Open Access Article
Open Access ArticleThree-component synthesis of β-sulfonyl enamines and dienamines enabled by silver(I) acetate†
Jakub Koudelka and
Tomáš Tobrman *
*
Department of Organic Chemistry, University of Chemistry and Technology, Prague, Technická 5, 166 28 Prague 6, Czech Republic. E-mail: tomas.tobrman@vscht.cz
First published on 4th February 2025
Abstract
We have developed a novel three-component synthesis of sulfonyl enamines by reacting secondary and tertiary amines with sodium sulfinic acid salt, a reaction that is mediated by silver acetate. The choice of solvent determines whether sulfonyl enamines or dienamines are obtained. The overall atom economy of this multicomponent reaction was further improved by isolating the resulting elemental silver and reconverting it into silver acetate.
Introduction
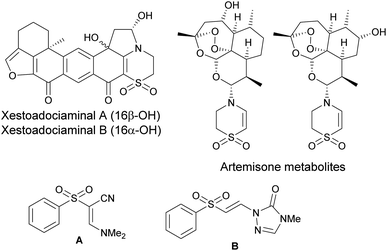
Organic synthesis has undergone rapid development over the last few decades, leading to the creation of new procedures for the preparation of amines,1 ethers,2 heterocyclic compounds,3 and both tetrasubstituted4 and trisubstituted5 alkenes. In addition to the above-mentioned substances, vinyl sulfones represent an important class of compounds that are the subject of intense study in terms of their synthesis and application. For example, rigosertib6 and other vinyl sulfones exhibit significant antitumor activity,7 neuroprotective effects against Parkinson's disease,8 cysteine protease inhibition,9 and antiparasitic activity.10 Moreover, significant attention has been paid to sulfonyl enamines due to their medicinal applications. In this regard, cyclic sulfonyl enamines form the key structural motif of Xestoadociaminals A and B, compounds that have been isolated from the Indonesian marine sponge Xestospongia sp. (Fig. 1).11 Some cyclic sulfonyl enamines are formed during the microsomal metabolism of artemisone.12 Furthermore, the artificially synthesized sulfonyl enamines A and B have been characterized based on their antimicrobial activity13 and their role as activators of nuclear factor erythroid 2-related factor 2 (Nrf2).14The practical significance of both vinyl sulfones and sulfonyl enamines is closely tied to the development of efficient methods for their preparation. Traditional methods for the preparation of sulfonyl enamines include the conjugate addition of amines to sulfonylacetylenes15 and the C–H sulfonylation of enamides.16 However, a distinct approach to the formation of sulfonyl enamines involves the oxidative sulfonylation of cyclic amines,17 formal C–H activation with the insertion of sulfur dioxide,18 and the direct reaction of tertial amines with sulfonyl chlorides19 or sulfonyl hydrazides.20
From a practical perspective, it is advantageous to perform the synthesis of sulfonyl enamines using the method described by Gui et al., who developed the tetrabutylammonium iodide-catalyzed synthesis of sulfonyl enamines in the presence of stoichiometric amounts of tert-butyl hydroperoxide (TBHP) (Scheme 1a).21 By contrast, Yuan observed the significant effect of solvents on the course of the reaction between sodium sulfinates and tertiary amines. In this respect, water favored the formation of sulfonamides, while dimethyl sulfoxide (DMSO) favored the formation of sulfonyl enamines (Scheme 1b).22 In both cases, the iminium salt Im1 and enamine Im2 were proposed as intermediates during the preparation of sulfonyl enamines.21,22 Our research interest in the synthesis of alkenes,23 along with the predicted formation of the iminium salt Im1 and the significant effect of N-substitution on the stability of iminium salts,24 led us to propose a new multicomponent synthesis procedure of β-sulfonyl enamines (Scheme 1, this work). In this new multicomponent reaction, we expected the formation of the more stable iminium salt Im4 by means of transimination from iminium salt Im3.
Result and discussion
In terms of the proposed multicomponent reaction, we aimed to optimize the reaction conditions. We quickly discovered that most oxidants, including CuI, I2/TBHP, I2, FeCl3, and MnO2, when used in either stoichiometric or catalytic amounts, were ineffective, meaning that the desired product 4aaa was not formed (see the ESI† for further details). However, using three equivalents of silver acetate in dimethylformamide (DMF) or dimethylsulfoxide (DMSO) yielded the enamine 4aaa, albeit in a low yield (Table 1, entries 1 and 2). Through solvent variation, we found that the most effective transformation occurred in tetrahydrofuran (THF) with six equivalents of silver acetate (Table 1, entries 3–6). It is important to note that lithium benzenesulfinate (1aLi) and potassium benzenesulfinate (1aK) yielded the enamine 4aaa in a lower yield (Table 1, entries 7 and 8). Further solvent variation revealed that significant amounts of diene 5aaa were formed in acetonitrile and ethanol, respectively (Table 1, entries 9–11). Ultimately, a mixture of ethanol and acetonitrile produced only diene 5aaa, although in a moderate isolated yield (Table 1, entry 12). The effect of the solvent on the formation of enamine 4aaa and dienamine 5aaa remains unclear and will be explored in future studies.| Entry | 1a (equiv.) | 2a (equiv.) | AgOAc (equiv.) | Solvent | 4aaa/5aaaa (%) |
|---|---|---|---|---|---|
a 1H NMR yields.b Isolated yield.c PhSO2Li (1aLi) was used instead of 1a.d PhSO2K (1aK) was used instead of 1a.e A mixture of MeCN and EtOH in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v was used. 3 v/v was used. |
|||||
| 1 | 1.2 | 1.2 | 3 | DMF | 14/— |
| 2 | 1.2 | 1.2 | 3 | DMSO | 14/3 |
| 3 | 1.2 | 1.2 | 3 | Toluene | 22/4 |
| 4 | 1.2 | 1.2 | 3 | CpOMe | 11/— |
| 5 | 1.2 | 1.2 | 3 | THF | 63/— |
| 6 | 2.4 | 2.4 | 6 | THF | 90 (67b)/— |
| 7 | 2.4c | 2.4 | 6 | THF | −(46b)/— |
| 8 | 2.4d | 2.4 | 6 | THF | −(55b)/— |
| 9 | 1.2 | 2.2 | 3 | MeCN | 15/25 |
| 10 | 1.2 | 2.2 | 3 | EtOH | 0/30 |
| 11 | 1.2 | 4.2 | 3 | MeCN/EtOHe | 0/53 (22b) |
| 12 | 2.4 | 4.2 | 6 | MeCN/EtOHe | 0/— (35b) |
After identifying the optimal reaction conditions, we evaluated their scope (Scheme 2). Both the cyclic and acyclic aliphatic secondary amines reacted as expected, forming enamines 4aaa–4aae. Similar reactivity was observed with N-methylallylamine (2f) and N-methyl(benzyl)amine (2g). Additionally, the secondary amines with bulky cyclohexyl and isopropyl substituents yielded the corresponding enamines 4aah, 4aai and 4aaj in satisfactory yields. For selected secondary amines, we also obtained dienamines 5aaa, 5aac, 5aaf, 5aag, and 5aaj, albeit in lower isolated yields, which is consistent with the optimization experiments. The primary amines and certain secondary amines did not yield the expected products. Moreover, no reactivity was observed for N-ethylaniline and indole.
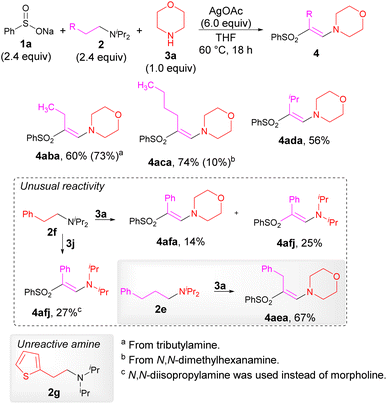
The optimized reaction conditions are not limited to the preparation of disubstituted alkenes. Notably, in the case of more complex tertiary amines, we successfully prepared trisubstituted alkenes (Scheme 3). Excellent reactivity was observed for amines with aliphatic substituents, yielding enamines 4aba, 4aca and 4ada. In contrast, a tertiary amine containing the homobenzyl group 2f yielded a mixture of the diisopropylamine 4afj and the morpholine derivative 4afa. Substituting morpholine (3a) with diisopropylamine (3j) resulted in the exclusive formation of amine 4afj in a 27% isolated yield, while the tertial amine 2e containing a three-carbon spacer resulted in the clean formation of transimination product 4aea. These results suggest that the exchange of the NiPr2 for the morpholine moiety occurs significantly slower than in other cases. In addition, the tertial amine 2g bearing a 2-thienyl group did not react at all.
The structure of the sulfinic acid sodium salt also plays a significant role in the course of the multicomponent reaction (Scheme 4). Sodium salts derived from substituted benzenesulfinic acids reacted satisfactorily, yielding alkenes 4baa, 4caa, and 4daa. Similar isolated yields of the enamines 4eaa and 4faa were obtained for even 1-naphthyl- and 1-pyrenylsulfinic acid sodium salts. However, n-butylsulfinic acid sodium salt produced the enamine 4gaa in only a 9% yield. The reactivity of the styrenyl and heterocyclic sulfinic acid sodium salts differed markedly. Here, 3-pyridylsulfinic acid sodium salt did not react at all, whereas 2-thienylsulfinic acid sodium salt yielded a mixture of sulfonamide 6 and enamine 4iaa, with no observed transimination. Styrenylsulfinic acid sodium salt only produced sulfone 7 in a low isolated yield.
To further expand the portfolio of trisubstituted alkenes, we performed the gram-scale synthesis of alkene 4aaa, achieving a 70% isolated yield (Scheme 5). In addition to the target alkene 4aaa, elemental silver was recovered and converted back into silver acetate using nitric acid and sodium acetate. The recycled silver acetate provided the alkene 4aaa with a 57% yield on a 0.5 mmol scale, thereby improving the overall atom economy of the developed multicomponent reaction. In addition, the prepared alkene 4aaa was lithiated to the alkene 4aaaLi using n-butyllithium, and the subsequent reactions with organohalides produced the trisubstituted enamines 4aha, 4aea, and 4aia in high yields. This approach successfully expanded the scope of the trisubstituted enamines available via novel three-component synthesis, starting from the readily available disubstituted enamine 4aaa. The mechanism of this novel multicomponent reaction will be further explored; however, we hypothesize that the key step involves the transimination of the iminium salt from Im3 to Im4 (Scheme 1, this work). Experimental evidence suggests that enamines 4 are not generated through nucleophilic substitution of the NiPr2 group by a secondary amine, as demonstrated by the reaction of the enamine 4aaj with morpholine under typical reaction conditions, which did not yield the anticipated product 4aaa (Scheme 5c).
 | ||
| Scheme 5 (a) Gram-scale synthesis of alkene 4aaa, (b) late-stage modification of enamine 4aaa and (c) attempted conversion of 4aaj to 4aaa. | ||
Conclusions
In conclusion, we developed a new multicomponent reaction for the preparation of di- and trisubstituted sulfonyl enamines. The optimized reaction conditions involve reacting tertiary and secondary amines with the sodium salt of arylsulfinic acid in the presence of silver acetate in THF at 60 °C. Substituting THF with an ethanol–acetonitrile mixture can yield the corresponding dienamines. These optimized conditions allow for the gram-scale synthesis of enamines. The overall atom economy of the multicomponent reaction is improved by isolating the silver by-product and reconverting it into silver acetate. Late-stage modification of disubstituted enamines further expands the portfolio of prepared trisubstituted enamines.Data availability
The data supporting this article (additional optimization experiments, experimental procedures, analytical data of synthesized compounds, copies of 1H and 13C NMR spectra) have been included as part of the ESI.†Author contributions
Investigation, methodology, visualization, writing – original draft, writing – review & editing (JK); funding, conceptualization, writing – original draft, writing – review & editing (TT).Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work is supported by Operational Programme Johannes Amos Comenius financed by European Structural and Investment Funds and the Czech Ministry of Education, Youth and Sports (Project No. SENDISO – CZ.02.01.01/00/22_008/0004596).References
- (a) P. A. Forero-Cortés and A. M. Haydl, Org. Process Res. Dev., 2019, 23, 1478 CrossRef; (b) M. M. Heravi, Z. Kheilkordi, V. Zadsirjan, M. Heydari and M. Malmir, J. Organomet. Chem., 2018, 861, 17 CrossRef CAS.
- (a) S. Mandal, S. Mandal, S. K. Ghosh, P. Sar, A. Ghosh, R. Saha and B. Saha, RSC Adv., 2016, 6, 69605 RSC; (b) E. N. Pitsinos, V. P. Vidali and E. A. Couladouros, Eur. J. Org Chem., 2011, 1207 CrossRef CAS; (c) D. J. Winternheimer, R. E. Shade and C. A. Merlic, Synthesis, 2010, 2497 CAS.
- (a) W. R. Bowman, M. O. Cloonan and S. L. Krintel, J. Chem. Soc., Perkin Trans. 1, 2001, 2885 RSC; (b) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Acc. Chem. Res., 2016, 49, 1911 CrossRef CAS PubMed; (c) N. T. Patil and Y. Yamamoto, Chem. Rev., 2008, 108, 3395 CrossRef CAS PubMed.
- (a) T. Edlová, M. Čubiňák and T. Tobrman, Synthesis, 2021, 53, 255 CrossRef; (b) P. Polák, H. Váňová, D. Dvořák and T. Tobrman, Tetrahedron Lett., 2016, 57, 3684 CrossRef; (c) T. Tobrman and S. Mrkobrada, Organics, 2022, 3, 210 CrossRef CAS; (d) F. Buttard, J. Sharma and P. A. Champagne, Chem. Commun., 2021, 57, 4071 RSC; (e) A. B. Flynn and W. W. Ogilvie, Chem. Rev., 2007, 107, 4698 CrossRef CAS PubMed; (f) N. Mukherjee, S. Planer and K. Grela, Org. Chem. Front., 2018, 5, 494 RSC.
- (a) A. H. Hoveyda, C. Qin, X. Z. Sui, Q. Liu, X. Li and A. Nikbakht, Acc. Chem. Res., 2023, 56, 2426 CrossRef CAS PubMed; (b) R. Manikandan and M. Jeganmohan, Org. Biomol. Chem., 2015, 13, 10420 RSC; (c) E.-i. Negishi, G. Wang, H. Rao and Z. Xu, J. Org. Chem., 2010, 75, 3151 CrossRef CAS PubMed; (d) K. A. Ouzounthanasis, S. R. Rizos and A. E. Koumbis, Eur. J. Org Chem., 2023, 26, e202300626 CrossRef CAS.
- K. Gumireddy, M. V. R. Reddy, S. C. Cosenza, R. B. Nathan, S. J. Baker, N. Papathi, J. Jiang, J. Holland and E. P. Reddy, Cancer Cell, 2005, 7, 275 CrossRef CAS PubMed.
- (a) T. Lu, C. A. Laughton, S. Wang and T. D. Bradshaw, Mol. Pharmacol., 2015, 87, 18 CrossRef; (b) L. Tang, T. Chen, H. Yang, X. Wen, Y. Sun, S. Liu, T. Peng, S. Zhang and L. Wang, RSC Adv., 2021, 11, 37462 RSC.
- S. Y. Woo, J. H. Kim, M. K. Moon, S.-H. Han, S. K. Yeon, J. W. Choi, B. K. Jang, H. J. Song, Y. G. Kang, J. W. Kim, J. Lee, D. J. Kim, O. Hwang and K. D. Park, J. Med. Chem., 2014, 57, 1473 CrossRef CAS PubMed.
- B. D. Fennell, J. M. Warren, K. K. Chung, H. L. Main, A. B. Arend, A. Tochowicz and M. G. Götz, J. Enzyme Inhib. Med. Chem., 2013, 28, 468 CrossRef CAS PubMed.
- I. D. Kerr, J. H. Lee, C. J. Farady, R. Marion, M. Rickert, M. Sajid, K. C. Pandey, C. R. Caffrey, J. Legac, E. Hansell, J. H. McKerrow, C. S. Craik, P. J. Rosenthal and L. S. Brinen, J. Biol. Chem., 2009, 284, 25697 CrossRef CAS PubMed.
- F. He, L. H. Mai, A. Longeon, B. R. Copp, N. Loaëc, A. Bescond, L. Meijer and M.-L. Bourguet-Kondracki, Mar. Drugs, 2015, 13, 2617 CrossRef CAS PubMed.
- L. Grobler, A. Grobler, R. Haynes, C. Masimirembwa, R. Thelingwani, P. Steenkamp and H. S. Steyn, Expert Opin. Drug Metab. Toxicol., 2014, 10, 313 CrossRef CAS PubMed.
- M. R. Shaaban, Heterocycles, 2008, 75, 3005–3014 CrossRef CAS.
- V. Markovtsov, M. A. J. Duncton, A. Bagos, S. Yi, S. Braselmann, S. Bhamidipati, I. S. Darwish, J. Yu, A. M. Owyang, B. Fernandez, B. Samant, G. Park, E. S. Masuda and S. J. Shaw, ACS Med. Chem. Lett., 2023, 14, 1700 CrossRef CAS PubMed.
- (a) S. Cossu, O. De Lucchi and R. Durr, Synth. Commun., 1996, 26, 4597 CrossRef CAS; (b) E. Petit, L. Bosch, J. Font, L. Mola, A. M. Costa and J. Vilarrasa, J. Org. Chem., 2014, 79, 8826 CrossRef CAS PubMed.
- H. Jiang, X. Chen, Y. Zhang and S. Yu, Adv. Synth. Catal., 2013, 355, 809 CrossRef CAS.
- (a) R. J. Griffiths, W. C. Kong, S. A. Richards, G. A. Burley, M. C. Willis and E. P. A. Talbot, Chem. Sci., 2018, 9, 2295 RSC; (b) K. Muralirajan, R. Kancherla and M. Rueping, Angew. Chem., Int. Ed., 2018, 57, 14787 CrossRef CAS PubMed; (c) X. Rong, J. Guo, Z. Hu, L. Huang, Y. Gu, Y. Cai, G. Liang and Q. Xia, Eur. J. Org Chem., 2020, 701 Search PubMed; (d) J. Tong, H. Li, Y. Zhu, P. Liu and P. Sun, Green Chem., 2022, 24, 1995 RSC.
- Y. He, J. Yang, Q. Liu, X. Zhang and X. Fan, J. Org. Chem., 2020, 85, 15600 CrossRef CAS.
- (a) M. Chen, Z. T. Huang and Q. Y. Zheng, Org. Biomol. Chem., 2014, 12, 9337 RSC; (b) H. Wei, G. Wang, B. Li, J. Huang, H. Li, O. P. Pereshivko and V. A. Peshkov, Heterocycl. Commun., 2016, 22, 333 CrossRef CAS; (c) R. Zhang, Y. Cai, D. Sun, S. Xu and Q. Zhou, Synlett, 2017, 28, 1630 CrossRef; (d) S. Firoozi and M. Hosseini-Sarvari, J. Org. Chem., 2021, 86, 2117 CrossRef CAS PubMed.
- H. S. Kim and S. Lee, Eur. J. Org Chem., 2019, 6951 CrossRef CAS.
- H. Jiang, X. Tang, Z. Xu, H. Wang, K. Han, X. Yang, Y. Zhou, Y.-L. Feng, X.-Y. Yu and Q. Gui, Org. Biomol. Chem., 2019, 17, 2715 RSC.
- J. Lai, L. Chang and G. Yuan, Org. Lett., 2016, 18, 3194 CrossRef CAS PubMed.
- (a) J. Koudelka and T. Tobrman, Eur. J. Org Chem., 2021, 3260 CrossRef CAS; (b) T. Edlová, H. Dvořáková, V. Eigner and T. Tobrman, J. Org. Chem., 2021, 86, 5820 CrossRef PubMed.
- A. M. Costa, A. Castro-Alvarez, D. Fillot and J. Vilarrasa, Eur. J. Org Chem., 2022, e202200627 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08480b |
| This journal is © The Royal Society of Chemistry 2025 |