 Open Access Article
Open Access ArticleUnexpected increase in water solubility by the introduction of hydrophobic units into imidazolium-based polymeric ionic liquids with carboxylate counteranions†
Nene
Maruyama
,
Sadahito
Aoshima
 * and
Arihiro
Kanazawa
* and
Arihiro
Kanazawa
 *
*
Department of Macromolecular Science, Graduate School of Science, Osaka University, Toyonaka, Osaka 560-0043, Japan. E-mail: kanazawaa11@chem.sci.osaka-u.ac.jp; aoshima@chem.sci.osaka-u.ac.jp
First published on 31st March 2025
Abstract
Water solubility of polymers is affected by hydrophobic or hydrophilic moieties introduced into polymer chains. In this study, the introduction of hydrophobic units into polymeric ionic liquids (ILs) unexpectedly resulted in an increase in the water solubility of polymers. A vinyl ether (VE) homopolymer bearing imidazolium-type IL moieties with nonanoate counteranions and poly(isobutyl VE) [poly(IBVE)] are both insoluble in water, while statistical copolymers of these VEs dissolved in water. Moreover, statistical copolymers with appropriate IBVE contents exhibited lower critical solution temperature (LCST)-type thermoresponsive behavior. The water solubility of an upper critical solution temperature (UCST)-type thermoresponsive polymeric IL with 2-naphthoate counteranions was also increased by the introduction of hydrophobic units.
Polymers exhibiting thermoresponsivity in water are classified into the following two types: lower critical solution temperature (LCST)-type thermoresponsive polymers,1–7 such as poly(N-isopropylacrylamide) (PNIPAAm) and oxyethylene-containing poly(vinyl ether)s [poly(VE)s], and upper critical solution temperature (UCST)-type thermoresponsive polymers,8–15 such as polysulfobetaines and imidazolium salt-type ionic liquid (IL) moiety-containing poly(VE)s. LCST-type thermoresponsive behavior in water is usually caused by dehydration and hydrophobic interaction among polymer chains on heating. Therefore, the introduction of hydrophobic units into LCST-type thermoresponsive polymers leads to decreased solubility and a decrease in cloud points.16–20
Unlike the non-ionic, LCST-type thermoresponsive polymers, the introduction of hydrophobic groups into UCST-type thermoresponsive polysulfobetaines occasionally contributes to not a decrease but an increase in water solubility.21–23 For example, the introduction of N-butylacrylamide units into poly[3-((3-acrylamidopropyl)dimethylammonio)propane-1-sulfonate] having a cloud point at 8.5 °C of the UCST-type thermoresponsive behavior resulted in copolymers with lower cloud points.21 Moreover, the replacement of a methyl group with an ethyl, propyl, or butyl group in poly[3-((2-methacryloyloxyethyl)dimethylammonio)propane-1-sulfonate], which is a UCST-type thermoresponsive polysulfobetaine exhibiting a cloud point of 31.4 °C, resulted in polymers soluble in water at 0 to 100 °C.23 The increase in solubility in water with an increase in hydrophobic content seems a strange phenomenon. The introduction of hydrophobic groups is considered to weaken the attractive electrostatic forces between the zwitterionic groups and cause the interaction between the zwitterionic groups and water. A decrease in water solubility with an increase in hydrophilic sugar content was also reported in the study of poly(2-oxazoline) glycopolymers with LCST-type thermoresponsivity.24
Recently, our group reported that poly(VE)s with pendent imidazolium carboxylate-type IL moieties exhibit different solubility and thermoresponsive behavior in water depending on the alkyl length or the aromatic ring structure of the alkyl or aryl carboxylate counteranions, respectively.25 Polymers with shorter alkyl groups were soluble in water at 0 to approximately 90 °C, while a polymer with octanoate counteranions (p[MeIm][C8]) exhibited LCST-type thermoresponsive behavior. A further increase in the alkyl length resulted in insoluble polymers. Unlike the LCST-type behavior of a polymer with aliphatic carboxylates, a polymer with 2-naphthoate counteranions (p[MeIm][NA]) exhibited UCST-type thermoresponsive behavior in water.
In this study, we aimed to examine the effects of hydrophobic units on the solubility of IL moiety-containing poly(VE) in water. Hydrophobic units were introduced into polymer chains by living cationic copolymerization of 2-chloroethyl VE (CEVE), which is a precursor of IL unit-containing VE, and hydrophobic VE (Scheme 1). The hydrophobic contents in the statistical copolymers were elaborately tuned by adjusting the monomer concentration and polymerization time. Interestingly, the introduction of a hydrophobic VE resulted in increased solubility of the IL moiety-containing poly(VE)s in water. In particular, water-soluble copolymers were synthesized from monomers whose homopolymers were insoluble in water. Increased solubility was observed in both aliphatic and aromatic counteranion-containing polymers.
 | ||
| Scheme 1 Synthesis of statistical copolymers consisting of imidazolium carboxylate-type IL moiety-containing VE and hydrophobic VE. | ||
Statistical copolymers of IL moiety-containing VE and hydrophobic VE (p[R1-VEn-stat-([MeIm][R2COO])m]) were synthesized by three-step reactions consisting of living cationic copolymerization of CEVE and hydrophobic VE, substitution of the CEVE-derived 2-chloroethyl groups with imidazolium groups, and a counteranion exchange reaction (Scheme 1). First, living cationic copolymerization of CEVE and isobutyl VE (IBVE) was conducted with the 1-isobutoxyethyl acetate (IBEA)/Et1.5AlCl1.5/SnCl4 initiating system in the presence of 1,4-dioxane and 2,6-di-tert-butylpyridine (DTBP) in toluene at 0 °C.25–28 As summarized in Table 1, copolymers with various CEVE/IBVE ratios were successfully produced by copolymerization at different initial monomer concentrations. IBVE exhibits higher reactivity than CEVE28–30 (the monomer reactivity ratios were determined to be 2.3 (IBVE) and 0.40 (CEVE), respectively, using the Meyer–Lowry method31,32 [Fig. S2†]; the values are roughly comparable to the reported values29,30); hence, polymerization was quenched at not high but moderate monomer conversion to obtain statistical copolymers with desired CEVE/IBVE ratios. A representative result is shown in Fig. 1A. IBVE was consumed at a faster rate than CEVE (Fig. 1A). The product copolymers had very narrow molecular weight distributions (MWDs) (Fig. 1B) and Mn values that increased with an increase in monomer conversion. The 1H NMR spectrum of the copolymer exhibited peaks assigned to both CEVE and IBVE units (Fig. 2A). The number of monomer units incorporated into copolymer chains was determined from the integral ratios of the main chain and chain ends in the 1H NMR spectra. 2-Ethylhexyl VE (EHVE) was also used instead of IBVE for the synthesis of a statistical copolymer (entry 9 in Table 1; Fig. 1C).
 | ||
| Fig. 1 (A) Time–conversion plots of the copolymerization (entry 8 in Table 1) and (B) the MWD curves of p(IBVE-stat-CEVE) (entry 8 in Table 1) and (C) p(EHVE-stat-CEVE) (entry 9 in Table 1). | ||
 | ||
| Fig. 2 1H NMR spectra of (A) p(IBVE-stat-CEVE) (in CDCl3 at 30 °C; entry 4 in Table 1) and (B) p[IBVE-stat-([MeIm][C9])] (in DMSO-d6 at 100 °C; entry 5 in Table 2). *Grease, water, CHCl3, DMSO, Me4Si, etc. | ||
| Entry | Copolymer | Time (min) | IBVE or EHVE conv. (%) | CEVE conv. (%) | IBVE or EHVE ratiob (mol%) |
M
n × 10−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c c |
M w/Mnc |
|---|---|---|---|---|---|---|---|
| a Polymerization conditions: [IBVE]0 = 0.050 (entry 1), 0.10 (entry 2), 0.17 (entries 3 and 8), 0.40 (entry 4), 0.60 (entry 6), or 0.80 (entries 5 and 7) M; [EHVE]0 = 0.20 (entry 9) M; [CEVE]0 = 0.40 (entries 6 and 7) or 0.80 (entries 1–5, 8, and 9) M; [IBEA]0 = 4.0 mM, [Et1.5AlCl1.5]0 = 10 mM, [DTBP]0 = 5.0 (entries 5 and 7), 8.0 (entry 1), or 10 (entries 2–4, 6, 8, and 9) mM, [SnCl4]0 = 10 (entries 1–8) or 15 (entry 9) mM, [1,4-dioxane] = 1.2 M, in toluene at 0 °C. See Fig. S1† for the MWD curves. b Determined by 1H NMR. c By size exclusion chromatography (SEC) (polystyrene calibration). | |||||||
| 1 | p(IBVE10-stat-CEVE95) | 7.5 | 78 | 63 | 10 | 11.4 | 1.04 |
| 2 | p(IBVE14-stat-CEVE70) | 30 | 78 | 45 | 17 | 15.2 | 1.03 |
| 3 | p(IBVE27-stat-CEVE83) | 10 | 77 | 48 | 25 | 13.8 | 1.03 |
| 4 | p(IBVE44-stat-CEVE42) | 2 | 42 | 22 | 51 | 10.0 | 1.05 |
| 5 | p(IBVE67-stat-CEVE39) | 1.7 | 59 | 30 | 63 | 17.9 | 1.08 |
| 6 | p(IBVE56-stat-CEVE23) | 0.8 | 49 | 31 | 71 | 10.2 | 1.07 |
| 7 | p(IBVE74-stat-CEVE23) | 1.5 | 49 | 27 | 76 | 15.7 | 1.06 |
| 8 | p(IBVE27-stat-CEVE73) | 15 | 75 | 45 | 27 | 13.7 | 1.03 |
| 9 | p(EHVE47-stat-CEVE139) | 10 | 60 | 45 | 25 | 23.1 | 1.11 |
Imidazolium moieties were introduced into the obtained statistical copolymers via a substitution reaction of the 2-chloroethyl groups with 1-methylimidazole in the presence of NaI in DMF or DMF/methyl isobutyl ketone (2/1 v/v) at 80 °C for 72 h. Subsequently, the iodide counteranions were changed into carboxylate anions with an excess of sodium carboxylate in methanol. The 1H NMR spectrum of the product obtained with sodium nonanoate is shown in Fig. 2B as an example. Peaks assignable to the imidazolium moiety and the carboxylate anion were observed, indicating successful incorporation of IL moieties. The incorporation ratios of imidazolium groups and carboxylate anions are summarized in Table S1.† Quantitative incorporation of imidazolium groups and carboxylate anions was not achieved in some cases. In our previous study, quantitative incorporation was achieved when a CEVE homopolymer was used; hence, the presence of the isobutoxy groups derived from IBVE in the copolymers likely prevented a quantitative reaction of the copolymers.
Water solubility of the obtained copolymers bearing various carboxylate anions was examined with a focus on the hydrophobic-unit ratios. As our group reported before, a [MeIm][C9] homopolymer is insoluble in water (entry 1 in Table 2; purple curve in Fig. 3A).25 Interestingly, the copolymers with IBVE contents of 10% and 17% were soluble in water at room temperature (entries 2 and 3). Moreover, the solution became turbid at 23 °C (IBVE content = 10%, blue curve in Fig. 3A) and 55 °C (17%, red curve in Fig. 3A) on heating, indicating that the copolymers exhibited LCST-type thermoresponsive behavior. Hysteresis was observed in the cooling scans (Fig. S4†), which suggests that relatively strong aggregation occurred among polymer chains. A further increase in the hydrophobic content to 25–63% resulted in copolymers that were soluble even at high temperature (0–90 °C; entries 4–6; Fig. 3 and S5†). A copolymer with an IBVE content of 71% (entry 7; green curve in Fig. 3A) also exhibited LCST-type thermoresponsive behavior, whereas a further increase in the IBVE content (76%) resulted in an insoluble copolymer (entry 8) as expected from the insolubility of the IBVE homopolymer in water (entry 10). An increase in water solubility by the introduction of hydrophobic units was observed even when EHVE, which has a larger hydrophobic group, was used instead of IBVE. A copolymer with an EHVE content of 25% was soluble in water even on heating (entry 9; Fig. S7†).
 | ||
| Fig. 3 (A) Turbidity measurement of p[IBVEn-stat-([MeIm][C9])m]s in water (entries 1, 2, 3, 6, and 7 in Table 2; polymer concentration: 3 wt%; scan rate = 1 °C min−1; see Fig. S4† for the cooling scan). (B) The relationship between solubility and thermoresponsivity in water of the statistical copolymers and IBVE content (the data are listed in Table 2, polymer concentration: 3 wt%). | ||
| Entry | Polymer | M n × 10−3 (precursor) | M w/Mn (precursor) | Hydrophobic unit ratio (mol%) | Solubility and thermoresponsivityb |
|---|---|---|---|---|---|
| a See Fig. S3 and S6† for the 1H NMR spectra and Table S1† for the imidazolium and carboxylate incorporation ratios. b 3 wt% for entries 1–9. | |||||
| 1 | p([MeIm][C9]) | 13.4 | 1.07 | 0 | Insoluble |
| 2 | p[IBVE10-stat-([MeIm][C9])95] | 11.4 | 1.04 | 10 | LCST-type |
| 3 | p[IBVE14-stat-([MeIm][C9])70] | 15.2 | 1.03 | 17 | LCST-type |
| 4 | p[IBVE27-stat-([MeIm][C9])83] | 13.8 | 1.03 | 25 | Soluble |
| 5 | p[IBVE44-stat-([MeIm][C9])42] | 10.0 | 1.05 | 51 | Soluble |
| 6 | p[IBVE67-stat-([MeIm][C9])39] | 17.9 | 1.08 | 63 | Soluble |
| 7 | p[IBVE56-stat-([MeIm][C9])23] | 10.2 | 1.07 | 71 | LCST-type |
| 8 | p[IBVE74-stat-([MeIm][C9])23] | 15.7 | 1.06 | 76 | Insoluble |
| 9 | p[EHVE47-stat-([MeIm][C9])139] | 23.1 | 1.11 | 25 | Soluble |
| 10 | p(IBVE) | — | — | 100 | Insoluble |
| 11 | p(EHVE) | — | — | 100 | Insoluble |
Copolymers bearing carboxylate anions with shorter or longer alkyl groups were also synthesized and subjected to solubility testing. The results are summarized in Table 3. p([MeIm][C8]) is soluble in water at room temperature (entry 1 in Table 3),25 while it becomes insoluble on heating (LCST-type behavior). As expected from the solubility of the [MeIm][C9]-containing copolymers, the copolymers of IBVE and [MeIm][C8] were soluble in water even on heating (entries 2 and 3), which indicates that solubility increased due to the incorporation of the hydrophobic units. Similar results were obtained when [MeIm][C10] was used. p([MeIm][C10]) was insoluble in water (entry 4),25 while p(IBVE-stat-[MeIm][C10]) with an IBVE content of 54% was soluble in water (entry 6).
| Entry | Polymer | M n × 10−3 (precursor) | M w/Mn (precursor) | Hydrophobic unit ratio (mol%) | Solubility and thermoresponsivityb |
|---|---|---|---|---|---|
| a See Fig. S8 and S9† for the 1H NMR spectra and Table S2† for the imidazolium and carboxylate incorporation ratios. b 5 wt% for entries 1–6. | |||||
| 1 | p([MeIm][C8]) | 13.5 | 1.05 | 0 | LCST-type |
| 2 | p[IBVE18-stat-([MeIm][C8])85] | 11.5 | 1.03 | 17 | Soluble |
| 3 | p[IBVE57-stat-([MeIm][C8])29] | 11.9 | 1.05 | 66 | Soluble |
| 4 | p([MeIm][C10]) | 14.5 | 1.04 | 0 | Insoluble |
| 5 | p[IBVE18-stat-([MeIm][C10])90] | 12.8 | 1.03 | 17 | Insoluble |
| 6 | p[IBVE45-stat-([MeIm][C10])39] | 13.6 | 1.05 | 54 | Soluble |
| 7 | p(IBVE) | — | — | 100 | Insoluble |
The unexpected increase in solubility via the introduction of hydrophobic units likely resulted from the subtle change of the balance among different interactions. p([MeIm][C8]) is considered to dissolve in water by hydration around the imidazolium moiety at low temperature. On heating, aggregation is caused by dehydration, ionic interaction among the ionic moieties, and hydrophobic interaction among the alkyl groups of the carboxylate counteranions and the main and side groups of the copolymer chains. Considering the suggested effects of hydrophobic units on polysulfobetaine solubility,21–23 the introduction of hydrophobic units likely weakened the ionic interaction among the ionic moieties of the imidazolium-type IL moiety-containing VE copolymers. This effect facilitates favorable hydration of the ionic moieties, resulting in increased solubility of the hydrophobic unit-introduced copolymers. Indeed, p([MeIm][C9]) is insoluble in water, while the introduction of hydrophobic units contributed to increased solubility, resulting in LCST-type thermoresponsive (IBVE content = 10, 17, or 71%) and soluble (25, 51, or 63%) copolymers. When a large amount of hydrophobic units are introduced, copolymers are insoluble in water likely due to more dominant hydrophobic interaction than hydration. In the 1H NMR spectra of p[IBVE14-stat-([MeIm][C9])70] in D2O at different temperatures (Fig. S10†), the methyl peak of the nonanoate anion slightly broadened at 90 °C, which suggests that the aliphatic counteranions are likely involved in polymer aggregation at high temperature. However, more detailed information was not obtained from the 1H NMR analysis (see the note for Fig. S10 in the ESI†).
The solution behavior depended on polymer concentration as in the case of the polymeric ILs examined in our previous studies.10,25 A dynamic light scattering (DLS) measurement of the copolymer with an IBVE content of 17% was conducted at 20–70 °C at a polymer concentration of 0.1 wt% (Fig. S11†). The diameters of the detected particles were less than 10 nm (comparable diameters to those of the copolymer with an IBVE content of 63% [Fig. S12†]). In addition, the transmittance of a polymer solution at a polymer concentration of 0.1 wt% was approximately 100% at low temperature (Fig. S13†), which was in contrast to the transmittance of approximately 60% at 3 wt% (Fig. 3A). From these results, the polymer chains are in a single chain state at a low concentration, while likely in an assembled state at a high concentration even at a temperature lower than the cloud point.
The introduction of hydrophobic units into UCST-type thermoresponsive polymers also resulted in increased solubility in water (Table 4 and Fig. 4B). A polymeric IL bearing 2-naphthoate (NA) counteranions (p[MeIm][NA]) is soluble in water at high temperature, while becomes insoluble at approximately 60 °C on cooling (entry 1 in Table 4; purple curve in Fig. 4A).25 p(IBVE-stat-[MeIm][NA])s with an IBVE content of 16% or 27% exhibited a cloud point of approximately 48 °C or 20 °C, respectively (entries 2 and 3; green and blue curves in Fig. 4A), which indicates increased solubility compared to the homopolymer. Moreover, a copolymer with an IBVE content of 48% was soluble even when cooled at approximately 5 °C (entry 4; red curve in Fig. 4A). A further increase in IBVE content resulted in a decrease in solubility. A copolymer with an IBVE content of 58% exhibited a cloud point of 32 °C (entry 5; orange curve in Fig. 4A) and those with 72% and 78% were insoluble even at high temperature (entries 6 and 7). The increase in solubility was observed when EHVE was used instead of IBVE as a hydrophobic VE. p[EHVE-stat-([MeIm][NA])] with an EHVE content of 15% exhibited UCST-type thermoresponsive behavior in water (entry 8; Fig. S16†). The cloud point was 40 °C, which was lower than that of the [MeIm][NA] homopolymer.
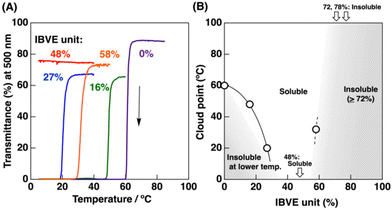 | ||
| Fig. 4 (A) Turbidity measurement of (A) p[IBVEn-stat-([MeIm][NA])m]s in water (entries 1–5 in Table 4; polymer concentration: 2 wt%; scan rate = 1 °C min−1). (B) The relationship between solubility and thermoresponsivity in water of the statistical copolymers and IBVE content (the data are listed in Table 4, polymer concentration: 2 wt%). | ||
| Entry | Polymer | M n × 10−3 (precursor) | M w/Mn (precursor) | Hydrophobic unit ratio (mol%) | Solubility and thermoresponsivityb |
|---|---|---|---|---|---|
| a See Fig. S14 and S15† for the 1H NMR spectra and Table S3† for the imidazolium and carboxylate incorporation ratios. b 2 wt% for entries 1–8. | |||||
| 1 | p([MeIm][NA]) | 11.9 | 1.05 | 0 | UCST-type |
| 2 | p[IBVE14-stat-([MeIm][NA])71] | 14.7 | 1.03 | 16 | UCST-type |
| 3 | p[IBVE27-stat-([MeIm][NA])73] | 13.7 | 1.03 | 27 | UCST-type |
| 4 | p[IBVE38-stat-([MeIm][NA])41] | 13.1 | 1.04 | 48 | Soluble |
| 5 | p[IBVE71-stat-([MeIm][NA])51] | 16.7 | 1.04 | 58 | UCST-type |
| 6 | p[IBVE76-stat-([MeIm][NA])30] | 13.5 | 1.06 | 72 | Insoluble |
| 7 | p[IBVE74-stat-([MeIm][NA])21] | 13.9 | 1.06 | 78 | Insoluble |
| 8 | p[EHVE18-stat-([MeIm][NA])103] | 13.7 | 1.03 | 15 | UCST-type |
| 9 | p(IBVE) | — | — | 100 | Insoluble |
| 10 | p(EHVE) | — | — | 100 | Insoluble |
A possible cause of the UCST-type thermoresponsive behavior is the π–π interaction among aromatic groups. Indeed, the π–π interaction between the imidazolium group and the aromatic counteranion was suggested in the study of not polymeric but low-molecular ILs.33 In this study, the incorporation of hydrophobic units into polymeric ILs likely disturbed the π–π interaction, which led to the increase in water solubility. Further investigation is required to elucidate the mechanism of thermoresponsivity.
The narrow MWDs of the polymers, which were achieved by living cationic polymerization, potentially contribute to very sensitive thermoresponsivity as in the case of the LCST-type thermoresponsive poly(VE)s with oxyethylene side chains.4 Indeed, most of the thermoresponsive polymers examined in this study exhibited very sensitive LCST- and UCST-type thermoresponsivity (Fig. 3A and 4A).
In conclusion, the introduction of hydrophobic units into imidazolium-type IL moiety-containing VE polymers resulted in an increase in water solubility. A [MeIm][C9] homopolymer was insoluble in water, while statistical copolymers consisting of IBVE and [MeIm][C9] were soluble (IBVE content = 25, 51, or 63%) or exhibited LCST-type thermoresponsivity (IBVE content = 10, 17, or 71%) in water. A statistical copolymer with a high IBVE content (76%) was insoluble in water. The solubility of p([MeIm][NA]), which exhibits UCST-type thermoresponsivity in water, also increased with the introduction of hydrophobic units. The results obtained in this study expand the possibility of polymeric ILs and thermoresponsive polymers. We will investigate the mechanism of the unexpected behavior in more detail.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by JSPS KAKENHI Grants 21K05168 and 23K26703. We thank Mr Naoki Matsuo (Osaka University) for the data of the IL moiety-containing homopolymers.References
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173–1222 CAS.
- D. Crespy and R. M. Rossi, Polym. Int., 2007, 56, 1461–1468 CAS.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 CAS.
- S. Aoshima, H. Oda and E. Kobayashi, J. Polym. Sci., Part A: Polym. Chem., 1992, 30, 2407–2413 CAS.
- S. Aoshima and S. Kanaoka, Adv. Polym. Sci., 2008, 210, 169–208 CAS.
- R. Hoogenboom and H. Schlaad, Polym. Chem., 2017, 8, 24–40 CAS.
- Y. Kohno, S. Saita, Y. Men, J. Yuan and H. Ohno, Polym. Chem., 2015, 6, 2163–2178 RSC.
- D. N. Schulz, D. G. Peiffer, P. K. Agarwal, J. Larabee, J. J. Kaladas, L. Soni, B. Handwerker and R. T. Garner, Polymer, 1986, 27, 1734–1742 CrossRef CAS.
- J. Seuring and S. Agarwal, Macromol. Chem. Phys., 2010, 211, 2109–2117 Search PubMed.
- H. Yoshimitsu, A. Kanazawa, S. Kanaoka and S. Aoshima, Macromolecules, 2012, 45, 9427–9434 Search PubMed.
- S. Glatzel, A. Laschewsky and J. Lutz, Macromolecules, 2011, 44, 413–415 CAS.
- J. Seuring and S. Agarwal, Macromol. Rapid Commun., 2012, 33, 1898–1920 CAS.
- J. Seuring and S. Agarwal, ACS Macro. Lett., 2013, 2, 597–600 CAS.
- J. Niskanen and H. Tenhu, Polym. Chem., 2017, 8, 220–232 CAS.
- K. K. Bansal, P. K. Upadhyay, G. K. Saraogi, A. Rosling and J. M. Rosenholm, eXPRESS Polym. Lett., 2019, 13, 974–992 Search PubMed.
- F. Stoica, A. F. Miller, C. Alexander and A. Saiani, Macromol. Symp., 2007, 251, 33–40 CAS.
- C. Zhao, Z. Ma and X. X. Zhu, Prog. Polym. Sci., 2019, 90, 269–291 Search PubMed.
- L. D. Taylor and L. D. Cerankowski, J. Polym. Sci., Polym. Chem., 1975, 13, 2551–2570 CAS.
- H. Y. Liu and X. X. Zhu, Polymer, 1999, 40, 6985–6990 CAS.
- S. Huber and R. Jordan, Colloid Polym. Sci., 2008, 286, 395–402 CAS.
- P. A. Woodfield, Y. Zhu, Y. Pei and P. J. Roth, Macromolecules, 2014, 47, 750–762 Search PubMed.
- V. Hildebrand, A. Laschewsky, M. Päch, P. Müller-Buschbaumd and C. M. Papadakis, Polym. Chem., 2017, 8, 310–322 Search PubMed.
- N. Wang, B. T. Seymour, E. M. Lewoczko, E. W. Kent, M. Chen, J. Wan and B. Zhao, Polym. Chem., 2018, 9, 5257–5261 Search PubMed.
- K. Kempe, T. Neuwirth, J. Czapleswka, M. Gottschaldt, R. Hoogenboom and U. S. Schubert, Polym. Chem., 2011, 2, 1737–1743 Search PubMed.
- N. Matsuo, M. Ueda, A. Kanazawa and S. Aoshima, Polym. Chem., 2023, 14, 4804–4808 Search PubMed.
- D. Yokota, A. Kanazawa and S. Aoshima, RSC Adv., 2020, 10, 42378–42387 CAS.
- Y. Seki, A. Kanazawa, S. Kanaoka, T. Fujiwara and S. Aoshima, Macromolecules, 2018, 51, 825–835 CrossRef CAS.
- D. Yokota, A. Kanazawa and S. Aoshima, Polym. Chem., 2018, 9, 5080–5085 RSC.
- S. Okamura, N. Kanoh and T. Higashimura, Makromol. Chem., 1961, 47, 35–47 Search PubMed.
- D. D. Eley and J. Saunders, J. Chem. Soc., 1954, 1677–1680 Search PubMed.
- V. E. Meyer and G. G. Lowry, J. Polym. Sci. Part A, 1965, 3, 2843–2851 Search PubMed.
- N. A. Lynd, R. C. Ferrier Jr and B. S. Beckingham, Macromolecules, 2019, 52, 2277–2285 Search PubMed.
- W. Xu, T. Wang, N. Cheng, Q. Hu, Y. Bi, Y. Gong and L. Yu, Langmuir, 2015, 31, 1272–1282 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, NMR spectra of polymers, and turbidity measurements. See DOI: https://doi.org/10.1039/d5py00139k |
| This journal is © The Royal Society of Chemistry 2025 |
