Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Artificial intelligence in smart drug delivery systems: a step toward personalized medicine
Mitali
Panchpuri
a,
Ritu
Painuli
b and
Chetan
Kumar
 *a
*a
aSchool of Pharmaceutical and Population Health Informatics, Faculty of Pharmacy, DIT University, Dehradun, Uttarakhand 248009, India. E-mail: kumarbelwal@gmail.com; chetan.kumar@dituniversity.edu.in
bDepartment of Chemistry, School of Applied and Life Sciences, Uttaranchal University, Dehradun, Uttarakhand 248007, India
First published on 22nd May 2025
Abstract
One of the most interesting applications of artificial intelligence is in the design of drug delivery systems. Smart drug delivery systems can transfer drugs to specific tissues and cells, enhancing therapeutic effects while reducing undesirable side effects. Attention is focused on the main concepts and techniques of AI such as machine learning, deep learning, and genetic algorithms. In addition to this, genetic algorithms can be used for the selection of the best numerical models, which are able to predict biological processes or optimize the activity of new drugs. Besides the powerful impact of AI on drug design, its combination with new biotechnologies for personalized medicine, sometimes called theragnostics, brings novel diagnostic tools together with targeted therapy, which could ensure quality and effectiveness during clinical research on new drugs. Artificial intelligence (AI) techniques are finding their application in almost all disciplines, with particular success in healthcare. AI-based algorithms can solve complex problems related to diagnosis, prediction, control, and prevention of diseases that are beyond the scope of human abilities. At the same time, the Internet of Things (IoT) revolution has added value to the healthcare sector. The resulting combination of IoT and AI platforms presents a promising fusion to provide healthcare delivery innovations like digital drug delivery, online healthcare consultancy platforms, and virtual healthcare assistants. Personalized medicine is well-suited, regardless of potential disadvantages, to creating drug delivery systems that can respond to the exact needs and other special requirements of patients. The development of smart drug delivery systems is a potential response to the unimodal properties of drugs and the discordance between patient requirements and patient outcomes achieved by currently prescribed medications. The potential and actual positive economic and health-related impacts of advanced drug delivery technologies have created strong demand for new advanced delivery forms.
1. Introduction
Over the years, traditional drug delivery systems have been developed based on the specific need to deliver therapeutics in an effective and safe manner. Such systems are already available as marketed products and these can be generally placed in one of the following groups: (a) oral or transdermal delivery systems, (b) injectable systems, (c) inhalation or topical creams or ointments, (d) partially or totally bio-adhesive systems, (e) nanoscale drug delivery systems, and (f) controlled release systems.1 However, despite the clinical successes of the marketed products, traditional drug delivery methods possess several limitations that are particularly noteworthy for proteins and nucleic acids. Proteins have complex 3D structures that enable them to perform their specific functions, and these proteins must be administered as active agents to the patients because these molecules cannot be synthesized by human cells after administration. A variety of factors can compromise the activity of therapeutic proteins, such as proteolysis, aggregation, or denaturation.1.1. Emergence of personalized medicine and its significance
The principle underlying personalized medicine is the capability to create therapies that are more precise and effective by identifying genetically distinct patients who can achieve improved efficacy.2 Genome-scale measurements of biological processes in patients can recognize differences in the structure of complex diseases and predict whether a disease will benefit from a particular treatment.3 As a result, genomic information can be utilized to better comprehend susceptibilities and strengths. This allows for early identification of those factors that provide higher probabilities of effective treatment.4 Furthermore, these factors can be employed to help patients determine the best courses of action. The effect can be greater efficacy and decreased adverse reactions in patient care. However, personalized medicine not only encompasses the medical field but also multiple other fields, including diagnostics, pharmaceuticals, and the delivery of medicine. With the development of advanced technology, the prevention and even prediction of adverse drug-related health issues are possible.5 In contrast to one-size-fits-all therapeutic designs, personalized medicine can offer new medicines that are adaptable to the needs of distinct patient groups. The delivery of new drug products can range from changes in formulation to complementary diagnostic tools that could be part of the therapy administered by various physicians. With significant implications for medical practices and the healthcare system, this technology provides the potential for early implementation.6AI is a transformative tool, and it can help modernize several aspects of the healthcare sector, from drug discovery to different aspects of clinical work.7 The role of AI in personalized medicine is vital, since the advent of genomics and other omics has created a monstrous amount of data, which are way beyond the scope of processing by traditional statistical methods.8 The ability of AI to identify patterns in vast amounts of data makes it the most suitable for personalized medicine, which requires analyzing patients’ genetic and clinical data to diagnose, treat, and even predict the risk of certain diseases.9 In general, AI can assist in the development and efficient operation of personalized medicine by integrating different data types, which include clinical data, medical imaging data, omics data, etc., and by providing patient stratification, diagnostics, and highly targeted treatment to bring about successful patient outcomes.10 Data integration helps to provide insights for targeted therapies. AI models trained on large, diverse datasets are useful in providing treatment for all patients with different disease risks, as AI-driven tools can take into consideration all possible traits of a disease and the genetic makeup of an individual.11
Also, AI-driven machine learning models can be trained on omics data to improve predictions of drug response and prognosis and will be superior in terms of reducing the number of patients required for clinical trials and for cost reductions.12 Requirements for data privacy are few in medical diagnostics and they can be shared for the development of public tools to diagnose rare diseases and conditions. Many believe that AI plays a decisive role in a multitude of fields. In medicine, AI is applied for solving complex problems where expert decision-making is combined with diagnosis in areas such as radiology and pathology, where findings are based on images, sounds, or texts.13 Analysis of radiological images of several different body parts highly benefits from deep learning models, which learn features and diagnose diseases automatically. The design and development of decision support systems to assist in radiology is a major force behind AI research.14 The advantages of using AI in the healthcare sector are widely accepted, and opportunities and challenges for researchers are identified. AI methods have shown enormous capacity to improve healthcare areas, ranging from planning treatments for chronic diseases to psychiatric disorders, modeling and predicting diseases, and fighting against rare diseases.15 Its potential to revolutionize medicine and greatly improve human health should be widely recognized, and researchers should carefully examine which AI techniques merit further exploitation and serious consideration for widespread clinical use.
2. Overview of AI technologies
2.1. Tools for AI technologies
Deep learning (DL) can also be categorized as a subtype of ML and can be applied to a wide variety of domains.18 DL is, in fact, an algorithm that enables ML to make decisions, executing a series of functions using parameters learned from large amounts of labeled data and employing simple modules like the ones inspired by the function and structure of the human brain.19 Different deep learning models may be more useful when treated with specific kinds of data or tasks.20 Deep feedforward neural networks have a simple three-layer architecture (input, hidden, and output), characterized by the absence of cycles and a virtually unlimited number of units, which may be used to model intricate relationships.21 A recurrent neural network (RNN) is another popular DL model that can capture patterns and trends in sequential data, which makes it a useful resource, especially for time series predictions.22 The transformer, which behaves similarly to an RNN model but has no limiting structures that confine information propagation in time or space, has been applied in document sound and language modeling, as well as in serving models for question-and-answer platforms.23
Despite the success of deep learning in other fields, its application in biomedicine often encounters methodological and theoretical challenges due to the high cost of labeled data, low cost of high-throughput data, and corresponding highly variable quality of molecular bio-profiling results, intrinsic sample variability in human subjects, and ethical constraints of animal studies.27 For example, the signal of complex annotations from different pathophysiological processes sampled at different spatial locations and temporal stages in medical imaging data incurs high false positive and false negative risks due to semantic mismatch. Multiple variables from different animal cohorts or subjects impose a burden on experimental design.28 Biological event-derived conditions often suffer from intrinsic distribution shift problems due to the confounding effects of both the among-subject and within-subject cycles of multiple observations. These challenges lead deep learning method developers to focus not only on new, well-generated interpretable models from various perspectives but also on robust, adaptively and transparently robust models with controllable parameters for custom adaptation and model calibration through novel theoretical perspectives.29
Knowledge discovery in clinical notes is associated with the creation and use of tools and methodologies for examining clinical notes to find new information about patients, diseases, or treatments.33 When it comes to customizing care plans that are right for individual patients, obtaining scientific knowledge is key. It is vitally important for businesses to build powerful, efficient NLP approaches to realize the promise of big data in delivering knowledge from unstructured EHR data.34 With the advancement of EHRs, we have the chance to finally obtain actionable knowledge from large-scale clinical notes. The increasing number and consistency of patient-encounter records combined with EHR popularity have allowed many studies to be conducted, establishing principles and techniques, and many helpful applications using clinical notes as research topics. Sharing data availability and such resources could help transform future patient care.35,36
In the pharmaceutical industry, package and prescribing errors can be prevented through machine learning that deploys NNs for clinical decision-making. For successful diagnosis and efficient prognosis of different diseases, brain–computer interfaces, analysis of blood, endoscopies, heart and lung tones, skin, etc.,40 NNs are capable of learning about individual patient medication. In order to ensure the delivery of suitable and necessary treatment for patients in need of both acute and long-term care to maintain their survival, e-prescribing was carefully introduced and improved. In addition, neural learning will effectively categorize health data that address frequent disease types and provide efficient and essential healthcare solutions during outbreaks like health crises, which potentially occur at a record rate.41
2.2. The role of ML, NLP, and deep learning in data denoising
The realm of data denoising has witnessed a transformative evolution through the advent of various technologies, each contributing unique methodologies and insights. The term “denoising” itself evokes a process reminiscent of clarifying a muddled message, akin to distilling the essence of noise. In the landscape of machine learning, a myriad of algorithms have emerged that are designed to sift through data clutter with remarkable precision. Machine learning (ML), a cornerstone of contemporary data science, has redefined the parameters of data analysis. By leveraging intricate patterns within datasets, ML techniques enable the identification and removal of anomalies that obscure clarity. Natural language processing (NLP), another critical component, extends this paradigm to textual data, employing linguistic models to refine and enhance the quality of communication.42 Here, the focus lies on eliminating syntactical noise and semantic ambiguities, paving the way for more coherent interpretations. Moreover, neural networks are at the forefront of this endeavor, functioning as intricate webs of interconnected nodes that emulate human cognitive processes. These networks are adept at learning from vast quantities of data, making them invaluable for denoising tasks that require a deep contextual understanding.43 Deep learning, a subset of this technology, further amplifies these capabilities, allowing for the extraction of features at multiple levels of abstraction. This layered approach facilitates the discernment of subtle signals amidst the cacophony of irrelevant information. In summary, the convergence of machine learning, natural language processing, neural networks, and deep learning has forged a robust framework for the denoising of data. Each technology contributes distinctive strengths, collectively enhancing our capacity to achieve clarity and precision in an increasingly complex data landscape (Table 1).44| Software | Interpretation | Characteristics | Ref. |
|---|---|---|---|
| DeepMind AlphaFold (Google, Mountain View, CA, USA) https://deepmind.google/technologies/alphafold/, accessed on 10 October 2024 | Protein structure prediction by deep learning model | Forecasts protein structures with high accuracy | 45 |
| Atomwise (Atomwise Inc., San Francisco, CA, USA) https://www.atomwise.com/, accessed on 10 October 2024 | AI-driven drug discovery platform | Virtual screening, lead optimization | 45 |
| Recursion Pharmaceuticals (Recursion, Salt Lake City, UT, USA) https://www.recursion.com/, accessed on 10 October 2024 | High-throughput screening platform | Cellular phenotypic analysis, rare diseases | 46 |
| BenevolentAI (Benevolent AI, London, UK) https://www.benevolent.com/, accessed on 10 October 2024 | Drug discovery and development platform | Predictive modelling, target identification | 47 |
| Schrödinger Maestro (Schrödinger, New York, NY, USA) https://www.schrodinger.com/, accessed on 10 October 2024 | Molecular modelling and simulations | Molecular docking, QSAR modelling | 48 |
| Insilico Medicine (Insilico Medicine, Hong Kong) https://insilico.com/, accessed on 10 October 2024 | Drug discovery and biomarker development | Generative modelling, drug repurposing, and aging research | 49 |
| XtalPi (QuantumPharm Inc., Boston, MA, USA) https://www.xtalpi.com, accessed on 10 October 2024 | AI-driven drug crystal prediction | Predicts drug crystal forms, stability | 50 |
| Cyclica (Cyclica, Toronto, ON, Canada) https://cyclicarx.com/science/, accessed on 10 October 2024 | AI-driven drug discovery platform | Polypharmacology prediction, target deconvolution | 51 |
3. Applications of AI in drug development
3.1. Drug discovery and design
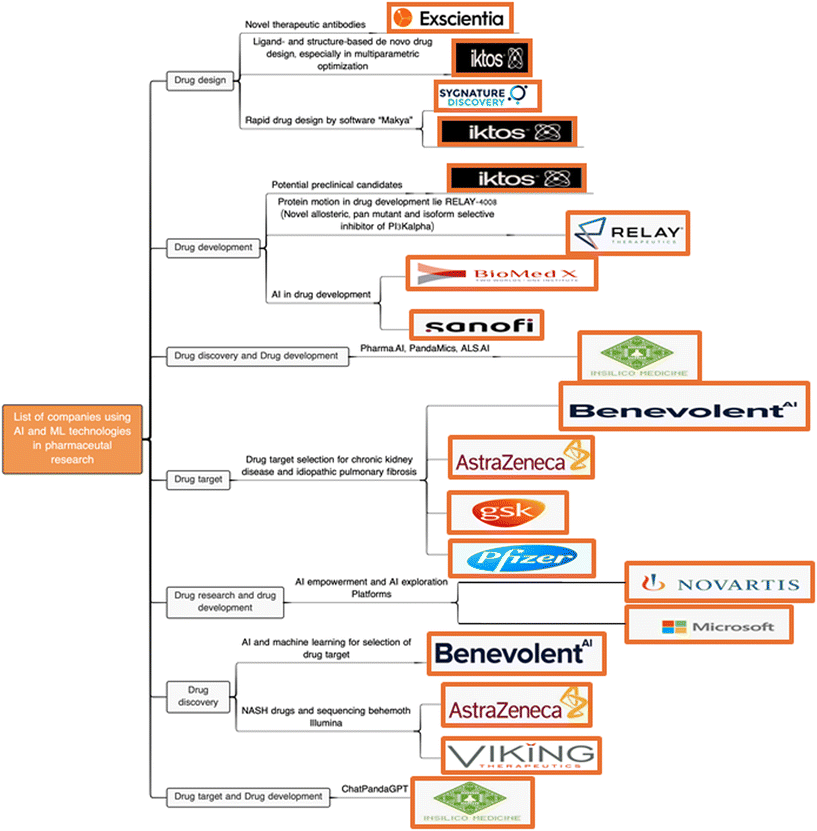
There are several highly technical review articles that discuss the use of artificial intelligence (AI) in drug design, though nearly all of them are specifically targeted at algorithms or areas.52 Here we present a brief overview of the main areas of applying AI to drug discovery and design. Central to AI in drug discovery is the concept of ‘in silico drug discovery’, where the vast amounts of genomic, chemical, and pharmacological data available are used to computationally describe biological systems and chemical processes with the goal of designing and discovering new compounds of therapeutic value.53 As a result, this technology has the potential to fundamentally change the way in which drugs for the treatment of many diseases are discovered and developed (Fig. 1).53The first applications of AI in drug discovery are mostly in computer-aided drug design, such as the often-discussed docking of molecules using machine learning or molecular description and prediction using deep learning.54 This includes the creation of libraries of chemical properties and structural information about drugs, the analysis of structural properties of drug target proteins such as proteomics research, the study of interactions between drug molecules and their corresponding endogenous protein targets such as in the determination of QSAR, enzyme–substrate interactions, and the prediction of binding constants.55 These applications have a significant impact on understanding the complexity of the human genome and in proposing new biological mechanisms that could not previously be envisaged for drug intervention.56In silico drug discovery has also had an expanded impact on finding new indications for drugs already on the market, to propose, for example, the repurposing of some drugs for the treatment of cancer or in the elucidation of the off-target effects of some drugs.57,58
3.2. Predictive modeling for efficacy and toxicity
Predictive modeling approaches, machine learning algorithms, and QSAR are widely employed for generating predictive models to integrate large amounts of data from diverse sources and types.59 However, predicting and optimizing the efficacy of personalized drug combinations is still very challenging. Investigations directed at optimizing drug combinations predominantly focus on chemical pleiotropy and signaling pathway crosstalk.59 However, the development of facile predictive algorithms, sophisticated systems biology models, and big data analytical approaches enables insights into a more complete set of molecular consequences of drug exposure, which could improve drug combination selection for efficacy, influence the direction of drug development, and identify potentially overlooked toxicities.60 QSAR, pharmacokinetic models, and PBPK models have been developed to predict the joint effects of therapeutic interventions.61There is enormous potential for advancing precision medicine by leveraging the growing power of technology for drug combination selection through precision medicine research. This may, however, require an improved view of the nodes that mediate drug–drug interactions and expanded human data banks.62 Logic circuits, signaling and regulatory networks, and derived decision trees can uncover the complexities of drug-induced changes and lead to the elucidation of combinations of reagent interventions. The design of novel combinations can be driven by a joint desire to minimize the probability of success while limiting adverse effects and enhancing therapeutic outcomes.63 Machine learning can guide the development of the multidrug microbiome system or suggest potential novel regimens by the discordance of optimizing cancer drug combinations in cells versus xenograft mice, or by identifying drug–target–pathway connections in certain cell types. Existing experimental and bioinformatics approaches can provide the gold standard training sets for the scrutiny of unexplored cells and tissues. The ideal learning paradigm may not exist, and multifaceted, multiconstraint workflows may be necessary for different situations.
3.3. Optimizing clinical trials
The use of artificial intelligence algorithms to select the appropriate patient population and optimal dosing is expected to raise the rate of clinical trial success. 90% of novel anticancer compounds entering phase I clinical trials never reach the market.64 Among these drugs, many are efficacious but just for a small fraction of patients, while most of the non-lethal side effects are not acceptable. Companies are working together to optimize trial recruitment, and several startups are involved in the AI-based selection of patients for their inclusion in clinical trials on patient-centric protocol design65 (Table 2). Optimization of the patient cohort may also lead to improved outcomes of the clinical trial. A challenge demonstrated how the AI algorithm led to more accurate re-assessment of breast cancer risk. Optimized clinical trials with enriched cohorts may result in shorter trials, saving time and money, and may reduce the dropout rate due to adverse events, thus speeding up clinical development and marketing.66 Moreover, with proven efficacy, the new therapeutic formulation or packaging option can be approved as a bioequivalent of its listed counterpart. Since the optimized patient population and very positive results can boost the price and thus profitability, investments will be easier to find while marketing expenses may be lower.67,68| Trial ID (NCT/DOI) | Condition/disease | AI application | Purpose of AI |
|---|---|---|---|
| NCT06059378 | Optical polyp detection | Using AI-assisted optical polyp diagnosis for diminutive colorectal polyps (AI-OD) | To show the accuracy of intracolonoscopy |
| NCT05178095 | Colonic polyp detection | Artificial intelligence in colonic polyp detection | Detection of colonic polyps during outpatient colonoscopy |
| NCT04358198 | Gastric intestinal metaplasia diagnosis | Usefulness of artificial intelligence (AI) for GIM | Diagnosing gastric intestinal metaplasia |
| NCT05489471 | Lung cancer | Impact of an artificial intelligence (AI) system on chest X-ray reporting | Nodule detection and malignancy prediction |
| NCT06093217 | Acute pulmonary embolism (AID-PE) (AID-PE) | Artificial intelligence to improve detection and risk stratification of AID-PE/AID-PE | Detection of acute pulmonary embolism (PE) in patients who undergo computed tomography pulmonary angiogram |
| NCT04918992 | Pelvic cancers | Post radiotherapy MRI based AI system to predict radiation proctitis for pelvic cancers | Predict radiation proctitis for patients with pelvic cancers who underwent radiotherapy |
| NCT06456203 | Respiratory tract infections, infections, lung diseases, respiratory tract diseases, pneumonia | Trial of artificial intelligence for chest radiography (ACER) | An economic analysis of the impact of AI decision support on radiology service delivery |
| NCT06934239 | Breast cancer | Impact of artificial intelligence on breast cancer screening (PRISM) | To compare patient-centered outcomes when 3D screening mammograms are interpreted with or without a leading FDA-cleared AI decision-support tool in real-world U.S. settings |
| NCT05018663 | Pancreatic solid lesions | Artificial intelligence (AI) cytopathology trial | To compare the accuracy of preliminary diagnosis results between ROSE and AI at the bedside versus final pathology report |
| NCT05241483 | Laboratory critical values, predictive value of tests, reference values, relative value scales, vital signs | Remote patient monitoring and detection of possible diseases with artificial intelligence telemedicine system (AI – diseases) | Possible disease detection with artificial intelligence from the patient's vital values; possible disease detection from the patient's examination records |
| NCT05423964 | Adenoma, adenoma colon, colorectal cancer | Impact of AI on trainee ADR | To determine the impact of AI based endoscopy on the rate of recording of quality improvement metrics versus historical performance in our program |
| NCT06527378 | Edentulous alveolar ridge, edentulous mouth, tooth loss | Artificial intelligence in dental implant planning (AIDENT) | Offering new opportunities to improve the precision and efficiency of implantology |
| NCT06877988 | Visual impairment | Artificial intelligence (AI) – assisted visual impairment screening model: community-based implementation and evaluation of performance, feasibility and costs | To evaluate the performance, operational efficiency, acceptability, feasibility, and cost-effectiveness of an AI-assisted screening model for visual impairment in a community setting |
| NCT06301945 | Thymic carcinoma, thymic epithelial tumor, thymoma, thymoma and thymic carcinoma | Artificial intelligence prediction tool in thymic epithelial tumors (INTHYM) | To improve the accuracy of histopathological classification of thymic epithelial tumors, and to better predict the risk of recurrence |
| NCT05438576 | Cardiomyopathy, pregnancy related | Screening for pregnancy related heart failure in Nigeria | To evaluate the effectiveness of an artificial intelligence-enabled ECG (AI-ECG) for cardiomyopathy detection in an obstetric population in Nigeria |
| NCT04580095 | Heart diseases | Artificial intelligence for improved echocardiography | To assess the effect of artificial intelligence algorithms on image quality in echocardiography |
| NCT06763952 | Diabetes, vision | Leveraging artificial intelligence to prevent vision loss from diabetes among socioeconomically disadvantaged communities | To investigate whether a novel artificial intelligence-based screening strategy improves screening and follow-up care rates across race/ethnicity groups and reduces racial/ethnic disparities in screening |
| NCT05339750 | Allergic contact dermatitis | Allergy skin patch artificial intelligence (AI) | To assess human and artificial intelligence performance in grading contact dermatitis reactions in healthy volunteers |
| NCT06790134 | Pancreatic diseases | Validation of an AI-assisted pancreatic EUS system for training improvement: a prospective, multi-center, randomized trial | To verify the auxiliary role of the artificial intelligence (AI) system in pancreatic endoscopic ultrasound (EUS) scans |
| NCT06584305 | Body dysmorphic disorder | AI screening for BDD in aesthetic surgery: enhancing safety and outcomes (AI) | To evaluate the effectiveness of an AI-powered screening tool for body dysmorphic disorder (BDD) among patients seeking aesthetic surgery |
| NCT05557162 | Cardiac amyloidosis | Artificial intelligence enhanced ECG to detect cardiac amyloidosis | To assess a novel artificial intelligence (AI)-enabled electrocardiogram (ECG)-based screening tool for improving the diagnosis of cardiac amyloidosis (CA) |
| NCT06397820 | Coronary artery disease, coronary artery stenosis | Relationship between AI-QCA and cardiac PET (AI-CARPET) | To evaluate the clinical implications of artificial intelligence (AI)-assisted quantitative coronary angiography (QCA) and positron emission tomography (PET)-derived myocardial blood flow in clinically indicated patients |
| NCT06412900 | Kidney stone, ureteral obstruction, renal colic, ureteral stone | Radiomics and image segmentation of urinary stones by artificial intelligence (RISUS_AI) | To personalize and improve treatment and follow-up of patients with kidney stones using radiomics and the development of an artificial intelligence tool for CT examination assessment |
4. Smart drug delivery systems
4.1. Controlled release systems
Since pharmacokinetic parameters for drug release should be highly controlled and allowed to be determined for a certain target, release of a particular drug in the part of the body where it is needed eliminates the inconvenience of numerous drug administrations, enhances simply structured therapy, and guarantees patient compliance.70 This led to the creation of controlled release systems. Artificial intelligence methods and control theory are gaining increasing recognition, and their implications in this direction have grown vastly. These insightful insights suggest that the interdisciplinary approach has a more profound effect on resolving the highly practical issues that are inherent in this field. Some challenging points of drug pharmacokinetics, dynamics, and modeling that are enhanced or limited by incorporating specific processes or applications are presented.71 Controlled release systems or multiple dosing regimens are self-associative, crystalline, polycrystalline, amorphous, and microporous drug carriers, drug–polymer conjugates, or osmotic and electronic pumps delivering drugs that possess a particular pharmacokinetic and pharmacodynamic profile. These profiles could be different from those produced by established prolonged-action drugs and have a similar range of therapeutic effects.72 The pharmacokinetic and pharmacodynamic times for such drug exposure should be determined in a certain target. Then, to be of interest, controlled release dosage forms might reasonably affect certain changes in the pharmacokinetic and pharmacodynamic processes.73 A specific controlled release involves stopping drug release, drug reprocessing, and adaptation of the most important actions. Such research, development, and production of controlled release systems have led to great interest in this subject.4.2. Targeted delivery mechanisms
The precise identification of suitable targets using an appropriate molecular recognition system, and the release of active therapeutic agents in the right dose at the right place, is a crucial feature of any practical smart drug delivery system.74 Nanoparticles designed for use in vivo can also incorporate targeting moieties that recognize and interact specifically with certain cell types or structures. The function of the tissue/cell-specific ligand on the nanoconstruct is to confer cell-specific properties to the nanoconstruct, allowing it to selectively target and accumulate in its target location. Ligands also reduce the take up of nanoconstructs by tissues not constitutively expressing the target antigen. Such ligands reduce the level of nanoconstruct accumulation in less-targeted tissues while increasing the circulation of these particles in the body.75 This interaction can improve the delivery of the drug and allow its controlled release with minimum side effects. Overall, the use of ligand-targeted nanoconstructs in vivo results in improved drug delivery and drug efficacy at the target site.76,77A guideline block of text in a column of the scientific literature shows that in vitro and in vivo studies report that targeted delivery systems improve delivery and take up. However, researchers have variously referred to different materials, structures, and configurations, as well as ligand attachment methodologies. A comprehensive and systematic survey is required, using advanced information collection techniques and scientific knowledge discovery methods.78 Such a study will provide researchers with a broad perspective on which particles or systems are often mentioned, why, and to what extent nanomaterial ligand attachment influences the property and function in these works. This study will enable researchers to grasp the current research status and to identify further research needs.79 Such a study in relation to smart drug delivery systems is a key reason for conducting the proposed work. Users can find out which systems are often machine-readable, which molecules are attached and get an overview of the techniques currently in use.80
4.3. Bio-responsive systems
The design of stimuli-responsive or bio-responsive systems could be seen as an intelligent approach for dealing with the drug delivery challenge. A general strategy aims to locally apply energy to control release kinetics, elimination, or spatial resolution.81 The use of, for example, light, sound waves, magnetic fields, or variations in temperature have been reported. Phototherapy methods currently play a significant role in the treatment of cancer.82 Hence, a local energy impulse could trigger the response to the dose of an applied therapeutic or supporting agent. Besides photodynamic therapy, the use of types of particles could be strongly promising towards photothermal and/or sonodynamic therapies.83 Moreover, upon such a locally applied set of external conditions, some smart liposomes and polymeric carriers could undergo subsequent transformations, enhance their encapsulating potential, or release the loaded agent.84While designing these smart nanocarriers,85 the approach of “planning for a long time of operation, considering many possible target molecules for action as much as possible”, as seen from the point of view of the number of functions per system established, seems to be just a “pure science” overstatement for an engineer, less relevant for the realistic range of opportunities awaiting medical use.86 In this sense, the use of these smart nanocarriers as hosts for therapeutic agent functions requiring some substitution of defective proteins and cell functions using different types of oligomers and polymers could represent a promising groundbreaking use concerning personalized medicine applications.87
5. AI-driven innovations in drug delivery
5.1. Predictive analytics for formulation design
Machine learning has seen a surge in popularity of research on study formulation design in recent years as it can enable rapid and high-throughput material discovery due to the improved prediction accuracy of AI models.88 Furthermore, this approach allows for the customization of drug delivery systems (e.g., tailoring release rates, increased stability, which can prolong drug shelf life). In one example, formulation design software has been implemented to innovate drug-loaded nanostructured lipid carriers with the desired spray drying characteristics, drug encapsulation, and drug release profiles for application in dry powder inhalation.89 Using this software to optimize NLCs for dry powder inhalation enabled greatly enhanced aerosol deposition and an increased dissolution rate. This transformative approach will enable a personalized, adjustable drug release system tailored to each patient's unique macromolecular composition for the treatment of various drug indications as we unravel new drug distribution mechanisms and develop reliable predictive capabilities.90,91In another example, a unified adaptive design optimization of an mRNA-based vaccine formulation was described that would cover the whole vectorial/combinatorial composition space of an mRNA formulation in as few lab experiments as possible.92 The model search technique was then applied to find the most efficacious personalized mRNA vaccine formulation. Follow-up wet lab characterization experiments validated the model predictions. In this work, the personalized process would rule out all specific antigens, enabling the evaluation of a large pool of candidates for all respondents by delivering a personalized mRNA vaccine to all participants.93 Although these studies have demonstrated the potential of predictive analytics for drug formulation design and material discovery, it is important to stress that there are still major challenges to overcome: (1) obtaining high-quality data and models, (2) how to transfer models across settings and into the clinic, and (3) the cost of goods sold that are necessary to implement AI-guided strategies in a living cell or establish recommendations and quality standards for regenerative medicine.94
5.2. Optimization of dosage and release profiles
To ensure that the administered dose of a drug is the most efficacious, it is often necessary to tightly control the release kinetics of the drug cargo. Parenteral routes of administration for most drugs deliver a constant, low-dose background level, with a bolus of additional drug after administration.95 This may not be a biologically relevant mimic of the peak-and-trough release profile for orally administered drugs, leading to inefficient drug utilization and a risk of adverse effects. Therefore, for many drugs, it would be beneficial to develop formulations with release kinetics that better mimic those of non-parenteral routes of administration.96 Optimization of complex drug-release profiles has already been demonstrated using proof-of-concept setups and algorithms, showing the potential for reduced time-to-market, money, time, and waste in the development of proposed formulations with desired release profiles.97Tailoring the release profile of a given therapeutic compound over time to deliver the drug most effectively and efficiently is of high relevance and interest, offering a fascinating combination of goal-driven research, challenges.98 Exploration of AI-based systems can be expected to lead to innovative and, most probably, unconventional solutions. In this review a brief overview of how AI is currently used to actively optimize the dosage and release profiles of existing drug delivery systems, as well as to develop new drug delivery systems that can be used to optimize the release profiles of known therapeutic compounds for any given effect specification, is provided.99 It must be considered that the optimization of active pharmaceutical ingredients within current mainstream dosage forms, followed by an exploration of how AI can be theoretically extended to the design and optimization of non-parenteral, nonoral drug delivery systems that offer the possibility of unique release profiles, rivaling or augmenting those which result from initial drug discovery, thereby offer the possibility of eliciting novel drug effects.100
5.3. Integration with nanotechnology and biosensors
The development of various artificial intelligence (AI) techniques has its roots entangled with specialized disciplines within nanotechnology such as nanomaterials, nanoelectronics, nanobiotechnology, and nanocomputing.101 On the other hand, AI integrated with nanotechnology is the formulation of AI-driven nanotechniques consisting of AI-based modeling, synthesis, characterization, testing, and quality control. AI can create thinking machines that could simulate biological neurons. Nano-biocomputing systems include memory, processors, and others that are dedicated to the consistent performance of computing within bioinformatics.AI, integrated with medical research and the administration of drugs, also plays a pivotal role in the field of pharmacy. For several years, research and studies have been evolving with the perfect match of AI and nanotechnology, which has ushered in the design and fabrication of nanoparticles, exploiting the intrinsic properties of the nanostructured material.102 Most of the work has been concentrated on the drug delivery of spare material. At the same time, some of the work is focused on the targeted distribution of biofunctionalized nanoparticles for cancer treatment and diagnostic imaging (Table 3).103
| AI model tools | Summary | Application area | Example/use case |
|---|---|---|---|
| DeepChem | Deep learning models for molecular property prediction, virtual screening, and generative chemistry are among the many tools and models for drug development offered by this open-source library | Predictive modeling, QSAR, multitask learning | Predicting bioavailability and solubility in nanoparticle drug formulations |
| RDKit | A popular open-source cheminformatics library with a number of features including handling molecules, searching substructures, and calculating descriptors. Drug discovery software can incorporate it with machine learning methods | Molecule manipulation, descriptor calculation | Generating molecular fingerprints for drug-likeness evaluation |
| ChemBERTa | A conceptual model developed especially for tasks involving drug development. It can produce molecular structures, predict characteristics, and aid with lead optimization because it is pre-trained on a sizable corpus of chemical and biomedical literature and is based on the transformer architecture | NLP-based molecular property prediction | Predicting ADMET properties from SMILES without handcrafted features |
| GraphConv (graph convolutional models) | A molecular graph-based deep learning model architecture. By using the structural information contained in the graph representation of molecules, it proved to be successful at forecasting molecular characteristics like toxicity and bioactivity | Structure-based prediction of drug activity | Predicting IC50 of drugs on cancer cell lines using molecular graphs |
| AutoDock Vina | A well-known docking program that predicts the binding affinity between small compounds and protein targets using machine learning approaches. It can help with lead optimization and virtual screening for drug discovery | Molecular docking and virtual screening | Identifying drug candidates for COVID-19 main protease |
| SMILES transformer | A deep learning model that creates molecular structures from simplified molecular input line entry system (SMILES) strings. Lead optimization and de novo drug design are two applications for it | Molecular representation learning (NLP) | Pretraining on SMILES for generative drug design and property prediction |
| Schrödinger suite | A complete drug discovery software suite that includes a number of AI-powered capabilities. Predictive modeling, ligand-based and structure-based drug design, virtual screening, and molecular modeling are among its modules | Molecular dynamics, docking, binding affinity | Simulation of protein–ligand complexes for kinase inhibitors |
| IBM RXN for chemistry | An artificial intelligence model for chemical reaction prediction. It helps with drug synthesis and the development of new synthetic pathways by generating possible reaction outcomes using deep learning algorithms and sizable reaction databases | Reaction prediction, synthesis planning | Designing retrosynthesis pathways for custom prodrugs |
| Scape-DB | A database called scape-DB (extraction of chemical and physical properties from the literature – DrugBank) uses machine learning and natural language processing to extract biological and chemical information from scholarly publications. It offers useful data for studies on medication discovery | Scaffolding and bioisosteric replacement | Identifying alternative scaffolds for known therapeutic compounds |
| GENTRL (generative tensorial reinforcement learning) | A deep learning model that creates new molecules with desired characteristics by fusing generative chemistry and reinforcement learning. De novo drug design and optimization have made use of it | Generative molecule design with reinforcement | Designing novel opioid analgesics with desired potency and low abuse potential |
| Genetic algorithms | Genetic algorithms are optimization methods that draw inspiration from the concepts of genetics and natural selection. To obtain the required dosage form properties, they can be used to optimize formulation compositions, drug release patterns, and process parameters | Feature selection, formulation optimization | Optimizing nanoparticle composition for sustained release |
| Artificial neural networks (ANNs) | Drug release kinetics from various dose forms have been modeled and optimized using artificial neural networks (ANNs). They can help identify the best formulations and forecast how active pharmaceutical ingredients (APIs) will be released under different circumstances | QSAR, release profile prediction | Predicting release rate of drugs from hydrogels based on polymer properties |
| Support vector machines (SVMs) | To forecast and model interactions between formulation variables, including excipient composition, processing parameters, and drug release profiles, SVMs have been employed in dosage form optimization. They facilitate formulation design space optimization | Classification of active/inactive compounds | Predicting drug-likeness and toxicity of new compounds |
| Particle swarm optimization (PSO) | For the purpose of optimizing dose forms, PSO is a population-based optimization algorithm. It has been used to optimize dissolution profiles, particle size distribution, and other formulation factors | Parameter optimization, hybrid modeling | Optimizing ANN weights for drug release modeling |
| Artificial intelligence-based expert systems | Expert systems mimic human experts’ decision-making processes by using AI approaches such as fuzzy logic and rule-based systems. Taking into account various formulation and process variables, they can be used for dosage form optimization | Decision support for formulation & synthesis | Recommending excipient selection for personalized oral dosage forms |
| Monte Carlo simulation | By taking into account the uncertainties and variability in formulation and process factors, Monte Carlo simulation techniques have been utilized to optimize the performance of drug products. They support process design and strong formulation | Probabilistic modeling, pharmacokinetics | Modeling absorption variability in transdermal drug delivery |
| Computational fluid dynamics (CFD) | The optimization of fluid flow and mixing in dosage form production processes, including granulation, coating, and drying, is made possible by CFD models. They aid in the creation of consistent and effective procedures | Simulating drug transport in biological systems | Modeling blood flow-mediated drug delivery in microvessels |
| Response surface methodology (RSM) | Through the modeling and analysis of the interaction between various variables and their impact on formulation responses, RSM is a statistical technique that aids in the optimization of dosage form formulations. It facilitates comprehension and formulation parameter optimization | Experimental design, formulation optimization | Optimizing liposomal formulation for maximal entrapment efficiency |
| Artificial neural network–genetic algorithm (ANN-GA) hybrid models | To optimize dose forms, hybrid models that combine ANN and GA approaches have been utilized. To find the best solutions and forecast formulation properties, they can effectively search the formulation space | Release kinetics modeling, optimization | Modeling and optimizing in situ gel formulations for ocular drug delivery |
| Multivariate analysis techniques | Dosage form optimization has made use of multivariate analysis techniques including partial least squares (PLS) and principal component analysis (PCA). They help with dimensionality reduction, formulation performance optimization, and the identification of crucial formulation factors | Chemometrics, PCA, PLS for data reduction | Analyzing HPLC profiles of drugs for quality control |
6. AI applications in implantable drug delivery devices
6.1. Role of feedback mechanisms
A significant aspect associated with the systems encompassing artificial intelligence is feedback. In systems driven by data, the importance of feedback is significantly amplified. The inclusion of feedback in smart delivery systems would enable the dose and frequency of agent administration to be adjusted according to individual characteristics and dosing targets, thus improving therapeutic effects, reducing toxicities, and minimizing ADR risks.108 The use of feedback in systems necessitates a shift away from the self-healing systems described previously in favor of prescribed healing mechanisms. This reliance on prescribed healing mechanisms necessitates the use of responsive materials and devices.109 Materials responsive to various stimuli, ranging from environmental factors to those associated with the therapeutic target, hold promise for incorporating feedback into the drug release mechanism. Such a development would demand the convergence of materials chemistry, responsive polymers, responsive amphiphiles, and responsive composite materials, such as pH-responsive nanoparticles. Additionally, appropriate devices and assembly techniques capable of altering drug dose delivery rates or switching drug release on and off would need to be engineered with a high degree of precision.110 Research on responsive polymers and pharmaceutical excipients is classified as responsive materials relevant to drug release modulation. Drug delivery systems featuring good flexibility in the modulation of agent release patterns, such as drug-eluting stents, can incorporate both iontophoretic and transport machine feedback schemes.111 These advanced smart drug delivery systems can revolutionize current clinical practice by virtue of their capability to offer therapeutic doses of the drug in response to the real needs of the patient, without demanding that the patient is physically treated in a hospital.6.2. Adaptation to patient-specific requirements
Pharmacological treatment in drug delivery design is typically delivered in fixed doses to patients of variable physio-pathological characteristics. For instance, patients may exhibit distinct disease progressions, such as slowed vascular blood flow in the vicinity of cholesterol plaque deposits in the context of inflammatory macrophage recruitment for atherosclerosis, which can affect the preferred particle type, size, coating chemistry, and site of release.112 Another aspect is the complex interaction of particle properties with the human body, from the protein corona that forms upon injection to the targeting and transportation capabilities that are dictated by the complex biological forces that control particle–particle and particle–tissue interactions. Together, this implies that a personalized approach toward particle design will become ever more relevant as we strive to treat patients in the most non-toxic, cost-effective, and successful manner.113 AI can significantly aid this development by capturing and utilizing vast amounts of knowledge of existing drug delivery systems, either used in their target context or in various other applications.1147. The concept of personalization in medicine
7.1. Genetic and phenotypic considerations
Genetics essentially determines not only the physiological and behavioral traits of an individual but also their propensity to develop diseases. The knowledge of specific genetic information may be pivotal for therapeutic decisions at an individual level.12 Pharmacogenetics and genotyping have already shown promise in individualized drug treatment by identifying genetic links to variations in therapeutic response to drugs. The defining elements associated with drug metabolism and individual-to-individual differences in targets such as drug receptors offer the ability to tailor treatment regimens with the greatest likelihood of positive benefits and reduced likelihood of toxicity due to drugs. Inherited genetic information describes only a portion of the drug response, and additional factors like diet, the microbiome, acquired genetic information, disease status, concomitant medication, and pharmacoeconomic issues can have substantial effects on drug response.115Personalization, based on a variety of phenotypic and genotypic assessments, is an advance of present drug selection strategies. Single nucleotide polymorphisms (SNPs) alter the response of some drugs and thus should influence several drug treatments in clinical practice.116 The role of SNPs in terms of linking specific drugs to specific diseases has not yet been fully appreciated. Pharmacogenetics is defined as the research of all inherited factors that affect drug actions in families and populations. The association of genotypic differences with inter-individual fluctuation in drug efficacy and toxicity outcomes is also known.117 Through analyzing genetic variation, we plan for a personalized medicine approach and convey the right dose and the correct drug to the right patient. In terms of inherited factors as well as prior genetic illnesses and other positive characteristics important for medical decisions such as disease diagnosis, clinical evaluation, and gene function evaluation, pharmacogenetics has evolved significantly.118 The analysis of the genetic variants influencing the reaction to a medication may be realized through both genome-wide association studies and clinical pharmacogenetics implementation (Fig. 2).119
7.2. Importance in chronic and rare diseases
There is a consensus among healthcare professionals that any medical treatment approach is patient-specific, but it is heterogeneous in disease and health state. Thus, the pharmacokinetics and pharmacodynamics are altered from person to person or may differ due to simultaneous administration of medication for other diseases.120 With respect to the varying pharmaceutical characteristics and the design of specific treatments for such rare diseases, considering sporadic patient data creates a challenge for management and therapy compliance.121 Additionally, most chronic diseases are accompanied by comorbidity features, such as side effects that result from the interaction of multiple factors using combined therapeutic agents. Maximum medication response with minimal deterioration of the health state needs to be addressed to improve the patient's quality of life.122 From pilot studies using cancer treatment to gene-targeted clinical trials, researchers have started to focus on personalized therapeutic regimens according to their recent findings and relevant databases. The present status of genetic and non-genetic factors of the disease needs to be discussed from initial disease prediction towards the treatment scenario and its prognosis.123 Precision medicine research is presently restricted to patients who are in good health, extensive medical technology, excellent public healthcare, and efficient data management systems, i.e., smart health devices, big data technology, and data-based models mostly used in treatment personalization.124 Accelerated advancements in the search for pharmacological treatments for peptic ulcers, the natural eradication of H. pylori, and the use of antibiotics to treat chronic hepatitis and certain types of cancer are other major factors in this growth. More extensive pathologies, including cancer, chronic pancreatitis, inflammatory bowel disease, and hepatic cryoglobulinemia targeting the pancreas, stomach, intestines, liver, and other organs with the same success have recently emerged in gastric and liver drug delivery systems with fewer chemotherapeutic drugs.125 As a result, treatment personalization also necessitates the ability to develop drug delivery systems targeting these areas. With novel vaccine and drug delivery, nanoparticles, nanoemulsions, and their combination kits can address both rare and chronic pathologies, and the design of smart devices is urgently required.8. AI tools for personalization
8.1. Genomics data analysis
Recent technological advancements have launched the new field of “genomic medicine” and its focus is on the influence of genetic differences on the development and progression of human diseases.126 There is growing evidence to substantiate that genetic differences exist among patients in their response to drugs and their susceptibility to drug-induced toxicity. Pharmacogenomics, a branch of personalized medicine, identifies patient profiles that subject them to drug responses, thereby optimizing drug therapy, with competencies for clinical decision-making and improvements in drug safety and outcomes. In addition, treatment suggestions based on patients’ genetic characterization are necessary to solve the issues of adverse drug reactions and the lack of pharmaceutical efficacy.127 Valuable information from whole genomes can be stored by diverse high-throughput functional genomics platforms employed for the comprehension of the function of genes. These potentially curative strategies are only showing substantial clinical success with the development of genome-sequencing methods, resulting in a wealth of protein variants, new therapy targets, and some therapies for rare Mendelian diseases that do not have other effective treatment options.128 This requires the adoption of personalized care and the efficient delivery of safe, genome-edited cells to patients.8.2. Patient stratification using AI algorithms
Highly heterogeneous disease biology is a problem that may not be resolved by targeting individual biomarkers. A growing trend in the clinical management of cancer patients is reclassifying patients into groups of similar prognosis and treatment efficacy, and more optimal therapeutic use of medicine.129 Through clinically actionable population homogeneity, tumor stratification creates subsets of cancer patients based on the heterogeneity of cellular and molecular features. Although biomarkers such as estrogen or HER2 expression in breast cancer, the activity of tyrosine kinase inhibitors in non-small cell lung cancer, and mutation testing before anti-EGFR treatment in colorectal cancer have demonstrated both clinical relevance and cost-effectiveness, additional biomarkers could predict if a particular drug were likely to have superior efficacy or disease-modulating activity in patients with a predefined genetic, proteomic, or metabolomic signature.130,131When a patient initially arrives at the clinic, advanced predictive algorithms may be used to correct them and direct them right away to the cluster with the best predicted outcome.132 This can be achieved by attempting to learn from populations of pre-labeled patients by machines. Modeling patient behavior and the course of the disease can result in predictive models that can group patients in specific clusters, calling them strata, for which specific treatment decisions can be recommended. Although enabling this may still be a dream for healthcare regulators, AI has just finished entering the clinic.133 AI has also been used to identify patient groupings for colorectal cancer, endocrine therapy in breast cancer, and drug effectiveness in systemic sclerosis.
To refine the potential benefits of using AI models for treatment guidance, clinical care must gradually become more personalized. The new treatment approach that has gained much attention in recent years is personalized medicine, also known as precision medicine.134 In contrast to the one-size-fits-all treatment of decades past or patients classified by the stage of a disease, clinicians and researchers now integrate clinical, molecular, and patient readiness data to predict individual risk factors and to optimize treatment options.135 The patient's clinical pathway, diagnostic testing, qualification for participation in a clinical trial, treatment intervention, and support after treatment are tailored and more focused on the individual. In particular, the immune system of patients is the centerpiece of personalized medicine, and no two patients have the same immune profile at the same moment.136 Such personalized treatment plans can be designed uniquely by integrating a patient's own molecular makeup, aggregates of molecular data, and AI technology.137
8.3. Development of tailored therapeutics
The development of personalized therapeutic agents capable of targeting features or mutations in an individual is an appealing form of individualized medication that may help optimize healthcare.138 Inherent to this approach is the capability to produce tailored drugs on a patient-to-patient basis with the same level of production efficiency as that currently experienced in the mass production of drugs.139 Advanced manufacturing techniques, now including techniques for gene editing and printing at the nanoscale, are increasingly being used in the pharmaceutical sector, accelerating the development of tailored therapeutics.140 Even the development of drugs tailored to a particular target population that can take advantage of economies of scale associated with large patient groups is advantageous.The development of tailored therapeutics can also be enhanced using artificial intelligence. Of specific interest is the concept of using intelligent software algorithms to help find optimal molecular therapeutics and geometric arrangements that best achieve a desired biological effect. The use of intelligent algorithms for the development of potential drug candidates can help to optimize attributes required of a potential drug, aiming to minimize typical poor in vivo drug performance, and help select drug leads that are more likely to lead to genuine improvement in the targeted therapeutic outcome. Allocating more efforts to identifying lead drug candidates that are smaller and more diverse in structure and mechanism, addressing the current popularity for repurposing existing drugs, offers several advantages. Small molecules and groups of molecules with properties other than those associated with traditional drugs, and which have lower known safety concerns may attract heightened interest while advancing our understanding of the behavior of molecules to support future drug lead optimization.141
9. AI-enhanced precision therapies
9.1. Case study: AI in oncology
Artificial intelligence (AI) has contributed substantially to cancer resolution in recent years. Cancer is the leading cause of death in developed countries. However, advances in early detection and improvements in therapeutics contribute to the decrease in cancer mortality and increase in the number of cancer survivors. Evidence-based medicine, based on a patient-centric approach, is rapidly replacing experience-based medicine. AI could revolutionize medicine, as the key driver of the transformation of healthcare to precision and personalized medicine. In oncology, there are major barriers to AI implementation, such as biased data, the lack of standardized collection, insufficient clinical validation, or outdated regulatory frameworks. Big data is extremely useful in the digitization of healthcare. Traditional software approaches are not suitable for the challenges imposed by digital healthcare. Automated algorithms can help to process complex data and extract meaningful patterns, changing treatment evaluations and patient classifications. AI and ‘machine learning’ (ML) have achieved several important medical advances. In oncology, the question is ‘How will AI improve the outcomes for patients with cancer?’ Major advances in technology have produced large-scale, multidimensional data for cancer research. Cancers are now understood as multifactorial diseases requiring unique treatment and management. New cancer diagnoses are focused on complex methods such as the measurement of molecular features to match individuals to targeted treatment plans. Analysis and sharing of clinical data have become paramount as our knowledge of cancer heterogeneity grows.142 The application of AI algorithms has the potential to transform health and healthcare delivery. Common applications of AI in healthcare include identifying conditions, risk factors, and patterns, which can support clinical decision making and improve treatment outcomes. The complexity of oncological diseases presents an opportunity for AI to impact oncology-related problems. However, few AI tools have had a significant impact on oncology. The goal of this study is to present an AI-based solution tool for oncology problems validated at a medical institution in Spain.143AI is expected to alter the treatment type greatly from exploratory treatment to mechanism guided treatment with the enhancement of health record data completeness. Treatment suggestion engines look for similar patients' prior treatment outcomes in databases in relation to the exploratory therapy type, which helps identify a larger pool of potential therapies. Most recently proposed recommendation systems are not capable of or are unsuitable for consulting again and to highlight insights from memory. A desired recommendation system should be patient level aware based on the validation method developed and a sample system should demonstrably consider the treatment candidate set in the context of incorporated local patient histories and treatment recommendations. AI empowered prediction tools could open the door to different treatment types and recommend an appealing patient cohort who would tolerate better targeted medicines via data mining on genotypic, epigenetic, lifestyle, social networks and interactions, and environmental heterogeneities on drug metabolism, reactivity and biological activity.144
AI assistance is paramount in better targeted therapy through pharmacogenomic analysis and predictive pharmacology. The number of publicly available pharmacogenomic data resources has been updated and merged with the pharmacogenomic knowledge base of drug treatment and targets in a user-friendly way in the PGP “Pharmacogenomics Database and Platform”. An analysis of AI's potential in precision pharmacology-focusing on task execution, outcome prediction, and feature identification-highlights its transformative role in advanced medical treatments. However, current limitations in the accessibility and implementation of these applications are also discussed. Through a combination of ML and NLP approaches, notable drugs in the COVID-19 context and associated potential target proteins have been identified based on a merged database of globally available attributes for drug repurposing.144,145 While AI excels at drug repurposing based on initial data, it faces challenges in maintaining important parameter weights and providing interpretability in counterfactual scenarios.
9.2. Case study: AI in cardiovascular medicine
Precision medicine is an evolving healthcare trend that aims to deliver personalized treatment protocols to every patient, particularly in cardiovascular medicine. The traditional one-size-fits-all healthcare approach has focused on generalization: every individual with hypertension is prescribed isosorbide dinitrate and/or metoprolol, while all coronary artery disease patients with hypertension are treated the same. Cardiovascular medicine has numerous branches; for example, a patient with hypertension not accompanied by atherosclerotic cardiovascular disease, congestive heart failure, or post-myocardial infarction will be treated differently primarily just based on the symptoms related to that particular branch. On the other hand, a young patient with two-vessel coronary artery disease who develops an acute myocardial infarction without prior history will have an entirely different management approach than a middle-aged man with three-vessel coronary artery disease with prior history. Therefore, this approach requires appropriate understanding and processing of large amounts of real-world patient data sampled over time. Precision cardiovascular medicine aims to identify and analyze the right intervention for the right set of patients at the right time with quantifiable outcome assessment, which is time-stamped and persists in a raw data format.146 Analysis of the data performed by human physicians is limited in the volume of data, the number of features involved in the analysis, and processing speed; this is time-consuming and error-prone. There is scope for AI-based methods to assist human physicians in understanding and optimizing the assessment of large amounts of patient data. Issues related to the input variable, extracting features, processing models, and understanding predicted outcomes require the implementation of several different AI paradigms. An alarming implication for the healthcare provider is that machine-learning and deep-learning based algorithms employing hundreds of thousands or even millions of input parameters provide prediction scores that do not offer a real understanding of the processed data. The black-box nature of these models and complexity of the data yield biophysical and medical implausibility of the predicted outcomes, which raises the need for research on interpretable AI and the underlying biophysical process for the prediction scores.Recently, AI-based methods have been evolving in precision cardiovascular medicine, attempting to improve patient care by analyzing patient data over time with quantifiable outcome assessments. As a result, the strategy involving medical analysis of patient data with the involvement of AI means providing a patient-centric data-assisted approach for human physicians. A systematic literature survey has been performed, by searching the most popular databases for the terms “precision medicine”, “cardiovascular”, and “AI” from January 1, 2010, up to July 10, 2023. Findings relevant to cardiovascular medicine, precision medicine, and patient care were considered. The focus was on AI implementations, biophysical models of predictions, and benefits of improved patient care. The data sources reviewed suggest that there has been an increasing trend for research on AI implementation for precision medicine in the cardiovascular medicine domain in the last five years. The United States has reported the most research trends in precision medicine for cardiovascular medicine with a total of 16 papers, suggesting that these trends will continue to grow over time. These papers have been classified into three broad categories, namely, cardiovascular branches, the precision medicine branch, and AI algorithms.
In this study, the availability of better-trained and supervised AI systems due to growing data volume and quality is highlighted. State-of-the-art research efforts based on journal papers and patent analyses are also addressed in this regard. Therefore, potential data sources that enable something similar to be done for the development of a better risk assessment model, including healthcare organizations, online patient health data aggregation, and analysis, literature mining, text and image data, etc., are highlighted. Efforts made with academic collaboration to address some of these challenges such as the development of AI guidelines and evaluation metrics are identified.
The potential threat to health and safety faced by a poorly implemented AI algorithm is stressed. The need for the establishment of an organization, similar to the FDA, for AI systems, to ensure the validity, reliability, and ethical usage of the algorithm prior to any marketing and commercial use, is also emphasized. The ever-increasing reliance on AI in health and society demands wider recognition of the uniqueness of AI algorithms, addressing this challenge with prospective forethought rather than retrospective rectification. Machine learning (ML) and artificial intelligence (AI) techniques are being increasingly incorporated into computer-aided diagnosis systems. These AI-based systems significantly improve the accuracy and reliability of breast cancer diagnosis and risk assessment. Many investigators have used their personal health data to identify breast cancer risk factors. Logistic regression, linear discriminant analysis, naive Bayes, and feed-forward neural network algorithms are utilized to predict the risk of breast cancer in 5 years’ time.147
The proper realization of precision medicine requires a progressive environment that facilitates the informatization of observational and experimental studies, so that the immense difficulties in analyzing big data are taken care of by powerful tools and technologies. Towards this end, a self-contained biomedical health data cube consisting of healthcare plans, in-patient and out-patient records, clinical data, genomics, and metabolomics has been constructed and tools for data analysis have been developed. The self-contained cube allows for unbiased heterogeneity detection and discovery as predefined user criteria can be taken into account for data queries. Data predictive analytical tasks are completed using local modeling-based and knowledge-driven method families that are characterized by a mode of explainable intelligence and ease of usage. Combining predictions from a data-quality-based human ensemble leads to more reliable and accurate results, reducing the effort needed for effective decision-making.148
9.3. Case study: AI in rare diseases
AI technologies have made major strides in recent years, and expectations for future applications are huge. AI is expected to more efficiently detect early signs of rare diseases by analyzing different types of medical data and identifying patients whose symptoms resemble those of diagnosed rare diseases. AI is also expected to help test new candidates for drug development. This indicates that there is a massive need for systems capable of screening a huge number of compounds against many targets and predicting a huge space of pharmacological interactions.There are excellent case studies on AI applications for drug treatment of rare diseases, one of them being protein misfolding diseases. There are also studies on the general detection of diseases by looking at images and texts. Some AI models generate molecular graphs and images of drugs with predicted affinity for targets based on previous knowledge. Some AI models are trained on sequences and 3D targets to perform drug repurposing without any assumptions regarding the functioning mechanism. There are AI models for mapping known drugs to new targets. Some algorithms merge existing data sources with novel data sources to build composite resources, yielding machine learning models with improved accuracy. It is now understood that well-defined learning tasks play an important role in machine learning model performance. Still, widely used self-supervised methods have no learning tasks to guide model learning.
There are only limited data available for the meaningful training of a model tasked with the identification of rare diseases. Some progress has been made in developing statistical methods validating the adequacy of a training dataset for a specific machine learning task. Detailed insights into state-of-the-art drug development approaches are given. Different AI methodologies are put in the context of selected rare diseases from the reviewed categories; state-of-the-art AI methodologies adapted to rare disease targets. Significant new developments have taken place for a wide spectrum of rare disease treatment applications. The computational feasibility of AI treatments for ultra-rare diseases should also benefit a few more common rare diseases.
Although precision oncology shows vast promise for many tumor types in an era of targeted agents, it has yet to deliver broadly in clinical practice. Camarillo's case involved a 43-year-old woman with stage IIIC ovarian cancer who was treated at multiple leading academic medical centers for whom all treatment options failed. Deep phenotyping in a patient-derived but genetically defined syngeneic organoid model identified the sensitivity of rapidly progressive cancer to combination therapy with poly-ADP ribose polymerase and immune checkpoint blockade. In vivo, this combination yielded profound tumor regression, prolonged remission, and simultaneous immune-mediated rejection of disseminated metastases.149 A wider appreciation of treatment paradigms across combinations of targeted therapies in breast, endometrial, pancreatic, and other cancers invigorate the development of de novo combinatorial therapies for these tumors.
Disease-specific platform technologies providing individualized precision medicine are also being combined with machine learning to discover previously unrecognized opportunities for drug repurposing. Camarillo's individual case study coupled targeted next-generation sequencing and droplet digital PCR of exometabolomics to inform lead compound selection for a novel, newly discovered WT1-p53 protein–protein targeting strategy for malignant pleural mesothelioma that was exploited for de novo combination therapy with paclitaxel. A new ex vivo drug combination platform to guide treatment in patients with relapsed/refractory DLBCL is also under development.150
| AI application | Overview | Case example | Ref |
|---|---|---|---|
| Synthesis route prediction | AI predicts optimal synthesis routes to APIs, examining chemical databases and the literature to suggest efficient pathways | IBM's “Rxn for Chemistry” tool predicts chemical reaction pathways, used to streamline synthesis | 151 |
| Robotic synthesis | Chemical synthesis is automated using AI-driven robotics, facilitating high-throughput testing and expediting the drug discovery process | The University of Glasgow's “Chemputer” automates the production of medicinal molecules | 152 and 153 |
| Drug design | AI identifies druggable targets by forecasting the molecular characteristics and structures of possible drug candidates | In just 18 months, Insilico Medicine used AI to create a new medication for idiopathic pulmonary fibrosis | 154 and 155 |
| Drug discovery | CRSIP technology and AI algorithms make it possible to determine which genes, when removed, result in cancer medication resistance or sensitization | To find new targets for developing better drugs, AstraZeneca applied AI to CRISPR gene-editing technology | 156 |
| Compound selection | To find potential drug candidates based on characteristics like solubility, permeability, and toxicity, AI evaluates chemical databases | Exscientia discovered a novel compound for the treatment of immunomodulatory and inflammatory disorders using artificial intelligence | 157 |
| Process optimization | By examining production line data to find inefficiencies and suggest fixes, artificial intelligence (AI) optimizes industrial operations | To increase yield and decrease production time for their COVID-19 vaccine, Pfizer utilized artificial intelligence | 158 and 159 |
| Continuous manufacturing and PAT technology | From acquiring raw materials to packaging the finished product, AI-driven optimization improves several aspects of pharmaceutical production | AI was used by pharmaceutical companies to increase efficiency in continuous manufacturing | 160 |
| Medical imaging | By streamlining workflows, improving detection, and automating time-consuming operations, AI systems have been developed to assist radiologists | AI algorithms are being used by Bayer to minimize burden and provide patients with quicker decision-making | 161 |
| Digital twin technology | To mimic, track, and optimize processes in real-time without interfering with actual production, artificial intelligence (AI) builds a digital twin, or virtual version, of the manufacturing process | Johnson & Johnson increased productivity by simulating and optimizing their production processes using digital twins | 162 |
| Predictive maintenance | Artificial intelligence (AI) models evaluate sensor data from equipment to forecast when maintenance is required, preventing unplanned malfunctions and efficiently scheduling maintenance tasks | Pfizer decreased maintenance expenses and downtime in its manufacturing facilities by implementing AI for predictive maintenance | 46 |
| Supply chain optimization | By forecasting demand, controlling inventory, and streamlining logistics using performance data and market trends, artificial intelligence (AI) improves the pharmaceutical supply chain | Novartis used artificial intelligence (AI) to handle supply chain logistics, which improved inventory control and cut expenses | 163 |
10. Synergistic role of AI in smart drug delivery and personalized medicine
10.1. Combining drug delivery systems with real-time data from AI models
One of the major limitations of current drug delivery systems is the inability to receive feedback on their effectiveness over time. This could be due to varied responses in drug activity, disease changes, and individual responses.166 However, with advances in diagnostics and imaging techniques, it is possible to monitor the drug delivery process and receive feedback on the drug's effectiveness in real time. Incorporating response data with AI models could have several positive implications, including but not limited to adjusting the drug dose, altering drug therapy, and modifying the delivery strategy for personalized drug delivery.167 All these possibilities call for a more patient-centric and precision medicine-based approach. At the same time, AI models usually require very large amounts of data to achieve successful results, whereas data acquisition in this field, especially through the examination of clinical and preclinical entities for personalized medicine, is a challenging activity.168 Combining drug formulations with AI algorithms is a promising strategy toward combating these issues. Indeed, tailored drug delivery systems that can both respond to external signals and collect relevant data with built-in sensors are considered a proactive way to enable personalized therapy strategies. By analyzing information collected with machine learning algorithms, the response of the drug delivery system can be predicted for various scenarios. Such designed systems will significantly expedite and optimize healthcare and enable personalized drug therapy for chronic diseases, especially in cases with different patient response rates or different disease phases.16910.2. Patient monitoring and adaptive treatment plans
Patient health can be constantly monitored through wireless connected devices. Patient monitoring is already a key application of smart wearable sensors and microfluidic devices integrated into garments.170 Advanced wearables development and artificial intelligence enable the introduction of context awareness based on the patient's environment and lifestyle, and personalized models for each patient for predictive association.171 Industry is quickly applying this technology to injectable medical devices. Even if some of these advanced sensors and body systems have not yet been integrated into commercial products, several companies are testing wearable microfluidic products. These liquids are properly combined with the drug in the microfluidic process.172 Other companies are developing artificial pancreas systems that can monitor plasma glucose concentrations. In this way, they aim to help the patient optimize their own pancreatic production of insulin. Such artificial intelligence systems are just the beginning of what individual patient health monitoring and diagnostic tests have to offer.The consequence is that, soon, if a patient's wearable device detects the symptoms of a health problem, a pre-trained algorithm will personalize the patient's precise medication at a specific dose. The health of the patient can be safely and automatically monitored outside of professional clinical environments and the patient's drug delivery management.173 The patient's compliant medication may significantly decrease, and the algorithms will adapt the therapeutic plan to the current condition of the patient. This medication may minimize diabetes and cancer effects in some cases through natural extracts or reduce chronic drug administration side effects.174 Individual-specific real-time predictive monitoring is the next phase of smart connected drug delivery enabled by the integration of microfluidics into drug delivery devices. In this context, artificial intelligence in connection with individual monitoring is processing the information gathered.175 The aim is to make such decisions and advise on medication for the patient so that their health condition can be maintained at the best possible level.
10.3. Addressing pharmacokinetic and pharmacodynamic variability
High pharmacokinetic and pharmacodynamic variability between individuals is an important reason why patients need different doses and treatment regimens to achieve optimal therapeutic outcomes. However, the fixed dose commonly used in the clinic does not consider the variability between individuals.176 The variability in drug concentrations in the body is determined by changes in pharmacokinetic parameters, such as reduced drug metabolism and reduced renal clearance. The current approach for addressing pharmacokinetic variability is not patient specific. Clinicians consider the patient's weight and BMI, as well as disease status and comorbidities, to adjust the dosage per protocol or based on effective medication.177 Although the patient's genetic background can indeed be used to roughly predict the pharmacokinetic parameters of certain drugs, pharmacokinetic modeling and simulation technology can better predict the pharmacokinetics of drugs in patients, but it requires future blood concentration data.178,179In summary, pharmacokinetic variability is the leading cause of improper treatment. However, various factors and covariates are not currently considered in dosing regimens. As a result, limited consideration is given to the different doses and dosing schedules needed for individuals to achieve the desired therapeutic effect.180 Since the genetic background of the patient can reveal many pharmacokinetic–pharmacodynamic relationships, it would be possible to develop a model to predict the pharmacokinetics of a target drug in vivo through the patient's DNA, and then deliver the drug in a personalized manner.181 Such an approach might also help to identify patients prone to adverse effects before they undergo therapy, allowing the dosage of the drug to be more customized for their use based on real-time pharmacokinetic information.182 In addition, personalized monitoring information is also important to determine the biomarkers that best reflect the work of the drug and the patient's eligibility for medications.183
11. Challenges and limitations
By using AI to analyze patient databases, we are training algorithms on the data produced in these smart systems. These data hold every detail of the patient, including diagnosis, co-morbidities, drug treatment and its effects, as well as other personal details.184 While the development of AI is crucial for the improvement of medicines, we must also ensure that we maintain patient privacy.185 Anonymization is not enough, as training datasets using state-of-the-art models can lead to accuracy improvements in rendering data ‘de-identified’. Personalization achieved by advanced data analytics techniques also requires the sharing of patient data and sometimes patient tissue at the sample level to implement the algorithm in clinical practice. Maintaining patient privacy during the lifetime of the field will require a fine balance to be struck between maintaining the power required for the AI to work effectively and anonymity.186 This field is a current area of active concern.The data needed to develop and use innovative drug delivery systems are rich and a perfect resource for data mining. The information will be used by a patient and by a future patient through machine-learning algorithms.187 Overcoming patient health as an object in use on a smart drug delivery system raises data privacy and security issues and ethical concerns related to informed consent, data ownership, fiduciary responsibility, patient transparency, data security and integrity, intellectual property, and societal and individual rights among others.188 Ethical considerations and innovations in materials and integration are important to bear in mind when developing personalized medical systems over smart drug delivery system platforms.189
11.1. Regulatory and ethical challenges
The development of AI components in biomedical algorithms not only encounters these technical issues but also other challenges from both regulatory and ethical perspectives. One of the biggest regulatory challenges to AI algorithm development concerns clinical validation.190 To obtain marketing approval or clearance from regulatory bodies, medical technology developers need to undertake empirical validation studies across a range of different environments and real-world users to demonstrate the safety and effectiveness of the technology.191 The incorporation of AI into regulated medical technologies introduces an additional layer of complexity to the validation process, from both technical and logistical standpoints.192 A resulting regulatory challenge is how to properly account for the unique issues that arise from an AI system that learns over time from a range of different real-world sources of data.193,194Developing ethical AI-based medical systems also presents many other contemporary bioethical issues, including accountability for AI's behavior and decisions; transparency to disclose the machine learning process and algorithm; preventing unfairness in the sense of harmful unintended bias; explainability and interpretability of an AI-based system's decisions; and reliability and stability in terms of unassertiveness or error.195 The precision of algorithms in complex environments is of particular concern. Complications arising from misunderstandings of how machine learning tools work may affect the required knowledge of the tools, resulting in concerns relating to privacy, autonomy, and whether these tools unjustifiably challenge autonomy.196 The intricate ways in which AI-related bioethics and self-governance, even autonomy in relation to people with changing goals and values, adds to the depth of complexity regarding the design of and reliance on AI tools such as drug delivery systems.
11.2. Risk of using AI in drug delivery and personalized medicine
Artificial intelligence (AI) is revolutionizing precision medicine and drug delivery systems. It offers immense potential to personalize immune responses, predict drug delivery kinetics, enhance pharmacological systems, and develop therapies for cancer and neurological disorders. By supporting drug design, chemical synthesis, biological evaluations, and decision-making in drug discovery, AI is an invaluable resource.197 The advantages of AI include predicting drug-likeness, exploring vast chemical libraries, and identifying synergistic drug combinations. It also aids in understanding treatments for rare diseases and facilitates drug repurposing. AI excels at extracting relevant biomarkers, improving data accuracy in epigenomics and genomics, and predicting protein–DNA interactions, which enhance future clinical trial designs.198 AI systems filter out data noise to prioritize compounds likely to succeed therapeutically, while safety models update toxicological databases, ensuring reliable information throughout drug development. Therefore, integrating AI into healthcare marks a transformative period, promising advancements in treatment precision and efficacy.The domain of artificial intelligence in healthcare faces numerous challenges requiring careful consideration. A major issue is the lack of extensive, well-annotated cancer datasets, significantly undermining machine learning effectiveness.199 The rise in false-positive melanoma detection rates, which can increase ten-fold compared to clinical diagnoses, highlights the urgent need for thorough validation.200 As AI applications in health technology assessments grow, they outpace available data, raising important questions about potential consequences. Data privacy and security are critical concerns that demand careful attention.201 The inherent trade-offs in sensitivity analysis complicate this balance between innovation and risk. Smart systems’ reliance on algorithmic decision-making makes them vulnerable to security breaches, which could have serious repercussions. The risk of producing erroneous outcomes also calls for strong oversight mechanisms. Ignoring new relational dynamics can lead to a loss of knowledge, while overlooked side effects from flawed algorithms can intensify existing vulnerabilities. The lack of human touch in AI-driven healthcare solutions raises significant issues, especially considering cultural differences in understanding mental health. Furthermore, algorithmic bias poses a threat by potentially perpetuating and exacerbating current inequalities in healthcare.202 Therefore, addressing these multifaceted challenges requires a focused and proactive approach.
Entities (data operators) tasked with the processing of personal data—defined as any information that can be linked to an identifiable individual—bear the responsibility for such processing. The General Data Protection Regulation (GDPR) endorses a principle of privacy by design and by default, mandating that data operators safeguard against unlawful processing, as well as accidental loss, destruction, or damage, while ensuring the availability and accessibility of data. Most principles established for the management of conventional personal data extend their applicability to data categorized under AI.204
To accurately assess the risk associated with the processing of personal data in the context of AI, existing risk assessment tools tailored to personal data must be enhanced through the incorporation of novel methodologies that address the unique characteristics inherent to AI systems. Given the lucrative prospects associated with AI, the extensive collection of personal data is further amplified by intense competition among data operators striving to acquire more personal information.205 Consequently, the processing of personal data not only introduces the risk of re-identification when an individual is acknowledged but also the danger of unfair discrimination, as it facilitates the discernment of particular individual attributes, both protected and unprotected, in terms of discrimination.
In pharmaceuticals, automated ML systems can process vast datasets to develop new medications rapidly. This efficiency, while promising potential cures for diseases like cancer, poses risks, such as the emergence of superbugs and the possibility of malicious entities deliberately releasing pathogens. Although no current pharmaceutical consortium is nearing this fast-paced research speed, such risks necessitate proactive measures to prevent dystopian outcomes. Additionally, as AI integration into daily life deepens, questions arise about ethical considerations in AI recommendations. Even though AI can suggest choices based on various values, it fails to provide data-driven solutions to ethical dilemmas, highlighting an ongoing complexity.
The increasing presence of AI systems may distort human perceptions of value, encouraging unhealthy attitudes and potentially promoting violent or unethical behavior. Moreover, AI capable of generating harmful code poses threats to users by exploiting their devices or networks. Advanced models can create realistic synthetic data, enabling malicious actors to produce convincing imagery or text with minimal coding skills. Such capabilities can expose security vulnerabilities and aid hackers, complicating the AI landscape. Deep learning models trained on code repositories may devise sophisticated exploits, further enhancing the challenges faced in AI governance and security.209
12. Future directions
12.1. Advancements in AI algorithms for better predictions
Artificial intelligence (AI) has increasingly enabled the development of intelligent systems and has been incorporated into public health studies with a high degree of reliability across interdisciplinary fields.210 In commonly adopted research models, various AI algorithms have been demonstrated to be effective.211 AI models can be trained, optimized, validated, and used on different scales and have been shown to be superior at capturing non-linear trends, analyzing vast amounts of complex data, and performing disease or drug compound predictions in big data. Different AI algorithms inherently contain specific principles or are suitable for diverse applications. The motivation for this work is that many AI algorithms could be better used but are possibly underemployed. Generally, AI algorithms, from traditional statistical and mathematical modeling methods to recently trending deep learning methods, have been widely and effectively implemented in various drug discovery or development predictive analyses or trials.212 Different AI algorithms contain distinct requirements and internal algorithms, and they are configured differently. The most used AI algorithms suitable for predictive analyses include random forest, support vector machine, convolutional neural network, and deep learning. When these methods are suitably configured, they can quickly achieve a high performance. Understanding the operational principles, strengths, and limitations of different AI algorithms is helpful for interdisciplinary professionals to execute a topic-sensitive design of reserved models.72 The AI algorithms can be better employed in terms of improved prediction results and reduced time-consuming or numerically aimed experimental designs.12.2. Potential of wearable technologies in real-time data integration
Several wearable tools and sensors have been designed to monitor numerous body-specific parameters in real-time. Personalized medicine can be greatly enhanced by wearable technologies integrated into smart drug delivery systems.213 Wearable devices collect and convey real-time biomarker and physiological indicator data to a doctor continually.214 These gadgets can provide real-time alerts, possess tiny form factors, are easy to operate and fix, and provide contact-free monitoring of the patient. The attendant sensors are capable of transducing data of the biochemical, electrical, or mechanical type. Being light in weight, these add a high level of comfort and do not skew the data obtained. A broad range of body-specific parameters can be directly monitored through wearable technology, i.e., heart rate, pH, body temperature, blood pressure, movement, etc. Many diseases can be identified early on with the help of these types of devices.215 These can be as diverse as self-health management in fitness enthusiasts, chronic disease management, COVID-19 detection, or heart health and syncope monitoring. Since wearable gadget technology is low-cost or widely accessible, it opens up limitless opportunities for innovation and creative applications.216 Certain clothing can have integrated skin sensors.12.3. Collaborative frameworks between AI researchers and clinicians
There is no simple answer for to how to initiate the implementation of AI frameworks in the clinic. A key challenge faced by many researchers is the realization that moving a proof-of-concept machine learning model into the clinical setting involves not just building a model, but the careful assessment of all individual components that together form the system that will be validated in a clinical trial.217 Therefore, it is suggested to make machine learning algorithms more accessible to the clinical domain. By encouraging results to adhere to AI-driven protocols, domain-specific language requirements would regulate newly developed algorithms and delegate compliance to the AI agents.218 The best approach for achieving this is the development of a standardized semantic knowledge framework to guide the development of future AI approaches and prioritize clinical needs. Similarly, for average clinicians to understand and critique AI-driven research, we need forward-thinking AI researchers to translate their work into language that is understandable without a degree in computer science. This reciprocal environment is extremely difficult to achieve, which is why we see interprofessional collaboration as the most important success factor for successful AI implementation in personalized medicine. Interprofessional collaboration requires frequent communication among AI researchers, domain-educated users, and decision-makers, as well as future AI developers and users of the system. Whether AI-driven management and triage, AI-driven therapeutics, or AI-driven augmented diagnostics are at stake, the role that AI will play within healthcare is growing.219 Only through interprofessional collaboration can this growth be managed effectively.12.4. Role of quantum computing in drug delivery and personalized medicine
Inspired by the large number of drugs approved daily by regulatory authorities around the world, scientists are engaged in customizing the necessary treatment that an individual patient may require and designing it precisely to reach the target site in a timely manner and quickly release functional doses.220 With the advancement of medical technology in recent years, continuous progress in the field of drug delivery has led to the conclusion that the introduction of quantum optimization of proteins (quantum optimization of proteins highlights the convergence of quantum mechanics and biochemistry; using quantum methods to optimize protein structures is gaining traction, potentially reshaping our comprehension of molecular interactions as researchers explore quantum states’ roles in protein folding and stability) and pharmacovigilance can pave the way to meet this challenge. With the introduction of quantum computing, novel ideas should be released immediately that can support the development of strategies to optimize prototype synthesis or facilitate combinatorial library selection.221 Subsequently, we present a quantum computing model that optimizes the protein selection problem for open-loop drug delivery (the term “open-loop drug delivery” refers to administering therapeutic agents without real-time feedback or adjustments based on the patient's response).222 The concept of a quantum computer is a new one, and we seek to evaluate its effects in computer technology and the software industry. The relationship between the process of drug discovery and quantum computing is still in its infancy. Researchers, however, realize that the two areas are compatible and work to merge quantum computing and chemical problems.223 The accelerated, disruptive technologies of quantum computing and quantum optimization begin to merge with strong interest in the future drug discovery market. The benefits that quantum computing can provide result from its nature as a real-time artificial intelligence algorithm.224 It provides real-time information by analyzing one molecule at a time, so one can quickly intervene when the forecasts show undesired results, which is positive in the field of drugs.22513. Conclusion
AI has revolutionized smart drug delivery and personalized medicine by enabling better characterization of drugs, real-time monitoring, and accurate therapeutic interventions. The use of AI-based strategies helps in patient stratification, pharmacovigilance, and multimodal diagnostics with the objective of the timely identification of potential problems and optimization of nanoparticle-based therapeutics. Real-time personalized point-of-care diagnostics and predictive models to personalize dosing accuracy, minimize adverse effects, and ensure treatment adherence will lead to better, safer delivery of drugs. The integration of AI with genomics, proteomics, and biomarkers has definitely set the foundation for personalized treatment protocols. However, the realization of the potential of digital health will call for international collaboration, policy support, and strategic investments. Inspired by past technological revolutions, digital health must be prioritized to ensure equitable access to precision medicine and proactive disease management. Future innovations in AI-driven healthcare include combining sensory-based health monitoring with AI, IoT, robotics, and advanced medical devices. This integration will make early disease detection, personalized digital therapeutics, and remote monitoring possible, ultimately saving hospital costs and the burden that chronic diseases inflict on society. Despite challenges that arise from the AI-driven nature of medical devices and digital therapeutics, attention must be paid to real-world applications rather than theoretical models. AI-driven medical technology must focus on real-time diagnostics, self-management systems, and adaptive interventions to enhance patient outcomes and revolutionize healthcare delivery. With AI, the future of healthcare is changing toward a proactive, personalized, and technologically integrated approach, marking the beginning of a digital health revolution aimed at improving global health and well-being.Data availability
This article does not contain any original data. All data discussed and analyzed in this review are derived from previously published studies, which are appropriately cited within the manuscript.Conflicts of interest
There are no conflicts to declare.References
- H. Park, A. Otte and K. Park, Evolution of Drug Delivery Systems: From 1950 to 2020 and Beyond, J. Controlled Release, 2022, 342, 53–65, DOI:10.1016/j.jconrel.2021.12.030.
- D. Ho, S. R. Quake, E. R. B. McCabe, W. J. Chng, E. K. Chow, X. Ding, B. D. Gelb, G. S. Ginsburg, J. Hassenstab, C. M. Ho, W. C. Mobley, G. P. Nolan, S. T. Rosen, P. Tan, Y. Yen and A. Zarrinpar, Enabling Technologies for Personalized and Precision Medicine, Trends Biotechnol., 2020, 497–518, DOI:10.1016/j.tibtech.2019.12.021.
- K. B. Johnson, W. Q. Wei, D. Weeraratne, M. E. Frisse, K. Misulis, K. Rhee, J. Zhao and J. L. Snowdon, Precision Medicine, AI, and the Future of Personalized Health Care, Clin. Transl. Sci., 2021, 86–93, DOI:10.1111/cts.12884.
- A. M. Tsimberidou, E. Fountzilas, M. Nikanjam and R. Kurzrock, Review of Precision Cancer Medicine: Evolution of the Treatment Paradigm, Cancer Treat. Rev., 2020, 86, 102019, DOI:10.1016/J.CTRV.2020.102019.
- F. Ciardiello, D. Ciardiello, G. Martini, S. Napolitano, J. Tabernero and A. Cervantes, Clinical Management of Metastatic Colorectal Cancer in the Era of Precision Medicine, CA Cancer J. Clin., 2022, 72(4), 372–401, DOI:10.3322/caac.21728.
- M. E. Prendergast and J. A. Burdick, Recent Advances in Enabling Technologies in 3D Printing for Precision Medicine, Adv. Mater., 2020, 32, 1902516, DOI:10.1002/adma.201902516.
- J. R. Beitler, B. T. Thompson, R. M. Baron, J. A. Bastarache, L. C. Denlinger, L. Esserman, M. N. Gong, L. M. LaVange, R. J. Lewis, J. C. Marshall, T. R. Martin, D. F. McAuley, N. J. Meyer, M. Moss, L. A. Reineck, E. Rubin, E. P. Schmidt, T. J. Standiford, L. B. Ware, H. R. Wong, N. R. Aggarwal and C. S. Calfee, Advancing Precision Medicine for Acute Respiratory Distress Syndrome, Lancet Respir. Med., 2022, 10(1), 107–120, DOI:10.1016/S2213-2600(21)00157-0.
- Z. Ahmed, K. Mohamed, S. Zeeshan and X. Q. Dong, Artificial Intelligence with Multi-Functional Machine Learning Platform Development for Better Healthcare and Precision Medicine, Database, 2020, 2020, baaa010, DOI:10.1093/database/baaa010.
- R. Hamamoto, K. Suvarna, M. Yamada, K. Kobayashi, N. Shinkai, M. Miyake, M. Takahashi, S. Jinnai, R. Shimoyama, A. Sakai, K. Takasawa, A. Bolatkan, K. Shozu, A. Dozen, H. Machino, S. Takahashi, K. Asada, M. Komatsu, J. Sese and S. Kaneko, Application of Artificial Intelligence Technology in Oncology: Towards the Establishment of Precision Medicine, Cancers, 2020, 1–32, DOI:10.3390/cancers12123532.
- B. Bhinder, C. Gilvary, N. S. Madhukar and O. Elemento, Artifi Cial Intelligence in Cancer Research and Precision Medicine, Cancer Discovery, 2021, 900–915, DOI:10.1158/2159-8290.CD-21-0090.
- S. A. Alowais, S. S. Alghamdi, N. Alsuhebany, T. Alqahtani, A. I. Alshaya, S. N. Almohareb, A. Aldairem, M. Alrashed, K. Bin Saleh, H. A. Badreldin, M. S. Al Yami, S. Al Harbi and A. M. Albekairy, Revolutionizing Healthcare: The Role of Artificial Intelligence in Clinical Practice, BMC Med. Educ., 2023, 23, 689, DOI:10.1186/s12909-023-04698-z.
- M. Singha, L. Pu, G. Srivastava, X. Ni, B. A. Stanfield, I. K. Uche, P. J. F. Rider, K. G. Kousoulas, J. Ramanujam and M. Brylinski, Unlocking the Potential of Kinase Targets in Cancer: Insights from CancerOmicsNet, an AI-Driven Approach to Drug Response Prediction in Cancer, Cancers, 2023, 15(16), 4050, DOI:10.3390/cancers15164050.
- Z. Tanoli, M. Vähä-Koskela and T. Aittokallio, Artificial Intelligence, Machine Learning, and, Drug Repurposing in Cancer, Expert Opin. Drug Discovery, 2021, 977–989, DOI:10.1080/17460441.2021.1883585.
- J. G. Greener, S. M. Kandathil, L. Moffat and D. T. Jones, A Guide to Machine Learning for Biologists, Nat. Rev. Mol. Cell Biol., 2022, 23, 40–55 Search PubMed.
- N. Sapoval, A. Aghazadeh, M. G. Nute, D. A. Antunes, A. Balaji, R. Baraniuk, C. J. Barberan, R. Dannenfelser, C. Dun, M. Edrisi, R. A. L. Elworth, B. Kille, A. Kyrillidis, L. Nakhleh, C. R. Wolfe, Z. Yan, V. Yao and T. J. Treangen, Current Progress and Open Challenges for Applying Deep Learning across the Biosciences, Nat. Commun., 2022, 13, 1728, DOI:10.1038/s41467-022-29268-7.
- S. Zhong, K. Zhang, M. Bagheri, J. G. Burken, A. Gu, B. Li, X. Ma, B. L. Marrone, Z. J. Ren, J. Schrier, W. Shi, H. Tan, T. Wang, X. Wang, B. M. Wong, X. Xiao, X. Yu, J. J. Zhu and H. Zhang, Machine Learning: New Ideas and Tools in Environmental Science and Engineering, Environ. Sci. Technol., 2021, 55(19), 12741–12754, DOI:10.1021/acs.est.1c01339.
- A. Holzinger, K. Keiblinger, P. Holub, K. Zatloukal and H. Müller, AI for Life: Trends in Artificial Intelligence for Biotechnology, New Biotechnol., 2023, 74, 16–24, DOI:10.1016/J.NBT.2023.02.001.
- I. H. Sarker, Machine Learning: Algorithms, Real-World Applications and Research Directions, SN Comput. Sci., 2021, 2, 160, DOI:10.1007/s42979-021-00592-x.
- P. Carracedo-Reboredo, J. Liñares-Blanco, N. Rodríguez-Fernández, F. Cedrón, F. J. Novoa, A. Carballal, V. Maojo, A. Pazos and C. Fernandez-Lozano, A Review on Machine Learning Approaches and Trends in Drug Discovery, Comput. Struct. Biotechnol. J., 2021, 19, 4538–4558, DOI:10.1016/J.CSBJ.2021.08.011.
- I. H. Sarker, Deep Learning: A Comprehensive Overview on Techniques, Taxonomy, Applications and Research Directions, SN Comput. Sci., 2021, 2, 420, DOI:10.1007/s42979-021-00815-1.
- S. Dong, P. Wang and K. Abbas, A Survey on Deep Learning and Its Applications, Comput. Sci. Rev., 2021, 40, 100379, DOI:10.1016/J.COSREV.2021.100379.
- S. Dargan, M. Kumar, M. R. Ayyagari and G. Kumar, A Survey of Deep Learning and Its Applications: A New Paradigm to Machine Learning, Arch. Comput. Methods Eng., 2020, 27(4), 1071–1092, DOI:10.1007/s11831-019-09344-w.
- N. Sharma, R. Sharma and N. Jindal, Machine Learning and Deep Learning Applications-A Vision, Glob. Transit. Proc., 2021, 2(1), 24–28, DOI:10.1016/J.GLTP.2021.01.004.
- I. Galić, M. Habijan, H. Leventić and K. Romić, Machine Learning Empowering Personalized Medicine: A Comprehensive Review of Medical Image Analysis Methods, Electronics, 2023, 12, 4411, DOI:10.3390/electronics12214411.
- M. Tsuneki, Deep Learning Models in Medical Image Analysis Author Names and Affiliations, 2022 Search PubMed.
- G. K. Thakur, A. Thakur, S. Kulkarni, N. Khan and S. Khan, Deep Learning Approaches for Medical Image Analysis and Diagnosis, Cureus, 2024, 6, e59507, DOI:10.7759/cureus.59507.
- J. Duan, J. Xiong, Y. Li and W. Ding, Deep Learning Based Multimodal Biomedical Data Fusion: An Overview and Comparative Review, Inf. Fusion, 2024, 112, 102536, DOI:10.1016/J.INFFUS.2024.102536.
- F. Piccialli, V. Di Somma, F. Giampaolo, S. Cuomo and G. Fortino, A Survey on Deep Learning in Medicine: Why, How and When?, Inf. Fusion, 2021, 66, 111–137, DOI:10.1016/J.INFFUS.2020.09.006.
- B. Chai, C. Efstathiou, H. Yue and V. M. Draviam, Opportunities and Challenges for Deep Learning in Cell Dynamics Research, Trends Cell Biol., 2024, 34, 955–967, DOI:10.1016/j.tcb.2023.10.010.
- D. Khurana, A. Koli, K. Khatter and S. Singh, Natural Language Processing: State of the Art, Current Trends and Challenges, Multimedia Tools Appl., 2023, 82(3), 3713–3744, DOI:10.1007/s11042-022-13428-4.
- I. Lauriola, A. Lavelli and F. Aiolli, An Introduction to Deep Learning in Natural Language Processing: Models, Techniques, and Tools, Neurocomputing, 2022, 470, 443–456, DOI:10.1016/J.NEUCOM.2021.05.103.
- P. M. Mah, I. Skalna and J. Muzam, Natural Language Processing and Artificial Intelligence for Enterprise Management in the Era of Industry 4.0, Appl. Sci., 2022, 12(18), 9207, DOI:10.3390/app12189207.
- H. Gao, X. Qin, R. J. D. Barroso, W. Hussain, Y. Xu and Y. Yin, Collaborative Learning-Based Industrial IoT API Recommendation for Software-Defined Devices: The Implicit Knowledge Discovery Perspective, IEEE Trans. Emerging Top. Comput. Intell., 2022, 6(1), 66–76, DOI:10.1109/TETCI.2020.3023155.
- T. Shaik, X. Tao, N. Higgins, L. Li, R. Gururajan, X. Zhou and U. R. Acharya, Remote Patient Monitoring Using Artificial Intelligence: Current State, Applications, and Challenges, Wiley Interdiscip. Rev.: Data Min. Knowl. Discovery, 2023, 13, e1485, DOI:10.1002/widm.1485.
- E. Hyvönen, Using the Semantic Web in Digital Humanities: Shift from Data Publishing to Data-Analysis and Serendipitous Knowledge Discovery, Semant. Web, 2020, 11(1), 187–193, DOI:10.3233/SW-19038.
- L. Cui, H. Seo, M. Tabar, F. Ma, S. Wang and D. Lee, DETERRENT: Knowledge Guided Graph Attention Network for Detecting Healthcare Misinformation, in Proceedings of the ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; Association for Computing Machinery, 2020, pp. 492–502. DOI:10.1145/3394486.3403092.
- M. Bansal, A. Goyal and A. Choudhary, A Comparative Analysis of K-Nearest Neighbor, Genetic, Support Vector Machine, Decision Tree, and Long Short Term Memory Algorithms in Machine Learning, Decis. Anal. J., 2022, 3, 100071, DOI:10.1016/J.DAJOUR.2022.100071.
- A. Goel, A. K. Goel and A. Kumar, The Role of Artificial Neural Network and Machine Learning in Utilizing Spatial Information, Spat. Inf. Res., 2023, 275–285, DOI:10.1007/s41324-022-00494-x.
- V. Nasir and F. Sassani, A Review on Deep Learning in Machining and Tool Monitoring: Methods, Opportunities, and Challenges, Int. J. Adv. Des. Manuf. Technol., 2021, 115, 2683–2709, DOI:10.1007/s00170-021-07325-7.
- H. Dahrouj, R. Alghamdi, H. Alwazani, S. Bahanshal, A. A. Ahmad, A. Faisal, R. Shalabi, R. Alhadrami, A. Subasi, M. T. Al-Nory, O. Kittaneh and J. S. Shamma, An Overview of Machine Learning-Based Techniques for Solving Optimization Problems in Communications and Signal Processing, IEEE Access, 2021, 9, 74908–74938, DOI:10.1109/ACCESS.2021.3079639.
- L. Alzubaidi, J. Bai, A. Al-Sabaawi, J. Santamaría, A. S. Albahri, B. S. N. Al-dabbagh, M. A. Fadhel, M. Manoufali, J. Zhang, A. H. Al-Timemy, Y. Duan, A. Abdullah, L. Farhan, Y. Lu, A. Gupta, F. Albu, A. Abbosh and Y. Gu, A Survey on Deep Learning Tools Dealing with Data Scarcity: Definitions, Challenges, Solutions, Tips, and Applications, J. Big Data, 2023, 10(1), 46, DOI:10.1186/s40537-023-00727-2.
- O. Kehinde, Machine Learning in Predictive Modelling: Addressing Chronic Disease Management through Optimized Healthcare Processes, Int. J. Res. Publ. Rev., 2025, 6, 1525–1539. https://www.ijrpr.com Search PubMed.
- D. Kumar, M. Soumya and R. Jena, NLP For Sentiment Analysis, 2024. DOI:10.5281/zenodo.14178332.
- R. Singh, R. Manohar and D. Kumar, Genomic Intelligence, 2025. https://www.researchgate.net/publication/390520078 Search PubMed.
- A. J. Oyejide, A. A. Adekunle, O. D. Abodunrin and E. O. Atoyebi, Artificial Intelligence, Computational Tools and Robotics for Drug Discovery, Development, and Delivery, Intell. Pharm., 2025 DOI:10.1016/J.IPHA.2025.01.001.
- D. R. Serrano, F. C. Luciano, B. J. Anaya, B. Ongoren, A. Kara, G. Molina, B. I. Ramirez, S. A. Sánchez-Guirales, J. A. Simon, G. Tomietto, C. Rapti, H. K. Ruiz, S. Rawat, D. Kumar and A. Lalatsa, Artificial Intelligence (AI) Applications in Drug Discovery and Drug Delivery: Revolutionizing Personalized Medicine, Pharmaceutics, 2024, 16(10), 1328, DOI:10.3390/pharmaceutics16101328.
- Complex Biology, Unlocked. https://www.benevolent.com.
- B. Z. Zsidó, R. Börzsei, V. Szél and C. Hetényi, Determination of Ligand Binding Modes in Hydrated Viral Ion Channels to Foster Drug Design and Repositioning, J. Chem. Inf. Model., 2021, 61(8), 4011–4022, DOI:10.1021/acs.jcim.1c00488.
- P. Kamya, I. V. Ozerov, F. W. Pun, K. Tretina, T. Fokina, S. Chen, V. Naumov, X. Long, S. Lin, M. Korzinkin, D. Polykovskiy, A. Aliper, F. Ren and A. Zhavoronkov, PandaOmics: An AI-Driven Platform for Therapeutic Target and Biomarker Discovery, J. Chem. Inf. Model., 2024, 64(10), 3961–3969, DOI:10.1021/acs.jcim.3c01619.
- R. Paul and A. Hossain, Integrating Genomic Data with AI Algorithms to Optimize Personalized Drug Therapy: A Pilot Study, 2024. https://www.bpasjournals.com Search PubMed.
- F. Mirakhori and S. K. Niazi, Harnessing the AI/ML in Drug and Biological Products Discovery and Development: The Regulatory Perspective, Pharmaceuticals, 2025, 18(1), 47, DOI:10.3390/ph18010047.
- R. Gupta, D. Srivastava, M. Sahu, S. Tiwari, R. K. Ambasta and P. Kumar, Artificial Intelligence to Deep Learning: Machine Intelligence Approach for Drug Discovery, Mol. Diversity, 2021, 25(3), 1315–1360, DOI:10.1007/s11030-021-10217-3.
- S. Singh, N. Kaur and A. Gehlot, Application of Artificial Intelligence in Drug Design: A Review, Comput. Biol. Med., 2024, 179, 108810, DOI:10.1016/J.COMPBIOMED.2024.108810.
- A. B. Gurung, M. A. Ali, J. Lee, M. A. Farah and K. M. Al-Anazi, An Updated Review of Computer-Aided Drug Design and Its Application to COVID-19, BioMed Res. Int., 2021, 8853056, DOI:10.1155/2021/8853056.
- S. K. Niazi and Z. Mariam, Computer-Aided Drug Design and Drug Discovery: A Prospective Analysis, Pharmaceuticals, 2024, 17(1), 22, DOI:10.3390/ph17010022.
- D. Bassani and S. Moro, Past, Present, and Future Perspectives on Computer-Aided Drug Design Methodologies, Molecules, 2023, 28(9), 3906, DOI:10.3390/molecules28093906.
- J. L. Medina-Franco, Grand Challenges of Computer-Aided Drug Design: The Road Ahead, Front. Drug Discovery, 2021, 1, 728551, DOI:10.3389/fddsv.2021.728551.
- P. Jia, J. Pei, G. Wang, X. Pan, Y. Zhu, Y. Wu and L. Ouyang, The Roles of Computer-Aided Drug Synthesis in Drug Development, Green Synth. Catal., 2022, 3(1), 11–24, DOI:10.1016/J.GRESC.2021.11.007.
- G. Battineni, G. G. Sagaro, N. Chinatalapudi and F. Amenta, Applications of Machine Learning Predictive Models in the Chronic Disease Diagnosis, J. Pers. Med., 2020, 10(2), 21, DOI:10.3390/jpm10020021.
- K. C. Santosh, COVID-19 Prediction Models and Unexploited Data, J. Med. Syst., 2020, 44(9), 170, DOI:10.1007/s10916-020-01645-z.
- A. A. H. de Hond, A. M. Leeuwenberg, L. Hooft, I. M. J. Kant, S. W. J. Nijman, H. J. A. van Os, J. J. Aardoom, T. P. A. Debray, E. Schuit, M. van Smeden, J. B. Reitsma, E. W. Steyerberg, N. H. Chavannes and K. G. M. Moons, Guidelines and Quality Criteria for Artificial Intelligence-Based Prediction Models in Healthcare: A Scoping Review, npj Digital Med., 2022, 5, 2, DOI:10.1038/s41746-021-00549-7.
- P. Mohseni and A. Ghorbani, Exploring the Synergy of Artificial Intelligence in Microbiology: Advancements, Challenges, and Future Prospects, Comput. Struct. Biotechnol. Rep., 2024, 1, 100005, DOI:10.1016/J.CSBR.2024.100005.
- M. C. R. Melo, J. R. M. A. Maasch and C. de la Fuente-Nunez, Accelerating Antibiotic Discovery through Artificial Intelligence, Commun. Biol., 2021, 4, 1050, DOI:10.1038/s42003-021-02586-0.
- S. Kolluri, J. Lin, R. Liu, Y. Zhang and W. Zhang, Machine Learning and Artificial Intelligence in Pharmaceutical Research and Development: A Review, AAPS J., 2022, 24(1), 19, DOI:10.1208/s12248-021-00644-3.
- M. Nagendran, Y. Chen, C. A. Lovejoy, A. C. Gordon, M. Komorowski, H. Harvey, E. J. Topol, J. P. A. Ioannidis, G. S. Collins and M. Maruthappu, Artificial Intelligence versus Clinicians: Systematic Review of Design, Reporting Standards, and Claims of Deep Learning Studies in Medical Imaging, BMJ, 2020, 368, m689, DOI:10.1136/bmj.m689.
- E. H. Weissler, T. Naumann, T. Andersson, R. Ranganath, O. Elemento, Y. Luo, D. F. Freitag, J. Benoit, M. C. Hughes, F. Khan, P. Slater, K. Shameer, M. Roe, E. Hutchison, S. H. Kollins, U. Broedl, Z. Meng, J. L. Wong, L. Curtis, E. Huang and M. Ghassemi, The Role of Machine Learning in Clinical Research: Transforming the Future of Evidence Generation, Trials, 2021, 22, 537, DOI:10.1186/s13063-021-05489-x.
- S. Cruz Rivera, X. Liu, A. W. Chan, A. K. Denniston, M. J. Calvert, H. Ashrafian, A. L. Beam, G. S. Collins, A. Darzi, J. J. Deeks, M. K. ElZarrad, C. Espinoza, A. Esteva, L. Faes, L. Ferrante di Ruffano, J. Fletcher, R. Golub, H. Harvey, C. Haug, C. Holmes, A. Jonas, P. A. Keane, C. J. Kelly, A. Y. Lee, C. S. Lee, E. Manna, J. Matcham, M. McCradden, D. Moher, J. Monteiro, C. Mulrow, L. Oakden-Rayner, D. Paltoo, M. B. Panico, G. Price, S. Rowley, R. Savage, R. Sarkar, S. J. Vollmer and C. Yau, Guidelines for Clinical Trial Protocols for Interventions Involving Artificial Intelligence: The SPIRIT-AI Extension, Lancet Digital Health, 2020, e549–e560, DOI:10.1016/S2589-7500(20)30219-3.
- M. J. Iqbal, Z. Javed, H. Sadia, I. A. Qureshi, A. Irshad, R. Ahmed, K. Malik, S. Raza, A. Abbas, R. Pezzani and J. Sharifi-Rad, Clinical Applications of Artificial Intelligence and Machine Learning in Cancer Diagnosis: Looking into the Future, Cancer Cell Int., 2021, 21(1), 270, DOI:10.1186/s12935-021-01981-1.
- https://clinicaltrials.gov .
- C. Sarkar, B. Das, V. S. Rawat, J. B. Wahlang, A. Nongpiur, I. Tiewsoh, N. M. Lyngdoh, D. Das, M. Bidarolli and H. T. Sony, Artificial Intelligence and Machine Learning Technology Driven Modern Drug Discovery and Development, Int. J. Mol. Sci., 2023, 24(3), 2026, DOI:10.3390/ijms24032026.
- W. Wang, Z. Ye, H. Gao and D. Ouyang, Computational Pharmaceutics - A New Paradigm of Drug Delivery, J. Controlled Release, 2021, 338, 119–136, DOI:10.1016/J.JCONREL.2021.08.030.
- R. Gupta, D. Srivastava, M. Sahu, S. Tiwari, R. K. Ambasta and P. Kumar, Artificial Intelligence to Deep Learning: Machine Intelligence Approach for Drug Discovery, Mol. Diversity, 2021, 25(3), 1315–1360, DOI:10.1007/s11030-021-10217-3.
- O. Adir, M. Poley, G. Chen, S. Froim, N. Krinsky, J. Shklover, J. Shainsky-Roitman, T. Lammers and A. Schroeder, Integrating Artificial Intelligence and Nanotechnology for Precision Cancer Medicine, Adv. Mater., 2020, 32(13), 1901989, DOI:10.1002/adma.201901989.
- S. Mishra, T. Bhatt, H. Kumar, R. Jain, S. Shilpi and V. Jain, Nanoconstructs for Theranostic Application in Cancer: Challenges and Strategies to Enhance the Delivery, Front. Pharmacol., 2023, 14, 1101320, DOI:10.3389/fphar.2023.1101320.
- C. Bhandari, M. Guirguis, N. A. Savan, N. Shrivastava, S. Oliveira, T. Hasan and G. Obaid, What NIR Photodynamic Activation Offers Molecular Targeted Nanomedicines: Perspectives into the Conundrum of Tumor Specificity and Selectivity, Nano Today, 2021, 36, 101052, DOI:10.1016/j.nantod.2020.101052.
- H. Ma, Z. Jiang, J. Xu, J. Liu and Z. N. Guo, Targeted Nano-Delivery Strategies for Facilitating Thrombolysis Treatment in Ischemic Stroke, Drug Delivery, 2021, 28(1), 357–371, DOI:10.1080/10717544.2021.1879315.
- H. A. Hussein and M. A. Abdullah, Novel Drug Delivery Systems Based on Silver Nanoparticles, Hyaluronic Acid, Lipid Nanoparticles and Liposomes for Cancer Treatment, Appl. Nanosci., 2022, 3071–3096, DOI:10.1007/s13204-021-02018-9.
- M. T. Manzari, Y. Shamay, H. Kiguchi, N. Rosen, M. Scaltriti and D. A. Heller, Targeted Drug Delivery Strategies for Precision Medicines, Nat. Rev. Mater., 2021, 351–370, DOI:10.1038/s41578-020-00269-6.
- P. Ghasemiyeh and S. Mohammadi-Samani, Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages, Drug Des., Dev. Ther., 2020, 14, 3271–3289, DOI:10.2147/DDDT.S264648.
- D. Ramadon, M. T. C. McCrudden, A. J. Courtenay and R. F. Donnelly, Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications, Drug Delivery Transl. Res., 2022, 12(4), 758–791, DOI:10.1007/s13346-021-00909-6.
- S. Municoy, M. I. Álvarez Echazú, P. E. Antezana, J. M. Galdopórpora, C. Olivetti, A. M. Mebert, M. L. Foglia, M. V. Tuttolomondo, G. S. Alvarez, J. G. Hardy and M. F. Desimone, Stimuli-Responsive Materials for Tissue Engineering and Drug Delivery, Int. J. Mol. Sci., 2020, 1–39, DOI:10.3390/ijms21134724.
- S. H. Pham, Y. Choi and J. Choi, Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery, Pharmaceutics, 2020, 1–19, DOI:10.3390/pharmaceutics12070630.
- S. Ahmadi, N. Rabiee, M. Bagherzadeh, F. Elmi, Y. Fatahi, F. Farjadian, N. Baheiraei, B. Nasseri, M. Rabiee, N. T. Dastjerd, A. Valibeik, M. Karimi and M. R. Hamblin, Stimulus-Responsive Sequential Release Systems for Drug and Gene Delivery, Nano Today, 2020, 34, 100914, DOI:10.1016/j.nantod.2020.100914.
- A. Bratek-Skicki, Towards a New Class of Stimuli-Responsive Polymer-Based Materials – Recent Advances and Challenges, Appl. Surf. Sci. Adv., 2021, 4, 100068, DOI:10.1016/J.APSADV.2021.100068.
- F. Sabir, M. Zeeshan, U. Laraib, M. Barani, A. Rahdar, M. Cucchiarini and S. Pandey, Dna Based and Stimuli-Responsive Smart Nanocarrier for Diagnosis and Treatment of Cancer: Applications and Challenges, Cancers, 2021, 13(14), 3396, DOI:10.3390/cancers13143396.
- K. B. Kumar, A. Rajitha, A. K. Rao, K. Alam, A. Albawi and G. Sethi, SMART Materials for Biomedical Applications: Advancements and Challenges, in E3S Web of Conferences, EDP Sciences, 2023, vol. 430, pp. 01133. DOI:10.1051/e3sconf/202343001133.
- L. Alzoubi, A. A. A. Aljabali and M. M. Tambuwala, Empowering Precision Medicine: The Impact of 3D Printing on Personalized Therapeutic, AAPS PharmSciTech, 2023, 24, 228, DOI:10.1208/s12249-023-02682-w.
- N. S. Eyke, B. A. Koscher and K. F. Jensen, Toward Machine Learning-Enhanced High-Throughput Experimentation, Trends Chem, 2020, 3(2), 120–132, 2020 Search PubMed.
- P. Bannigan, M. Aldeghi, Z. Bao, F. Häse, A. Aspuru-Guzik and C. Allen, Machine Learning Directed Drug Formulation Development, Adv. Drug Delivery Rev., 2021, 175, 113806, DOI:10.1016/J.ADDR.2021.05.016.
- R. A. Patel, S. Colmenares and M. A. Webb, Sequence Patterning, Morphology, and Dispersity in Single-Chain Nanoparticles: Insights from Simulation and Machine Learning, ACS Polym. Au, 2023, 3(3), 284–294 Search PubMed.
- E.-W. Huang, W.-J. Lee, S. Singh, P. Kumar, C.-Y. Lee, T.-N. Lam, H.-H. Chin, B.-H. Lin, P. K. Liaw and S. S. Singh, Machine-Learning and High-Throughput Studies for High-Entropy Materials, Mater. Sci. Eng., R, 2021, 147, 100645. DOI:10.1016/j.mser.2021.100645.
- S. M. Moosavi, K. M. Jablonka and B. Smit, The Role of Machine Learning in the Understanding and Design of Materials, J. Am. Chem. Soc., 2020, 20273–20287, DOI:10.1021/jacs.0c09105.
- J. Jiménez-Luna, F. Grisoni, N. Weskamp and G. Schneider, Artificial Intelligence in Drug Discovery: Recent Advances and Future Perspectives, Expert Opin. Drug Discovery, 2021, 16(9), 949–959, DOI:10.1080/17460441.2021.1909567.
- J. Cai, X. Chu, K. Xu, H. Li and J. Wei, Machine Learning-Driven New Material Discovery, Nanoscale Adv., 2020, 3115–3130, 10.1039/d0na00388c.
- R. L. Milliken, T. Quinten, S. K. Andersen and D. A. Lamprou, Application of 3D Printing in Early Phase Development of Pharmaceutical Solid Dosage Forms, Int. J. Pharm., 2024, 653, 123902, DOI:10.1016/J.IJPHARM.2024.123902.
- G. K. Jena, C. N. Patra, S. Jammula, R. Rana and S. Chand, Artificial Intelligence and Machine Learning Implemented Drug Delivery Systems: A Paradigm Shift in the Pharmaceutical Industry, J. Bio-X Res., 2024, 7, 0016, DOI:10.34133/jbioxresearch.0016.
- H. Dey, N. Arya, H. Mathur, N. Chatterjee and R. Jadon, Exploring the Role of Artificial Intelligence and Machine Learning in Pharmaceutical Formulation Design, Int. J. Newgen Res. Pharm. Healthcare, 2024, 30–41, DOI:10.61554/ijnrph.v2i1.2024.67.
- A. J. Gormley, Machine Learning in Drug Delivery, J. Controlled Release, 2024, 373, 23–30, DOI:10.1016/J.JCONREL.2024.06.045.
- J. Jiang, X. Ma, D. Ouyang and R. O. Williams, Emerging Artificial Intelligence (AI) Technologies Used in the Development of Solid Dosage Forms, Pharmaceutics, 2022, 14(11), 2257, DOI:10.3390/pharmaceutics14112257.
- B. M. Castro, M. Elbadawi, J. J. Ong, T. Pollard, Z. Song, S. Gaisford, G. Pérez, A. W. Basit, P. Cabalar and A. Goyanes, Machine Learning Applied to over 900 3D Printed Drug Delivery SystemsJ. Control Release 2021337530–545, DOI:10.1016/j.jconrel.2021.07.046.
- M. Chisanga and J.-F. Masson, Machine Learning-Driven SERS Nanoendoscopy and Optophysiology, Annu. Rev. Anal. Chem., 2025, 34, 27, DOI:10.1146/annurev-anchem-061622.
- S. J. Trenfield, A. Awad, L. E. McCoubrey, M. Elbadawi, A. Goyanes, S. Gaisford and A. W. Basit, Advancing Pharmacy and Healthcare with Virtual Digital Technologies, Adv. Drug Delivery Rev., 2022, 182, 114098, DOI:10.1016/j.addr.2021.114098.
- A. Blanco-González, A. Cabezón, A. Seco-González, D. Conde-Torres, P. Antelo-Riveiro, Á. Piñeiro and R. Garcia-Fandino, The Role of AI in Drug Discovery: Challenges, Opportunities, and Strategies, Pharmaceuticals, 2023, 16(6), 891, DOI:10.3390/ph16060891.
- Y. Song, T. Zhou, R. Bai, M. Zhang and H. Yang, Review of CFD-DEM Modeling of Wet Fluidized Bed Granulation and Coating Processes, Processes, 2023, 11(2), 382, DOI:10.3390/pr11020382.
- D. Paul, G. Sanap, S. Shenoy, D. Kalyane, K. Kalia and R. K. Tekade, Artificial Intelligence in Drug Discovery and Development, Drug Discovery Today, 2021, 80–93, DOI:10.1016/j.drudis.2020.10.010.
- R. I. Mukhamediev, Y. Popova, Y. Kuchin, E. Zaitseva, A. Kalimoldayev, A. Symagulov, V. Levashenko, F. Abdoldina, V. Gopejenko, K. Yakunin, E. Muhamedijeva and M. Yelis, Review of Artificial Intelligence and Machine Learning Technologies: Classification, Restrictions, Opportunities and Challenges, Mathematics, 2022, 10(15), 2552, DOI:10.3390/math10152552.
- A. P. K. Mahapatra, R. Saraswat, M. Botre, B. Paul and N. Prasad, Application of Response Surface Methodology (RSM) in Statistical Optimization and Pharmaceutical Characterization of a Patient Compliance Effervescent Tablet Formulation of an Antiepileptic Drug Levetiracetam, Future J. Pharm. Sci., 2020, 6(1), 82, DOI:10.1186/s43094-020-00096-0.
- J. Wang, C. Lan, C. Liu, Y. Ouyang, T. Qin, W. Lu, Y. Chen, W. Zeng and P. S. Yu, Generalizing to Unseen Domains: A Survey on Domain Generalization. 2021 Search PubMed.
- A. L. B. Seynhaeve, M. Amin, D. Haemmerich, G. C. van Rhoon and T. L. M. ten Hagen, Hyperthermia and Smart Drug Delivery Systems for Solid Tumor Therapy, Adv. Drug Delivery Rev., 2020, 163–164, 125–144, DOI:10.1016/J.ADDR.2020.02.004.
- H. Ma, Z. Jiang, J. Xu, J. Liu and Z. N. Guo, Targeted Nano-Delivery Strategies for Facilitating Thrombolysis Treatment in Ischemic Stroke, Drug Delivery, 2021, 28(1), 357–371, DOI:10.1080/10717544.2021.1879315.
- K. Elumalai, S. Srinivasan and A. Shanmugam, Review of the Efficacy of Nanoparticle-Based Drug Delivery Systems for Cancer Treatment, Biomed. Technol., 2024, 5, 109–122, DOI:10.1016/J.BMT.2023.09.001.
- S. Z. Alshawwa, A. A. Kassem, R. M. Farid, S. K. Mostafa and G. S. Labib, Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence, Pharmaceutics, 2022, 14, 883, DOI:10.3390/pharmaceutics14040883.
- J. Gao, J. M. Karp, R. Langer and N. Joshi, The Future of Drug Delivery, Chem. Mater., 2023, 359–363, DOI:10.1021/acs.chemmater.2c03003.
- D. Raijada, K. Wac, E. Greisen, J. Rantanen and N. Genina, Integration of Personalized Drug Delivery Systems into Digital Health, Adv. Drug Delivery Rev., 2021, 176, 113857, DOI:10.1016/J.ADDR.2021.113857.
- O. Destiny Balogun, O. Ayo-Farai, O. Ogundairo, C. Paschal Maduka, C. Chinaemelum Okongwu, A. Olaide Babarinde and O. Tolulope Sodamade, The role of pharmacists in personalised medicine: a review of integrating pharmacogenomics into clinical practice, Int. Med. Sci. Res. J., 2024, 4(1), 19–36, DOI:10.51594/imsrj.v4i1.697.
- R. C. Wang and Z. Wang, Precision Medicine: Disease Subtyping and Tailored Treatment, Cancers, 2023, 15, 3837, DOI:10.3390/cancers15153837.
- K. A. Malsagova, T. V. Butkova, A. T. Kopylov, A. A. Izotov, N. V. Potoldykova, D. V. Enikeev, V. Grigoryan, A. Tarasov, A. A. Stepanov and A. L. Kaysheva, Pharmacogenetic Testing: A Tool for Personalized Drug Therapy Optimization, Pharmaceutics, 2020, 1–23, DOI:10.3390/pharmaceutics12121240.
- S. Vadapalli, H. Abdelhalim, S. Zeeshan and Z. Ahmed, Artificial Intelligence and Machine Learning Approaches Using Gene Expression and Variant Data for Personalized Medicine, Briefings Bioinf., 2022, 23, bbac191, DOI:10.1093/bib/bbac191.
- S. Rezayi, R. Niakan Kalhori and S. Saeedi, Effectiveness of Artificial Intelligence for Personalized Medicine in Neoplasms: A Systematic Review, BioMed Res. Int., 2022, 2022, 7842566, DOI:10.1155/2022/7842566.
- H. Liu, S. Chen, M. Liu, H. Nie and H. Lu, Comorbid Chronic Diseases Are Strongly Correlated with Disease Severity among COVID-19 Patients: A Systematic Review and Meta-Analysis, Aging Dis., 2020, 668–678, DOI:10.14336/AD.2020.0502.
- I. Djaharuddin, S. Munawwarah, A. Nurulita, M. Ilyas, N. A. Tabri and N. Lihawa, Comorbidities and Mortality in COVID-19 Patients, Gac. Sanit., 2021, 35, S530–S532, DOI:10.1016/J.GACETA.2021.10.085.
- C. D. Russell, N. I. Lone and J. K. Baillie, Comorbidities, Multimorbidity and COVID-19, Nat. Med., 2023, 334–343, DOI:10.1038/s41591-022-02156-9.
- A. Fanouriakis, N. Tziolos, G. Bertsias and D. T. Boumpas, Update in the Diagnosis and Management of Systemic Lupus Erythematosus, Ann. Rheum. Dis., 2021, 14–25, DOI:10.1136/annrheumdis-2020-218272.
- M. D′ascanio, M. Innammorato, L. Pasquariello, D. Pizzirusso, G. Guerrieri, S. Castelli, A. Pezzuto, C. De vitis, P. Anibaldi, A. Marcolongo, R. Mancini, A. Ricci and S. Sciacchitano, Age Is Not the Only Risk Factor in COVID-19: The Role of Comorbidities and of Long Staying in Residential Care Homes, BMC Geriatr., 2021, 21(1), 63, DOI:10.1186/s12877-021-02013-3.
- F. Mauvais-Jarvis, N. B. Merz, P. J. Barnes, R. D. Brinton, J.-J. Carrero, D. L. Demeo, G. J. De Vries, N. Epperson, R. Govindan, S. L. Klein, A. Lonardo, P. M. Maki, L. D. Mccullough, V. Regitz-Zagrosek, J. G. Regensteiner, J. B. Rubin, K. Sandberg and A. Suzuki, Sex and Gender: Modifiers of Health, Disease, and Medicine, The Lancet, 2020, 396, 565–582 Search PubMed.
- J. A. McCulloch, D. Davar, R. R. Rodrigues, J. H. Badger, J. R. Fang, A. M. Cole, A. K. Balaji, M. Vetizou, S. M. Prescott, M. R. Fernandes, R. G. F. Costa, W. Yuan, R. Salcedo, E. Bahadiroglu, S. Roy, R. N. DeBlasio, R. M. Morrison, J. M. Chauvin, Q. Ding, B. Zidi, A. Lowin, S. Chakka, W. Gao, O. Pagliano, S. J. Ernst, A. Rose, N. K. Newman, A. Morgun, H. M. Zarour, G. Trinchieri and A. K. Dzutsev, Intestinal Microbiota Signatures of Clinical Response and Immune-Related Adverse Events in Melanoma Patients Treated with Anti-PD-1, Nat. Med., 2022, 28(3), 545–556, DOI:10.1038/s41591-022-01698-2.
- R. M. Cooper-DeHoff, M. Niemi, L. B. Ramsey, J. A. Luzum, E. K. Tarkiainen, R. J. Straka, L. Gong, S. Tuteja, R. A. Wilke, M. Wadelius, E. A. Larson, D. M. Roden, T. E. Klein, S. W. Yee, R. M. Krauss, R. M. Turner, L. Palaniappan, A. Gaedigk, K. M. Giacomini, K. E. Caudle and D. Voora, The Clinical Pharmacogenetics Implementation Consortium Guideline for SLCO1B1, ABCG2, and CYP2C9 Genotypes and Statin-Associated Musculoskeletal Symptoms, Clin. Pharmacol. Ther., 2022, 111(5), 1007–1021, DOI:10.1002/cpt.2557.
- M. Garcia-Cortes, M. Robles-Diaz, C. Stephens, A. Ortega-Alonso, M. I. Lucena and R. J. Andrade, Drug Induced Liver Injury: An Update, Arch. Toxicol., 2020, 3381–3407, DOI:10.1007/s00204-020-02885-1.
- F. Wu, J. Fan, Y. He, A. Xiong, J. Yu, Y. Li, Y. Zhang, W. Zhao, F. Zhou, W. Li, J. Zhang, X. Zhang, M. Qiao, G. Gao, S. Chen, X. Chen, X. Li, L. Hou, C. Wu, C. Su, S. Ren, M. Odenthal, R. Buettner, N. Fang and C. Zhou, Single-Cell Profiling of Tumor Heterogeneity and the Microenvironment in Advanced Non-Small Cell Lung Cancer, Nat. Commun., 2021, 12(1), 2540, DOI:10.1038/s41467-021-22801-0.
- A. Zambelli, C. Tondini, G. Munkácsy, L. Santarpia and G. B. Orffy, Gene Expression Profiling in Early Breast Cancer-Patient Stratification Based on Molecular and Tumor Microenvironment Features, 2022. DOI:10.3390/biomedicines.
- L. K. Chan, Y. M. Tsui, D. W. H. Ho and I. O. L. Ng, Cellular Heterogeneity and Plasticity in Liver Cancer, Semin. Cancer Biol., 2022, 82, 134–149, DOI:10.1016/J.SEMCANCER.2021.02.015.
- A. Mitsala, C. Tsalikidis, M. Pitiakoudis, C. Simopoulos and A. K. Tsaroucha, Artificial Intelligence in Colorectal Cancer Screening, Diagnosis and Treatment. A New Era, Curr. Oncol., 2021, 1581–1607, DOI:10.3390/curroncol28030149.
- K. Freeman, J. Geppert, C. Stinton, D. Todkill, S. Johnson, A. Clarke and S. Taylor-Phillips, Use of Artificial Intelligence for Image Analysis in Breast Cancer Screening Programmes: Systematic Review of Test Accuracy, BMJ, 2021, 374, n1872, DOI:10.1136/bmj.n1872.
- P. W. Franks, E. Melén, M. Friedman, J. Sundström, I. Kockum, L. Klareskog, C. Almqvist, S. E. Bergen, K. Czene, S. Hägg, P. Hall, K. Johnell, A. Malarstig, A. Catrina, H. Hagström, M. Benson, J. Gustav Smith, M. F. Gomez, M. Orho-Melander, B. Jacobsson, J. Halfvarson, D. Repsilber, M. Oresic, C. Jern, B. Melin, C. Ohlsson, T. Fall, L. Rönnblom, M. Wadelius, G. Nordmark, Å. Johansson, R. Rosenquist and P. F. Sullivan, Technological Readiness and Implementation of Genomic-Driven Precision Medicine for Complex Diseases, J. Intern. Med., 2021, 602–620, DOI:10.1111/joim.13330.
- D. Reska, M. Czajkowski, K. Jurczuk, C. Boldak, W. Kwedlo, W. Bauer, J. Koszelew and M. Kretowski, Integration of Solutions and Services for Multi-Omics Data Analysis towards Personalized Medicine, Biocybern. Biomed. Eng., 2021, 41(4), 1646–1663, DOI:10.1016/j.bbe.2021.10.005.
- E. Faulkner, A. P. Holtorf, S. Walton, C. Y. Liu, H. Lin, E. Biltaj, D. Brixner, C. Barr, J. Oberg, G. Shandhu, U. Siebert, S. R. Snyder, S. Tiwana, J. Watkins, M. J. Ijzerman and K. Payne, Being Precise About Precision Medicine: What Should Value Frameworks Incorporate to Address Precision Medicine? A Report of the Personalized Precision Medicine Special Interest Group, Value Health, 2020, 23(5), 529–539, DOI:10.1016/J.JVAL.2019.11.010.
- M. Bizzarri, V. Fedeli, N. Monti, A. Cucina, M. Jalouli, S. H. Alwasel and A. H. Harrath, Personalization of Medical Treatments in Oncology: Time for Rethinking the Disease Concept to Improve Individual Outcomes, EPMA J., 2021, 545–558, DOI:10.1007/s13167-021-00254-1.
- Y. H. Li, Y. L. Li, M. Y. Wei and G. Y. Li, Innovation and Challenges of Artificial Intelligence Technology in Personalized Healthcare, Sci. Rep., 2024, 14(1), 18994, DOI:10.1038/s41598-024-70073-7.
- A. Blasiak, J. Khong and T. Kee, CURATE.AI: Optimizing Personalized Medicine with Artificial Intelligence, SLAS Technol., 2020, 95–105, DOI:10.1177/2472630319890316.
- S. Zeb, N. Fnu, N. Abbasi and M. Fahad, AI in Healthcare: Revolutionizing Diagnosis and Therapy, Int. J. Multidiscip. Sc. Arts, 2024, 3(3), 118–128, DOI:10.47709/ijmdsa.v3i3.4546.
- D. Q. Wang, B. Qiu, H. Q. He, S. H. Yin, K. Q. Peng, N. Hu, J. Y. Guo, Q. W. Li, N. B. Chen, C. Chu, F. J. Liu, C. M. Xie and H. Liu, Tumor Response Evaluation by Combined Modalities of Chest Magnetic Resonance Imaging and Computed Tomography in Locally Advanced Non-Small Cell Lung Cancer after Concurrent Chemoradiotherapy, Radiother. Oncol., 2022, 168, 211–220, DOI:10.1016/J.RADONC.2022.01.042.
- I. S. Chua, M. Gaziel-Yablowitz, Z. T. Korach, K. L. Kehl, N. A. Levitan, Y. E. Arriaga, G. P. Jackson, D. W. Bates and M. Hassett, Artificial Intelligence in Oncology: Path to Implementation, Cancer Med., 2021, 4138–4149, DOI:10.1002/cam4.3935.
- M. Torrente, P. A. Sousa, R. Hernández, M. Blanco, V. Calvo, A. Collazo, G. R. Guerreiro, B. Núñez, J. Pimentao, J. C. Sánchez, M. Campos, L. Costabello, V. Novacek, E. Menasalvas, M. E. Vidal and M. Provencio, An Artificial Intelligence-Based Tool for Data Analysis and Prognosis in Cancer Patients: Results from the Clarify Study, Cancers, 2022, 14(16), 4041, DOI:10.3390/cancers14164041.
- Z. Ahmed, K. Mohamed, S. Zeeshan and X. Q. Dong, Artificial Intelligence with Multi-Functional Machine Learning Platform Development for Better Healthcare and Precision Medicine, Database, 2020, 2020, baaa010, DOI:10.1093/database/baaa010.
- S. K. Rath, A. K. Dash, N. Sarkar and M. Panchpuri, A Glimpse for the Subsistence from Pandemic SARS-CoV-2 Infection, Bioorg. Chem., 2025, 154, 107977, DOI:10.1016/j.bioorg.2024.107977.
- F. Mohsen, B. Al-Saadi, N. Abdi, S. Khan and Z. Shah, Artificial Intelligence-Based Methods for Precision Cardiovascular Medicine, J. Pers. Med., 2023, 13, 1268, DOI:10.3390/jpm13081268.
- G. F. Stark, G. R. Hart, B. J. Nartowt and J. Deng, Predicting Breast Cancer Risk Using Personal Health Data and Machine Learning Models, PLoS One, 2019, 14(12), e0226765, DOI:10.1371/journal.pone.0226765.
- C. Carini and A. A. Seyhan, Tribulations and Future Opportunities for Artificial Intelligence in Precision Medicine, J. Transl. Med., 2024, 22, 411, DOI:10.1186/s12967-024-05067-0.
- P. Wang, Q. Y. Leong, N. Y. Lau, W. Y. Ng, S. P. Kwek, L. Tan, S. W. Song, K. You, L. M. Chong, I. Zhuang, Y. H. Ong, N. Foo, X. Tadeo, K. S. Kumar, S. Vijayakumar, Y. Sapanel, M. N. Raczkowska, A. Remus, A. Blasiak and D. Ho, N-of-1 Medicine, Singapore Med. J., 2024, 167–175, DOI:10.4103/singaporemedj.SMJ-2023-243.
- T. G. Soldatos, S. Kaduthanam and D. B. Jackson, Precision Oncology—The Quest for Evidence, J. Pers. Med., 2019, 9, 43, DOI:10.3390/jpm9030043.
- P. Schwaller, T. Laino, T. Gaudin, P. Bolgar, C. A. Hunter, C. Bekas and A. A. Lee, Molecular Transformer: A Model for Uncertainty-Calibrated Chemical Reaction Prediction, ACS Cent. Sci., 2019, 5(9), 1572–1583, DOI:10.1021/acscentsci.9b00576.
- K. M. Jablonka, D. Ongari, S. M. Moosavi and B. Smit, Big-Data Science in Porous Materials: Materials Genomics and Machine Learning, Chem. Rev., 2020, 8066–8129, DOI:10.1021/acs.chemrev.0c00004.
- A. I. Leonov, A. J. S. Hammer, S. Lach, S. H. M. Mehr, D. Caramelli, D. Angelone, A. Khan, S. O'Sullivan, M. Craven, L. Wilbraham and L. Cronin, An Integrated Self-Optimizing Programmable Chemical Synthesis and Reaction Engine, Nat. Commun., 2024, 15(1), 1240, DOI:10.1038/s41467-024-45444-3.
- T. Hornick, C. Mao, A. Koynov, P. Yawman, P. Thool, K. Salish, M. Giles, K. Nagapudi and S. Zhang, In Silico Formulation Optimization and Particle Engineering of Pharmaceutical Products Using a Generative Artificial Intelligence Structure Synthesis Method, Nat. Commun., 2024, 15(1), 9622, DOI:10.1038/s41467-024-54011-9.
- A. Zhavoronkov, Y. A. Ivanenkov, A. Aliper, M. S. Veselov, V. A. Aladinskiy, A. V. Aladinskaya, V. A. Terentiev, D. A. Polykovskiy, M. D. Kuznetsov, A. Asadulaev, Y. Volkov, A. Zholus, R. R. Shayakhmetov, A. Zhebrak, L. I. Minaeva, B. A. Zagribelnyy, L. H. Lee, R. Soll, D. Madge, L. Xing, T. Guo and A. Aspuru-Guzik, Deep Learning Enables Rapid Identification of Potent DDR1 Kinase Inhibitors, Nat. Biotechnol., 2019, 37(9), 1038–1040, DOI:10.1038/s41587-019-0224-x.
- J. Mökander and L. Floridi, Operationalising AI Governance through Ethics-Based Auditing: An Industry Case Study, AI Ethics, 2023, 3(2), 451–468, DOI:10.1007/s43681-022-00171-7.
- W. Xu, Current Status of Computational Approaches for Small Molecule Drug Discovery, J. Med. Chem., 2024, 67, 18633–18636, DOI:10.1021/acs.jmedchem.4c02462.
- A. Sharma, T. Virmani, V. Pathak, A. Sharma, K. Pathak, G. Kumar and D. Pathak, Artificial Intelligence-Based Data-Driven Strategy to Accelerate Research, Development, and Clinical Trials of COVID Vaccine, BioMed Res. Int., 2022, 2022, 7205241, DOI:10.1155/2022/7205241.
- A. Sharma, T. Virmani, V. Pathak, A. Sharma, K. Pathak, G. Kumar and D. Pathak, Artificial Intelligence-Based Data-Driven Strategy to Accelerate Research, Development, and Clinical Trials of COVID Vaccine, BioMed Res. Int., 2022, 2022, 7205241, DOI:10.1155/2022/7205241.
- Y. Roggo, M. Jelsch, P. Heger, S. Ensslin and M. Krumme, Deep Learning for Continuous Manufacturing of Pharmaceutical Solid Dosage Form, Eur. J. Pharm. Biopharm., 2020, 153, 95–105, DOI:10.1016/J.EJPB.2020.06.002.
- L. K. Vora, A. D. Gholap, K. Jetha, R. R. S. Thakur, H. K. Solanki and V. P. Chavda, Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design, Pharmaceutics, 2023, 15, 1916, DOI:10.3390/pharmaceutics15071916.
- T. Purcărea, V. Ioan-Franc, Ş.A Ionescu, I. M. Purcărea, V. L. Purcărea, I. Purcărea, M. C. Mateescu-Soare, O. E. Platon and A. O. Orzan, Major Shifts in Sustainable Consumer Behavior in Romania and Retailers’ Priorities in Agilely Adapting to It, Sustainability, 2022, 14(3), 1627, DOI:10.3390/su14031627.
- A. G. Novartis, Novartis in Society – Integrated Report 2023; 2023.
- N. H. Aysa, S. W. Aziz and R. Al-Assaly, Novel in silico nano-drug design and delivery systems employing the density functional theory: a review, Rev. Clin. Pharmacol. Pharmacokinet. Int. Ed., 2024, 38(Sup2), 193–196, DOI:10.61873/FGXZ4557.
- L. K. Vora, A. D. Gholap, K. Jetha, R. R. S. Thakur, H. K. Solanki and V. P. Chavda, Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design, Pharmaceutics, 2023, 15, 1916, DOI:10.3390/pharmaceutics15071916.
- Q. V. Pham, D. C. Nguyen, T. Huynh-The, W. J. Hwang and P. N. Pathirana, Artificial Intelligence (AI) and Big Data for Coronavirus (COVID-19) Pandemic: A Survey on the State-of-the-Arts, IEEE Access, 2020, 130820–130839, DOI:10.1109/ACCESS.2020.3009328.
- S. E. Whang, Y. Roh, H. Song and J.-G. Lee, Data Collection and Quality Challenges in Deep Learning: A Data-Centric AI Perspective. 2021 Search PubMed.
- G. Menghani, Efficient Deep Learning: A Survey on Making Deep Learning Models Smaller, Faster, and Better. 2021. DOI:10.1145/3578938.
- S. A. Bhat and N. F. Huang, Big Data and AI Revolution in Precision Agriculture: Survey and Challenges, IEEE Access, 2021, 9, 110209–110222, DOI:10.1109/ACCESS.2021.3102227.
- S. Li, Z. Ma, Z. Cao, L. Pan and Y. Shi, Advanced Wearable Microfluidic Sensors for Healthcare Monitoring, Small, 2020, 16, 1903822, DOI:10.1002/smll.201903822.
- S. Beg, M. Handa, R. Shukla, M. Rahman, W. H. Almalki, O. Afzal and A. S. A. Altamimi, Wearable Smart Devices in Cancer Diagnosis and Remote Clinical Trial Monitoring: Transforming the Healthcare Applications, Drug Discovery Today, 2022, 27(10), 103314, DOI:10.1016/J.DRUDIS.2022.06.014.
- F. Khoshmanesh, P. Thurgood, E. Pirogova, S. Nahavandi and S. Baratchi, Wearable Sensors: At the Frontier of Personalised Health Monitoring, Smart Prosthetics and Assistive Technologies, Biosens. Bioelectron., 2021, 176, 112946, DOI:10.1016/J.BIOS.2020.112946.
- S. Bhoi, M. L. Lee, W. Hsu, H. S. A. Fang and N. C. Tan, Personalizing Medication Recommendation with a Graph-Based Approach, ACM Trans. Inf. Syst., 2022, 40(3), 55, DOI:10.1145/3488668.
- E. Wallace, M. Gardner and S. Singh, Interpreting Predictions of NLP Models, in EMNLP 2020 – Conference on Empirical Methods in Natural Language Processing, Tutorial Abstracts; Association for Computational Linguistics (ACL), 2020, pp. 20–23. DOI:10.18653/v1/P17.
- G. K. Ramachandran, K. Lybarger, Y. Liu, D. Mahajan, J. J. Liang, C. H. Tsou, M. Yetisgen and Ö. Uzuner, Extracting Medication Changes in Clinical Narratives Using Pre-Trained Language Models, J. Biomed. Inf., 2023, 139, 104302, DOI:10.1016/J.JBI.2023.104302.
- O. Estradé, V. Vozmediano, N. Carral, A. Isla, M. González, R. Poole and E. Suarez, Key Factors in Effective Patient-Tailored Dosing of Fluoroquinolones in Urological Infections: Interindividual Pharmacokinetic and Pharmacodynamic Variability, Antibiotics, 2022, 11, 641, DOI:10.3390/antibiotics11050641.
- A. O'Jeanson, R. Larcher, C. Le Souder, N. Djebli and S. Khier, Population Pharmacokinetics and Pharmacodynamics of Meropenem in Critically Ill Patients: How to Achieve Best Dosage Regimen According to the Clinical Situation, Eur. J. Drug Metab. Pharmacokinet., 2021, 46(5), 695–705, DOI:10.1007/s13318-021-00709-w.
- E. Chatelut, J. J. M. A. Hendrikx, J. Martin, J. Ciccolini and D. J. A. R. Moes, Unraveling the Complexity of Therapeutic Drug Monitoring for Monoclonal Antibody Therapies to Individualize Dose in Oncology, Pharmacol. Res. Perspect., 2021, 9, e00757, DOI:10.1002/prp2.757.
- I. K. Minichmayr, E. Dreesen, M. Centanni, Z. Wang, Y. Hoffert, L. E. Friberg and S. G. Wicha, Model-Informed Precision Dosing: State of the Art and Future Perspectives, Adv. Drug Delivery Rev., 2024, 215, 115421, DOI:10.1016/J.ADDR.2024.115421.
- K. K. Sen, D. Sinha, A. K. Nayak and S. O. Sen, Contribution of Biopharmaceutics and Pharmacokinetics to Improve Drug Therapy, Physico-Chemical Aspects of Dosage Forms and Biopharmaceutics, 2024, pp. 231–249. DOI:10.1016/B978-0-323-91818-3.00023-2.
- G. Fu, W. Sun, Z. Tan, B. Liang and Y. Cai, An Insight into Pharmacokinetics and Dose Optimization of Antimicrobials Agents in Elderly Patients, Front. Pharmacol., 2024, 15, 1396994, DOI:10.3389/fphar.2024.1396994.
- L. Meijer, G. Hery-Arnaud, C. Leven, E. Nowak, S. Hillion, Y. Renaudineau, I. Durieu, R. Chiron, A. Prevotat, I. Fajac, D. Hubert, M. Murris-Espin, S. Huge, I. Danner-Boucher, B. Ravoninjatovo, S. Leroy, J. Macey, T. Urban, G. Rault, D. Mottier and R. Le Berre, Safety and Pharmacokinetics of Roscovitine (Seliciclib) in Cystic Fibrosis Patients Chronically Infected with Pseudomonas Aeruginosa, a Randomized, Placebo-Controlled Study, J. Cystic Fibrosis, 2022, 21(3), 529–536, DOI:10.1016/J.JCF.2021.10.013.
- H. Y. Yow, K. Govindaraju, A. H. Lim and N. Abdul Rahim, Optimizing, Antimicrobial Therapy by Integrating Multi-Omics With Pharmacokinetic/Pharmacodynamic Models and Precision Dosing, Front. Pharmacol., 2022, 13, 915355, DOI:10.3389/fphar.2022.915355.
- P. Manickam, S. A. Mariappan, S. M. Murugesan, S. Hansda, A. Kaushik, R. Shinde and S. P. Thipperudraswamy, Artificial Intelligence (AI) and Internet of Medical Things (IoMT) Assisted Biomedical Systems for Intelligent Healthcare, Biosensors, 2022, 12, 562, DOI:10.3390/bios12080562.
- S. Kaur, J. Singla, L. Nkenyereye, S. Jha, D. Prashar, G. P. Joshi, S. El-Sappagh, M. S. Islam and S. M. Riazul Islam, Medical, Diagnostic Systems Using Artificial Intelligence (AI) Algorithms: Principles and Perspectives, IEEE Access, 2020, 8, 228049–228069, DOI:10.1109/ACCESS.2020.3042273.
- M. Nasr, M. M. Islam, S. Shehata, F. Karray and Y. Quintana, Smart Healthcare in the Age of AI: Recent Advances, Challenges, and Future Prospects, IEEE Access, 2021, 9, 145248–145270, DOI:10.1109/ACCESS.2021.3118960.
- Y. H. Bae and K. Park, Advanced Drug Delivery 2020 and beyond: Perspectives on the Future, Adv. Drug Delivery Rev., 2020, 4–16, DOI:10.1016/j.addr.2020.06.018.
- H. Zhang, T. Fan, W. Chen, Y. Li and B. Wang, Recent Advances of Two-Dimensional Materials in Smart Drug Delivery Nano-Systems, Bioact. Mater., 2020, 5(4), 1071–1086, DOI:10.1016/J.BIOACTMAT.2020.06.012.
- T. Sahu, Y. K. Ratre, S. Chauhan, L. V. K. S. Bhaskar, M. P. Nair and H. K. Verma, Nanotechnology Based Drug Delivery System: Current Strategies and Emerging Therapeutic Potential for Medical Science, J. Drug Delivery Sci. Technol., 2021, 63, 102487, DOI:10.1016/J.JDDST.2021.102487.
- S. Ebrahimian, M. K. Kalra, S. Agarwal, B. C. Bizzo, M. Elkholy, C. Wald, B. Allen and K. J. Dreyer, FDA-Regulated AI Algorithms: Trends, Strengths, and Gaps of Validation Studies, Acad. Radiol., 2022, 29(4), 559–566, DOI:10.1016/J.ACRA.2021.09.002.
- D. B. Larson, H. Harvey, D. L. Rubin, N. Irani, J. R. Tse and C. P. Langlotz, Regulatory Frameworks for Development and Evaluation of Artificial Intelligence–Based Diagnostic Imaging Algorithms: Summary and Recommendations, J. Am. Coll. Radiol., 2021, 18(3), 413–424, DOI:10.1016/j.jacr.2020.09.060.
- S. Askin, D. Burkhalter, G. Calado and S. El Dakrouni, Artificial, Intelligence Applied to Clinical Trials: Opportunities and Challenges, Health Technol., 2023, 203–213, DOI:10.1007/s12553-023-00738-2.
- S. H. Park, J. Choi and J. S. Byeon, Key Principles of Clinical Validation, Device Approval, and Insurance Coverage Decisions of Artificial Intelligence, Korean J. Radiol., 2021, 442–453, DOI:10.3348/kjr.2021.0048.
- J. Y. Cheng, J. T. Abel, U. G. J. Balis, D. S. McClintock and L. Pantanowitz, Challenges in the Development, Deployment, and Regulation of Artificial Intelligence in Anatomic Pathology, Am. J. Pathol., 2021, 191(10), 1684–1692, DOI:10.1016/J.AJPATH.2020.10.018.
- C. Mennella, U. Maniscalco, G. De Pietro and M. Esposito, Ethical and Regulatory Challenges of AI Technologies in Healthcare: A Narrative Review, Heliyon, 2024, 10, e26297, DOI:10.1016/j.heliyon.2024.e26297.
- T. Vandemeulebroucke, The Ethics of Artificial Intelligence Systems in Healthcare and Medicine: From a Local to a Global Perspective, and Back, Pflugers Arch., 2025, 477, 591–601, DOI:10.1007/s00424-024-02984-3.
- C. Fu and Q. Chen, The Future of Pharmaceuticals: Artificial Intelligence in Drug Discovery and Development, J. Pharm. Anal., 2025, 101248, DOI:10.1016/J.JPHA.2025.101248.
- A. D. Gholap and A. Omri, Advances in Artificial Intelligence-Envisioned Technologies for Protein and Nucleic Acid Research, Drug Discovery Today, 2025, 30(5), 104362, DOI:10.1016/J.DRUDIS.2025.104362.
- A. Adegbesan, A. Akingbola, O. Ojo, O. U. Jessica, U. H. Alao, U. Shagaya, O. Adewole and O. Abdullahi, Ethical Challenges in the Integration of Artificial Intelligence in Palliative Care, J. Med. Surg. Public Health, 2024, 4, 100158, DOI:10.1016/J.GLMEDI.2024.100158.
- S. M. R. Swapno, S. M. N. Nobel, P. K. Meena, V. P. Meena, J. Bahadur and A. Appaji, Accelerated and Precise Skin Cancer Detection through an Enhanced Machine Learning Pipeline for Improved Diagnostic Accuracy, Results Eng., 2025, 25, 104168, DOI:10.1016/J.RINENG.2025.104168.
- L. Pantanowitz, M. Hanna, J. Pantanowitz, J. Lennerz, W. H. Henricks, P. Shen, B. Quinn, S. Bennet and H. H. Rashidi, Regulatory Aspects of Artificial Intelligence and Machine Learning, Mod. Pathol., 2024, 37(12), 100609, DOI:10.1016/J.MODPAT.2024.100609.
- P. S. Park, S. Goldstein, A. O'Gara, M. Chen and D. Hendrycks, AI Deception: A Survey of Examples, Risks, and Potential Solutions, Patterns, 2024, 5(5), 100988, DOI:10.1016/J.PATTER.2024.100988.
- H. Mondal and S. Mondal, Ethical and Social Issues Related to AI in Healthcare, Methods Microbiol., 2024, 55, 247–281, DOI:10.1016/BS.MIM.2024.05.009.
- Guide to the General Data Protection Regulation (GDPR).
- M. Kattnig, A. Angerschmid, T. Reichel and R. Kern, Assessing Trustworthy AI: Technical and Legal Perspectives of Fairness in AI, Comput. Law Secur. Rev., 2024, 55, 106053, DOI:10.1016/J.CLSR.2024.106053.
- N. H. Conradie and S. K. Nagel, Digital Sovereignty and Smart Wearables: Three Moral Calculi for the Distribution of Legitimate Control over the Digital, J. Responsible Technol., 2022, 12, 100053, DOI:10.1016/J.JRT.2022.100053.
- O. J. Gstrein and A. Beaulieu, How to Protect Privacy in a Datafied Society? A Presentation of Multiple Legal and Conceptual Approaches, Philos. Technol., 2022, 35(1), 3, DOI:10.1007/s13347-022-00497-4.
- S. Yadav, A. Singh, R. Singhal and J. P. Yadav, Revolutionizing Drug Discovery: The Impact of Artificial Intelligence on Advancements in Pharmacology and the Pharmaceutical Industry, Intell. Pharm., 2024, 2(3), 367–380, DOI:10.1016/J.IPHA.2024.02.009.
- A. Mulahuwaish, B. Qolomany, K. Gyorick, J. B. Abdo, M. Aledhari, J. Qadir, K. Carley and A. Al-Fuqaha, A Survey of Social Cybersecurity: Techniques for Attack Detection, Evaluations, Challenges, and Future Prospects, Comput. Hum. Behav. Rep., 2025, 18, 100668, DOI:10.1016/J.CHBR.2025.100668.
- A. Nayarisseri, R. Khandelwal, P. Tanwar, M. Madhavi, D. Sharma, G. Thakur, A. Speck-Planche and S. K. Singh, Artificial Intelligence, Big Data and Machine Learning Approaches in Precision Medicine & Drug Discovery, Curr. Drug Targets, 2021, 22(6), 631–655, DOI:10.2174/1389450122999210104205732.
- J. Deng, Z. Yang, I. Ojima, D. Samaras and F. Wang, Artificial Intelligence in Drug Discovery: Applications and Techniques. 2021 Search PubMed.
- S. Dara, S. Dhamercherla, S. S. Jadav, C. M. Babu and M. J. Ahsan, Machine Learning in Drug Discovery: A Review, Artif. Intell. Rev., 2022, 55(3), 1947–1999, DOI:10.1007/s10462-021-10058-4.
- J. Tyler, S. W. Choi and M. Tewari, Real-Time Personalized Medicine through Wearable Sensors and Dynamic Predictive Modeling: A New Paradigm for Clinical Medicine, Curr. Opin. Syst. Biol., 2020, 20, 17–25, DOI:10.1016/J.COISB.2020.07.001.
- D. A. Domingo-Lopez, G. H. J. Lattanzi, L. Schreiber, E. J. Wallace, R. Wylie, J. O'Sullivan, E. B. Dolan and G. P. Duffy, Medical Devices, Smart Drug Delivery, Wearables and Technology for the Treatment of Diabetes Mellitus, Adv. Drug Delivery Rev., 2022, 185, 114280, DOI:10.1016/J.ADDR.2022.114280.
- J. Manikkath and J. A. Subramony, Toward Closed-Loop Drug Delivery: Integrating Wearable Technologies with Transdermal Drug Delivery Systems, Adv. Drug Delivery Rev., 2021, 179, 113997, DOI:10.1016/J.ADDR.2021.113997.
- A. Kar, N. Ahamad, M. Dewani, L. Awasthi, R. Patil and R. Banerjee, Wearable and Implantable Devices for Drug Delivery: Applications and Challenges, Biomaterials, 2022, 283, 121435, DOI:10.1016/j.biomaterials.2022.121435.
- D. W. Kim, E. Zavala and J. K. Kim, Wearable Technology and Systems Modeling for Personalized Chronotherapy, Curr. Opin. Syst. Biol., 2020, 21, 9–15, DOI:10.1016/J.COISB.2020.07.007.
- B. Teferi, M. Omar, T. Jeyakumar, R. Charow, C. Gillan, J. Jardine, J. Mattson, A. Dhalla, S. A. Kocak, M. Salhia, B. Davies, M. Clare, S. Younus and D. Wiljer, Accelerating the Appropriate Adoption of Artificial Intelligence in Health Care: Prioritizing IDEA to Champion a Collaborative Educational Approach in a Stressed System, Educ. Sci., 2024, 14(1), 39, DOI:10.3390/educsci14010039.
- S. Y. Liaw, J. Z. Tan, S. Lim, W. Zhou, J. Yap, R. Ratan, S. L. Ooi, S. J. Wong, B. Seah and W. L. Chua, Artificial Intelligence in Virtual Reality Simulation for Interprofessional Communication Training: Mixed Method Study, Nurse Educ. Today, 2023, 122, 105718, DOI:10.1016/J.NEDT.2023.105718.
- N. Jeyaraman, M. Jeyaraman, S. Yadav, S. Ramasubramanian and S. Balaji, Revolutionizing Healthcare: The Emerging Role of Quantum Computing in Enhancing Medical Technology and Treatment, Cureus, 2024, 16, e67486, DOI:10.7759/cureus.67486.
- G. Kumar, S. Yadav, A. Mukherjee, V. Hassija and M. Guizani, Recent Advances in Quantum Computing for Drug Discovery and Development, IEEE Access, 2024, 12, 64491–64509, DOI:10.1109/ACCESS.2024.3376408.
- M.-L. How and S.-M. Cheah, Business Renaissance: Opportunities and Challenges at the Dawn of the Quantum Computing Era, Businesses, 2023, 3(4), 585–605, DOI:10.3390/businesses3040036.
- A. Pyrkov, A. Aliper, D. Bezrukov, Y. C. Lin, D. Polykovskiy, P. Kamya, F. Ren and A. Zhavoronkov, Quantum Computing for Near-Term Applications in Generative Chemistry and Drug Discovery, Drug Discovery Today, 2023, 28(8), 103675, DOI:10.1016/J.DRUDIS.2023.103675.
- K. Batra, K. M. Zorn, D. H. Foil, E. Minerali, V. O. Gawriljuk, T. R. Lane and S. Ekins, Quantum Machine Learning Algorithms for Drug Discovery Applications, J. Chem. Inf. Model., 2021, 2641–2647, DOI:10.1021/acs.jcim.1c00166.
- H. Doga, A. Bose, M. E. Sahin, J. Bettencourt-Silva, A. Pham, E. Kim, A. Andress, S. Saxena, L. Parida, J. L. Robertus, H. Kawaguchi, R. Soliman and D. Blankenberg, How Can Quantum Computing Be Applied in Clinical Trial Design and Optimization?, Trends Pharmacol. Sci., 2024, 45, 880–891, DOI:10.1016/j.tips.2024.08.005.
| This journal is © The Royal Society of Chemistry 2025 |