 Open Access Article
Open Access ArticleSolid lipid nanoparticles in cervical cancer: a comprehensive review of a decade of progress and prospects
Pooja
Tiwary†
 a,
Krishil
Oswal†
a,
Krishil
Oswal†
 a,
Ryan
Varghese
a,
Ryan
Varghese
 *b,
Ravi Vamsi
Peri
b and
Pardeep
Gupta
*b
*b,
Ravi Vamsi
Peri
b and
Pardeep
Gupta
*b
aDepartment of Pharmacy Practice, Poona College of Pharmacy, Bharati Vidyapeeth (Deemed to be) University, Pune, 411038, India. E-mail: poojatiwary455@gmail.com; krishiloswal@gmail.com
bDepartment of Pharmaceutical Sciences, Philadelphia College of Pharmacy, Saint Joseph's University, Philadelphia, PA 19104, USA. E-mail: ryanvarghese14@gmail.com; peri.ravivamsi@gmail.com; pgupta@sju.edu
First published on 25th June 2025
Abstract
Background: Cervical cancer is the second most commonly diagnosed cancer worldwide and the third leading cause of death among women, with approximately 604![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 new cases being reported in 2020. Conventional treatment methods, such as chemotherapy, radiation therapy, surgery, and hormonal therapy, often face significant challenges, including systemic toxicity and reduced efficacy, particularly in the advanced stages of the disease. The treatment of cervical cancer is further complicated by tumor heterogeneity, resistance mechanisms to chemotherapeutic drugs, and the persistent presence of HPV. However, in recent years, nanotechnological interventions, particularly solid lipid nanoparticles (SLNs), have gained increasing attention owing to their robust potential to effectively deliver chemotherapeutic agents while minimizing systemic toxicity. SLNs present a compelling solution for reducing side effects, enhancing drug solubility, improving stability and bioavailability, and overcoming the limitations and resistance associated with conventional treatment strategies. Methods: To provide the context and evidence, relevant publications were searched on Google Scholar, PubMed, ScienceDirect, Dimensions AI, and EBSCO host, using specific keywords such as “cervical cancer”, “drug loading”, “encapsulation efficiency”, “HPV”, “sustained drug release”, and “solid lipid nanoparticles (SLNs)”. We did not impose any restrictions on the publication date during the selection of papers. However, it is imperative to highlight that the initial reports containing specified keywords began publication in 2013. Conclusion: SLNs represent a promising frontier in drug delivery, particularly within cervical cancer therapeutics, because of their ability to facilitate the targeted delivery of chemotherapeutic agents and genetic materials. The potential of SLNs to encapsulate and protect vital therapeutic compounds presents significant opportunities for developing innovative treatment strategies including DNA and peptide vaccines. However, the lack of approved SLN-encapsulated vaccines for cervical cancer underscores the need for rigorous in vivo research and clinical trials to validate their safety and efficacy. Future studies should not only optimize SLNs for various agents but also explore diverse combination therapies to enhance therapeutic outcomes.
127 new cases being reported in 2020. Conventional treatment methods, such as chemotherapy, radiation therapy, surgery, and hormonal therapy, often face significant challenges, including systemic toxicity and reduced efficacy, particularly in the advanced stages of the disease. The treatment of cervical cancer is further complicated by tumor heterogeneity, resistance mechanisms to chemotherapeutic drugs, and the persistent presence of HPV. However, in recent years, nanotechnological interventions, particularly solid lipid nanoparticles (SLNs), have gained increasing attention owing to their robust potential to effectively deliver chemotherapeutic agents while minimizing systemic toxicity. SLNs present a compelling solution for reducing side effects, enhancing drug solubility, improving stability and bioavailability, and overcoming the limitations and resistance associated with conventional treatment strategies. Methods: To provide the context and evidence, relevant publications were searched on Google Scholar, PubMed, ScienceDirect, Dimensions AI, and EBSCO host, using specific keywords such as “cervical cancer”, “drug loading”, “encapsulation efficiency”, “HPV”, “sustained drug release”, and “solid lipid nanoparticles (SLNs)”. We did not impose any restrictions on the publication date during the selection of papers. However, it is imperative to highlight that the initial reports containing specified keywords began publication in 2013. Conclusion: SLNs represent a promising frontier in drug delivery, particularly within cervical cancer therapeutics, because of their ability to facilitate the targeted delivery of chemotherapeutic agents and genetic materials. The potential of SLNs to encapsulate and protect vital therapeutic compounds presents significant opportunities for developing innovative treatment strategies including DNA and peptide vaccines. However, the lack of approved SLN-encapsulated vaccines for cervical cancer underscores the need for rigorous in vivo research and clinical trials to validate their safety and efficacy. Future studies should not only optimize SLNs for various agents but also explore diverse combination therapies to enhance therapeutic outcomes.
1. Introduction
Cervical cancer (CC) develops from a malignant epithelial tumor that leads to the proliferation of cells in the cervix. The predominant cause is persistent infection of Human papillomavirus (HPV), particularly HPV 16 and 18.1 Moreover, HIV-infected females have a 6-fold increased risk of developing CC compared to the general population.2 Emerging evidence suggests that HIV and HPV co-infection significantly contributes to cervical carcinogenesis through multifactorial viral interactions, encompassing immunosuppression, chronic inflammation, and the induction of epithelial–mesenchymal transition (EMT)3 (Table 1). The disease commonly presents as either less common adenocarcinoma (20–30%) or more common squamous cell carcinoma (70–80%).4,5 Additionally, factors such as multiple sexual partners,6,7 consistent use of oral contraceptives,6 early initiation of sexual activity,7 and a higher number of vaginal deliveries8,9 are also associated with the development of CC. The risk factors associated with the development of cervical cancer are illustrated in Fig. 1.10 Prognostic factors, including depth of cervical stromal invasion, tumor size, lymph node involvement, histological features, and International Federation of Gynecology and Obstetrics (FIGO) cancer stage, influence the prognosis of CC.11–13 Higher FIGO stage, increased node involvement, larger tumors, and deeper invasion are associated with poorer prognosis, leading to worsened overall survival (OS) and disease-free survival (DFS).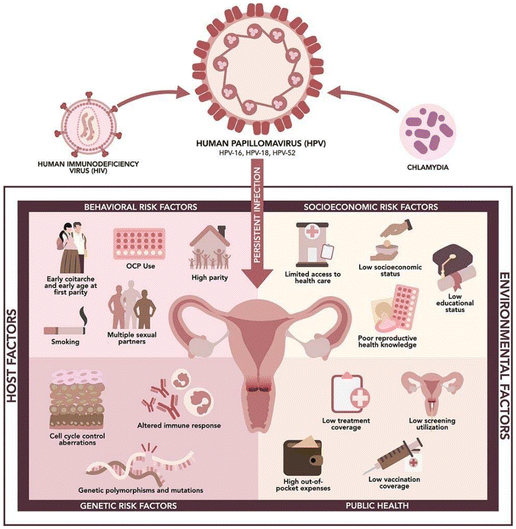 | ||
| Fig. 1 Risk factors associated with CC. Reproduced from Lintao et al.10 licensed under the terms of the Creative Commons Attribution License (CC BY). | ||
| Aspect | Description | Impact on disease progression |
|---|---|---|
| Immune system dysfunction | HIV impairs the immune system primarily by depleting CD4+ T cells, leading to apoptosis and reduced functionality. This suppression extends to specific T lymphocytes against HPV oncoproteins E6 and E7, crucial for controlling tumor progression in HPV-infected cervical cells | Increased susceptibility to high-grade squamous intraepithelial lesions (HSILs) due to reduced immune surveillance and inability to control HPV infection, thus facilitating progression to CC |
| Dendritic cell (DC) dynamics | HIV infection results in an increased number of immature DCs in cervical tissues, characterized by low expression of maturation markers CD83 and CD86. These immature DCs are less effective in antigen presentation and initiating a robust T-cell-mediated response | Persistent HPV infection due to inadequate activation of immune responses against HPV, leading to sustained viral infection and increased risk of cervical lesion progression |
| Vaginal microbiome and inflammation | HIV-related changes in the vaginal microbiome lead to dysbiosis, characterized by an imbalance in microbial species that promotes a pro-inflammatory state. This inflammation can exacerbate HPV persistence and lesion progression | Chronic inflammation associated with microbial dysbiosis enhances HPV persistence and promotes the progression of precancerous lesions to CC due to a continuously inflamed environment |
| Epithelial–mesenchymal transition (EMT) | HIV proteins gp120 and Tat induce EMT in cervical epithelial cells. This process involves the loss of epithelial characteristics and gain of mesenchymal traits, facilitating tumor cell invasion, migration, and resistance to apoptosis | Accelerates the progression of cervical lesions, increases the invasiveness and metastatic potential of CC cells, and may lead to resistance to conventional treatments |
CC ranks as the third leading cause of death in women globally and is the second most frequently diagnosed cancer in women, especially those under 25 years of age.14 In 2020, there were 341![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833 reported cases of CC worldwide, with a total of 604
833 reported cases of CC worldwide, with a total of 604![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 new cases.15 According to Global Cancer Statistics, it is projected that there will be approximately 13
127 new cases.15 According to Global Cancer Statistics, it is projected that there will be approximately 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 820 new cases and 4360 deaths in 2024 due to CC in the US.16 The incidence and mortality of CC vary based on the geographic and socioeconomic status of the country, with a heavy burden on low-middle-income countries (LMICs) and those with a lower Human Development Index (HDI).17 Astonishingly, 88% of all deaths and 83% of new CC cases occur in LMICs, including India, Sub-Saharan Africa, and Latin America.15,18
820 new cases and 4360 deaths in 2024 due to CC in the US.16 The incidence and mortality of CC vary based on the geographic and socioeconomic status of the country, with a heavy burden on low-middle-income countries (LMICs) and those with a lower Human Development Index (HDI).17 Astonishingly, 88% of all deaths and 83% of new CC cases occur in LMICs, including India, Sub-Saharan Africa, and Latin America.15,18
The treatment of CC depends on the stage and extent of spread. This includes surgery, radiotherapy, and chemotherapy, with increasing research on immunotherapy and targeted therapy.19 The National Comprehensive Cancer Network (NCCN) clinical practice guidelines recommend specific surgical procedures, such as conization, loop electrosurgical excision procedure (LEEP), or radical trachelectomy, for fertility preservation in women diagnosed with early stage disease.5 For women of childbearing age, radical hysterectomy or total hysterectomy with or without salpingo-oophorectomy is the treatment of choice.20,21 Radical hysterectomy using the open technique is the preferred option for tumors greater than 2 cm and larger cervical lesions.22 In the case of locally advanced stages (FIGO IIB/IIIB and FIGO IVA), concurrent chemotherapy with radiation therapy (RT) [external beam radiation therapy (EBRT), brachytherapy (internal RT), and intensity-modulated radiotherapy (IMRT)] followed by surgery is the most widely used method for managing CC.23,24 RT alone is not used for treatment due to its association with numerous adverse effects such as abdominal cramps, diarrhea, pelvic pain, skin toxicity, and lymphedema, which reduce the quality of life (QoL).25 Moreover, the treatment of locally advanced disease with chemoradiation has been associated with a high failure rate of 30–50%.26 In treating metastatic CC (Stage FIGO IVB) and locally advanced cervical cancer, pelvic exenteration for localized recurrence and chemotherapy alone, or in combination with radiation therapy, followed by surgery are crucial treatment options.27 Cisplatin is the most effective single agent, and the European Society for Medical Oncology (ESMO) recommends cisplatin over radiotherapy for local control and survival.28 Research has shown that the efficacy of cisplatin increases when it is combined with other chemotherapeutic agents.29,30 In a phase III study conducted by a gynecologic oncologic group, it was reported that combining cisplatin with topotecan and paclitaxel resulted in response rates of 39% and 29%, respectively.31 However, the median progression-free survival (mPFS) and median overall survival (mOS) for metastatic and recurrent CC remain low despite advances in treatment.28
The combination of targeted therapies, such as Bevacizumab with Cisplatin, has significantly improved progression-free survival (PFS) and overall survival (OS) in metastatic diseases.32,33 Additionally, immunotherapy targeting HPV oncogenes has been found to effectively target dysplastic precancerous lesions and malignant epithelial cervical cells.34 This exploration has led to the development of various checkpoint blockades, vaccines, and adoptive T-cell therapies, each with varying rates of success and many currently under clinical trials.35
Despite the wide range of treatment options, CC still faces numerous obstacles, particularly in the treatment of metastatic and locally advanced disease. There is a significant need to improve the survival outcomes of patients with stages IB3 to IVA undergoing chemoradiation.36 Moreover, the long-term side effects of these therapies are a major concern due to the potential risks of bladder, bowel, and sexual dysfunction, severely impacting prognosis.37 This highlights the urgent need for novel therapeutic approaches for the treatment of CC, particularly to achieve modest benefits for mOS and mPFS. Therefore, it is imperative to explore innovative delivery platforms, such as nanostructured lipid carriers (NLC), polymeric nanocarriers, and solid lipid nanoparticles (SLNs), which offer promising advantages in terms of drug bioavailability, tumor selectivity, and toxicity reduction.38–40 The following section discusses the evolution and potential of nanotechnology, especially SLNs, as a transformative approach in CC therapy. This transformative approach can provide personalized therapy, minimize adverse effects, and optimize treatment efficacy. Current research is primarily focused on utilizing nanotechnological tools to enhance drug delivery, reduce side effects, ensure accurate diagnosis, and improve overall survival in the management of CC.41,42 Extensive studies have been conducted on nanocarriers, such as lipid nanoparticles, dendrimers, and liposomes, because of their potential to cross the cell membrane and accumulate at the tumor site, thereby increasing the concentration of the drug at the target.43,44 Furthermore, these nanocarriers can also play a crucial role in developing cost-effective HPV vaccines to enhance vaccine efficacy for the successful prevention of CC in women at higher risk.45,46
2. Nanotech as a transformative tool
Nanotech-based strategies have brought a substantial shift in therapeutic applications in various medical fields, including oncology.47 The integration of nanomedicine, which employs nanoscale components to achieve therapeutic effects, has played a crucial role in driving this change. Their targeted drug delivery capabilities, stability, non-invasiveness, extended blood-circulation time, and lower toxicity profile have contributed to their appeal.48The 21st century efforts have prioritized nanotechnology-based therapeutics as the leading approach to treat cancer.48 This is largely attributed to the challenges of conventional treatments, which often struggle to cross biological membranes effectively and are prone to biological degradation.49,50 Moreover, nanotechnology holds great promise for the delivery of small molecules, genes, and biologics. For instance, it has been shown to effectively deliver antitumor drugs to various cancer cell lines, such as HeLa cells, human breast adenocarcinoma (MCF-7), human lung cancer cells, and human hepatocellular liver carcinoma cell line (HepG2).51 Furthermore, Durán-Lobato et al. conducted a three-decade research to develop a nanotechnological formulation containing peptides, proteins, and monoclonal antibodies (mAbs) and reported significant advantages of this formulation, suggesting its potential shortly.52 Nanotechnology is poised to revolutionize gene therapy by offering non-viral vector systems that can be tailored for specific functional transformations using peptides and protein ligands.53 The emergence of multifunctional envelope-type nanodevices (MEND) has marked a significant leap forward, enabling precise control over intracellular trafficking. This capability is crucial for the advancement of next-generation therapies, particularly in the realm of gene therapy.
Nanotech presents a transformative approach to drug delivery in CC therapy, overcoming the limitations and challenges associated with conventional therapies. Their enhanced drug delivery mechanism improves the precision of targeted drug delivery reducing systemic toxicity.54 Moreover, stimuli-responsive nanocarriers are designed to release their therapeutic payload according to the conditions of the tumor microenvironment.55 The application of nano-delivery systems significantly enhances the pharmacokinetics and effectiveness of drug delivery compared to traditional dosage forms.54,56 Among the different types of nanotechnologies, including liposomes, polymeric nanospheres, niosomes, and nano micelles, SLNs have become a leading and efficient nanotechnological option. They demonstrate excellent biocompatibility, a reduced toxicity profile, and increased stability. Their capability to incorporate both lipophilic and hydrophilic medications enhances their encapsulation efficiency and makes them efficient drug delivery systems. SLNs offer a promising avenue for advancing the treatment of CC through innovative drug delivery systems. This review provides an in-depth exploration of SLNs specifically tailored for CC therapy, with a focus on the development and optimization of these formulations. It addresses the various challenges encountered in the formulation process and highlights the potential opportunities for future research and applications. The primary objective is to assess how SLNs can serve as an effective method for delivering therapeutic agents in the management of CC, potentially improving treatment outcomes and minimizing side effects associated with conventional therapies.
3. Fundamentals of SLNs
SLNs are unique colloidal drug delivery systems that are distinguished by their solid core and are capable of incorporating both hydrophobic and hydrophilic drugs.57,58 Biodegradable and biocompatible solid lipids are used in their formulation with low toxicity, making them suitable for oral, parenteral, and topical administration.57 They offer potential for improved drug stability, enhanced drug delivery, and biocompatibility.59 Furthermore, they combine the advantages of emulsions and liposomes with those of polymeric nanoparticles.60 SLNs provide numerous advantages over conventional drug delivery systems, such as biocompatibility and biodegradability, versatile route of administration, enhanced stability, low toxicity profile, straightforward production, and scalability.59 The interaction of SLNs with biological barriers is determined by their physicochemical properties such as particle size, lipid composition, and surface charge. They provide the benefit of sustained drug release, thereby reducing the need for repeated administration.61The major portion of SLNs is composed of solid lipids, typically including fatty acids, triglycerides, and waxes.62 To maintain the stability of this core, it is surrounded by a layer of surfactants, which also reduce the surface tension and prevent aggregation. Commonly employed surfactants include polyoxyethylene (20) sorbitan monooleate, poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol), phosphatidylcholine, polyoxyethylene (10)-oleyl ether.63,64 Emulsifiers play a crucial role in influencing various physicochemical parameters of SLNs, such as drug release kinetics and surface charge, and aid in the mixing of the aqueous and liquid phases.65 Additionally, co-emulsifiers and co-surfactants, such as sodium cholate and sodium deoxycholate, are used to further stabilize the system and enhance the functionality and physicochemical properties of the nanoparticles.66 Furthermore, other additives such as surface modification with polyethylene glycol (PEG) stearate were included to optimize the performance of drug encapsulation.67
The drug-loading mechanism of SLNs depends on the physicochemical properties of the lipid matrix and drug. There are three important types of mechanisms to consider: enriched core model, homogenous matrix model, and enriched shell model, as shown in Fig. 2.65,68 In the enriched core model, a highly lipophilic drug is loaded onto the lipid core that is solubilized during the formation of the nanoparticle, making it suitable for drugs with a high affinity for the lipid core.59 The homogeneous matrix model involves the distribution of drugs throughout the lipid matrix through cold homogenization, when both have the same solubility, allowing uniform dispersion of the drug in the matrix.69 In the enriched shell model, the drug is affixed to the surfactant, which interacts with the lipid surface and localizes to the outer shell.70
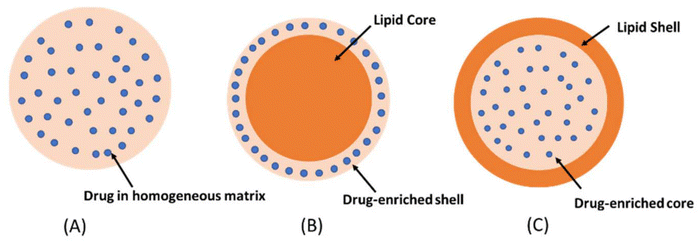 | ||
| Fig. 2 The drug loading mechanism of SLNs (A) homogeneous matrix model (B) enriched shell model (C) enriched Core model. Reproduced from Chutoprapat et al.71 under the terms and conditions of the Creative Commons Attribution (CC BY) license. | ||
SLNs can be produced through high-pressure homogenization (HPH) using either cold homogenization at room temperature or hot homogenization at elevated temperatures. Hot homogenization involves utilizing hot melt extrusion (HME) to uniformly pump the raw material.72 The melted lipid is then combined with an aqueous solution of surfactant and subjected to HPH to reduce the size of SLNs. In cold homogenization, the solidified lipid–drug mixture is ground and subjected to HPH in a cold aqueous solution of surfactant.73 Other methods for manufacturing SLNs are also employed, such as solvent emulsification–evaporation, the microemulsion technique, and the solvent injection method, which are beyond the scope of this review.
SLNs act by enhancing the drug delivery mechanisms and improving drug absorption and bioavailability. The reduced particle size and lipidic nature of SLNs enable their administration via various routes. Additionally, the solid lipid matrix facilitates controlled release of the drug, and the drug release mechanism can be modified by altering the composition of the lipid matrix and surfactant used.74 Moreover, they prevent drug degradation, particularly for peptides and proteins, allowing for controlled release.58 Orally administered SLNs are absorbed through M cell uptake, whereas intravenously administered SLNs are taken up by the liver and spleen.62 Surface modification with PEG can redirect them to other tissues and organ references. When delivering biologics, active targeting is mainly employed.75 The delivery of genetic material can occur through various mechanisms. Electrostatic binding involves the binding of cationic SLN to negatively charged nucleic acids, preventing enzymatic degradation and ensuring cellular uptake.76 The clearance and elimination of SLNs depend on the route of administration. For example, elimination occurs via the hepatobiliary and fecal excretion of oral and parenterally administered drugs. SLNs administered via pulmonary delivery are cleared by the mucociliary escalator, and eventually through fecal elimination,77 whereas ocular drugs are eliminated via lacrimal secretion.78
4. SLNs in cancer
The contemporary decade has seen a significant evolution in the development of cancer therapeutics.79 Inventions such as small-molecule inhibitors,79 immunotherapies,80 chimeric antigen receptor [CAR]-T cell therapy,81 and PROTACs82 are transforming the field of oncology, providing significant therapeutic advantages for cancer patients. Despite these developments, substantial limitations persist, including treatment resistance,83 high costs,84,85 complex tumor microenvironment (TME),86 and limited availability in low- and middle-income countries.87 Moreover, owing to the heterogenic nature of the tumor, malignant cells are more likely to develop survival mechanisms.88 This underscores the necessity for enhancing the currently used cancer therapies through the adoption of nanotechnological tools.SLNs have emerged as a versatile nanoplatform for the delivery of anticancer agents across multiple malignancies, establishing foundational design strategies that are increasingly being adapted to CC therapy. For instance, Rahman et al.89 developed SLNs containing curcumin (Cur-SLNs) to study their effective delivery and anticancer potential in lung cancer. Cur-SLNs displayed optimal properties (particle size of 114.9 ± 1.36 nm, zeta potential of −32.3 ± 0.30 mV, encapsulation efficiency of 69.74 ± 2.03%, and drug loading of DL, 0.81 ± 0.04%). In vitro studies on the A549 cell line demonstrated a significantly higher cellular uptake with substantial cytotoxicity (IC50 = 26.12 ± 1.24 μM) when compared to the control (Cur) group (IC50 = 35.12 ± 2.33 μM). In another study, Wang et al.90 formulated resveratrol (Res)-embedded D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS)-Res-SLNs to combat multi-drug resistance in breast cancer (BC). TPGS-Res-SLNs showed greater cytotoxicity than Res-SLNs and free resveratrol in SKBR3/PR cells. In addition, a potent apoptotic profile (57.40%), reduced cellular migration and invasion, and strong antitumor efficacy were found. The nanoformulation displayed a substantial reduction in drug efflux transporters, such as P-gp and BCRP expression. In vivo analysis of SKBR3/PR tumor-bearing mice showed stronger tumor growth inhibition and apoptotic profiles. Hatami et al.91 developed Quercetin-loaded SLNs (QC-SLN) to investigate their anti-cancer effects on the triple-negative breast cancer (TNBC) cell line MDA-MB231. The QC-SLN modulated the expression of Bax and Bcl-2 at both the gene and protein levels, resulting in a considerable increase in the apoptotic rate with a significant reduction in cell viability, colony formation, and angiogenesis. Furthermore, PEGylated 5-FU-loaded SLNs reported profound effectiveness against HCT-116 cancer cells. Among the six batches assessed, PEGylated 5FU-SLN4 displayed the most effective results. These include a particle size of 263 ± 3 nm, zeta potential of 0.1 ± 0.02, EE of 81 ± 10%, enhanced suppression of tumor growth, downregulation of HER2 receptor expression, high cytotoxicity, and no reported hepatic and renal tissue toxicity.92
SLNs, through surface modification with specific targeting moieties, can be functionalized to selectively bind to overexpressed receptors in cancerous tissues. This approach facilitates the targeted delivery of encapsulated anticancer agents, resulting in enhanced local concentrations at the designated cancerous site.75 For instance, Akanda and colleagues bioconjugated transferrin and curcumin in SLNs for effective delivery to prostate cancer cells. Their results indicated profound chemopreventive activity characterized by enhanced cellular uptake and an increased apoptotic profile.93 In another study, transferrin-decorated tamoxifen citrate-loaded SLNs displayed a greater cellular uptake profile than tamoxifen citrate-loaded SLNs in MCF-7 BC cells, indicating the potential for targeted therapeutic approaches.94 Additionally, folic acid-grafted oxaliplatin (OA) SLNs enhanced cellular uptake, resulting in substantial cytotoxicity, with a potent anticancer profile and significant sensitivity to HT-29 cells.95 Furthermore, surface modification with folic acid actively delivers anticancer drugs in lung,96 breast,97,98 and colorectal cancers.99,100 Although functionalization of SLNs with ligands such as transferrin and folic acid has demonstrated promising results in preclinical studies, their clinical translation remains challenging. Key obstacles include ensuring long-term stability of the ligand-modified SLNs, minimizing batch-to-batch variability in conjugation efficiency, and maintaining the integrity of active targeting formulations during storage and administration.101,102 Despite these limitations, such surface modifications highlight the potential of SLNs to revolutionize cervical cancer therapy by enabling targeted drug delivery while minimizing systemic toxicity.
SLNs exhibit remarkable promise not only in therapeutic applications but also in the domain of tumor diagnostics. The incorporation of diagnostic agents such as superparamagnetic iron oxide and technetium-99 (99mTc) has proven to be highly effective and can be employed as a CNS MRI contrast and lymphoscintigraphic agent.103 Furthermore, surface modification with specific compounds enhances the contrast and resolution, thereby enabling precise tumor mapping and characterization.104
The delivery of SLNs to the targeted cancer site is followed by their uptake and subsequent entry into the tumor, along with their interactions with the TME. Broadly, uptake mechanisms are classified into energy-dependent endocytosis (including clathrin-mediated, caveolae-mediated, clathrin- and caveolae-independent endocytosis, and micropinocytosis) and energy-independent non-endocytosis.105,106 Endocytosis is a cellular internalization mechanism characterized by invagination of the plasma membrane in response to cellular receptor interactions. This inward budding process leads to the formation of endocytic vesicles, which undergo a cascade of biochemical processes that facilitate the release of the contents into the cellular environment.107–110 While the current literature is limited to in vitro cell line studies that demonstrate clathrin-mediated endocytosis as the primary route, the lack of in vivo animal and human studies has prevented a strong conclusion. For instance, Martins et al. studied the uptake mechanism of four human glioma cell lines (U87, U251, A172, and U373) and one human monocytic cell line (THP1) and reported clathrin-mediated endocytosis as the primary pathway.106 Similarly, Arana et al.111 Cavaco and colleagues,112 Granja and co-workers,113 and Garanti et al.114 also confirmed clathrin-mediated endocytosis in their experiments.
Notably, the uptake mechanisms are influenced by several factors, including their particle size, lipid composition, surface characteristics, and target cell type.115–118 Following endocytosis, these nanoparticles navigate the intracellular environment through two main pathways: endosomal routing or endosomal escape. In the case of endosomal routing, the internalized SLNs undergo a series of maturation stages, starting with the formation of early endosomes that eventually mature into lysosomes.119 The enzymatic and acidic conditions present within lysosomes promote the degradation of SLNs, leading to the release of the encapsulated molecules.119 Conversely, during endosomal escape, some SLNs circumvent the endosomal pathway before reaching lysosomal degradation, allowing them to release their cargo directly into the cytosol.120
SLNs leverage the EPR effect within the TME, taking advantage of the abnormal vasculature and compromised lymphatic drainage to preferentially accumulate in tumor tissues. This passive targeting strategy enables prolonged drug release and minimizes systemic toxicity. Additionally, surface modifications like PEGylation can further improve tumor-specific interactions and circulation time.117,121 However, the interaction of SLNs with TME lacks available data, posing a potential barrier to its clinical translation and demanding in-depth analyses.
These mechanistic insights and performance metrics derived from various cancer models provide an essential framework for applying SLNs to CC. In the following section, we shift focus to the specific application of SLNs in CC, examining current preclinical data, formulation strategies, and therapeutic outcomes.
5. SLNs in CC: targeting and drug delivery applications
SLNs are essential in the treatment of various cancers, and their benefits in cervical cancer are particularly notable. They have demonstrated impressive results in enhancing drug delivery to target cells, improving stability and biocompatibility, and achieving significant cytotoxic effects on cervical cells. Furthermore, their capability to incorporate both small molecules and genetic materials has led to remarkable outcomes.5.1. SLNs for small molecule delivery
SLNs act as potential carriers, safeguarding drugs from the extracellular environment by facilitating passive or active drug delivery. They play a pivotal role in targeted drug delivery by upregulating the receptor and enhancing drug specificity. SLNs can be tailored with chemotherapeutic agents to overcome challenges, such as low solubility, stability, and bioavailability. A study by Karamchedu and colleagues122 demonstrated a three-fold increase in the cytotoxicity of morin hydrate, an anticancer bioflavonoid, when loaded into SLNs. The optimized SLNs exhibited a particle size of 92 nm, zeta potential of −23.5 ± 1.6 mV, 27.5% cell viability, and 87% encapsulation efficiency, improving intracellular delivery in HeLa CC cells. Additionally, Wang et al.123 developed chitosan-coated cisplatin-loaded SLNs (as shown in Fig. 3A) to provide sustained drug release and increased stability in HeLa cervical carcinoma, minimizing side effects. The surface modification with chitosan improved the drug release profile of cisplatin (as shown in Fig. 3B), resulting in excellent dispersity and a significant reduction in cell viability with an IC50 value of 0.6125 μg ml−1, leading to enhanced internalization and higher apoptosis. | ||
| Fig. 3 Schematic representation of chitosan-loaded cisplatin-loaded SLN and the drug release profile of chitosan-loaded cisplatin-loaded SLNs. Reproduced from ref. 123, under the terms of the Creative Commons CC BY license. | ||
Similarly, Chen et al. conducted a study on the development and evaluation of topotecan-loaded SLNs.124 They prepared twenty SLN batches and optimized them using a face-centered 33-factorial experimental design. The optimized SLNs exhibited a mean particle size of 262.5 ± 8.32 nm, a PDI of 0.151, and a mean zeta potential of +20 mV. Cytotoxicity analyses performed on HeLa and SiHa cervical cancer cell lines showed significantly reduced cell viability (P > 0.05) compared to the control group. Additionally, the optimized SLNs demonstrated considerably higher IC50 values in cancer cells compared to normal cells. These optimized SLNs also showed an optimal stability profile when stored under refrigeration for up to six months. However, when exposed to room temperatures of 25 °C, the drug concentration within the SLNs decreased.
To achieve synergistic antitumor activity against CC, Liu et al.125 developed trans-activating transcriptional activator (TAT)-functionalized SLNs for the co-delivery of paclitaxel (PTX) and α-tocopherol succinate-cisplatin prodrug (TOS-CDDP) (TAT PTX/TOS-CDDP SLNs). These SLNs were meticulously prepared using a solvent evaporation and emulsification method, which facilitated their successful internalization within HeLa cells. The formulation exhibited ideal physicochemical properties, featuring a particle size of 108.6 ± 3.1 nm, a zeta potential of −31.2 ± 2.7 mV, and an encapsulation efficiency (EE) of approximately 90% for both PTX and CDDP. In vitro release studies indicated a slower release from the TAT PTX/TOS-CDDP SLNs compared to the PTX/TOS-CDDP SLNs (P < 0.05). Additionally, a faster drug release was observed at pH 5.0 than at pH 7.4 (P < 0.05). The cellular uptake efficiency of the TAT-modified SLNs was significantly higher than other SLNs (P < 0.05). Furthermore, treatment with TAT PTX/TOS-CDDP SLNs resulted in significant reductions in tumor volume in cervical cancer-bearing mice compared to other SLNs (P < 0.05). These SLNs demonstrated an impressive tumor inhibition rate (TIR) of 72.2%, surpassing that of the PTX/TOS-CDDP SLNs, thereby emphasizing the importance of TAT modification (as shown in Fig. 4). Following in vivo administration of TAT PTX/TOS-CDDP SLNs, drug concentrations within the tumor remained relatively stable at all measured time points until 24 and 48 hours, while the concentrations in the PTX/TOS-CDDP group significantly decreased.
 | ||
| Fig. 4 Tumor inhibition rate of various formulations on cervical cancer-bearing mice. Reproduced from Liu et al.,125 with permission from Dove Medical Press Limited under the terms of the Creative Commons Attribution-Non-commercial License (CC-BY-NC) (2017). (Figure legends: CDDP, cisplatin; PTX, paclitaxel; SD, standard deviation; SLNs, solid lipid nanoparticles; TAT, trans-activating transcriptional activator; TIR, tumor inhibition rate; TOS-CDDP, α-tocopherol succinate-cisplatin prodrug). | ||
In a separate study, Wang et al. introduced hyaluronic acid-decorated Pluronic 85-coated SLNs loaded with paclitaxel (HA-PTX-P85-SLN) to combat multidrug resistance in CC.126 Pluronic-85 is a potent inhibitor of P-glycoprotein (P-gp), that causes multiple drug resistance (MDR) in cancer cells. The surface modification with hyaluronic acid enabled active targeting of overexpressed receptors CD44 and CD168, facilitating enhanced delivery of paclitaxel to tumor cells, even those with multidrug resistance. HA-PTX-P85-SLN displayed desirable particle size, zeta potential, and EE. The cellular uptake efficiency of the formulation was found to be significantly greater compared to other SLNs (P < 0.05). Additionally, HA-PTX-P85-SLN demonstrated considerable cytotoxicity towards cervical cancer cell lines than other formulations. For HeLa/PTX cells, the formulation displayed IC50 of around 0.2 nmol mL−1, which was exceptionally low compared to other formulations, such as PTX-P85-SLN (0.9 nmol mL−1), PTX-SLN (2.7 nmol mL−1) and free PTX (8.2 nmol mL−1). The resistance index of HA-PTX-P85-SLN (i.e. 2.5) was nearly 5.5-fold lower than that of free PTX (i.e. 13.7). The in vivo antitumor efficacy of the formulation exhibited the highest tumor growth inhibition among other treatment groups. Furthermore, the mice within this group displayed no weight loss, whereas in those treated with free PTX or saline, the weight loss was observed.
The recent study conducted by Adeyemi and colleagues focused on the delivery of 5-Fluorouracil (5-FU) loaded SLNs using thermo-sonic-nano-organogel (TNO) variants to achieve a rate-modulated intracervical drug release.127 The in vitro assessment revealed an initial burst of drug release on day 1 followed by consistent and sustained release for 14 days. Notably, TNO variant 1 demonstrated superior sustained release over 15 days compared to TNO-2 and TNO-3. It was observed that the SLN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TNO ratio influenced the biodegradation and release rate, with a higher ratio decreasing the swelling ability of TNO formulations. Additionally, essential oils (EOs) have been recognized as important anti-cancer agents in cervical cancer cell lines, specifically HeLa (HPV 18) and SiHa (HPV 16). Studies have highlighted their effectiveness in reducing tumor volume and angiogenesis.128 Despite their potential to inhibit cell proliferation and induce apoptosis, the bioavailability of essential oils is limited due to their poor stability, high volatility, and susceptibility to degradation when exposed to oxygen, light, and heat.129 Encapsulation of EOs offers a promising approach for successful delivery into cancer cells. For instance, encapsulation of Eucalyptus globulus EO has demonstrated an enhanced cytotoxic effect with an IC50 of 21.30 μg mL−1 against HeLa cell lines.130 However, it is imperative to note that the FDA has only classified a few EOs as “Generally recognized as safe” (GRAS), and there is a dearth of animal studies, emphasizing the necessity for future research to establish their safety and efficacy in CC treatment. In another study, a temperature-sensitive gel-loaded SLN was prepared to enhance in vivo drug absorption and reduce local irritation after vaginal delivery.131 It exhibited a superior anti-proliferative action compared to conventional powder, along with S and G0/G1 phase arrest in HeLa cancer cells. Moreover, it proved to enhance biocompatibility and sustained drug release by adhering to the vaginal mucosa.
TNO ratio influenced the biodegradation and release rate, with a higher ratio decreasing the swelling ability of TNO formulations. Additionally, essential oils (EOs) have been recognized as important anti-cancer agents in cervical cancer cell lines, specifically HeLa (HPV 18) and SiHa (HPV 16). Studies have highlighted their effectiveness in reducing tumor volume and angiogenesis.128 Despite their potential to inhibit cell proliferation and induce apoptosis, the bioavailability of essential oils is limited due to their poor stability, high volatility, and susceptibility to degradation when exposed to oxygen, light, and heat.129 Encapsulation of EOs offers a promising approach for successful delivery into cancer cells. For instance, encapsulation of Eucalyptus globulus EO has demonstrated an enhanced cytotoxic effect with an IC50 of 21.30 μg mL−1 against HeLa cell lines.130 However, it is imperative to note that the FDA has only classified a few EOs as “Generally recognized as safe” (GRAS), and there is a dearth of animal studies, emphasizing the necessity for future research to establish their safety and efficacy in CC treatment. In another study, a temperature-sensitive gel-loaded SLN was prepared to enhance in vivo drug absorption and reduce local irritation after vaginal delivery.131 It exhibited a superior anti-proliferative action compared to conventional powder, along with S and G0/G1 phase arrest in HeLa cancer cells. Moreover, it proved to enhance biocompatibility and sustained drug release by adhering to the vaginal mucosa.
Despite notable advancements, SLNs encounter substantial challenges in vaginal drug delivery, particularly concerning pH compatibility, mucoadhesion, and mucosal retention time.116,132,133 The vaginal environment, with its acidic pH range of 3.8–4.5, necessitates the development of SLNs specifically tailored to maintain stability and therapeutic efficacy under such conditions.132 For instance, chitosan-coated SLNs have emerged as promising candidates for vaginal application due to their pH-responsive mucoadhesive properties.132 Chitosan exhibits enhanced adhesion in acidic environments, primarily through electrostatic interactions between its positively charged amino groups and the negatively charged mucin glycoproteins present in the vaginal mucus.116,132 This enhances SLN retention at the site of action, potentially improving therapeutic outcomes. However, excessive mucoadhesion can be counterproductive, leading to entrapment of the SLNs within the superficial mucus layer, which is subject to continuous turnover and clearance.116,134 To overcome this limitation, strategies involving the development of mucus-penetrating particles (MPPs) have been adopted. These include surface modifications such as PEGylation or the design of nanoparticles with diameters below 200 nm, facilitating their diffusion through the mucus barrier and promoting interaction with the underlying epithelial tissues.116
5.2. SLNs for gene drug delivery
SLNs are particularly well-suited for delivering therapeutic genes due to their capacity to encapsulate genetic material and protect it from degradation. They also provide sustained release profiles, which enhance therapeutic outcomes. Developed vaginal suppositories that combined small molecules with genetic material for the localized treatment of cervical cancer. They incorporated paclitaxel and Bcl-2 siRNA into the SLNs to target specific oncogenes.135 The study evaluated the transfection efficiency and physicochemical properties of the formulations on cancer cells, demonstrating their potential for simultaneous drug delivery at reduced doses, even in cells resistant to paclitaxel.Shah et al. demonstrated the uptake of stearic acid-based SLNs in human epithelial cells through the clathrin-mediated endocytic pathway.120 This energy-dependent mechanism efficiently transports the nanoparticles via the formation of vesicles. Other pathways such as micropinocytosis and ligand–receptor interaction are poorly understood in CC. Furthermore, understanding the interaction of SLNs with the TME is crucial, considering its unique characteristics such as the acidic environment, hypoxia, and the presence of matrix metalloproteinase, which enhance their efficacy and delivery. Current literature primarily focuses on targeted drug delivery with chemotherapeutic agents and genetic material in CC. However, to optimize delivery and personalize treatment approaches, comprehending the interaction of SLN with TME is imperative. A profound understanding of TME can facilitate its modulation to make it less conducive for tumor growth. Based on TME characteristics, patients can be stratified for effective treatment regimens, thereby improving their overall survival.
6. Formulation development and optimization
The formulation of SLNs involves key aspects ranging from lipid selection and their optimization to toxicity and safety profiles. Lipids are selected based on their melting points, ability to form stable formulations, and compatibility with the drug because their selection directly affects drug solubility, polydispersity, and particle size.136,137 Lipids with optimal solubilizing capacities, such as glyceryl monooleate and glyceryl monostearate,138 are preferred because they provide optimum miscibility with multiple drugs, enhance stability, and result in smaller particle sizes.139 Achieving low polydispersity, typically below 0.3, results in an excellent homologous particle size distribution in a reproducible SLN formulation.73 Ultrasonication and high-shear homogenization are commonly employed to achieve low polydispersity.140Additionally, maintaining a zeta potential of >+30 mV or < −30 mV prevents aggregation, thereby ensuring colloidal stability and playing a vital role in cellular uptake by tumor cells.141 The surface charge in cancer cells is typically negative owing to increased glycolysis-associated lactate formation and substantial expression of sialic acid.142,143 Therefore, formulating SLNs with cationic lipids will generate a magnetic effect towards the targeted tumor, boosting drug uptake and leading to an optimal formulation, as proven by Carbone et al.144 and Luo et al.145
The size range of SLNs varies depending on the preparation method and formulation parameters. For instance, Charcosset and co-workers utilized the membrane contractor method for SLN preparation and obtained sizes ranging from 70 nm to 215 nm.146 Another group of researchers used sonication methods147 and reported a particle size range of 200–300 nm. Moreover, decreasing the lipid concentration and increasing the solvent-to-lipid ratio can result in smaller particle sizes.148 Additionally, the choice and concentration of surfactants play a significant role in influencing the particle size and stability. For instance, increasing the concentration of cationic surfactants (dioctadecyldimethylammonium bromide and cetylpyridinium chloride) increases the zeta potential, resulting in smaller particles and enhanced stability.149 The addition of emulsifiers, such as polyethylene glycol sorbitan monolaurate, has also been reported to decrease particle size owing to their increased surface activity.150
The potential of SLN as nanotechnological tools in clinical scenarios necessitates their successful in vitro and in vivo applications. In vitro analyses utilizing cell lines encompass crucial parameters, such as particle size, zeta potential, EE, DL, drug release patterns, cellular uptake, cytotoxicity, and apoptosis.151,152 On the other hand, in vivo studies involve essential assessment of pharmacokinetic parameters, biodistribution, and antitumor efficacy.151,153,154 For instance, Rodenak-Kladniew and colleagues153 determined the mean diameter, size distribution, and zeta potential using photon correlation spectroscopy and laser Doppler anemometry. Stability studies were carried out to investigate the mean particle size, PDI, zeta potential, and EE, while the release patterns were assessed using a dialysis membrane. The MTT assay was employed to determine cell viability, and uptake studies were performed using fluorescence microscopy in A549 (human alveolar adenocarcinoma basal epithelial cells) and HepG2 (human liver carcinoma cells) cell lines.153 SLNs displayed a particle size of 90–130 nm, zeta potential of −4.0 mV, PDI lower than 0.2, and EE greater than 80%. Furthermore, Jang et al. conducted in vivo tissue distribution and antitumor efficacy studies in tumor-bearing mice, and in vivo pharmacokinetic studies of camptothecin (CPT)-SLNs in rats. CPT-SLNs displayed significant CPT concentrations within tumor thigh, considerable anti-tumor efficacy, and substantial CPT plasma concentrations compared to free CPT.155
SLNs are generally considered safe and biocompatible nanocarriers. However, their overall safety profile can be substantially influenced by factors such as the lipid matrix composition, the choice of surfactants, and the nature of excipients used.136 Additionally, SLNs undergo rigorous regulatory assessment due to various toxicological concerns, including surfactant-related toxicity, the presence of degradation byproducts, and unresolved long-term safety risks. Collectively, these actors pose major barriers to their successful clinical translation.156–161
To illustrate, cationic surfactants can induce potential toxicities. The systemic administration of 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) has been shown to provoke inflammatory reactions.156 Similarly, the use of Cetyltrimethylammonium bromide (CTAB) has been reported to activate neutrophils and induce oxidative stress, leading to profound inflammation and ultimately cell death.157 Secondly, polymorph transformation of lipid matrices, particularly from α- to β-crystal forms, can result in premature drug expulsion and erratic drug release profiles.117,162 Emerging evidence further indicates that degradation byproducts from SLN formulations may activate the immune system and trigger inflammatory signaling pathways.163,164 Despite these findings, current formulation optimization strategies often lack comprehensive characterization of lipid–surfactant interaction byproducts, making regulatory approval more challenging.117,158 Thirdly, the employment of PEGylated nanoformulation may lead to the production of anti-PEG antibodies.159,160 These antibodies tend to accelerate the clearance of the formulation from the bloodstream, compromising therapeutic efficacy.159,160 Furthermore, they alter the biodistribution of the encapsulated drug, potentially increasing the risk of off-target effects and adverse events.159,160
7. Studies
We have conducted a thorough analysis of all the research on the use of SLNs for drug delivery in CC and have summarized the findings in Tables 2 and 3. Furthermore, our search did not yield significant information on biologics and patents.| S.no. | Drug name | Structure | Utility | Excipients used | Advantages | Limitations | Result | Model | Cell culture model | Evaluation protocol | Intracellular uptake studies | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | Cisplatin |

|
CC | Glyceryl dibehenate and monooleate of sorbitan ethoxylated | Decreased cell viability at low concentration, superior efficacy over CSLN, enhanced internalization and drug release | Lack of in vivo studies | ↑ cytotoxicity and apoptosis rate (40%), ↓ IC50 (0.6125 mg ml−1), ↓ particle size (80–300 nm) | In vitro | HeLa cell line | MTT assay for cytotoxicity. Cell viability, apoptosis rate and IC50 | Confocal laser scanning microscopy (CLSM) | 123 |
| 2. | Topotecan |

|
CC | Cetyl palmitate, Polysorbate 80, chloroform, methanol, water | Enhanced bioavailability, efficient encapsulation of hydrophilic, hydrophobic and amphiphilic drug | Lack of in vivo studies | ↓ toxicity of drug, ↑ stability, mean drug loading (PDL) value was observed to be 92.03 ± 3.65%, indicating efficient loading of topotecan into the SLNs, ↓ PDI (0.151), and ↓ mean particle size (262.5 ± 8.32 nm) | In vitro | HeLa and SiHa cell line | Particle morphology, PDI, zeta potential and in vitro release | Not specified | 124 |
| 3. | Morin hydrate |
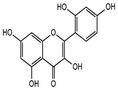
|
CC | Glyceryl monostearate, lecithin soy, polysorbate 80 and chloroform | High drug loading capacity, controlled drug release, improved biocompatibility | Lack of in vivo studies | ↑ encapsulation efficiency (87.1%), ↓ zeta potential (−23.5 mV), and ↓ particle size (92 nm) | In vitro | HeLa cell line | Particle size, zeta potential, stearic stability, encapsulation efficiency, in vitro drug release | Not specified | 122 |
| 4. | 5-FU |

|
CC | Poly-L-lactic acid, palmitic acid, PVA, dichloromethane, methanol, ethylene glycol, ethanol, dimethyl sulfoxide | Site-specific stimuli-responsive drug delivery, controlled drug release | Lack of in vivo studies, single drug model, lack of clinical validation | Rate modulated release (44.29% under single stimuli and 67.13% under combined stimuli after 15 days), ↓ zeta potential (−23.2 mV), indicating good stability of the SLNs, ↓ particle size (450.9 nm), PDI value (0.541), EE (33%) reflecting the amount of 5-FU successfully encapsulated in the SLNs | In vitro | Not specified | Particle size, PDI, biodegradation analysis, 5-FU release | Not specified | 127 |
| 5. | Paclitaxel+α-tocopherol succinate-cisplatin prodrug |

|
CC | Glyceryl monostearate, soybean lecithin, TAT peptide with terminal cysteine, 4-dimethyl aminopyridine, DSPE-PEG-Maleimide, N,N′-dicyclohexyl-carbodimide | High tumor accumulation, superior anti-tumor efficiency, enhanced internalization with HeLa cells, synergistic effect | — | ↓ toxicity in vivo, ↑ tumor inhibition rate (72.2%) of TAT-modified SLNs, zeta potential of TAT PTXTOS-CDDP SLNs ↓ to −31 mV, indicating stability in the formulation, ↓ size of 109 nm, ↑ EE (90%), the drug loading (DL) of PTX ranged between 3.5% and 5.9%, while the DL of CDDP ranged from 2.3% to 4.4% | In vitro and In vivo | HeLa cell lines | Physicochemical properties (size, morphology, release profile), cytotoxicity evaluation, IC50 value | Cellular uptake efficiency was analyzed using fluorescent probe and coumarin-6 | 125 |
| S.no. | Genetic Sequence PLUS Drug | Use | Excipients used | Advantages | Limitations | Model | Cell culture model | Result | Evaluation parameter | Intracellular uptake studies | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | siRNA + Paclitaxel | CC | Gelucire® 50/13, Span® 85, Tween® 80, dichloromethane, DOTAP, dimethylsulfoxide, PEG 6000 | Reduced dose in systemic circulation, less toxicity, possibility of self-administration, potential for local drug delivery | Lack of in vivo studies, lack of study on long-term stability and adverse effect | In vitro | HeLa cell lines | Simultaneous release of Bcl-2 siRNA and paclitaxel in vaginal suppository | Particle size, PDI, zeta potential, cytotoxicity, in vitro drug release, and transfection ability | Not specified | 135 |
8. Clinical trials and translational studies
After conducting an extensive search using the keywords “Solid lipid nanoparticles” and “Cervical cancer” using the website clinicaltrial.gov, it is clear that no SLN-based therapies have progressed to clinical trials for managing cervical cancer. This gap in data highlights significant barriers across multiple domains, including technical, regulatory, and financial challenges.165–169Notably, from a technical perspective, limitations in manufacturing and scalability are particularly pronounced. During the manufacturing process, SLN can undergo polymorphic transitions within lipid matrices, which may result in drug expulsion and consequent loss of therapeutic efficacy.165,166 Furthermore, processes such as HPH require stringent temperature control; even slight deviations can contribute to batch-to-batch variability.167 Moreover, lipids and thermally unstable molecules lose their functional integrity when subjected to steam-based sterilization or gamma irradiation techniques, highlighting sterilization as another crucial technical limitation.166 At an industrial scale, producing SLNs presents significant challenges. Variability in equipment design, the absence of standardized process parameters, and difficulties in achieving reproducibility and uniformity across batches greatly impede scalability.169 Additionally, the lack of standardized protocols for analyzing lipid polymorphism, assessing long-term stability, and thoroughly characterizing nanoparticles poses additional obstacles to gaining regulatory approval.165,167,168 On the financial front, insufficient funding during the preclinical phase, combined with the substantial costs associated with compliance to Good Manufacturing Practice (GMP), has impeded the translational progression of SLN-based systems into clinical settings.165,168 These various challenges highlight the urgent need for future translational studies that can address these gaps, facilitating the clinical adoption of SLNs in the treatment of cervical cancer.165,168
9. Challenges and future opportunities
Nano-drug delivery systems, particularly SLNs, have achieved considerable progress in developing effective anti-cancer formulations. The advantageous properties of SLNs compared to other delivery systems, along with their widespread application have revolutionized the field of oncology.170 Despite their potential, the clinical translation of SLNs remains hindered by several critical limitations, including low drug-loading capacity, physical instability, potential toxicity, and manufacturing complexities. These challenges collectively impede regulatory approval and limit their commercial viability.162,171 One of the primary barriers to SLN progression into late-stage clinical trials is their propensity for polymorphic transitions during storage.162 For example, SLNs formulated with stearic acid often undergo a transition from the metastable α-form to the more stable β-form, leading to core destabilization and expulsion of encapsulated drugs, such as progesterone, into the external dispersion medium.162Furthermore, the highly ordered crystalline matrix of SLNs significantly restricts their drug-loading capacity, typically capping it at approximately 10%, especially for hydrophilic compounds.57,162 This limitation has shifted the focus towards NLCs, which incorporate liquid lipids into the matrix to enhance drug entrapment efficiency.57 For instance, NLCs containing oleic acid have demonstrated up to 30% higher encapsulation of antifungal agents compared to their SLN counterparts, thereby offering greater promise for systemic antifungal therapies.57 As of 2025, no SLN-based systemic therapeutic has progressed beyond Phase II clinical trials, with the majority of approved SLN products restricted to topical applications, such as clotrimazole-loaded SLNs for localized fungal infections.116,171 These ongoing challenges highlight the pressing need for innovative formulation approaches, including the development of hybrid SLN–NLC systems, to overcome current limitations and facilitate successful clinical translation.
SLNs have been reported to be thermodynamically unstable during storage. The lipid composition and the method of SLN preparation may cause microphase separation leading to lipid segregation and resulting in a variable drug release pattern.172–175 Moreover, these formulations demonstrate unacceptable drug-loading efficiency. The ordered crystalline lattice of the formulation allows a smaller space for drug incorporation in between the fatty acid chains. Polymorphic transitions during the production and storage of SLNs often result in drug expulsion and particle growth which affects the release pattern and overall bioavailability of the formulations.57,174,176–178
The employment of different production techniques followed by significant discrepancies in the selection of lipid, surfactant concentration, and mixing methods often lead to substantial variations in the particle size, surface characteristics, and drug encapsulation efficiency. These differences produce batch-to-batch variabilities, which negatively affect the pharmacokinetics and pharmacodynamics of the formulations and ultimately clinical translation and industrial scale-up.179,180 Another limitation which may delay the preclinical to clinical transition process is the utilization of mouse models for studying the effects of SLNs on cancer. These models often fail to accurately describe the morphology of intratumoral vasculature over time. Additionally, studies have highlighted significant structural and functional variations between orthotopic and ectopic mouse models, as well as human tumors,181,182 which collectively affect the translation process. As a result, it is advisable to incorporate a more representative model such as the “prime editor mouse”. This innovative model allows for the precise engineering of various mutations in cell lines and organoids obtained from primary tissues. By doing so, it effectively mimics cellular heterogeneity and the TME, which could be particularly beneficial for studying lethal cancers, such as cervical cancer.183 Furthermore, using novel techniques such as tumour-on-a-chip can forecast the behavior of SLNs in CC within a controlled environment that closely mimics human tissue responses.184–187
Moreover, understanding the interaction of SLNs with the TME is essential, given TMEs’ unique characteristics such as the acidic environment, hypoxia, and the presence of matrix metalloproteinase, that may enhance the efficacy and delivery of SLNs.188–192 Current literature primarily focuses on targeted drug delivery with chemotherapeutic agents and genetic material in CC. However, to optimize delivery and personalize treatment approaches, comprehending the interaction of SLN with TME is imperative. A profound understanding of TME can facilitate its modulation to make it less conducive for tumor growth. Based on TME characteristics, patients can be stratified for effective treatment regimens, thereby improving their overall survival. Additionally, future research should focus on optimizing vaginal SLNs. The local action via vaginal delivery followed by active targeting with suitable overexpressed receptors can provide a synergistic targeted action. Furthermore, recent advances in nanotechnology may allow the development of personalized SLN formulations tailored to meet individual patient needs by addressing specific tumor characteristics.189
10. Conclusion
SLNs represent a transformative drug delivery system with advanced mechanisms for targeted drug delivery. Their effectiveness in administering various chemotherapeutic agents, coupled with genetic strategies, has revolutionized cancer therapeutics. Moreover, significant theranostic opportunities are awaiting exploration following a thorough and prompt analysis of CC. Future research should focus on the optimization and characterization of SLNs for specific chemotherapeutic agents to bolster their controlled drug-release profiles. It is essential to investigate combination therapies beyond paclitaxel and cisplatin to achieve a synergistic effect on antitumor activity. A broader exploration of novel anti-cancer compounds, such as targeted kinase inhibitors, immune checkpoint inhibitors, and next-generation alkylating agents, will be crucial in diversifying SLN applications. In particular, SLN formulations can be designed to overcome drug resistance mechanisms by incorporating multidrug-resistant (MDR) inhibitors to improve intracellular drug retention and therapeutic efficacy. The development of MEND represents a major advancement, allowing precise control of intracellular trafficking, which is vital for next-generation therapies, especially gene therapy. Additionally, further research should focus on tuning the physicochemical properties of SLNs, such as particle size, lipid composition, and surface charge, to enhance cell uptake, tumor penetration, and bioavailability.The development of synergistic combination therapies is a major frontier of SLN research. In addition to conventional chemotherapy, combining SLNs with immunotherapy, gene therapy, and radiotherapy can enhance anti-tumor effects. For example, SLNs that co-encapsulate immune checkpoint inhibitors (e.g., PD-1/PD-L1 inhibitors) with chemotherapeutic drugs may boost immune system activity while reducing systemic toxicity. Moreover, SLNs can be tailored for personalized medicine by incorporating patient-specific genetic and molecular markers. Predictive models driven by AI can assist in designing individualized SLN formulations based on a patient's tumor genetic profile, optimizing treatment efficacy, while minimizing adverse effects.
Although SLN-based therapeutics and vaccines hold immense promise, their translation from bench to bedside requires overcoming key hurdles. Rigorous in vivo studies, along with extensive preclinical and clinical trials, are necessary to validate the safety, efficacy, and pharmacokinetics of SLN-based therapies. Furthermore, establishing a robust regulatory framework with stringent quality control measures is imperative for guaranteeing the safety and efficacy of SLN-based formulations. Addressing concerns regarding immunogenicity, off-target effects, and long-term stability of SLN formulations will be critical in ensuring regulatory approval and widespread clinical adoption. Regulatory agencies, including the U.S. FDA requires comprehensive characterization of physicochemical properties, safety profiles, and stability data for approval of drug delivery systems. SLNs, in particular, must meet stringent criteria, including controlled particle size distribution, optimal zeta potential, low PDI, and appropriate ligand density, to ensure consistency, efficacy, and safety. Despite meeting these criteria, SLNs may still face regulatory rejection owing to critical formulation-related challenges. These include suboptimal bioequivalence, physical instability, and unresolved toxicity concerns such as macrophage-induced apoptosis or cytokine storm induction observed in preclinical models. Addressing these issues through robust preclinical evaluations and strategic formulation optimization is essential for regulatory success.
Despite the potential to encapsulate DNA, mRNA, and peptides and protect them from degradation, there are currently no SLN-encapsulated vaccines specifically approved for CC. These SLNs can be tailored to deliver DNA vaccines encoding HPV oncogenes such as E6 and E7 oncoproteins. The encapsulated SLNs would be taken up by antigen-presenting cells (APCs), eliciting a robust immune response. Additionally, peptides can be encapsulated in molecular adjuvants to enhance their uptake by the innate immune response. While ongoing research on nanotherapeutics for vaccine delivery is promising, none of them have been approved for clinical use, highlighting the critical need for further studies. Future research must focus on overcoming the existing formulation and stability challenges to develop effective SLN-based vaccine.
Author contributions
Pooja Tiwary, Krishil Oswal, Ryan Varghese, Ravi Vamsi Peri: conceptualization, data curation, formal analysis; Ryan Varghese: project administration, supervision; Pooja Tiwary, Krishil Oswal: writing – original draft, writing – review and editing, Pardeep Gupta: supervision. All authors commented on subsequent revisions and provided references.Conflicts of interest
The authors declare that there are no competing interests.Data availability
The data in this Review article is not sensitive in nature and is accessible in the public domain. The data is therefore available and not of a confidential nature.References
- K. S. Okunade, Human papillomavirus and cervical cancer, J. Obstet. Gynaecol., 2020, 40(5), 602–608 CrossRef CAS PubMed.
- A. G. Abraham, G. D'Souza and Y. Jing, et al., Invasive cervical cancer risk among HIV-infected women: a North American multicohort collaboration prospective study, JAIDS, J. Acquired Immune Defic. Syndr., 2013, 62(4), 405–413 CrossRef PubMed.
- G. Pavone, A. Marino and V. Fisicaro, et al., Entangled Connections: HIV and HPV Interplay in Cervical Cancer—A Comprehensive Review, Int. J. Mol. Sci., 2024, 25(19), 10358 CrossRef CAS PubMed.
- E. J. Crosbie, M. H. Einstein, S. Franceschi and H. C. Kitchener, Human papillomavirus and cervical cancer, Lancet, 2013, 382(9895), 889–899 CrossRef PubMed.
- W. J. Koh, N. R. Abu-Rustum and S. Bean, et al., Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology, J. Natl. Compr. Cancer Network, 2019, 17(1), 64–84 CrossRef CAS.
- J. Green, A. Berrington de Gonzalez and S. Sweetland, et al., Risk factors for adenocarcinoma and squamous cell carcinoma of the cervix in women aged 20–44 years: the UK National Case–Control Study of Cervical Cancer, Br. J Cancer, 2003, 89(11), 2078–2086 CrossRef CAS PubMed.
- D. Yang, J. Zhang, X. Cui, J. Ma, C. Wang and H. Piao, Risk factors associated with human papillomavirus infection, cervical cancer, and precancerous lesions in large-scale population screening, Front. Microbiol., 2022, 13, 914516 CrossRef PubMed.
- S. Franceschi, T. Rajkumar and S. Vaccarella, et al., Human papillomavirus and risk factors for cervical cancer in Chennai, India: A case–control study, Int. J. Cancer, 2003, 107(1), 127–133 CrossRef CAS PubMed.
- C. Y. Hsieh, S. L. You, C. L. Kao and C. J. Chen, Reproductive and infectious risk factors for invasive cervical cancer in Taiwan, Anticancer Res., 1999, 19(5C), 4495–4500 CAS.
- R. C. V. Lintao, L. F. T. Cando and G. A. S. Perias, et al., Current status of human papillomavirus infection and cervical cancer in the Philippines, Front. Med., 2022, 9, 929062 CrossRef PubMed.
- S. Kang, J. Wu, J. Li, Q. Hou and B. Tang, Prognostic significance of clinicopathological factors influencing overall survival and event-free survival of patients with cervical cancer: A systematic review and meta-analysis, Med. Sci. Monit. Int. Med. J. Exp. Clin. Res., 2022, 28, e934588–e934581 CAS.
- L. Xie, R. Chu and K. Wang, et al., Prognostic assessment of cervical cancer patients by clinical staging and surgical-pathological factor: a support vector machine-based approach, Front. Oncol., 2020, 10, 1353 CrossRef PubMed.
- X. Z. Xie, K. Song and B. Cui, et al., Clinical and Pathological Factors Related to the Prognosis of Chinese Patients with Stage Ib To IIb Cervical Cancer, Asian Pac. J. Cancer Prev., 2012, 13(11), 5505–5510 CrossRef PubMed.
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet–Tieulent and A. Jemal, Global cancer statistics, 2012, Ca-Cancer J. Clin., 2015, 65(2), 87–108 CrossRef PubMed.
- H. Sung, J. Ferlay and R. L. Siegel, et al., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, Ca-Cancer J. Clin., 2021, 71(3), 209–249 CrossRef PubMed.
- R. L. Siegel, A. N. Giaquinto and A. Jemal, Cancer statistics, 2024, Ca-Cancer J. Clin., 2024, 74(1), 12–49 CrossRef PubMed.
- D. Singh, J. Vignat and V. Lorenzoni, et al., Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative, Lancet Global Health, 2023, 11(2), e197–e206 CrossRef CAS PubMed.
- M. Arbyn, E. Weiderpass and L. Bruni, et al., Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis, Lancet Global Health, 2020, 8(2), e191–e203 CrossRef PubMed.
- C. A. Burmeister, S. F. Khan and G. Schäfer, et al., Cervical cancer therapies: Current challenges and future perspectives, Tumour Virus Res., 2022, 13, 200238 CrossRef CAS PubMed.
- F. Kokka, A. Bryant, A. Olaitan, E. Brockbank, M. Powell and D. Oram, Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer, Cochrane Database Syst. Rev., 2022,(8) Search PubMed.
- Y. Suzuki, Y. Huang, J. Ferris, A. Kulkarni, D. Hershman and J. D. Wright, Prescription of hormone replacement therapy among cervical cancer patients with treatment-induced premature menopause, Int. J. Gynecol. Cancer, 2023, 33(1), 26–34 CrossRef PubMed.
- I. Konishi, The Okabayashi radical hysterectomy: basic principle and step-by-step procedure, Surg. J., 2021, 7(S 02), S57–S69 CrossRef PubMed.
- J. Chino, C. M. Annunziata and S. Beriwal, et al., Radiation therapy for cervical cancer: executive summary of an ASTRO clinical practice guideline, Pract. Radiat. Oncol., 2020, 10(4), 220–234 CrossRef PubMed.
- P. J. Eifel, Concurrent chemotherapy and radiation therapy as the standard of care for cervical cancer, Nat. Clin. Pract. Oncol., 2006, 3(5), 248–255 CrossRef CAS PubMed.
- K. Wang and J. E. Tepper, Radiation therapy–associated toxicity: Etiology, management, and prevention, Ca-Cancer J. Clin., 2021, 71(5), 437–454 CrossRef PubMed.
- S. Markovina, K. A. Rendle, A. C. Cohen, L. M. Kuroki, S. Grover and J. K. Schwarz, Improving cervical cancer survival–A multifaceted strategy to sustain progress for this global problem, Cancer, 2022, 128(23), 4074–4084 CrossRef PubMed.
- A. Gadducci, R. Tana, S. Cosio and L. Cionini, Treatment options in recurrent cervical cancer, Oncol. Lett., 2010, 1(1), 3–11 CrossRef CAS PubMed.
- C. Marth, F. Landoni, S. Mahner, M. McCormack, A. Gonzalez-Martin and N. Colombo, Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Ann. Oncol., 2017, 28, iv72–iv83 CrossRef CAS PubMed.
- Y. Aoki, K. Ochiai and S. Lim, et al., Phase III study of cisplatin with or without S-1 in patients with stage IVB, recurrent, or persistent cervical cancer, Br. J. Cancer, 2018, 119(5), 530–537 CrossRef CAS PubMed.
- J. Y. Moon, I. C. Song, Y. B. Ko and H. J. Lee, The combination of cisplatin and topotecan as a second-line treatment for patients with advanced/recurrent uterine cervix cancer, Medicine, 2018, 97(14), e0340 CrossRef CAS PubMed.
- K. S. Tewari and B. J. Monk, Gynecologic oncology group trials of chemotherapy for metastatic and recurrent cervical cancer, Curr. Oncol. Rep., 2005, 7, 419–434 CrossRef CAS PubMed.
- M. Frumovitz, G. B. Chisholm and A. Jhingran, et al., Combination therapy with topotecan, paclitaxel, and bevacizumab improves progression-free survival in patients with recurrent high-grade neuroendocrine cervical cancer: a Neuroendocrine Cervical Tumor Registry (NeCTuR) study, Am. J. Obstet. Gynecol., 2023, 228(4), 445.e1–445.e8 CrossRef CAS PubMed.
- K. S. Tewari, M. W. Sill and H. J. Long III, et al., Improved survival with bevacizumab in advanced cervical cancer, N. Engl. J. Med., 2014, 370(8), 734–743 CrossRef CAS PubMed.
- M. Kagabu, T. Nagasawa and C. Sato, et al., Immunotherapy for uterine cervical cancer using checkpoint inhibitors: future directions, Int. J. Mol. Sci., 2020, 21(7), 2335 CrossRef CAS PubMed.
- L. Ferrall, K. Y. Lin, R. B. S. Roden, C. F. Hung and T. C. Wu, Cervical cancer immunotherapy: facts and hopes, Clin. Cancer Res., 2021, 27(18), 4953–4973 CrossRef CAS PubMed.
- J. S. Mayadev, G. Ke, U. Mahantshetty, M. D. Pereira, R. Tarnawski and T. Toita, Global challenges of radiotherapy for the treatment of locally advanced cervical cancer, Int. J. Gynecol. Cancer, 2022, 32(3), 436–445 CrossRef PubMed.
- K. S. Pfaendler, L. Wenzel, M. B. Mechanic and K. R. Penner, Cervical cancer survivorship: long-term quality of life and social support, Clin. Ther., 2015, 37(1), 39–48 CrossRef PubMed.
- G. Srinivasan, Cervical cancer: Novel treatment strategies offer renewed optimism, Pathol. Pract., 2024, 254, 155136 CrossRef PubMed.
- S. Anjum, A. Akhtar and S. M. Aldaqal, et al., Enhanced targeted treatment of cervical cancer using nanoparticle-based doxycycline delivery system, Sci. Rep., 2025, 15(1), 2318 CrossRef CAS PubMed.
- N. Kumar and M. Mangla, Nanotechnology and nanobots unleashed: pioneering a new era in gynecological cancer management–a comprehensive review, Cancer Chemother. Pharmacol., 2025, 95(1), 18 CrossRef PubMed.
- L. M. Himiniuc, B. F. Toma and R. Popovici, et al., Update on the use of nanocarriers and drug delivery systems and future directions in cervical cancer, J. Immunol. Res., 2022, 2022, 1–11 CrossRef PubMed.
- R. Yadav, P. P. Das, S. Sharma, S. Sengupta, D. Kumar and R. Sagar, Recent advancement of nanomedicine-based targeted delivery for cervical cancer treatment, Med. Oncol., 2023, 40(12), 347 CrossRef CAS PubMed.
- F. U. Din, W. Aman and I. Ullah, et al., Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors, Int. J. Nanomed., 2017, 7291–7309 CrossRef.
- L. Sun, H. Liu and Y. Ye, et al., Smart nanoparticles for cancer therapy, Signal Transduction Targeted Ther., 2023, 8(1), 418 CrossRef PubMed.
- M. J. Rupar, P. Golusinski, W. Golusinski and M. M. Masternak, Human Papillomavirus and the use of nanoparticles for immunotherapy in HPV-related cancer: A review, Rep. Pract. Oncol. Radiother., 2019, 24(6), 544–550 CrossRef PubMed.
- X. Zhao, Y. Zhang and O. Trejo-Cerro, et al., A safe and potentiated multi-type HPV L2-E7 nanoparticle vaccine with combined prophylactic and therapeutic activity, npj Vaccines, 2024, 9(1), 119 CrossRef CAS PubMed.
- B. Wang, S. Hu and Y. Teng, et al., Current advance of nanotechnology in diagnosis and treatment for malignant tumors, Signal Transduction Targeted Ther., 2024, 9(1), 200 CrossRef PubMed.
- L. Zhang, F. X. Gu, J. M. Chan, A. Z. Wang, R. S. Langer and O. C. Farokhzad, Nanoparticles in medicine: therapeutic applications and developments, Clin. Pharmacol. Ther., 2008, 83(5), 761–769 CrossRef CAS PubMed.
- A. Awada and P. G. Aftimos, Targeted therapies of solid cancers: new options, new challenges, Curr. Opin. Oncol., 2013, 25(3), 296–304 CrossRef CAS PubMed.
- Y. Diesendruck and I. Benhar, Novel immune check point inhibiting antibodies in cancer therapy—Opportunities and challenges, Drug Resistance Updates, 2017, 30, 39–47 CrossRef PubMed.
- Y. Hu, J. Song and A. Feng, et al., Recent Advances in Nanotechnology-Based Targeted Delivery Systems of Active Constituents in Natural Medicines for Cancer Treatment, Molecules, 2023, 28(23), 7767 CrossRef CAS PubMed.
- M. Durán-Lobato, A. M. López-Estévez and A. S. Cordeiro, et al., Nanotechnologies for the delivery of biologicals: Historical perspective and current landscape, Adv. Drug Delivery Rev., 2021, 176, 113899 CrossRef PubMed.
- S. Er, U. Laraib and R. Arshad, et al., Amino acids, peptides, and proteins: implications for nanotechnological applications in biosensing and drug/gene delivery, Nanomaterials, 2021, 11(11), 3002 CrossRef CAS PubMed.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Engineering precision nanoparticles for drug delivery, Nat. Rev. Drug Discovery, 2021, 20(2), 101–124 CrossRef CAS PubMed.
- Y. Zhou, E. Rassy and A. Coutte, et al., Current standards in the management of early and locally advanced cervical cancer: update on the benefit of neoadjuvant/adjuvant strategies, Cancers, 2022, 14(10), 2449 CrossRef PubMed.
- X. Cheng, Q. Xie and Y. Sun, Advances in nanomaterial-based targeted drug delivery systems, Front. Bioeng. Biotechnol., 2023, 11, 1177151 CrossRef PubMed.
- P. Ghasemiyeh and S. Mohammadi-Samani, Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages, Res. Pharm. Sci., 2018, 13(4), 288–303 CrossRef PubMed.
- M. K. Satapathy, T. L. Yen and J. S. Jan, et al., Solid lipid nanoparticles (SLNs): an advanced drug delivery system targeting brain through BBB, Pharmaceutics, 2021, 13(8), 1183 CrossRef CAS PubMed.
- O. A. Madkhali, Perspectives and prospective on solid lipid nanoparticles as drug delivery systems, Molecules, 2022, 27(5), 1543 CrossRef CAS PubMed.
- N. Dhiman, R. Awasthi, B. Sharma, H. Kharkwal and G. T. Kulkarni, Lipid nanoparticles as carriers for bioactive delivery, Front. Chem., 2021, 9, 580118 CrossRef CAS PubMed.
- N. Naseri, H. Valizadeh and P. Zakeri-Milani, Solid lipid nanoparticles and nanostructured lipid carriers: structure, preparation and application, Adv. Pharm. Bull., 2015, 5(3), 305 CrossRef CAS PubMed.
- J. Qi, Y. Lu and W. Wu, Absorption, disposition and pharmacokinetics of solid lipid nanoparticles, Curr. Drug Metab., 2012, 13(4), 418–428 CrossRef CAS PubMed.
- D. L. Pink, O. Loruthai and R. M. Ziolek, et al., On the structure of solid lipid nanoparticles, Small, 2019, 15(45), 1903156 CrossRef CAS PubMed.
- K. Rajpoot, Solid lipid nanoparticles: a promising nanomaterial in drug delivery, Curr. Pharm. Des., 2019, 25(37), 3943–3959 CrossRef CAS PubMed.
- S. Pandey, F. Shaikh, A. Gupta, P. Tripathi and J. S. Yadav, A recent update: solid lipid nanoparticles for effective drug delivery, Adv. Pharm. Bull., 2022, 12(1), 17 CAS.
- P. Talele, S. Sahu and A. K. Mishra, Physicochemical characterization of solid lipid nanoparticles comprised of glycerol monostearate and bile salts, Colloids Surf., B, 2018, 172, 517–525 CrossRef CAS PubMed.
- M. Garcia-Fuentes, M. J. Alonso and D. Torres, Design and characterization of a new drug nanocarrier made from solid–liquid lipid mixtures, J. Colloid Interface Sci., 2005, 285(2), 590–598 CrossRef CAS PubMed.
- V. Mishra, K. K. Bansal and A. Verma, et al., Solid lipid nanoparticles: Emerging colloidal nano drug delivery systems, Pharmaceutics, 2018, 10(4), 191 CrossRef CAS PubMed.
- K. M. Rosenblatt and H. Bunjes, Evaluation of the drug loading capacity of different lipid nanoparticle dispersions by passive drug loading, Eur. J. Pharm. Biopharm., 2017, 117, 49–59 CrossRef CAS PubMed.
- J. Ezzati Nazhad Dolatabadi, H. Hamishehkar and H. Valizadeh, Development of dry powder inhaler formulation loaded with alendronate solid lipid nanoparticles: solid-state characterization and aerosol dispersion performance, Drug Dev. Ind. Pharm., 2015, 41(9), 1431–1437 CrossRef CAS PubMed.
- R. Chutoprapat, P. Kopongpanich and L. W. Chan, A mini-review on solid lipid nanoparticles and nanostructured lipid carriers: topical delivery of phytochemicals for the treatment of acne vulgaris, Molecules, 2022, 27(11), 3460 CrossRef CAS PubMed.
- H. Patil, V. Kulkarni, S. Majumdar and M. A. Repka, Continuous manufacturing of solid lipid nanoparticles by hot melt extrusion, Int. J. Pharm., 2014, 471(1–2), 153–156 CrossRef CAS PubMed.
- E. Musielak, A. Feliczak-Guzik and I. Nowak, Optimization of the conditions of solid lipid nanoparticles (SLN) synthesis, Molecules, 2022, 27(7), 2202 CrossRef CAS PubMed.
- M. Geszke-Moritz and M. Moritz, Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies, Mater. Sci. Eng., C, 2016, 68, 982–994 CrossRef CAS PubMed.
- J. German-Cortés, M. Vilar-Hernández, D. Rafael, I. Abasolo and F. Andrade, Solid lipid nanoparticles: multitasking nano-carriers for cancer treatment, Pharmaceutics, 2023, 15(3), 831 CrossRef PubMed.
- I. Gómez-Aguado, J. Rodríguez-Castejón, M. Vicente-Pascual, A. Rodríguez-Gascón, A. del Pozo-Rodríguez and M.Á Solinís Aspiazu, Nucleic acid delivery by solid lipid nanoparticles containing switchable lipids: plasmid DNA vs. messenger RNA, Molecules, 2020, 25(24), 5995 CrossRef PubMed.
- S. Haque, M. Whittaker, M. P. McIntosh, C. W. Pouton, S. Phipps and L. M. Kaminskas, A comparison of the lung clearance kinetics of solid lipid nanoparticles and liposomes by following the 3H-labelled structural lipids after pulmonary delivery in rats, Eur. J. Pharm. Biopharm., 2018, 125, 1–12 CrossRef CAS PubMed.
- W. Poon, Y. N. Zhang and B. Ouyang, et al., Elimination pathways of nanoparticles, ACS Nano, 2019, 13(5), 5785–5798 CrossRef CAS PubMed.
- B. Liu, H. Zhou, L. Tan, K. T. H. Siu and X. Y. Guan, Exploring treatment options in cancer: tumor treatment strategies, Signal Transduction Targeted Ther., 2024, 9(1), 175 CrossRef PubMed.
- A. Chow, K. Perica, C. A. Klebanoff and J. D. Wolchok, Clinical implications of T cell exhaustion for cancer immunotherapy, Nat. Rev. Clin. Oncol., 2022, 19(12), 775–790 CrossRef PubMed.
- M. Hong, J. D. Clubb and Y. Y. Chen, Engineering CAR-T cells for next-generation cancer therapy, Cancer Cell, 2020, 38(4), 473–488 CrossRef CAS PubMed.
- X. Li and Y. Song, Proteolysis-targeting chimera (PROTAC) for targeted protein degradation and cancer therapy, J. Hematol. Oncol., 2020, 13, 1–14 CrossRef PubMed.
- P. Cooray, Comment on “Redefining cancer research for therapeutic breakthroughs”, Br. J. Cancer, 2024, 1–2 Search PubMed.
- G. Choi, G. Shin and S. Bae, Price and prejudice? The value of chimeric antigen receptor (CAR) T-cell therapy, Int. J. Environ. Res. Public Health, 2022, 19(19), 12366 CrossRef CAS PubMed.
- A. Haslam and V. Prasad, Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs, JAMA Netw. Open, 2019, 2(5), e192535–e192535 CrossRef PubMed.
- J. Zugazagoitia, C. Guedes, S. Ponce, I. Ferrer, S. Molina-Pinelo and L. Paz-Ares, Current challenges in cancer treatment, Clin. Ther., 2016, 38(7), 1551–1566 CrossRef PubMed.
- A. Kurtovic-Kozaric, S. Vranic and S. Kurtovic, et al., Lack of access to targeted cancer treatment modalities in the developing world in the era of precision medicine: real-life lessons from Bosnia, J. Glob. Oncol., 2018, 4, 1–5 Search PubMed.
- J. A. Kemp and Y. J. Kwon, Cancer nanotechnology: current status and perspectives, Nano Converg., 2021, 8(1), 34 CrossRef CAS PubMed.
- M. A. Rahman, A. Ali and M. Rahamathulla, et al., Fabrication of sustained release curcumin-loaded solid lipid nanoparticles (cur-SLNs) as a potential drug delivery system for the treatment of lung cancer: Optimization of formulation and in vitro biological evaluation, Polymers, 2023, 15(3), 542 CrossRef CAS PubMed.
- W. Wang, M. Zhou and Y. Xu, et al., Resveratrol-loaded tpgs-resveratrol-solid lipid nanoparticles for multidrug-resistant therapy of breast cancer: in vivo and in vitro study, Front. Bioeng. Biotechnol., 2021, 9, 762489 CrossRef PubMed.
- M. Hatami, M. Kouchak, A. Kheirollah, L. Khorsandi and M. Rashidi, Quercetin-loaded solid lipid nanoparticles exhibit antitumor activity and suppress the proliferation of triple-negative MDA-MB 231 breast cancer cells: implications for invasive breast cancer treatment, Mol. Biol. Rep., 2023, 50(11), 9417–9430 CrossRef CAS PubMed.
- T. Smith, K. Affram and E. L. Nottingham, et al., Application of smart solid lipid nanoparticles to enhance the efficacy of 5-fluorouracil in the treatment of colorectal cancer, Sci. Rep., 2020, 10(1), 16989 CrossRef CAS PubMed.
- M. Akanda, G. Getti, U. Nandi, M. S. Mithu and D. Douroumis, Bioconjugated solid lipid nanoparticles (SLNs) for targeted prostate cancer therapy, Int. J. Pharm., 2021, 599, 120416 CrossRef CAS PubMed.
- G. S. Bhagwat, R. B. Athawale and R. P. Gude, et al., Formulation and development of transferrin targeted solid lipid nanoparticles for breast cancer therapy, Front. Pharmacol., 2020, 11, 614290 CrossRef CAS PubMed.
- K. Rajpoot and S. K. Jain, Colorectal cancer-targeted delivery of oxaliplatin via folic acid-grafted solid lipid nanoparticles: preparation, optimization, and in vitro evaluation, Artif. Cells, Nanomed., Biotechnol., 2018, 46(6), 1236–1247 CrossRef CAS PubMed.
- R. Rosiere, M. Van Woensel and M. Gelbcke, et al., New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation, Mol. Pharm., 2018, 15(3), 899–910 CrossRef CAS PubMed.
- H. Pawar, S. K. Surapaneni and K. Tikoo, et al., Folic acid functionalized long-circulating co-encapsulated docetaxel and curcumin solid lipid nanoparticles: in vitro evaluation, pharmacokinetic and biodistribution in rats, Drug Delivery, 2016, 23(4), 1453–1468 CrossRef CAS PubMed.
- S. F. Tabatabaeain, E. Karimi and M. Hashemi, Satureja khuzistanica essential oil-loaded solid lipid nanoparticles modified with chitosan-folate: Evaluation of encapsulation efficiency, cytotoxic and pro-apoptotic properties, Front. Chem., 2022, 10, 904973 CrossRef CAS PubMed.
- C. S. Kumar, R. Thangam, S. A. Mary, P. R. Kannan, G. Arun and B. Madhan, Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells, Carbohydr. Polym., 2020, 231, 115682 CrossRef PubMed.
- K. Rajpoot and S. K. Jain, Oral delivery of pH-responsive alginate microbeads incorporating folic acid-grafted solid lipid nanoparticles exhibits enhanced targeting effect against colorectal cancer: A dual-targeted approach, Int. J. Biol. Macromol., 2020, 151, 830–844 CrossRef CAS PubMed.
- M. G. Fuster, J. Wang, O. Fandiño, G. Víllora and A. J. Paredes, Folic acid-decorated nanocrystals as highly loaded trojan horses to target cancer cells, Mol. Pharm., 2024, 21(6), 2781–2794 CrossRef CAS PubMed.
- G. Zhang, F. Liu, E. Jia, L. Jia and Y. Zhang, Folate-modified, cisplatin-loaded lipid carriers for cervical cancer chemotherapy, Drug Delivery, 2016, 23(4), 1393–1397 CrossRef CAS PubMed.
- S. V. Mussi and V. P. Torchilin, Recent trends in the use of lipidic nanoparticles as pharmaceutical carriers for cancer therapy and diagnostics, J. Mater. Chem. B, 2013, 1(39), 5201–5209 RSC.
- M. Conte, M. Carofiglio, G. Rosso and V. Cauda, Lipidic formulations inspired by COVID vaccines as smart coatings to enhance nanoparticle-based cancer therapy, Nanomaterials, 2023, 13(15), 2250 CrossRef CAS PubMed.
- T. B. Gandek, L. van der Koog and A. Nagelkerke, A comparison of cellular uptake mechanisms, delivery efficacy, and intracellular fate between liposomes and extracellular vesicles, Adv. Healthcare Mater., 2023, 12(25), 2300319 CrossRef CAS PubMed.
- S. Martins, S. Costa-Lima, T. Carneiro, A. Cordeiro-da-Silva, E. B. Souto and D. C. Ferreira, Solid lipid nanoparticles as intracellular drug transporters: an investigation of the uptake mechanism and pathway, Int. J. Pharm., 2012, 430(1–2), 216–227 CrossRef CAS PubMed.
- I. Canton and G. Battaglia, Endocytosis at the nanoscale, Chem. Soc. Rev., 2012, 41(7), 2718–2739 RSC.
- S. Christoforidis, H. M. McBride, R. D. Burgoyne and M. Zerial, The Rab5 effector EEA1 is a core component of endosome docking, Nature, 1999, 397(6720), 621–625 CrossRef CAS PubMed.
- A. Gautreau, K. Oguievetskaia and C. Ungermann, Function and regulation of the endosomal fusion and fission machineries, Cold Spring Harbor Perspect. Biol., 2014, 6(3), a016832 CrossRef PubMed.
- J. G. Joseph and A. P. Liu, Mechanical regulation of endocytosis: new insights and recent advances, Adv. Biosyst., 2020, 4(5), 1900278 CrossRef PubMed.
- L. Arana, L. Bayón-Cordero, L. I. Sarasola, M. Berasategi, S. Ruiz and I. Alkorta, Solid lipid nanoparticles surface modification modulates cell internalization and improves chemotoxic treatment in an oral carcinoma cell line, Nanomaterials, 2019, 9(3), 464 CrossRef CAS PubMed.
- M. C. Cavaco, C. Pereira and B. Kreutzer, et al., Evading P-glycoprotein mediated-efflux chemoresistance using Solid Lipid Nanoparticles, Eur. J. Pharm. Biopharm., 2017, 110, 76–84 CrossRef CAS PubMed.
- A. Granja, C. Nunes, C. T. Sousa and S. Reis, Folate receptor-mediated delivery of mitoxantrone-loaded solid lipid nanoparticles to breast cancer cells, Biomed. Pharmacother., 2022, 154, 113525 CrossRef CAS PubMed.
- T. Garanti, A. Stasik, A. J. Burrow, M. A. Alhnan and K. W. Wan, Anti-glioma activity and the mechanism of cellular uptake of asiatic acid-loaded solid lipid nanoparticles, Int. J. Pharm., 2016, 500(1–2), 305–315, DOI:10.1016/j.ijpharm.2016.01.018.
- A. S. Al Khafaji and M. D. Donovan, Endocytic uptake of solid lipid nanoparticles by the nasal mucosa, Pharmaceutics, 2021, 13(5), 761 CrossRef CAS PubMed.
- S. Scioli Montoto, G. Muraca and M. E. Ruiz, Solid lipid nanoparticles for drug delivery: pharmacological and biopharmaceutical aspects, Front. Mol. Biosci., 2020, 7, 587997 CrossRef PubMed.
- M. Akanda, M. D. S. H. Mithu and D. Douroumis, Solid lipid nanoparticles: An effective lipid-based technology for cancer treatment, J. Drug Delivery Sci. Technol., 2023, 104709 CrossRef CAS.
- E. Lee, L. N. Dang, J. Choi, H. Kim, L. Bastatas and S. Park, Elasticity–Driven Nanomechanical Interaction to Improve the Targeting Ability of Lipid Nanoparticles in the Malignant Tumor Microenvironment, Adv. Sci., 2025, 2502073 CrossRef PubMed.
- T. Moraes-Lacerda and M. B. de Jesus, Mechanisms of solid lipid nanoparticles-triggered signaling pathways in eukaryotic cells, Colloids Surf., B, 2022, 220, 112863 CrossRef CAS PubMed.
- R. M. Shah, D. Rajasekaran, M. Ludford-Menting, D. S. Eldridge, E. A. Palombo and I. H. Harding, Transport of stearic acid-based solid lipid nanoparticles (SLNs) into human epithelial cells, Colloids Surf., B, 2016, 140, 204–212, DOI:10.1016/j.colsurfb.2015.12.029.
- I. Waheed, A. Ali, H. Tabassum, N. Khatoon, W. F. Lai and X. Zhou, Lipid-based nanoparticles as drug delivery carriers for cancer therapy, Front. Oncol., 2024, 14, 1296091 CrossRef CAS PubMed.
- S. Karamchedu, L. Tunki, H. Kulhari and D. Pooja, Morin hydrate loaded solid lipid nanoparticles: Characterization, stability, anticancer activity, and bioavailability, Chem. Phys. Lipids, 2020, 233, 104988, DOI:10.1016/j.chemphyslip.2020.104988.
- J. Y. Wang, Y. Wang and X. Meng, Chitosan Nanolayered Cisplatin-Loaded Lipid Nanoparticles for Enhanced Anticancer Efficacy in Cervical Cancer, Nanoscale Res. Lett., 2016, 11(1), 524, DOI:10.1186/s11671-016-1698-9.
- Z. J. Chen, Z. Zhang, B. B. Xie and H. Y. Zhang, Development and evaluation of topotecan loaded solid lipid nanoparticles: A study in cervical cancer cell lines, J. Photochem. Photobiol., B, 2016, 165, 182–188, DOI:10.1016/j.jphotobiol.2016.10.019.
- B. Liu, L. Han, J. Liu, S. Han, Z. Chen and L. Jiang, Co-delivery of paclitaxel and TOS-cisplatin via TAT-targeted solid lipid nanoparticles with synergistic antitumor activity against cervical cancer, Int. J. Nanomed., 2017, 955–968 CrossRef CAS PubMed.
- F. Wang, L. Li, B. Liu, Z. Chen and C. Li, Hyaluronic acid decorated pluronic P85 solid lipid nanoparticles as a potential carrier to overcome multidrug resistance in cervical and breast cancer, Biomed. Pharmacother., 2017, 86, 595–604, DOI:10.1016/j.biopha.2016.12.041.
- S. A. Adeyemi, Z. Az-Zamakhshariy and Y. E. Choonara, In Vitro Prototyping of a Nano-Organogel for Thermo-Sonic Intra-Cervical Delivery of 5-Fluorouracil-Loaded Solid Lipid Nanoparticles for Cervical Cancer, AAPS PharmSciTech, 2023, 24(5), 123, DOI:10.1208/s12249-023-02583-y.
- N. Abd Rashid, N. H. Mohamad Najib, N. A. Abdul Jalil and S. L. Teoh, Essential Oils in Cervical Cancer: Narrative Review on Current Insights and Future Prospects, Antioxidants, 2023, 12(12), 2109, DOI:10.3390/antiox12122109.
- G. Tang, Z. Zhou and X. Zhang, et al., Fabrication of supramolecular self-assembly of the Schiff base complex for improving bioavailability of aldehyde-containing plant essential oil, Chem. Eng. J., 2023, 471, 144471 CrossRef CAS.
- R. Azadmanesh, M. Tatari, A. Asgharzade, S. F. Taghizadeh and A. Shakeri, GC/MS profiling and biological traits of Eucalyptus globulus L. essential oil exposed to solid lipid nanoparticle (SLN), J. Essent. Oil Bear. Plants, 2021, 24(4), 863–878 CrossRef CAS.
- S. Zhang, Y. Zhang and Z. Wang, et al., Temperature-sensitive gel-loaded composite nanomedicines for the treatment of cervical cancer by vaginal delivery, Int. J. Pharm., 2020, 586, 119616, DOI:10.1016/j.ijpharm.2020.119616.
- H. A. Abou-Taleb, Z. Fathalla, D. M. Naguib, A. A. Fatease and H. Abdelkader, Chitosan/solid-lipid nanoparticles hybrid gels for vaginal delivery of estradiol for management of vaginal menopausal symptoms, Pharmaceuticals, 2023, 16(9), 1284 CrossRef CAS PubMed.
- B. Le-Vinh, C. Steinbring, R. Wibel, J. D. Friedl and A. Bernkop-Schnürch, Size shifting of solid lipid nanoparticle system triggered by alkaline phosphatase for site specific mucosal drug delivery, Eur. J. Pharm. Biopharm., 2021, 163, 109–119 CrossRef CAS.
- M. A. Mirza, A. K. Panda and S. Asif, et al., A vaginal drug delivery model, Drug Delivery, 2016, 23(8), 3123–3134 CrossRef CAS PubMed.
- G. Büyükköroğlu, B. Şenel and E. Yenilmez, Vaginal Suppositories with siRNA and Paclitaxel-Incorporated Solid Lipid Nanoparticles for Cervical Cancer: Preparation and In Vitro Evaluation, Methods Mol. Biol., 2019, 1974, 303–328, DOI:10.1007/978-1-4939-9220-1_22.
- J. R. Campos, P. Severino and A. Santini, et al., Solid lipid nanoparticles (SLN): prediction of toxicity, metabolism, fate and physicochemical properties, Nanopharmaceuticals, 2020, 1–15 Search PubMed.
- A. Deshpande, M. Mohamed, S. B. Daftardar, M. Patel, S. H. S. Boddu and J. Nesamony, Solid lipid nanoparticles in drug delivery: Opportunities and challenges, Emerging Nanotechnol. Diagn., Drug Delivery Med. Devices, 2017, 291–330 CAS.
- A. Trivino, A. Gumireddy and H. Chauhan, Drug-lipid-surfactant miscibility for the development of solid lipid nanoparticles, AAPS PharmSciTech, 2019, 20, 1–9 CrossRef PubMed.
- K. Göke and H. Bunjes, Drug solubility in lipid nanocarriers: Influence of lipid matrix and available interfacial area, Int. J. Pharm., 2017, 529(1–2), 617–628 CrossRef PubMed.
- C. D. Pizzol, A. O. Beringhs, E. Winter, D. Sonaglio, A. M. de Campos and T. B. Creczynski-Pasa, Application of response surface methodology for the technological improvement of solid lipid nanoparticles, J. Nanosci. Nanotechnol., 2016, 16(2), 1238–1247 CrossRef PubMed.
- J. D. Clogston and A. K. Patri, Zeta potential measurement, Charact. NPs Intend. Drug Delivery, 2010, 63–70 Search PubMed.
- B. Chen, W. Le and Y. Wang, et al., Targeting negative surface charges of cancer cells by multifunctional nanoprobes, Theranostics, 2016, 6(11), 1887 CrossRef CAS PubMed.
- Z. Deng, J. Lin and S. L. Bud'ko, et al., Dual targeting with cell surface electrical charge and folic acid via superparamagnetic Fe3O4@ Cu2−xS for photothermal cancer cell killing, Cancers, 2021, 13(21), 5275 CrossRef CAS PubMed.
- C. Carbone, B. Tomasello, B. Ruozi, M. Renis and G. Puglisi, Preparation and optimization of PIT solid lipid nanoparticles via statistical factorial design, Eur. J. Med. Chem., 2012, 49, 110–117 CrossRef CAS PubMed.
- Y. Luo, Z. Teng, Y. Li and Q. Wang, Solid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake, Carbohydr. Polym., 2015, 122, 221–229 CrossRef CAS PubMed.
- C. Charcosset and H. Fessi, Preparation of nanoparticles with a membrane contactor, J. Membr. Sci., 2005, 266(1–2), 115–120 CrossRef CAS.
- M. Sznitowska, E. Wolska, H. Baranska, K. Cal and J. Pietkiewicz, The effect of a lipid composition and a surfactant on the characteristics of the solid lipid microspheres and nanospheres (SLM and SLN), Eur. J. Pharm. Biopharm., 2017, 110, 24–30 CrossRef CAS PubMed.
- C. Vitorino, F. A. Carvalho, A. J. Almeida, J. J. Sousa and A. A. C. C. Pais, The size of solid lipid nanoparticles: an interpretation from experimental design, Colloids Surf., B, 2011, 84(1), 117–130 CrossRef CAS PubMed.
- C. Botto, N. Mauro, E. Amore, E. Martorana, G. Giammona and M. L. Bondì, Surfactant effect on the physicochemical characteristics of cationic solid lipid nanoparticles, Int. J. Pharm., 2017, 516(1–2), 334–341 CrossRef CAS PubMed.
- T. Helgason, T. S. Awad, K. Kristbergsson, D. J. McClements and J. Weiss, Effect of surfactant surface coverage on formation of solid lipid nanoparticles (SLN), J. Colloid Interface Sci., 2009, 334(1), 75–81 CrossRef CAS PubMed.
- N. Mosallaei, M. R. Jaafari, M. Y. Hanafi-Bojd, S. Golmohammadzadeh and B. Malaekeh-Nikouei, Docetaxel-loaded solid lipid nanoparticles: preparation, characterization, in vitro, and in vivo evaluations, J. Pharm. Sci., 2013, 102(6), 1994–2004 CrossRef CAS PubMed.
- V. Sanna, E. Gavini, M. Cossu, G. Rassu and P. Giunchedi, Solid lipid nanoparticles (SLN) as carriers for the topical delivery of econazole nitrate: in vitro characterization, ex vivo and in vivo studies, J. Pharm. Pharmacol., 2007, 59(8), 1057–1064 CrossRef CAS PubMed.
- B. Rodenak-Kladniew, G. A. Islan, M. G. de Bravo, N. Durán and G. R. Castro, Design, characterization and in vitro evaluation of linalool-loaded solid lipid nanoparticles as potent tool in cancer therapy, Colloids Surf., B, 2017, 154, 123–132 CrossRef CAS PubMed.
- G. Suresh, K. Manjunath, V. Venkateswarlu and V. Satyanarayana, Preparation, characterization, and in vitro and in vivo evaluation of lovastatin solid lipid nanoparticles, AAPS PharmSciTech, 2007, 8, E162–E170 CrossRef PubMed.
- D. J. Jang, C. Moon and E. Oh, Improved tumor targeting and antitumor activity of camptothecin loaded solid lipid nanoparticles by preinjection of blank solid lipid nanoparticles, Biomed. Pharmacother., 2016, 80, 162–172 CrossRef CAS PubMed.
- V. Campani, G. Salzano, S. Lusa and G. De Rosa, Lipid nanovectors to deliver RNA oligonucleotides in cancer, Nanomaterials, 2016, 6(7), 131 CrossRef PubMed.
- T. L. Hwang, I. A. Aljuffali, C. F. Lin, Y. T. Chang and J. Y. Fang, Cationic additives in nanosystems activate cytotoxicity and inflammatory response of human neutrophils: lipid nanoparticles versus polymeric nanoparticles, Int. J. Nanomed., 2015, 371–385 Search PubMed.
- M. L. Chen, Lipid excipients and delivery systems for pharmaceutical development: a regulatory perspective, Adv. Drug Delivery Rev., 2008, 60(6), 768–777 CrossRef CAS PubMed.
- K. Knop, R. Hoogenboom, D. Fischer and U. S. Schubert, Poly (ethylene glycol) in drug delivery: pros and cons as well as potential alternatives, Angew. Chem., Int. Ed., 2010, 49(36), 6288–6308 CrossRef CAS PubMed.
- A. S. A. Lila, H. Kiwada and T. Ishida, The accelerated blood clearance (ABC) phenomenon: clinical challenge and approaches to manage, J. Controlled Release, 2013, 172(1), 38–47 CrossRef PubMed.
- K. Nagaraj, Surfactant-based drug delivery systems for cancer therapy: Advances, challenges, and future perspectives, Int. J. Pharm., 2025, 125655 CrossRef CAS PubMed.
- S. S. Hallan, M. Sguizzato, E. Esposito and R. Cortesi, Challenges in the physical characterization of lipid nanoparticles, Pharmaceutics, 2021, 13(4), 549 CrossRef CAS PubMed.
- J. Wang, Y. Ding and K. Chong, et al., Recent advances in lipid nanoparticles and their safety concerns for mRNA delivery, Vaccines, 2024, 12(10), 1148 CrossRef CAS PubMed.
- X. Hou, T. Zaks, R. Langer and Y. Dong, Lipid nanoparticles for mRNA delivery, Nat. Rev. Mater., 2021, 6(12), 1078–1094 CrossRef CAS PubMed.
- M. M. Lalu, J. Montroy and C. G. Begley, et al., Identifying and understanding factors that affect the translation of therapies from the laboratory to patients: a study protocol, F1000Research, 2020, 9, 485 CAS.
- S. Sangeetha and D. Narayanasamy, The Science of Solid Lipid Nanoparticles: From Fundamentals to Applications, Cureus, 2024, 16(9) Search PubMed.
- A. S. Chauhan, P. Chand and T. Parashar, Lipid-based Nanoparticles: Strategy for Targeted Cancer Therapy, BIO Integr., 2025, 6(1), 994 Search PubMed.
- D. Rosenblum, N. Joshi, W. Tao, J. M. Karp and D. Peer, Progress and challenges towards targeted delivery of cancer therapeutics, Nat. Commun., 2018, 9(1), 1410 CrossRef PubMed.
- S. V. Khairnar, P. Pagare and A. Thakre, et al., Review on the scale-up methods for the preparation of solid lipid nanoparticles, Pharmaceutics, 2022, 14(9), 1886 CrossRef CAS PubMed.
- D. Sivadasan, K. Ramakrishnan, J. Mahendran, H. Ranganathan, A. Karuppaiah and H. Rahman, Solid lipid nanoparticles: applications and prospects in cancer treatment, Int. J. Mol. Sci., 2023, 24(7), 6199 CrossRef CAS PubMed.
- V. Harish, S. Mohd and D. Tewari, et al., Unravelling the role of solid lipid nanoparticles in drug delivery: Journey from laboratory to clinical trial, J. Drug Delivery Sci. Technol., 2023, 85, 104616 CrossRef CAS.
- K. Glaubitt, M. Ricci and S. Giovagnoli, Exploring the nano spray-drying technology as an innovative manufacturing method for solid lipid nanoparticle dry powders, AAPS PharmSciTech, 2019, 20, 1–14 CrossRef CAS PubMed.
- D. G. Lopes, I. Koutsamanis and K. Becker, et al., Microphase separation in solid lipid dosage forms as the cause of drug release instability, Int. J. Pharm., 2017, 517(1–2), 403–412 CrossRef CAS PubMed.
- V. Makwana, R. Jain, K. Patel, M. Nivsarkar and A. Joshi, Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach, Int. J. Pharm., 2015, 495(1), 439–446 CrossRef CAS PubMed.
- S. Yeo, S. Jung, H. Kim, J. H. Ahn and S. J. Hwang, 4-Hexylresorcinol Loaded Solid Lipid Nanoparticles for Enhancing Anticancer Activity, Pharmaceuticals, 2024, 17(10), 1296 CrossRef CAS PubMed.
- Y. Duan, A. Dhar and C. Patel, et al., A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems, RSC Adv., 2020, 10(45), 26777–26791 RSC.
- V. K. Venishetty, R. Komuravelli, M. Kuncha, R. Sistla and P. V. Diwan, Increased brain uptake of docetaxel and ketoconazole loaded folate-grafted solid lipid nanoparticles, Nanomedicine, 2013, 9(1), 111–121 CrossRef CAS PubMed.
- K. H. Bae, J. Y. Lee, S. H. Lee, T. G. Park and Y. S. Nam, Optically traceable solid lipid nanoparticles loaded with siRNA and paclitaxel for synergistic chemotherapy with in situ imaging, Adv. Healthcare Mater., 2013, 2(4), 576–584 CrossRef CAS PubMed.
- H. W. Chan, S. Chow, X. Zhang, P. C. L. Kwok and S. F. Chow, Role of particle size in translational research of nanomedicines for successful drug delivery: Discrepancies and inadequacies, J. Pharm. Sci., 2023, 112(9), 2371–2384 CrossRef CAS PubMed.
- S. M. Kamali Shahri, S. Sharifi and M. Mahmoudi, Interdependency of influential parameters in therapeutic nanomedicine, Expert Opin. Drug Delivery, 2021, 18(10), 1379–1394 CrossRef CAS PubMed.
- U. Ben-David, G. Ha and Y. Y. Tseng, et al., Patient-derived xenografts undergo mouse-specific tumor evolution, Nat. Genet., 2017, 49(11), 1567–1575 CrossRef CAS PubMed.
- M. V. Guerin, V. Finisguerra, B. J. Van den Eynde, N. Bercovici and A. Trautmann, Preclinical murine tumor models: a structural and functional perspective, Elife, 2020, 9, e50740 CrossRef CAS PubMed.
- Z. A. Ely, N. Mathey-Andrews and S. Naranjo, et al., A prime editor mouse to model a broad spectrum of somatic mutations in vivo, Nat. Biotechnol., 2024, 42(3), 424–436 CrossRef CAS PubMed.
- A. Albanese, A. K. Lam, E. A. Sykes, J. V. Rocheleau and W. C. W. Chan, Tumour-on-a-chip provides an optical window into nanoparticle tissue transport, Nat. Commun., 2013, 4(1), 2718 CrossRef PubMed.
- M. R. Carvalho, D. Barata and L. M. Teixeira, et al., Colorectal tumor-on-a-chip system: A 3D tool for precision onco-nanomedicine, Sci. Adv., 2019, 5(5), eaaw1317 CrossRef CAS PubMed.
- C. Tian, S. Zheng, X. Liu and K. Kichiro, Tumor-on-a-chip model for advancement of anti-cancer nano drug delivery system, J. Nanobiotechnol., 2022, 20(1), 338 CrossRef PubMed.
- H. F. Tsai, A. Trubelja, A. Q. Shen and G. Bao, Tumour-on-a-chip: microfluidic models of tumour morphology, growth and microenvironment, J. R. Soc. Interface, 2017, 14(131), 20170137 CrossRef PubMed.
- E. Boedtkjer and S. F. Pedersen, The acidic tumor microenvironment as a driver of cancer, Annu. Rev. Physiol., 2020, 82(1), 103–126 CrossRef CAS PubMed.
- K. R. Gajbhiye, R. Salve, M. Narwade, A. Sheikh, P. Kesharwani and V. Gajbhiye, Lipid polymer hybrid nanoparticles: a custom-tailored next-generation approach for cancer therapeutics, Mol. Cancer, 2023, 22(1), 160 CrossRef PubMed.
- K. Kessenbrock, V. Plaks and Z. Werb, Matrix metalloproteinases: regulators of the tumor microenvironment, Cell, 2010, 141(1), 52–67 CrossRef CAS PubMed.
- V. Petrova, M. Annicchiarico-Petruzzelli, G. Melino and I. Amelio, The hypoxic tumour microenvironment, Oncogenesis, 2018, 7(1), 10 CrossRef PubMed.
- M. J. Smyth, S. F. Ngiow, A. Ribas and M. W. L. Teng, Combination cancer immunotherapies tailored to the tumour microenvironment, Nat. Rev. Clin. Oncol., 2016, 13(3), 143–158 CrossRef CAS PubMed.
Footnote |
| † Equally contributing author. |
| This journal is © The Royal Society of Chemistry 2025 |
