Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photo-controllable cytotoxicity in cell culture using a diarylethene photoswitch†
Kimio
Sumaru
 *a,
Norikazu
Maruyama
b,
Kana
Morishita
a,
Satoshi
Yokojima
*a,
Norikazu
Maruyama
b,
Kana
Morishita
a,
Satoshi
Yokojima
 c and
Kingo
Uchida
c and
Kingo
Uchida
 *b
*b
aCellular and Molecular Biotechnology Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), AIST Tsukuba Central 5, 1-1-1 Higashi, Tsukuba, Ibaraki 305-8565, Japan. E-mail: k.sumaru@aist.go.jp
bDepartment of Materials Chemistry, Faculty of Science and Technology, Ryukoku University, Otsu, Shiga 520-2194, Japan. E-mail: uchida@rins.ryukoku.ac.jp
cSchool of Pharmacy, Tokyo University of Pharmacy and Life Sciences, 1432-1 Horinouchi, Hachioji, Tokyo 192-0392, Japan
First published on 29th May 2025
Abstract
For diarylethene (DAE) derivatives, whose dark cytotoxicity varies greatly depending on their isomerization state, we observe that the non-toxic open-ring isomer taken up by cells is converted to a toxic closed-ring isomer after only 10 seconds of UV light irradiation. This enables these derivatives to cause pronounced cell death after 1 day. Furthermore, when the closed-ring isomer is detoxified by opening the ring with green light irradiation after several intervals, cell damage increases with the period that DAE remains in the closed-ring state. The timescale at which the closed-ring isomer triggers the onset of cytotoxicity is considered in conjunction with the intercalation properties of DAE into DNA, and we also discuss the application of photonic molecular machines as a new technology for the control of biosystems.
Introduction
The ability to act on an object locally, remotely, and instantaneously makes light particularly suited for on-demand sterile control of microscopic biosystems. In practice, this property has led to the commercialization of automated systems for rapid processing of cultured cellular systems by combining light-responsive culture substrates and lasers.1 In addition to cell killing, photo-responsive control of cell differentiation or detachment has been implemented using photo-functional substrates.2,3 On the other hand, several attempts have been made to incorporate photoresponsive molecules into cells to control them by light from the outside. This technology kills cells by leveraging molecules that produce reactive oxygen species as sensitizers when exposed to light inside the cells, and it has already been widely applied clinically as photodynamic therapy (PDT).4,5 Recently, combined with antibody targeting, cancer photoimmunotherapy has attracted attention as the latest therapeutic technology.6,7 In addition, research is also underway to control biosystems by inducing photochromic molecules that change their structure under light to work inside cells based on a different mechanism from sensitization.8–10 In the first example of such an attempt, Branda et al. demonstrated that the movement of C. elegans that have taken up diarylethene (DAE) can be remotely controlled by externally inducing isomerization of DAE with light.11 And some diarylethenes have been applied in photopharmacology.12–14 Subsequently, in cell culture systems to which DAE derivatives were added at very low concentrations (<0.1 ppm), we found that the intercalation of DAE can be triggered by irradiation with UV light at 365 nm, which closes the open-ring form, and that irradiation of the molecules in this state with blue light at 436 nm causes pronounced phototoxicity, leading to rapid apoptosis of the cells.9 The cytotoxicity was found to be increasingly dependent on the concentration of DAE incorporated as a closed-ring isomer and the dose of blue light irradiation; moreover, it was observed that light irradiation on a timescale of 100 s at light intensities on the order of 100 mW cm−2 is required to induce cell death.10In this study, we investigate the photo-switching of cytotoxicity of a different type newly found for the DAE derivative 1,15 based on the dark toxicity which varies greatly depending on the isomerization state. We discuss the timescale required for the transient ring closure to doom the cells to death after 1 day, along with the intercalation properties of DAE into DNA.
Results and discussion
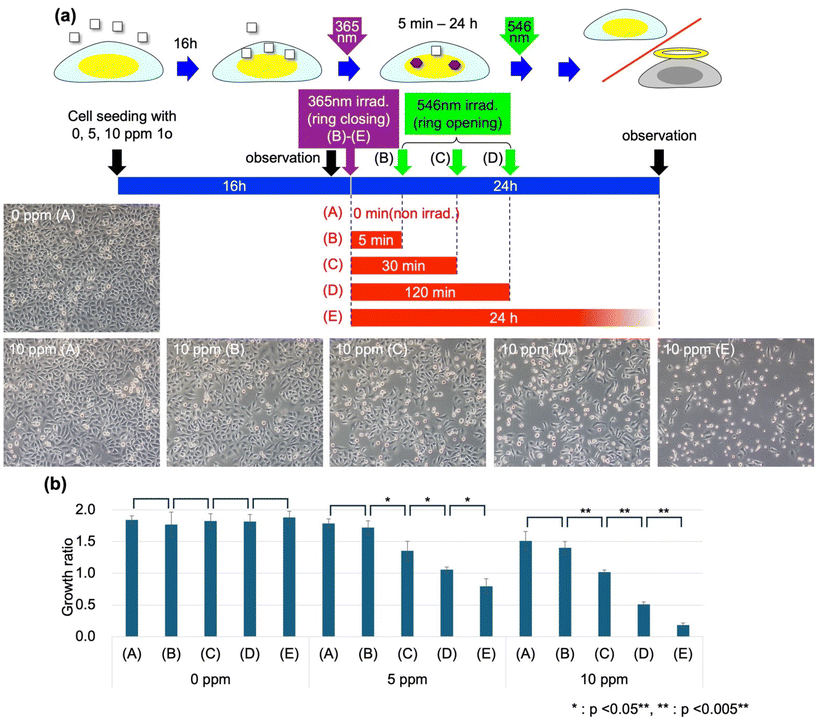
The structures and cellular effects of the two photoisomers of DAE used in this study are shown in Fig. 1(a). Prior to this experiment, we found that 1c, the closed-ring isomer, exhibited dark toxicity at concentrations of 5–10 ppm, whereas the corresponding open-ring isomer, 1o, had little effect on cell viability at these concentrations. In addition, we observed that the dark toxicity effects exhibited by 1c were not observed until about half a day after addition. This slow response is in contrast to the rapid (<1 h) cellular damage that occurs upon photoirradiation of dye molecules incorporated into cells, including our previous report on DAE (see the ESI†).9,10 UV-vis absorption spectra of 1o, 1c and the photostationary state under UV irradiation at 365 nm are shown in Fig. 1(b). Here, irradiation of 1o with UV light resulted in a 55.5% conversion to 1c and irradiation with a sufficient amount of green light at 500–550 nm resulted in a complete conversion to 1o.Fig. 2(a) shows an illustration of the overall experimental flow used to investigate the effects of photoswitching on the dark toxicity of DAE on cells. Experiments were conducted under three conditions (0, 5, and 10 ppm) for DAE coexistence concentration and five conditions ((A)–(E)) for the light irradiation schedule, as shown in Table 1, with a total of 15 conditions. HeLa cells were seeded on culture dishes in a culture medium containing 0, 5, or 10 ppm 1o. After 16 h of incubation, we observed the cells under a microscope, replaced the culture medium with a DAE-free one, and irradiated the samples with UV light at 365 nm for 10 s for conditions (B)–(E). The cells were then incubated for the prescribed time (ti, (B): 5 min, (C): 30 min, and (D): 120 min) and then irradiated with green light at 546 nm for 30 s. Twenty-four hours after the first observation, the cells were gently flushed with the culture medium to remove the cells detached due to damage, and microscopic observation was performed again. Fig. 2(a) shows the microscopy images of the cells in the second observation.
| Condition | UV irradiation | t i (min) | Green irradiation |
|---|---|---|---|
| Irradiation with UV and green light carried out at 16 and 40 h, respectively, after cell seeding. | |||
| (A) | No | — | No |
| (B) | Yes | 5 | Yes |
| (C) | Yes | 30 | Yes |
| (D) | Yes | 120 | Yes |
| (E) | Yes | — | No |
Under conditions without light irradiation, the coexistence of 10 ppm 1o had only a slight effect on cell density, but when UV light irradiation activated the toxicity by converting 1o to 1c, most cells were dead in 24 h (10 ppm (E)). Since no DAE was present in the culture medium at the time of UV irradiation due to the preceding medium change, this effect was thought to be due to the action of DAE already incorporated into the cells. 1o is nearly insoluble in water, and when added to an aqueous solution system after dissolving in ethanol, it was observed to gradually precipitate out. On the other hand, when added to cell culture systems, it was taken up efficiently by cells that are rich in lipids. The effects of UV light irradiation did not appear immediately and the irradiation dose was necessary and sufficient to convert more than half of the 1o to 1c; these observations clearly indicate that this cytotoxicity was due to the dark toxicity of 1c, which was totally different from the dose-dependent one reported in our previous papers.8,10 We have summarized the characteristics of the photo-switching of cytotoxicity studied in this work and that reported previously in Fig. S3 and Table S3 in the ESI.†Fig. 2(b) shows the cell growth rate (number of cells in the second observation/number of cells in the first observation) in 24 hours in the presence of three coexisting concentrations of DAE under each irradiation condition. It was confirmed that none of the light irradiation conditions alone without DAE (0 ppm) had any effect on cell viability.
On the other hand, it was confirmed that the toxicity activated by the conversion of 1o to 1c could be nearly cancelled by irradiation with green light after 5 min (B). Even under the condition of 10 ppm DAE concentration, which killed most of the cells within 24 h after UV light irradiation, irradiation with green light after 5 min maintained the same viability as under the condition with no light irradiation at all (A). Fig. 3 shows the results of micropatterned irradiation performed to clearly demonstrate this situation: HeLa cells cultured in a 1o-containing medium and incorporating 1o were irradiated with UV light of 365 nm wavelength (Fig. 3(a), rectangular area), and immediately after that, a micropattern (Fig. 3(b), “546” area) was irradiated with green light of 546 nm wavelength. Then, many cells that experienced only UV light irradiation were damaged and detached from the substrate surface in 24 h, while the cells that received overlapping green light irradiation escaped damage (Fig. 3(c)). The ON/OFF (activation/cancellation) of cell damage was localized to the irradiated area for each corresponding condition, clearly indicating that the toxicity of the DAE molecule can be controlled on-demand at this spatiotemporal scale, and the effects on the cells appeared in 24 h.
 | ||
| Fig. 3 Demonstration of micropatterned irradiation of cell culture. UV irradiation area (a), green light irradiation area (b), and experimental result (c). | ||
However, it was also found that the cancellation of this toxicity by green light irradiation decreased as ti (time elapsed since UV irradiation) increased (Fig. 2, 10 ppm, (C) and (D)). In connection with this finding, we investigated the intercalation of DAE added as 1o into DNA. We discuss below the results of experiments in which 1o DMSO solution was added to an aqueous DNA solution and light irradiated under conditions corresponding to the above cell experiments ((A)–(E)). Fig. 4(a) shows the absorbance spectra of the solutions at each stage of light irradiation and after the elapse of time for the irradiation conditions (A), (B), (D), and (E). Under condition (A) without UV irradiation, the absorbance spectrum attributed to 1o gradually changed its profile over time, and the absorbance decreased significantly 40 h after the addition of 1o. This change was consistent with the change corresponding to the progression of aggregation and precipitation (decrease in concentration in solution) seen when 1o DMSO solution is added to water. Fig. 4(b) shows the decrease ratio (1 (residual ratio)) of DAE in the solution estimated from the absorbance decrease. Even under condition (B), in which the samples underwent UV irradiation followed by green light irradiation 5 min later, the decrease ratio 40 h after the addition of 1o was similar to that under condition (A), in which no irradiation was performed. However, we observed a decrease as the interval to green irradiation (ti: time that most DAEs remain in the 1c state) increases. In our previous study, we observed that 1c intercalates with DNA when added to aqueous DNA solution, thereby stabilizing the dispersion and preventing precipitation, whereas 1o hardly intercalates at all. From this finding, it was inferred that the experimental results shown in Fig. 4(b) were due to the gradual intercalation of DAE into DNA during ring closure, which improved dispersion stability in an aqueous system. Under condition (D), where ti = 120 min, the rate of decrease of DAE from the solution during the 22 hours following green light irradiation was predominantly smaller than that under conditions (A) and (B). In previous studies, we have observed that 1c-incorporated cells show sensitivity (dose-dependent damage) to blue light at a wavelength of 436 nm and that green light irradiation,10 which should cause 1c ring opening, does not reduce this sensitivity. All of these results suggest that once DAE is intercalated into DNA, DAE is not expelled from inside the DNA by green light irradiation but remains there. Furthermore, the timescale for the decrease of 1o from the DNA solution was on the order of 1 h, consistent with that of the ti for DAE to reduce cell viability. As ti lengthened, the slight absorbance bulge around the wavelength of 550 nm, which is attributed to 1c, gradually increased even after green light irradiation (Fig. 4(a) “40 h (A)–(D)”). This suggests that a small fraction of DAE intercalated into DNA in the 1c state was strongly stabilized inside the DNA, making it less likely to become ring-open.
Based on the above results, we have considered the following mechanism for the decreased viability of the cells after 10 s of UV irradiation (Fig. 5): DAE, which is taken up by cells as 1o and converted to 1c by light, is gradually transferred into the DNA on a timescale of 1 h in the cells due to its planar structure (ESI†). This, like other DNA intercalators, causes dark toxicity and dooms the cells to a decreased viability after about 1 day. The mechanisms of cell death due to doxorubicin, one of the DNA intercalators used for anticancer drugs, are complex and are not fully understood but topoisomerase II poisoning is considered to be one of the mechanisms.16–18 If a similar mechanism works for 1c, the slow response of the dark toxicity of 1c may be explained. However, even 1c that is converted from 1o in the cells by UV irradiation, if done prior to transfer to DNA, will return to the 1o state almost stoichiometrically by green light irradiation and will not damage the cells.
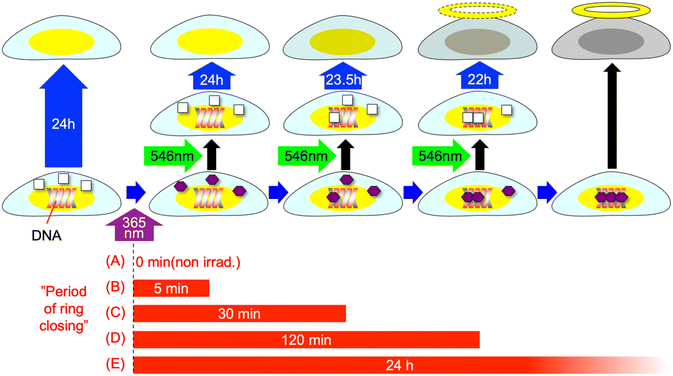 | ||
| Fig. 5 Schematic illustration of the mechanism of decreased viability of cells after UV irradiation. | ||
Conclusions
We introduced DAE, a molecular machine that exhibits photoisomerization, into cultured cells and demonstrated that its intercalation ability can be switched on by external light irradiation for as little as 10 s, thus dooming the cells to remarkable cell death after 1 day. This new scheme of photoinduced cell death stands in contrast to the dose-dependent phototoxic systems reported for PDT-related technologies as well as our previous work. In particular, dissolved oxygen plays an important role in the photosensitization-based systems used in PDT,5 but in many cases cancer tissue is placed in an anaerobic environment, which has been identified as a problem that reduces the efficiency of PDT.19 However, the scheme presented in this study, in which light is used to switch dark toxicity, can avoid such problems. Although major issues, such as the applicable concentration and wavelength of irradiating light, still need to be overcome for clinical application, our approach is expected to provide a new tool for controlling biosystems in vitro with light.Experimental
Materials and apparatus
The DAE used in this study was synthesized and purified by methods given in the existing literature.12 The HeLa cell line derived from human cervical adenocarcinoma was purchased from the Riken Cell Bank (no. RCB0007). Tissue culture polystyrene dishes (IWAKI no. 3000-035, AGC Techno glass Co., Ltd), minimum essential medium (MEM, no. 051-07615, Wako Pure Chemical Industries, Ltd) and FBS were used for cell culture. Light irradiation of the cell culture system was carried out using a PC-controlled micro-projection system (DESM-01, Engineering System Co.) installed in an inverted research microscope (IX70, Olympus Co.).20,21 DNA was obtained from salmon testes-derived DNA (sodium salts of DNA from salmon testes, average molecular weight: 1.3 × 106ca. 2000 bp, SIGMA). For UV irradiation, CL-1503 and CL-H1-365-9-1-B from Asahi Spectra Co. Ltd. were used. For green light irradiation, an AC-powered LED hand light, LED-EXHD/RFP (center wavelength: 540 nm) from Optocode Corporation, was used. A Hitachi spectrophotometer, UH-4150, was used to record absorption spectra in solution.Experimental methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (v/v), DNA: 75.8 μM (bp), DAE concentration: 25 μM). Light irradiation of the 1c-added system was performed immediately after the addition of 1c, and for conditions (B)–(D), to convert 1o to 1c, light irradiation of 365 nm wavelength and 200 mW cm−2 intensity was performed for 10 s to convert 1o to 1c. The DNA solution was kept in the dark for a predetermined time (ti = 5 min (B), 30 min (C), and 120 min (D)) and then irradiated with green light of 540 nm wavelength and 10 mW cm−2 intensity for 2 minutes to convert 1c to 1o. In both cases, absorbance spectra were recorded 40 h after the introduction of 1o into the DNA solution.
5 (v/v), DNA: 75.8 μM (bp), DAE concentration: 25 μM). Light irradiation of the 1c-added system was performed immediately after the addition of 1c, and for conditions (B)–(D), to convert 1o to 1c, light irradiation of 365 nm wavelength and 200 mW cm−2 intensity was performed for 10 s to convert 1o to 1c. The DNA solution was kept in the dark for a predetermined time (ti = 5 min (B), 30 min (C), and 120 min (D)) and then irradiated with green light of 540 nm wavelength and 10 mW cm−2 intensity for 2 minutes to convert 1c to 1o. In both cases, absorbance spectra were recorded 40 h after the introduction of 1o into the DNA solution.
Author contributions
KS, SY and KU designed and directed the project. NM performed DNA experiments. MK performed cell experiments. KS wrote the original draft.Data availability
The data that support the findings of this study are available from the corresponding authors (KS and KU) upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by JSPS KAKENHI grant number 22K18443 as a Grant-in-Aid for Challenging Research Pioneering and the Nanotechnology Platform of the Ministry of Education, Culture, Sports, Science and Technology (MEXT). We also thank Yamada Chemical for their supply of diarylethene 1o.References
- Y. Hayashi, J. Matsumoto, S. Kumagai, K. Morishita, L. Xiang, Y. Kobori, S. Hori, M. Suzuki, T. Kanamori, K. Hotta and K. Sumaru, Commun. Biol., 2018, 1, 218 CrossRef CAS PubMed.
- A. M. Kloxin, A. M. Kasko, C. N. Salinas and K. S. Anseth, Science, 2009, 324, 59–63 CrossRef CAS PubMed.
- K. Sumaru, T. Takagi, K. Morishita and T. Kanamori, Biomacromolecules, 2018, 19, 2913–2922 CrossRef CAS PubMed.
- D. E. Dolmans, D. Fukumura and R. K. Jain, Nat. Rev. Cancer, 2003, 3, 380–387 Search PubMed.
- D. Kessel and N. L. Oleinick, Photochem. Photobiol., 2018, 94, 213–218 Search PubMed.
- M. Mitsunaga, M. Ogawa, N. Kosaka, L. T. Rosenblum, P. L. Choyke and H. Kobayashi, Nat. Med., 2011, 17, 1685 Search PubMed.
- M. Tahara, S. Okano, T. Enokida, Y. Ueda, T. Fujisawa, T. Shinozaki, T. Tomioka, W. Okano, M. A. Biel, K. Ishida and R. Hayashi, Int. J. Clin. Oncol., 2021, 26, 1812–1821 CrossRef CAS PubMed.
- J. Okuda, Y. Tanaka, R. Kodama, K. Sumaru, K. Morishita, T. Kanamori, S. Yamazoe, K. Hyodo, S. Yamazaki, T. Miyatake, S. Yokojima, S. Nakamura and K. Uchida, Chem. Commun., 2015, 51, 10957–10960 RSC.
- Y. Nakagawa, T. Hishida, E. Hatano, K. Sumaru, K. Morishita, M. Morimoto, S. Yokojima, S. Nakamura and K. Uchida, Org. Biomol. Chem., 2022, 20, 3211–3217 RSC.
- Y. Nakagawa, T. Hishida, K. Sumaru, K. Morishita, K. Kirito, S. Yokojima, Y. Sakamoto, S. Nakamura and K. Uchida, J. Med. Chem., 2023, 66, 5937–5949 CrossRef CAS PubMed.
- U. Al-Atar, R. Fernandes, B. Johnsen, D. Baillie and N. R. Branda, J. Am. Chem. Soc., 2009, 131, 15966–15977 CrossRef CAS PubMed.
- K. Horbatok, T. Makhnii, V. Kosach, V. Danko, A. Kovalenko, S. Fatiushchenkov, P. Borysko, I. Pishel, O. Babii, A. S. Ulrich, T. Schober, S. Afonin and I. V. Komarov, J. Visualized Exp., 2023, 199, e64902 Search PubMed.
- A. Presa, R. Brissos, A. B. Caballero, I. Borilovic, L. Korrodi-Gregório, R. Pérez-Tomás, O. Roubeau and P. Gamez, Angew. Chem., Int. Ed., 2015, 54, 4561–4565 CrossRef CAS PubMed.
- O. Babii, S. Afonin, A. Y. Ishchenko, T. Schober, A. O. Negelia, G. M. Tolstanova, L. V. Garmanchuk, L. I. Ostapchenko, I. V. Komarov and A. S. Ulrich, J. Med. Chem., 2018, 61, 10793–10813 CrossRef CAS PubMed.
- K. Uchida, T. Ishikawa, M. Takeshita and M. Irie, Tetrahedron, 1998, 54, 6627–6638 CrossRef CAS.
- R. Mattioli, A. Ilari, B. Colotti, L. Mosca, F. Fazi and G. Colotti, Mol. Aspects Med., 2023, 93, 101205 Search PubMed.
- M. Kciuk, A. Gielecińska, S. Mujwar, D. Kołat, Ż Kałuzińska-Kołat, I. Celik and R. Kontek, Cells, 2023, 12, 659 CrossRef CAS PubMed.
- J. L. Delgado, C.-M. Hsieh, N.-L. Chan and H. Hiasa, Biochem. J., 2018, 475, 373–398 Search PubMed.
- L. Yu, Z. Liu, W. Xu, K. Jin, J. Liu, X. Zhu, Y. Zhang and Y. Wu, Acta Pharm. Sin. B, 2024, 14, 1111–1131 Search PubMed.
- K. Sumaru, J. Edahiro, Y. Ooshima, T. Kanamori and T. Shinbo, Biosens. Bioelectron., 2007, 22, 2356–2359 Search PubMed.
- K. Sumaru and T. Kanamori, Method in Cell Biology Micro- patterning in Cell Biology, Part B, in Method in Cell Biology, ed. P. Matthieu and T. Manuel, Elsevier, 2014, vol. 120, pp. 185–197 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob02087a |
| This journal is © The Royal Society of Chemistry 2025 |