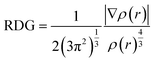Open Access Article
Open Access ArticleAIEE-driven highly sensitive fluorescent probe for Fe3+ sensing in aqueous and solid phases: application in interference-free biological media†
Rida Khalida,
Tayyeba Javida,
Aqsa Pervaiza,
Mohammed A. Assiribc,
Zulfiqar Ali Khand,
Saniaa and
Sohail Anjum Shahzad *a
*a
aDepartment of Chemistry, COMSATS University Islamabad, Abbottabad Campus, University Road, Abbottabad 22060, Pakistan. E-mail: sashahzad@cuiatd.edu.pk
bCentral Labs, King Khalid University, AlQura'a, P.O. Box 960, Abha, 61413, Saudi Arabia
cDepartment of Chemistry, Faculty of Science, King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia
dDepartment of Chemistry, Faculty of Physical Sciences, Government College University Faisalabad, Faisalabad-38000, Pakistan
First published on 18th March 2025
Abstract
Herein, a novel fluorescein-based fluorescent probe FHP was systematically designed and synthesised, which exhibited aggregation-induced emission enhancement (AIEE) properties. FHP showed the maximum emission response at a wavelength (λmax) of 516 nm. Using probe FHP, convenient and cost-effective sensing of Fe3+ in solution and solid states was accomplished with notable sensitivity and selectivity. Quenching of the FHP fluorescence intensity was observed owing to the chelation between the electron-rich probe and electron-deficient Fe3+, with a detection limit of 253 nM. The FHP–Fe3+ interaction was studied using UV-visible and fluorescence spectroscopies, dynamic light scattering (DLS), 1H-NMR titration and density functional theory (DFT) calculations. Theoretical analysis was carried out using DFT to justify the non-covalent type of interaction in the FHP–Fe3+ complex and to study the electronic properties of probe FHP and FHP–Fe3+ complex. The practical application of the FHP probe in Fe3+ sensing was evaluated using biological samples.
Introduction
Iron is an abundant transition metal found in the Earth's core, and it is one of the necessary micronutrients for almost all living beings.1 It acts as a basic core for the structure and function of a variety of proteins such as haemoglobin (to carry oxygen in the body) and myoglobin (to ease the diffusion of oxygen in muscles).2 Iron is also important for various cofactors that help in enzyme functioning and is a component of the prosthetic group in various enzymes,3 thus serving as a central role in the biosphere.4 In plants, a variety of biochemical, physiological and metabolic pathways require iron as it is an integral part of various enzymes. For instance, iron acts as an important component in the electron transport chain during respiration and photosynthesis, nitrogen fixation,5 chlorophyll synthesis and DNA synthesis.6 Iron can be obtained from a variety of dietary sources, including rice, milk, meat, fruits and water. Among vegetables, spinach and broccoli have high iron content. Seafoods such as shellfish and oysters also have a high content of iron. Concentration of any nutrient below or above a certain level is deleterious,7 and the same is the case for iron; because it is one of the heavy metals, it can cause toxic effects in living systems and to the environment. It is termed a “heavy metal” on account of its high specific gravity8 and poisonous effects even at low concentrations.9 For instance, increased concentration of iron in the body is related to pancreas dysfunction and Alzheimer's disease.10 Studies have shown that a high level of plasma iron in males is related to an increased risk of coronary artery disease.11 Hemochromatosis is another condition related to excessive build-up of iron in the liver, skin, joints, pancreas or pituitary gland.12 Hemochromatosis increases the risk of hepatocellular carcinoma, which is the most common liver malignancy and a leading cause of cancer-related deaths worldwide, by about 100–200 times.13 The risk of primary liver cancer increases with iron-overloaded states, such as thalassemia, which contribute to both hemochromatosis and hepatocellular carcinoma.14 Different oxidation states of iron have varying effects on the efficiency of its function, transport, metabolism and toxicity in the body. For instance, the reduced state of iron, Fe2+, is responsible for oxygen transport and storage via haemoglobin and myoglobin, respectively, while its oxidised state, Fe3+, generates methaemoglobin and metmyoglobin, which have no binding capability with oxygen.15 Another condition related to abnormal iron concentrations in the body is ferroptosis, which refers to the iron-dependent peroxidation of lipids, leading to cell death. The oxidation state of iron is important in ferroptosis as Fe2+ is linked with lipid peroxidation, while Fe3+ is inert and is usually stored as ferritin.16 Therefore, the determination of iron levels along with its oxidation state is important. Currently applied techniques for the detection of iron include capillary electrophoresis,17 electrochemical sensing,18 liquid chromatography-mass spectrometry,19 polarography,20 voltammetry21 and atomic absorption spectroscopy.22 These methods have limited practical applications because of the need for expensive instruments, difficult sample preparation process and limited efficiency. Fluorescence-based detection approach is emerging as an extensively used chemosensing technique because of its high sensitivity and selectivity, quick response time, rapid detection, wide-ranging applications and simplicity.23–25 Fluorescent sensors offer portability and easy on-site detection of a variety of materials, and hence, they have wide applications in sensing metal ions, pesticides, biomolecules, cancer cells, explosives and dynamite; they even find application in food safety and environmental pollution monitoring.26–31 Some organic compounds show good emission in the solution form, while their fluorescence emission is reduced upon aggregation due to π–π stacking, a phenomenon named aggregation-caused quenching (ACQ).32,33 Alternatively, some fluorophores have the tendency to show enhanced emission when in the solid form compared to the solution form. This phenomenon is referred to as aggregation-induced emission (AIE).34 AIE behaviour is observed when some molecular interactions, such as restricted intramolecular rotations (RIRs), motions (RIMs) and vibrations (RIVs), are at play.35–38 A variety of AIEE-active fluorophores have been developed lately, and they work at fairly low concentrations by showing “turn-on” fluorescence in the aggregated state.39–41 These AIEE-active fluorophores find practical applications because of their resistance to photobleaching, stability, low signal-to-noise ratio and remarkable Stokes shift.42,43 Biomedical applications of AIEE-active sensors are attributed to their ability to show minimum or no emission in their solution form but a fairly notable emission in their aqueous solution.44 Electronic circuits, organic light-emitting diodes (OLEDs) and molecular logic gates also take advantage of the AIEE property of these luminous compounds.24In continuation of the work by our research group45–52 on the development of novel fluorescent sensors with remarkable selectivity for toxic heavy metals and biologically important compounds, a new fluorescein-based probe FHP was developed in this work through a Schiff base reaction for selective detection of Fe3+. Probe FHP showed AIEE response with manifold-enhanced emission in aqueous media. These remarkable properties of the newly developed probe were used successfully for highly sensitive and selective sensing of Fe3+. A detailed study of the photophysical properties and mechanisms involved in Fe3+ sensing by probe FHP was carried out using UV-vis and fluorescence spectroscopy, 1H and 13C-NMR titration experiments and DFT studies to analyse the interactions and support the experimental findings.
Experimental methods
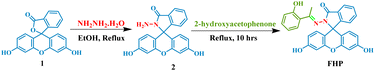
Synthesis of FHP
The reaction of fluorescein (332.31 mg, 1.0 mmol) with hydrazine hydrate (2.0 mL) in the presence of ethanol as solvent afforded fluorescein hydrazine 2, which was then subjected to a condensation reaction with 2-hydroxyacetophenone under reflux in ethanol for 10 hours in the presence of a few drops of glacial acetic acid, and the progress of the reaction was monitored via TLC. After the completion of the reaction, the excess solvent was evaporated, and the residue was purified by extracting it three times in a water–ethyl acetate system. The crude product obtained after solvent extraction was further purified by column chromatography. For column chromatography, silica gel was employed as the stationary phase, while hexane and ethyl acetate served as the mobile phase. The flow rate was adjusted to maintain the steady flow of the liquid mobile phase, and the separation of the desired product was checked by visualizing the TLC plate under a 365 nm UV light. A binary solution of n-hexane and ethyl acetate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was run in the column, which provided the purified off-white powder of FHP. Yield 78%; 1H-NMR (400 MHz, DMSO-d6): δ 11.64 (s, 2H), 9.94 (s, 2H, ArH), 7.92 (m, 1H, ArH), 7.62 (m, 3H, ArH), 7.30 (m, 1H), 7.17 (d, 2H, J = 7.6 Hz), 7.07 (t, 1H, J = 1.6 Hz), 6.86 (t, 2H, J = 7.5 Hz), 6.75 (d, 1H, J = 8.2 Hz), 6.59 (m, 4H), 6.51 (m, 3H), 2.28 (s, 3H). 13C-NMR (100 MHz, DMSO-d6): (δ = ppm): (1 × C) 17.96, (1 × C) 66.24, (2 × C) 102.86, (2 × C) 109.47, (2 × C) (1 × C) 113.04, (1 × C) 112.04, (1 × C) 117.74, (1 × C) 118.69, (1 × C) 119.30, (1 × C) 123.67, (1 × C) 124.43, (2 × C) 129.02, (1 × C) 129.28, (1 × C) 129.48, (1 × C) 129.60, (1 × C) 130.18, (1 × C) 133.34, (1 × C) 134.11, (1 × C) 151.53, (2 × C) 152.59, (2 × C) 159.08, (1 × C) 159.59, (1 × C) 161.55, and (1 × C) 174.70. DEPT-135 NMR (100 MHz, DMSO-d6): 17.96, (2 × C) 102.86, (2 × C) 113.04, 117.74, 134.11, 119.30, 123.67, 124.43, (2 × C) 129.02, 129.60, 130.18, 133.34.
1) was run in the column, which provided the purified off-white powder of FHP. Yield 78%; 1H-NMR (400 MHz, DMSO-d6): δ 11.64 (s, 2H), 9.94 (s, 2H, ArH), 7.92 (m, 1H, ArH), 7.62 (m, 3H, ArH), 7.30 (m, 1H), 7.17 (d, 2H, J = 7.6 Hz), 7.07 (t, 1H, J = 1.6 Hz), 6.86 (t, 2H, J = 7.5 Hz), 6.75 (d, 1H, J = 8.2 Hz), 6.59 (m, 4H), 6.51 (m, 3H), 2.28 (s, 3H). 13C-NMR (100 MHz, DMSO-d6): (δ = ppm): (1 × C) 17.96, (1 × C) 66.24, (2 × C) 102.86, (2 × C) 109.47, (2 × C) (1 × C) 113.04, (1 × C) 112.04, (1 × C) 117.74, (1 × C) 118.69, (1 × C) 119.30, (1 × C) 123.67, (1 × C) 124.43, (2 × C) 129.02, (1 × C) 129.28, (1 × C) 129.48, (1 × C) 129.60, (1 × C) 130.18, (1 × C) 133.34, (1 × C) 134.11, (1 × C) 151.53, (2 × C) 152.59, (2 × C) 159.08, (1 × C) 159.59, (1 × C) 161.55, and (1 × C) 174.70. DEPT-135 NMR (100 MHz, DMSO-d6): 17.96, (2 × C) 102.86, (2 × C) 113.04, 117.74, 134.11, 119.30, 123.67, 124.43, (2 × C) 129.02, 129.60, 130.18, 133.34.
UV-visible and fluorescence experiments
The fluorescence spectra of FHP (50 μM) were recorded on a spectrofluorometer using a 1 mM stock solution. The emission spectra of probe FHP were observed at 516 nm using an excitation wavelength of 450 nm. Solutions of various analytes (100 μM) i.e. Fe3+, Zn2+, K+, Cu2+, Ca2+, Ag+, Mg2+, Hg2+, Na+, and Ni2+ were scanned against the probe to check its selectivity. The spectral data were recorded with increasing concentrations of Fe3+ and all the other analytes using a quartz cuvette to record the absorption and emission parameters.Computational studies
The proposed Fe3+ detection mechanism of the synthesised probe FHP was further supported by DFT studies, and the simulations were performed on the Gaussian 09 program.53 For the visualization of results, GaussView 5.0 was used along with Multiwfn 3.7,54 while the VMD55 and GaussSum56 hybrid density function B3LYP (Becke–Lee–Yang–Parr composite of exchange correction functional) method was employed for structure optimization along with the 6-31G(d,p) basis set for mapping C, H, O and N and LANL2DZ57 for mapping the heavier atom Fe3+. The below equation was used to calculate the interaction energy of probe FHP with the analyte:| Eint = Ec − (Ep + Ea) + EBSSE |
In the above equation, “∇” is the gradient operator, (ρ) indicates the bond strength and |∇ρ| shows the electronic density. To get better insights into the dynamics of the intermolecular interactions of probe FHP with analyte Fe3+, Bader's QTAIM analysis was performed.58 The bond critical point (BCP) obtained from QTAIM was used to explore a variety of topological parameters, including Laplacian of electron density ∇2ρ(r), electron density ρ(r), Lagrangian kinetic energy G(r), potential energy density V(r) and energy density H(r). All these parameters demonstrated a particular type of bond and the nature of the interaction between probe FHP and analyte Fe3+.
Results and discussion
Synthetic chemistry of probe FHP
Fluorescein hydrazine was synthesized via condensation reaction, which involved the conversion of the lactone form of fluorescein into its amide form i.e. fluorescein hydrazine, with the formation of a water molecule. This condensation reaction was carried out in the ethanol solvent under reflux. Nucleophilic nitrogen from hydrazine attacks the oxygen–acyl bond in fluorescein. In consequence, the lactone ring opens, making oxygen a good leaving group. Oxygen leaves with the protons transferred from hydrazine, and the amide ring is formed. Under similar conditions, probe FHP was synthesised in ethanol solvent under reflux via a condensation reaction. The amino group in fluorescein hydrazine being nucleophilic in nature attacks on the carbonyl centre of 2-hydroxyacetophenone. This results in the formation of carbinolamine intermediate, and Schiff base FHP is formed with the elimination of water (Scheme 1). 1H-NMR spectroscopy confirmed the formation of probe FHP. The peak of NH2 at 4.38 ppm in fluorescein hydrazide disappeared in 1H-NMR spectra of FHP and new peaks are observed for CH3 and the aromatic hydrogens of 2-hydroxyacetophenone. The detailed NMR spectra (1H, 13C and DEPT-135 NMR) and information about instrument and reagents are provided in ESI.†Optimization for emission intensity
After optimization, 50 μM of probe FHP in DMF was considered the optimised concentration because it resulted in the maximum intensity and minimum inner filter effect (IFE), as confirmed by the absence of overlapping of the absorption and emission spectra of FHP accompanied by a Stokes shift of 190 nm (Fig. S1†). Probe FHP displayed the maximum emission at a wavelength (λmax) of 516 nm when it was excited using radiation of 450 nm wavelength.Water aggregation studies and AIEE characteristics
Some organic compounds show less or no emission in non-polar or organic solvents but become emissive due to aggregation in a solvent of low solubility, such as water.59 The optical behaviour of probe FHP with increasing water content was studied in a binary system (DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water). As the concentration of water (fw) was increased from 0–90%, the fluorescence emission intensity of probe FHP also increased (Fig. 1a). This significant increase in emission intensity is attributed to the restriction of rotations along the single bonds. This restriction in intramolecular motion causes radiative decay of the molecule, thus leading to enhanced fluorescence emission intensity, contributing to the AIEE characteristics of probe FHP. The relative emission intensity plot of the probe against increasing water fractions showed an increase in emission intensity with the water fractions (Fig. 1b). Based on the results, 80% fw was selected for further photophysical studies because probe FHP showed the maximum intensity in this fraction, demonstrating its practical applicability in biological samples. The polarity of biological systems is in between the polarity of water and organic solvents. Therefore, this mixture provides intermediate polarity that resembles the cellular environment and hence is suitable for studying the possible interactions between molecules. Probe FHP showed the highest value of intensity at 90% water, but it was not selected to avoid the maximum emission limit of the spectrofluorometer while maintaining the suitable concentration of the samples.
water). As the concentration of water (fw) was increased from 0–90%, the fluorescence emission intensity of probe FHP also increased (Fig. 1a). This significant increase in emission intensity is attributed to the restriction of rotations along the single bonds. This restriction in intramolecular motion causes radiative decay of the molecule, thus leading to enhanced fluorescence emission intensity, contributing to the AIEE characteristics of probe FHP. The relative emission intensity plot of the probe against increasing water fractions showed an increase in emission intensity with the water fractions (Fig. 1b). Based on the results, 80% fw was selected for further photophysical studies because probe FHP showed the maximum intensity in this fraction, demonstrating its practical applicability in biological samples. The polarity of biological systems is in between the polarity of water and organic solvents. Therefore, this mixture provides intermediate polarity that resembles the cellular environment and hence is suitable for studying the possible interactions between molecules. Probe FHP showed the highest value of intensity at 90% water, but it was not selected to avoid the maximum emission limit of the spectrofluorometer while maintaining the suitable concentration of the samples.
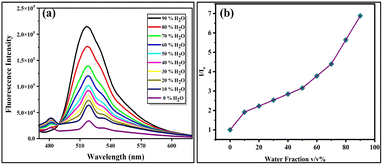 | ||
| Fig. 1 Emission spectrum of FHP with increasing water fraction (0–90%) (a) and relative emission intensity of the probe in increasing water fractions (0–90%) (b). | ||
Fluorescence sensing of Fe3+
Various biologically important ions were examined in aqueous media (H2O/DMF; 4/1, v/v) through fluorescence titration experiments to investigate the sensing potential of probe FHP (50 μM). Ions that were spiked in the solution of FHP for fluorescence studies included Fe3+, Zn2+, K+, Cu2+, Ca2+, Ag+, Mg2+, Hg2+, Na+ and Ni2+. A selective quenching response of probe FHP was observed with the gradual increase in the concentration of Fe3+. The response of probe FHP towards analytes other than Fe3+ was insignificant, demonstrating the selective behaviour of probe FHP towards Fe3+ only (Fig. 2a).A strong fluorescence emission at 516 nm was observed for probe FHP. When probe FHP was titrated only against Fe3+ from 0–100 μM, the emission intensity of FHP decreased gradually with increasing concentrations of Fe3+ (Fig. 2b). A 2D Stern–Volmer plot was drawn to illustrate the binding efficacy of FHP with Fe3+, which displayed a continuous upward trend. The Stern–Volmer constant was calculated using the equation given below, which further verified the sensitivity of probe FHP to Fe3+ (Fig. 2c).
| Io/I = 1 + Ksv[Q] |
Probable sensing mechanism
The plausible mechanism of fluorescence sensing was investigated based on the experimental results obtained. The sensing mechanism of Fe3+ ions by the FHP probe was evaluated by performing 1H-NMR titration experiments to elucidate whether the interaction was covalent or non-covalent in nature. For this purpose, 1H-NMR spectra were obtained by dissolving 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 equivalents of FHP and Fe3+ in the DMSO-d6 solvent.
1 equivalents of FHP and Fe3+ in the DMSO-d6 solvent.
The degree of change in the chemical shift, multiplicity and integration gives information about the interaction type. The appearance of new peaks, significant changes in the chemical shift (δ) or multiplicity and integration change can demonstrate covalent interactions between the probe and the analyte. However, in the case of the FHP–Fe3+ complex, neither a new peak appeared nor a change in the integration or multiplicity was observed. Only a negligible shift in the spectral peaks from 11.64, 9.95 and 7.93 in FHP to 11.60, 9.93 and 7.89, respectively, in FHP–Fe3+ complex was observed, clearly demonstrating the presence of non-covalent type interaction between probe FHP and Fe3+ (Fig. 3a). Furthermore, the fluorescence titration experiments of FHP and Fe3+ showed quenching of the emission intensity of the FHP probe upon interaction with increasing concentrations (0–100 μM) of Fe3+ ions. No new peak appeared in the emission spectra, further verifying the non-covalent type interaction between the FHP probe and Fe3+ ions. Similar behaviour was also observed in UV-vis titration experiments. Only quenching was observed without the generation of new peaks (Fig. S3†). The absence of new peaks in both the UV and emission spectra illustrates that ICT is not responsible for quenching the FHP molecule upon interaction with Fe3+. Fe3+ is a well-known fluorescence quencher that is electron deficient and can accept electrons from electron-rich atoms. The carbonyl and hydroxyl groups present at the terminal ends of probe FHP make it an electron-rich entity that can donate electrons to the metal Fe3+ ions. Therefore, probe FHP might have chelated the Fe3+ metal ions, which in turn opens the non-radiative decay pathway, due to which the fluorescence emission intensity of FHP reduces during interaction with Fe3+ ions (Fig. 3b). Moreover, the binding stoichiometry was calculated by Job's plot, which validated the binding capability of probe FHP with Fe3+. By varying the mole fraction from 0 to 1, different concentrations of probe FHP and Fe3+ were utilized to construct the Job's plot. The binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which depicts the binding of one molecule of FHP with one ion of Fe3+, yielded the maximum relative emission intensity value at 0.5 mole fraction (Fig. S4†). In addition to this, the DLS analysis results also verified the binding of FHP with Fe3+ based on the increase in particle size of FHP from 323.3 nm to 520.4 nm (Fig. S5†). During the interaction between the AIEE-active probe and the analyte, Fe3+ gets into the layers of the aggregates of probe FHP, and due to complex formation, the overall size appears to increase. Moreover, the presence of non-covalent interactions between FHP and Fe3+ and the charge transfer from FHP to Fe3+ were theoretically supported by DFT studies, in which the non-covalent interactions were visualised through NCI analysis, and the numerical value of charge transfer was calculated by NBO analysis, which showed that 1.01823 e− charge was transferred from FHP to Fe3+.
1, which depicts the binding of one molecule of FHP with one ion of Fe3+, yielded the maximum relative emission intensity value at 0.5 mole fraction (Fig. S4†). In addition to this, the DLS analysis results also verified the binding of FHP with Fe3+ based on the increase in particle size of FHP from 323.3 nm to 520.4 nm (Fig. S5†). During the interaction between the AIEE-active probe and the analyte, Fe3+ gets into the layers of the aggregates of probe FHP, and due to complex formation, the overall size appears to increase. Moreover, the presence of non-covalent interactions between FHP and Fe3+ and the charge transfer from FHP to Fe3+ were theoretically supported by DFT studies, in which the non-covalent interactions were visualised through NCI analysis, and the numerical value of charge transfer was calculated by NBO analysis, which showed that 1.01823 e− charge was transferred from FHP to Fe3+.
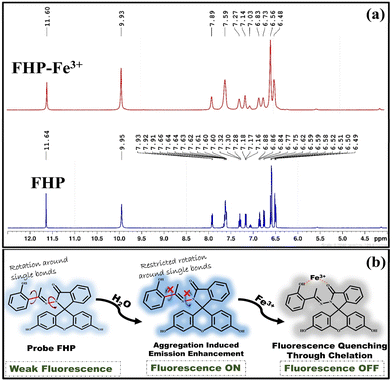 | ||
| Fig. 3 1H-NMR (400 MHz, DMSO-d6) titration spectra of FHP and FHP–Fe3+ (a) and the Fe3+ sensing strategy of FHP (b). | ||
Interference studies
To investigate the selectivity of FHP, sensing studies were carried out in the presence of different interfering analytes, including K+, Cu2+, Ca2+, Ag+, Mg2+, Hg+, Na+, Ni+, NO3+, Cl−, CN−, H2O2, and N2H4. The fluorescence response of probe FHP was unaffected in the presence of interfering species and remained selective for Fe3+ (Fig. S6†). The sensing capability of FHP was investigated under varying pH conditions ranging from 3–11 to explore its applicability for on-site detection. Despite changing pH, the quenching response of probe FHP by Fe3+ was unaffected (Fig. S7a†). The effect of temperature was also studied by changing the temperature from 20–60 °C in a DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water system. The temperature was not increased to 100 °C to avoid boiling of water. The sensing response of FHP remained unaffected with increasing temperatures (Fig. S7b†). The quenching response of FHP was observed, and the quenching efficiency remained constant for Fe3+ from 20–60 s, demonstrating the quick response time of probe FHP (Fig. S8a†). A photostability test was carried out, and probe FHP was exposed to high-energy radiations. Probe FHP was fairly stable and showed no fluctuations in Fe3+ sensing capability (Fig. S8b†). Probe FHP was found suitable for practical use as its fluorescence response was independent of pH changes, varying temperature and photobleaching and it showed a quick response time.
water system. The temperature was not increased to 100 °C to avoid boiling of water. The sensing response of FHP remained unaffected with increasing temperatures (Fig. S7b†). The quenching response of FHP was observed, and the quenching efficiency remained constant for Fe3+ from 20–60 s, demonstrating the quick response time of probe FHP (Fig. S8a†). A photostability test was carried out, and probe FHP was exposed to high-energy radiations. Probe FHP was fairly stable and showed no fluctuations in Fe3+ sensing capability (Fig. S8b†). Probe FHP was found suitable for practical use as its fluorescence response was independent of pH changes, varying temperature and photobleaching and it showed a quick response time.
DFT studies
 | ||
| Fig. 5 QTAIM analysis of FHP–Fe3+ (a) and fluorescence spectra of FHP in biological samples (0–50 μM) (b). | ||
| BCPs | FHA–Fe3+ | ρ(r) (a.u.) | ∇2ρ(r) (a.u.) | G(r) (a.u.) | V(r) (a.u.) | H(r) (a.u.) | −V/G | Eint (kcal mol−1) |
|---|---|---|---|---|---|---|---|---|
| 1 | O⋯Fe3+ | 0.004 | 0.008 | 0.002 | −0.002 | −0.001 | 1.00 | 0.627 |
| 2 | C⋯Fe3+ | 0.006 | 0.016 | 0.004 | −0.005 | −0.0004 | 1.25 | 1.56 |
| 3 | O⋯Fe3+ | 0.003 | 0.006 | 0.001 | −0.002 | −0.0003 | 2.00 | 0.627 |
Practical applications
The fluorescence spectra of probe FHP showed efficient fluorescence quenching response in the plasma samples with increasing concentrations of Fe3+ (Fig. 5b). A calibration curve was drawn to compare the results of biological samples with the lab samples (Fig. S10†), and the calculated recovery percentages were between 96.5% and 105% (Table S2†). This shows that FHP exhibits quenching behaviour in the presence of Fe3+ and can be used for Fe3+ detection in biological samples.
Conclusion
A fairly good yield (78%) of FHP was obtained under simple and convenient reaction conditions. FHP was then developed as a probe for the detection of Fe3+. FHP displayed AIEE properties with increasing fw in a DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v) system with a λmax of 516 nm. FHP could effectively sense Fe3+ ions with an LOD of 253 nM. The 1H and 13C titration NMR results justified the presence of non-covalent interactions. The Job's plot of FHP displayed a binding stoichiometry of 1
4, v/v) system with a λmax of 516 nm. FHP could effectively sense Fe3+ ions with an LOD of 253 nM. The 1H and 13C titration NMR results justified the presence of non-covalent interactions. The Job's plot of FHP displayed a binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with Fe3+. Furthermore, DLS showed that the particle size of the FHP–Fe3+ complex was 520.3 nm and that of the FHP probe was 323.4 nm. Quenching of FHP fluorescence emission intensity in the presence of Fe3+ could be attributed to the chelation between the electron-rich centre of FHP and electron-deficient Fe3+. Probe FHP was able to successfully sense Fe3+ in the presence of various interfering species, varying pH ranges and even temperature changes, demonstrating excellent sensitivity and selectivity towards FHP. Solid-state fluorescence spectroscopy provided insights into the solid-state detection of Fe3+ by FHP. On exposure to high-energy radiations, FHP remained perfectly stable. In electronic analysis, a reduction in the HOMO–LUMO gap from 3.89 eV to 1.97 eV and the appearance of a new virtual energy peak provided evidence of the great sensitivity of FHP for Fe3+. The NCI analysis indicated non-covalent interactions between FHP and Fe3+, and QTAIM analysis further elucidated those three interacting points as BCPs. FHP could even sense Fe3+ in real biological samples with remarkable efficacy, which exemplifies its potential for practical usage in the real world.
1 with Fe3+. Furthermore, DLS showed that the particle size of the FHP–Fe3+ complex was 520.3 nm and that of the FHP probe was 323.4 nm. Quenching of FHP fluorescence emission intensity in the presence of Fe3+ could be attributed to the chelation between the electron-rich centre of FHP and electron-deficient Fe3+. Probe FHP was able to successfully sense Fe3+ in the presence of various interfering species, varying pH ranges and even temperature changes, demonstrating excellent sensitivity and selectivity towards FHP. Solid-state fluorescence spectroscopy provided insights into the solid-state detection of Fe3+ by FHP. On exposure to high-energy radiations, FHP remained perfectly stable. In electronic analysis, a reduction in the HOMO–LUMO gap from 3.89 eV to 1.97 eV and the appearance of a new virtual energy peak provided evidence of the great sensitivity of FHP for Fe3+. The NCI analysis indicated non-covalent interactions between FHP and Fe3+, and QTAIM analysis further elucidated those three interacting points as BCPs. FHP could even sense Fe3+ in real biological samples with remarkable efficacy, which exemplifies its potential for practical usage in the real world.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Rida Khalid: methodology, investigation, validation, writing – original draft. Tayyeba Javid: methodology, investigation, formal analysis, writing – original draft. Aqsa Pervaiz: investigation, methodology, software. Mohammed A. Assiri: visualization, methodology, investigation, funding acquisition. Zulfiqar Ali Khan: investigation, visualization. Sania: methodology. Sohail Anjum Shahzad: supervision, conceptualization, methodology, visualization, validation, investigation, project administration, funding acquisition, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors extend their appreciation to University Higher Education Fund for funding this research work under Research Support Program for Central Labs at King Khalid University through the project number CL/PRI/RP/10.Notes and references
- D. Meynard, J. L. Babitt and H. Y. Lin, Blood, 2014, 123, 168–176 CrossRef CAS PubMed.
- V. K. Gupta, A. Jain, S. Agarwal and G. Maheshwari, Talanta, 2007, 71, 1964–1968 CrossRef CAS PubMed.
- G. R. Rout and S. Sahoo, Rev. Agric. Sci., 2015, 3, 1–24 CrossRef.
- F. A. Cotton, G. Wilkinson, C. A. Murillo and M. Bochmann, Advanced inorganic chemistry, John Wiley & Sons, 6th edn, 1999 Search PubMed.
- J. Kim and D. Rees, Science, 1992, 257, 1677–1682 CrossRef CAS PubMed.
- S. Puig, L. Ramos-Alonso, A. M. Romero and M. T. Martínez-Pastor, Metallomics, 2017, 9, 1483–1500 CrossRef PubMed.
- R. Bharti and R. Sharma, Mater. Today: Proc., 2022, 51, 880–885 Search PubMed.
- K. J. Appenroth, Acta Physiol. Plant., 2010, 32, 615–619 CrossRef CAS.
- R. K. Sharma and M. Agrawal, J. Environ. Biol., 2005, 26, 301–313 CAS.
- A. Y. A. Alzahrani, K. O. Khan, S. Rafique, H. Irshad, Khadija, A. M. Khan and S. A. Shahzad, Spectrochim. Acta, Part A, 2023, 297, 122745 CrossRef CAS PubMed.
- Z. Li, X. Li, Y. Zhang, X. Feng, F. Yang, D. Su, J. Qiu, W. Ling and Y. Yang, Food Funct., 2013, 4, 1535–1542 RSC.
- J. Balogh, D. Victor III, E. H. Asham, S. G. Burroughs, M. Boktour, A. Saharia, X. Li, R. M. Ghobrial and H. P. Monsour Jr, J. Hepatocell. Carcinoma, 2016, 41–53 CrossRef.
- C. Ko, N. Siddaiah, J. Berger, R. Gish, D. Brandhagen, R. K. Sterling, S. J. Cotler, R. J. Fontana, T. M. McCashland and S. H. Han, Liver Int., 2007, 27, 1394–1401 CrossRef PubMed.
- K. Hino, I. Yanatori, Y. Hara and S. Nishina, FEBS J., 2022, 289, 7810–7829 CrossRef CAS PubMed.
- A. Safavi and H. Abdollahi, Microchem. J., 1999, 63, 211–217 CrossRef CAS.
- T. Chen, L. Liang, Y. Wang, X. Li and C. Yang, Apoptosis, 2024, 29, 289–302 CrossRef PubMed.
- H. J. Lin, K. P. Hsieh, S. S. Chiou, H. S. Kou and S. M. Wu, J. Pharm. Biomed. Anal., 2016, 131, 497–502 CrossRef CAS PubMed.
- J. W. Oh, T. H. Kim, S. W. Yoo, Y. O. Lee, Y. Lee, H. Kim, J. Kim and J. S. Kim, Sens. Actuators, B, 2013, 177, 813–817 CrossRef CAS.
- M. Y. Lu, N. Wang, W. H. Wu, C. W. Lai, P. H. Kuo, P. H. Chiang, K. H. Lin and T. H. Wu, Clin. Ther., 2015, 37, 1751–1760 CrossRef CAS PubMed.
- S. Wang, L. Du, A. Zhang and B. Li, Anal. Lett., 1997, 30, 2099–2107 CrossRef CAS.
- A. Bobrowski, K. Nowak and J. Zarębski, Anal. Bioanal. Chem., 2005, 382, 1691–1697 CrossRef CAS PubMed.
- A. Ohashi, H. Ito, C. Kanai, H. Imura and K. Ohashi, Talanta, 2005, 65, 525–530 CrossRef CAS PubMed.
- S. Xia, S. Y. Xiao, Q. Q. Hong, J. R. Zou, S. Yang, M. X. Zhang and H. Zuo, RSC Adv., 2015, 5, 5244–5249 RSC.
- Khadija, H. Irshad, S. Rafique, A. M. Khan, S. Nawazish, H. ur Rehman, M. Imran, S. A. Shahzad and U. Farooq, Spectrochim. Acta, Part A, 2023, 290, 122273 CrossRef CAS PubMed.
- S. Rafique, A. Y. A. Alzahrani, Khadija, H. Irshad, A. M. Khan and S. A. Shahzad, Spectrochim. Acta, Part A, 2023, 300, 122946 CrossRef CAS PubMed.
- J. H. Jung, D. S. Cheon, F. Liu, K. B. Lee and T. S. Seo, Angew. Chem., Int. Ed., 2010, 49, 5708–5711 CrossRef CAS PubMed.
- A. Majeed, M. A. Assiri, H. Irshad, Khadija, M. Z. Ullah and S. A. Shahzad, Microchem. J., 2024, 201, 110640 CrossRef CAS.
- K. O. Khan, M. A. Assiri, H. Irshad, S. Rafique, A. M. Khan, A. K. Khan, M. Imran and S. A. Shahzad, J. Photochem. Photobiol., A, 2023, 442, 114805 CrossRef CAS.
- H. Irshad, Khadija and K. Qvortrup, J. Mol. Liq., 2024, 126049 CrossRef CAS.
- M. A. Assiri, S. Hanif, H. M. Junaid, A. Hamad, H. Irshad, M. Yar, W. Rauf and S. A. Shahzad, J. Photochem. Photobiol., A, 2023, 438, 114514 CrossRef CAS.
- A. Pervaiz, S. A. Shahzad, M. A. Assiri, T. Javid, H. Irshad and K. Qvortrup, J. Hazard. Mater. Lett., 2024, 5, 100132 CrossRef CAS.
- W. Z. Yuan, P. Lu, S. Chen, J. W. Lam, Z. Wang, Y. Liu, H. S. Kwok, Y. Ma and B. Z. Tang, Adv. Mater., 2010, 22, 2159–2163 CrossRef CAS PubMed.
- J. Qi, X. Hu, X. Dong, Y. Lu, H. Lu, W. Zhao and W. Wu, Adv. Drug Delivery Rev., 2019, 143, 206–225 CrossRef CAS PubMed.
- J. Zhang, H. Zhang, J. W. Lam and B. Z. Tang, Chem. Res. Chin. Univ., 2021, 37, 1–15 CrossRef CAS.
- M. Kang, Z. Zhang, N. Song, M. Li, P. Sun, X. Chen, D. Wang and B. Z. Tang, Aggregate, 2020, 1, 80–106 CrossRef.
- S. Liu, G. Feng, B. Z. Tang and B. Liu, Chem. Sci., 2021, 12, 6488–6506 RSC.
- R. Abassi, F. Abassi, A. Mosavizadeh, H. Sadeghi and A. Keshtkari, Journal of Clinical Care and Skills, 2020, 1, 127–132 CrossRef.
- M. A. Assiri, F. Munir, M. T. Waseem, H. Irshad, W. Rauf and S. A. Shahzad, Microchem. J., 2023, 193, 109220 CrossRef CAS.
- X. Sun, B. M. Chapin, P. Metola, B. Collins, B. Wang, T. D. James and E. V. Anslyn, Nat. Chem., 2019, 11, 768–778 CrossRef CAS PubMed.
- T. Javid, M. A. Assiri, A. Pervaiz, H. Irshad, K. Qvortrup and S. A. Shahzad, J. Mol. Liq., 2024, 409, 125526 CrossRef CAS.
- M. A. Assiri, S. Rafique, H. Irshad, Z. A. Khan, F. A. Khan and S. A. Shahzad, J. Mol. Struct., 2024, 1307, 137963 CrossRef.
- J. Yang, Z. Chi, W. Zhu, B. Z. Tang and Z. Li, Sci. China:Chem., 2019, 62, 1090–1098 CrossRef CAS.
- N. Jiang, T. Shen, J. Z. Sun and B. Z. Tang, Sci. China Mater., 2019, 62, 1227–1235 CrossRef.
- Z. Liu, Q. Wang, W. Qiu, Y. Lyu, Z. Zhu, X. Zhao and W. H. Zhu, Chem. Sci., 2022, 13, 3599–3608 RSC.
- K. Khurshid, S. A. Shahzad, M. A. Assiri, A. Shabbir, T. Javid and H. Irshad, RSC Adv., 2024, 14, 21682–21691 RSC.
- H. Rabale, H. Irshad, A. Pervaiz, S. S. Almujri, A. Y. A. Alzahrani, M. Z. Ullah and S. A. Shahzad, Microchem. J., 2024, 205, 111312 CrossRef CAS.
- R. Khalid, S. A. Shahzad, M. A. Assiri, T. Javid, H. Irshad and M. Z. Ullah, Microchem. J., 2024, 200, 110264 CrossRef CAS.
- I. Ullah, S. A. Shahzad, M. A. Assiri, M. Z. Ullah, H. Irshad and U. Farooq, Spectrochim. Acta, Part A, 2024, 314, 124224 CrossRef CAS PubMed.
- A. Pervaiz, S. A. Shahzad, M. A. Assiri, T. Javid, H. Irshad and K. O. Khan, Spectrochim. Acta, Part A, 2024, 313, 124121 CrossRef CAS PubMed.
- M. Z. Ullah, S. A. Shahzad, M. A. Assiri, H. Irshad, S. Rafique, S. A. Shakir and A. Mumtaz, Spectrochim. Acta, Part A, 2024, 306, 123607 CrossRef CAS PubMed.
- T. Javid, S. A. Shahzad, M. A. Assiri, A. Pervaiz, Khadija and H. Irshad, Microchem. J., 2024, 199, 109934 CrossRef CAS.
- S. Rafique, H. Irshad, S. Majeed, Khadija, R. Rubab, M. Imran, A. M. Khan and S. A. Shahzad, J. Photochem. Photobiol., A, 2023, 437, 114459 CrossRef CAS.
- M. Biczysko, P. Panek, G. Scalmani, J. Bloino and V. Barone, J. Chem. Theory Comput., 2010, 6, 2115–2125 CrossRef CAS PubMed.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, J. Mol. Graphics, 1996, 14, 33–38 CrossRef CAS PubMed.
- N. M. O'boyle, A. L. Tenderholt and K. M. Langner, J. Comput. Chem., 2008, 29, 839–845 CrossRef PubMed.
- P. J. Hay and W. R. Wadt, J. Chem. Phys., 1985, 82, 270–283 CrossRef CAS.
- R. Bader, Acc. Chem. Res., 1985, 18, 9 CrossRef CAS.
- H. Tian Jr, A. C. Sedgwick, H. H. Han, S. Sen, G.-R. Chen, Y. Zang, J. L. Sessler, T. D. James, J. Li and X. P. He, Coord. Chem. Rev., 2021, 427, 213577 CrossRef.
- M. Sohail, F. Khaliq, T. Mahmood, K. Ayub, S. Tabassum and M. A. Gilani, Radiat. Phys. Chem., 2021, 184, 109457 CrossRef CAS.
- X. Quan, X. Xu and B. Yan, J. Hazard. Mater., 2022, 427, 127869 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra07824a |
| This journal is © The Royal Society of Chemistry 2025 |