 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Introduction, classification and applications of 3D bioprinted hydrogels for cancer treatment: a review
Anusha
Thumma

Department of Pharmaceutical Sciences, College of Pharmacy, Nova Southeastern University, Fort Lauderdale, FL, USA. E-mail: at1385@mynsu.nova.edu
First published on 20th June 2025
Abstract
Polymeric hydrogels have become effective materials in cancer therapy because of their biocompatibility, biodegradability and tunable chattels. This review presents a thorough investigation of the synthesis and medicinal uses of different naturally occurring and synthetic hydrogels, for cancer therapy, mainly via 3D modeling and printing. The exceptional biocompatibility of hydrogels, coupled with their remarkable potential for replicating the intricate extracellular matrix (ECM), positions them as ideal materials for constructing scaffolds used in the synthesis of in vitro 3D tumor constructs. Hydrogels can also be used for 3D printing to treat cancer by aiding in accurate control over the composition of hydrogel scaffolds. 3D modeling and printing play an important role in cancer treatment by enabling drug screening. This review distinguishes itself by integrating a comparative analysis of both conventional and emerging hydrogel systems—including natural, synthetic, and hybrid types particularly designed for 3D bioprinting in cancer modeling. This study paves the path for new researchers to explore cancer treatment by combining hydrogel-based materials with advanced techniques.
1. Introduction
Hydrogels are hydrophilic polymers with a three-dimensional (3D) structure. For a material to be called a hydrogel, it must contain a minimum of 10% water by weight. The presence of a significant proportion of water molecules makes the polymeric material swell and renders softness and flexibility. The structural integrity of a hydrogel is due to the physical or chemical linkages of the polymer chains.1,2 The hydrophilicity can be attributed to the presence of hydrophilic functional groups on its surface including the amino group (–NH2), carboxylic group, (–COOH), hydroxyl group (–OH), amide group (–CONH2), imide group (–CONH) and sulfonic acid group (–SO3H).3,4 Their soft, tissue-like structure enables them to integrate with biological tissues, without any antagonistic reaction.5 Wichterle and Lim's development of soft contact lenses in 1960 using natural hydrogels marked a significant breakthrough in the biomedical field.6 A significant advancement in this area involves the development of multi-responsive hydrogels, exemplified by materials like propyl acrylamide, chitosan and polyvinyl alcohol. These materials exhibit high sensitivity to environmental changes, making them suitable for applications in smart sensors, actuators, and targeted drug delivery systems for cancer treatment.7,8 In this context, several researchers have explored the use of hydrogels in cancer treatment.9–13 This review paper aims to bridge the gap between bench studies and clinical practice for the application of both natural and synthetic polymer hydrogels in cancer treatment. The study indicates some key challenges in utilizing laboratory-based studies in practical applications. It further suggests an optimized application of these materials, discussing the latest technological advancements.Keeping in view the above discussion, the study aims to (i) explore the utilization of 3D-printed hydrogels in cancer treatment, (ii) investigate the synthesis, characterization, and classification of both natural and synthetic hydrogels, (iii) analyse the integration of these hydrogels in 3D modeling and bioprinting techniques to accurately replicate the complex tumor microenvironment, (iv) study the advantages of combining natural and synthetic hydrogels to optimize drug delivery and improve their mechanical properties, (v) discuss the advancements in hydrogel-based therapies as photothermal and magnetic hyperthermic agents for targeted cancer treatment and (vi) provide valuable insights into the challenges and opportunities associated with scaling up hydrogel-based cancer therapies for clinical application. This review offers a unique contribution by providing an integrated comparative analysis of both established and cutting-edge hydrogel systems encompassing natural, synthetic, and hybrid forms specifically engineered for 3D bioprinting applications in cancer modeling. The paper consolidates recent advancements in stimuli-responsive hydrogels, such as those that react to reactive oxygen species (ROS) and hypoxia, and investigates their incorporation into constructs designed to precisely replicate the tumor microenvironment (TME). Furthermore, it details various hydrogel types, crosslinking strategies, and their specific uses in different cancer models, offering a structured overview that is often absent in previous academic works, thereby providing invaluable direction for future translational research in hydrogel-based cancer therapies.
In the field of polysaccharide science, particularly concerning their application in biomedical engineering, a pertinent hypothesis derived from the provided review is that combining natural polymeric hydrogels, such as those made from polysaccharides like alginate, chitosan, and hyaluronic acid, with synthetic polymers can effectively address the inherent limitations of natural hydrogels. While natural hydrogels are highly valued for their biocompatibility, biodegradability, and non-toxic nature, they often exhibit weaker mechanical properties and limited versatility. The hypothesis posits that integrating synthetic components can enhance crucial characteristics like mechanical strength and enable more precise control over drug release, thereby improving their overall suitability and efficacy for various biomedical applications, including cancer treatment. This approach aims to leverage the benefits of both natural and synthetic materials to create optimized hydrogel systems.
2. Classification of polymeric hydrogels
Polymeric hydrogels can be grouped into two categories i.e., natural and synthetic.14–17 Natural polymeric hydrogels are obtained from biological sources like plants, animals, or microorganisms.18 They are biocompatible, biodegradable, and inherently bioactive, which promotes their usage in biomedical applications.19 These hydrogels can encapsulate cells and can be used as therapeutic agents. Their bioactive nature may be enhanced by modifying their structures through physical and chemical crosslinking methods.20 Physical methods like ionic crosslinking and hydrogen bonding depend on weak polymeric chain interactions. The formation of alginate hydrogels by crosslinking alginate chains with calcium ions is a common example. Chemical methods rely on the formation of covalent bonds within the polymeric chains and free radical polymerization. The choice of method depends upon the desired properties of the hydrogel.21 The bioactive nature of natural hydrogels has led various researchers to work on their extraction. Sohrabi22 focused on biometric hydrogels like collagen, fibrin, and hyaluronic acid having an extracellular matrix. Gong et al.23 further revealed that these materials effectively improve cell adhesion, proliferation, and differentiation. Elsabahy and Wooley24 worked on functional nanomaterials and hydrogels for controlled drug delivery using the polymerization-induced self-assembly (PISA) technique. Peppas25 used alginate and chitosan for the release of drugs to specific body tissues. The study by Kang et al.26 focused on obtaining stimuli-responsive hydrogels, which are sensitive to changes in system pH, temperature or light. Liao et al.27 worked on regenerative medicines.28 Wu et al. used silk fibroin and gelatin to produce biodegradable hydrogels having adjustable mechanical properties. These polymers can be used in tissue engineering and organ implants.29Synthetic polymeric hydrogels are synthesized in laboratories from monomers to obtain better control of their structural and chemical properties.30,31 These hydrogels can further be modified to obtain precise drug release and are sensitive to pH and temperature.32,33 These polymers mostly used in hydrogels include polyethylene glycol (PEG), poly acrylic acid (PAA), polyvinyl alcohol (PVA), poly N-isopropyl acrylamide (PNIPAA) and poly hydroxyethyl methacrylate (pHEMA).34–37 Miyata et al. (1999) developed polyacrylamide hydrogels by chemical cross-linking and modified it using rabbit IgG antibodies.38 It is worth noting that the characterization of polymers post-synthesis and post-modification is important to explore the properties and nature of the synthesized product.39–42 Hydrogels based on polysaccharides like chitosan, dextrin, fibrin, and gelatin can be synthesized using covalent crosslinking and polymerization techniques.43
Natural polymeric hydrogels, although biocompatible and biodegradable, possess weak mechanical and non-versatile properties, limiting their applications. In this aspect, synthetic hydrogels promote added mechanical strength and other properties. However, unlike natural polymers, synthesized hydrogels are not environmentally friendly.44 This problem can be addressed by combining natural polymeric hydrogels with the synthetic ones.36 As an example, chitosan-based natural hydrogels can be combined with those based on synthetic polymers like polymethacrylic acid or polyacrylic acid to improve their properties. Such combinations with synthetic hydrogels may improve the mechanical strength and drug release control of natural hydrogels. A practical method for combining natural and synthetic hydrogels is to use interpenetrating network (IPN) hydrogels obtained by crosslinking different polymeric materials. IPNs show enhanced mechanical properties and more precise drug release control compared to single-network hydrogel polymers. Natural polymers like alginate, carrageenan, and gum arabic may also be combined with synthetic polymers to improve the control over the delivery of drugs to specific organs and offer improved tissue engineering.34 The work by Nair et al. can be regarded as one of the foundational research on multi-functional materials for controlled drug release and tissue engineering. They successfully developed strong and biocompatible hybrid hydrogels using thiol-Michael addition click chemistry.45 The use of non-covalent, supramolecular interactions like π–π interactions and hydrogen bonding to synthesize self-assembling hybrid hydrogels was explored by Hu et al. The self-healing abilities and stimuli responsiveness of these hybrid hydrogels render them a good candidate for biomedical and environmental applications.46,47 The development of hydrogels using 3-D printing, as investigated by Zhang et al. helps achieve precise control of the structure and multifunctional properties of hydrogels (Table 1).48
| Types | Examples | Advantages | Disadvantages | Ref. | |
|---|---|---|---|---|---|
| Natural polymeric hydrogels | Polysaccharides | Alginate, chitosan, hyaluronic acid | Biocompatible, biodegradable, non-toxic | Lower mechanical strength, batch-to-batch variability | 49–52 |
| Proteins | Collagen, gelatin, fibrin | Biocompatible, cell adhesive, biodegradable | Less stable, can be immunogenic | 53–55 | |
| Synthetic polymeric hydrogels | Acrylic acid-based | Poly(acrylic acid) (PAA) | Tunable swelling properties, pH sensitivity | Can be toxic, less biodegradable | 30 |
| Vinyl alcohol-based | Poly(vinyl alcohol) (PVA) | Good mechanical properties, high water content | Less biocompatible, difficult to crosslink | 56 | |
| Polyethylene glycol-based | Poly(ethylene glycol) (PEG) | Biocompatible, non-toxic, highly water-soluble | Low mechanical strength, difficult to crosslink | 50 |
3. Industrial and medical applications of polymeric hydrogels
Polymeric hydrogels, due to their salient features, may be widely used in different industrial, medical and biomedical applications. Some of these possible uses are discussed below:Polymeric hydrogels are excellent adsorbents.57,58 They can be used in wastewater treatment to adsorb the organic and inorganic pollutants like dyes,59–61 heavy metals and pharmaceuticals from polluted water.40,62 In agriculture, acrylic acid–acrylamide–guar gum hydrogels have shown efficacy in improving the growth of guava plants affected by drought.43 In the medical and bio-medical fields, as already discussed, the ability of polymeric hydrogels for the controlled release of drugs to specific targets can effectively be utilized in the encapsulation of drugs.63 They are sensitive to changes in pH, temperature and concentration of the substance. This property, especially pH sensitivity, is very helpful for drug delivery, as different tissues and cellular components in the body are at different pH levels.64
In engineering applications, like bone tissue engineering, hybrid polymers based on calcium carbonate and alginate have shown effective performance being biocompatible.65 Similarly, nanomaterials obtained from xanthan gum or chitosan impregnated with ferric oxide nanoparticles have shown improved mechanical strength. This combination has been used in developing magnetically responsive tissue engineering systems.66 Mokhtari et al. developed an injectable hydrogel of Kappa-carrageenan functionalized with C-phycocyanin which is mechanically strong and promotes cell growth. The synthesized hydrogel possesses anti-oxidant, anti-microbial and anti-inflammatory properties, thus helping in quick wound recovery.67 Similarly, the research by Dev et al. was focused on κ-carrageenan-C-phycocyanin-based injectable hydrogels for quick recovery of wounds, especially for patients with intensive tissue damage due to burning or diabetes. C-Phycocyanin accelerates hemostasis in severe injuries, which helps in quick wound healing.68 Another wide application of polymeric hydrogels is cancer treatment, through 3-D modeling. The controlled release of drugs to the target cancer cells and improving the immune response, these hydrogels promote therapeutic ability and also minimize side effects of the treatment drugs.69
Hydrogels and their biological performance is profoundly influenced by their chemical composition and the specific functional groups they possess. For example, the inclusion of moieties like amino (–NH2), carboxyl (–COOH), and hydroxyl (–OH) groups significantly affects their degradation, bioactivity, and cellular interactions.70,71 Amino groups, with their positive charge, enhance electrostatic interactions with negatively charged cell membranes, thereby facilitating cell adhesion and proliferation, while also playing roles in buffering intracellular pH and aiding endosomal escape in drug delivery. Carboxyl groups, conversely, contribute to pH responsiveness and degradability, especially in acidic tumor microenvironments, enabling hydrogen bonding and ionic crosslinking vital for stimuli-responsive drug release. Meanwhile, hydroxyl (–OH) and sulfonic acid (–SO3H) groups improve hydrophilicity, swelling behavior, and facilitate protein adsorption, modulating cellular uptake and immune responses.33 These functional groups also influence enzymatic degradation; for instance, gelatin and collagen-based hydrogels, rich in natural –COOH and –NH2 groups, are more susceptible to matrix metalloproteinase (MMP)-mediated degradation, a process highly relevant in tumor microenvironments.3,18
The very design of these hydrogels is intricately tied to their crosslinking chemistry, a fundamental aspect that controls their architecture, mechanical integrity, degradation rate, and cellular compatibility. Three major crosslinking strategies are commonly employed: photocrosslinking, which uses UV or visible light and photoinitiators to activate polymerization in materials like GelMA (gelatin methacryloyl), offering precise spatial and temporal control for fabricating complex cancer models with tunable stiffness and porosity.72 Another approach is click chemistry, particularly thiol-Michael addition and azide-alkyne cycloaddition, enabling mild, efficient, and cytocompatible gelation ideal for bio-orthogonal and stimuli-responsive hydrogels, such as PEG-thiol and vinyl sulfone-based systems that incorporate bioactive cues with minimal cytotoxicity.45,73 Finally, enzymatic crosslinking utilizes biologically compatible enzymes like horseradish peroxidase (HRP) and transglutaminase to form hydrogels under physiological conditions, often mimicking the natural extracellular matrix (ECM) and proving suitable for cell-laden constructs.74
Beyond their chemical makeup and crosslinking, the structural properties of hydrogels, including their porosity, crosslinking density, and degradation rate, also profoundly influence their biological interactions. Porosity, for example, directly impacts nutrient diffusion, oxygen transfer, and cell migration; highly porous scaffolds might promote angiogenesis and faster tissue ingrowth but could compromise mechanical robustness. Conversely, crosslinking density is inversely related to degradation rate and permeability; hydrogels with a high density exhibit prolonged drug retention and sustained release profiles, which are beneficial for chemotherapeutic delivery in cancer. The degradation rate itself must be precisely synchronized with tissue regeneration or treatment timeframes. While natural hydrogels like gelatin or alginate degrade enzymatically or hydrolytically, synthetic ones like PEG or PVA offer better control over breakdown through targeted structural modifications, allowing for tailored performance in diverse biomedical applications.75,76
This intricate interplay of the composition and structure often culminates in advanced designs such as interpenetrating polymer networks (IPNs), which integrate two or more polymer systems to provide a synergistic blend of mechanical robustness and biological functionality, effectively simulating the mechanical heterogeneity and signaling complexity of native ECMs. For instance, gelatin–alginate IPNs have been extensively used in breast and colorectal cancer models, where gelatin supports cell adhesion while alginate provides structural stability and ionic crosslinking, mimicking in vivo tumor stiffness and heterogeneity.77–79 Similarly, PEG–chitosan IPNs merge the pH responsiveness and biodegradability of chitosan with the structural flexibility and non-immunogenicity of PEG, enabling responsive drug delivery in acidic tumor environments.34 These sophisticated ECM-mimetic systems offer advanced capabilities for spatial patterning and matrix stiffness tuning, and thus are essential tools for studying cancer cell invasion, metastasis, and drug resistance within complex 3D platforms.80
4. Cancer: a disease of uncontrolled cell growth
Cancer is a disease caused by abnormal cell growth and division. Genetic mutations can lead to uncontrolled cell proliferation, forming tumors that may invade surrounding tissues or spread to distant parts of the body through a process called metastasis.81 Various factors, including lifestyle, environmental exposure, and genetic predisposition, can increase cancer risk. Cancer can develop in various tissues and organs.82,83 Some of the most common types of cancer are discussed below:● Carcinoma: This type of cancer originates in epithelial tissues, which line organs and cavities. Examples include lung cancer, breast cancer, and colon cancer.84
● Sarcoma: Sarcomas develop in connective tissues, such as bone, muscle, and cartilage. Examples include osteosarcoma and liposarcoma.85
● Leukemia: This cancer affects blood-forming tissues, primarily bone marrow. It leads to the overproduction of abnormal white blood cells.
● Lymphoma: This cancer develops in the lymphatic system, a network of vessels and glands that help fight infection. Hodgkin lymphoma and non-Hodgkin lymphoma are its two main types.86
Treatment options, such as surgery, chemotherapy, radiation therapy, immunotherapy, and targeted therapy, depend on the specific type and stage of cancer. The stage of cancer refers to the extent of disease, which helps determine the best course of treatment and predict the patient's prognosis.87 Cancer is typically staged from 0 to IV:88
(a) Stage 0 (carcinoma in situ): abnormal cells are present but are not spread.
(b) Stage I: cancer is small and localized.
(c) Stage II and III: cancer has spread to nearby lymph nodes or tissues.
(d) Stage IV (metastatic): cancer has spread to distant parts of body.
Cancer is a dangerous disease because it disrupts normal growth and division of cells. When abnormal cells grow uncontrollably, they can form tumors that invade nearby tissues or spread to distant parts of the body through a process called metastasis. This can lead to severe health problems, organ failure, and ultimately, death.89
4.1. Conventional cancer treatment methods
Cancer treatment involves a combination of therapies, tailored to a specific type in addition to the stage of disease. Traditional methods (Fig. 1) include surgery to physically remove tumors, chemotherapy to target rapidly dividing cancer cells, and radiation therapy to damage cancer cells with high-energy rays. In recent years, more advanced therapies have emerged, such as immunotherapy, which harnesses the body's immune system to fight against cancer cells, and targeted therapy, which specifically targets cancer cells and minimizes damage to healthy tissues.90 Additionally, emerging fields like gene therapy and nanotechnology offer promising avenues for future cancer treatments. The patient's health, type, stage of cancer, and possible side effects of various treatments are some of the key variables that affect treatment type selection.91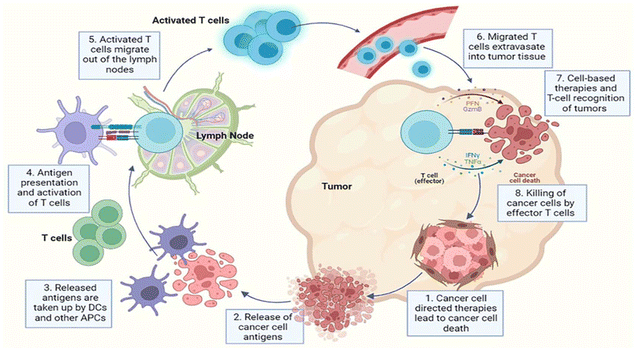 | ||
| Fig. 1 Diagrammatic overview of cancer treatment showing the multifaceted approaches for combating cancer. Reproduced from ref. 92 with permission from Frontiers, copyright 2025. | ||
4.2. Polymeric hydrogels for cancer treatment
Polymeric hydrogels, with their exceptional properties such as biocompatibility and biodegradability, are promising materials for the treatment of cancer owing to improved drug delivery, reduced side effects, and improved therapeutic efficiency.12 There are several benefits to treating cancer with 3D printing and 3D modelling that uses hydrogels as hydrogels offer a 3D environment that closely resembles the extracellular matrix (ECM) found naturally in the human body owing to its high-water content and biocompatibility. Furthermore, when compared with traditional 2D cell culture systems, the 3D environment makes it possible to create more accurate tumour models.93–95 | ||
| Fig. 2 (a) Hypoxia within tumor sites affects cancer cell response to therapy. (b) Hydrogels and cell culture media aid in facilitating oxygenation to the tumor site, possibly enhancing therapeutic efficiency. Reproduced from ref. 102 with permission from RSC copyright 2025. | ||
Hypoxia-inducible factor-1 (HIF-1) is a key factor in hypoxia, that is characterized by low oxygen levels and is a characteristic of advanced malignancies. Because oxygen gradients within tumours are vital, scientists have now worked on the synthesis of O2-controllable hydrogels to investigate how hypoxia affects sarcoma cell invasion and migration. A useful tool for assessing the early phases of the metastatic process are these synthetic hydrogels.103 Additionally, it has been confirmed that 3D collagen scaffolds are more resistant to cisplatin having larger amounts of reactive oxygen species (ROS) when compared with 2D models. A more physiologically appropriate microenvironment that resembles the circumstances faced by tumour cells in vivo can lead to enhanced ROS generation in 3D cultivated cells.104,105 The efficient distribution of therapeutic medicines is hampered by elements such as cancer cell adherence and cellular packing density. Nowadays, researchers use 3D hydrogel-based models for simulating the physiological conditions inside the TME for addressing this limitation.106 3D hydrogels, made from alginate, are widely employed to study anticancer agents and drug resistance pathways. These models offer a more physiologically relevant platform when compared with conventional 2D culture systems. They also better study the unique characteristics of tumor necrosis factor (TNF), as cancer stem cells (CSCs), in their specialized niches.107
A major area of preclinical study has been breast cancer, a cancer that is common in women. Creating 3D models of breast cancer can speed up research resulting in more accurate results by offering valuable information.108 A study reported the use of an engineered hydrogel-based human adipose/collagen model for investigating breast cancer cell migration and exploring the effect of adipocytes on this migration. The model highlights the improved cell migration when its efficiency was compared with the empty scaffold controls revealing its cancer therapeutic efficacy in personalized medicine approaches.109 Furthermore, 3D hydrogel-based platforms were used to model different kinds of cancers beyond pancreatic ductal adenocarcinoma. For example, 3D hydrogel models have been developed to replicate the microenvironment of ovarian cancer.110 For lung cancer, 3D hydrogel models have been shown to more accurately mimic cell invasion and metastasis when compared with traditional 2D models.111,112
Researchers have employed hydrogels to mimic the pancreatic ductal adenocarcinoma (PDAC) tumor microenvironment (TME) by incorporating components such as hyaluronic acid (HA), collagen, and Matrigel, which are derived from or inspired by the TME. Osteosarcoma (OS) is the most common primary malignant bone tumor. Studies have shown that both scaffold-free and scaffold-based 3D OS culture models are valuable tools for replicate complex TME, with spheroids being particularly well-suited for this purpose.113 A 3D endothelialized vesicle equivalent, developed using a bladder collagen-based cancer model95 can potentially reduce animal experimentation and aid in toxicological evaluation of anti-cancer drugs. To accurately model cancer progression and human tumors, a 3D environment that mimics the extracellular matrix (ECM) is crucial. 3D hydrogels facilitate in vitro study of various tumor cells and modeling of tumor angiogenesis.114 By manipulating conditions and parameters, researchers can more easily conduct drug screening and obtain reliable results.115
Reactive oxygen species (ROS) exhibit a complex, dual nature in cancer progression and treatment. While tumor microenvironments often exhibit elevated ROS levels due to heightened metabolic activity and mitochondrial dysfunction, these same ROS can be strategically exploited for therapeutic gain. Specifically, ROS-sensitive hydrogels offer a promising avenue; they undergo cleavage, swelling, or degradation in response to oxidative stress, enabling precise, controlled drug release.116–118 Consider, for instance, hydrogels that incorporate disulfide bonds. These materials effectively degrade when exposed to both glutathione (GSH) and ROS, facilitating on-demand drug release directly within oxidative tumor sites.104,119 Similarly, thioketal-based polymers have been integrated into hydrogel matrices to release agents like doxorubicin or siRNA. Their cleavage by ROS in inflamed or cancerous tissues allows for targeted delivery.120 Such systems are vital for minimizing systemic toxicity by ensuring drug delivery is localized to these ROS-rich cancerous regions. 3D hydrogels provide a robust platform for simulating in vivo-like tumor conditions, including critical hypoxia and oxidative stress gradients. By embedding ROS-sensitive probes or reporter systems within these hydrogels, researchers can visually assess drug efficacy based on subsequent ROS generation. For example, 3D collagen scaffolds have been effectively used to evaluate the varied ROS responses and cisplatin resistance in both ovarian and lung cancer cells.104 Notably, higher ROS levels observed in 3D models correlated with greater drug resistance compared to 2D models. This underscores the significant utility of these advanced models in screening drugs under realistic stress conditions. Furthermore, ROS-inducible fluorescence reporters can be leveraged for real-time monitoring of antioxidant or pro-oxidant drug activities within 3D matrices. This methodology allows for dynamic assessment of redox-modifying therapeutics, such as paclitaxel, curcumin, or selenium nanoparticles.121
Hydrogels are versatile for 3D bioprinting in cancer research. Their adaptable characteristics, including mechanics, degradation, and optics, along with cell-supporting capabilities, make them well-suited for constructing 3D tumor models. Table 2 compares properties of natural and synthetic hydrogels polymers for cancer treatment.124 Natural polymers while offering good biocompatibility (e.g., alginate, collagen) often suffer from poor mechanical properties that can hinder bioprinting. Semi-synthetic polymers like GelMA address these limitations by enhancing stability and tunability while maintaining biocompatibility. Synthetic polymers, such as PEG, demonstrate excellent bio-printability and shape fidelity but may exhibit limited biocompatibility. The choice of crosslinking method significantly impacts hydrogel properties. Chemical crosslinking, involving irreversible covalent bonds, often results in strong hydrogels but may introduce cytotoxicity due to the use of chemical crosslinking agents. On the other hand, physical crosslinking, relying on non-covalent interactions (e.g., thermal or ionic), is generally more cell-friendly but may produce weaker hydrogels. By carefully selecting the polymer and the crosslinking method, researchers can tailor hydrogel properties to specific 3D cancer bioprinting applications.125
| Hydrogel | Origin | Properties | Crosslinker | Application in cancerous models |
|---|---|---|---|---|
| Alginate | Natural (brown algae) | Biocompatible, forms stable gels, easy to handle126 | Ionic crosslinking (calcium ions), enzymatic crosslinking | Breast cancer, ovarian cancer, prostate cancer, pancreatic cancer78,127 |
| Gelatin | Animal derived protein (from collagen) | Biodegradable, support cell, attachment and growth | Chemical crosslinking | Used for 3D culture model & breast cancer, liver cancer, glioblastoma, melanoma128 |
| Hyaluronic acid (HA) | Natural (animal tissues) | Biocompatible, anti-inflammatory properties, found in extracellular matrix | Chemical crosslinking (hydrazide chemistry), photopolymerization | Breast cancer, ovarian cancer, skin cancer, brain cancer, colorectal cancer model79 |
| Collagen | Natural (animal sources) | Excellent cell–matrix interactions, high biocompatibility | Chemical crosslinking (EDC/NHS), enzymatic crosslinking | Breast cancer, liver cancer, lung cancer, head and neck cancer, pancreatic cancer model129 |
| Fibrin | Natural (blood plasma protein) | Promotes cell adhesion and tissue growth | Covalently crosse linked | Tissue engineering for cancer therapy130 |
| Polyethylene glycol (PEG) | Synthetic | Biocompatible, tunable properties, low immunogenicity | Photopolymerization | Cancer of breast, liver, prostate, pancreas, lung adenocarcinoma model131 |
| Poly(vinyl alcohol) | Synthetic | Biocompatible, good mechanical strength, stable | Freeze–thaw cycles, chemical crosslinking | Glioblastoma, brain cancer, pancreatic cancer132 |
| Poly(lactic-co-glycolic acid) PLGA | Synthetic | Biodegradable, adjustable degradation rate & mechanical properties | Chemical crosslinking | Scaffolds for cancer therapy133 |
| GelMA | Semisynthetic (modified form of gelatin) | Maintained biocompatibility, biodegradable, increased stability and tunability in comparison with unmodified form | Free radical polymerization | Bladder cancer model134 |
Breast cancer remains a significant global health concern, with metastasis and recurrence being major contributors to mortality. While advancements in screening and awareness have improved survival rates, the complex interplay between tumor cells and the surrounding microenvironment poses significant challenges in modeling this disease. 3D bioprinting has emerged as a valuable tool for developing sophisticated breast cancer models.135 Utilizing patient-derived materials, such as human mammary-derived extracellular matrix (ECM), has further enhanced the physiological relevance of these models. Photothermal therapy, which utilizes infrared radiation, has emerged as a valuable modality for cancer treatment. To effectively implement photothermal therapy, the development of scaffolds/hydrogels with robust photothermal properties is crucial. One study demonstrated the potential of 3D printing (3DP) to fabricate porous scaffolds (dopamine-modified alginate and PDA) that mimic the mechanical properties of breast tissue while exhibiting impressive photothermal effects. Elba E. Serrano et al.136,137 developed methodologies for the ultrastructural analysis of hepatocellular carcinoma 70 (HCC70) cells cultured in both monolayer and 3D hydrogel environments. The triple-negative breast cancer (TNBC) cell line HCC70 was maintained in a commercially available membrane matrix hydrogel for a 2D monolayer culture. In contrast to a disorderly spread of flattened cells in the 2D culture (Fig. 3A–C), cells cultured in 3D hydrogels formed multi-layered spheroids (Fig. 3D–F). This approach provides a valuable tool for studying and analyzing transmission electron microscopy (TEM) images of both cancer and healthy cell line hydrogel cultures.
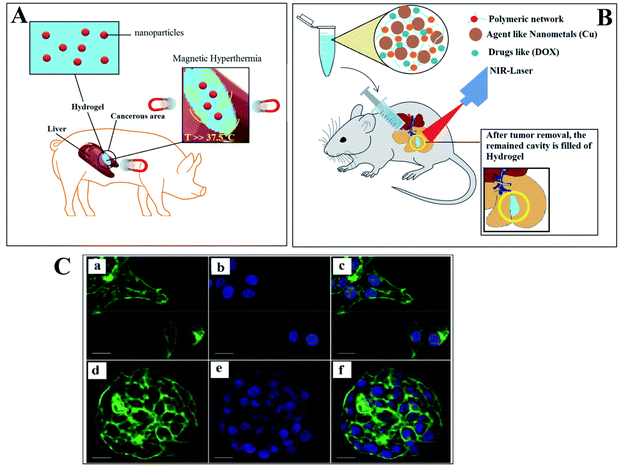 | ||
| Fig. 3 (A) Hydrogels loaded with nanoparticles for enhanced magnetic hyperthermia. (B) Scaffold/hydrogel with photothermal properties. (C) Confocal microscopy of HCC70 cells: (a–c) in monolayer culture and (d–f) in a hydrogel environment. Reproduced from ref. 102 with permission from RSC copyright 2025. | ||
Lung cancer remains a leading cause of cancer-related deaths worldwide, with late diagnosis significantly impacting mortality rates, particularly in aggressive small-cell lung cancer. 3D bioprinted models enable the study of various aspects of lung cancer, such as cellular metabolism, metastatic progression, muscle cachexia, and drug response. Common cell lines used in these models include A549 and NL20, while patient-derived tumor cells can be utilized to create personalized preclinical models. Compared to traditional tumor organoid cultures, 3D bioprinting offers potential advantages such as faster development, reduced technical complexity, and increased success rates. This study investigated the impact of scaffold architecture on liver cancer model development. Gelatin-based 3D liver models were bioprinted with varying angles between adjacent layers: 90 degrees (A) and 60 degrees (B). The study assessed cell viability of HUH7 liver cancer cells after three days of culture within these scaffolds and evaluated the formation of bile canaliculi, which are crucial structures for liver function.138 Colorectal cancer, a leading cause of cancer-related deaths worldwide, predominantly arises as an adenocarcinoma originating from the inner lining of the colon or rectum. Accurate modeling of this disease presents significant challenges due to the complex interplay between cancer cells, the gut microbiota, and the intricate layered structure of the gastrointestinal tract.139 A recent study has shed light on cell of colon cancer migration through leveraging 3D printing (3DP) technology. By combining hydrogels with 3DP, researchers can conduct more accurate and cost-effective studies. For instance, 3D-printed mandible templates can accelerate product development and reduce manufacturing and clinical costs after cancer treatment. Despite significant advancements in this field, there remains a pressing need to further develop and integrate these methods to establish a comprehensive strategy for cancer treatment.140
5. Challenges and clinical translation of 3D-bioprinted hydrogels
5.1. Technical limitations
A paramount concern in 3D bioprinting is ensuring cells remain viable throughout the entire printing process. Various factors, such as high extrusion pressure, exposure to UV light during photocrosslinking, or elevated temperatures, can lead to cellular damage or even apoptosis. Research indicates that printing pressures exceeding 100 kPa and high concentrations of photoinitiators substantially reduce cell survival rates.141 To combat this, optimizing nozzle diameter and extrusion speed, alongside incorporating protective additives like gelatin or trehalose, has proved effective in boosting post-printing cell viability. Another limitation in 3D bioprinting is bioink rheological behaviour. The successful printability of bioinks is intrinsically linked to their rheological characteristics, including shear-thinning behavior, viscosity, and gelation kinetics. Hydrogels like alginate and gelatin-methacryloyl (GelMA) are frequently chosen for their customizable rheological profiles. Rapid crosslinking immediately after deposition is crucial for preserving the printed structure's fidelity. If the rheological properties aren't properly tuned, issues such as nozzle clogging or the collapse of the printed construct can occur.142,143 Batch-to-batch inconsistency is another limitation of 3D bioprinting. Biological components commonly used in hydrogels, such as gelatin, Matrigel, and collagen, often exhibit variability in their composition, which depends on their source and processing methods. Employing synthetic or semi-synthetic bioinks, such as PEG or GelMA, synthesized under stringent, controlled conditions, helps in reduction of batch-to-batch inconsistency.126,143,1445.2. Clinical translation and regulatory limitations
Scaling up hydrogel production for either clinical application or industrial use demands highly reproducible processes that maintain consistent viscosity, crosslinking, and biological performance. Sterilization methods must also be carefully chosen to preserve the hydrogel's integrity. While advancements such as automated extrusion and robotic bioprinting platforms have improved the process, the associated costs and need for greater standardization continue to present significant challenges.145 In the U.S., the Food and Drug Administration (FDA) classifies hydrogel systems that combine a biological matrix with embedded drugs, growth factors, or cells as combination products.146–148 The regulatory review process thoroughly evaluates manufacturing processes (CMC), sterility, biodegradation, and potential device–drug interactions. Early engagement with regulatory bodies, often through mechanisms like the FDA's INTERACT meetings, is highly recommended for developers.149,1506. Conclusion
In conclusion, this comprehensive review has underscored the transformative potential of polymeric hydrogels, particularly when integrated with 3D bioprinting technologies, in revolutionizing cancer modeling and therapy. 3D bioprinting enables precise manipulation of hydrogel scaffolds, controlling their architecture and composition. This allows for the creation of intricate structures that mimic the tumor microenvironment, advancing cancer research and treatment. We have explored the synthesis and diverse medicinal applications of both naturally occurring and man-made hydrogels, demonstrating their critical role in advancing cancer research through precise compositional control afforded by 3D bioprinting, which is crucial for high-throughput drug screening. By engineering stimuli-responsive hydrogels with enhanced mechanical and biochemical properties, researchers can develop more effective and personalized therapies. To ensure widespread adoption of hydrogel-based treatments, scalability and affordability are critical considerations. Addressing these aspects will position hydrogels as foundational technologies for next-generation cancer therapies. While holding significant promise in cancer treatment, the clinical translation of polymeric hydrogels faces several hurdles. These include the need to develop hydrogels with improved mechanical properties, controlled degradation, and efficient drug delivery. Additionally, successful clinical translation requires careful consideration of biocompatibility, toxicity, and regulatory approval. Despite these challenges, the future of polymeric hydrogels in cancer treatment remains promising.Data availability
The data are already available within the article.Conflicts of interest
There are no conflicts to declare.References
- N. A. Peppas and A. S. Hoffman, Hydrogels, in Biomaterials science, Elsevier, 2020, pp. 153–166 Search PubMed.
- Y. S. Zhang and A. Khademhosseini, Advances in engineering hydrogels, Science, 2017, 356(6337), eaaf3627 CrossRef PubMed.
- B. S. Kaith, A. Singh, A. K. Sharma and D. Sud, Hydrogels: synthesis, classification, properties and potential applications—a brief review, J. Polym. Environ., 2021, 29(12), 3827–3841 CrossRef CAS.
- M. H. Alves, B. E. Jensen, A. A. Smith and A. N. Zelikin, Poly (vinyl alcohol) physical hydrogels: new vista on a long serving biomaterial, Macromol. Biosci., 2011, 11(10), 1293–1313 CrossRef CAS PubMed.
- M. Bustamante-Torres, D. Romero-Fierro, B. Arcentales-Vera, K. Palomino, H. Magaña and E. Bucio, Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials, Gels, 2021, 7(4), 182 CrossRef CAS PubMed.
- O. Wichterle and D. Lim, Hydrophilic gels for biological use, Nature, 1960, 185(4706), 117–118 CrossRef.
- M. H. Ayoubi-Joshaghani, K. Seidi, M. Azizi, M. Jaymand, T. Javaheri, R. Jahanban-Esfahlan and M. R. Hamblin, Potential applications of advanced nano/hydrogels in biomedicine: static, dynamic, multi-stage, and bioinspired, Adv. Funct. Mater., 2020, 30(45), 2004098 CrossRef CAS.
- R. V. Ulijn, N. Bibi, V. Jayawarna, P. D. Thornton, S. J. Todd, R. J. Mart, A. M. Smith and J. E. Gough, Bioresponsive hydrogels, Mater. Today, 2007, 10(4), 40–48 CrossRef CAS.
- P. Pallavi, K. Girigoswami, P. Gowtham, K. Harini, A. Thirumalai and A. Girigoswami, Encapsulating Rhodamine 6G in Oxidized Sodium Alginate Polymeric Hydrogel for Photodynamically Inactivating Cancer Cells, Curr. Pharm. Des., 2024, 30(35), 2801–2812 CrossRef CAS PubMed.
- Z. Sun, C. Song, C. Wang, Y. Hu and J. Wu, Hydrogel-based controlled drug delivery for cancer treatment: a review, Mol. Pharm., 2019, 17(2), 373–391 Search PubMed.
- M. Marzi, M. R. Chijan and E. Zarenezhad, Hydrogels as promising therapeutic strategy for the treatment of skin cancer, J. Mol. Struct., 2022, 1262, 133014 CrossRef CAS.
- Z.-Q. Zhang and S.-C. Song, Multiple hyperthermia-mediated release of TRAIL/SPION nanocomplex from thermosensitive polymeric hydrogels for combination cancer therapy, Biomaterials, 2017, 132, 16–27 CrossRef CAS PubMed.
- A. Sachdev, I. Matai and P. Gopinath, Carbon dots incorporated polymeric hydrogels as multifunctional platform for imaging and induction of apoptosis in lung cancer cells, Colloids Surf., B, 2016, 141, 242–252 CrossRef CAS PubMed.
- P. Sikdar, M. M. Uddin, T. M. Dip, S. Islam, M. S. Hoque, A. K. Dhar and S. Wu, Recent advances in the synthesis of smart hydrogels, Mater. Adv., 2021, 2(14), 4532–4573 RSC.
- N. H. Thang, T. B. Chien and D. X. Cuong, Polymer-based hydrogels applied in drug delivery: An overview, Gels, 2023, 9(7), 523 CrossRef CAS PubMed.
- M. Sepantafar, R. Maheronnaghsh, H. Mohammadi, F. Radmanesh, M. M. Hasani-Sadrabadi, M. Ebrahimi and H. Baharvand, Engineered hydrogels in cancer therapy and diagnosis, Trends Biotechnol., 2017, 35(11), 1074–1087 CrossRef CAS PubMed.
- A. Mandal, J. R. Clegg, A. C. Anselmo and S. Mitragotri, Hydrogels in the clinic, Bioeng. Transl. Med., 2020, 5(2), e10158 CrossRef CAS PubMed.
- L. Zhao, Y. Zhou, J. Zhang, H. Liang, X. Chen and H. Tan, Natural polymer-based hydrogels: From polymer to biomedical applications, Pharmaceutics, 2023, 15(10), 2514 CrossRef CAS PubMed.
- Z. Shi, X. Gao, M. W. Ullah, S. Li, Q. Wang and G. Yang, Electroconductive natural polymer-based hydrogels, Biomaterials, 2016, 111, 40–54 CrossRef CAS PubMed.
- A. H. Karoyo and L. D. Wilson, A review on the design and hydration properties of natural polymer-based hydrogels, Materials, 2021, 14(5), 1095 CrossRef CAS PubMed.
- B. Kaczmarek, K. Nadolna and A. Owczarek, The physical and chemical properties of hydrogels based on natural polymers, in Hydrogels based on natural polymers, 2020, pp. 151–172 Search PubMed.
- A. Sohrabi, Brain-Mimetic, Three-Dimensional Hyaluronic Acid-Based Hydrogels to Investigate Effects of the Tumor Microenvironment on Glioblastoma Progression, University of California, Los Angeles, 2021 Search PubMed.
- D. Gong, F. Yu, M. Zhou, W. Dong, D. Yan, S. Zhang, Y. Yan, H. Wang, Y. Tan and Y. Chen, Ex vivo and in vivo properties of an injectable hydrogel derived from acellular ear cartilage extracellular matrix, Front. Bioeng. Biotechnol., 2021, 9, 740635 CrossRef PubMed.
- M. Elsabahy and K. L. Wooley, Design of polymeric nanoparticles for biomedical delivery applications, Chem. Soc. Rev., 2012, 41(7), 2545–2561 RSC.
- N. A. Peppas, Biomedical applications of hydrogels handbook, Springer Science & Business Media, 2010 Search PubMed.
- Y. Kang, J. Guo and Q. Yuan, in An International Conference of Biomedical Information Perception & Microsystems will be hosted in one of the most glamorous cities in China, Chengdu, in June 13–15 of 2018. This conference aims to provide an international forum for biomedical scientists and engineers worldwide to share the latest advances in the booming fields of bioinformation.
- J. Liao and H. Huang, Review on magnetic natural polymer constructed hydrogels as vehicles for drug delivery, Biomacromolecules, 2020, 21(7), 2574–2594 CrossRef CAS PubMed.
- S. W. Liao, T.-B. Yu and Z. Guan, De novo design of saccharide− peptide hydrogels as synthetic scaffolds for tailored cell responses, J. Am. Chem. Soc., 2009, 131(48), 17638–17646 CrossRef CAS PubMed.
- J. Wu, Microbeads from Crystalline Nanocellulose: Regulating Physical Properties by Chemical Modification and Hybridization, McGill University, Canada, 2020 Search PubMed.
- U. S. Madduma-Bandarage and S. V. Madihally, Synthetic hydrogels: Synthesis, novel trends, and applications, J. Appl. Polym. Sci., 2021, 138(19), 50376 CrossRef CAS.
- A. C. Kumar and H. Erothu, Synthetic polymer hydrogels, in Biomedical applications of polymeric materials and composites, 2016, pp. 141–162 Search PubMed.
- I. Gibas and H. Janik, Synthetic polymer hydrogels for biomedical applications, 2010 Search PubMed.
- S. Bashir, M. Hina, J. Iqbal, A. Rajpar, M. Mujtaba, N. Alghamdi, S. Wageh, K. Ramesh and S. Ramesh, Fundamental concepts of hydrogels: Synthesis, properties, and their applications, Polymers, 2020, 12(11), 2702 CrossRef CAS PubMed.
- M. Patenaude and T. Hoare, Injectable, mixed natural-synthetic polymer hydrogels with modular properties, Biomacromolecules, 2012, 13(2), 369–378 CrossRef CAS PubMed.
- M. F. Akhtar, M. Hanif and N. M. Ranjha, Methods of synthesis of hydrogels. A review, Saudi Pharm. J., 2016, 24(5), 554–559 CrossRef PubMed.
- D. A. Gyles, L. D. Castro, J. O. C. Silva Jr. and R. M. Ribeiro-Costa, A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations, Eur. Polym. J., 2017, 88, 373–392 CrossRef CAS.
- K. Roa, E. Oyarce, A. Boulett, M. Alsamman, D. Oyarzún, G. D. C. Pizarro and J. Sánchez, Lignocellulose-based materials and their application in the removal of dyes from water: A review, Sustainable Mater. Technol., 2021, 29, e00320 CrossRef CAS.
- T. Miyata, T. Uragami and K. Nakamae, Biomolecule-sensitive hydrogels, Adv. Drug Delivery Rev., 2002, 54(1), 79–98 CrossRef CAS PubMed.
- M. Vigata, C. Meinert, D. W. Hutmacher and N. Bock, Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques, Pharmaceutics, 2020, 12(12), 1188 CrossRef CAS PubMed.
- W. A. Laftah, S. Hashim and A. N. Ibrahim, Polymer hydrogels: A review, Polym.-Plast. Technol. Eng., 2011, 50(14), 1475–1486 CrossRef CAS.
- C. Alberton, T. A. D. Colman, J. A. de Souza, C. S. de Oliveira, M. M. P. Andrade and E. Schnitzler, Thermal analysis, rheology, X-ray diffractometry and atomic force microscopy in the evaluation of binary mixtures of “starch-hydrocolloids”, J. Microbiol., Biotechnol. Food Sci., 2014, 3(4), 305–309 Search PubMed.
- M. Talikowska, X. Fu and G. Lisak, Application of conducting polymers to wound care and skin tissue engineering: A review, Biosens. Bioelectron., 2019, 135, 50–63 CrossRef CAS PubMed.
- K. Gul, R.-Y. Gan, C.-X. Sun, G. Jiao, D.-T. Wu, H.-B. Li, A. Kenaan, H. Corke and Y.-P. Fang, Recent advances in the structure, synthesis, and applications of natural polymeric hydrogels, Crit. Rev. Food Sci. Nutr., 2022, 62(14), 3817–3832 CrossRef CAS PubMed.
- C. Vasile, D. Pamfil, E. Stoleru and M. Baican, New developments in medical applications of hybrid hydrogels containing natural polymers, Molecules, 2020, 25(7), 1539 CrossRef CAS PubMed.
- D. P. Nair, M. Podgorski, S. Chatani, T. Gong, W. Xi, C. R. Fenoli and C. N. Bowman, The thiol-Michael addition click reaction: a powerful and widely used tool in materials chemistry, Chem. Mater., 2014, 26(1), 724–744 CrossRef CAS.
- J.-H. Hu, Y. Huang, C. Redshaw, Z. Tao and X. Xiao, Cucurbit [n] uril-based supramolecular hydrogels: Synthesis, properties and applications, Coord. Chem. Rev., 2023, 489, 215194 CrossRef CAS.
- A. Zhang, F. Wang, L. Chen, X. Wei, M. Xue, F. Yang and S. Jiang, 3D printing hydrogels for actuators: A review, Chin. Chem. Lett., 2021, 32(10), 2923–2932 CrossRef CAS.
- C. Liu, N. Xu, Q. Zong, J. Yu and P. Zhang, Hydrogel prepared by 3D printing technology and its applications in the medical field, Colloid Interface Sci. Commun., 2021, 44, 100498 CrossRef CAS.
- Q. Gu, E. Tomaskovic-Crook, R. Lozano, Y. Chen, R. M. Kapsa, Q. Zhou, G. G. Wallace and J. M. Crook, Functional 3D neural mini-tissues from printed gel-based bioink and human neural stem cells, Adv. Healthcare Mater., 2016, 5(12), 1429–1438 CrossRef CAS PubMed.
- A. C. Daly, S. E. Critchley, E. M. Rencsok and D. J. Kelly, A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage, Biofabrication, 2016, 8(4), 045002 CrossRef PubMed.
- D. F. Duarte Campos, A. Blaeser, A. Korsten, S. Neuss, J. Jäkel, M. Vogt and H. Fischer, The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages, Tissue Eng., Part A, 2015, 21(3–4), 740–756 CrossRef CAS PubMed.
- G. Gao, A. F. Schilling, T. Yonezawa, J. Wang, G. Dai and X. Cui, Bioactive nanoparticles stimulate bone tissue formation in bioprinted three-dimensional scaffold and human mesenchymal stem cells, Biotechnol. J., 2014, 9(10), 1304–1311 CrossRef CAS PubMed.
- A. Skardal, D. Mack, E. Kapetanovic, A. Atala, J. D. Jackson, J. Yoo and S. Soker, Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds, Stem Cells Transl. Med., 2012, 1(11), 792–802 CrossRef CAS PubMed.
- R. Gaetani, P. A. Doevendans, C. H. Metz, J. Alblas, E. Messina, A. Giacomello and J. P. Sluijter, Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells, Biomaterials, 2012, 33(6), 1782–1790 CrossRef CAS PubMed.
- S. H. Ahn, H. J. Lee, J.-S. Lee, H. Yoon, W. Chun and G. H. Kim, A novel cell-printing method and its application to hepatogenic differentiation of human adipose stem cell-embedded mesh structures, Sci. Rep., 2015, 5(1), 13427 CrossRef CAS PubMed.
- E. A. Kamoun, E.-R. S. Kenawy and X. Chen, A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings, J. Adv. Res., 2017, 8(3), 217–233 CrossRef CAS PubMed.
- Z. H. Abdulhusain, L. S. Jasim and M. Batool, Azur C Dye Removal using GO/P (CMC-Co-Am) Nanocomposite: Adsorption and Kinetic Studies, J. Nanostruct., 2024, 14(4), 1225–1238 CAS.
- Q. K. M. Alshamusi, K. A. A. Hameed, A. M. Taher, M. Batool and L. S. Jasim, Efficiency of Chitosan-Grafted Poly (Carboxymethyl Cellulose-Co-Acrylamide) Nano Hydrogel for Cadmium(II) Removal: Batch Adsorption Study, J. Nanostruct., 2024, 14(4), 1122–1133 Search PubMed.
- A. A. M. Alzayd, A. N. Zghair, A. M. Essa, A. S. Jawad, M. J. Abed, M. Batool and L. S. Jasim, Isotherm and Thermodynamic Analysis of Azur C Dye Adsorption on GO/P (CMC-Co-Am) Nanocomposite, J. Nanostruct., 2024, 14(3), 845–856 Search PubMed.
- H. O. Jamel, M. H. Jasim, M. A. Mahdi, S. H. Ganduh, M. Batool, L. S. Jasim and M. N. Haider, Adsorption of Rhodamine B dye from solution using 3-((1-(4-((1H-benzo [d] imidazol-2-yl) amino) phenyl) ethylidene) amino) phenol (BIAPEHB)/P (AA-co-AM) composite, Desalin. Water Treat., 2025, 101019 CrossRef CAS.
- H. J. Majeed, T. J. Idrees, M. A. Mahdi, M. J. Abed, M. Batool, S. R. Yousefi, A. Thumma and L. S. Jasim, Synthesis and application of novel sodium carboxy methyl cellulose-g-poly acrylic acid carbon dots hydrogel nanocomposite (NaCMC-g-PAAc/CDs) for adsorptive removal of malachite green dye, Desalin. Water Treat., 2024, 320, 100822 CrossRef CAS.
- H. A. Mohammad, B. W. Mahde, L. S. Jasim and M. Batool, Adsorption of Diclofenac Sodium (DS) Drug from Water on CMC-g-P(AAc-AAm) Nano-Hydrogel: Isotherm and Thermodynamic Study, J. Nanostruct., 2024, 14(1), 232–244 CAS.
- F. Ullah, M. B. H. Othman, F. Javed, Z. Ahmad and H. M. Akil, Classification, processing and application of hydrogels: A review, Mater. Sci. Eng., C, 2015, 57, 414–433 CrossRef CAS PubMed.
- D. Buenger, F. Topuz and J. Groll, Hydrogels in sensing applications, Prog. Polym. Sci., 2012, 37(12), 1678–1719 CrossRef CAS.
- A. Sharma, G. R. Kokil, Y. He, B. Lowe, A. Salam, T. A. Altalhi, Q. Ye and T. Kumeria, Inorganic/organic combination: inorganic particles/polymer composites for tissue engineering applications, Bioact. Mater., 2023, 24, 535–550 CAS.
- S. Liu, F. Yao, M. Kang, S. Zhao, Q. Huang and G. Fu, Hierarchical xanthan gum/graphene oxide nanocomposite film induced by ferric ions coordination, Mater. Des., 2017, 113, 232–239 CrossRef CAS.
- H. Mokhtari, M. Kharaziha, F. Karimzadeh and S. Tavakoli, An injectable mechanically robust hydrogel of Kappa-carrageenan-dopamine functionalized graphene oxide for promoting cell growth, Carbohydr. Polym., 2019, 214, 234–249 CrossRef CAS PubMed.
- A. Dev, S. J. Mohanbhai, A. C. Kushwaha, A. Sood, M. N. Sardoiwala, S. R. Choudhury and S. Karmakar, κ-carrageenan-C-phycocyanin based smart injectable hydrogels for accelerated wound recovery and real-time monitoring, Acta Biomater., 2020, 109, 121–131 CrossRef CAS PubMed.
- S. Braccini, C. Tacchini, F. Chiellini and D. Puppi, Polymeric hydrogels for in vitro 3D ovarian cancer modeling, Int. J. Mol. Sci., 2022, 23(6), 3265 CrossRef CAS PubMed.
- M. Batool, M. N. Haider and T. Javed, Applications of spectroscopic techniques for characterization of polymer nanocomposite: A review, J. Inorg. Organomet. Polym. Mater., 2022, 32(12), 4478–4503 CrossRef CAS.
- M. Batool, M. A. Abid, T. Javed and M. N. Haider, Applications of biodegradable polymers and ceramics for bone regeneration: a mini-review, Int. J. Polym. Mater. Polym. Biomater., 2025, 74(1), 39–53 CrossRef CAS.
- K. Yue, G. Trujillo-de Santiago, M. M. Alvarez, A. Tamayol, N. Annabi and A. Khademhosseini, Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels, Biomaterials, 2015, 73, 254–271 CrossRef CAS PubMed.
- Y. Wang, S. Zhang and J. Wang, Photo-crosslinkable hydrogel and its biological applications, Chin. Chem. Lett., 2021, 32(5), 1603–1614 CrossRef CAS.
- R. Chapla and J. L. West, Hydrogel biomaterials to support and guide vascularization, Prog. Biomed. Eng., 2020, 3(1), 012002 CrossRef.
- P. V. Londhe, M. V. Londhe, A. B. Salunkhe, S. S. Laha, O. T. Mefford, N. D. Thorat and V. M. Khot, Magnetic hydrogel (MagGel): An evolutionary pedestal for anticancer therapy, Coord. Chem. Rev., 2025, 522, 216228 CrossRef CAS.
- M. M. H. Rumon, M. S. Rahman, A. A. Akib, M. S. Sohag, M. R. A. Rakib, M. A. R. Khan, F. Yesmin, M. S. Shakil and M. M. Rahman Khan, Progress in hydrogel toughening: Addressing structural and crosslinking challenges for biomedical applications, Discover Mater., 2025, 5(1), 1–29 CrossRef.
- J. Wu, W. Xue, Z. Yun, Q. Liu and X. Sun, Biomedical applications of stimuli-responsive “smart” interpenetrating polymer network hydrogels, Mater. Today Bio, 2024, 25, 100998 CrossRef CAS PubMed.
- M. Cavo, M. Caria, I. Pulsoni, F. Beltrame, M. Fato and S. Scaglione, A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”, Sci. Rep., 2018, 8(1), 5333 CrossRef PubMed.
- F. Cadamuro, L. Marongiu, M. Marino, N. Tamini, L. Nespoli, N. Zucchini, A. Terzi, D. Altamura, Z. Gao and C. Giannini, 3D bioprinted colorectal cancer models based on hyaluronic acid and signalling glycans, Carbohydr. Polym., 2023, 302, 120395 CrossRef CAS PubMed.
- S.-J. Huang, T.-H. Wang, Y.-H. Chou, H.-M. D. Wang, T.-C. Hsu, J.-L. Yow, B.-S. Tzang and W.-H. Chiang, Hybrid PEGylated chitosan/PLGA nanoparticles designed as pH-responsive vehicles to promote intracellular drug delivery and cancer chemotherapy, Int. J. Biol. Macromol., 2022, 210, 565–578 CrossRef CAS PubMed.
- R. A. Weinberg, How cancer arises, Sci. Am., 1996, 275(3), 62–70 CrossRef CAS PubMed.
- P. Nenclares and K. Harrington, The biology of cancer, Medicine, 2020, 48(2), 67–72 CrossRef.
- D. M. Gress, S. B. Edge, F. L. Greene, M. K. Washington, E. A. Asare, J. D. Brierley, D. R. Byrd, C. C. Compton, J. M. Jessup and D. P. Winchester, Principles of cancer staging, in AJCC cancer staging manual, 2017, vol. 8, pp. 3–30 Search PubMed.
- R. Tibshirani, T. Hastie, B. Narasimhan and G. Chu, Diagnosis of multiple cancer types by shrunken centroids of gene expression, Proc. Natl. Acad. Sci. U. S. A., 2002, 99(10), 6567–6572 CrossRef CAS PubMed.
- M. J. Williams, B. Werner, C. P. Barnes, T. A. Graham and A. Sottoriva, Identification of neutral tumor evolution across cancer types, Nat. Genet., 2016, 48(3), 238–244 CrossRef CAS PubMed.
- K. Sak, Cytotoxicity of dietary flavonoids on different human cancer types, Pharmacogn. Rev., 2014, 8(16), 122 CrossRef CAS PubMed.
- D. T. Debela, S. G. Muzazu, K. D. Heraro, M. T. Ndalama, B. W. Mesele, D. C. Haile, S. K. Kitui and T. Manyazewal, New approaches and procedures for cancer treatment: Current perspectives, SAGE Open Med., 2021, 9, 20503121211034366 CrossRef PubMed.
- A. S. Trigos, R. B. Pearson, A. T. Papenfuss and D. L. Goode, How the evolution of multicellularity set the stage for cancer, Br. J. Cancer, 2018, 118(2), 145–152 CrossRef CAS PubMed.
- G. P. Dunn, L. J. Old and R. D. Schreiber, The immunobiology of cancer immunosurveillance and immunoediting, Immunity, 2004, 21(2), 137–148 CrossRef CAS PubMed.
- J. Zugazagoitia, C. Guedes, S. Ponce, I. Ferrer, S. Molina-Pinelo and L. Paz-Ares, Current challenges in cancer treatment, Clin. Ther., 2016, 38(7), 1551–1566 CrossRef PubMed.
- Z. Abbas and S. Rehman, An overview of cancer treatment modalities, Neoplasm, 2018, 1, 139–157 Search PubMed.
- K. Shah, S. B. Mallik, P. Gupta and A. Iyer, Targeting tumour-associated fibroblasts in cancers, Front. Oncol., 2022, 12, 908156 CrossRef CAS PubMed.
- Z. Zahedi-Tabar, S. Bagheri-Khoulenjani, H. Mirzadeh and S. Amanpour, 3D in vitro cancerous tumor models: Using 3D printers, Med. Hypotheses, 2019, 124, 91–94 CrossRef PubMed.
- Y. Luo, X. Wei, Y. Wan, X. Lin, Z. Wang and P. Huang, 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair, Acta Biomater., 2019, 92, 37–47 CrossRef CAS PubMed.
- H.-H. G. Song, K. M. Park and S. Gerecht, Hydrogels to model 3D in vitro microenvironment of tumor vascularization, Adv. Drug Delivery Rev., 2014, 79, 19–29 CrossRef PubMed.
- M. T. Spang and K. L. Christman, Extracellular matrix hydrogel therapies: In vivo applications and development, Acta Biomater., 2018, 68, 1–14 CrossRef CAS PubMed.
- V. Brancato, J. M. Oliveira, V. M. Correlo, R. L. Reis and S. C. Kundu, Could 3D models of cancer enhance drug screening?, Biomaterials, 2020, 232, 119744 CrossRef CAS PubMed.
- M. G. Gonzalez, I. Cichon, A. Scislowska-Czarnecka and E. Kolaczkowska, Challenges in 3D culturing of neutrophils: assessment of cell viability, J. Immunol. Methods, 2018, 457, 73–77 CrossRef PubMed.
- V. Brancato, F. Gioiella, G. Imparato, D. Guarnieri, F. Urciuolo and P. A. Netti, 3D breast cancer microtissue reveals the role of tumor microenvironment on the transport and efficacy of free-doxorubicin in vitro, Acta Biomater., 2018, 75, 200–212 CrossRef CAS PubMed.
- D. Rodenhizer, D. Cojocari, B. G. Wouters and A. P. McGuigan, Development of TRACER: tissue roll for analysis of cellular environment and response, Biofabrication, 2016, 8(4), 045008 CrossRef PubMed.
- X. Xin, H. Yang, F. Zhang and S.-T. Yang, 3D cell coculture tumor model: A promising approach for future cancer drug discovery, Process Biochem., 2019, 78, 148–160 CrossRef CAS.
- J. Esmaeili, A. Barati, J. Ai, V. T. Nooshabadi and Z. Mirzaei, Employing hydrogels in tissue engineering approaches to boost conventional cancer-based research and therapies, RSC Adv., 2021, 11(18), 10646–10669 RSC.
- T. Tan, Y. Wang, J. Wang, Z. Wang, H. Wang, H. Cao, J. Li, Y. Li, Z. Zhang and S. Wang, Targeting peptide-decorated biomimetic lipoproteins improve deep penetration and cancer cells accessibility in solid tumor, Acta Pharm. Sin. B, 2020, 10(3), 529–545 CrossRef CAS PubMed.
- Q. Liu, Z. Zhang, Y. Liu, Z. Cui, T. Zhang, Z. Li and W. Ma, Cancer cells growing on perfused 3D collagen model produced higher reactive oxygen species level and were more resistant to cisplatin compared to the 2D model, J. Appl. Biomater. Funct. Mater., 2018, 16(3), 144–150 CAS.
- A. Khurana and C. Godugu, Alginate-based three-dimensional in vitro tumor models: A better alternative to current two-dimensional cell culture models, in Alginates and their biomedical applications, 2018, pp. 157–183 Search PubMed.
- D. Mennerich, K. Kubaichuk and T. Kietzmann, DUBs, hypoxia, and cancer, Trends Cancer, 2019, 5(10), 632–653 CrossRef CAS PubMed.
- D. M. Lewis, S. Gerecht and T. K. Eisinger-Mathason, Hydrogels to study ECM-oxygen gradient interactions for sarcoma cell migration, Cancer Res., 2017, 77(13_Supplement), 5477 CrossRef.
- C. Liu, D. L. Mejia, B. Chiang, K. E. Luker and G. D. Luker, Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion, Acta Biomater., 2018, 75, 213–225 CrossRef CAS PubMed.
- R. D. Hume, L. Berry, S. Reichelt, M. D'Angelo, J. Gomm, R. E. Cameron and C. J. Watson, An engineered human adipose/collagen model for in vitro breast cancer cell migration studies, Tissue Eng., Part A, 2018, 24(17–18), 1309–1319 CrossRef CAS PubMed.
- E. A. White, H. A. Kenny and E. Lengyel, Three-dimensional modeling of ovarian cancer, Adv. Drug Delivery Rev., 2014, 79, 184–192 CrossRef PubMed.
- C.-C. Lin and M. Korc, Designer hydrogels: Shedding light on the physical chemistry of the pancreatic cancer microenvironment, Cancer Lett., 2018, 436, 22–27 CrossRef CAS PubMed.
- H. Cao, M. K. H. Lee, H. Yang, S. K. Sze, N. S. Tan and C. Y. Tay, Mechanoregulation of cancer-associated fibroblast phenotype in three-dimensional interpenetrating hydrogel networks, Langmuir, 2018, 35(23), 7487–7495 CrossRef PubMed.
- A. Suo, W. Xu, Y. Wang, T. Sun, L. Ji and J. Qian, Dual-degradable and injectable hyaluronic acid hydrogel mimicking extracellular matrix for 3D culture of breast cancer MCF-7 cells, Carbohydr. Polym., 2019, 211, 336–348 CrossRef CAS PubMed.
- A. De Luca, L. Raimondi, F. Salamanna, V. Carina, V. Costa, D. Bellavia, R. Alessandro, M. Fini and G. Giavaresi, Relevance of 3d culture systems to study osteosarcoma environment, J. Exp. Clin. Cancer Res., 2018, 37, 1–15 CrossRef PubMed.
- H. T. Ta, C. R. Dass, I. Larson, P. F. Choong and D. E. Dunstan, A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy, Biomaterials, 2009, 30(27), 4815–4823 CrossRef CAS PubMed.
- G.-Y. Liou and P. Storz, Reactive oxygen species in cancer, Free Radical Res., 2010, 44(5), 479–496 CrossRef CAS PubMed.
- H. Nakamura and K. Takada, Reactive oxygen species in cancer: Current findings and future directions, Cancer Sci., 2021, 112(10), 3945–3952 CrossRef CAS PubMed.
- C. R. Reczek and N. S. Chandel, The two faces of reactive oxygen species in cancer, Annu. Rev. Cancer Biol., 2017, 1(1), 79–98 CrossRef PubMed.
- Y. Huang, B. S. Canup, S. Gou, N. Chen, F. Dai, B. Xiao and C. Li, Oral nanotherapeutics with enhanced mucus penetration and ROS-responsive drug release capacities for delivery of curcumin to colitis tissues, J. Mater. Chem. B, 2021, 9(6), 1604–1615 RSC.
- D. S. Wilson, G. Dalmasso, L. Wang, S. V. Sitaraman, D. Merlin and N. Murthy, Orally delivered thioketal nanoparticles loaded with TNF-α–siRNA target inflammation and inhibit gene expression in the intestines, Nat. Mater., 2010, 9(11), 923–928 CrossRef CAS PubMed.
- M. Y. Lee, D. Lee, D. Choi, K. S. Kim and P. M. Kang, Targeting Reactive Oxygen Species for Diagnosis of Various Diseases, J. Funct. Biomater., 2024, 15(12), 378 CrossRef CAS PubMed.
- N. Ahmad, G. Packirisamy and R. Dutta, 3D printing technology in nanomedicine, Elsevier, 2019 Search PubMed.
- A. Herreros-Pomares, X. Zhou, S. Calabuig-Fariñas, S.-J. Lee, S. Torres, T. Esworthy, S. Y. Hann, E. Jantus-Lewintre, C. Camps and L. G. Zhang, 3D printing novel in vitro cancer cell culture model systems for lung cancer stem cell study, Mater. Sci. Eng., C, 2021, 122, 111914 CrossRef CAS PubMed.
- A. Sharma, D. Sasaki, D. W. Rickey, A. Leylek, C. Harris, K. Johnson, J. E. A. Aviles, B. McCurdy, A. Egtberts and R. Koul, Low-cost optical scanner and 3-dimensional printing technology to create lead shielding for radiation therapy of facial skin cancer: First clinical case series, Adv. Radiat. Oncol., 2018, 3(3), 288–296 CrossRef PubMed.
- H. Mou, J. Wang, H. Hu, W. Xu and Q. Chen, Non-small cell lung cancer 95D cells co-cultured with 3D-bioprinted scaffold to construct a lung cancer model in vitro, Zhonghua Zhongliu Zazhi, 2015, 37(10), 736–740 Search PubMed.
- T. K. Merceron and S. V. Murphy, Hydrogels for 3D bioprinting applications. Essentials of 3D biofabrication and translation, Elsevier, 2015, pp. 249–270 Search PubMed.
- A. Gomot, Toxic effects of cadmium on reproduction, development, and hatching in the freshwater snaillymnaea stagnalisfor water quality monitoring, Ecotoxicol. Environ. Saf., 1998, 41(3), 288–297 CrossRef CAS PubMed.
- K. J. Curtis, J. Schiavi, M. J. Mc Garrigle, V. Kumar, L. M. McNamara and G. L. Niebur, Mechanical stimuli and matrix properties modulate cancer spheroid growth in three-dimensional gelatin culture, J. R. Soc., Interface, 2020, 17(173), 20200568 CrossRef CAS PubMed.
- R. Curvello, V. Kast, M. H. Abuwarwar, A. L. Fletcher, G. Garnier and D. Loessner, 3D collagen-nanocellulose matrices model the tumour microenvironment of pancreatic cancer, Front. Digit. Health, 2021, 3, 704584 CrossRef PubMed.
- J. Ahn, J. Lim, N. Jusoh, J. Lee, T.-E. Park, Y. Kim, J. Kim and N. L. Jeon, 3D microfluidic bone tumor microenvironment comprised of hydroxyapatite/fibrin composite, Front. Bioeng. Biotechnol., 2019, 7, 168 CrossRef PubMed.
- F. Del Bufalo, T. Manzo, V. Hoyos, S. Yagyu, I. Caruana, J. Jacot, O. Benavides, D. Rosen and M. K. Brenner, 3D modeling of human cancer: A PEG-fibrin hydrogel system to study the role of tumor microenvironment and recapitulate the in vivo effect of oncolytic adenovirus, Biomaterials, 2016, 84, 76–85 CrossRef CAS PubMed.
- C. Ricci, C. Mota, S. Moscato, D. D'Alessandro, S. Ugel, S. Sartoris, V. Bronte, U. Boggi, D. Campani and N. Funel, Interfacing polymeric scaffolds with primary pancreatic ductal adenocarcinoma cells to develop 3D cancer models, Biomatter, 2014, 4(1), e955386 CrossRef PubMed.
- W. H. Abuwatfa, W. G. Pitt and G. A. Husseini, Scaffold-based 3D cell culture models in cancer research, J. Biomed. Sci., 2024, 31(1), 7 CrossRef PubMed.
- M. J. Kim, B. H. Chi, J. J. Yoo, Y. M. Ju, Y. M. Whang and I. H. Chang, Structure establishment of three-dimensional (3D) cell culture printing model for bladder cancer, PLoS One, 2019, 14(10), e0223689 CrossRef CAS PubMed.
- P. Ahangar, E. Akoury, A. S. Ramirez Garcia Luna, A. Nour, M. H. Weber and D. H. Rosenzweig, Nanoporous 3D-printed scaffolds for local doxorubicin delivery in bone metastases secondary to prostate cancer, Materials, 2018, 11(9), 1485 CrossRef PubMed.
- J. W. Park, H. G. Kang, K. M. Lim, D. W. Park, J. H. Kim and H. S. Kim, Bone tumor resection guide using three-dimensional printing for limb salvage surgery, J. Surg. Oncol., 2018, 118(6), 898–905 CrossRef PubMed.
- M. P. Jogalekar and E. E. Serrano, Morphometricanalysis of a triple negative breast cancer cell line in hydrogel and monolayer culture environments, PeerJ, 2018, 6, e4340 CrossRef PubMed.
- J. Zhong, Y. Zhang, J. Chen, R. Huang, Y. Yang, H. Chen, Y. Huang, W. Tan and Z. Tan, In vitro study of colon cancer cell migration using E-Jet 3D printed cell culture platforms, Macromol. Biosci., 2018, 18(11), 1800205 CrossRef PubMed.
- A. Dupret-Bories, S. Vergez, T. Meresse, F. Brouillet and G. Bertrand, Contribution of 3D printing to mandibular reconstruction after cancer, Eur. Ann. Otorhinolaryngol., Head Neck Dis., 2018, 135(2), 133–136 CrossRef CAS PubMed.
- H. Zhang, G. Liu, X. Tong and W. Hang, Application of three-dimensional printing technology in the surgical treatment of nasal skull base tumor, Zhonghua Erbi Yanhouke Zazhi, 2018, 53(10), 780–784 CAS.
- S. V. Murphy and A. Atala, 3D bioprinting of tissues and organs, Nat. Biotechnol., 2014, 32(8), 773–785 CrossRef CAS PubMed.
- A. Schwab, R. Levato, M. D'Este, S. Piluso, D. Eglin and J. Malda, Printability and shape fidelity of bioinks in 3D bioprinting, Chem. Rev., 2020, 120(19), 11028–11055 CrossRef CAS PubMed.
- X. Chen, A. F. Anvari-Yazdi, X. Duan, A. Zimmerling, R. Gharraei, N. Sharma, S. Sweilem and L. Ning, Biomaterials/bioinks and extrusion bioprinting, Bioact. Mater., 2023, 28, 511–536 CAS.
- H. Budharaju, D. Sundaramurthi and S. Sethuraman, Embedded 3D bioprinting–An emerging strategy to fabricate biomimetic & large vascularized tissue constructs, Bioact. Mater., 2024, 32, 356–384 CAS.
- I. Matai, G. Kaur, A. Seyedsalehi, A. McClinton and C. T. Laurencin, Progress in 3D bioprinting technology for tissue/organ regenerative engineering, Biomaterials, 2020, 226, 119536 CrossRef CAS PubMed.
- C. M. Witten, R. D. McFarland and S. L. Simek, Concise review: the US Food and Drug Administration and regenerative medicine, Stem Cells Transl. Med., 2015, 4(12), 1495–1499 CrossRef PubMed.
- H. Hameed, S. Faheem, A. C. Paiva-Santos, H. S. Sarwar and M. Jamshaid, A comprehensive review of hydrogel-based drug delivery systems: classification, properties, recent trends, and applications, AAPS PharmSciTech, 2024, 25(4), 64 CrossRef PubMed.
- A. Ferrarotti, J. A. Lugtu and A. Malhotra, Hydrogel-Based Delivery of Biologics in Cancer and Cardiovascular Diseases: Proof-of-Concept, in Biologics and Biosimilars, CRC Press, 2022, pp. 165–180 Search PubMed.
- L. Gutierrez, N. S. Cauchon, T. R. Christian, M. J. Giffin and M. J. Abernathy, The confluence of innovation in therapeutics and regulation: Recent CMC considerations, J. Pharm. Sci., 2020, 109(12), 3524–3534 CrossRef CAS PubMed.
- M. Di Prima, J. Coburn, D. Hwang, J. Kelly, A. Khairuzzaman and L. Ricles, Additively manufactured medical products–the FDA perspective, 3D Print. Med., 2016, 2, 1–6 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

