 Open Access Article
Open Access ArticleVegetative and microbial proteins for bioplastics applications – a review in the indian context
Aditya Patela,
Rushabh Muralia,
Heena Choudharyb,
D. Purnimac and
Ramendra Kishor Pal *c
*c
aDepartment of Chemical Engineering, Birla Institute of Technology and Science, Pilani, Pilani Campus, Vidya Vihar, Pilani, Rajasthan 333031, India
bDepartment of Pharmacy, Birla Institute of Technology and Science, Pilani, Hyderabad Campus, Jawahar Nagar, Kapra Mandal, Medchal District, 500 078 Telangana, India
cDepartment of Chemical Engineering, Birla Institute of Technology and Science, Pilani, Hyderabad Campus, Jawahar Nagar, Kapra Mandal, Medchal District, 500 078 Telangana, India. E-mail: ramendra.pal@hyderabad.bits-pilani.ac.in
First published on 20th May 2025
Abstract
The generation of plastic waste is around 400 million tons per year. The non-degradable nature of fossil-derived plastics creates pollution, one of the most concerning environmental challenges faced by society, agencies, and governments today. A promising alternative to plastics is bioplastics. Bioplastics are biopolymer-based plastics derived from biomass or manufactured from the processing of monomers derived from biomass. Proteins are naturally occurring biomolecules that are one of the most suitable natural polymers for making bioplastics. In the form of films, proteins possess various desirable properties such as mechanical strength, gas impermeability, and durability. They are also renewable and easily accessible. Making bioplastics from wasted or unused protein sources is the ideal scenario. This review discusses the opportunities that come along with vegetative and microbial proteins to make bioplastics. It covers various sources for protein extraction, such as gluten, whey, zein, and soy from terrestrial sources and water hyacinth and duckweed from aquatic sources. It also discusses the methods of processing vegetative proteins to make bio-plastic products, the current challenges in employing bioplastics for typical applications, and the prospects to steer us towards a clean and sustainable future.
1. Introduction
The generation of plastic waste and consequent plastic pollution is one of the greatest threats to humans and nature. According to the United Nations Environment Program, the generation of plastic waste has crossed 400 million tons per year; moreover, only 10% of plastic waste generated to date underwent recycling.1 Plastics are versatile, affordable, and durable and apply to almost everything around us. Among these, single-use plastics (SUPs), used once and then trashed, are of major concern. Most packaging applications use SUPs. Due to its convenience, SUPs have become an integral part of our life. However, these are now responsible for ∼43% of total plastic waste in India.2 A few examples of SUPs include low-density polyethylene, polypropylene, polystyrene, polyethylene terephthalate, and polyvinyl chloride, which can remain in nature for more than 450 years and emit greenhouse gases (GHGs); thus, there is a growing concern regarding their contribution to environmental pollution. Such issues associated with SUPs call for a transition towards sustainable solutions.India is the world's fifth-largest generator of plastic waste and plans to achieve net-zero emissions by 2070. Many countries, including India, impose restrictions on using SUPs to curb plastic pollution at different levels. Nonetheless, these restrictions have a meagre impact on the usage of SUPs due to the unavailability of alternatives on a large scale and at a competitive price. Among many proposed solutions, biodegradable and eco-friendly bioplastics are the leading ones, mainly derived from relatively fast-renewing resources such as first-generation feedstocks (potentially edible sources) and second-generation feedstocks (non-edible biowastes). Materials required for bioplastics come from various vegetative, non-vegetative, or microbial sources. These materials are natural polymers such as polysaccharides (starch, cellulose, pectins, hemicellulose) and proteins (pea protein, casein, zein, gluten, gelatin). They are processable to form films, membranes, and various other shapes. During film formation, the polymeric chains present in these materials interact intra- and inter-molecularly and form cross-links. These interactions and crosslinks lead to a partially rigid three-dimensional polymer matrix, which provides barrier properties and mechanical strength required for packaging applications.
According to the United Nations Food and Agriculture Organization (FAO), the Global generation of food-based biowastes is about 1300 million tonnes per year.3 According to the Indian Council of Agriculture Research, India produces about 350 million tonnes of agriculture biomass waste annually.4 This biomass waste contains ∼0.25–38 wt% of proteins, ∼30–60 wt% of polysaccharides, and 10–40 wt% of lipids.5,6 Traditionally, biomass waste is either discarded, burnt, or used for animal feeding. The circular economy model encourages the development of sustainable technologies that can turn biowaste into valuable products. One such example is the production of biodegradable SUPs from polymers extracted from biowastes and non-edible food sources.
Proteins are naturally occurring biomacromolecules that are suitable for making bioplastics. In the form of films, proteins possess various desirable properties such as mechanical strength, gas impermeability, and durability (after suitable processing or adding additives).7–11 They are also renewable and easily accessible. The ideal possibility is to produce bioplastics from wasted or unused protein sources. However, proteins can come from both animals and plants. Recently, the use of plant-based and microbe-derived proteins for developing protein-based films has garnered interest due to their lower environmental impact and higher contributions to sustainability.12
Several reviews exist that focus on different aspects of the processing of proteins. Nonetheless, the complete picture of the production of bioplastics using plant-based protein sources is scattered. Beyond the survey of the current state-of-the-art to define opportunities and challenges present for protein-based bioplastics, the review aims to provide detailed information about all the major steps involved in producing plant-based protein bioplastics. Towards this end, the review first sheds light on the available and potential sources of vegetative and microbial proteins (i.e., wheat gluten, potato, zein, soy, rapeseed, sunflower, protein, casein, whey, and proteins derived from algae, duckweed and water hyacinth) as per the Indian context. We also discuss extraction techniques to recover proteins from natural sources and the different processes (such as wet and dry) to produce protein-based bioplastics. Then, we detail the important processing methods and additives used for tuning the properties of bioplastics for desired end applications. In this regard, we also describe the main characteristics (properties) of protein-based bioplastics and characterisation techniques such as mechanical, rheological, thermal, and optical. In addition, we also illustrate the potential application areas and life-cycle analysis of protein-based bioplastics. Thus, the review aims to be a starting point for readers who wish to innovate protein-based bioplastics to replace fossil-derived polymers for typical daily applications.
It is important to note that the International Union of Pure and Applied Chemistry (IUPAC) defines bioplastics as “Biobased polymer derived from the biomass or issued from monomers derived from the biomass and which, at some stage in its processing into finished products, can be shaped by flow.”13 IUPAC cautions that all the materials derived from biomass may not be environment-friendly and suggests using “bio-based polymers” instead of bioplastics. However, we choose to use the term “bioplastic” in this review as the usage of the term is more common than the term “bio-based polymer”. This review discusses the bioderived and biodegradable protein-based materials and refers to them as “protein-based bioplastics”.
2. Opportunities: protein as bioplastics
Proteins are complex macromolecules constructed from long chains of 20 distinct amino acids composed of carbon, hydrogen, oxygen, nitrogen, and sulfur.14 Proteins self-assemble to establish an internal structural order at multiple length scales and create secondary and tertiary structures; thus, proteins differ from common polymers. The secondary structure (α-helices or β-strands) arises due to the tendency of the protein chains to loop or coil together to form a stable structure via forming hydrogen bonds. Specific biological properties of proteins occur due to the tertiary structure of the protein, which is a collection of organised and unorganised sections of secondary structures. Due to the hierarchical organisation of proteins, their structures vary considerably and allow for self-assembly. The diverse amino acids present in proteins allow both functionalisation and structural tuning. Such modifications enable modulation of the protein's strength, stability, solubility, biocompatibility, and biodegradability; thus, they are promising candidates for creating bioplastics with advanced capabilities such as mechanical strength, gas impermeability, durability, nutritional values, stimuli responsiveness, and fabricability applied to develop high-tech, functional, and smart applications.15,16 These applications include active packaging of foods, edible coatings, 3D printing, and biomedical tools.Techniques for extracting plant protein at an industrial scale are well-established.17 However, extracting proteins from plant biomass may denature the proteins and affect the functional properties of proteins.18 Beyond plant proteins, proteins extracted from single-cell organisms or microbes (such as algae, bacteria, and yeast) can be a significant source of non-animal protein. Biosynthetic engineering approaches can modify microbes such as bacteria to produce high-strength proteins that mimic the structure of spider silk (one of the strongest proteins found in nature); such processes have the potential for scaling to industrial levels.19,20 Further, biosynthetic approaches have the ability to produce proteins of defined internal sequences and structures exhibiting specific strength, stability, solubility, biocompatibility, and biodegradability. Engineered proteins obtained from microbes and crosslinked with smartly chosen crosslinkers are mechanically robust, with a fracture stress of ∼54 MPa, better than common plastics such as polyvinyl chloride and polyvinyl alcohol.21 Furthermore, the extraction of proteins from food wastes is also under investigation. We will discuss a few crucial processes in the later sections. Thus, providing physicochemical modifications or biosynthetically designed and expressed proteins and scalable production capabilities offer unique opportunities to produce bioplastics with proteins with tailored properties for different applications.
Synthetic plastic-based food packages may contain and shed microplastics in the foodstuffs.22 Regular consumption of such foods may lead to serious health issues. On the other hand, humans and animals can efficiently metabolise proteins. Therefore, using protein-based bioplastics for food packaging offers a safe and sustainable alternative to synthetic bioplastics. Further, protein-based plastics are also suitable for the active-packaging of food, which includes immobilisation and controlled release of ingredients such as natural antimicrobials (essential oils, botanical extracts, herbs, and antimicrobial peptides), natural antioxidants (vitamins C and E, carotenoids, flavonoids, phenolic acids, lignans, stilbenes, and essential oils), specific enzymes (glucose oxidase, lysozyme, laccase, and lipase) or provide gas selective permeability.23–26 Such protein-based active-packaging will help extend the shelf-life of food products, improve food safety, reduce spoilage, and increase food security.
Most of the current efforts in developing bioplastics focus on carbohydrates, PVA, and PLA, which have many successful products out on the market.27 However, there is a growing interest in utilising proteins to manufacture SUP packages mainly due to their excellent film-formation ability, biodegradability, and nutritional value.28 Proteins have certain advantages due to several key reasons:
(1) Structural versatility: proteins possess a diverse and complex molecular structure, allowing for a broader range of potential bioplastic properties. This versatility is valuable for tailoring bioplastics to specific applications, such as packaging films.29
(2) Strength and durability: proteins generally exhibit high tensile strength, gas impermeability, and durability, which can be further enhanced using appropriate processing methods and additives.7–11 These properties make protein-based bioplastics better suited for applications requiring sturdiness and resistance to wear and tear.
(3) Biodegradability: proteins often biodegrade faster than carbohydrates. If ingested by animals, they get digested, unlike non-biodegradable plastics that accumulate.30 Many microorganisms can readily metabolise protein-based bioplastics, reducing their environmental impact when disposed of in a natural environment.
(4) Hygroscopic properties: protein-based bioplastics have tunable water retention properties. Depending on the protein's nature and the selected thermal treatment, water absorption values range from 40–320%. Rice protein bioplastics show the lowest water absorption values.31 Soy-protein bioplastic samples typically show high water uptake values (200–700%). The high absorption capacity is due to the hydrophilic character of soy proteins, which absorb water into their structure.32 This property is useful in developing water-absorbent materials for healthcare, agriculture, and horticulture applications, where water absorbency and retention are essential.31 Proper processing of vegetative proteins can lead to superabsorbent materials.32 On the other hand, hydrophobic proteins or hydrophobisation of proteins with lipids can lead to water-resistant protein-based SUPs.29,33
(5) Potential for bioengineering: proteins can be genetically modified to achieve specific properties, which can also be advantageous for customising bioplastics with desired characteristics.33–35
(6) Developing economic ecosystem: currently, many industrial wastes contain proteins. Some common examples are wheat gluten, which is a byproduct in the bio-ethanol industry, and rice bran, which is one of the main byproducts in the process of rice milling, usually discarded as waste – making them a sustainable choice for bioplastic production.36 Using such protein sources will allow us to add value to wastes and by-products, encouraging a sustainable ecosystem around them.
While carbohydrates, like starches, are currently the prime choice to create bioplastics, proteins offer a broader range of options for producing environmentally friendly materials with properties tailored to different applications. However, the choice between proteins and carbohydrates for bioplastic production should consider the specific requirements of the intended use and the sustainability goals.
3. From plants to bioplastics
3.1 Overview
Protein is an essential biomacromolecule in all living things, such as plants (terrestrial and aquatic), single-cell species, and animals. The mechanical strength of the proteins derived from animal sources is higher than those derived from plant sources. Further, most plant-derived proteins dissolve in a limited number of solvents; thus, their processing is challenging.37 Nonetheless, this review only discusses proteins obtained from non-animal sources: plants and single-cell species. Why not animal proteins for bioplastics and SUPs? Most of the population living in third world countries, including India, are protein-deficient, with insufficient protein reaching the plates daily to fulfil even the basic nutritional requirements.38,39 Most of the protein that we consume comes from animal sources. Furthermore, ∼25 kilocalories of fossil energy are required to produce 1 kilocalories of animal protein.40 Therefore, using animal-derived proteins for bioplastic production is not a viable solution. Animal-derived proteins are prone to animal-borne diseases, and consumers may reject the bio-plastics made out of them due to their religious beliefs, ethical beliefs, or personal preferences.41,42 Conversely, plant-derived and single-cell-derived proteins do not face these issues. Plant-derived proteins are more cost-effective and low on greenhouse gas emissions than animal-based proteins.43 Plant-derived proteins are suitable for large-scale production from edible, non-edible, agri-waste, bio-refinery, and food waste. It is important to note that animal-derived proteins (e.g. keratin), which usually end up as waste, may find bioplastic applications.44 Nonetheless, we limit our discussions in this review to proteins derived from protein-rich plants, plant products, and single-cell species, followed by a brief discussion on their extraction and processing. From source to bioplastics, the entire production process may consist of three major steps: (1) sourcing of raw materials (protein-rich plants and plant products), (2) extraction of proteins, and (3) their processing.3.2 Sourcing of raw material
Cultivation of protein-rich plants or sourcing protein-rich plant residues as a by-product from industries can be a sustainable source of raw materials for producing protein-based bioplastics. Fig. 1 shows a few important protein-rich sources discussed in this review.| Source | Protein content | Osborne fractionation | References | |||
|---|---|---|---|---|---|---|
| Albumin | Globulin | Prolamines | Glutelin | |||
| Wheat | 8–15 | 3–5 | 6–10 | 40–50 | 30–40 | 45 and 46 |
| Pea protein | 23–31 | 20 | 65 | 15 | 47 and 48 | |
| Zein from corn | 6–12 | Mostly prolamines | 49 | |||
| Kafirin from sorghum | 8–9 | Mostly prolamines | 50 | |||
| Soybean | 33–49 | 10 | 90 | 48 and 51 | ||
| Rice bran | 10–15 | 4–37 | 5–36 | 1–6 | 11–80 | 46 and 52 |
| Sunflower seeds | 10–27 | 10–30 | 40–90 | ∼5 | ∼15 | 48 and 53 |
| Cottonseed | 30–40 | 21–32 | 33–64 | 9–28 | 54–56 | |
3.2.1.1 Wheat gluten. Gluten is the rubbery residue obtained after washing the wheat dough. The residue contains 75–85% of protein (Table 1) and 5–10% of lipids; the remaining is primarily carbohydrates (starch and non-starch).57 The wheat kernel contains 8–15% protein, of which 85–90% is gluten and 10–15% is albumin/globulin.45 Gluten protein is a complex mixture of distinct proteins, mainly gliadin and glutenin components.45 Again, these are monomers, oligomers and high molecular weight polymers linked by disulphide bonds.57 Wheat gluten is often a by-product of the bio-ethanol industries.58 It is a promising material due to its relative abundance, low cost, and favourable material properties. It also possesses good film-forming ability, with selective gas-barrier properties, UV-blocking properties, insolubility in water, and biodegradability.59,60 Low-grade or unused wheat is used mainly as animal feed, and its use as raw material for bioplastic production would also increase its value. Thus, there is a growing interest in its potential application as a packaging material for the food industry. However, usage is somewhat restricted as the films formed are sensitive to humidity and absorb water when submerged.15 Another issue limiting its usage is its association with celiac disease in humans, which is an autoimmune disorder affecting about 1% of the worldwide population.61
3.2.1.2 Pea protein. Peas have tremendous potential for protein extraction as they have the highest protein content of any legume. For instance, grass pea (Lathyrus sativus) contains around 23 to 31 wt% protein (Table 1).47 Pea protein consists of four major protein classes: globulin, albumin, prolamin, and glutelin. Globulin and albumin contents are higher than the other two.62
Several established methods, including flocculation, exist for extracting pea protein,63 in which dry pea seeds are ground into flour to obtain the protein. India grows peas on a large scale for human consumption, which are available at low prices. Pea protein has the potential to make edible bioplastics because of its low allergenicity,64 which allows the manufacture of bioplastics for storing food without the fear of allergies. The constituents of peas are fibre, protein, and starch; all of these can have varied applications, which further increase the value of the final products.65,66 Pea protein-based bioplastics are compatible with the injection moulding process. Further, different crosslinking methods can enhance its mechanical properties, such as deformability and antimicrobial properties (the review discusses this in later sections).67
3.2.1.3 Zein. Zein is a prolamin protein (composed of α-zein, β-zein, and γ-zein) primarily found and extracted from the endosperm of corn, a commonly grown crop in India.68 Zein contains a high proportion (50%) of non-polar hydrophobic amino acid residues, such as glutamine, leucine, proline, and alanine; thus, it is insoluble in water, contributing to the water vapour barrier properties of films, but it is soluble in alcohol.69 Zein comprises 50–60 wt% of the protein in the corn kernel, where the protein content may range from 6–12% on a dry basis based on corn verities (Table 1).49 The potential application of zein as a biomaterial is due to its relative hydrophilicity and biocompatibility.70,71 They also have a moderate moisture barrier, oxygen barrier and mechanical properties, resulting in potential applications in textile and adhesive industries, where petroleum-based plastics are predominant.72 Chemical crosslinking, such as treatment with glutaraldehyde, can improve zein-based films' strength and barrier properties.73 Further, physical treatments such as irradiation can fine-tune the properties of zein films to meet product specifications.8
3.2.1.4 Kafirin. Kafirin is a sorghum prolamin protein. India, one of the world's top 10 sorghum producers, produced around 4.8 million tonnes of sorghum in 2021.74,75 Sorghum contains ∼11% protein, of which 70–80% is kafirin (Table 1).50 Though kafirin is relatively more hydrophobic and less digestible than zein,76 kafirin has superior water, gas, and lipid barrier properties.77 Reports suggest that the kafirin has a glass transition temperature (Tg) but varies significantly from 40 °C to 233.8 °C.78,79 A limited number of reports discuss bioplastics made from kafirin. A few reports explored the solution casting method to create kafirin films, which include the dissolution of the protein in an organic solvent or acidic aqueous solutions, pouring the solution on a hydrophobic smooth surface, and evaporation of the solvent to produce a film.78,79 However, kafirin can find applications in food packaging where gluten-free materials are required.
3.2.1.5 Soy protein. The soybean is a species of legume commonly grown for its edible bean. It is one of the most cultivated plants in the world. In India, it is grown in Maharashtra, Rajasthan, Madhya Pradesh, and Karnataka. It finds its uses in various preparations, such as soy milk, tofu, tofu skin, soy sauce, and fermented bean paste. Soy protein is a protein in soybeans that undergoes processing into three commercial products: soy flour, concentrates, and isolates. Soy protein isolate has been used commonly in foods for its functional properties for a long time. Soybeans are rich in proteins (33 to 49 wt%) (Table 1);51 fat-free soybean meal is a significant source of protein for animal feeds. Protein extraction is an essential part of this industry. Alkaline extraction and isoelectric precipitation are the standard methods to extract pea protein. New extraction techniques have been developed, including enzyme-assisted extraction.80 Soy protein contains high percentages of glutamic and aspartic acids, allowing the formation of hydrogen bonds, and it may find applications as superabsorbent material.81 Reports also suggest that soy proteins are compatible with conventional processing methods such as injection moulding and extrusion.82,83 A recent report demonstrated the feasibility of making soy protein-based bioplastics using two prime byproducts of the soybean industry: soy whey and soybean pulp (okara) at an industrial scale.84
3.2.1.6 Rice bran. Rice bran comprises the outer brown layer of rice. It is a by-product when rice is processed to remove rice bran to obtain white rice. India produced 10.8 million tons of rice bran in 2017.85 Rice bran is primarily used in making oil and as fodder for livestock. It contains 10 to 15% protein and is available cheaply (Table 1).52 Rice bran protein precipitates when rice bran is suspended in an alkaline hexane solution. Extraction of protein from rice bran can further valorise this by-product. Several research efforts have focused on improving the product characteristics of rice bran protein-based bioplastics. Limited reports have shown bioplastics made from proteins extracted from rice bran. However, several works have explored the manufacture of bioplastics from defatted rice bran, which contains proteins and starches. These works indicate that the rice bran can undergo thermomechanical processes such as injection moulding and extrusion.86,87 Further, mechanical properties such as the stiffness of rice bran-based bioplastics can elevate ∼40% by incorporating minute amounts of natural filler materials (∼2%) such as cellulose, flax and hazelnut shell.88 A recent report showed that adding carboxymethyl cellulose can improve the film-forming properties of rice bran protein. Further, the addition of photocatalytic agents such as Zirconia can provide antibacterial properties to the bioplastics.89
3.2.1.7 Sunflower protein. Sunflower seeds find applications mainly in oil production. These seeds contain a high amount of protein (10–27 wt%) (Table 1),53 thus justifying interest in this as a source of protein for the manufacture of bioplastics. As the primary use of sunflower seeds is in oil extraction, protein extraction from the residual cakes can reduce the waste generated. However, oil extraction uses organic solvents at high pressure and temperatures, which can modify the structure and functionality of proteins from sunflower seeds.90 Further, phenolic compounds such as chlorogenic acid are still present in the protein due to interactions of phenolic compounds with proteins. Phenolic compounds possess excellent antioxidant properties.90 Thus, protein from sunflowers is a potential candidate for bioplastic production for food packaging applications. The solution casting method is the prime method for producing sunflower protein films.90,91
3.2.1.8 Cottonseed. Cotton is a major cash crop, with around 25.89 million tonnes produced in 2021–22.92 India is one of the major producers of cotton, and it produced 5.84 million tonnes during the year 2022–23, which is around 23% of the world's cotton production.93 Cotton grows in a protective shell called a ‘boll.’ Inside a boll, cotton fibres cover the cottonseeds. A boll of cotton contains two parts of cotton fibres and three parts of cottonseeds by weight. The primary product from cottonseeds is oil, which is around 16% by weight. Other components of the cottonseeds are meal (45%), hull (25%), and linters (8%).94 The cottonseed kernel contains 30–40% protein, which can find further applications (Table 1).54,55 A harmful terpenoid (gossypol), which is cardio- and hepatotoxic to humans and other monogastric animals, may be present in cottonseed meals; thus, it is unsuitable for food or feed applications. Cottonseed proteins also have a high plasticiser efficiency (>5), making it a suitable raw material for producing protein-based bioplastics for non-food applications.95 Readers can find the definition of plasticiser efficiency in Section 4. Reports also indicate that the cottonseed proteins are compatible with solution-cast, compression moulding, and hot-press moulding methods.96–98
3.2.2.1 Water hyacinth. Though not an indigenous species to India, water hyacinth is an invasive aquatic weed that grows in water bodies across India. Water hyacinth is a floating aquatic plant that blocks the air–water interface, reducing dissolved oxygen levels and leading to the degradation of the water quality, reducing the species richness of the aquatic ecosystem. Since this plant invades large areas of open water, it produces enormous quantities of wasteful biomass. This weed is eradicated by removing it from water and discarding it as waste on land. Eradicating hyacinths is difficult, mainly due to environmental and financial challenges. Hence, there is a need for alternative solutions that are scalable, sustainable, and value-adding.
Locals in many regions of India, like Bengal and Kerala, have started using these resources to generate energy and produce biodegradable products.103,104 Proximate analysis showed that the protein fraction in water hyacinth is about 8%, and in water hyacinth leaf protein concentrate (WHLPC) is about 50% of its nutrients. At the same time, carbohydrates, fat, ash and fibre comprise the remaining nutrients.105,106 Further analysis showed that WHLPC contains 17 of 20 standard amino acids.106 Hence, water hyacinth is a good source of protein and a potential raw material for bioplastics manufacturing. Packaging companies shifting towards seaweed-based packaging have also shown increasing interest towards other abundant, economically viable sources, such as hyacinths.107 To date, no reports show the fraction of RuBisCo proteins present in water hyacinth and the applications of protein extracted from water hyacinth.
3.2.2.2 Duckweed. Duckweed is a fast-growing (doubling time 1.34 to 4.54 days) aquatic macrophyte,108 and production rates can reach up to 193 tonnes/hectareyear of dried duckweed.109 It grows on the surface of water bodies such as lakes and ponds. Like hyacinths, it covers the surfaces, causing oxygen depletion in the water. On the other hand, its fast growth could make it a useful resource that converts CO2 into biomass. Also, duckweed has a high protein concentration (40 wt% of dry biomass) under nitrogen-rich growing conditions.110 Current US and European investigations suggest it is a protein-rich superfood.
Nonetheless, duckweed grown in wastewater bodies will be inedible. These qualities make it an attractive raw material for making bioplastic films. A few reports showed dried duckweed biomass as a filler material to create bioplastic using biodegradable thermoplastics such as poly(lactic acid), polyhydroxyalkanoates, and cassava starch.110–112 To date, no reports have shown the fraction of RuBisCo proteins present in water duckweed and the development of bioplastics from duckweed proteins.
Below, we briefly describe proteins from various single-cell sources.
3.2.3.1 Bacterial sources. Bacterial SCP contains high protein content (60–80 wt%) and essential amino acids.118 Further, genetically modified bacteria can produce proteins with excellent mechanical properties.20 Bacterial SCP may have several advantages over plant proteins: (1) under suitable conditions, single-cell species grow faster than plants; thus, they can produce proteins faster than plants. These bacterial SCPs can find applications as bioplastics without stressing the food supply chain; (2) microorganisms can grow on food wastes and do not require fertile lands or freshwater; thus, they do not compete with traditional agriculture but support waste management and waste valorisation; (3) cultivation of microorganisms does not require nitrogenous fertilisers; hence, they do not increase carbon footprints, (4) microbial growth is least affected by climatic conditions, and they can grow throughout the year in desired quantities, (5) fermentation and bioreactor technologies are well developed for both aerobic and anaerobic microbes; thus process modulation depending on requirements are possible, (6) bacterial SCP production can integrate well with waste treatment plants of the food industry or bio-refineries. One unique advantage of bacterial sources is that they can be genetically modified using biosynthetic engineering approaches to produce high-strength proteins that mimic the structure of spider silk (one of the strongest proteins found in nature); such processes have the potential for scaling to industrial levels.19,20 Further, biosynthetic approaches can tune the genes of bacterial species to produce proteins with specific internal sequences and structures exhibiting specific strength, stability, solubility, biocompatibility, and biodegradability. A recent techno-economic analysis of SCP production using industrial off-gas through acetate-to-SCP fermentation processes suggests the production cost may be ∼2.78 USD per kg of SCP.119 Thus, they add value and promote circular bio-economy concepts.118
3.2.3.2 Microalgae and spirulina algal biomass. Many different algae have emerged as novel raw material sources for bioplastics. Algae species are classified into macroalgae and microalgae. Microalgae are microscopic organisms that utilise solar energy and live in fresh, saline waters. Crops such as corn, wheat, and soy compete for resource distribution between bioplastic manufacturing and human consumption. Also, using polymeric compounds from plants to synthesise bioplastics is difficult due to the multi-layered cell walls.120 Bioplastics made from food crops like corn also face the issues of poor water resistance and mechanical properties. On the other hand, algae can grow on waste resources and does not compete with traditional food sources. However, it is gaining popularity as a dietary supplement.121 They also tolerate harsh environmental conditions and utilise carbon dioxide as a nutrient source for biomass production. Hence, they emerge as better alternatives for bioplastic production.122
Spirulina comprises a group of cyanobacteria (blue-green algae). Spirulina is cultivated worldwide as a whole food, dietary supplement, or feed supplement in the aquaculture, aquarium, and poultry industries.123 Although it can grow in fresh and saline water, it grows more than other algae and microorganisms in alkaline bodies.124 Spirulina is less susceptible to contamination than other algal species when grown as cultures.
The current production of spirulina is around 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tonnes (dry biomass) per year, and consumed as a superfood.125 Typically, such spirulina is cultivated in scientifically designed algae farms, whereas industries produce it on a large scale in raceway ponds, where wastewater may also be utilised.126
000 metric tonnes (dry biomass) per year, and consumed as a superfood.125 Typically, such spirulina is cultivated in scientifically designed algae farms, whereas industries produce it on a large scale in raceway ponds, where wastewater may also be utilised.126
In India, research and commercial production on Spirulina started in the mid-1900s. It is marketed mainly as developed products, like tablets and capsules, by many pharmaceutical companies. Setting up a small-scale spirulina farm is particularly easy, and it has become common in many regions of India.127
Spirulina grows fast (doubling time is up to 2–3 hours), contains 60–70% (dry weight) protein and comprises almost all the amino acids, including the essential ones.128 A recent work reported the Osborne fractionation of the extracted proteins: albumins 51.5%, globulins 2.4%, prolamins 46.1% and no glutelin.129 Thus, it is an excellent raw material source to produce protein-based bioplastics. Recent works demonstrate the application of spirulina to make bioplastics and different techniques to enhance the mechanical properties of spirulina-derived bioplastics.130
Chlorella vulgaris is another important green microalgae that contains 42–58% (dry weight) of protein and is primarily used as a fish meal.131 This algae can be another excellent source of protein for creating protein-based bioplastics.
3.2.3.3 Fungal biomass. Fungi play an important role in the industrial fermentation processes. Many day-to-day products, including alcohol, glycerol, carbon dioxide, citric acid, gluconic acid, antibiotics, vitamin B12, and riboflavin are manufactured using yeast or mould.132 Fungal-derived mycoproteins can be an important source of protein for bioplastics applications as they have high protein content (30 to 50%) on a dry matter basis;116 cultivation of fungal species occurs at a low cost on agricultural or food residues. Fungal species are resilient to landscapes and can grow in harsh environments. Currently, S. cerevisiae is one of the largely available protein sources at a low cost as it is used during the fermentation process to produce ethanol. A report suggests that around 49% of protein extraction is possible from the residue of the beer manufacturing process.132 Other important fungal species that can produce protein are K. marxianus, C. utilis, Y. lipolytica, and the moulds F. venenatum, A. oryzae, and M. purpureus.117 To date, no reports showcased bioplastics from the proteins obtained from fungal sources.
3.3 Production of protein-based bioplastics
The sourced raw materials undergo major processing steps, which convert them into usable bioplastics (Fig. 2). A detailed description of these steps follows below.Crop plant leaves are a potential protein source. For most industrialised crops, only specific parts of the plants (e.g. roots, flowers and fruits) are harvested and processed. At the same time, the leaves are left unused in large quantities. Some farmers use these as fodder, while others decompose these unused parts. Often, this leads to buildup. Instead, this biomass can be processed and converted to bioplastics. Several crops are ideal candidates for leaf protein extraction. The protein content ranges between 16–29% on a dry basis. Protein extraction from these sources follows a general process of disrupting cell walls to release soluble proteins, followed by fractionation to isolate specific proteins, dissolution and forming/moulding.46
In microalgae, the dissociation of proteins from pigments and polysaccharides is complex. Breaking the microalgal cell wall is challenging and usually expensive, and the process involves one or more combinations of physical, enzymatic, or chemical treatments. Next, the solubilisation of proteins in alkaline solutions occurs. Isoelectric precipitation can extract dissolved proteins.
Similarly, proteins from leaves of aquatic plants are extracted after mechanical disruption to release soluble components, followed by pH treatments or thermal treatments. The resulting extract is finally concentrated or dried. This procedure only extracts half of the total protein in the leaves, while the rest is discarded. A significant fraction of the protein is chemically associated with the cell wall in aquatic plants.
Protein fractionation from seaweeds is intricate due to cell wall mucilage and phenolic compounds, which hinder mass transfer and lower protein extractability. Improved results are obtained when seaweed is processed unconventionally using the techniques used for processing leafy biomass (starting with fresh biomass and applying mechanical pressing and solubilisation of cell contents). Other methods of improving protein yields from seaweed include using fresh seaweed, alternative methods such as supercritical extraction, ultrasound- and microwave-assisted extraction, and enzymatic treatments.
Various methods are employed to extract the proteins present in the plants. Each source of protein requires a different method of extraction and processing. The previous section offered a brief idea of the various potential protein sources; this section aims to discuss different extraction methods. The overall process consists of the following steps:46 (1) disruption of plant tissues, (2) solubilisation of proteins, (3) separation from unusable plant matter, (4) precipitation/purification of proteins, and (5) concentrating proteins. The overall process consists of the following steps.
3.3.1.1 Disruption of plant tissues. Plant cells have rigid and recalcitrant cell walls containing cellulose and lignin, requiring strong mechanical force to disrupt the plant tissues. The diversity of source materials calls for different approaches to disrupting plant tissues. For instance, mechanical dehulling and milling are suitable for plant seeds. At the same time, high-shear blending works better for grasses and leaves. Other physical treatments include autoclaving, microwaves, ultrasonication, cryogenic fracturing, osmotic shock, and pulsed electric fields.46,133 Chemical and enzymatic hydrolysis are also potent techniques for disrupting cell walls. In many cases, a combination of these methods provides satisfactory results.
3.3.1.2 Solubilisation of proteins. Often, biomass contains significant amounts of fats/lipids, which may hinder the protein extraction process unless the biomass is the vegetable oil industry wastes; these require a defatting step before solubilisation of proteins. Defatting or extracting oil from biomass involves using organic solvents such as petroleum ether, ethanol, acetone, n-hexane and n-pentane. Various reported methods exist for the solubilisation of proteins trapped in the tissue matrix of biomass.134–136 A few important ones are discussed below and listed in Table 2.
| Alkaline method | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proteins | Assistive technologies | Solid loading | pH | Temp. (°C) | Process time (h) | Precipitation methods | Yields (%) | Advantages | Disadvantages | Ref. |
| a NR = Not reported.b RT = Room temperature. | ||||||||||
| Rice bran protein concentrate | No | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 41 41 |
9.55 | bRT | 0.5 | Isoelectric precipitation (HCl, pH 4.5, refrigeration, 24 hours) | 11.76 | Relatively easy process without the use of complicated tools | Extracted protein concentrate only has only 36.3% protein; process time is > 24 hours | 137 |
| Rice bran protein concentrate | EAE | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
9 | 90.6 | 4.5 | Isoelectric precipitation (HCl, pH 3.5 to 4.5) | 15.9 | Improved protein yields and extraction | More complicated and expensive than the method listed above | 138 |
| Soy protein isolate | No | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
8 | 70 | 0.75 | Isoelectric precipitation (HCl, pH 4.5, 24 hours) | 49.79 | Alkaline medium was a sanitiser industry waste; thus, cost of chemicals may be low | 139 | |
| Soy protein isolate | No | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
8 | 50 | 3 | Isoelectric precipitation (HCl, pH 4.5) | 41.03 ± 1.96 | EAE reduces possible protein damage and formation of undesired products over conventional alkaline treatment | Possible protein damage and formation of undesired products | 140 |
| Soy protein isolate | EAE | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
12.7 | 50 | 2(enzyme treatment) + 1(alkaline treatment) | Isoelectric precipitation (HCl, pH 4.5) | 45.93 | More expensive process due to the use of an enzyme cocktail | 140 | |
| Duckweed | No | 2 to 4% | 11 | 80 | 2 | Isoelectric precipitation (HCl, pH 4) | 60 | Protein concentrate contained ∼85.0% RubisCO | 141 | |
| Cottonseed | No | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
10.4 | 34 | 2.4 | Isoelectric precipitation (HCl, pH 4.5, 12 hours) | 28 | Protein concentrate contained ∼88.7% protein | 142 | |
| Rice bran protein | PEF (250 pulses/min and voltage 8 kV) | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
10 | aNR | 1 | Isoelectric precipitation (HCl, pH 4.5, 24 hours) | 20.71 ± 3.03 to 22.80 ± 2.04 (% increase in yields over alkaline treatment) | Improved protein recovery without increasing treatment time | Requires PEF equipment, which is expensive | 143 |
| Proteins | Assistive technologies | Process | Temp. (°C) | Process time (h) | Precipitation methods | Yields (%) | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Salt solutions | |||||||||
| Pea protein isolate | No | Pea flour (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) in 0.1 M phosphate buffer, 6.4% KCl; pH 8.00; mixing (500 rpm) 10) in 0.1 M phosphate buffer, 6.4% KCl; pH 8.00; mixing (500 rpm) |
RT | 96 | Supernatant dialyzed; freeze-drying | 81.6 | Simple process; extracted proteins have better solubility over the alkaline method | Lower yields over the alkaline method | 144 |
| Soy protein isolate | No | Soy flour (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) 0.05 M sodium phosphate buffer, 0.8 M NaCl; pH 8.00; mixing (500 rpm) 10) 0.05 M sodium phosphate buffer, 0.8 M NaCl; pH 8.00; mixing (500 rpm) |
RT | 72 | Supernatant dialyzed; freeze-drying | 72.6 | 144 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Organic solvents | |||||||||
| Kafirin | No | Presoak (16 h) 1.0% sodium metabisulphite; then add glacial acetic acid | 25 | 17 | Isoelectric precipitation (HCl, pH 5, 12 hours) | 61 | ∼90% of kafirin recovery | 161 | |
| Kafirin | No | Ethanol (70%), 10% cysteine, 5% SDS, liquor ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, pH 10 7, pH 10 |
60 | 4 | Isoelectric precipitation (40% ethanol, HCl, 1g l−1 H2O2 pH 4.5, 12 hours) | 83% (kafirin); 83% (glutelin) | Recovery of two types of protein. 93% of kafirin; 93% of glutelin recovery | Multistep process | 162 |
| Zein | UAE | 65% ethanol solution, 100 mg ml−1 solid loading, ultrasonic homogenizer (frequency 40 kHz) | RT | 0.25 | NR | 2.09 mg ml | Fast process | Study was done at a very small scale | 163 |
| Zein | No | 70% ethanol solution, 0.25% Na2CO3, HCl to adjust pH to 1, solid loading 100g l−1 | 80 | 5 | Adding water to a 40% concentration | ∼50% | Acidic pH leads to low denaturation of zein and more yield | 164 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Aqueous two-phase systems (ATPS) | |||||||||
| Corn protein | No | 10 g Corn germ in the 50 ml of PEG–NaCl–Na2SO4 system in 0.05 M PBS. | RT | 1.5 | NR | NA | Possibility to tune to process to obtain more hydrophilic or hydrophobic proteins. Specific protein yields of 100% may be achieved | It is a complicated process, mostly used for extracting high-value products from crude protein obtained from other methods | 165 |
| α-amylase from soybean extracts | No | Solid loading (10%) in 18.4% PEG and 21.2% potassium phosphate | NR | Continuous operation (residence time 0.2 h) | NR | 87 | Continuous operation, which may lower the cost | Only suitable for recovery of high-value products | 166 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Subcritical water | |||||||||
| Sunflower protein | No | Solvent-to-feed ratio of 20 (pH 5–6) | 150 | 0.25 | NR | 43 | An environmentally friendly and fast method to obtain water-soluble proteins | Require specialized high-pressure reactors | 167 |
| Soy protein from heat-denatured meal | No | Solid loading 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (w/v) 10 (w/v) |
120 | 0.33 | Isoelectric precipitation (pH 4.5) | 59.5 | Protein purity ∼80%; improved protein recovery over alkaline extraction (16.4%) | 168 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Ionic liquids (ILs) | |||||||||
| Protein from macroalgae | No | Ethyl methyl imidazolium dibutyl phosphate [EMIM][DBP] | 25 | 0.17 | Partitioning by dipotassium phosphate followed by ultrafiltration | 64.6 | Fast process | ILs are expensive; thus, the process is not viable until recyclability is established | 169 |
| Proteins from microalgae | MAE (700 W and frequency 2450 MHz) | Solid loading 2% w/v in choline acetate | 40 | 0.5 | Partitioning by methanol/chloroform solution; aqueous layer was protein-rich | 26.35 | 170 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Deep eutectic solvents (DESs) | |||||||||
| Protein from brewer's spent grain | No | DES (sodium acetate/urea); 10% solid loading | 80 | 2 | NR | 79 | >50 wt% protein in the concentrate | The recyclability of DES was not examined in this study. Require expensive microwave reactor | 171 |
| Protein from microalgae | MAE (160 W) | DES (ChCl/Urea); spirulina loading (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40); chlorella loading (1 40); chlorella loading (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30); pH 12 30); pH 12 |
NR | 0.17 | Isoelectric precipitation (pH 4.5) | 30.48 (spirulina) and 15.53 (chlorella) | Green solvents | 172 | |
| Improved yields over alkaline extraction at similar pH | |||||||||
3.3.1.2.1 Alkaline medium. Proteins are the least soluble near their isoelectric point. At an isoelectric point, protein molecules do not carry any net charges. Proteins gain net positive or negative charges when the pH is away (higher or lower) from the isoelectric point and become soluble.144 Most plant proteins' isoelectric points are in acidic ranges. Therefore, solubilising such proteins in an alkaline medium is the most used method. In such cases, isoelectric precipitation is achieved by reducing the medium's pH (5 to 4.5). Combining these techniques can lead to an efficient process providing maximum recovery of functional, stable, and lipid-free proteins (Table 2).145 On the flip side, highly alkaline conditions may lead to the denaturation of proteins. A recent report suggests that raising the alkaline medium's pH and temperature increases protein solubilisation rates.146 Nonetheless, high temperatures may also precipitate and denature proteins.
3.3.1.2.2 Salt solutions. This method has applications in extracting pea proteins. In this method, proteins are solubilised in a phosphate buffer medium having neutral salts such as sodium chloride or potassium chloride.136 This method is only effective in recovering water-soluble proteins. Dialysis of solution or micellar precipitation gives purified protein solution, which may further undergo concentration steps. The salt-extraction process leads to low denaturation and aggregation of proteins, which are thus more soluble (Table 2).147
3.3.1.2.3 Organic solvents. When the biomass contains lipid-binding proteins and when proteins have a plethora of nonpolar side chains, the organic solvent extraction method may be useful as the organic solvents such as ethanol, methanol, acetone, and butanol show strong lipophilicity and hydrophilicity (Table 2).148 Nonetheless, the organic solvents are toxic and lead to protein denaturation.
3.3.1.2.4 Aqueous two-phase systems (ATPS). ATPS forms when two water-soluble polymers or salt and a polymer are dissolved in water at a higher than their critical concentration, which is why two immiscible phases form.149 Some common ATPS are polyethylene glycol (PEG) with a citrate salt, sulphate or phosphate or mixing two polymers (PEG/dextran system) with water. Partitioning between two phases occurs once the disrupted plant tissues mix in solvents to form ATPS. Partitioning depends on the surface properties of proteins and the nature of ATPS. Most soluble matter partitions to the lower, more polar phase, whilst proteins partition to the top, less polar and more hydrophobic phase, such as PEG. To achieve efficient protein extraction by ATPS, the hydrophobicity of the phase system, the electrical potential between phases, molecular size, and bio-affinity of the proteins can be exploited. This method is suitable for extracting high-value proteins from sources (Table 2).
3.3.1.3 Subcritical water. Subcritical water treatment is an upcoming technique to extract proteins. The process is eco-friendly and does not produce toxic waste. Here, hot compressed water dissolves proteins. Hot compressed water has a higher ionisation constant with heating, has a solution rich in H+ and OH− ions, and acts as an acid/base catalyst, providing high reactivity (Table 2).150
3.3.1.4 Ionic liquids (ILs). ILs are salts or mixtures of salts, often composed of organic cations and organic or inorganic anions that stay molten under 100 °C.151 Physical and chemical properties such as thermal stability, viscosity, and solubility in polar and nonpolar solvents are highly tuneable by varying the composition of cations and anions. Low vapour pressure, high thermal stability, and low flammability of ILs make them nearly ideal solvents for extracting proteins.152 The detailed discussion of this topic is beyond the scope of this review. Nonetheless, the authors recommend recent reviews by Bharmoria et al. and Nunce et al. to the readers for a detailed understanding of the topic.152,153 Though ILs possess excellent extraction capabilities, their large-scale applications are still limited due to their higher production costs and complicated recycling-cum-purification processes (Table 2).154
3.3.1.4.1 Deep eutectic solvents (DESs). DES is a combination of hydrogen bond acceptor (HBA) and hydrogen bond donor (HBD) that form a eutectic mixture via the formation of hydrogen bonds.154 DESs are gaining popularity due to their inherent biodegradability, biocompatibility and low toxicity. DESs depict similar physical properties, including lower volatility, higher viscosity, thermal stability, and non-flammability. Further, the DESs are easy to prepare and show moderate protein extraction capability.154 Recent work suggests that the DES-based protein extraction depends on a few key factors: (1) raw material, (2) liquid-to-solid ratio, (3) type of DES system and its physiochemical properties, (4) molar ratio, (5) extraction temperature, (6) pH, (7) time and (8) the water content.155 Though the DES-based extraction process of proteins shows great promise, more studies are needed to make it comparable to the alkaline extraction processes (Table 2).
Apart from the extraction methods, assistive technologies can improve extraction efficiency by disrupting the cellular walls of plants. We briefly discuss those below.
3.3.1.5 Ultrasound-assisted extraction (UAE). The ultrasound waves (20 kHz to 1000 kHz) generate tiny vacuum bubbles or voids in the liquid, which implode and produce high shear stress (∼50 MPa) and temperature ∼4500 °C. These localised forces disrupt cell membranes, facilitating the extraction of intercellular materials and promoting sonolysis, which can disintegrate organic polymers.156 Application of UAE can improve extraction efficiency by minimising extraction time, energy costs, and solvent consumption, producing more homogeneous mixtures, increasing energy transfer rates, reducing temperature gradients, providing selective extraction, reducing extractor size, enabling faster response and improving process control (Table 2). Nonetheless, the process also comes with several disadvantages, such as it can change the structure of proteins or denature the proteins.
3.3.1.5.1 Microwave-assisted extraction (MAE). In this assistive technology, microwave energy (300 MHz to 300 GHz) pulses heat up the solvents containing biomass, facilitating protein extraction (Table 2).157 The efficacy of MAE depends on solvent properties, the type of biomass, the nature of proteins to be extracted and their dielectric constants. Therefore, the process requires extensive optimisation of the amount and polarity of extracting solvent, biomass loading, extraction temperature, microwave power, pulse duration, and treatment time.158
3.3.1.5.2 Pulsed electric field (PEF)-assisted extraction. PEF is a low-temperature extraction process that uses short-duration, high-intensity pulsed electric fields (0.5 to 40 kV cm−1) from a high current flow inducing electroporation of cell membranes, which destabilises the lipid-bilayer of cells, making it permeable to solvents, which in turn leads to extraction of intracellular materials (Table 2).159 Specific energy input, extraction temperature and particle size are crucial optimisation parameters influencing protein extraction. Nonetheless, further studies may provide an improved understanding of the process.
3.3.1.5.3 Enzyme-assisted extraction (EAE). The EAE utilises enzymes that cleave the intercellular materials that hinder the penetration of solvents into the cells. The process optimisation parameters are particle size, time, pH, and temperature. Enzymatic processes occur effectively at a near room temperature and moderate pH and do not require expensive equipment. However, they may take longer (up to a few hours) than the above-discussed methods (Table 2). A few well-known classes of enzymes are cellulases, pectinases, hemicellulases, and proteolytic enzymes.160
3.3.1.6 Separation from unusable plant matter. Several physical separation methods can effectively remove insoluble plant matter from the solution. The few important ones are decanting, filtration, ultrafiltration, and centrifugation.
3.3.1.7 Precipitation/purification of proteins. Precipitation involves varying the solution conditions such that the protein transitions from a soluble to an insoluble state, allowing the separation of proteins. Several methods exist to precipitate proteins; these target protein solubility, ionic strength, pH modifications, denaturation, and intermolecular interaction modulation. The method of choice depends on the properties of the target proteins, such as their solubility, isoelectric point, and structural stability. Widely used techniques for protein precipitation are isoelectric point precipitation, ammonium sulphate precipitation (salting out-salting in), organic solvent precipitation, thermal precipitation, and polymer precipitation.173
3.3.1.7.1 Isoelectric precipitation. One standard method to precipitate proteins from solution is based on their isoelectric point. At the isoelectric point (pI), the negative and positive charges on the protein surface are in balance.144 This balance enables attractive forces between the positive and negative surface charges, which triggers protein aggregation and precipitation. The pI values of most proteins are in between the pH range of 4 to 7 (Table 2). Adjusting the pH of the solution to the isoelectric point leads to protein precipitation. Mineral acids, including hydrochloric and sulfuric acids, are the most common precipitants for isoelectric point precipitation. However, this method irreversibly denatures protein due to the use of mineral acids.174 Thus, the precipitated proteins are not in their native or biologically active form.
3.3.1.7.2 Salting out using ammonium sulphate. This method exploits the modulation of protein solubility by the ammonium sulphate.175 The solubility of proteins increases when the salt is present in minute quantities (<0.15 M), which is salting-in. But at higher salt concentrations, protein solubility decreases, leading to precipitation; this effect is salting-out. This method is extensively used to recover proteins from bacterial sources.19
3.3.1.7.3 Organic solvent precipitation. Organic solvents such as ethanol, methanol, and acetone decrease the solubility of proteins soluble in aqueous systems as these solvents disrupt the water–protein interactions.176 Further centrifugation or filtration can separate the proteins from the solvent. The process typically works better at lower temperatures, leading to lower denaturation and aggregation. The pH and ionic strength of the solution are also important parameters that affect precipitation efficiency.
3.3.1.7.4 Thermal precipitation. The process exploits the sensitivity of proteins at elevated temperatures.177 When subjected to higher temperatures, proteins denature and exfoliate to expose their hydrophobic regions hidden in the native state. The newly exposed hydrophobic regions then interact with the hydrophilic regions of a protein, leading to the formation of aggregates, which are less soluble and eventually precipitate.
3.3.1.7.5 Polymer-induced precipitation. Similar to organic solvent precipitations, specific water-soluble polymers alter the solution's physicochemical environment such that the proteins precipitate out due to aggregation.178 The common polymers are polyethylene glycol (PEG) and dextran, which induce precipitation.
3.3.2.1 Pre-processing of proteins. Protein conditioning makes them amenable to different commercial moulding techniques. Such conditioning involves the addition of plasticisers, binding agents, crosslinkers, and compatibilisers into the extracted proteins. A detailed description of these additives is available in the next section.
3.3.2.2 Moulding/shaping the proteins into products. Several moulding/shaping processes are currently available (Table 3). The choice of method primarily depends on the nature and shape of the products. These processing methods can handle materials with specific properties; thus, the properties/processibility of proteins must be tweaked by pre-processing to adapt to the particular moulding process. Further, processing conditions play an important role in determining the properties of bioplastics formed; some crucial properties of the material, e.g. strength, get altered based on the processing conditions. Physical characteristics, such as the material's colour, can also be varied by techniques, such as adding colouring agents. The bioplastic blends/doughs undergo mechanical/thermal/electrical moulding to manufacture various shapes and sizes of bioplastics. Some methods might not be efficient, sustainable or economically viable for the available protein. Thus, more research efforts towards developing new combinations of protein-based materials and processing methods are necessary to make their range of applications comprehensive. Below, we discuss a few important moulding processes reported as compatible with protein-based bioplastics.
| Processing techniques | Processable proteins materials | Solvents | Additives | Interaction of product with water | Possible shapes/applications | References |
|---|---|---|---|---|---|---|
| a NR = Not reported. | ||||||
| Injection moulding | Soy protein isolate (91.8% protein) | NRa | Plasticizer (glycerol) | Water uptake in 24 hours ranged between 150 to 270% | Complex shapes such as plates, cups, automobile panels, cases, toothbrush handles | 82 |
| Rice protein concentrate (80% protein) | NR | Plasticizer (glycerol); reducing agent (sodium bisulfite); cross-linking agents (glyoxal and L-cysteine) | NR | 181 | ||
| Pea protein concentrate (89.5% protein) | NR | Glycerol | Water uptake in 2 hours was around 100% | 182 | ||
| Foil extrusion/Cast film extrusion | Wheat gluten (77.7% protein) | NR | Salicylic acid, glycerol, ammonium hydroxide | Normalized solubility of the protein in 0.5% sodium dodecyl sulfate–phosphate buffer and sonication of 3 minutes was 0.2 | Thin sheets, films | 183 |
| Zein | Ethanol (75%) | Oleic acid; distilled mono-glyceride | NR | 184 | ||
| Soy protein isolate (91%) | Plasticizer (glycerol); montmorillonite (nanoclay) to enhance barrier properties; 4-dodecylbenzenesulfonic acid and ethanolamine to adjust pH | 185 | ||||
| Blow moulding | Zein | Ethanol (75%) | Oleic acid; distilled mono-glyceride | NR | Thin-walled bottles, beakers | 184 |
| Soy protein | Water | Starch filler; plasticizer (glycerol); reducing agent (sodium sulfite or sodium bisulfite) | Resistant to water | 186 | ||
| Fibre spinning | Wheat gluten (76.6%), zein (91.6%), soy protein (72.7%), pea protein (80.4%), and rice bran protein (81.2%) | [Cu(NH3)4(H2O)2]SO4 solution | Filler (cellulose nanofibers); coagulation bath (5% H2SO4) | NR | Fibres | 187 |
| Thermo-forming | Wheat gluten (gliadin rich) | Ethanol (70%) | Glycerol | Processed proteins at 150 °C had 76.5 ± 2.5% water uptake and 22.0 ± 1.1% soluble mass loss in 24 hours | Panels of complex contours, luggage trolley bags | 188 |
| Fused deposition moulding | Zein | Glycerol | NR | 3D printed products | 189 | |
| Pea protein isolate solution (86.4%) | Water | NaCl, sodium carboxymethyl cellulose, xanthan gum (XG), carrageenan (car), corn oil; cold plasma treated | NR | 190 | ||
| Compression moulding | Soy protein isolate (90%) | Gelatine, glycerol | NR | Shapes of complex geometries such as video game controller casing, kitchen tools, respirator masks | 191 | |
| Potato protein concentrate (8.1% ± 0.4) | Glycerol | For moulding at 150C, the water uptake was ∼50% and soluble mass loss was ∼40% in 24 hours | 192 | |||
| Solution casting | Cottonseed protein (57.2%) | Water | Denaturing agent (urea); crosslinking agents (formaldehyde, glyoxal, glutaraldehyde); plasticizer (glycerol) | Water uptake was 30 to 40% in 1000 minutes | Optical lenses, medical tubings, prosthetics | 97 |
| Soy protein isolate (92%) | 30% acetic acid aqueous solution | Glycerol | SPI coated paper board showed ∼60% water uptake in 0.5 hours | 193 | ||
3.3.2.2.1 Injection moulding. Injection moulding is a common processing method used to process synthetic polymers.181 It involves melting thermoplastic polymers and injecting the molten polymers into a mould cavity under high pressure. Ejection of the moulded part follows once cooled and solidified. This process is preferred to produce plastic parts for the automotive, electronics, and packaging industries due to its ability to produce complex shapes with excellent dimensional accuracy and minimal waste. Key factors influencing the quality of injection-moulded products include mould temperature, injection pressure, cooling time, and material properties.194
In the context of bioplastics, injection moulding has been used to produce pea protein-based and rice bran-based bioplastics. A homogeneous blend of pea-protein isolate and glycerol is obtained by mixing in a mixer and injecting the dough-like blend into moulds to obtain bioplastics.67 First, a bioplastic blend is produced by adding solvents such as water, plasticizers, reducing agents, compatibilizers, and crosslinking agents into the extracted protein to obtain good processibility of blends. Then, injection moulding at an elevated temperature follows to favour filling the mould cavity and crosslinking of bioplastic blend in the mould.87,181
3.3.2.2.2 Foil extrusion/cast film extrusion. Extrusion is a crucial polymer processing technique widely employed in modern manufacturing. It is commonly used for processing synthetic plastics such as low-density polyethylene (LDPE) films. An extruder functions as a specialized continuous high-temperature short-time (HTST) reactor, where raw materials are continuously fed into a hopper, transported by a rotating screw, and forced through a die to achieve the desired shape. The process may involve various operations, including heating, cooling, feeding, conveying, compression, shearing, chemical reactions, mixing, melting, homogenization, amorphization, cooking, and shaping.195
Foil extrusion and cast film extrusion are specialized processes within polymer extrusion that produce thin plastic sheets. They are as follows.
∙ Foil extrusion: produces very thin plastic sheets (foils) with high precision, often used in packaging, insulation, and electrical applications. The extruded polymer is typically calendered (rolled) to achieve the desired thickness and surface properties.
∙ Cast film extrusion: in this method, molten polymer is extruded through a flat die and then rapidly cooled on a chilled roller to form a uniform film. Cast films are widely used in food packaging, medical applications, and protective coatings due to their excellent clarity, gloss, and mechanical properties.
Extrusion has been used to produce bioplastics from wheat gluten, whey protein, soy protein and sunflower protein isolate (SFPI).195 For instance, SFPI obtained by alkaline extraction and centrifugation can form a bioplastic blend with water and glycerol. This blend can undergo a screw extrusion to produce the film under carefully maintained conditions.196
3.3.2.2.3 Blow moulding/film blowing. Film-blowing techniques have moulded many common synthetic polymers. The blow moulding process requires polymers to have good melt strength, thermal stability, and limited swelling. The parison (the hollow bulb of plastic used in blow moulding) must endure its weight as it will hang and expand before the hollow plastic surface reaches the mould.197 A recent report suggests that bioplastics made from zein protein can use this technique to produce films with low thicknesses and suitable mechanical properties for packaging applications.198
3.3.2.2.4 Fibre spinning. Fibre spinning transforms raw materials, often liquids or solids, into continuous fibres, which can find applications from textiles to industrial products. Traditional fibre spinning involves processing synthetic and natural polymers into fibres through dry, wet or melt spinning. These processes include drawing out and stretching the material until it forms a continuous thread.199
Fibre spinning can also be apt for preparing protein-based fibres. Current spinning technologies allow the spinning of fibres from cellulose nanofibers and various plant-derived proteins, including wheat gluten, zein, soy protein, pea protein, and rice bran protein. The microfluidic spinning technique produced plant protein fibres with smooth surfaces, strong mechanical properties, high thermal stability, antioxidative activity, good digestibility, and low sensitization.187
3.3.2.2.5 Thermoforming. Thermoforming is a process used to shape thermoplastic materials by heating them until they become soft and pliable, followed by moulding them into a desired shape using a mould. Once the material cools, it retains the shape of the mould. Bioplastics can be processed using this technique to obtain the required shapes. The bioplastic material obtained, usually as a sheet, is heated till it becomes soft and flexible. Once the material is pliable, it is either vacuum-formed, pressure-formed, or mechanically-formed. As the material takes the shape of the mould, it also cools and hardens; after that, the part is retrieved from the mould.
Thermal compacting is a widely used method to process soy protein isolate (SPI) – a highly refined soy protein made from soy flour. The process involves the preparation of an appropriate blend of protein and plasticisers, filling the blend into the cavity of the stainless steel moulds, and moulding blends into shapes by a bench-top press at high temperatures and pressure. After the pressure release, the moulded sample cools for a predetermined time, and the final product comes from steel moulds.8
Hot press moulding involves high pressure to compress the material into a mould. It is suited for thicker 3D shapes or parts that need structural strength. Bioplastics made from cottonseed protein can be processed using this technique. Cottonseed flour is first dissolved in deionised water, followed by the addition of urea solution. The mixture is then agitated to obtain the denatured cottonseed protein (DCP). A cross-linking agent (formaldehyde, glyoxal, or glutaraldehyde) is added. The resulting mixture is dried, plasticizer (glycerol) is added to the dried denatured cross-linked protein, and the mixture is homogenised. The mixture is then ground, processed, and conditioned before hot-press moulding. The mould formed is then cooled to room temperature.97
Thermo-mechanical moulding of the samples is performed to prepare the bioplastics from proteins obtained from microalgae and spirulina algal biomass. A bench-top press with electrically heated and water-cooled platens is used. Compression moulding of samples is carried out at 150 °C followed by a 10-minute cooling period, and both are performed under high pressure.200
3.3.2.2.6 Fused deposition moulding (FDM). This method also popular as additive manufacturing. This technique involves a layer-by-layer deposition of the bioplastic polymer in a mould to develop a solid 3D structure using a computer program. The process involves extruding a thermoplastic protein blend through a nozzle at temperatures greater than its glass transition temperature onto a substrate. This deposited material is then cooled quickly below the glass transition temperature, after which an additional layer is deposited on this cooled material, forming the final solid mass. Bioplastics made from zein protein have been used to create 3D shapes using the FDM approach. Zein is plasticised using a suitable plasticiser and extruded through a nozzle at 130 °C, a temperature above the glass transition temperature of the bioplastic, following which the deposition process was carried out. The zein plasticised blend displayed suitable thermochemical properties to employ this method to produce various shapes.189
3.3.2.2.7 Compression moulding. A recent report has shown the application of compression moulding to prepare bioplastic samples from duckweed blends. The equipment had electrical heating and water-cooled plates. Duckweed is harvested and dried at high temperatures to reduce its moisture content. The samples undergo a milling process to end up as a powder, which then gets thoroughly mixed with high-purity glycerol. The dough gets compressed and takes shape in the stainless steel moulds at an elevated temperature. After cooling, the as-prepared products come out from the mould.110
Compression moulding has been utilised to produce SCP films. The flaky protein sample is first ground to make a powder using a mortar and pestle. Glycerol is added to the powder and ground thoroughly to ensure uniform mixing. The mixture is then allowed to dry at room temperature for a few hours. After that, the material is again ground with the mortar and pestle to obtain a fine powder of the final protein/glycerol mixture. The powder undergoes a compression moulding in a press. Brown, translucent, and flexible films are obtained.118
3.3.2.2.8 Solution casting. Casting exploits the chemicals' ability to disrupt disulfide bonds, which improves intermolecular interactions and enhances mechanical properties. This process allows the formation of a new three-dimensional structure.201,202
Casting can be used to form bioplastic from various proteins. Proteins in an appropriate solvent are first heated, resulting in partial or complete protein denaturation. Applying heat, shear, or extreme pH often achieves partial protein denaturation. These weakens the 3D structure of the protein, exposing previously hidden functional groups such as carbonyl, amide, and disulphide. Functional groups become available for intermolecular interactions, forming a 3D protein network entrapping film components during protein aggregation and drying. After dissolution, the mixture is spread on nonsticky platforms and allowed to dry to form the film.8,72
Despite the ease of operation, there are several casting methods. It can sometimes lead to films of varying thicknesses. Due to the evaporation of volatile solvents, the thickness of the film reduces after drying, and this difference needs to be accounted for any specific applications.
4. Additives to tailor properties of protein-based bioplastics
The mechanical properties of a polymer provide information to assess the suitability of the polymer for the intended purpose. Here, we compare the mechanical properties such as tensile strength, elongation at break and modulus of elasticity of various plant-based protein bioplastics and traditional petroleum-based plastics (e.g. polypropylene), allowing us to judge the appropriateness of the material for use in multiple applications. The criteria for the design and construction, as well as the usage and lifetime of the product, can be predicted with the help of these properties. They also show us the feasibility of replacing traditional plastics with protein-based bioplastics.4.1 Plasticisers
Plasticisers are essential in bioplastic manufacturing, as they play a crucial role in engineering the material's physical properties, including viscosity, strength, and elasticity. Raw protein-based bioplastics are often rigid and crystalline due to high amounts of hydrogen bonds, making them susceptible to cracking under stress. We can adjust these properties by adding plasticisers to improve ductility for meeting specific performance requirements. Plasticisers play the following roles:203It is necessary to select a plasticiser that satisfies specific criteria to optimise the performance of the plasticiser:206
The plasticiser efficiency parameter generally informs about the relative amount of plasticiser required to tune a specific property into desirable limits, such as a reduction in glass transition temperature. The plasticiser efficiency parameter may be obtained using the equation:207
Plasticisers may be of two types: internal plasticisers and external plasticisers. Internal plasticisers incorporate functional groups into the protein matrix via acetylation, succinylation and Maillard reactions with monosaccharides.208 These functional groups create steric hindrance among different protein chains, which leads to greater free volume and better flexibility than pure proteins. External plasticisers are small molecular weight additives that solvate and lubricate the protein matrix. Common external plasticisers reported for protein-based bioplastics are polar plasticisers and amphiphilic plasticisers.
Polar plasticiser molecules form hydrogen bonds with amide groups of protein molecules. Polyols (glycerol, propylene glycol, polypropylene glycol, sorbitol), sucrose, and water are common polar plasticisers. By their highly hydrophilic nature, polar plasticisers reduce internal hydrogen bonds among protein molecules and, thus, increase the spacing and reduce attraction forces between protein molecules. Glycerol is the most widely reported polar and biodegradable plasticiser, especially for food packaging applications. Due to the weak hydrogen bonding with protein molecules, glycerol readily migrates through the protein matrix, comes to the surface, and leaches out of the protein matrix readily. Further, the hydrophilic nature of polar plasticisers increases the hygroscopic nature of protein films, which may limit the applications of protein-based bioplastics.209
Amphiphilic molecules consist of hydrophobic and hydrophilic groups on either end (e.g., lipids, phenolic compounds, and surfactants) or regions (e.g., biological macromolecules). Protein molecules first adsorb these plasticisers due to hydrophilic interactions of their polar groups and later develop binding with protein via hydrophobic interactions. Fatty acids such as oleic, palmitic, stearic and linoleic acids are effective plasticizers in protein-based bioplastics.209 They reduce the water vapour permeability of protein films and impart antioxidant and antimicrobial characteristics to protein films.210 Nonetheless, due to their limited compatibility with protein, they reduce the strength of protein films beyond a certain percentage.211
4.2 Compatibilisation
Discussion in the previous section shows that the performance of protein-based biopolymers must be increased considerably, and no single protein discussed possesses all the required properties needed for specific applications. Thus, combining different proteins and additives can synergistically augment the bioplastic properties at the desired levels. Physical blending, such as melt compounding, solution blending, and latex mixing by rolls, is relatively cheap, fast, and commercially used to create polymer blends. However, many biopolymers are incompatible due to coarse morphology and limited interfacial adhesion, leading to phase-separated polymer blends. Therefore, compatibilisers are added to the mix to tailor the second phase size and distribution, the interfacial adhesion, and the macroscopic physical-mechanical properties to achieve the desired macroscopic properties. Several reviews discuss the compatibilisers in detail.212,213 Here, we aim to provide a brief overview of the compatibilisation. The methods to achieve compatibilisation are divided mainly into three groups: (i) non-reactive compatibilisation, which involves co-solvents or block-copolymers or graft-copolymers; (ii) reactive compatibilisation, where in situ processing leads to the formation of copolymers; and (iii) nanofiller-induced compatibilisation (Fig. 3). The understanding of the efficacy of compatibilisers for protein-based bioplastics production is still limited. | ||
| Fig. 3 Types of compatibilisation and their mechanism of action. (A) Non-reactive compatibilisation, (B) Reactive compatibilisation, and (C) nanofiller-induced compatibilisation. | ||
Nonetheless, a recent study showed the use of glycerol monostearate (GMS) and soy lecithin as compatibilisers in the blend of 60% poly(butylene succinate) (PBS) and 40% of plasticised whey protein (PWP). The work also modified the whey protein with oleate and laurate groups. In this study, the authors found that the Soy lecithin and the modified whey protein were effective compatibilisers for the PWP/PBS blend and resulted in a significant increase in elastic modulus, tensile strength and elongation at break over the not compatibilised blend.214 Another study reported the reactive compatibilisation using dibenzoyl peroxide (BPO) and hexanediol diacrylate (HDDA) in the polymer blend of PBS and soy protein isolate (SPI). This compatibilisation led to a 46% increase in tensile strength and a 55% increase in Young's modulus over the not compatibilised blend.215 Thus, compatibilsation can be an effective strategy to achieve desired properties with blended protein-based bioplastics.
4.3 Crosslinkers of proteins
The crosslinking of proteins is an essential process that produces structural changes by interconnecting the segments of different protein chains to improve their mechanical and microscopic properties and incorporate functional or application-oriented modifications.216 There are four reported methods to incorporate crosslinks in proteins.A previous effort showed that the water vapour annealing of silk protein films can increase the relative content of beta-sheet structures from 23 ± 2% (for untreated coatings) to 58 ± 5% (for coatings exposed to water vapour for 12 hours). Water vapour annealing promotes beta-sheet formation, thus increasing the crystallinity and reducing water vapour and gas permeability.
Physically crosslinked proteins are generally weaker than chemically crosslinked proteins and break under deformation. Such breakage of the physical crosslinks dissipates strain energy that improves the mechanical toughness of polymers.218 Under cyclic loading and unloading, the dynamic rupture of the physical crosslinks gives rise to residual strains and mechanical hysteresis. Creating a physically crosslinked protein with high elasticity (low mechanical hysteresis) and toughness is one of the significant design challenges.
| Natural crosslinkers | |||||
|---|---|---|---|---|---|
| Crosslinkers | Source | Chemical structure | Working mechanism | Reported proteins | Ref. |
| Genipin | Fruits of genipa americana, gardenia fructus and gardenia jasminoides |  |
Genipin acts upon primary amine groups. First, it reacts to an amine group in the polymer chain to α, β-unsaturated, leading to an open ring of genipin. Next, another amine group from the polymer chain attacks the methoxycarbonyl group to produce a secondary amide-type linkage, leading to a crosslink | Pea protein, whey protein isolate | 220–222 |
| Citric acid | Citrus fruits | 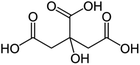 |
A slightly alkaline pH promotes the crosslinking of proteins with citric acid. The mechanism of crosslinking is still unclear. Previous work suggests that the carboxyl group of a citric acid molecule and the nucleophilic amine groups and carboxyl groups present in proteins lead to the formation of amide bonds | Wheat gluten | 223 and 224 |
| Nordihydroguaiaretic acid (NDGA)/masoprocol | Leaves of Larrea tridentata |  |
The two catechols in NDGA oxidise at neutral/alkaline pH producing reactive quinones, which couple via aryloxy-free radical formation and oxidative coupling, forming bisquinone crosslinks at each end | Collagen | 225 |
| Procyanidins | They are condensed tannins of oligomeric nature found in many fruits, vegetables, seeds, nuts, flowers, and barks |  |
It binds to the proline residues, through hydrogen bonding and hydrophobic interactions between carbonyl and hydroxyl groups | Pea protein isolate, faba protein isolate, lentil protein isolate, soy protein concentrate | 226 and 227 |
| Tannic acid | Tannic acid is a polyphenol found in most of the aerial plant tissues |  |
Phenolic hydroxyl groups of tannic acid can react with lysine, tyrosine, and cysteine. The covalent bonding between phenolic compounds and proteins occurs via multiple steps. First, the oxidation of phenolic groups occurs under alkaline conditions, forming quinone intermediates, which then react with nucleophiles from the amino acid groups and create a network of chemical crosslinks by the formation of new C–N bonds by Michael addition or schiff-base reactions | Soy protein, corn protein, pea protein | 228–232 |
| Vanillin | Vanilla bean extract |  |
Aldehyde groups in vanillin react with the primary amine of the proteins or polysaccharides having amine pendant groups through a schiff-base reaction. In contrast, its hydroxyl group forms hydrogen bonds with the hydroxyl or the amino groups in another protein molecule forming the crosslinking network | Chitosan/gelatin composite | 233 and 234 |
| Phytic acid | Plant seeds, cereal grains, nuts, and legumes |  |
It has many anions (O−) that can react with metal ions and proteins having a positive charge (such as gelatin) to form ionic bonds | Gelatine, collagen | 228 and 235 |
| Caffeic acid | Coffee, wine, tea, and propolis resin |  |
The hydroxyl groups present in the benzene ring of caffeic acid oxidise by molecular oxygen to form a quinone, which reacts with the N-terminal α-NH2 or ε-NH2 side chains from arginine, lysine and hydroxylysine residues in proteins to form covalent bonds of C–N, with the regeneration of hydroquinone. The hydroquinone re-oxidises and binds to another protein molecule, leading to a crosslink | Soy protein isolate | 236 and 237 |
| Epigallocatechin gallate (EGCG) | Green tea |  |
EGCG depicts the cross-linking effects on proteins by hydrogen bonds, which change the secondary and tertiary structures of protein macromolecule | Soy protein isolate | 238 and 239 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Synthetic crosslinkers | |||||
| Carbodiimide agents (EDC/NHS) |  |
EDC activates the carboxylate moiety of aspartate or glutamate residues, forming the O-acylisourea group. NHS converts O-acylisourea group into an NHS-activated carboxylic acid group, which readily reacts with amine groups and other amino acids | Wheat gluten | 240 and 241 | |
| Epoxy compounds (examples: PEGDE, BDDGE) |  |
Epoxy compounds having two or more epoxy functional groups can react with amino, carboxyl, and hydroxyl groups depending upon the pH of the protein solution and crosslink proteins | Collagen, gelatine, silk fibroin | 242 and 243 | |
| Polysaccharide derivatives containing aldehyde groups (example: dialdehyde starch) |  |
Aldehyde groups in the polysaccharide chain react with free amino groups of the protein molecules during the cross-linking reaction | Soy protein isolate | 244 and 245 | |
| N-Hydroxysulfosuccinimide and aryl sulfonyl fluoride (NHSF) |  |
N-Hydroxysuccinimide (NHS) ester reacts to the amine present in lysine, planting the reagent at specific localisations on protein. When the pendant aryl sulfonyl fluoride is present in proximity to the target residues, the increased effective concentration enables aryl sulfonyl fluoride to react with multiple weakly nucleophilic amino acid residues (histidine, serine, threonine, tyrosine, and lysine) | Single-cell protein | 246 | |
| Glutaraldehyde |  |
It also has two aldehyde groups, which can react with amine groups present in the protein chain | Wheat gluten, soy protein | 247–249 | |
| (Hydroxymethyl)phosphines (examples: β-[tris(hydroxymethyl) phosphino] propanoic acid (THPP) |  |
(hydroxymethyl)phosphines (HMPs) undergo Mannich-type condensation with primary and secondary amines of amino acids to form protein–protein crosslinks | Single-cell protein | 250 and 251 | |
| Tetrakis(hydroxymethyl) phosphonium chloride) (THPC) |  |
||||
| Cyclic anhydrides |  |
Cyclic anhydrides react with an amine group to form an amide through nucleophilic acyl substitution. Anhydride reacts with alcohol to form an ester known as an acylation reaction | Gelatin | 252 and 253 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Photo-crosslinkers | |||||
| Methacrylate chemistry | Chemical conjugation of methacrylate groups in proteins is possible by reacting proteins with chemicals such as 2-isocyanatoethyl methacrylate and glycidyl methacrylate. Methacrylate pendent groups readily react and form in the presence of suitable photoinitiators such as 2-hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone or lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) and UV light | Silk fibroin | 254 and 255 | ||
| Diazirine-based photo-crosslinkers |  |
Diazirine reacts with polar residues upon the UV irradiation of ∼365 nm wavelength. Diazirine-conjugated amino acids (such as leucine, isoleucine, and methionine) are used for protein–protein crosslinking | Single-cell protein | 256 and 257 | |
| Quinone Methides (QMs) |  |
QMs are electrophilic reactive intermediates that react promptly with nucleophilic amino acids (Cys, Lys, His, Asp, Glu, Tyr, Ser, Thr and Hyp) present in proteins via the Michael reaction. A previous work reported a heterobifunctional cross-linker denoted as ‘NHQM’ realised by reacting a photocaged quinone methide (PQM) and NHS-ester. NHS-ester reacts with the lys residues and quinone methide reacts to the adjacent nucleophilic residues upon exposure to the UV light | Single-cell protein | 258 | |
The enzymatic method of crosslinking allows highly selective intermolecular covalent bond formation under mild reaction conditions. Thus, enzymatic crosslinking finds major applications in the food and nutraceutical industry to change the food texture and enhance food stability. As listed, many enzymes can crosslink proteins; however, microbial transglutaminase (MTGases), often called ‘meat glue’, is a commercially available and employed enzyme in the food industry. It acts on glutamine and lysine residues of proteins and peptides at an optimum pH of 5 to 8 and at 50 °C (Fig. 4).261 A recent report suggests that MTGases effectively crosslink plant proteins such as pea and whey.262 MTGases are calcium-independent enzymes that are often expressed in the cultures of Streptoverticillium mobaraense, which is a spore-forming bacterium.263 However, reports suggest that Streptoverticillium cinnamoneum, Actinomadura sp., Streptoverticillium ladakanum, Bacillus circulans, Streptomyces sp. can also express MTGases.264 For detailed information about MTGase, please refer to the review by Fatima et al.265 USA FDA (Food and Drug Administration) has considered MTGase as ‘Generally Recognised As Safe’ (GRAS) since 1998 and allowed its consumption as a food additive.266
 | ||
| Fig. 4 MTGase catalyses lysine and glutamine residues of protein to yield ε-(γ-glutamyl) lysine cross-link, isopeptide amide bond. | ||
A recent report suggests that the MTGase-treated hemp protein films have four times higher Young's modulus than untreated films. The treated films also display higher hydrophobicity, lower water solubility, and lower swelling than untreated films.267 Another work showed that the amylose blended with MTGase-treated argon protein films had better barrier properties against water and carbon dioxide.268 Though enzymatic crosslinking is not widely popular for the manufacture of protein-based bioplastics, the report suggests that it can be a useful technique for the manufacturing of protein-based bioplastics as the use of chemical crosslinking reagents may become less viable due to their potential toxicity when used for the production of food-grade bioplastic films.
Previous work compared collagen's fibre-like aggregation and gelling in a neutral solution by applying heat and UV irradiation.275 The authors used 37 °C temperature for thermal crosslinking and at 254 nm wavelength at an intensity of 5.0 × 10−3 W cm−2 UV lamp for UV irradiation. They radiated UV (5 times) for 30 minutes each with a 60-minute gap between exposures to ensure the solution temperature did not shoot over 10 °C. The authors found that the thermal crosslinking of collagen was better and faster than UV-based irradiation. Another work showed that e-beam radiation can crosslink silk protein films to make them act as a negative photoresist (it crosslinks at receiving an appropriate radiation) via water radiolysis, which induces the amorphous-to-helix structural change of the silk protein, making it water-insoluble.276 Another group of researchers studied the mechanism of bi-tyrosine formation at different pH solutions using GYG-model peptide and pulse electron beam irradiation.272 Though several reports have put forth different protein crosslinking pathways using ionising radiations, variations exist depending on the type of protein and solvent/co-solvent systems used, along with the kind of radiation employed and pH of the solution.272 The understanding of radiation chemistry that forms protein crosslinks is still in its infancy. Nonetheless, radiation-based approaches can be conducive to the continuous manufacturing of protein-based bioplastics because it is a purely physical process, making it a chemical/reduce-free technology. Radiations are tuneable and organisable; thus, they allow modulation of crosslinking parameters to achieve product-specific crosslinking.277
5. Important properties of bioplastics
5.1 Tensile strength
Tensile strength measures the pulling force a material can withstand before breaking apart. It is one of the most important and studied properties of bioplastics, including the derivation of stress–strain curves. Using this property, one can predict the conditions under which the material undergoes a rapid decrease in elongation of break, thereby specifying the parameters for the safe use of materials.The tensile strength of bioplastics is generally lower than that of synthetic polymers such as polypropylene (Table 5). Rice bran and wheat gluten-based bioplastics display the lowest tensile strength among protein-based bioplastics. In contrast, soy-based bioplastics have a higher tensile strength than them.58,87,285 However, a trade-off exists between the tensile strength and stretchability of the bioplastics. The higher the tensile strength of the bioplastic, the lower the elongation at break of the bioplastic. Adding a plasticiser to the bioplastics increases the ductility of the bioplastic while reducing the tensile strength.285–287 The optimised addition of plasticisers to the bioplastics creates bioplastics with reasonable ductility while retaining their tensile strength (Table 5).
| Bioplastic (at room temperature) | Tensile strength (MPa) | Modulus of elasticity or Young's modulus (MPa) | Elongation at break (%) | References |
|---|---|---|---|---|
| a NR = Not reported. | ||||
| Polypropylene for reference | 31.0–41.4 | 1140–1550 | 100–600 | 283 |
| Wheat gluten (glycerol/water) | 0.5–1.6 | 6–20 | 50–250 | 58 |
| Pea protein (glycerol) | 3–5 | 50–180 | 50–75 | 11 |
| Rice bran (glycerol) | 0.057–0.27 | 4.33–31.3 | 2.1–2.8 | 36 |
| Rice bran (sorbitol) | 0.263–1.27 | 30–200 | 0.65–3.93 | 36 |
| Cottonseed (glycerol/Sisal fibre/dialdehyde starch) | 3–22 | 15–150 | 7–75 | 284 |
| Soy protein flour | 8–13 | 200–800 | 4–23 | 285 |
| Zein (oleic acid/distilled mono-glyceride/ethanol) | 3–4.7 | 90–215 | 22.5–127 | 184 |
| Sunflower protein (glycerol/water/NaOH) | ∼4 | 0.58 | ∼24 | 90 |
| Sunflower protein (potato starch/Glycerol/water) | 3.6–5.7 | NRa | 3.7–5.4 | 91 |
| Elastin-like protein expressed from bioengineered bacteria (glycerol/CHO-PEG-CHO | 290–45 | NRa | 7–110 | 21 |
| Single cell protein (glycerol) | 0.5–1.4 | 22–25 | 4–9 | 118 |
| Soy protein and zein (olive stone powder/Microfibrillated cellulose/ | 10–45 | 310–556 | 5–165 | 34 |
5.2 Elongation at break
The elongation at break is a measure of the maximum strain that the material can withstand. Along with the tensile strength of the material and the modulus of elasticity, we can describe the stress–strain behaviour of the bioplastic using this property, which in turn defines the operating conditions of bioplastic material. The elongation at which bioplastics break is considerably less than that of polypropylene (Table 5). Soy-based bioplastics with glycerol as plasticiser have an elongation at break of only 4%. Bioplastics made out of sorbitol and a combination of plasticisers using sorbitol and glycerol have shown higher elongation at break at 16% and 23%, respectively. Wheat gluten-based bioplastics have the highest elongation at break 250% for bioplastics.An inverse correlation exists between tensile strength and elongation at break. Soy-based bioplastics display a high tensile strength but remain brittle even with an added plasticiser like glycerol or sorbitol. Previous work showed the effect of the plasticiser concentration on the mechanical properties of the protein-based bioplastics made from the proteins extracted from the algae Spirulina platensis with glycerol at varying concentrations.287 The study showed a ∼4% decrease in the tensile strength with an increase in the plasticiser concentration of 5% while simultaneously showing a ∼112% increase in the elongation at the break of the bioplastic.
The addition of compatibilisers such as maleic anhydride and PVA, poly(butylene adipate-co-terephthalate) with 4,4′-methylene diphenyl diisocyanate can improve the mechanical properties of bioplastic by increasing the adhesion or reducing the interfacial tension between two phases of immiscible polymer blends or between the polymeric chains and smaller particle dispersions.212,213,288
5.3 Glass transition temperature
The glass transition temperature (Tg) is an essential property of thermoplastics and defines its moulding conditions. The glass transition temperature is the temperature below which the material is glassy and rigid and above which the material is flexible and rubbery. Glass transition temperature effectively acts as the limiting temperature for moulding and production of various items using a bioplastic.Proteins to behave like thermoplastics would require protein chains to denature, disassociate, unravel, and re-align, which is possible only if the amino acid chains in protein are free to move or mobile – enabling proteins to flow under thermal and shear stress, moulding to the different shapes. Proteins are semi-crystalline in nature. Thus, they show a glassy transition and melting point. Nonetheless, most proteins have very close glass transition and decomposition temperatures. Table 6 shows the critical factors that dictate the glass transition temperature of a protein. The table shows that adding moisture or plasticisers can increase the mobility in protein chains. Studies indicate that the moisture content of the bioplastic-post moulding reduces the glass transition temperature of the bioplastic. Placing the bioplastic in an environment with high relative humidity (RH) also decreases the glass transition temperature of the bioplastic polymer. Water molecules in the bioplastics modify the three-dimensional organisation of the polymer, reducing the intermolecular forces of attraction while increasing free volume and chain mobility.87 The Tg decreases with the logarithmic increase in the percentage of moisture content.290 The plasticiser also influences the glass transition temperature of a bioplastic added to the bioplastics. The work from P. Tummula et al. suggests that glycerol and sorbitol lower the glass transition temperatures of soy-based bioplastics.285 Other additives, including reducing agents, such as sodium sulfite, can disrupt cysteine/cysteine disulfide linkages; surfactants, such as sodium dodecyl sulphate, can disturb hydrophobic interactions, and protein denaturants, such as urea, can unfold native structures.289
| Mobility-reducing factors in proteins (↑Tg) | Mobility-increasing factors in proteins (↓Tg) |
|---|---|
| Bulky side residues | Additives like plasticisers and moisture |
| Stiffening groups | Flexible main groups |
| Chain symmetry | Dissymetry |
| Polar groups | Non-polar groups |
| Crosslinking |
5.4 Water uptake capacity
A bioplastic's water uptake capacity measures the amount of water it absorbs. The hydrophilicity of the proteins, the processing temperature and the plasticisers used affect the water uptake capacity of the bioplastic.32 Soy bioplastics, due to their hydrophilic nature, have a higher water uptake capacity than wheat gluten bioplastics and rice bran bioplastics.31,32 For soy-based bioplastics, the greater the processing temperature, the lower the water uptake capacity.32 However, processing pressure does not influence this property.31,32 Zein protein is sparingly solubility in water, mainly because of its high content of hydrophobic amino acid residues like leucine, alanine, and proline.291The water uptake capacity, in turn, influences multiple mechanical properties of the bioplastic, like its tensile strength, glass transition temperature, and lifetime, and, as a result, the suitability of that bioplastic for a specific application. Thus, the water uptake capacity is vital information for optimal utility in commercial settings.
5.5 Biodegradability
Petroleum-based plastics that are currently widely used are not biodegradable. Often, recycling of these plastics is inefficient, expensive, and uncommon. At the end of lifespan, these plastics either end up buried in a landfill or incinerated, and both options are detrimental to the environment. The OECD estimates that only 9% of global plastic waste is recycled globally. At the same time, the rest is disposed of mainly through large-scale dumping in landfills, leading to the accumulation of plastic and the pollution of the ecosystem.On the other hand, bioplastics produced using plant matter are biodegradable. The degradability is governed by factors such as the source of raw material used, the chemical makeup of the bioplastic,292 the processes used for formulation and processing, the ambient conditions to which the bioplastic is exposed, such as temperature, the microorganisms present in the soil, and the pH value of the soil.293 Generally, biopolymers higher in molecular weight, crosslinking, hydrophobicity, degree of substitution of functional groups per monomer unit, and crystallinity are also more resilient to biodegradation.294,295 Table 7 lists major factors that may affect the biodegradation of biopolymers.294,295 Often, some factors affect a material more than others. For instance, the biodegradability of seaweed films remains unaffected by the amount of plasticiser used for formulation, but it depends on the other factors mentioned previously.286,296
| Material factors | Medium related factors | |
|---|---|---|
| Physicochemical factors | Biological factors | |
| Molar mass | Moisture content pH value | Microbial activity |
| Polymer composition | ||
| Steric configuration | Temperature | Microbial diversity |
| Size, shape and surface area | ||
| Melting and glass transition temperature | Availability of oxygen | Microbial population density |
| Polymer crystallinity | ||
| Porosity | Availability of nutrients | |
| Material thickness | ||
| Additives | Redox potential | |
| Fillers | ||
The presence or absence of oxygen can also influence the rate of decomposition of bioplastics, depending on whether the degradation of the bioplastic takes place aerobically or anaerobically. Generally, anaerobic biodegradation of bioplastics is a faster process than aerobic decomposition. However, bioplastics like PCL are found to decompose only aerobically.297 Another critical factor affecting the degradability rate is protein concentration in the bioplastic. Bioplastics made from soy have a higher protein concentration than pea protein, affecting biodegradability. Soy-based bioplastics have a higher rate of degradability than pea-protein-based bioplastics.298,299
The moulding conditions of the bioplastics can increase the strength of crosslinking between the bioplastic polymer stands, thereby increasing the mechanical stability of the polymer and, in turn, slowing down the degradation rate.298 There are two broad stages in the biodegradation of bioplastics. First, there is a breakdown of the structure of the bioplastic due to the attack of agents of degradation on the surface of the bioplastic, resulting in the fragmentation of the long chains of proteins into smaller chains and then soluble molecules of smaller molecular weight forms. The final breakdown of the smaller molecules into water, methane (in case of anaerobic decomposition), carbon dioxide (in case of aerobic decomposition), and biomass.300 Proteins usually degrade faster than other bioplastic candidates, such as polysaccharides, PLA, and biodegradable polyesters. The ease of water penetration in proteins allows microorganisms to penetrate inside the protein matrix. Proteins decompose to amino acids under the action of proteolytic enzymes secreted by microorganisms. Amino acids are also the nutrients for the microbiome in the soil; therefore, proteins also stimulate the microbiome, further expediting the degradation process.
The proteolytic activity of enzymes depends on the medium's pH, such as soil. The most common proteases are neutral (pH 5 to 8) and alkaline (pH ∼10). Proteolytic degradation kinetics is a strong function of temperature. Usually, an optimal temperature exists at which microbes' growth is the fastest, and the enzyme activity is the greatest. At high temperatures, microbes may not survive, and enzymes may denature. Metal ions such as Ca2+, Mg2+, Mn2+, Zn2+, and Cu2+ can affect enzyme production.301 Metal ions also protect the enzymes against thermal denaturation.
6. Life-cycle analysis/environmental impact/circular economy of protein-based bioplastics
Approximately 2.5 billion tons of food are discarded annually on a global scale. The United States discards approximately 60 million tons of waste annually, while India discards around 74 million tons yearly. India generates ∼74 million tonnes of food waste annually.302 This food waste ends up in landfills where it rots and emits greenhouse gases, particularly methane, which is ∼80 times more potent than carbon dioxide. Food waste holds around 6–8% of all anthropogenic emissions worldwide.303 Additionally, crop residue has also contributed significantly to greenhouse gas emissions.304 In India, crop residue accounts for approximately tw−hirds of the total 683 million tons of residue generated annually.305 Among these, 500 million tons are recycled, although 175 million tons are still left, and around 80 million tons are burnt in an unregulated manner. This unregulated burning is concerning, and we need to act on it either by recycling the crop residue and food waste or using it for energy production. Bioplastic production from food waste, biomass, and crop residue has gained popularity globally. No doubt, bioplastic production also emits greenhouse gases. However, the rate and extent of emission are low compared to petro-plastic production and crop residue incineration.306 The greenhouse gas emissions range from 0.354 to 0.623 kg CO2 eq. per kg for bioplastic, in contrast to 2.37 kg CO2 eq. per kg for polypropylene as a petro-plastic.307 These results show that bioplastic can replace petro-plastic, which will reduce greenhouse gas emissions and harm to the environment.Protein-based bioplastics go through a whole cycle, from the growth of plants rich with raw materials to the biodegradation of those bioplastics. The overall life cycle of bioplastics includes the following steps (Fig. 5): (1) cultivation of plants/microorganisms, (2) recovery of plant residue, (3) refining of raw materials, (4) recovery of proteins, (5) manufacturing of bioplastic, (6) distributing and using the bioplastics for various purposes, (7) disposal of bioplastics, and (8) composting/biodegradation/use as an animal feed. The compost can again return to earth and help grow new plants and complete the cycle. If the protein-based bioplastics are edible (made with natural and edible ingredients), they may be a nutritious animal feed.
Life cycle analysis is a comprehensive research methodology that examines and evaluates energy requirements and harmful environmental effects associated with every phase of the life cycle of manufactured goods, starting from the extraction of raw materials and continuing through manufacturing, warehousing, filling up, distribution, use, disposal, and reprocessing.307
7. Potential uses of protein-based bioplastics
The first 80 years of the plastics industry produced products primarily from biopolymers, such as cellulose, casein, shellac, and ebonite. One can track the early applications and uses of protein-based bioplastics to the early 1900s, when casein, soy, and gelatine were used to create protein-based bioplastics. The ability of casein proteins to form hydrophobic films was known from historical times and found uses in paints and coatings. In the late 1800s, Adolf Spittler patented the technology of creating hard plastic by crosslinking casein protein and manufactured products such as buttons, boxes, cases, and umbrella grips.308 In the early 1900s, until World War II, several products based on plant proteins, such as films, coating, and textiles (commercially azlons), were commercially available. Wool-like fibres were created from casein, soy, corn zein and peanut protein.309 It is interesting to note that in 1936, Ford Motors produced a million cars; each had around 15 pounds (∼7 kg s) of soy-plastic parts—in gearshift knobs, window frames, electrical switches, horn buttons, and distributor caps.310 Nonetheless, with the arrival of petroleum-based products, which were easier to produce, low-cost, and relatively superior properties to protein-based bioplastics, production and use of protein-based bioplastics became obsolete after WWII. Though limited products from protein-based bioplastics are commercially available today, the early applications and commercial success bolster the idea of replacing fossil-derived plastics with protein-based bioplastics. Protein-based bioplastics may have varied uses. A few of them are listed below (Fig. 6).7.1 Packaging materials
Most packaging materials come under SUPs, such as food wrappers, edible packaging solutions, packaging materials for clothes, gift packaging solutions, or shopping bags to carry fruits and vegetables. A recent report showed that coating a blend of soybean protein isolate and montmorillonite on paper improved oil resistance, barrier performances, and mechanical properties.311 Another work showcased that small additions of curcumin (∼2%) led to a pH-responsive material with improved mechanical strength, surface hydrophobicity, UV barrier property, antimicrobial activity and water vapour permeability, making it a valuable functional biomaterial for food packaging applications.312 These studies indicate that blending proteins with functional materials can lead to innovative packaging solutions.7.2 Edible coatings
An emerging application of protein-based bioplastics is in the coating of fresh produce to improve its taste, texture, appearance, and shelf-life by protecting against mechanical abuse, regulating moisture and gas transport, and providing barriers to UV and visible radiation.28 A recent research effort showed that whey protein, alginate, and curcumin blends are effective edible coatings. It demonstrated its effectiveness for the preservation of apples. In this blend, alginate provided the coating matrix, and whey protein imparted hydrophobicity and antioxidant properties. Curcumin helped increase the film's opacity, hydrophobicity, antioxidant activity, and UV-blocking efficiency.3137.3 Disposable products
Disposable cutlery such as plastic spoons, bottles for storing personal hygiene products like shampoo soap, and medical disposables can be made using bioplastic materials or bioplastics blended with fossil-derived plastics.102,314–316 To date, no reports show the applications of protein-based bioplastics to produce disposable products. Given the history of protein-based bioplastics and their processability in injection moulding and thermomoulding processes,310 we envision that protein-based bioplastics or protein-coated paper-based biodegradable, disposable products will soon become a reality.7.4 Agricultural products
Mulching films and vine clips. Mulch films provide soil cover and promote suitable growth opportunities for plants by impeding moisture evaporation from the soil. Protein-based films with low water vapour permeability can be an apt solution for mulching as they will naturally degrade over time without removing plastic covers. Protein-based films and coatings can also help reduce soil erosion control to barren or erodible soils, providing temporary cover and protection in environmental restoration projects. Protein sheets decompose over time via an amicrobial process and replenish nutrients in the soil.317 Hydrolyzed protein-based mulching coatings performances were comparable with the low-density polyethylene mulching film.318 Furthermore, proteins-based systems can help in the controlled delivery of nutrients, pesticides, and antibiotics in plants/crops to ensure high growth rates and achieve superior produce quality while minimizing the waste of resources and environmental impact.319,3207.5 Containers and boxes for storing and transporting goods
To date, no report shows the development of containers for storing and transporting goods. Pea protein-based automobile parts were a reality, suggesting that protein-based bioplastics products such as containers and boxes can be tough and durable.3107.6 Coating and adhesives for non-edibles
Bioplastic coatings have many favourable properties, like excellent grease barring, good sealing properties, stability, and drawability. These coatings can thus be used in carton board packaging, drinking cups, and confectionery boxes. For instance, coatings made from soy protein hydrolysate and gelatin protein crosslinked by tannic acid show stability under aqueous washings and are effective against bacteria and biofilm formation. These antimicrobial coatings can be applied on food-contacting surfaces in food processing industries.3217.7 Encapsulation and packaging of pharmaceutical drugs and cosmetics
Proteins are biocompatible, bioresorbable, and minimally immunogenic with great functionalisation possibilities. Thus, proteins are prime candidates for encapsulating and protecting active pharmaceutical ingredients, cosmetics, and fragrances. Further, encapsulation masks the taste, extends the shelf-life of encapsulated substances, transports them to the regions of interest in the body, and facilitates controlled release.322 Many plant or algae protein fragments are physiologically important and called bioactive compounds. They play a great role in developing new cosmetic formulations.323 Thus, we see great potential for plant-based protein bioplastics in encapsulating and packaging drugs and cosmetics.7.8 Other applications
Though not reported, we believe that the protein-based bioplastics have great potential to replace fossil-derived plastics used in other commonplace products such as pens, sports equipment like cricket stumps, balls, bats, helmets, mouth guards, shin pads and face shields, footwear and childcare products like milk bottles.8. Challenges: proteins as bioplastics
Although bioplastic research dates back to the 1980s, large-scale implementation is in its infancy due to several challenges encountered during the implementation phase. A multifaceted approach includes identifying appropriate protein sources, optimising processing conditions, developing effective testing protocols, and matching proteins-based materials for apt applications. Research and development of bioplastics demand a heavy investment of time, effort, and money. Some major problems faced while implementing bioplastics are as follows.8.1 Competition against petroleum-derived plastics
Competition from fossil-derived plastics is fierce. Protein-based bioplastics must possess mechanical properties at par with commonly used fossil-derived plastics. This primary material selection criterion often requires additional raw materials and processing, leading to inflated costs.8.2 Raw material sourcing
The limited availability of specific protein sources in certain regions limits the scalability of bioplastic production. The use of food proteins is not a viable option for a country like India, where insufficient protein reaches citizens' plates. Hence, development using alternative protein sources is essential. The ideal scenario is manufacturing bioplastics from untapped or waste sources, such as unused agro wastes, dried leaves, aquatic weeds, food wastes, or silk industry wastes. Still, some of these resources might be scarce in regions such as Rajasthan in India. Hence, new strategies need to be developed. Proteins themselves are heterogeneous polymers, and their properties vary widely. Moreover, proteins obtained from different sources or regions often have different compositions, properties, and behaviours; therefore, maintaining quality will be challenging. Such variations limit the application of protein-based bioplastics for specific applications that require strict quality control. Cost of production: the cost of production is a limiting factor for the wide adoption of bioplastics in price-sensitive markets of middle and low-income countries such as India. Cost analysis of bioplastics produced from carbohydrates is only available. Still, commercial production of protein-based bioplastics has yet to be realised; thus, we currently lack a proper cost analysis of protein-based bioplastics.The factors that come into play during cost analysis are the costs of the raw material, the thickness of the product required, the costs of reagents and the equipment used for production. Hence, it is difficult to pinpoint the exact costs involved in producing a particular bioplastic. A recent techno-economic analysis study of whey protein-based bioplastic production showed that the plant may achieve break-even in 2.4 to 3.7 years.324 A similar study discusses the cost competitiveness of sustainable bioplastic feedstocks by comparing PLA production from corn starch and corn stover.325 Though both studies are not for plant-based protein bioplastics, they provide some insight into the costs involved in the production of bioplastics.
The cost and scalability of sourcing and extracting proteins from various sources can be challenging. Some protein sources may be expensive or difficult to obtain in large quantities, limiting the scalability of bioplastic production.
8.3 Sensitivity of bioplastics to external factors
Protein-based bioplastics are sensitive to environmental factors such as temperature and humidity. This sensitivity can affect their properties, making them unreliable compared to fossil-derived plastics. Improper decomposition: owing to the lack of proper knowledge and awareness, bioplastics often end up in landfills, deprived of the oxygen needed for decomposition. Improper disposal can cause a release of methane. When bioplastics are not discarded separately, they can contaminate batches of recycled plastic and harm recycling infrastructure.8.4 Environmental impacts
Harvesting large amounts of plants to obtain bioplastics can disrupt ecosystems. For example, large-scale surface seaweed cultivation and harvesting can block light and reduce dissolved oxygen in the water, which is vital for aquatic organisms.8.5 Social issues
Food and nutrition-related rules are necessarily stringent. Regulatory approval of protein-based bioplastics can be lengthy, adding time and costs to the process and slowing the implementation of protein-based bioplastics for food packaging solutions. Educating consumers and businesses on the benefits of bioplastics and associated challenges is critical to driving adoption and creating demand. Awareness is still evolving, and the demand for sustainable products is growing, but there is an urgent need to accelerate this process to prevent further harm to the planet.The government has shown support for propelling sustainability in the nation; thus far, it has been inadequate. The policies undertaken have not been effective. A lack of such support has hindered the shift from petroleum-derived plastics to biodegradable materials. Without financial and policy support, many have found it challenging to invest or scale up the production of bioplastics, which resulted in a lack of standardisation and regulation in the bioplastics industry, further impeding the growth and spread of this industrial sector.
9. Future prospects: the way forward
The future prospects for bioplastics are promising. With the increasing focus on sustainability and environmental consciousness, there is a growing demand for eco-friendly alternatives to traditional plastics. As research and development in the bioplastics sector continue to advance, there is potential for bioplastics to become a viable and competitive alternative. To achieve this, the industry must address many challenges, such as sourcing raw materials sustainably, optimising production processes, and scaling up production to meet demand. One of the most important reasons bioplastics have been unsuccessful in replacing conventional plastics is because of their cheap and easy availability. Bioplastic manufacturing costs are exuberant, and sufficient demand is non-existent to offset these costs by working out economies of scale. The manufacturing cost must be lowered by innovating new processes. Sources for the bioplastics must be biocompatible and eco-friendly. During manufacturing, one also needs to ensure that the manufacturing process is non-polluting so that the environment is safeguarded and one evil is not replaced.The processing conditions used to create bioplastics can significantly impact the functionality and behaviour of proteins. Hence, processing conditions need to be optimised to develop bioplastics with desirable properties, and certain additives and processing techniques discussed earlier can help achieve this. They are under testing to produce products with better mechanical properties such as strength, durability and permeability to expand into new application areas. Further development in protein extraction, processing modification and moulding is required to enhance the efficiency of the production process.
The policy-makers play a crucial role in the transition to bioplastics. The government must encourage research and development by incentivising and providing funding. Additionally, governments can facilitate this revolution for sustainability by implementing policies that prioritise using environmentally friendly materials. Regulatory bodies need to ensure that bioplastic production meets safety and quality standards, which will encourage their adoption by consumers and businesses. Overall, the way forward for bioplastics lies in continued innovation, collaboration, and investment from both the public and private domains.
10. Conclusion
Tackling the problems of pollution and plastic accumulation is a vast job. One effective solution is to replace single-use plastics with entirely recyclable or biodegradable choices before irreversible harm is done to the environment. Bioplastics that are biodegradable, durable, and clean might soon dominate various plastic-based sectors and become viable alternatives to plastics. Vegetative proteins are widely available and can be used to manufacture bioplastics. Raw material for this purpose can be sourced from waste products or untapped sources, as these do not compromise the country's food resources. Additives and processing techniques help us fine-tune the properties of protein-based bioplastics. They are under testing to produce products with better mechanical properties such as strength, durability and permeability to expand into new application areas. Further development in protein extraction, processing, modification and moulding is required to meet product specifications for industrial applications.There have been many challenges hindering the implementation of bioplastics. Production cost has been a major limiting factor in the spread of bioplastics. Thus, they fail to compete with inexpensive petroleum-based plastics. Process optimisation is a need of the hour to reduce costs and make these sustainable materials economically viable. Though government policies push for improvements, it is also up to the public to be aware of innovations and adapt them to their daily lifestyles. Corporations can be specifically expected to recognise the problem and revise their ways by integrating sustainability within their developments.
Data availability
This review article did not include any primary research results, software or code. The authors did not generate any new data or analyse any data as part of this review.Author contributions
Aditya Patel: conceptualization, visualization, validation, investigation, methodology, formal analysis, writing – original draft, and writing – review & editing. Rushabh Murali: writing – original draft, writing – review & editing, visualization. Heena Choudhary: writing – original draft, writing – review & editing, visualization, formal analysis. D. Purnima: supervision, writing – review & editing. Ramendra Kishor Pal: conceptualization, visualization, validation, investigation, methodology, formal analysis, writing – original draft, writing – review & editing, resources, supervision.Conflicts of interest
The authors of this review confirm that this manuscript has not been previously published and is not currently being considered by any other journal. To our knowledge, none of the authors have any conflict of interest, financial or otherwise.Acknowledgements
The authors acknowledge the Department of Chemical Engineering, BITS Pilani, Hyderabad Campus and Pilani Campus for providing facilities, resources, and guidance.References
- Visual Feature | Beat Plastic Pollution, http://unep.org/interactive/beat-plastic-pollution/, accessed 10 May 2023.
- Is India the World's Largest Plastic Polluter?, https://www.plasticsforchange.org/blog/india-emerges-as-the-worlds-largest-plastic-polluter-what-went-wrong-and-whats-next, accessed 30 November 2024.
- FAO - News Article, https://www.fao.org/news/story/en/item/196402/icode/, accessed 31 May 2023.
- Creating Wealth From Agricultural Waste, https://icar.org.in/content/creating-wealth-agricultural-waste, accessed 31 May 2023.
- H. Kamal, C. F. Le, A. M. Salter and A. Ali, Compr. Rev. Food Sci. Food Saf., 2021, 20, 2455–2475 CrossRef PubMed.
- D. Pleissner and C. S. K. Lin, Sustain. Chem. Process., 2013, 1, 21 CrossRef.
- Y. Zhang, Y. Nian, Q. Shi and B. Hu, J. Mater. Chem. A, 2023, 11, 9884–9901 RSC.
- H. Chen, J. Wang, Y. Cheng, C. Wang, H. Liu, H. Bian, Y. Pan, J. Sun and W. Han, Polymers, 2019, 11, 2039 CrossRef CAS PubMed.
- Y. A. Shah, S. Bhatia, A. Al-Harrasi, M. Afzaal, F. Saeed, M. K. Anwer, M. R. Khan, M. Jawad, N. Akram and Z. Faisal, Polymers, 2023, 15, 1724 CrossRef CAS PubMed.
- W. Gu, Y. Tan, H. Pang, Q. Ye, X. Li and J. Li, Macromol. Mater. Eng., 2024, 309, 2300224 CrossRef CAS.
- V. M. Perez-Puyana, M. Jiménez-Rosado, I. Martínez and A. Romero, ACS Appl. Polym. Mater., 2024, 6, 1891–1899 CrossRef CAS.
- A. Senthilkumaran, A. Babaei-Ghazvini, M. T. Nickerson and B. Acharya, Polymers, 2022, 14, 1065 CrossRef CAS PubMed.
- M. Vert, Y. Doi, K.-H. Hellwich, M. Hess, P. Hodge, P. Kubisa, M. Rinaudo and F. Schué, Pure Appl. Chem., 2012, 84, 377–410 CrossRef CAS.
- D. Voet, J. G. Voet and C. W. Pratt, Fundamentals of Biochemistry, https://www.wileyplus.com/chemistry/voet-fundamentals-of-chemistry-5e-eprof18108/, accessed 7 June 2023.
- S. J. Calva-Estrada, M. Jiménez-Fernández and E. Lugo-Cervantes, Food Eng. Rev., 2019, 11, 78–92 CrossRef CAS.
- Y. Li, G. Yang, L. Gerstweiler, S. H. Thang and C.-X. Zhao, Adv. Funct. Mater., 2023, 33, 2210387 CrossRef CAS.
- M. Kumar, M. Tomar, J. Potkule, R. Verma, S. Punia, A. Mahapatra, T. Belwal, A. Dahuja, S. Joshi, M. K. Berwal, V. Satankar, A. G. Bhoite, R. Amarowicz, C. Kaur and J. F. Kennedy, Food Hydrocoll., 2021, 115, 106595 CrossRef CAS.
- A. S. Chandran, P. Kashyap and M. Thakur, eFood, 2024, 5, e151 CrossRef.
- G. Bhattacharyya, P. Oliveira, S. T. Krishnaji, D. Chen, M. Hinman, B. Bell, T. I. Harris, A. Ghazitabatabaei, R. V. Lewis and J. A. Jones, Protein Expr. Purif., 2021, 183, 105839 CrossRef CAS PubMed.
- X.-X. Xia, Z.-G. Qian, C. S. Ki, Y. H. Park, D. L. Kaplan and S. Y. Lee, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 14059–14063 CrossRef CAS PubMed.
- J. Su, K. Zhao, Y. Ren, L. Zhao, B. Wei, B. Liu, Y. Zhang, F. Wang, J. Li, Y. Liu, K. Liu and H. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202117538 CrossRef CAS PubMed.
- M. Sewwandi, H. Wijesekara, A. U. Rajapaksha, S. Soysa and M. Vithanage, Environ. Pollut., 2023, 317, 120747 CrossRef CAS PubMed.
- V. M. Rangaraj, K. Rambabu, F. Banat and V. Mittal, Food Biosci., 2021, 43, 101251 CrossRef.
- V. K. Sharma, M. Sharma, Z. Usmani, A. Pandey, B. N. Singh, M. Tabatabaei and V. K. Gupta, Trends Biotechnol., 2022, 40, 1004–1017 CrossRef CAS PubMed.
- T. Song, S. Qian, T. Lan, Y. Wu, J. Liu and H. Zhang, Foods, 2022, 11, 2228 CrossRef CAS PubMed.
- N. Zhen, X. Wang, X. Li, J. Xue, Y. Zhao, M. Wu, D. Zhou, J. Liu, J. Guo and H. Zhang, Microb. Biotechnol., 2021, 15, 1324–1338 CrossRef PubMed.
- P. Torres Lepe, K. V. Heredia, E. Cárdenas Namur, G. C. Sandoval Fabián and S. García-Enriquez, in Bioplastics for Sustainability, ed. A. K. Mishra and C. M. Hussain, Elsevier, 2024, pp. 271–309 Search PubMed.
- M. N. Khin, S. Ahammed, Md. M. Kamal, M. N. Saqib, F. Liu and F. Zhong, Food Hydrocolloids Health, 2024, 6, 100182 CrossRef CAS.
- T. Li, J. Kambanis, T. L. Sorenson, M. Sunde and Y. Shen, Biomacromolecules, 2024, 25, 5–23 CrossRef CAS PubMed.
- P. Wolf, M. Reimer, M. Maier and C. Zollfrank, Polym. Degrad. Stab., 2023, 217, 110538 CrossRef CAS.
- A. Jerez, P. Partal, I. Martínez, C. Gallegos and A. Guerrero, Rheol. Acta, 2007, 46, 711–720 CrossRef CAS.
- L. Fernández-Espada, C. Bengoechea, F. Cordobés and A. Guerrero, J. Appl. Polym. Sci., 2016, 133, 43524 CrossRef.
- Z. Qazanfarzadeh and V. Kumaravel, Trends Food Sci. Technol., 2023, 138, 27–43 CrossRef CAS.
- T. Shevtsova, K. Iduoku, K. Patnode Setien, I. Olabode, G. M. Casanola-Martin, B. Rasulev and A. Voronov, ACS Sustain. Chem. Eng., 2024, 12, 15948–15960 CrossRef CAS.
- F. De Marchis, T. Vanzolini, E. Maricchiolo, M. Bellucci, M. Menotta, T. Di Mambro, A. Aluigi, A. Zattoni, B. Roda, V. Marassi, R. Crinelli and A. Pompa, Biotechnol. J., 2024, 19, 2300363 CrossRef CAS PubMed.
- M. Alonso-González, M. Felix and A. Romero, Resour. Conserv. Recycl., 2024, 208, 107713 CrossRef.
- M. B. Stie, K. Kalouta, V. Vetri and V. Foderà, J. Controlled Release, 2022, 344, 12–25 CrossRef CAS PubMed.
- Are you among the 90% who don't know the body's daily protein requirement?, https://www.hindustantimes.com/lifestyle/health/are-you-among-the-90-who-don-t-know-the-body-s-daily-protein-requirement-101669633917053.html, accessed 24 June 2023.
- OECD, OECD-FAO Agricultural Outlook 2021-2030, Organisation for Economic Co-operation and Development, Paris, 2021 Search PubMed.
- D. Pimentel and M. Pimentel, Am. J. Clin. Nutr., 2003, 78, 660S–663S CrossRef CAS PubMed.
- M. a. Asgar, A. Fazilah, N. Huda, R. Bhat and A. a. Karim, Compr. Rev. Food Sci. Food Saf., 2010, 9, 513–529 CrossRef CAS PubMed.
- N. Heredia and S. García, Anim. Nutr., 2018, 4, 250–255 CrossRef PubMed.
- J. Poore and T. Nemecek, Science, 2018, 360, 987–992 CrossRef CAS PubMed.
- H. Chen, S. Gao, Y. Li, H.-J. Xu, W. Li, J. Wang and Y. Zhang, Int. Res. J. Publ. Environ. Health, 2022, 19, 6681 CrossRef CAS PubMed.
- J. R. Biesiekierski, J. Gastroenterol. Hepatol., 2017, 32, 78–81 CrossRef CAS PubMed.
- A. Tamayo Tenorio, K. E. Kyriakopoulou, E. Suarez-Garcia, C. van den Berg and A. J. van der Goot, Trends Food Sci. Technol., 2018, 71, 235–245 CrossRef CAS.
- A. L. Khandare, J. J. Babu, M. Ankulu, N. Aparna, A. Shirfule and G. S. Rao, Indian J. Med. Res., 2014, 140, 96–101 CAS.
- D. Chéreau, P. Videcoq, C. Ruffieux, L. Pichon, J.-C. Motte, S. Belaid, J. Ventureira and M. Lopez, OCL, 2016, 23, D406 CrossRef.
- R. Shukla and M. Cheryan, Ind. Crops Prod., 2001, 13, 171–192 CrossRef CAS.
- T. P. Castro-Jácome, L. E. Alcántara-Quintana and E. G. Tovar-Pérez, Biores. Open Access, 2020, 9, 198–208 CrossRef PubMed.
- T. Hymowitz, F. I. Collins, J. Panczner and W. M. Walker, Agron. J., 1972, 64, 613–616 CrossRef CAS.
- C. Fabian and Y.-H. Ju, Crit. Rev. Food Sci. Nutr., 2011, 51, 816–827 CrossRef CAS PubMed.
- S. González-Pérez and J. M. Vereijken, J. Sci. Food Agric., 2007, 87, 2173–2191 CrossRef.
- W. H. Martinez, L. C. Berardi and L. A. Goldblatt, J. Agric. Food Chem., 1970, 18, 961–968 CrossRef CAS.
- R. A. Buffo and J. H. Han, in Innovations in Food Packaging, ed. J. H. Han, Academic Press, London, 2005, pp. 277–300 Search PubMed.
- M. Kumar, M. Tomar, S. Punia, S. Grasso, F. Arrutia, J. Choudhary, S. Singh, P. Verma, A. Mahapatra, S. Patil, Radha, S. Dhumal, J. Potkule, S. Saxena and R. Amarowicz, Trends Food Sci. Technol., 2021, 111, 100–113 CrossRef CAS.
- H. Wieser, Food Microbiol., 2007, 24, 115–119 CrossRef CAS PubMed.
- M. Jiménez-Rosado, L. S. Zarate-Ramírez, A. Romero, C. Bengoechea, P. Partal and A. Guerrero, J. Clean. Prod., 2019, 239, 117994 CrossRef.
- Y. Chen, Y. Li, S. Qin, S. Han and H. Qi, Composites, Part B, 2022, 238, 109868 CrossRef CAS.
- S. Domenek, P. Feuilloley, J. Gratraud, M.-H. Morel and S. Guilbert, Chemosphere, 2004, 54, 551–559 CrossRef CAS PubMed.
- I. Parzanese, D. Qehajaj, F. Patrinicola, M. Aralica, M. Chiriva-Internati, S. Stifter, L. Elli and F. Grizzi, World J. Gastrointest. Pathophysiol., 2017, 8, 27–38 CrossRef PubMed.
- Z. X. Lu, J. F. He, Y. C. Zhang and D. J. Bing, Crit. Rev. Food Sci. Nutr., 2020, 60, 2593–2605 CrossRef CAS PubMed.
- B. Baraniak, M. Niezabitowska, J. Pielecki and W. Wójcik, Food Chem., 2004, 85, 251–257 CrossRef CAS.
- A. C. Y. Lam, A. Can Karaca, R. T. Tyler and M. T. Nickerson, Food Rev. Int., 2018, 34, 126–147 CrossRef CAS.
- M. Aider, M. Sirois-Gosselin and J. Boye, J. Food Res., 2012, 1, p160 CrossRef.
- S. M. Beck, K. Knoerzer, M. Foerster, S. Mayo, C. Philipp and J. Arcot, J. Food Eng., 2018, 231, 61–71 CrossRef CAS.
- V. Perez-Puyana, P. Cuartero, M. Jiménez-Rosado, I. Martínez and A. Romero, Food Packag. Shelf Life, 2022, 32, 100836 CrossRef CAS.
- J. Gorham, N Engl J Med or N. Engl. J. Med., 1820, 9, 320–328 Search PubMed.
- X. Wang and M. Fan, RSC Adv., 2019, 9, 5748–5755 RSC.
- J. Taylor, J. O. Anyango and J. R. N. Taylor, Cereal Chem., 2013, 90, 344–357 CrossRef CAS.
- S. Tortorella, M. Maturi, V. Vetri Buratti, G. Vozzolo, E. Locatelli, L. Sambri and M. Comes Franchini, RSC Adv., 11, 39004–39026 RSC.
- T. J. Anderson and B. P. Lamsal, Cereal Chem., 2011, 88, 159–173 CrossRef CAS.
- D. J. Sessa, A. Mohamed and J. A. Byars, J. Agric. Food Chem., 2008, 56, 7067–7075 CrossRef CAS PubMed.
- FAOSTAT, https://www.fao.org/faostat/en/#data/QCL/visualize, accessed 29 July 2023.
- M. V. Nagesh Kumar, V. Ramya, M. Govindaraj, A. Dandapani, S. Maheshwaramma, K. N. Ganapathy, K. Kavitha, M. Goverdhan and R. Jagadeeshwar, Front. Plant Sci. Search PubMed.
- J. Xiao, Y. Chen and Q. Huang, Food Funct., 2017, 8, 1402–1413 RSC.
- T. Huang, J. Lin, Z. Fang, W. Yu, Z. Li, D. Xu, W. Yang and J. Zhang, Food Bioprocess Technol., 2020, 13, 522–532 CrossRef CAS.
- D. A. Patil, S. Tated and S. T. Mhaske, Polym. Bull., 2021, 78, 1721–1733 CrossRef CAS.
- J. R. N. Taylor and J. Taylor, Cereal Chem., 2023, 100, 539–555 CrossRef CAS.
- Y. Zhao, R. Tian, Z. Xu, L. Jiang and X. Sui, J. Am. Oil Chem. Soc., 2023, 100, 187–195 CrossRef CAS.
- M. Jiménez-Rosado, E. Bouroudian, V. Perez-Puyana, A. Guerrero and A. Romero, J. Clean. Prod., 2020, 262, 121517 CrossRef.
- E. Álvarez-Castillo, G. Caballero, A. Guerrero and C. Bengoechea, J. Polym. Environ., 2021, 29, 2789–2796 CrossRef.
- M. Jiménez-Rosado, J.-E. Maigret, V. Perez-Puyana, A. Romero and D. Lourdin, J. Polym. Environ., 2022, 30, 1587–1599 CrossRef.
- M. Bagnani, M. Peydayesh, T. Knapp, E. Appenzeller, D. Sutter, S. Kränzlin, Y. Gong, A. Wehrle, S. Greuter, M. Bucher, M. Schmid and R. Mezzenga, Biomacromolecules, 2024, 25, 2033–2040 CrossRef CAS PubMed.
- A. Ranjan, S. Kumar, N. P. Sahu, K. K. Jain and A. D. Deo, Aquac. Int., 2022, 30, 99–114 CrossRef CAS.
- M. Alonso-González, M. Felix, A. Romero, C. Sergi, I. Bavasso and F. Sarasini, J. Polym. Environ., 2025, 33, 512–527 CrossRef.
- M. Alonso-González, M. Felix, A. Guerrero and A. Romero, Polymers, 2021, 13, 398 CrossRef PubMed.
- M. Alonso-González, M. Felix, A. Romero, C. Sergi, I. Bavasso and F. Sarasini, Resour. Conserv. Recycl., 2025, 212, 107990 CrossRef.
- R. Li, W. Ma, Y. Feng, M. Zhang, H. Zhang and J. Wang, Food Chem., 2025, 465, 142022 CrossRef CAS PubMed.
- P. R. Salgado, S. E. Molina Ortiz, S. Petruccelli and A. N. Mauri, Food Hydrocoll., 2010, 24, 525–533 CrossRef CAS.
- M.-N. Efthymiou, E. Tsouko, A. Papagiannopoulos, I.-G. Athanasoulia, M. Georgiadou, S. Pispas, D. Briassoulis, T. Tsironi and A. Koutinas, Sci. Rep., 2022, 12, 6935 CrossRef CAS PubMed.
- ICAC, https://icac.org/Publications/PastIssues?Id=81, accessed 28 July 2023.
- Textile Data | Ministry of Textiles | GoI, https://texmin.nic.in/textile-data, accessed 28 July 2023.
- Products – National Cottonseed Products Association, https://www.cottonseed.com/products/, accessed 28 July 2023.
- H. Yue, G. Yin, Y. Cui, H. Yue, G. Yin and Y. Cui, in Cotton Research, IntechOpen, 2016 Search PubMed.
- G. W. Selling, M. P. Hojilla-Evangelista, W. T. Hay, K. D. Utt and G. D. Grose, Ind. Crops Prod., 2022, 178, 114615 CrossRef CAS.
- H.-B. Yue, Y.-D. Cui, P. S. Shuttleworth and J. H. Clark, Green Chem., 2012, 14, 2009–2016 RSC.
- Y. Shao, B. Mu, X. Yu, L. Xu, R. Dhandapani and Y. Yang, Int. J. Biol. Macromol., 2024, 282, 137251 CrossRef CAS PubMed.
- É. Domokos-Szabolcsy, S. R. Yavuz, E. Picoli, M. G. Fári, Z. Kovács, C. Tóth, L. Kaszás, T. Alshaal and N. Elhawat, Life, 2023, 13, 307 CrossRef PubMed.
- A.-L. Nynäs, E. Berndtsson, W. R. Newson, H. P. Hovmalm and E. Johansson, ACS Food Sci. Technol., 2024, 4, 126–138 CrossRef.
- S. A. Santos, World Intellectual Property Organization, WO2013173434A1, 2013.
- Home - Zerocircle, https://www.zerocircle.in/, accessed 31 March 2025.
- How a noxious aquatic weed was used to make eco-friendly products, generate employment in rural Bengal, https://www.downtoearth.org.in/blog/agriculture/how-a-noxious-aquatic-weed-was-used-to-make-eco-friendly-products-generate-employment-in-rural-bengal-83388, accessed 11 August 2023.
- Now, Kuttanad in Kerala all set to profit from water hyacinth, https://www.newindianexpress.com/states/kerala/2023/jun/22/now-kuttanad-in-kerala-all-set-to-profit-from-water-hyacinth-2587359.html, accessed 11 August 2023.
- M. Suleiman, A. Y. Khadija, Y. Nasiru, A. A. Garba, M. Alhassan and H. J. Bello, Earthline J. Chem. Sci., 2020, 3, 51–59 CAS.
- O. Adeyemi and C. C. Osubor, Egypt. J. Aquat. Res., 2016, 42, 269–272 CrossRef.
- HyaPak – HyaPak, https://hyapak.com/, accessed 11 August 2023.
- P. Ziegler, K. Adelmann, S. Zimmer, C. Schmidt and K.-J. Appenroth, Plant Biol., 2015, 17, 33–41 CrossRef PubMed.
- G. Chen, Y. Yu, W. Li, B. Yan, K. Zhao, X. Dong, Z. Cheng, F. Lin, L. Li, H. Zhao and Y. Fang, Bioresour. Technol., 2020, 317, 124033 CrossRef CAS PubMed.
- M. A. Zeller, R. Hunt and S. Sharma, J. Appl. Polym. Sci., 2013, 127, 375–386 CrossRef CAS.
- S. Sharma, R. W. Hunt and M. A. Zeller, US Pat., US9765205B2, United States, 2017 Search PubMed.
- R. Yoksan, A. Boontanimitr, N. Klompong and T. Phothongsurakun, Int. J. Biol. Macromol., 2022, 203, 369–378 CrossRef CAS PubMed.
- G. D. Najafpour, in Biochemical Engineering and Biotechnology, ed. G. D. Najafpour, Elsevier, Amsterdam, 2007, pp. 332–341 Search PubMed.
- K. Spalvins, L. Zihare and D. Blumberga, Energy Procedia, 2018, 147, 409–418 CrossRef CAS.
- B. C. Bratosin, S. Darjan and D. C. Vodnar, Sustainability, 2021, 13, 9284 CrossRef CAS.
- Y. P. Li, F. Ahmadi, K. Kariman and M. Lackner, npj Sci. Food, 2024, 8, 66 CrossRef PubMed.
- A. Ritala, S. T. Häkkinen, M. Toivari and M. G. Wiebe, Front. Microbiol., 2017, 8, 2009 CrossRef PubMed.
- S. Singha, M. Mahmutovic, C. Zamalloa, L. Stragier, W. Verstraete, A. J. Svagan, O. Das and M. S. Hedenqvist, ACS Sustain. Chem. Eng., 2021, 9, 6337–6346 CrossRef CAS.
- E. Vlaeminck, E. Uitterhaegen, K. Quataert, T. Delmulle, S.-S. Kontovas, N. Misailidis, R. G. Ferreira, D. Petrides, K. De Winter and W. K. Soetaert, Fermentation, 2023, 9, 771 CrossRef CAS.
- W. Y. Chia, D. Y. Ying Tang, K. S. Khoo, A. N. Kay Lup and K. W. Chew, Environ. Sci. Ecotechnology, 2020, 4, 100065 CrossRef PubMed.
- P. D. Karkos, S. C. Leong, C. D. Karkos, N. Sivaji and D. A. Assimakopoulos, Evidence-Based Complementary Altern. Med., 2011, 2011, 531053 CrossRef CAS PubMed.
- S. Onen Cinar, Z. K. Chong, M. A. Kucuker, N. Wieczorek, U. Cengiz and K. Kuchta, Int. Res. J. Publ. Environ. Health, 2020, 17, 3842 CrossRef PubMed.
- M. Zeenat, S. Sharmin, M. T. Islam, K. M. Sujan, M. I. Haque, M. K. Islam and J. Bangladesh, Vet. Med., 2019, 17, 71–75 Search PubMed.
- N. K. Z. AlFadhly, N. Alhelfi, A. B. Altemimi, D. K. Verma and F. Cacciola, Plants, 2022, 11, 3063 CrossRef CAS PubMed.
- C. Ye, D. Mu, N. Horowitz, Z. Xue, J. Chen, M. Xue, Y. Zhou, M. Klutts and W. Zhou, Algal Res., 2018, 34, 154–163 CrossRef.
- B. Thevarajah, G. K. S. H. Nishshanka, M. Premaratne, P. H. V. Nimarshana, D. Nagarajan, J.-S. Chang and T. U. Ariyadasa, Biochem. Eng. J., 2022, 185, 108541 CrossRef CAS.
- D. Selvendran, in Algal Biorefinery: an Integrated Approach, ed. D. Das, Springer International Publishing, Cham, 2015, pp. 151–167 Search PubMed.
- B. W. Jester, H. Zhao, M. Gewe, T. Adame, L. Perruzza, D. T. Bolick, J. Agosti, N. Khuong, R. Kuestner, C. Gamble, K. Cruickshank, J. Ferrara, R. Lim, T. Paddock, C. Brady, S. Ertel, M. Zhang, A. Pollock, J. Lee, J. Xiong, M. Tasch, T. Saveria, D. Doughty, J. Marshall, D. Carrieri, L. Goetsch, J. Dang, N. Sanjaya, D. Fletcher, A. Martinez, B. Kadis, K. Sigmar, E. Afreen, T. Nguyen, A. Randolph, A. Taber, A. Krzeszowski, B. Robinett, D. B. Volkin, F. Grassi, R. Guerrant, R. Takeuchi, B. Finrow, C. Behnke and J. Roberts, Nat. Biotechnol., 2022, 40, 956–964 CrossRef CAS PubMed.
- S. Benelhadj, S. Douiri, A. Ghouilli, R. B. Hassen, S. M. A. S. Keshk, A. El-kott, H. Attia and D. Ghorbel, J. Food Compos. Anal., 2023, 115, 104984 CrossRef CAS.
- H. Iyer, P. Grandgeorge, A. M. Jimenez, I. R. Campbell, M. Parker, M. Holden, M. Venkatesh, M. Nelsen, B. Nguyen and E. Roumeli, Adv. Funct. Mater., 2302067 Search PubMed.
- N. M. Albaqami, Aquaculture, 2025, 594, 741404 CrossRef CAS.
- K. Pobiega, J. Sękul, A. Pakulska, M. Latoszewska, A. Michońska, Z. Korzeniowska, Z. Macherzyńska, M. Pląder, W. Duda, J. Szafraniuk, A. Kufel, Ł. Dominiak, Z. Lis, E. Kłusek, E. Kozicka, A. Wierzbicka, M. Trusińska, K. Rybak, A. M. Kot and M. Nowacka, Appl. Sci., 2024, 14, 6259 CrossRef CAS.
- S. Y. J. Sim, A. Srv, J. H. Chiang and C. J. Henry, Foods, 2021, 10, 1967 CrossRef CAS PubMed.
- B. Kopilovic, A. I. Valente, A. M. Ferreira, M. R. Almeida, A. P. M. Tavares, M. G. Freire and J. A. P. Coutinho, RSC Sustain., 2023, 1, 1314–1331 RSC.
- A. S. Chandran, S. Suri and P. Choudhary, Sustainable Food Technol., 2023, 1, 466–483 RSC.
- C. Tanger, J. Engel and U. Kulozik, Food Hydrocoll., 2020, 107, 105949 CrossRef CAS.
- D. Akter, R. Begum, M. N. Rahman, N. Talukder and M. J. Alam, Curr. Res. Nutr. Food Sci. J., 2020, 8, 596–608 Search PubMed.
- L. T. K. Loan, Q. H. Minh, T. N. Minh, N. T. Nhung, T. D. Xuan, V. X. Duong, K. H. Trung, L. H. N. Minh, T. D. Khanh and T. T. T. Ha, J. Exp. Biol. Agric. Sci., 2023, 11, 290–296 CrossRef CAS.
- E. Ovando, L. Rodríguez-Sifuentes, L. M. Martínez and C. Chuck-Hernández, Appl. Sci., 2022, 12, 3113 CrossRef CAS.
- M. N. Perović, Z. D. Knežević Jugović and M. G. Antov, J. Food Eng., 2020, 276, 109894 CrossRef.
- T. Muller, M.-È. Bernier and L. Bazinet, Foods, 2023, 12, 3424 CrossRef CAS PubMed.
- K. Ware, P. Kashyap, P. M. Gorde, R. Yadav and V. Sharma, Food Bioprod. Process., 2025, 150, 63–77 CrossRef CAS.
- S. Thongkong, W. Klangpetch, K. Unban, P. Tangjaidee, Y. Phimolsiripol, P. Rachtanapun, K. Jantanasakulwong, R. Schönlechner, P. Thipchai and S. Phongthai, Foods, 2023, 12, 835 CrossRef CAS PubMed.
- A. C. Karaca, N. Low and M. Nickerson, Food Res. Int., 2011, 44, 2742–2750 CrossRef CAS.
- A. Sasidharan and V. Venugopal, Waste Biomass Valorization, 2020, 11, 5647–5663 CrossRef CAS.
- A. Ozkan, T. Dufour, A. Bogaerts and F. Reniers, Plasma Sources Sci. Technol., 2016, 25, 045016 CrossRef.
- S. Shrestha, L. van ’t Hag, V. S. Haritos and S. Dhital, Food Hydrocoll., 2023, 135, 108142 CrossRef CAS.
- Q. Cui, X. Ni, L. Zeng, Z. Tu, J. Li, K. Sun, X. Chen and X. Li, Hortic. Plant J., 2017, 3, 172–176 CrossRef.
- J. A. Asenjo and B. A. Andrews, J. Chromatogr. A, 2011, 1218, 8826–8835 CrossRef CAS PubMed.
- A. A. Galkin and V. V. Lunin, Russ. Chem. Rev., 2005, 74, 21 CrossRef CAS.
- W. Silva, M. Zanatta, A. S. Ferreira, M. C. Corvo and E. J. Cabrita, Int. J. Mol. Sci., 2020, 21, 7745 CrossRef CAS PubMed.
- J. C. F. Nunes, M. R. Almeida, J. L. Faria, C. G. Silva, M. C. Neves, M. G. Freire and A. P. M. Tavares, J. Solut. Chem., 2022, 51, 243–278 CrossRef CAS.
- P. Bharmoria, A. A. Tietze, D. Mondal, T. S. Kang, A. Kumar and M. G. Freire, Chem. Rev., 2024, 124, 3037–3084 CrossRef CAS PubMed.
- H. Bowen, R. Durrani, A. Delavault, E. Durand, J. Chenyu, L. Yiyang, S. Lili, S. Jian, H. Weiwei and G. Fei, Front. Chem., 2022, 10, 912411 CrossRef PubMed.
- A. Hewage, O. O. Olatunde, C. Nimalaratne, J. D. House, R. E. Aluko and N. Bandara, Food Hydrocoll., 2024, 147, 109283 CrossRef CAS.
- K. Kumar, S. Srivastav and V. S. Sharanagat, Ultrason. Sonochem., 2021, 70, 105325 CrossRef CAS PubMed.
- D. L. Nonglait and J. S. Gokhale, Food Bioprocess Technol., 2024, 17, 1681–1705 CrossRef.
- T. Varghese and A. Pare, J. Food Eng., 2019, 262, 92–99 CrossRef CAS.
- R. Bocker and E. K. Silva, Food Chem.:X, 2022, 15, 100398 CAS.
- T. Kleekayai, M. Khalesi, M. Amigo-Benavent, M. Cermeño, P. Harnedy-Rothwell and R. J. FitzGerald, in Green Protein Processing Technologies from Plants: Novel Extraction and Purification Methods for Product Development, ed. A. J. Hernández-Álvarez, M. Mondor and M. G. Nosworthy, Springer International Publishing, Cham, 2023, pp. 131–178 Search PubMed.
- J. Taylor, J. R. N. Taylor, M. F. Dutton and S. de Kock, Cereal Chem., 2005, 82, 485–487 CrossRef CAS.
- B. Mu, Y. Shao, X. Yu, L. Xu and Y. Yang, Ind. Crops Prod., 2024, 222, 120046 CrossRef CAS.
- L. Darie-Ion, M. Jayathirtha, G. E. Hitruc, M.-M. Zaharia, R. V. Gradinaru, C. C. Darie, A. Pui and B. A. Petre, Biomolecules, 2021, 11, 1838 CrossRef CAS PubMed.
- H. Tan, H. Zhou, T. Guo, J. Li, C. Zhang, S. Wang, Y. Zhang and L. Ma, Food Chem., 2022, 374, 131563 CrossRef CAS PubMed.
- Z. Gu and C. E. Glatz, J. Chromatogr. B, 2007, 845, 38–50 CrossRef CAS PubMed.
- P. Vázquez-Villegas, E. Espitia-Saloma, M. Rito-Palomares and O. Aguilar, J. Sep. Sci., 2013, 36, 391–399 CrossRef PubMed.
- G. Náthia-Neves and E. Alonso, Biomass Convers. Biorefin., 2024, 14, 1637–1650 CrossRef.
- W. Lu, X.-W. Chen, J.-M. Wang, X.-Q. Yang and J.-R. Qi, J. Food Eng., 2016, 169, 250–258 CrossRef CAS.
- E. Suarez Garcia, C. F. Miranda, M. T. Cesario, R. H. Wijffels, C. van den Berg and M. H. M. Eppink, ACS Sustain. Chem. Eng., 2023, 11, 1752–1762 CrossRef CAS PubMed.
- S. R. Motlagh, A. A. Elgharbawy, R. Khezri, R. Harun and R. Omar, Biomass Convers. Biorefin., 2023, 13, 8327–8338 CrossRef CAS.
- R. Wahlström, K. Rommi, P. Willberg-Keyriläinen, D. Ercili-Cura, U. Holopainen-Mantila, J. Hiltunen, O. Mäkinen, H. Nygren, A. Mikkelson and L. Kuutti, ChemistrySelect, 2017, 2, 9355–9363 CrossRef.
- M. T. Zin, T. Kaewkod, J. Pekkoh, W. Pathom-aree, S. Chaipoot, G. Kanthakat, P. Seesuriyachan, Y.-Y. Chen, K. S. Khoo, B. Cheirsilp, S. Srinuanpan and J. Agric, Food Res., 2025, 19, 101673 CAS.
- A. Akyüz, İ. Tekin, Z. Aksoy and S. Ersus, J. Food Process Eng., 2024, 47, e14758 CrossRef.
- A.-L. Nynäs, W. R. Newson and E. Johansson, Foods, 2021, 10, 2533 CrossRef PubMed.
- K. C. Duong-Ly and S. B. Gabelli, in Methods in Enzymology, ed. J. Lorsch, Academic Press, 2014, vol. 541, pp. 85–94 Search PubMed.
- P. Periasamy, S. Rajandran, R. Ziegman, M. Casey, K. Nakamura, H. Kore, K. Datta and H. Gowda, Proteomics, 2021, 21, 2100152 CrossRef CAS PubMed.
- F. Macritchie, J. Polym. Sci., Polym. Symp., 1976, 55, 139–142 CrossRef CAS.
- D. H. Atha and K. C. Ingham, J. Biol. Chem., 1981, 256, 12108–12117 CrossRef CAS PubMed.
- R. Qing, S. Hao, E. Smorodina, D. Jin, A. Zalevsky and S. Zhang, Chem. Rev., 2022, 122(18), 14085–14179 CrossRef CAS PubMed.
- M. J. Webber, E. A. Appel, E. W. Meijer and R. Langer, Nat. Mater., 2016, 15, 13–26 CrossRef CAS PubMed.
- M. Félix, A. Lucio-Villegas, A. Romero and A. Guerrero, Ind. Crops Prod., 2016, 79, 152–159 CrossRef.
- V. Perez, M. Felix, A. Romero and A. Guerrero, Food Bioprod. Process., 2016, 97, 100–108 CrossRef CAS.
- N. H. Ullsten, M. Gällstedt, G. M. Spencer, E. Johansson, S. Marttila, R. Ignell and M. S. Hedenqvist, Polym. Renew. Resour., 2010, 1, 173–186 CAS.
- Y. Wang and G. W. Padua, Macromol. Mater. Eng., 2003, 288, 886–893 CrossRef CAS.
- M. Felix, I. Martinez, A. Romero, P. Partal and A. Guerrero, Composites, Part B, 2018, 140, 197–203 CrossRef CAS.
- J. Jane and S. Wang, US Pat., US5523293A, United States, 1996 Search PubMed.
- R. Li, Y. Feng, S. Zhang, H. Zhang and J. Wang, Food Biosci., 2024, 60, 104248 CrossRef CAS.
- M. P. Balaguer, J. Gomez-Estaca, J. P. Cerisuelo, R. Gavara and P. Hernandez-Munoz, LWT--Food Sci. Technol., 2014, 56, 161–167 CrossRef CAS.
- L. Chaunier, E. Leroy, G. D. Valle, M. Dalgalarrondo, B. Bakan, D. Marion, B. Madec and D. Lourdin, AIP Conf. Proc., 2017, 1914, 190003 CrossRef.
- Y. Liu, J. Sun, Z. Wen, J. Wang, M. S. Roopesh, D. Pan and L. Du, Food Res. Int., 2024, 197, 115267 CrossRef CAS PubMed.
- P. Guerrero, P. M. Stefani, R. A. Ruseckaite and K. de la Caba, J. Food Eng., 2011, 105, 65–72 CrossRef CAS.
- W. R. Newson, F. Rasheed, R. Kuktaite, M. S. Hedenqvist, M. Gällstedt, T. S. Plivelic and E. Johansson, RSC Adv., 2015, 5, 32217–32226 RSC.
- A. Kamada, M. Rodriguez-Garcia, F. S. Ruggeri, Y. Shen, A. Levin and T. P. J. Knowles, Nat. Commun., 2021, 12, 3529 CrossRef CAS PubMed.
- G. Singh and A. Verma, Mater. Today Proc., 2017, 4, 1423–1433 CrossRef.
- V. m. Hernandez-Izquierdo and J. m. Krochta, J. Food Sci., 2008, 73, R30–R39 CAS.
- J. Gómez-Estaca, R. Gavara, R. Catalá and P. Hernández-Muñoz, Packag. Technol. Sci., 2016, 29, 203–224 CrossRef.
- W. Rodríguez-Castellanos, F. Martínez-Bustos, D. Rodrigue and M. Trujillo-Barragán, Eur. Polym. J., 2015, 73, 335–343 CrossRef.
- M. Oliviero, E. Di Maio and S. Iannace, J. Appl. Polym. Sci., 2010, 115, 277–287 CrossRef CAS.
- L. Shang, Y. Yu, Y. Liu, Z. Chen, T. Kong and Y. Zhao, ACS Nano, 2019, 13, 2749–2772 CrossRef CAS PubMed.
- M. A. Zeller, R. Hunt, A. Jones and S. Sharma, J. Appl. Polym. Sci., 2013, 130, 3263–3275 CrossRef CAS.
- K. Bruyninckx, K. J. A. Jansens, B. Goderis, J. A. Delcour and M. Smet, Eur. Polym. J., 2015, 68, 573–584 CrossRef CAS.
- A. Jerez, P. Partal, I. Martínez, C. Gallegos and A. Guerrero, Biochem. Eng. J., 2005, 26, 131–138 CrossRef CAS.
- F. M. de Souza and R. K. Gupta, J. Polym. Environ., 2024, 32, 5499–5515 CrossRef CAS.
- J. K. Kaushik and R. Bhat, J. Phys. Chem. B, 1998, 102, 7058–7066 CrossRef CAS.
- V. Siracusa, P. Rocculi, S. Romani and M. D. Rosa, Trends Food Sci. Technol., 2008, 19, 634–643 CrossRef CAS.
- Y. Wu, R. Tang, A. Guo, X. Tao, Y. Hu, X. Sheng, P. Qu, S. Wang, J. Li and F. Li, Materials, 2023, 16, 5953 CrossRef CAS PubMed.
- G. Wypych, Handbook of Plasticizers, Elsevier, 2023 Search PubMed.
- K. Dangaran, P. M. Tomasula and P. Qi, in Edible Films and Coatings for Food Applications, ed. K. C. Huber and M. E. Embuscado, Springer, New York, NY, 2009, pp. 25–56 Search PubMed.
- M. G. A. Vieira, M. A. da Silva, L. O. dos Santos and M. M. Beppu, Eur. Polym. J., 2011, 47, 254–263 CrossRef CAS.
- M. Zubair, R. A. Pradhan, M. Arshad and A. Ullah, Macromol. Mater. Eng., 2021, 306, 2000799 CrossRef CAS.
- A. Lamp, M. Kaltschmitt and J. Dethloff, Molecules, 2022, 27, 446 CrossRef CAS PubMed.
- G. Fredi and A. Dorigato, Adv. Ind. Eng. Polym. Res., 2024, 7, 373–404 CAS.
- B. Imre and B. Pukánszky, Eur. Polym. J., 2013, 49, 1215–1233 CrossRef CAS.
- M.-B. Coltelli, L. Aliotta, V. Gigante, M. Bellusci, P. Cinelli, E. Bugnicourt, M. Schmid, A. Staebler and A. Lazzeri, Molecules, 2020, 25, 3313 CrossRef CAS PubMed.
- X. Zhou and Q. Dou, J. Polym. Environ., 2022, 30, 1847–1863 CrossRef CAS.
- F. Garavand, M. Rouhi, S. H. Razavi, I. Cacciotti and R. Mohammadi, Int. J. Biol. Macromol., 2017, 104, 687–707 CrossRef CAS PubMed.
- K. Mayumi, C. Liu, Y. Yasuda and K. Ito, Gels, 2021, 7, 91 CrossRef CAS PubMed.
- J. P. Gong, Science, 2014, 344, 161–162 CrossRef CAS PubMed.
- G. J. Lake, A. G. Thomas and D. Tabor, Proc. R. Soc. Lond. Ser. Math. Phys. Sci., 1997, 300, 108–119 Search PubMed.
- D. Utami Nike, N. I. Md Fadilah, N. Sallehuddin, A. Y. H. Nor Azlan, F. H. Imran, M. Maarof and M. B. Fauzi, Front. Bioeng. Biotechnol., 2022, 10, 865014 CrossRef PubMed.
- V. M. Perez-Puyana, E. Cortés-Triviño, M. Jiménez-Rosado, A. Romero and I. Martínez, J. Polym. Environ., 2024, 32, 31–44 CrossRef CAS.
- P. Laurujisawat, T. Dumrongchai, A. Rodklongtan and P. Chitprasert, LWT--Food Sci. Technol., 2025, 216, 117347 CrossRef.
- I. Dudeja, R. K. Mankoo, A. Singh and J. Kaur, Sustain. Chem. Pharm., 2023, 36, 101307 CrossRef CAS.
- H. Xu, L. Shen, L. Xu and Y. Yang, Ind. Crops Prod., 2015, 74, 234–240 CrossRef CAS.
- Y. M. Ju, B. Yu, T. J. Koob, Y. Moussy and F. Moussy, J. Biomed. Mater. Res., Part A, 2008, 87A, 136–146 CrossRef CAS PubMed.
- A. N. Petelski, S. C. Pamies and G. L. Sosa, Biophys. Chem., 2021, 276, 106627 CrossRef CAS PubMed.
- C. Chen, S. A. Althawab and J. M. Awika, Food Chem., 2025, 478, 143513 CrossRef CAS PubMed.
- A. C. Alavarse, E. C. G. Frachini, R. L. C. G. da Silva, V. H. Lima, A. Shavandi and D. F. S. Petri, Int. J. Biol. Macromol., 2022, 202, 558–596 CrossRef CAS PubMed.
- H. Robles, in Encyclopedia of Toxicology, ed. P. Wexler, Academic Press, Oxford, 3rd edn, 2014, pp. 474–475 Search PubMed.
- Z. Chang, Y. Shen, J. Xue, Y. Sun and S. Zhang, Chem. Eng. J., 2023, 457, 140984 CrossRef CAS.
- G. Schmidt, J. T. Woods, L. X.-B. Fung, C. J. Gilpin, B. R. Hamaker and J. J. Wilker, Adv. Sustainable Syst., 2019, 3, 1900077 CrossRef CAS.
- R. Li, T. Dai, Y. Tan, G. Fu, Y. Wan, C. Liu and D. J. McClements, Food Chem., 2020, 310, 125828 CrossRef CAS PubMed.
- C. Xu, W. Zhan, X. Tang, F. Mo, L. Fu and B. Lin, Polym. Test., 2018, 66, 155–163 CrossRef CAS.
- H. Yu, Y. Ge, H. Ding, Y. Yan and L. Wang, Int. J. Biol. Macromol., 2023, 253, 126726 CrossRef CAS PubMed.
- Z. Tashi, M. Zare and N. Parvin, Mater. Lett., 2020, 264, 127275 CrossRef CAS.
- H. Ji, H. Zhang, Y. Wang, Z. Qiu, J. Wu, J. Cao, K. Xu, Y. Zhang, Y. Jiang and M. Wang, Biochem. Biophys. Res. Commun., 2023, 677, 182–189 CrossRef CAS PubMed.
- H. Kang, Z. Wang, W. Zhang, J. Li and S. Zhang, Food Hydrocoll., 2016, 61, 923–932 CrossRef CAS.
- C. Tan, Q.-D. Xu, N. Chen, Q. He, Q. Sun and W.-C. Zeng, J. Food Biochem., 2022, 46, e14416 CrossRef CAS PubMed.
- C. Li, Y. Zheng, X. Xiong and F. Xue, Food Chem., 2025, 463, 141300 CrossRef CAS PubMed.
- G. T. Hermanson, in Bioconjugate Techniques, ed. G. T. Hermanson, Academic Press, Boston, 3rd edn, 2013, pp. 259–273 Search PubMed.
- V. Tropini, J.-P. Lens, W. J. Mulder and F. Silvestre, Cereal Chem., 2000, 77, 333–338 CrossRef CAS.
- B. Jayachandran, T. N. Parvin, M. M. Alam, K. Chanda and B. Mm, Molecules, 2022, 27, 8124 CrossRef CAS PubMed.
- Y. Wei, D. Sun, H. Yi, H. Zhao and J. Wang, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2014, 29, 1083–1089 CrossRef CAS.
- J. Skopinska-Wisniewska, K. Wegrzynowska-Drzymalska, A. Bajek, M. Maj and A. Sionkowska, J. Mater. Sci.:Mater. Med., 2016, 27, 67 CrossRef CAS PubMed.
- J.-W. Rhim, A. Gennadios, C. L. Weller, C. Cezeirat and M. A. Hanna, Ind. Crops Prod., 1998, 8, 195–203 CrossRef CAS.
- B. Yang, H. Wu, P. D. Schnier, Y. Liu, J. Liu, N. Wang, W. F. DeGrado and L. Wang, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 11162–11167 CrossRef CAS PubMed.
- M. Salem, Y. Mauguen and T. Prangé, Acta Crystallogr., Sect. F:Struct. Biol. Cryst. Commun., 2010, 66, 225–228 CrossRef CAS PubMed.
- M. Jin, S. Ikeda and Q. Zhong, LWT--Food Sci. Technol., 2013, 51, 23–29 CrossRef CAS.
- P. Hernández-Muñoz, R. Villalobos and A. Chiralt, Food Hydrocoll., 2004, 18, 403–411 CrossRef.
- C. Chung, K. J. Lampe and S. C. Heilshorn, Biomacromolecules, 2012, 13, 3912–3916 CrossRef CAS PubMed.
- D. W. Lim, D. L. Nettles, L. A. Setton and A. Chilkoti, Biomacromolecules, 2007, 8, 1463–1470 CrossRef CAS PubMed.
- M. V. Spanedda and L. Bourel-Bonnet, Bioconjug. Chem., 2021, 32, 482–496 CrossRef CAS PubMed.
- T. Loth, R. Hötzel, C. Kascholke, U. Anderegg, M. Schulz-Siegmund and M. C. Hacker, Biomacromolecules, 2014, 15, 2104–2118 CrossRef CAS PubMed.
- S. H. Kim, Y. K. Yeon, J. M. Lee, J. R. Chao, Y. J. Lee, Y. B. Seo, M. T. Sultan, O. J. Lee, J. S. Lee, S. Yoon, I.-S. Hong, G. Khang, S. J. Lee, J. J. Yoo and C. H. Park, Nat. Commun., 2018, 9, 1620 CrossRef PubMed.
- N. E. Kurland, T. Dey, S. C. Kundu and V. K. Yadavalli, Adv. Mater., 2013, 25, 6207–6212 CrossRef CAS PubMed.
- Y. Jiang, X. Zhang, H. Nie, J. Fan, S. Di, H. Fu, X. Zhang, L. Wang and C. Tang, Nat. Commun., 2024, 15, 6060 CrossRef CAS PubMed.
- C. Piotrowski, C. H. Ihling and A. Sinz, Methods, 2015, 89, 121–127 CrossRef CAS PubMed.
- J. Liu, L. Cai, W. Sun, R. Cheng, N. Wang, L. Jin, S. Rozovsky, I. B. Seiple and L. Wang, Angew. Chem., Int. Ed., 2019, 58, 18839–18843 CrossRef CAS PubMed.
- G. Matheis and J. R. Whitaker, J. Food Biochem., 1987, 11, 309–327 CrossRef CAS.
- M. Loi, L. Quintieri, E. De Angelis, L. Monaci, A. F. Logrieco, L. Caputo and G. Mulè, Int. Dairy J., 2020, 100, 104555 CrossRef CAS.
- M. Motoki and K. Seguro, Trends Food Sci. Technol., 1998, 9, 204–210 CrossRef CAS.
- W. Kim, Y. Wang, Q. Ye, Y. Yao and C. Selomulya, Food Bioprod. Process., 2023, 139, 204–215 CrossRef CAS.
- H. Ando, M. Adachi, K. Umeda, A. Matsuura, M. Nonaka, R. Uchio, H. Tanaka and M. Motoki, Agric. Biol. Chem., 1989, 53, 2613–2617 CAS.
- D. Zhang, Y. Zhu and J. Chen, Biotechnol. Genet. Eng. Rev., 2009, 26, 205–222 CrossRef PubMed.
- S. W. Fatima and S. K. Khare, Microbiol. Res., 2018, 215, 7–14 CrossRef CAS PubMed.
- B. K. Bernard, S. Tsubuku and S. Shioya, Int. J. Toxicol., 1998, 17, 703–721 CrossRef CAS.
- S. F. Mirpoor, C. V. L. Giosafatto, R. Di Girolamo, M. Famiglietti and R. Porta, Food Packag. Shelf Life, 2022, 31, 100779 CrossRef CAS.
- M. Famiglietti, D. Zannini, R. Turco and L. Mariniello, Int. J. Mol. Sci., 2023, 24, 3405 CrossRef CAS PubMed.
- D. V. Bent and E. Hayon, J. Am. Chem. Soc., 1975, 97, 2599–2606 CrossRef CAS PubMed.
- D. V. Bent and E. Hayon, J. Am. Chem. Soc., 1975, 97, 2612–2619 CrossRef CAS PubMed.
- D. V. Bent and E. Hayon, J. Am. Chem. Soc., 1975, 97, 2606–2612 CrossRef CAS PubMed.
- S. Sowiński, G. H. C. Varca, S. Kadłubowski, A. B. Lugão and P. Ulański, Radiat. Phys. Chem., 2021, 188, 109644 CrossRef.
- V. Raikos, L. Campbell and S. R. Euston, Food Hydrocoll., 2007, 21, 237–244 CrossRef CAS.
- H. Kchaou, N. Benbettaieb, M. Jridi, M. Nasri and F. Debeaufort, Food Hydrocoll., 2019, 97, 105196 CrossRef CAS.
- C. Xu, X. Wei, F. Shu, X. Li, W. Wang, P. Li, Y. Li, S. Li, J. Zhang and H. Wang, Int. J. Biol. Macromol., 2020, 153, 232–239 CrossRef CAS PubMed.
- S. Kim, B. Marelli, M. A. Brenckle, A. N. Mitropoulos, E.-S. Gil, K. Tsioris, H. Tao, D. L. Kaplan and F. G. Omenetto, Nat. Nanotechnol., 2014, 9, 306–310 CrossRef CAS PubMed.
- A. G. Chmielewski, M. Haji-Saeid and S. Ahmed, Nucl. Instrum. Methods Phys. Res., Sect. B, 2005, 236, 44–54 CrossRef CAS.
- Q. Ke, H. Wang, X. Huang, Y. Zhang, Q. Meng and X. Kou, Ind. Crops Prod., 2023, 206, 117653 CrossRef CAS.
- K. Tumu, K. Vorst and G. Curtzwiler, Compr. Rev. Food Sci. Food Saf., 2023, 22, 1337–1359 CrossRef PubMed.
- R. Jolanki, T. Estlander and L. Kanerva, Contact Dermatitis, 1987, 16, 87–92 CrossRef CAS PubMed.
- R. M. LoPachin and T. Gavin, Chem. Res. Toxicol., 2014, 27, 1081–1091 Search PubMed.
- H. F. Program, FDA.
- W. D. Callister Jr and D. G. Rethwisch, Materials Science and Engineering: an Introduction, John Wiley & Sons, Incorporated, 10th edn, 2017 Search PubMed.
- H. Yue, Y. Zheng, P. Zheng, J. Guo, J. P. Fernández-Blázquez, J. H. Clark and Y. Cui, Green Chem., 2020, 22, 8642–8655 RSC.
- P. Tummala, W. Liu, L. T. Drzal, A. K. Mohanty and M. Misra, Ind. Eng. Chem. Res., 2006, 45, 7491–7496 CrossRef CAS.
- K. L. Consebit, K. C. Dermil, E. Y. Magbanua, F. J. Racadio, S. V. Saavedra, H. Abusama and A. Valdez, Asian J. Sci. Eng., 2022, 2, 129–132 CrossRef.
- M. G. Dianursanti and C. Noviasari, E3S Web Conf., 2018, 67, 03048 CrossRef.
- P. Mohammadi, A. S. Aranko, C. P. Landowski, O. Ikkala, K. Jaudzems, W. Wagermaier and M. B. Linder, Sci. Adv., 2019, 5, eaaw2541 CrossRef CAS PubMed.
- J. M. Bier, C. J. R. Verbeek and M. C. Lay, Macromol. Mater. Eng., 2014, 299, 524–539 CrossRef CAS.
- P. Zhou and T. P. Labuza, Food Biophys., 2007, 2, 108–116 CrossRef.
- J. L. Kokini, A. M. Cocero, H. Madeka and E. de Graaf, Trends Food Sci. Technol., 1994, 5, 281–288 CrossRef CAS.
- O. A. Attallah, M. Mojicevic, E. L. Garcia, M. Azeem, Y. Chen, S. Asmawi and M. Brenan Fournet, Polymers, 2021, 13, 2155 CrossRef CAS PubMed.
- Y. Zoungranan, E. Lynda, K. K. Dobi-Brice, E. Tchirioua, C. Bakary and D. D. Yannick, J. Environ. Chem. Eng., 2020, 8, 104396 CrossRef CAS.
- J. R. Kim, J.-R. Thelusmond, V. C. Albright and Y. Chai, Sci. Total Environ., 2023, 890, 164338 CrossRef CAS PubMed.
- S. Kliem, M. Kreutzbruck and C. Bonten, Materials, 2020, 13, 4586 CrossRef CAS PubMed.
- A. Folino, A. Karageorgiou, P. S. Calabrò and D. Komilis, Sustainability, 2020, 12, 6030 CrossRef CAS.
- O. García-Depraect, R. Lebrero, S. Rodriguez-Vega, S. Bordel, F. Santos-Beneit, L. J. Martínez-Mendoza, R. Aragão Börner, T. Börner and R. Muñoz, Bioresour. Technol., 2022, 344, 126265 CrossRef PubMed.
- M. Jiménez-Rosado, J. F. Rubio-Valle, V. Perez-Puyana, A. Guerrero and A. Romero, J. Appl. Polym. Sci., 2021, 138, 50412 CrossRef.
- I. Santana, M. Félix, A. Guerrero and C. Bengoechea, Polymers, 2022, 14, 355 CrossRef CAS PubMed.
- G. Bhagwat, K. Gray, S. P. Wilson, S. Muniyasamy, S. G. T. Vincent, R. Bush and T. Palanisami, J. Polym. Environ., 2020, 28, 3055–3075 CrossRef CAS.
- J. Ewert, C. Glück, H. Strasdeit, L. Fischer and T. Stressler, Enzyme Microb. Technol., 2018, 110, 69–78 CrossRef CAS PubMed.
- S. S. Priya, S. K. Dixit, S. Kabiraj and M. S. Priya, Environ. Sci. Pollut. Res., 2023, 30, 124401–124406 CrossRef PubMed.
- Food loss and waste account for 8-10% of annual global greenhouse gas emissions; cost USD 1 trillion annually | UNFCCC, https://unfccc.int/news/food-loss-and-waste-account-for-8-10-of-annual-global-greenhouse-gas-emissions-cost-usd-1-trillion, accessed 11 November 2024.
- R. Lan, S. D. Eastham, T. Liu, L. K. Norford and S. R. H. Barrett, Nat. Commun., 2022, 13, 6537 CrossRef CAS PubMed.
- A. Dutta, A. Patra, K. K. Hazra, C. P. Nath, N. Kumar and A. Rakshit, Environ. Chall., 2022, 8, 100581 CrossRef.
- T. Semba, Y. Sakai, T. Sakanishi and A. Inaba, J. Clean. Prod., 2018, 195, 932–938 CrossRef CAS.
- M. Samer, O. Hijazi, B. A. Mohamed, E. M. Abdelsalam, M. A. Amer, I. H. Yacoub, Y. A. Attia and H. Bernhardt, Clean Technol. Environ. Policy, 2022, 24, 815–827 CrossRef CAS.
- A. Wells, Casein, https://plastiquarian.com/?page_id=14228, accessed 11 February 2025.
- M. M. Brooks, Forgotten Histories & Possible Futures: Learning from 20th century fibres and films made from waste regenerated protein sources. Presented at Plastics Heritage Congress, Lisbon, Portugal, 2020 Search PubMed.
- B. E. Ralston and T. A. Osswald, Plast. Eng., 2008, 64, 36–40 CrossRef.
- P. Li, X. Zhou, B. Jian, M. Zhou, R. Liu, B. Sun, X. Li, Y. Wang and B. Zhou, Ind. Crops Prod., 2024, 222, 119431 CrossRef CAS.
- K. P. Panthi, D. K. Shahi, M. L. Sharma, Z. Li, L. M. Pandey and M. K. Joshi, Food Bioprocess Technol., 2025, 18, 2882–2898 CrossRef CAS.
- A. Botalo, T. Inprasit, S. Ummartyotin, K. Chainok, S. Vatthanakul and P. Pisitsak, Polymers, 2024, 16, 447 CrossRef CAS PubMed.
- S. Patel, US Pat., US8852157B2, United States, 2014 Search PubMed.
- Mater-Bi | Original biodegradable and compostable bioplastics, https://materbi.com/en/, accessed 31 March 2025.
- The Plant Based Products Council, https://live-pbpccom.pantheonsite.io/, accessed 31 March 2025.
- S. S. Purewal, A. Kaur, S. P. Bangar, P. Singh and H. Singh, Coatings, 2024, 14, 32 CrossRef CAS.
- L. Sartore, E. Schettini, L. de Palma, G. Brunetti, C. Cocozza and G. Vox, Sci. Total Environ., 2018, 645, 1221–1229 CrossRef CAS PubMed.
- Y. Cao, S. S. Koh, Y. Han, J. J. Tan, D. Kim, N.-H. Chua, D. Urano and B. Marelli, Adv. Mater., 2023, 35, 2205794 CrossRef CAS PubMed.
- N. Jaramillo-Quiceno, D. M. Carmona, M. Torres-Taborda, G. A. Hincapié-Llanos and C. Álvarez-López, Horticulturae, 2024, 10, 273 CrossRef.
- J. Zou, J. Wong, C.-R. Lee, N. Nitin, L. Wang and G. Sun, ACS Appl. Bio Mater., 2024, 7, 1842–1851 CrossRef CAS PubMed.
- R. Ramos, J. Bernard, F. Ganachaud and A. Miserez, Small Sci., 2022, 2, 2100095 CrossRef CAS PubMed.
- F. Apone, A. Barbulova and M. G. Colucci, Front. Plant Sci., 2019, 10, 756 CrossRef PubMed.
- B. Chalermthai, M. T. Ashraf, J.-R. Bastidas-Oyanedel, B. D. Olsen, J. E. Schmidt and H. Taher, Polymers, 2020, 12, 847 CrossRef CAS PubMed.
- C. Wellenreuther, A. Wolf and N. Zander, Clean Eng. Technol., 2022, 6, 100411 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |






