 Open Access Article
Open Access ArticleLong-range magnetic interaction of native defects in transition metal dichalcogenides†
Prashant Vijay Gaikwad abd,
T. Thuy Hoang
abd,
T. Thuy Hoang ac,
Sungjin Parkab and
Junhyeok Bang
ac,
Sungjin Parkab and
Junhyeok Bang *ab
*ab
aDepartment of Physics, Chungbuk National University, Cheongju 28644, Republic of Korea. E-mail: jbang@cbnu.ac.kr
bResearch Institute for Nanoscale Science and Technology, Cheongju 28644, Republic of Korea
cBasic Science Research Institute, Chungbuk National University, Cheongju 28644, Republic of Korea
dDepartment of Physics, Savitribai Phule Pune University, Pune-411007, India
First published on 27th February 2025
Abstract
Recent experiments have revealed weak ferromagnetism in pristine transition metal dichalcogenides (TMDs), although the underlying mechanism remains unclear. In this work, we investigate the possibility of native defects inducing ferromagnetism in TMDs, specifically WS2 and MoS2. Among the various native defects, we have identified that cation antisites exhibit localized magnetic moments of 2μB. These localized moments tend toward ferromagnetic ordering via magnetic interactions facilitated by local spin density oscillations. While the strength of the magnetic interactions is comparable to that of magnetic dopants in TMD, they are significantly weakened when the distance between the two antisites exceeds 9 Å. Therefore, our results suggest that native-defect-induced ferromagnetism in TMD is feasible only in heavily defective TMD samples.
1. Introduction
The discovery of two-dimensional (2D) magnetic materials such as CrI3 and CrGeTe3 was a great scientific and practical advance.1,2 In the scientific aspect, the existence of the long-range magnetic order in the 2D systems has been a fundamental question, as the Mermin–Wagner theorem suggested that the 2D long-range orders are destroyed by the thermal fluctuations.3 Currently, 2D magnetism is understood based on the effect of the magnetic anisotropy by transferring the 2D system from the Heisenberg model to the Ising model.2 In the practical aspect, the realization of the 2D magnetic systems could lead to new 2D-based magnetic applications.4–9 Several 2D materials with exceptional electric and optical properties have been found over the past decades and are considered to be a new family for novel device applications.10–13 However, magnetism has been one property missing from 2D materials. The emergence of 2D magnets is likely to open new directions in 2D materials.Among the 2D materials, graphene and transition metal dichalcogenides (TMDs) have been intensively studied due to their exceptional properties. Although they are non-magnetic in their pristine forms, many studies have been performed to introduce magnetism in them by doping with transition metals such as Mn, V, Cr, Fe, CO, and P.14–20 In TMDs, the long-range ferromagnetic interaction can be established by super-exchange interaction among highly localized d-states of the transition metals by antiferromagnetically coupled delocalized p-states of chalcogenides. More interestingly, ferromagnetism has been experimentally observed in graphene without any intentional doping.21–25 Many theoretical works have studied this graphene ferromagnetism, and it is now understood to be based on the pseudo-spin-mediated long-range ferromagnetic coupling between local magnetic moments generated by native defects or hydrogen absorption.26–28
Recent experiments have also observed a weak but room-temperature ferromagnetism in pristine (but possibly defective) TMDs such as WS2 and MoS2.29–33 Because TMDs are semiconductors without the metallic pseudo-spin state that exists in graphene, the mechanism of the ferromagnetism of TMDs could be different from that in graphene. To explain the intrinsic ferromagnetism, several theoretical and experimental studies have been performed. Huo et al. proposed edge-state-induced ferromagnetism in WS2.29 Other groups have suggested that local metallic 1T phases inside the host 2H TMD can be ferromagnetically coupled and lead to ferromagnetism.32,34
As another possible mechanism, defects could also lead to ferromagnetism in TMDs, like in graphene. Native defects such as vacancies and interstitials are inevitably generated in the growth processes and can significantly affect the properties of TMDs.35,36 It is known that defects in TMDs could have local magnetic moments,35,37 and a previous work suggested the possibility of magnetically-active-defect-induced ferromagnetism.30 However, the detailed mechanism of defect-induced ferromagnetism, especially long-range magnetic coupling between the magnetic defects, has not been studied yet.
In this work, we have investigated the effects of native defects on the magnetic properties of TMDs such as WS2 and MoS2. First, we have examined isolated defects such as S vacancies (VS), transition metal anti-sites (MS, M = W or Mo), transition metal interstitials (Mi), and MS and VS complex defects (MS–VS), which have localized d-orbitals, and thus could have a localized magnetic moment. While VS and Mi have no magnetic moment, MS and MS–VS exhibit localized magnetic moments of 2μB. Second, we have studied the long-range magnetic interaction between MS defects with respect to their relative positions. The magnetic interaction is mediated by the local spin density oscillation, and it shows that ferromagnetic couplings are favorable for most of the relative positions. However, the magnetic interaction varies with the relative position and is significantly reduced over the fourth-nearest neighboring site. Our results suggest that defect-induced ferromagnetism can be generated in heavily defective TMDs.
2. Methodology
The optimized atomic structures and relevant total energies were calculated based on spin-polarized density functional theory (DFT),38,39 as implemented in the Vienna ab initio simulation package (VASP).40–43 The plane augmented wave method was used to represent the ion cores,44 and the generalized gradient approximation formulated by Perdew, Burke, and Ernzerhof was used for the exchange–correlation functional.45,46 The wavefunctions were expanded in a plane wave basis set up to a cut-off energy of 500 eV. To model the isolated defects and the magnetic coupling between two defects, we employed a periodic 7 × 7 supercell with a 15 Å vacuum region along the z-direction, which is sufficiently large to avoid artificial interaction between periodic images. For Brillouin-zone integration, a Γ-centered 3 × 3 × 1 k-point mesh was used for ionic relaxation, and a denser 6 × 6 × 1 k-point mesh was used for precise electronic structure calculations. Atomic structures were fully relaxed until the residual forces were less than 0.02 eV Å−1. The spin–orbit coupling (SOC) was incorporated using an approximation of relativistic effects by a scalar relativistic Hamiltonian with SOC in a perturbation treatment.47 However, the inclusion of SOC was found to have a minor effect on the energy of the magnetic coupling.The formation energy FE(D) of a defect D was calculated as
3. Results and discussion
3.1. Localized magnetic moments in native defects
We first considered native defects in WS2 that could generate a local magnetic moment. Among the various native defects, we focused on VS, WS, and Wi defects as shown in Fig. 1(a)–(c), respectively, because they have unpaired d-orbitals of W atoms, so they are likely to have a localized magnetic moment. This is not the case for the other isolated native defects such as W vacancies, S interstitials, and S anti-sites. All the defects we considered are likely to be formed in W-rich (or S-poor) growth conditions. In this regard, we assume W-rich WS2 samples in this work.Anion vacancies, i.e., VS in this case, have been widely observed in TMDs.35,36,48 Our calculations also showed that VS has the lowest formation energy, as shown in Table 1. However, VS has no local magnetic moments, despite the existence of the non-bonding d-orbitals of the three neighboring W atoms [see Fig. 1(a)]. This is because of the inward relaxation of the three W atoms and the formation of the fully occupied bonding levels of the non-bonding d-orbitals, leaving the fully unoccupied d-levels in the gap. In pristine WS2, the distance between two W atoms (DW–W) is 3.18 Å. However, near the VS defect, as shown in Fig. 1(a), DW–W is reduced by 0.14 Å. This relaxation leads to the formation of a bonding level inside the valence band, effectively quenching the local magnetic moment.
| WS2 | MoS2 | |||||
|---|---|---|---|---|---|---|
| EF (eV) | m (μB) | EF (eV) | m (μB) | |||
| W-rich | S-rich | Mo-rich | S-rich | |||
| VS | 1.56 | 2.79 | 0.0 | 1.33 | 2.63 | 0.0 |
| Mi | 5.30 | 7.77 | 0.0 | 3.86 | 6.47 | 0.0 |
| MS | 5.45 | 9.14 | 2.0 | 3.86 | 7.78 | 2.0 |
| MS-VS | 6.74 | 11.66 | 2.0 | 5.14 | 10.36 | 2.0 |
In the W-rich growth conditions, the sufficient W adatoms are likely to encounter the abundant VS sites on WS2, and the VS sites can be filled by the W atoms, forming cation anti-site defects WS, as shown in Fig. 1(b). The inclusion of a W atom at the VS site restores the DW–W (3.12 Å) near the WS defect [see Fig. 1(b)] to a value close to that of the pristine structure. Additionally, no significant changes were observed in other bonds, such as the W–S bonds. Our calculations showed that the WS defect exhibits a local magnetic moment of 2 μB, which is in good agreement with previous results.49 Due to the different atomic environment of the WS atom compared with that of the host W atoms, it shows different level splitting of the WS d-orbitals. As shown in Fig. 2(a), the three dXY, dX2−Y2, and dZ2 levels appear around the band gap, while the other two dXZ and dYZ levels are located below the valence band maximum (VBM). For the majority spin state, the dXY and dX2−Y2 levels are doubly degenerate at 0.91 eV, and the single dZ2 level is located at 1.16 eV with respect to the VBM, as shown in Fig. 2(a). Note that the minority spin dXY, dX2−Y2, and dZ2 levels are located near the conduction band minimum (CBM), showing large spin splitting. WS has effectively six valence electrons in the d-orbitals: four originate from the valence electrons of WS and two originate from the three neighboring W atoms. Among them, the four are filled into the dXZ and dYZ levels below VBM, and the doubly degenerate dXY and dX2−Y2 levels in the gap are occupied by the remaining two electrons. Therefore, this partial electron occupation of localized d-levels induces the large spin splitting by the exchange interaction,14 and WS can have a magnetic moment of 2μB.
The inset in Fig. 2(a) shows the spatial distribution of the spin density of WS. The majority spin is highly localized near the WS atom, resulting in an atomic magnetic moment 1.51μB. For the three nearest neighboring W atoms, however, the minority spin is distributed with an atomic magnetic moment of −0.09μB per each W atom. For the three next nearest neighboring W atoms, the majority spin is again distributed with a smaller local magnetic moment 0.09μB per each W atom. The spin density oscillation is caused by the super-exchange interaction through an S atom.49 Note that the magnetic moment on S atoms is very small because of the absence of d-orbitals. This spin density oscillation is induced by the WS defect. Similar spin density oscillations have been found in graphene due to defects, but the spin density is distributed in much longer range, leading to long-range magnetic coupling.26
Fig. 1(c) shows the atomic structure of Wi. The W adatom is stabilized on the host W site, forming a symmetrically separated two W dumbbell structure. The formation energy of Wi in the W-rich condition is similar to, but a bit smaller than that of WS (see Table 1). However, due to the bonding between the two W atoms, similar to VW, Wi also has no magnetic moment.
We have summarized the formation energies and magnetic moments of the isolated defects discussed above in Table 1. While WS has a localized magnetic moment, its formation energy is relatively high. Thus, under equilibrium growth conditions, the WS concentration [WS] may be too low to lead to ferromagnetism in WS2. However, under non-equilibrium growth conditions, [WS] can be significant due to a diffusion-limited kinetic process. In the process, WS can be formed by the migration and clustering of VS and Wi if the following two conditions are met: (i) attractive interaction between VS and Wi, and (ii) activation of VS or/and Wi diffusion.50,51 For condition (i), our calculations show that the binding energy between VS and Wi to form WS is quite large (about 1.41 eV), implying a strong attractive interaction. For condition (ii), previous results showed that the diffusion energy barrier is about 0.6 eV for transition metal interstitials in TMD.52 This is in good agreement with the fact that WS as well as VS are widely observed in experiments.35,36 Hence, we will consider the effect of the anti-site defect WS on the magnetic properties in TMD in the next section.
Until now, we have discussed isolated point defects in WS2. In the non-equilibrium process that we considered above, large complex defects as well as WS can be also formed by the kinetic processes of the isolated defects. The MS–VS complex [see Fig. 1(d)], which has been observed in experiments,35 is an example. Regarding the two conditions above, (i) the binding energy between MS and VS is about 0.27 eV, and (ii) the VS diffusion, for which the diffusion barrier is about 2.3 eV,53 can be activated at the sample growth temperature of ∼1000 K.15,29 While the binding energy is small, and so the WS–VS concentration is expected to be low even in non-equilibrium conditions, the WS−VS complex can exist and could lead a localized magnetic moment in WS2. As shown in Fig. 2(b), the doubly degenerate dXY and dX2−Y2 levels in the gap are occupied, and it has a local magnetic moment 2μB, similar to those of WS. Previous works54 have considered other large complex defects such as a W and two S vacancy complex and a six S vacancy cluster, which exhibit local magnetic moments. These results indicate that, although these complex defects could affect the magnetic properties of WS2, the concentration of the large complex defects is so low, their effect could be insignificant even under non-equilibrium conditions. In this regard, we ignored the possible effect of large complex defects in the next section, and we focused on the long-range magnetic coupling between MS defects, which are expected to be the most abundant magnetic defect in WS2.
Until now, we have focused on the various defects in WS2. We also studied the corresponding defects in MoS2, for which the results are summarized in Table 1. Most of the results are qualitatively similar to those in WS2. There is one thing to note: the formation energy of the Mo anti-site defect (MoS) is smaller than that of WS in WS2, so one can expect a higher MoS concentration. Thus, we believe that the qualitative magnetic properties induced by the native defects could be similar in both materials.
3.2. Long-range magnetic interaction of cation anti-site defects
To investigate the long-range ordering of the local magnetic moments in native defects and the defect-induced ferromagnetism in TMDs, the magnetic interaction between two MS defects (M = W for WS2 and Mo for MoS2) was considered in a 7 × 7 supercell, in which the MS concentration was 2% with respect to the total number of M atoms. This defect concentration is experimentally accessible in the TMDs.35 As a measure of the magnetic interaction ΔEm, we considered the energy differences between ferromagnetic (FM) and antiferromagnetic (AFM) ordering of the two MS defects, i.e., ΔEm = EAFM − EFM, where EFM and EAFM are the total energy of the FM and AFM orderings. Fig. 3(a) shows ΔEm as a function of the distance d between two MS defects, and the results are also summarized in Table 2. Here, the positive (negative) ΔEm indicates that the FM (AFM) ordering is stable.| WS2 | MoS2 | |||||
|---|---|---|---|---|---|---|
| d (Å) | ΔEm (meV) | EB (eV) | d (Å) | ΔEm (meV) | EB (eV) | |
| 2 MS-1NN | 3.18 | 239 | 0.27 | — | — | — |
| 2 MS-2NNAC | 5.51 | −17 | 0.12 | 5.52 | −2.0 | 0.06 |
| 2 MS-2NNZZ | 6.37 | 53 | 0.07 | 6.37 | 21 | 0.01 |
| 2 MS–3NNAC | 8.42 | 1.4 | 0.04 | 8.43 | 1.4 | 0.03 |
| 2 MS-3NNZZ | 9.55 | 0.6 | 0.02 | 9.55 | 0.6 | 0.01 |
| 2 MS-4NN | 11.48 | 0.2 | 0.02 | 11.48 | 0.1 | 0.00 |
We considered various relative positions between two MS defects up to the fourth nearest neighboring (NN) site, and the positions are represented in Fig. 3(b); with respect to the MS defect circled in black, the first, second, third, and fourth NN sites of the other MS defects are designated by blue, green, red, and pink circles. We denote the two n-th NN MS defects as 2MS-nNNX. If more than one n-th NN site exist, the different sites are distinguished using the subscript X, which can be ZZ or AC depending on whether the two defects are aligned along the zigzag or armchair direction, respectively [see Fig. 3(b)]. For 2WS-1NN in WS2, the two WS atoms were relaxed away from the S atoms but relaxed inward between them, forming a bond between the two W atoms. This lowers the total energy of the two defects by 0.27 eV. Because the distance between the two defects is close enough for their spin densities to interact [see inset of Fig. 2(a)], the magnetic interaction ΔEm is measured to be relatively large (239 meV), as shown in Fig. 3(a) and Table 2.
For 2WS-2NN, there are two inequivalent positions: two WS are separated along the armchair line (2WS-2NNAC) or zigzag line (2WS-2NNZZ) [see Fig. 3(b)]. The distance d between the two WS defects in 2WS-2NNAC is shorter by 0.5 Å than that in 2WS-2NNZZ. Among the relative positions that we have considered, only 2WS-2NNAC prefers the AFM coupling by 17 meV compared to the FM coupling. The favorable AFM coupling reflects the spin density oscillation in WS [see inset of Fig. 2(a)]. As we mentioned above, if the majority spin is distributed on the WS atom, the minority spin is distributed on the NN W atoms, and again the majority spin is distributed on the next NN W atoms. Thus, because of the relative position of the two WS defects in 2WS-2NNAC, the spin distribution of the AFM coupling becomes compatible with the spin distribution of each WS, as shown in Fig. 4(a). For 2WS-2NNZZ, on the other hand, the spin distributions are compatible in the FM coupling [see Fig. 4(b)], and our results thus show that the energy of the FM coupling is lower by 53 meV than that of the AFM coupling.
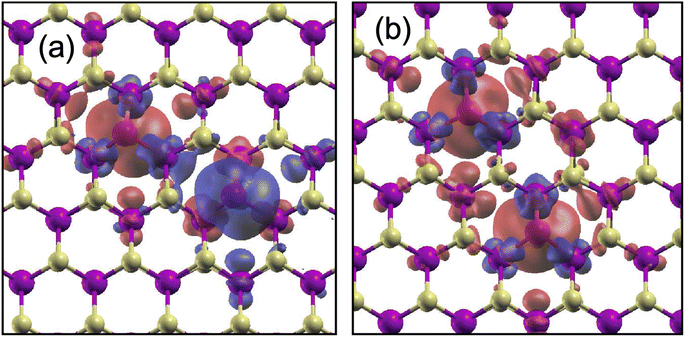 | ||
| Fig. 4 Spin density for (a) 2WS-2NNAC and (b) 2WS-2NNZZ in WS2. Red (blue) contours represent the spatial distribution of the majority (minority) spin. | ||
The magnetic interaction is mediated by the spin density of each WS defect. Because the spin density is localized around WS, as shown in the inset of Fig. 2(a), ΔEm decreases with respect to d [see Table 2 and the inset of Fig. 3(a)]. However, a finite and positive ΔEm (FM coupling) still exists for the third and fourth NN configurations, for which d is over 8 Å. The d-dependent magnetic interaction is also reflected in the splitting of the degenerate dXY and dX2−Y2 levels within the band gap. Fig. 5 shows the total and projected DOSs of 2WS-2NNZZ, 2WS-3NNAC, and 2WS-3NNZZ. As shown in Fig. 5(a), each WS defect has occupied dXY andd X2−Y2 levels and the empty dZ2 level in the gap. For 2WS-2NNZZ, the occupied dXY and dX2−Y2 levels, which are degenerate for the isolated case, exhibit a sizable energy splitting of about 0.17 eV due to the interaction between the defect levels. The electronic structures of 2WS-3NNAC and 2WS-3NNZZ [see Fig. 5(b) and (c)] are similar to that of 2WS-2NNZZ, but the level splittings are reduced to 0.09 and 0.07 eV, respectively, as d increases.
The strength of the magnetic interaction ΔEm is on the same order of magnitude as that of magnetic Mn dopants in TMDs, which have been considered as a source leading to ferromagnetism.14 For several TMDs, the calculated ΔEm of the two Mn dopants are about 130–200 meV for the 1NN configuration and 5–70 meV for the 2NNZZ configuration, which are comparable with the ΔEm of WS in WS2, as shown in Table 2 and Fig. 3(a). In this regard, we expect that the WS defect can induce the ferromagnetism experimentally observed in undoped WS2. However, as discussed above, ΔEm becomes small over the defect distance d of 8 Å, so long-range ferromagnetic ordering could be possible in highly defective systems.
In addition, we considered the ΔEm of the MoS defects in MoS2. The results are very similar to those of WS in WS2, but there are two differences. First, the 2MoS-1NN configuration is unstable, and during the ionic relaxation, the two MoS defects are relaxed inward each other, forming bonds. This relaxation quenches the magnetic moment in MoS. Second, the ΔEm is slightly smaller than that of WS in WS2. This difference may be caused by the small d-orbital radius of the Mo atoms. Thus, for the same defect density of MS, MoS2 is less likely to show ferromagnetism. However, as discussed above, the formation energy of MoS is smaller than that of WS (see Table 1). As such, more abundant MoS defects could lead to ferromagnetism in MoS2.
4. Conclusions
In summary, we investigated various native defects in WS2 and MoS2 and found that the MS defect is the most abundant magnetic defect, exhibiting a local magnetic moment of 2μB, under non-equilibrium W-rich growth conditions. The spin density oscillation associated with the local magnetic moments mediates a long-range magnetic interaction between the MS defects, which mostly leads to ferromagnetic ordering. The strength of this magnetic interaction is comparable to that of magnetic dopants in TMDs. However, the interaction decreases significantly when the defect distance exceeds 9 Å. Thus, our findings suggest that ferromagnetism in TMDs can be induced in heavily defective TMD samples with a high concentration of MS defects.Data availability
The data (the calculated atomic structures) supporting this article have been included as part of the ESI data.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) (NRF-2023R1A2C1006433 and RS-2023-00253716). This work was conducted during the research year of Chungbuk National University in 2023. We used the software VESTA to generate Fig. 1, 3, and 4.55References
- B. Huang, et.al., Nature, 2017, 546, 270–273 CrossRef CAS PubMed.
- C. Gong, et.al., Nature, 2017, 546, 265–269 CrossRef CAS PubMed.
- N. D. Mermin and H. Wagner, Phys. Rev. Lett., 1966, 17, 1133 CrossRef CAS.
- M. Gibertini, M. Koperski, A. Morpurgo and K. S. Novoselov, Nat. Nanotechnol., 2019, 14, 408–419 CrossRef CAS PubMed.
- A. Soumyanarayanan, N. Reyren, A. Fert and C. Panagopoulos, Nature, 2016, 539, 509–517 CrossRef CAS PubMed.
- A. Manchon, H. Koo, J. Nitta, S. Frolov and R. Duine, Nat. Mater., 2015, 14, 871–882 CrossRef CAS PubMed.
- D. Go, J.-P. Hanke, P. Buhl, F. Freimuth, G. Bihlmayer, H.-W. Lee, Y. Mokrousov and S. Blügel, Sci. Rep., 2017, 7, 46742 CrossRef PubMed.
- X. Liu and M. Hersam, Nat. Rev. Mater., 2019, 4, 669–684 CrossRef.
- B. Nikolić, K. Dolui, M. Petrović, P. Plecháč, T. Markussen and K. Stokbro, First-Principles Quantum Transport Modeling of Spin-Transfer and Spin-Orbit Torques in Magnetic Multilayers, Springer International Publishing, 2018 Search PubMed.
- G. Eda, H. Yamaguchi, D. Voiry, T. Fujita, M. Chen and M. Chhowalla, Nano Lett., 2011, 11, 5111–5116 CrossRef CAS PubMed.
- A. Durand, T. Clua-Provost, F. Fabre, P. Kumar, J. Li, J. H. Edgar, P. Udvarhelyi, A. Gali, X. Marie, C. Robert, J. M. Gérard, B. Gil, G. Cassabois and V. Jacques, Phys. Rev. Lett., 2023, 131, 116902 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- N. Mao, J. Tang, L. Xie, J. Wu, B. Han, J. Lin, S. Deng, W. Ji, H. Xu, K. Liu, L. Tong and J. Zhang, J. Am. Chem. Soc., 2016, 138, 300–305 CrossRef CAS PubMed.
- R. Mishra, W. Zhou, S. Pennycook, S. Pantelides and J.-C. Idrobo, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 144409 CrossRef.
- F. Zhang, et.al., Adv. Sci., 2020, 7, 2001174 CrossRef CAS PubMed.
- D. Duong, S. Yun, Y. Kim, S.-G. Kim and Y. Lee, Appl. Phys. Lett., 2019, 115, 242406 CrossRef.
- S. Fu, et.al., Nat. Commun., 2020, 11, 2034 CrossRef CAS PubMed.
- A. K. Jena, S. K. Mallik, M. C. Sahu, S. Sahoo, A. K. Sahoo, N. K. Sharma, J. Mohanty, S. K. Gupta, R. Ahuja and S. Sahoo, Sci. Rep., 2022, 12, 2593 CrossRef CAS PubMed.
- A. Singh, C. C. Price and V. B. Shenoy, ACS Nano, 2022, 16, 9452–9460 CrossRef CAS PubMed.
- Y. Sun, H. Zhang, Y. Zheng, P. Gao, C. Ye and F. Wang, J. Alloys Compd., 2024, 994, 174634 CrossRef CAS.
- O. V. Yazyev, Phys. Rev. Lett., 2008, 101, 037203 CrossRef PubMed.
- O. V. Yazyev and L. Helm, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125408 CrossRef.
- M. M. Ugeda, I. Brihuega, F. Guinea and J. M. Gómez-Rodríguez, Phys. Rev. Lett., 2010, 104, 096804 CrossRef CAS PubMed.
- J. J. Palacios, J. Fernández-Rossier and L. Brey, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 77, 195428 CrossRef.
- H. Ohldag, P. Esquinazi, E. Arenholz, D. Spemann, M. Rothermel, A. Setzer and T. Butz, New J. Phys., 2010, 12, 123012 CrossRef.
- H. Kim, J. Bang and J. Kang, Sci. Rep., 2018, 8, 13940 CrossRef PubMed.
- H. González-Herrero, J. M. Gómez-Rodríguez, P. Mallet, M. Moaied, J. J. Palacios, C. Salgado, M. M. Ugeda, J.-Y. Veuillen, F. Yndurain and I. Brihuega, Science, 2016, 352, 437–441 CrossRef PubMed.
- O. V. Yazyev and M. I. Katsnelson, Phys. Rev. Lett., 2008, 100, 047209 CrossRef PubMed.
- N. Huo, Y. Li, J. Kang, R. Li, Q. Xia and J. Li, Appl. Phys. Lett., 2014, 104, 202406 CrossRef.
- R. Sanikop and C. Sudakar, ACS Appl. Nano Mater., 2020, 3, 576–587 CrossRef CAS.
- X. Ding, T. Liu, S. Ahmed, N. Bao, J. Ding and J. Yi, J. Alloys Compd., 2019, 772, 740–744 CrossRef CAS.
- L. Cai, J. He, Q. Liu, T. Yao, L. Chen, W. Yan, F. Hu, Y. Jiang, Y. Zhao, T. Hu, Z. Sun and S. Wei, J. Am. Chem. Soc., 2015, 137, 2622–2627 CrossRef CAS PubMed.
- X. Mao, Y. Xu, Q. Xue, W. Wang and D. Gao, Nanoscale Res. Lett., 2013, 8, 430 CrossRef PubMed.
- S. Han, Y. Park, Y. Hwang, S. Jekal, M. Kang, W. Lee, W. Yang, G. D. Lee and S. Hong, Sci. Rep., 2016, 6, 38730 CrossRef CAS PubMed.
- J. Hong, et.al., Nat. Commun., 2015, 6, 6293 CrossRef CAS PubMed.
- W. Zhou, X. Zou, S. Najmaei, Z. Liu, Y. Shi, J. Kong, J. Lou, P. Ajayan, B. Yakobson and J.-C. Idrobo, Nano Lett., 2013, 13, 2615–2622 CrossRef CAS PubMed.
- D. Yang, X. Fan, F. Zhang, Y. Hu and Z. Luo, Nanoscale Res. Lett., 2019, 14, 192 CrossRef CAS PubMed.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- W. Kohn and L. J. Sham, Phys. Rev., 1965, 140, A1133–A1138 CrossRef.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Steiner, K. Khmelevskyi, M. Marsmann and G. Kresse, Phys. Rev. B, 2016, 93, 224425 CrossRef.
- L. M. V. Ildefonso, E. L. Buthers, B. S. Archanjo, C. Legnani, W. G. Quirino, D. V. P. Massote, I. O. Maciel and B. Fragneaud, J. Phys. Chem. C, 2024, 128, 7294–7305 CrossRef CAS.
- W.-F. Li, C. Fang and M. A. Van Huis, Phys. Rev. B, 2016, 94, 195425 CrossRef.
- J. Bang, Y.-S. Kim, C. Park, F. Gao and S. Zhang, Appl. Phys. Lett., 2014, 104, 252101 CrossRef.
- C. Si, D. Choe, W. Xie, H. Wang, Z. Sun, J. Bang and S. Zhang, Nano Lett., 2019, 19, 3612–3617 CrossRef CAS PubMed.
- H.-P. Komsa and A. V. Krasheninnikov, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 125304 CrossRef.
- J. Chaste, J. Bang, I. Hnid, L. Khalil, C. Si, A. Durnez, X. Lafosse, M. Q. Zhao, A. T. Charlie Johnson, S. Zhang and A. Ouerghi, ACS Nano, 2020, 14, 13611–13618 CrossRef CAS PubMed.
- X. Zhao, X. Dai, C. Xia and T. Wang, Superlattices Microstruct., 2015, 85, 339–347 CrossRef CAS.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08374a |
| This journal is © The Royal Society of Chemistry 2025 |





