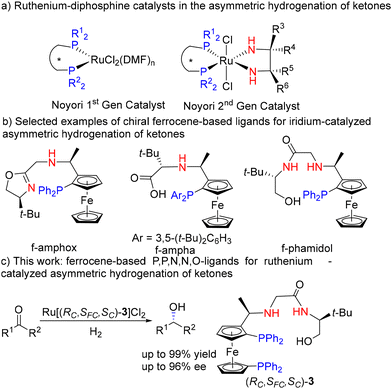
Development of chiral ferrocenyl P,P,N,N,O-ligands for ruthenium-catalyzed asymmetric hydrogenation of ketones†
Lei
Xu
a,
Gen-Qiang
Chen
 *b and
Xumu
Zhang
*b and
Xumu
Zhang
 *acd
*acd
aShenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, 1088 Xueyuan Road, Shenzhen 518055, China
bAcademy for Advanced Interdisciplinary Studies and Shenzhen Grubbs Institute, Southern University of Science and Technology, Shenzhen 518000, People's Republic of China. E-mail: chengq@sustech.edu.cn
cShenzhen Key Laboratory of Small Molecule Drug Discovery and Synthesis, Department of Chemistry, and Medi-Pingshan, Southern University of Science and Technology, Shenzhen 518000, People's Republic of China
dChemistry and Chemical Engineering Guangdong Laboratory, Shantou 515031, People's Republic of China. E-mail: zhangxm@sustech.edu.cn
First published on 8th November 2024
Abstract
A new type of ferrocenyl P,P,N,N,O-ligand has been developed through a one-step transformation. This represents a rare example of a ligand containing both chiral bisphosphine and diamine groups suitable for ruthenium-catalyzed asymmetric hydrogenation. Its ruthenium complex can be directly prepared by stirring the ligand and [Ru(benzene)Cl2]2 at 90 °C in DMF for 4 hours. The catalyst showed high reactivity and enantioselectivity in the hydrogenation (AH) of simple ketones and α,β-unsaturated ketones, providing the corresponding chiral aryl alkyl alcohols and chiral allyl alcohols with up to 99% yield and 96% ee.
In modern organic synthetic chemistry, chiral metal catalysts are of vital importance.1–10 Among them, diphosphine–ruthenium–diamine complexes11–14 (Noyori's second-generation catalysts) are landmark works (Fig. 1a). The use of chiral diphosphines such as BINAP, as well as certain chiral diamines, has led to the development of highly effective enantioselective catalysts.15–17 A significant advantage of these catalysts, compared to Ru(diphosphine)Xn salts (Noyori's first-generation catalysts), is their ability to be used for the asymmetric hydrogenation of ketones.18 With the development of a large number of chiral diphosphine ligands such as BICP, PhanePhos and etc.,1,19–24 many catalysts of this general type have been prepared. Although these catalysts have achieved great success, they still have significant drawbacks, including relatively poor stability.25 In some cases, the products may exhibit lower enantioselectivity (ee) values due to catalyst decomposition.25
Ferrocene-based chiral ligands represent a class of ligands widely applied in asymmetric synthesis.26 Since Hayashi developed the first example of such ligands (ppfa, N,N-dimethyl-1-[2-(diphenylphosphino) ferrocenyl]ethylamine, a asymmetric ligand for ruthenium) in 1974,27 a large number of ferrocene ligands such as Josiphos, Walphos, Taniaphos have been developed. In 2007, a ferrocenyl phosphine-thioether ligand suitable for iridium-catalyzed asymmetric hydrogenation of ketones was developed by Peruzzini and coworkers.28 Due to the chelating effect, multidentate ligands showed higher stability and selectivity compared with the traditional bidentate ligands.2 In 2013, Chen developed a tridentate P,N,N-ligand, which showed moderate enantioselectivity in iridium-catalyzed asymmetric hydrogenation of acetophenone derivatives (60%–85% ee).29 Our group has focused on the design and synthesis of efficient ligands for the asymmetric hydrogenation of ketones for over two decades.30 Since 2016, we have developed a series of multidentate ligands such as f-amphox,31 f-amphol,32 f-ampha,33 f-amphamide.34 Recently, we developed the tetradendate ligand f-phamidol, for iridium-catalyzed asymmetric hydrogenation of ketones, whose iridium complex is used for the asymmetric hydrogenation of acetophenone with a TON of up to 13 million (Fig. 1b).35
While there are many polydentate ligands suitable for iridium, there are few suitable for ruthenium.36–38 Herein, we report the design and synthesis of an air-stable ferrocenyl P,P,N,N,O-ligand for ruthenium-catalyzed asymmetric hydrogenation of simple ketones with good yields and high enantioselectivities, which represents a rare example of a ligand containing both chiral bisphosphines and diamine groups suitable for ruthenium-catalyzed asymmetric hydrogenation reactions (Fig. 1c).
At the beginning of this study, we attempted to introduce a glycyl tert-leucinol moiety into (SC,RFC)-PPFOAc [(Sc,RFC)-1] to synthesize the P,P,N,N,O-ligand (Scheme 1). A substitution reaction between (Sc,RFC)-PPFOAc [(Sc,RFC)-1] and (S)-glycyl tert-leucinol (2) proceeded smoothly to yield the target ligand (SC,RFC,SC)-3 with retention of enantioselectivity. Ligand (SC,RFC,SC)-3 is air-stable and it could be easily purified by column chromatography, accordingly, ligand (RC,SFC,SC)-3 can be prepared using the same method. The ruthenium complex 4 can be easily prepared by stirring ligand (SC,RFC,SC)-3 and [Ru(benzene)Cl2]2 at 90 °C in DMF for 4 hours, and complex 5 can be prepared using the same method.
With the ligand and catalyst in hand, we evaluated their efficacy in asymmetric hydrogenation with acetophenone (6a) as a model substrate and ruthenium complex 4 as the catalyst, and the results were depicted in Table 1. Using 0.1 mol% of 4 as the catalyst, t-BuOK as the base and i-PrOH as the solvent, 7a was obtained with 99% yield and 83% ee (entry 1). When i-PrOH was replaced with other solvents such as MeOH, THF and 1,4-dioxane, no target product was detected (entries 2–4). Subsequently, we examined the effect of the base on the reaction. With t-BuONa as the base, 99% yield, and 86% ee were achieved (entry 5). However, when K2CO3 or Cs2CO3 was used as the base, the target product could not be detected at all (entries 6 and 7). To our delight, when 5 was used as the catalyst, the ee value of the target alcohol was increased to −94% with 99% yield (entry 8). The reaction could not proceed in the absence of base, catalyst or hydrogen (entries 9–11).
| Entry | Catalyst | Solvent | Base | Yieldb (%) | ee |
|---|---|---|---|---|---|
| a Reaction condition: acetophenone 6a (240 mg, 2.0 mmol), solvent (2.0 mL), catalyst (1.8 mg, 0.002 mmol, 0.001 equiv.), t-BuONa (0.02 mmol, 0.01 equiv.), H2 (10 atm), 10 h. b Isolated yields. | |||||
| 1 | 4 | i-PrOH | t-BuOK | 99 | 83 |
| 2 | 4 | MeOH | t-BuOK | 0 | — |
| 3 | 4 | THF | t-BuOK | 0 | — |
| 4 | 4 | Dioxane | t-BuOK | 0 | — |
| 5 | 4 | i-PrOH | t-BuONa | 99 | 86 |
| 6 | 4 | i-PrOH | K2CO3 | 0 | — |
| 7 | 4 | i-PrOH | Cs2CO3 | 0 | — |
| 8 | 5 | i-PrOH | t-BuONa | 99 | −94 |
| 9 | — | i-PrOH | t-BuONa | 0 | — |
| 10 | 5 | i-PrOH | — | 0 | — |
| 11 | 5 | i-PrOH | t-BuONa | 0 | — |
Following the optimal reaction conditions, we then examined the scope of the ruthenium-catalyzed asymmetric hydrogenation (Table 2). For different acetophenone-derived substrates with various substituents on the aromatic ring, the conversion of all substrates was good, with ee values ranging from 83% to 96%. This catalytic system could also tolerate various substituents at the para or meta position of aromatic rings with different electronic properties. Substituted acetophenones bearing electron-donating groups, such as alkyl (7b–7e), dimethylamino (7g), and methylthio (7h), as well as electron-withdrawing groups like fluorine (7j, 7k), chlorine (7l, 7m), and bromine (7n, 7o), were well tolerated. Notably, acetophenone with two substituents (7f), 2-naphthalene ethyl ketone (7i), various derivatives of phenylacetone (7p–7r), 2-methyl-1-phenylpropan-1-one (7s) and cyclohexyl(phenyl)methanone (7t) were also compatible with this reaction. Generally, substrates with electron-donating substituents on aromatic rings or large alkyl substituents resulted in higher enantioselectivities of the products.
| a Reaction condition: ketone (2.0 mmol), i-PrOH (2.0 mL), 5 (1.8 mg, 0.002 mmol, 0.001 equiv.), t-BuONa (1.9 mg, 0.02 mmol, 0.01 equiv.), H2 (10 atm), 10 h, isolated yields. |
|---|

|
To further test the applicability of the catalyst, a range of substituted–unsaturated aryl ketones were evaluated, and the results were depicted in Table 3 (for the detailed optimization of the reaction conditions, see Table S1†). The conversion of all substrates was good, with ee values ranging from 67% to 82% (9a–9g). The introduction of substituents on the aromatic rings had a significant effect on the ee value of the product. Products with methyl (9b) or fluorine (9d) substituents at the para position of the aromatic ring, or chlorine (9e) at the meta position, were generally produced with increased ee. In contrast, the ee values of products with methoxy (9c) or chlorine (9f) substituents at the para position were significantly reduced. The reaction could also tolerate the 2-naphthalene substrate, producing 9g with 93% yield and 82% ee.
| a Reaction condition: α,β-unsaturated ketones (0.1 mmol), o-xylene (0.4 mL), 5 (1.8 mg, 0.002 mmol, 0.02 equiv.), DABCO (0.1 mmol, 1.0 equiv.), TMG (0.1 mmol, 1.0 equiv.), H2 (50 atm) stir for 10 hours, isolated yields. DABCO = triethylenediamine; TMG = tetramethylguanidine. |
|---|

|
In summary, an air-stable chiral ferrocenyl P,P,N,N,O-ligand (RC,SFC,SC)-3 suitable for ruthenium-catalyzed asymmetric hydrogenation has been developed. This ligand combines a chiral bisphosphine ligand and a chiral diamine. Its ruthenium complexes can be prepared by a straightforward one-step method, which is suitable for the asymmetric hydrogenation of simple ketones and α,β-unsaturated aryl ketones, and provides the target chiral alcohols with high yield and moderate to high enantioselectivity.
Author contributions
L. Xu, G.-Q. Chen and X. Zhang devised the project. L. Xu and G.-Q. Chen designed and discussed the experiments; L. Xu performed the experiments, compound characterization and data analysis. L. Xu and G.-Q. Chen prepared the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
X. Zhang is indebted to the financial support from the National Key R&D Program of China (2021YFA1500200), National Natural Science Foundation of China (No. 21991113), Chemistry and Chemical Engineering Guangdong Laboratory (Grant No. 2011006 and 2132013) and Innovative Team of Universities in Guangdong Province (No. 2020KCXTD016). G.-Q. Chen gratefully acknowledges the National Natural Science Foundation of China (No. 22171129), Shenzhen Science and Technology Innovation Committee (JCYJ20210324104202007) and the Guangdong Basic and Applied Basic Research Foundation (2022B1515020055) for financial support. The authors acknowledge the assistance of SUSTech Core Research Facilities.References
- K. Aikawa and K. Mikami, Angew. Chem., Int. Ed., 2003, 42, 5455–5458 CrossRef CAS PubMed.
- J.-B. Xie, J.-H. Xie, X.-Y. Liu, W.-L. Kong, S. Li and Q.-L. Zhou, J. Am. Chem. Soc., 2010, 132, 4538–4539 CrossRef CAS PubMed.
- J. H. Xie, X. Y. Liu, J. B. Xie, L. X. Wang and Q. L. Zhou, Angew. Chem., Int. Ed., 2011, 50, 7329–7332 CrossRef CAS.
- C.-J. Hou and X.-P. Hu, Org. Lett., 2016, 18, 5592–5595 CrossRef CAS PubMed.
- G.-Q. Chen, B.-J. Lin, J.-M. Huang, L.-Y. Zhao, Q.-S. Chen, S.-P. Jia, Q. Yin and X. Zhang, J. Am. Chem. Soc., 2018, 140, 8064–8068 CrossRef CAS.
- Z. Zheng, Y. Cao, Q. Chong, Z. Han, J. Ding, C. Luo, Z. Wang, D. Zhu, Q.-L. Zhou and K. Ding, J. Am. Chem. Soc., 2018, 140, 10374–10381 CrossRef CAS.
- F. H. Zhang, C. Wang, J. H. Xie and Q. L. Zhou, Adv. Synth. Catal., 2019, 361, 2832–2835 CrossRef CAS.
- F.-H. Zhang, F.-J. Zhang, M.-L. Li, J.-H. Xie and Q.-L. Zhou, Nat. Catal., 2020, 3, 621–627 CrossRef CAS.
- S. K. Sharma, A. S. R. Paniraj and Y. B. Tambe, J. Agric. Food Chem., 2021, 69, 14761–14780 CrossRef CAS PubMed.
- Y. E. You, C. Yin, L. Xu, G.-Q. Chen and X. Zhang, Green Synth. Catal., 2024, 5, 141–152 CrossRef CAS.
- H. Doucet, T. Ohkuma, K. Murata, T. Yokozawa, M. Kozawa, E. Katayama, A. F. England, T. Ikariya and R. Noyori, Angew. Chem., Int. Ed., 1998, 37, 1703–1707 CrossRef CAS.
- T. Ohkuma, H. Ooka, S. Hashiguchi, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1995, 117, 2675–2676 CrossRef CAS.
- T. Ohkuma, H. Ooka, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1995, 117, 10417–10418 CrossRef CAS.
- T. Ohkuma, H. Ooka, M. Yamakawa, T. Ikariya and R. Noyori, J. Org. Chem., 1996, 61, 4872–4873 CrossRef CAS.
- T. Ohkuma, M. Koizumi, H. Doucet, T. Pham, M. Kozawa, K. Murata, E. Katayama, T. Yokozawa, T. Ikariya and R. Noyori, J. Am. Chem. Soc., 1998, 120, 13529–13530 CrossRef CAS.
- T. Ohkuma, M. Koizumi, H. Ikehira, T. Yokozawa and R. Noyori, Org. Lett., 2000, 2, 659–662 CrossRef CAS PubMed.
- T. Ohkuma, M. Koizumi, M. Yoshida and R. Noyori, Org. Lett., 2000, 2, 1749–1751 CrossRef CAS PubMed.
- R. Noyori and T. Ohkuma, Angew. Chem., Int. Ed., 2001, 40, 40–73 CrossRef CAS.
- M. J. Burk, W. Hems, D. Herzberg, C. Malan and A. Zanotti-Gerosa, Org. Lett., 2000, 2, 4173–4176 CrossRef CAS PubMed.
- P. Cao and X. Zhang, J. Org. Chem., 1999, 64, 2127–2129 CrossRef CAS.
- R. Guo, X. Chen, C. Elpelt, D. Song and R. H. Morris, Org. Lett., 2005, 7, 1757–1759 CrossRef CAS.
- D. He, X. Xu, Y. Lu, M.-J. Zhou and X. Xing, Org. Lett., 2020, 22, 8458–8463 CrossRef CAS.
- A. Hu, H. L. Ngo and W. Lin, Org. Lett., 2004, 6, 2937–2940 CrossRef CAS PubMed.
- W. Li, X. Sun, L. Zhou, G. Hou, S. Yu and X. Zhang, J. Org. Chem., 2008, 74, 1397–1399 CrossRef.
- G.-Q. Chen and X. Zhang, in Adv. Catal, ed. M. Diéguez and A. Pizzano, Academic Press, 2021, vol. 68, pp. 291–339 Search PubMed.
- R. Gómez Arrayás, J. Adrio and J. C. Carretero, Angew. Chem., Int. Ed., 2006, 45, 7674–7715 CrossRef PubMed.
- T. Hayashi, K. Yamamoto and M. Kumad, Tetrahedron Lett., 1974, 15, 4405–4408 CrossRef.
- E. Le Roux, R. Malacea, E. Manoury, R. Poli, L. Gonsalvi and M. Peruzzini, Adv. Synth. Catal., 2007, 349, 309–313 CrossRef CAS.
- H. Nie, G. Zhou, Q. Wang, W. Chen and S. Zhang, Tetrahedron, 2013, 24, 1567–1571 CrossRef CAS.
- F. Wan and W. Tang, Chin. J. Chem., 2021, 39, 954–968 CrossRef CAS.
- W. Wu, S. Liu, M. Duan, X. Tan, C. Chen, Y. Xie, Y. Lan, X.-Q. Dong and X. Zhang, Org. Lett., 2016, 12, 2938–2941 CrossRef.
- J. Yu, M. Duan, W. Wu, X. Qi, P. Xue, Y. Lan, X. Q. Dong and X. Zhang, Chem. – Eur. J., 2016, 23, 970–975 CrossRef.
- J. Yu, J. Long, Y. Yang, W. Wu, P. Xue, L. W. Chung, X.-Q. Dong and X. Zhang, Org. Lett., 2017, 19, 690–693 CrossRef CAS PubMed.
- Z.-Q. Liang, T.-L. Yang, G. Gu, L. Dang and X. Zhang, Chin. J. Org. Chem., 2018, 36, 851–856 CrossRef CAS.
- C. Yin, Y.-F. Jiang, F. Huang, C.-Q. Xu, Y. Pan, S. Gao, G.-Q. Chen, X. Ding, S.-T. Bai, Q. Lang, J. Li and X. Zhang, Nat. Commun., 2023, 14, 3178 CrossRef PubMed.
- Y. Jiang, Q. Jiang and X. Zhang, J. Am. Chem. Soc., 1998, 120, 3817–3818 CrossRef CAS.
- S. D. Phillips, J. A. Fuentes and M. L. Clarke, Chem. – Eur. J., 2010, 16, 8002–8005 CrossRef CAS PubMed.
- T. Yamamura, H. Nakatsuka, S. Tanaka and M. Kitamura, Angew. Chem., Int. Ed., 2013, 52, 9313–9315 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob01679c |
| This journal is © The Royal Society of Chemistry 2025 |