties in the copolymer (≈2–3%). Instead, the destruction of the samples obtained from CD(Meth)5 (samples TiO2-P8Ag and TiO2-P3, curves 3 and 4) proceeded in two stages: the temperature range of 290–370°C corresponds to the destruction of β-CD and the methacrylate component, 370–460 °C is associated with the destruction of the aliphatic part of the copolymer, which for TMPTA links had a lower temperature (by ≈20–30°C) of the maximum thermal degradation rate than for sample TiO2-P1. According to the TGA data, the content of β-CD moieties in the copolymers was significantly lower during the polymerization of the maleoyl derivative than when methacrylate was used. For the pure β-CD, the maximum thermal degradation rate was 330 °C (Tonset = 316.8 °C).
When β-CD-(Meth)5 being applied, the cyclodextrin moieties in the samples degraded at almost the same temperature as the original β-CD-332–334 °C. Meanwhile, for the specimens prepared from 7-substituted acrylate (TiO2-P9) and methacrylate (TiO2-P10), the thermal stability of the macrocycle fragments increased by 16–22 °C.
The content of the organic component (copolymer) was determined based on the total weight loss and moisture, ranging from 9.9% for TiO2-P1 to 20.1% for TiO2-P10 (Table 4). The introduction of silver (samples TiO2-P8Ag, TiO2-P10Ag, TiO2-P11Ag, TiO2-P13Ag) slightly reduced the copolymer content, which may be due to a slight saponification of the ester groups since NaOH was utilized to reduce the silver ions. In addition, for the silver-containing composites, there is no distinguishable third stage in which the aliphatic component of the copolymer decomposes. The thermal analysis of TiO2-P14 and TiO2-P15Ag samples was performed at a lower heating rate (10 °C min−1) in a nitrogen atmosphere. For these composites, which were synthesized without TMPTA, the decomposition stage of the aliphatic component (methacrylate groups) was more pronounced and manifested over a wider temperature range (400–800 °C). The weight loss at this stage was consistent with the content of methacrylate groups in β-CD(Meth)7.
| Composite | Weight loss 50–150 °C, % | Tonset, °C | Tmax (DTG), °C | Total weight loss, % | |
|---|---|---|---|---|---|
| a The samples were recorded under a nitrogen atmosphere. | |||||
| TiO2 | 0.16 | — | — | — | 0.4 |
| TiO2-P1 | 0.21 | 398.6 | — | 459.5 | 10.1 |
| TiO2-P3 | 0.81 | 289.8 | 331.9 | 437.9 | 15.6 |
| TiO2-P3a | 1.12 | 302.9 | 334.8 | 462.2 | 15.2 |
| TiO2-P8Ag | 0.60 | 297.3 | 334.4 | — | 12.0 |
| TiO2-P9 | 0.29 | 284.7 | 346.6 | 442.4 | 11.6 |
| TiO2-P10 | 0.98 | 325.0 | 352.4 | 455.1 | 21.1 |
| TiO2-P10Ag | 0.77 | 324.8 | 352.1 | 373.6 | 20.0 |
| TiO2-P11Ag | 0.85 | 315.0 | 347.3 | 374.2 | 18.2 |
| TiO2-P13Ag | 0.87 | 320.9 | 348.3 | 437.5 | 18.2 |
| TiO2-P14a | 2.40 | 319.5 | 350.1 | — | 17.9 |
| TiO2-P15Aga | 1.60 | 324.4 | 354.4 | — | 16.1 |
| Sample | Surface area, m2 g−1 | Volume pore, cm3 g−1 | Pore radius, Å | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BET | BJH | DH | Vtotal | Vmeso | Vmicro SF | Micro-porous, %Vmicro/Vtotal | Rav | R | |||||
| BJH | DH | Rmicro | DH | DFT | |||||||||
| DR | DA | ||||||||||||
| TiO2 | 9.79 | 6.69 | 6.68 | 0.0296 | 0.0275 | 0.0267 | 0.00294 | 9.9 | 60.6 | 7.9 | 8.0 | 15.0 | 18.3 |
| TiO2-P10 | 6.24 | 2.86 | 2.90 | 0.0247 | 0.0230 | 0.0223 | 0.00181 | 7.3 | 79.3 | 11.3 | 8.9 | 48.2 | 12.1 |
| TiO2-P11Ag | 8.28 | 7.51 | 7.59 | 0.0623 | 0.0615 | 0.0597 | 0.00172 | 2.8 | 150.6 | 11.4 | 9.1 | 15.6 | 136.9 |
| TiO2-P15Ag | 10.14 | 7.53 | 7.61 | 0.0612 | 0.0593 | 0.0576 | 0.00264 | 4.3 | 120.6 | 12.3 | 9.2 | 14.8 | 18.3 |
The analysis of the pore distribution curves (Fig. S2†) shows that up to a pore size of 24–28 nm (radius 120–140 Å), the cumulative pore volume for all modified samples (TiO2-P10, TiO2-P11Ag, TiO2-P15Ag) is lower than that of the original TiO2. This indicates partial blocking of some micropores and mesopores (up to 28 nm). In the case of the TiO2-P10 sample containing only CD, the structure was probably compacted by filling the pores with polymer macromolecules. The observed increase in the cumulative volume of meso- and macropore regions for the samples with silver nanoparticles and CD polymers may be due to the formation of new pores in the polymer networks, but most likely it is caused by the unblocking of previously inaccessible pores in the range of 28–60 nm. This behavior could result from reduced particle aggregation or changes in surface wettability. These results indicate that the modification increases the accessible pore network, thereby improving the mass transport and photocatalytic properties.
3.3. Photocatalytic properties of the prepared composites
In this study, the photodegradation of MO was carried out at pH 2.5 because this is the most efficient method for initial TiO2 (ref. 39) degradation. In our opinion, the efficiency of MO decomposition at this pH is mainly due to the easier cleavage of the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– azo group, which is predominantly in the protonated quinoid form –N–N
N– azo group, which is predominantly in the protonated quinoid form –N–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) at pH 2.5, since the pKa value of MO is 3.4.40,41 Although the adsorption of MO on the surface of the photocatalyst is also important, previous studies have shown that no adsorption occurred on the initial TiO2 at 2.5 for 30 min with stirring in the dark, and at pH 6, the adsorption was 1%. The effect of dark adsorption for TiO2-P10, TiO2-P11Ag and TiO2-P15Ag on the subsequent photodestruction of MO is shown in Fig. S3.† A slight decrease in the photodegradation of MO was observed for the TiO2-P10 and TiO2-P15Ag samples, for which the amount of adsorbed MO during 30 min was 4.5 and 6.4%, respectively. For the initial TiO2 and TiO2-P11Ag, adsorption as well as significant changes in the photodegradation process did not occur. Thus, the photodegradation deterioration was observed only in the case in which MO was previously adsorbed on the photocatalyst.
at pH 2.5, since the pKa value of MO is 3.4.40,41 Although the adsorption of MO on the surface of the photocatalyst is also important, previous studies have shown that no adsorption occurred on the initial TiO2 at 2.5 for 30 min with stirring in the dark, and at pH 6, the adsorption was 1%. The effect of dark adsorption for TiO2-P10, TiO2-P11Ag and TiO2-P15Ag on the subsequent photodestruction of MO is shown in Fig. S3.† A slight decrease in the photodegradation of MO was observed for the TiO2-P10 and TiO2-P15Ag samples, for which the amount of adsorbed MO during 30 min was 4.5 and 6.4%, respectively. For the initial TiO2 and TiO2-P11Ag, adsorption as well as significant changes in the photodegradation process did not occur. Thus, the photodegradation deterioration was observed only in the case in which MO was previously adsorbed on the photocatalyst.
Fig. 9a and b illustrate the photodegradation curves of methyl orange at pH 2.5 in artesian water, and Fig. 10a–h the corresponding UV-vis spectra during irradiation for copolymers on TiO2, the best results were obtained with samples based on β-CD(Meth)7 (TiO2-P10), due to the presence of a greater number of macrocycles on the TiO2 surface than in composites prepared with other β-CD monomers. A comparison of the polymerization methods showed that the samples obtained by UV polymerization exhibited slightly higher photocatalytic activity (TiO2-P3 and TiO2-P4, respectively).
 | ||
| Fig. 9 Photocatalytic degradation curves of methyl orange with TiO2, TiO2/CDP, TiO2/AgNPs, and TiO2/CDP/AgNPs (conditions: C0 (MO) = 25 mg L−1, V = 80 ml, and pH 2.5). | ||
Two kinetic models were used to study the mechanism of MO photodegradation in the presence of prepared photocatalysts: pseudo-zero (1) and pseudo-first order (2) kinetic models, which are described by the following equations:
 | (1) |
 | (2) |
| Photocatalyst | Kinetic model | |||||
|---|---|---|---|---|---|---|
| Pseudo-first order | Pseudo-zero order | |||||
| Equation | K1 (min−1) | R2 | Equation | k0 mg l−1 min−1 | R2 | |
| TiO2 | y = 0.0367x − 0.1461 | 0.0367 | 0.9762 | y = −0.0151x + 0.9548 | 0.0151 | 0.9801 |
| TiO2/AgNitr/CD | y = 0.092x − 0.1318 | 0.092 | 0.9814 | y = −0.0311x + 0.913 | 0.0311 | 0.9439 |
| TiO2/AgCit/CD | y = 0.0912x − 0.1137 | 0.0912 | 0.9856 | y = −0.031x + 0.9066 | 0.031 | 0.9366 |
| TiO2/AgMeth/CD | y = 0.1136x − 0.0587 | 0.1136 | 0.992 | y = −0.0448x + 0.9438 | 0.0448 | 0.955 |
| TiO2-P1 | y = 0.0467x − 0.2333 | 0.0467 | 0.979 | y = −0.0129x + 0.8722 | 0.0129 | 0.9235 |
| TiO2-P2 | y = 0.0462x − 0.1086 | 0.0462 | 0.9638 | y = −0.0212x + 0.9755 | 0.0212 | 0.9941 |
| TiO2-P3 | y = 0.0539x − 0.1982 | 0.0539 | 0.9203 | y = −0.0225x + 1.0017 | 0.0225 | 0.9996 |
| TiO2-P4 | y = 0.0527x − 0.2047 | 0.0527 | 0.9206 | y = −0.0201x + 0.9714 | 0.0201 | 0.994 |
| TiO2-P5Ag | y = 0.1195x − 0.1577 | 0.1195 | 0.9504 | y = −0.0454x + 0.98 | 0.0454 | 0.9942 |
| TiO2-P6Ag | y = 0.1138x − 0.3137 | 0.1138 | 0.8734 | y = −0.0386x + 1.0069 | 0.0386 | 0.9989 |
| TiO2-P7Ag | y = 0.1467x − 0.233 | 0.1467 | 0.9297 | y = −0.0473x + 0.9791 | 0.0473 | 0.9942 |
| TiO2-P8Ag | y = 0.1534x − 0.0826 | 0.1534 | 0.9664 | y = −0.0619x + 0.9797 | 0.0619 | 0.9872 |
| TiO2-P9 | y = 0.0548x − 0.1413 | 0.0548 | 0.9614 | y = −0.0184x + 0.8974 | 0.0184 | 0.9432 |
| TiO2-P10 | y = 0.0843x − 0.1634 | 0.0843 | 0.9618 | y = −0.027x + 0.935 | 0.027 | 0.965 |
| TiO2-P10Ag | y = 0.1449x − 0.1374 | 0.1449 | 0.9737 | y = −0.0472x + 0.9424 | 0.0472 | 0.9573 |
| TiO2-P11Ag | y = 0.2132x − 0.2628 | 0.2132 | 0.9564 | y = −0.0493x + 0.9179 | 0.0493 | 0.9232 |
| TiO2-P12Ag | y = 0.1246x − 0.1568 | 0.1246 | 0.9547 | y = −0.0459x + 0.973 | 0.0459 | 0.9897 |
| TiO2-P13Ag | y = 0.2551x − 0.1364 | 0.2551 | 0.9668 | y = −0.0922x + 0.9605 | 0.0922 | 0.9785 |
| TiO2-P14 | y = 0.0571x − 0.0542 | 0.0571 | 0.9881 | y = −0.0273x + 0.9698 | 0.0273 | 0.984 |
| TiO2-P15Ag | 1st cycle y = 0.3314x − 0.15 | 0.3314 | 0.9361 | y = −0.1004x + 0.9748 | 0.1004 | 0.9795 |
| 5th cycle y = 0.1167x − 0.0699 | 0.1167 | 0.9953 | y = −0.0368x + 0.8613 | 0.0368 | 0.902 | |
To determine the effect of the β-CD macrocycle on the photocatalytic properties of silver-containing samples, the study was first carried out on samples containing only silver nanoparticles reduced from different silver salts in the presence and absence of β-CD. The presence of β-CD moieties during the reduction of silver ions on the TiO2 surface contributed to the formation of a material with better photocatalytic properties for all the studied salts and, to a greater extent, in the case of silver nitrate. In general, more efficient photodestruction was observed for the TiO2/AgNPs/meth/CD sample obtained from methacrylate in the presence of β-CD (Fig. 9b). Fig. 9b shows the results of the photodegradation studies of MO for samples containing both the copolymer and silver NPs. For the samples based on β-CD(Meth)7 and silver methacrylate (Fig. 9b), the destruction was the most effective, with the discoloration of MO occurring within 12 min, which is also evident from the corresponding UV spectra taken during the destruction process (Fig. 10e and f). A similar pattern was observed for TiO2-P15Ag prepared from silver methacrylate without TMPTA. The decomposition of the MO degradation by-products was also more efficient for these samples, as evidenced by the absence of a noticeable shoulder at 276 and 320 nm, as for the original copolymers. The degradation of products with maximum absorption at these wavelengths is also clearly visible from the UV-vis spectra at the 5th irradiation cycle (4 cycles of 25 min each) (Fig. 10h). The products formed because of the photodegradation of methyl orange significantly inhibit this process in the following cycles. By the 5th cycle, the decomposition time of the main dye molecule was twice as long. If the solution is separated from the photocatalyst or the degradation products are effectively decomposed, for instance by adding hydrogen peroxide,20,42 potassium peroxymonosulfate43 or peroxydisulfate,44 the photocatalytic efficiency will not decrease. Table 7 summarizes the results of the efficiency of silver-containing TiO2-based photocatalysts for the photodegradation of MO and other pollutants.
| Photocatalyst | Experimental conditions | Light source | Photodegradation efficiency | Ref. |
|---|---|---|---|---|
| Ag/MoO3/TiO2 | C (MO) = 10 mg L−1 | UV lamp 100 W | 95.6% | 42 |
| C (Catalyst) = 0.96 g L−1 | 99% (H2O2) | |||
| V = 125 ml | t = 330 min | |||
| pH = 3.0 | ||||
| TiO2–Ag(1%)-potassium persulfate 1.87 mM | C (MO) = 100 mg L−1 | UV lamp | 95,83% | 43 |
| C (Catalyst) = 0.66 g L−1 | t = 20 min | |||
| V = 100 ml | ||||
| pH = 2, 6 | ||||
| Ag@SiO2/TiO2 peroxydisulfate 0.45 mM | C (MO) = 5 mg L−1 | Xenon lamp | 99% | 44 |
| C (Catalyst) = 0.6 g L−1 | t = 120 min | |||
| V = 100 ml | ||||
| pH = 7.25 | ||||
| TiO2/40AgI | C (MO) = 5 mg L−1 | 10 W LED (λ = 430 nm) | 100% | 45 |
| C (Catalyst) = 1.5 g L−1 | t = 20 min | |||
| V = 20 ml | ||||
| TiO2/Ag (7.2%) composite microspheres | C (MO) = 10 mg L−1 | 15 W, λ = 365 nm, 395 nm | 96.84% and 98.65% | 46 |
| C (Catalyst) = 1.5 g L−1 | t = 120 min | |||
| V = 40 ml | ||||
| TiO2–AgI–cotton | C (MO) = 5 mg L−1 | 1000 W Xe lamp (λ > 400 nm) | 56% | 47 |
| C (Catalyst) = 1.25 g L−1 | t = 120 min | |||
| V = 50 ml | ||||
| Ag–AgI(4%)–TiO2/carbon nanofibers | C (MO) = 10 mg L−1 | 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W Xe arc lamp (λ W Xe arc lamp (λ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ≧ 400 nm ≧ 400 nm |
97% | 48 |
| C (Catalyst) = 1 g L−1 | t = 180 min | |||
| V = 100 ml | ||||
| AgI–TiO2/PAN | C (MO) = 10 mg L−1 | 300 W Xe arc lamp | 87.8% | 49 |
| C (Catalyst) = 2 g L−1 | t = 270 min | |||
| V = 100 ml | ||||
| g-C3N4/Ag-TiO2 | C (MO) = 10 mg L−1 | 300 W Xe lamp with a UV cut filter and an 18 W UV-A lamp | 74.92% and 92.82% | 50 |
| C (Catalyst) = 0.42 g L−1 | t = 60 min | |||
| V = 120 ml | ||||
| TiO2/0.3Ag//porous polymer | C (MO) = 10 mg L−1 | Xenon lamp | 81.4% | 51 |
| C (Catalyst) 0.5 g L−1 | 84.8% | |||
| V = 100 ml | t = 180 min | |||
| TiO2/0.5Ag/biochar | C (MO) = 20 mg L−1 | 500 W mercury-vapor lamp 360 nm | 97.48% | 52 |
| C (Catalyst) 0.25 g L−1 | t = 60 min | |||
| V = 40 ml | ||||
| TiO2/Ag (4%) | C (MO) = 16 mg L−1 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) W Hg vapor lamp (365 W Hg vapor lamp (365![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm) nm) |
98.9% (UV) | 53 |
| C (Catalyst) = 1 g L−1 | t = 60 min, 99.3% solar irradiation | |||
| V = 500 ml | t = 80 min | |||
| TiO2/β-cyclodextrin polymer/Ag (TiO2-P15Ag) | C (MO) = 25 mg L−1 | UV lamp 24 W 365/254 nm | 97% t = 10 min (1st cycle) | This work |
| C (Catalyst) = 0.5 g L−1 | 94.6% t = 25 min (5th cycle) | |||
| V = 80 ml, pH = 2.5 | ||||
| β-CDP/TiO2 | C (TC) = 50 mg L−1 | Solar light irradiation | 96% | 7 |
| C (Catalyst) = 0,5 g L−1 | t = 90 min | |||
| CMCD-Fe3O4@TiO2 | C (BPA, DBP) = 20 mg L−1 | Mercury vapor lamp (400 W) | 100% | 54 |
| C (Catalyst) 0,5 g L−1 pH 7–10, V = 15 ml | t = 105 min | |||
| TiO2/CMCD | C (BPA) = 20 mg L−1 | UV lamp 24 W | 100% | 55 |
| C (Catalyst) 0,25 g L−1 | t = 120 min | |||
| V = 40 ml | ||||
| TiO2–La 0.05%–CMCD | C (MB) = 10 mg L−1 | 80 W high pressure mercury lamp, 254 nm | 95% | 12 |
| C (Catalyst) 1 g L−1 pH 6, V = 15 ml | t = 15 min | |||
| β-CD/PNC/TiO2 | C (MB) = 5 mg L−1 | 500 W xenon lamp with 350 nm cut-off filter | 83.7% | 9 |
| C (Catalyst) = 0.5 g L−1 | t = 180 min | |||
| V = 100 ml | ||||
| β-CD/TiO2 | C (MO) = 32 mg L−1 | 250 W high-pressure mercury lamp | 100% | 56 |
| C (Catalyst) = 1 g L−1 | t = 20 min | |||
| V = 100 ml | ||||
| Ce/TiO2/CD polymer | C (RhB) = 5 mg L−1 | 500 W halogen lamp | 80% | 57 |
| V = 50 ml | t = 60 min | |||
| Fe–N–TiO2/β-CD | C (AO7) = 20 mg L−1 | 10 W 400–800 nm (daylight white light, UV-LED at 365 nm) | MB: 94% (UV) t = 60 min | 58 |
| C (MB) = 7 mg L−1 | 50% (visible light) t = 180 min | |||
| C (Catalyst) = 1 g L−1 | AO7: 99% (UV) | |||
| V = 100 ml | t = 60 min |
4. Conclusions
Hybrid composites TiO2/Ag and TiO2/β-cyclodextrin polymer/Ag were developed using β-CD methacrylate or maleate with different degrees of substitution, trimethylolpropane trimethacrylate as a crosslinking agent, and various silver salts (nitrate, methacrylate, citrate). Studies have shown that the use of TiO2/CD polymer composites doped with silver nanoparticles significantly enhances the efficiency of the photocatalytic decomposition of methyl orange dye. The photodegradation kinetics of methyl orange fit well to a pseudo-zero-order model, and the rate constant (k0) increases by up to 6.5 times compared with the initial TiO2. This improvement is attributed to the ability of cyclodextrin's cavity to stabilize silver nanoparticles and enhance pollutant adsorption on the catalyst surface. Using XRD and FTIR spectroscopy, it was found that the introduction of silver nanoparticles led to changes in the crystal structure of TiO2 and improved separation of electron-hole pairs, as confirmed by a shift in the valence vibrational frequencies of Ti–O bonds. This phenomenon also influences the photodegradation efficiency of the hybrid composites mentioned above, suggesting the possibility of optimizing the catalyst composition.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Serhii Kobylinskyi: investigation, writing – original draft, conceptualization, and formal analysis; Sergii Sinelnikov: investigation and resources; Larysa Kobrina: investigation and visualization; Yuliia Bardadym: investigation and visualization; Sergii Riabov: conceptualization, writing – review and editing, project administration, and supervision. All the authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely thank the Armed Forces of Ukraine for the opportunity to work and engage in science during this time.References
- S. Dutta, S. Adhikary, S. Bhattacharya, D. Roy, S. Chatterjee, A. Chakraborty, D. Banerjee, A. Ganguly, S. Nanda and P. Rajak, Contamination of textile dyes in aquatic environment: Adverse impacts on aquatic ecosystem and human health, and its management using bioremediation, J. Environ. Manage., 2024, 353, 120103, DOI:10.1016/j.jenvman.2024.120103.
- B. Pandey, P. Singh and V. Kumar, Photocatalytic-sorption processes for the removal of pollutants from wastewater using polymer metal oxide nanocomposites and associated environmental risks, Environ. Nanotechnol., Monit. Manage, 2021, 16, 100596, DOI:10.1016/j.enmm.2021.100596.
- T. Mao, J. Zha, Y. Hu, Q. Chen, J. Zhang and X. Luo, Research Progress of TiO2 Modification and Photodegradation of Organic Pollutants, Inorganics, 2024, 12(7), 178, DOI:10.3390/inorganics12070178.
- O. Sangeeta, N. Sandhu, C. Kumar, F. Mohajer and R. Tomar, Critical Review on Titania-Based Nanoparticles: Synthesis, Characterization, and Application as a Photocatalyst, Chem. Afr., 2024, 7(4), 1749–1768, DOI:10.1007/s42250-023-00875-1.
- S. Kumar and A. Chanana, TiO2 based nanomaterial: Synthesis, structure, photocatalytic properties, and removal of dyes from wastewater, Korean J. Chem. Eng., 2023, 40(8), 1822–1838, DOI:10.1007/s11814-023-1455-6.
- D. Kanakaraju, F. D. anak Kutiang, Y. C. Lim and P. S. Goh, Recent progress of Ag/TiO2 photocatalyst for wastewater treatment: Doping, co-doping, and green materials functionalization, Appl. Mater. Today, 2022, 27, 101500, DOI:10.1016/j.apmt.2022.101500.
- S. Zhang, L. Yong, N. Sun, Y. Chen, T. Zhang, Y. Fan, F. Ke and H. Zhang, In-situ construction of β-CD polymer/TiO2 with enhanced charge separation for boosting photocatalytic degradation of tetracycline, Inorg. Chem. Commun., 2024, 169, 113122, DOI:10.1016/j.inoche.2024.113122.
- H. Liu, J. Wang, L. Liu, Y. Xu, X. Meng, X. Guo and J. Wang, Cooperative catalysis between cyclodextrin and TiO2 nanoparticles, Mater. Lett., 2024, 365, 136453, DOI:10.1016/j.matlet.2024.136453.
- J. Jia, D. Wu, J. Yu, T. Gao, J. Li, L. Guo and F. Li, β-cyclodextrin-based adsorbents integrated with N-doped TiO2 photocatalysts for boosting dye elimination of industrial wastewater, Surf. Interfaces, 2024, 45, 103901, DOI:10.1016/j.surfin.2024.103901.
- E. García-Díaz, D. Zhang, Y. Li, R. Verduzco and P. J. J. Alvarez, TiO2 microspheres with cross-linked cyclodextrin coating exhibit improved stability and sustained photocatalytic degradation of bisphenol A in secondary effluent, Water Res., 2020, 183, 116095, DOI:10.1016/j.watres.2020.116095.
- N. U. Sangari, Role of β-Cyclodextrin in Enhanced Photocatalytic Decolorization of Metanil Yellow Dye with TiO2, Asian J. Chem., 2018, 30(10), 2294–2298, DOI:10.14233/ajchem.2018.21468.
- G. L. Colpani, J. T. Zanetti, F. Cecchin, A. Dal'Toé, M. A. Fiori, R. D. F. P. M. Moreira and C. Soares, Carboxymethyl-β-cyclodextrin functionalization of TiO2 doped with lanthanum: characterization and enhancement of photocatalytic activity, Catal. Sci. Technol., 2018, 8(10), 2636–2647, 10.1039/C7CY02115A.
- Y. Zhang, Q. Li, Q. Gao, S. Wan, P. Yao and X. Zhu, Preparation of Ag/β-cyclodextrin co-doped TiO2 floating photocatalytic membrane for dynamic adsorption and photoactivity under visible light, Appl. Catal., B, 2020, 267, 118715, DOI:10.1016/j.apcatb.2020.118715.
- H. Zhu, N. Goswami, Q. Yao, T. Chen, Y. Liu and Q. Xu, et al., Cyclodextrin–gold nanocluster decorated TiO2 enhances photocatalytic decomposition of organic pollutants, J. Mater. Chem. A, 2018, 6(3), 1102–1108, 10.1039/C7TA09443D.
- X. Chen, D. Liu, Z. Wu, G. Cravotto, Z. Wu and B. Ye, Microwave-assisted rapid synthesis of Ag-β-cyclodextrin/TiO2/AC with exposed {001} facets for highly efficient naphthalene degradation under visible light, Catal. Commun., 2018, 104, 96–100, DOI:10.1016/j.catcom.2017.10.026.
- A. Zamani, S. Asgharfi and M. Tajbakhsh, Synthesis of TiO2/CD and TiO2/Ag/CD nanocomposites and investigation of their visible light photocatalytic activities in the degradation of methylene blue, Chem. Method, 2024, 8, 177–199, DOI:10.48309/chemm.2024.432168.1753.
- J. R. Isasi and D. Olmos, Cyclodextrin-Grafted TiO2 Nanoparticles: Synthesis, Complexation Capacity, and Dispersion in Polymeric Matrices, Nanomaterials, 2018, 8(9), 642, DOI:10.3390/nano8090642.
- S. Rajalakshmi, S. Pitchaimuthu, N. Kannan and P. Velusamy, Photocatalytic effect of β-cyclodextrin on semiconductors for the removal of Acid Violet dye under UV light irradiation, Desalin. Water Treat., 2014, 52(16–18), 3432–3444, DOI:10.1080/19443994.2013.809024.
- O. A. Opanasenko, S. V. Riabov, S. I. Sinelnikov and Yu. Yu. Kercha, The influence of various factors on the photodegradation of methyl orange in the presence of titanium dioxide and β-cyclodextrin derivatives, Rep. Nat. Acad. Sci. Ukr., 2014, 1, 130–135, DOI:10.15407/dopovidi2014.01.130.
- S. Riabov, Y. Bardadym, S. Kobylinskyi and L. Kobrina, Peculiarities of photocatalytic system based on titanium dioxide, β-cyclodextrin and its derivatives in different water medium, Int. J. Environ. Sci. Technol., 2024, 21(4), 4519–4526, DOI:10.1007/s13762-023-05300-1.
- Ye. A. Opanasenko, S. V. Riabov and S. I. Sinelnikov, Synthesis and properties of cross-linked β-cyclodextrin-containing copolymers and their role in photocatalytic processes, Ukr. Chem. J., 2014, 80(5), 58–63 Search PubMed . https://jnas.nbuv.gov.ua/article/UJRN-0000664459.
- O. Radchenko, S. Sinelnikov, O. Moskalenko and S. Riabov, Nanocomposites based on titanium dioxide, modified by β-cyclodextrin containing copolymers, J. Appl. Polym. Sci., 2018, 135(25), 46373, DOI:10.1002/app.46373.
- Yu. V. Bardadym, S. M. Kobylinskyi, L. V. Kobrina and S. V. Riabov, The use of cyclodextrins to increase the efficiency of titanium dioxide in the heterogeneous catalysis, Ukr. Chem. J., 2020, 86(7), 32–52, DOI:10.33609/2708-129X.86.7.2020.32-52.
- S. Djokić, Synthesis and Antimicrobial Activity of Silver Citrate Complexes, Bioinorg. Chem. Appl., 2008, 1, 436458, DOI:10.1155/2008/436458.
- I. V. Babich, S. V. Ryabov, S. I. Sinelnikov, S. V. Laptiy and Y. Y. Kercha, Synthesis and study of sorption characteristics of molecularly imprinted polymers based on modified cyclodextrins, Ukr. Chem. J., 2012, 78(9), 64–68 CAS.
- Y. A. Opanasenko, S. V. Riabov, S. I. Sinelnikov and S. V. Laptiy, Synthesis and properties of modified titanium dioxide by β-cyclodextrin-containing polymers, Ukr. chem. J., 2015, 81(7), 68–73 CAS.
- A. H. Delgado and A. M. Young, Methacrylate peak determination and selection recommendations using ATR-FTIR to investigate polymerisation of dental methacrylate mixtures, PLoS One, 2021, 16(6), e0252999, DOI:10.1371/journal.pone.0252999.
- T. Bezrodna, G. Puchkovska, V. Shymanovska, J. Baran and H. Ratajczak, IR-analysis of H-bonded H2O on the pure TiO2 surface, J. Mol. Struct., 2004, 700(1–3), 175–181, DOI:10.1016/j.molstruc.2003.12.057.
- L. A. García-Contreras, J. O. Flores-Flores, J. Á. Arenas-Alatorre and J. Á. Chávez-Carvayar, Synthesis, characterization and study of the structural change of nanobelts of TiO2 (H2Ti3O7) to nanobelts with anatase, brookite and rutile phases, J. Alloys Compd., 2022, 923, 166236, DOI:10.1016/j.jallcom.2022.166236.
- I. Mironyuk, N. Danyliuk, T. Tatarchuk, I. Mykytyn and V. Kotsyubynsky, Photocatalytic degradation of Congo red dye using Fe-doped TiO2 nanocatalysts, Phys. Chem. Solid State, 2021, 22(4), 697–710, DOI:10.15330/pcss.22.4.697-710.
- R. Messemeche, H. Saidi, A. Attaf, Y. Benkhetta, S. Chala, R. Azizi and R. Nouadji, Elaboration and characterization of nano-crystalline layers of transparent titanium dioxide (Anatase-TiO2) deposited by a sol-gel (spin coating) process, Surf. Interfaces, 2020, 19, 100482, DOI:10.1016/j.surfin.2020.100482.
- B. Choudhury, R. Verma and A. Choudhury, Oxygen defect assisted paramagnetic to ferromagnetic conversion in Fe doped TiO2 nanoparticles, RSC Adv., 2014, 4(55), 29314–29323, 10.1039/C3RA45286G.
- K. Das, S. N. Sharma, M. Kumar and S. K. De, Morphology dependent luminescence properties of Co doped TiO2 nanostructures, J. Phys. Chem., 2009, 113(33), 14783–14792, DOI:10.1021/jp9048956.
- C. Yang, H. Zhang, W. Gu and C. Chen, Preparation and mechanism investigation of highly sensitive humidity sensor based on Ag/TiO2, Curr. Appl. Phys., 2022, 43, 57–65, DOI:10.1016/j.cap.2022.08.006.
- C. Khurana, O. P. Pandey and B. Chudasama, Synthesis of visible light-responsive cobalt-doped TiO2 nanoparticles with tunable optical band gap, J. Sol-Gel Sci. Technol., 2015, 75, 424–435, DOI:10.1007/s10971-015-3715-3.
- R. Hidayat, S. Wahyuningsih and G. Fadillah, Green synthesis approach to produce TiO2/rGO nanocomposite as voltammetric sensor for monitoring trace level bisphenol-A, Mater. Sci. Eng. B, 2022, 286, 116083, DOI:10.1016/j.mseb.2022.116083.
- T. D. Nguyen-Phan, V. H. Pham, E. W. Shin, H. D. Pham, S. Kim and J. S. Chung, et al., The role of graphene oxide content on the adsorption-enhanced photocatalysis of titanium dioxide/graphene oxide composites, Chem. Eng. J., 2011, 170(1), 226–232, DOI:10.1016/j.matlet.2017.05.070.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure Appl. Chem., 2015, 87(9–10), 1051–1069, DOI:10.1515/pac-2014-1117.
- Y. Bardadym, S. Kobylinskyi, L. Kobrina and S. Riabov, UV spectroscopy and kinetic research of photodegradation of methyl orange in the presence of titanium dioxide and β-cyclodextrin or its derivatives, Polym. J., 2021, 43(2), 103–112, DOI:10.15407/polymerj.43.02.103.
- A. A. Shalaby and A. A. Mohamed, Determination of acid dissociation constants of Alizarin Red S, Methyl Orange, Bromothymol Blue and Bromophenol Blue using a digital camera, RSC Adv., 2020, 10(19), 11311–11316, 10.1039/C9RA10568A.
- N. Bouanimba, N. Laid, R. Zouaghi and T. Sehili, Effect of pH and inorganic salts on the photocatalytic decolorization of methyl orange in the presence of TiO2 P25 and PC500, Desalin. Water Treat., 2015, 53(4), 951–963, DOI:10.1080/19443994.2013.848667.
- S. Kader, M. R. Al-Mamun, M. B. K. Suhan, S. B. Shuchi and M. S. Islam, Enhanced photodegradation of methyl orange dye under UV irradiation using MoO3 and Ag doped TiO2 photocatalysts, Environ. Technol. Innovation, 2022, 27, 102476, DOI:10.1016/j.eti.2022.102476.
- L. Lv, Y. Li, J. Tang and F. Zhang, Ag/TiO2 photocatalytic synergistic persulfate activation for degradation of methyl orange, Opt. Mater., 2025, 159, 116600, DOI:10.1016/j.optmat.2024.116600.
- X. Zuo, S. Zou, J. Wu, B. Ding and A. Jiang, Simulated sunlight-driven photocatalytic activation of peroxydisulfate by core–shell Ag@ SiO2/TiO2 nanocomposite for efficient methyl orange degradation, Desalin. Water Treat., 2024, 320, 100806, DOI:10.1016/j.dwt.2024.100806.
- I. Mironyuk, N. Danyliuk, L. Turovska, I. Mykytyn and V. Kotsyubynsky, Structural, morphological and photocatalytic properties of nanostructured TiO2/AgI photocatalyst, Phys. Chem. Solid State, 2023, 24(2), 374–384, DOI:10.15330/pcss.24.2.374-384.
- Q. Y. Li, S. D. Han, J. G. Liu, L. Y. Sun, Y. L. Wang, Q. Wei and S. P. Cui, Controllable preparation and photocatalytic performance of hollow hierarchical porous TiO2/Ag composite microspheres, Colloids Surf., A, 2023, 658, 130707, DOI:10.1016/j.colsurfa.2022.130707.
- D. Wu and M. Long, Enhancing visible-light activity of the self-cleaning TiO2-coated cotton fabrics by loading AgI particles, Surf. Coat. Technol., 2011, 206(6), 1175–1179, DOI:10.1016/j.surfcoat.2011.08.007.
- D. Yu, J. Bai, H. Liang, J. Wang and C. Li, Fabrication of a novel visible-light-driven photocatalyst Ag-AgI-TiO2 nanoparticles supported on carbon nanofibers, Appl. Surf. Sci., 2015, 349, 241–250, DOI:10.1016/j.apsusc.2015.05.019.
- D. Yu, J. Bai, H. Liang, T. Ma and C. Li, AgI-modified TiO2 supported by PAN nanofibers: A heterostructured composite with enhanced visible-light catalytic activity in degrading MO, Dyes Pigm., 2016, 133, 51–59, DOI:10.1016/j.dyepig.2016.05.036.
- T. Narkbuakaew, S. Sattayaporn, N. Saito and P. Sujaridworakun, Investigation of the Ag species and synergy of Ag-TiO2 and g-C3N4 for the enhancement of photocatalytic activity under UV–Visible light irradiation, Appl. Surf. Sci., 2022, 573, 151617, DOI:10.1016/j.apsusc.2021.151617.
- A. Sabir, T. A. Sherazi and Q. Xu, Porous polymer supported Ag-TiO2 as green photocatalyst for degradation of methyl orange, Surf. Interfaces, 2021, 26, 101318, DOI:10.1016/j.surfin.2021.101318.
- R. Shan, L. Lu, J. Gu, Y. Zhang, H. Yuan, Y. Chen and B. Luo, Photocatalytic degradation of methyl orange by Ag/TiO2/biochar composite catalysts in aqueous solutions, Mater. Sci. Semicond. Process., 2020, 114, 105088, DOI:10.1016/j.mssp.2020.105088.
- R. Saravanan, D. Manoj, J. Qin, M. Naushad, F. Gracia, A. F. Lee and M. A. Gracia-Pinilla, Mechanothermal synthesis of Ag/TiO2 for photocatalytic methyl orange degradation and hydrogen production, Process Saf. Environ. Prot., 2018, 120, 339–347, DOI:10.1016/j.psep.2018.09.015.
- R. Chalasani and S. Vasudevan, Cyclodextrin-functionalized Fe3O4@ TiO2: reusable, magnetic nanoparticles for photocatalytic degradation of endocrine-disrupting chemicals in water supplies, ACS Nano, 2013, 7(5), 4093–4104, DOI:10.1021/nn400287k.
- D. Zhang, C. Lee, H. Javed, P. Yu, J. H. Kim and P. J. Alvarez, Easily recoverable, micrometer-sized TiO2 hierarchical spheres decorated with cyclodextrin for enhanced photocatalytic degradation of organic micropollutants, Environ. Sci. Technol., 2018, 52(21), 12402–12411, DOI:10.1021/acs.est.8b04301.
- S. Lu, N. Sun and T. Wang, Research on Photocatalytic Degradation of Methyl Orange by a β-Cyclodextrin/Titanium Dioxide Composite, Gen. Chem., 2017, 3, 164–169, DOI:10.21127/yaoyigc20170007.
- J. Li, S. Jia, G. Sui and L. Du, Preparation of a cerium/titanium composite with porous structure and enhanced visible light photocatalytic activity using β-cyclodextrin polymer microspheres as the template, Chem. Pap., 2018, 72, 369–379, DOI:10.1007/s11696-017-0286-5.
- S. Mottola, A. Mancuso, O. Sacco, I. De Marco and V. Vaiano, Photocatalytic performance assessment of Fe-N co-doped TiO2/β-cyclodextrin hybrid systems prepared by supercritical antisolvent micronization for organic dyes removal, J. Supercrit. Fluids, 2023, 201, 106005, DOI:10.1016/j.supflu.2023.106005.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08901d |
| This journal is © The Royal Society of Chemistry 2025 |