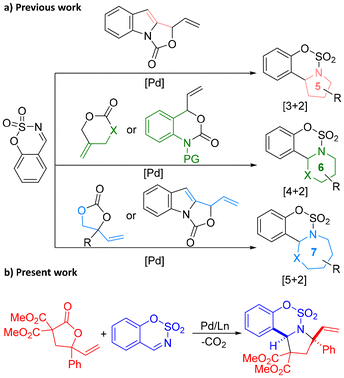
Palladium-catalyzed [3 + 2] cycloaddition of 4-vinyl-4-butyrolactones with sulfamate-derived cyclic imines: construction of sulfamate-fused pyrrolidines†
Honghao
Sun
ab,
Siyuan
Ding
a,
Bo
Wang
*b,
Jiaxing
Huang
*a and
Hongchao
Guo
 *a
*a
aDepartment of Chemistry and Innovation Center of Pesticide Research, China Agricultural University, Beijing 100193, P. R. China. E-mail: hchguo@cau.edu.cn; 05084@cau.edu.cn
bInstitute of Food Science and Technology, Chinese Academy of Agricultural Sciences, Beijing, 100193, China. E-mail: 362395987@qq.com
First published on 6th November 2024
Abstract
The palladium-catalyzed [3 + 2] decarboxylative cycloaddition of 4-vinyl-4-butyrolactones with sulfamate-derived cyclic imines has been developed, providing the sulfamate-fused pyrrolidine derivatives in high yields with good diastereoselectivities. The scale-up reaction and further derivation of the product worked well, demonstrating the potential application of the current reaction in organic synthesis. A plausible reaction mechanism was also proposed.
The sulfamate moiety1 is often found in the skeletons of natural products and pharmaceutical compounds having biological activities such as anticancer, antibiotic and antiviral properties. Pyrrolidine2 is the commonly used five-membered heterocyclic structure in organic synthesis and pharmaceutical chemistry. The structures of more than 50 drugs include pyrrolidine fragments. Due to the important value of the sulfamate moiety and pyrrolidine framework, it is very meaningful to develop a synthetic method for sulfamate-fused pyrrolidine derivatives.
Palladium-catalyzed decarboxylative cycloaddition reactions have been intensively studied in the past decades and have been proven to be one of the effective methods for the synthesis of valuable cyclic compounds.3 In recent years, a variety of novel palladium-catalyzed decarboxylative cycloaddition reactions have been discovered.4–7 Because the sulfamate moiety is a core structural part of some bioactive molecules, sulfamate-derived cyclic imines were often used as electron-deficient reaction partners in palladium-catalyzed cycloaddition reactions to produce bioactive sulfamate-fused heterocyclic compounds (Scheme 1a). In 2018, our group reported a palladium-catalyzed highly stereoselective [4 + 2] cycloaddition reaction of vinyl benzoxazinones with sulfamate-derived cyclic imines to access chiral tetrahydroquinazolines.8 In 2020, the Kim group accomplished an enantioselective [5 + 2] cycloaddition reaction of vinylethylene carbonates and cyclic imines, completing the synthesis of N-fused 1,3-oxazepines bearing one stereogenic center.9 In 2021, the Chen group developed asymmetric regiodivergent [5 + 2] and [3 + 2] annulations of vinyl indoloxazolidones under palladium catalysis, furnishing distinct azepino[4,3-b]indole and pyrrolo[3,4-b]indole frameworks.10 In 2023, the Mao group disclosed a palladium-catalyzed [4 + 2] cycloaddition of 2-methylidenetrimethylene carbonate or methylene cyclic carbamate with sulfamate-derived cyclic imines, affording pharmacologically interesting oxazine or hydropyrimidine derivatives in high yields.11 To the best of our knowledge, there are limited reports on the construction of the pyrrolidine framework through palladium-catalyzed decarboxylative cycloaddition reactions.
In the recent five years, our group focused on developing novel precursors of π-allyl palladium zwitterionic intermediates and their applications in palladium-catalyzed cycloadditions to construct novel structural skeletons.12 We designed and synthesized 4-vinyl-4-butyrolactones (VBLs) as precursors for π-allyl palladium zwitterionic intermediates,13 and recently applied them in palladium-catalyzed [3 + 2] cycloaddition with alkenes to afford various spirocyclopentane products.14 As our continuous efforts on cycloaddition reactions,15 in order to further expand the application scope of 4-vinyl-4-butyrolactones, we herein report a palladium-catalyzed [3 + 2] decarboxylative cycloaddition of 4-vinyl-4-butyrolactones with sulfamate-derived cyclic imines, providing the biologically important sulfamate-fused pyrrolidine derivatives.
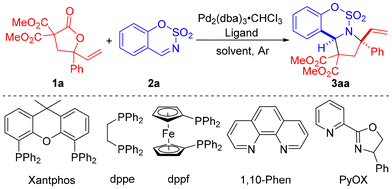
Initially, we studied the reaction between VBL 1a and sulfamate-derived cyclic imine 2a by screening a series of ligands in the presence of Pd2(dba)3·CHCl3 with dichloromethane (DCM) as the solvent (Table 1, entries 1–6). To our delight, the common phosphine ligands such as PPh3 and Xantphos were able to promote the reaction of VBL 1a and sulfamate-derived cyclic imine 2a, generating the [3 + 2] cycloaddition product 3aa (CCDC number: 2355129, detailed in the ESI†)16 in 88% yield with 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and in 62% yield with 7
1 dr and in 62% yield with 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, respectively (entries 1 and 2). Other diphosphine ligands such as dppe and dppf did not promote the reaction (entries 3 and 4). The dinitrogen ligands such as 1,10-Phen and PyOX displayed high catalytic activities, providing the [3 + 2] cycloaddition product 3aa in 95% yield with 19
1 dr, respectively (entries 1 and 2). Other diphosphine ligands such as dppe and dppf did not promote the reaction (entries 3 and 4). The dinitrogen ligands such as 1,10-Phen and PyOX displayed high catalytic activities, providing the [3 + 2] cycloaddition product 3aa in 95% yield with 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr and in 85% yield with 17
1 dr and in 85% yield with 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, respectively (entries 5 and 6). Subsequently, a quick screening of solvents such as CHCl3, 1,2-dichloroethane (DCE), toluene, EtOAc, and THF (entries 7–12) revealed that a high yield and excellent diastereoselectivity were achieved in DCE (93% yield, >20
1 dr, respectively (entries 5 and 6). Subsequently, a quick screening of solvents such as CHCl3, 1,2-dichloroethane (DCE), toluene, EtOAc, and THF (entries 7–12) revealed that a high yield and excellent diastereoselectivity were achieved in DCE (93% yield, >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Decreasing the loading of the ligand to 10 mol% did not deteriorate both yield and stereoselectivity (entry 13). The optimal reaction conditions were determined as the use of Pd2(dba)3·CHCl3 (5 mol%) and 1,10-Phen (10 mol%) in DCE at 25 °C.
1 dr). Decreasing the loading of the ligand to 10 mol% did not deteriorate both yield and stereoselectivity (entry 13). The optimal reaction conditions were determined as the use of Pd2(dba)3·CHCl3 (5 mol%) and 1,10-Phen (10 mol%) in DCE at 25 °C.
| Entry | Ligand | Solvent | t (h) | Yieldb (%) | drc |
|---|---|---|---|---|---|
| a All reactions were carried out with Pd2(dba)3·CHCl3 (5 mol%), ligand (20 mol%), 1a (0.12 mmol) and 2a (0.1 mmol) in 1 mL of solvent at 25 °C. b Isolated yield; NR: no reaction. c Determined by 1H NMR analysis. d 10 mol% of ligand was used. | |||||
| 1 | PPh3 | DCM | 12 | 88 | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | Xantphos | DCM | 12 | 62 | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | dppe | DCM | 48 | NR | — |
| 4 | dppf | DCM | 48 | NR | — |
| 5 | 1,10-Phen | DCM | 12 | 95 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | PyOX | DCM | 12 | 85 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 1,10-Phen | CHCl3 | 12 | 87 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 1,10-Phen | DCE | 12 | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 1,10-Phen | Toluene | 48 | 43 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 1,10-Phen | EtOAc | 12 | NR | — |
| 11 | 1,10-Phen | MeCN | 12 | 81 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 1,10-Phen | THF | 12 | NR | — |
| 13d | 1,10-Phen | DCE | 12 | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
With the optimal reaction conditions in hand, we set out to explore the generality of this catalytic system. The scope with respect to various VBLs 1 with various substituents was first examined. As shown in Scheme 2, a wide range of aryl substituted VBLs having different electronic and steric properties performed the reaction well, producing a variety of sulfamate-pyrrolidine derivatives (3ba–3ka) in 86–93% yields with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. There was no remarkable difference in the reactivity between electron-withdrawing and electron-donating group-substituted VBLs. All these substrates displayed high reactivities and good stereoselectivities. The introduction of a 2-naphthyl substituent into the VBL was also feasible for this reaction (3ma). It is worth noting that when R′ was ethyl, the product (3na) was still obtained in 86% yield with >20
1 dr. There was no remarkable difference in the reactivity between electron-withdrawing and electron-donating group-substituted VBLs. All these substrates displayed high reactivities and good stereoselectivities. The introduction of a 2-naphthyl substituent into the VBL was also feasible for this reaction (3ma). It is worth noting that when R′ was ethyl, the product (3na) was still obtained in 86% yield with >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. The 2-thiophenyl-substituted VBL displayed high reactivity, offering 84% yield of the product 3la, but low diastereoselectivity (1
1 dr. The 2-thiophenyl-substituted VBL displayed high reactivity, offering 84% yield of the product 3la, but low diastereoselectivity (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). Unfortunately, when VBL 1 had only one ester group, its reactivity was very poor, giving a trace amount of the product.
1 dr). Unfortunately, when VBL 1 had only one ester group, its reactivity was very poor, giving a trace amount of the product.
We next investigated the scope of sulfamate-derived cyclic imines 2 (Table 2). A series of sulfamate-derived cyclic imines with different substituents on the benzene ring were capable of undergoing [3 + 2] cycloaddition with 4-vinyl-4-butyrolactone 1a under standard reaction conditions, affording the corresponding sulfamate-fused pyrrolidine derivatives (3aa–3am) in high yields (87–95%, entries 1–13). The results showed that the introduction of different electron-withdrawing and electron-donating groups at the 6, 7 and 8 positions of sulfamate-derived cyclic imines 2 had no significant influence on the reaction (entries 1–13), and the corresponding products were obtained in high yields with excellent diastereoselectivities.
| Entry | R | 3 | Yieldb (%) | drc |
|---|---|---|---|---|
| a Reactions of 1a (0.12 mmol) and 2 (0.10 mmol) were performed in the presence of Pd2(dba)3·CHCl3 (5 mol%) and 1,10-phen (10 mol%) in 1 mL of DCE at 25 °C for 12 h. b Isolated yield. c Determined by 1H NMR analysis. | ||||
| 1 | H (2a) | 3aa | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | 7-F (2b) | 3ab | 89 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | 6-Cl (2c) | 3ac | 91 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 4 | 7-Br (2d) | 3ad | 95 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 5 | 8-Br (2e) | 3ae | 90 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 6 | 6-Me (2f) | 3af | 94 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 7 | 7-Me (2g) | 3ag | 89 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 8 | 8-Me (2h) | 3ah | 90 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 9 | 6-t-Bu (2i) | 3ai | 92 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 10 | 7-t-Bu (2j) | 3aj | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11 | 8-t-Bu (2k) | 3ak | 93 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12 | 7-OMe (2l) | 3al | 87 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13 | 8-OEt (2m) | 3am | 91 | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
In order to investigate the practicality of the current reaction, the scale-up reaction of VBL 1a (1.2 mmol) with sulfamate-derived cyclic imine 2a (1.0 mmol) was performed under the optimal reaction conditions, resulting in the corresponding product 3aa with 91% yield and >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Further transformation of the product was also carried out. The product 3aa was treated with DIBAL in DCM at −78 °C for 2 h to afford the alcohol derivative 4aa in 57% yield (Scheme 3).
1 dr. Further transformation of the product was also carried out. The product 3aa was treated with DIBAL in DCM at −78 °C for 2 h to afford the alcohol derivative 4aa in 57% yield (Scheme 3).
As shown in Scheme 4, a plausible reaction mechanism was proposed. Under the catalysis of palladium/1,10-Phen, VBL 1a produced the zwitterionic intermediate A through a decarboxylation ring-opening reaction. This intermediate A then underwent addition to sulfamate-derived cyclic imine 2a to generate the intermediate B. Subsequent intramolecular annulation led to the product 3aa.
In summary, we have successfully achieved a palladium-catalyzed [3 + 2] cycloaddition of VBLs with sulfamate-derived cyclic imines, giving various sulfamate-fused pyrrolidine derivatives in high yields with excellent diastereoselectivities. The combination of two active skeletons in these novel sulfamate-fused pyrrolidine derivatives has the potential to be biologically active and to improve medicinal chemistry.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Natural Science Foundation of China (No. 21871293 and 22071264).References
- (a) J. Y. Winum, A. Scozzafava, J. L. Montero and C. T. Supuran, Med. Res. Rev., 2005, 25, 186–228 CrossRef CAS PubMed; (b) W. Spillane and J. B. Malaubier, Chem. Rev., 2014, 114, 2507–2586 CrossRef CAS PubMed; (c) S. O. Zaraei, A. R. Abduelkarem, H. S. Anbar, S. Kobeissi, M. Mohammad, A. Ossama and M. I. El-Gamal, Eur. J. Med. Chem., 2019, 179, 257–271 CrossRef CAS; (d) M. Mustafa and J. Y. Winum, Expert Opin. Drug Discovery, 2022, 17, 501–512 CrossRef CAS.
- (a) P. Saraswat, G. Jeyabalan, M. Z. Hassan, M. U. Rahman and N. K. Nyola, Synth. Commun., 2016, 46, 1643–1664 CrossRef CAS; (b) W. J. Olivier, J. A. Smith and A. C. Bissember, Org. Biomol. Chem., 2018, 16, 1216–1226 RSC; (c) G. Li Petri, M. V. Raimondi, V. Spanò, R. Holl, P. Barraja and A. Montalbano, Top. Curr. Chem., 2021, 379, 1–46 CrossRef.
- For selected reviews, see: (a) B. D. Allen, C. P. Lakeland and J. P. A. Harrity, Chem. – Eur. J., 2017, 23, 13830–13857 CrossRef CAS PubMed; (b) W. Guo, J. E. Gómez, À. Cristòfol, J. Xie and A. W. Kleij, Angew. Chem., Int. Ed., 2018, 57, 13735–13747 CrossRef CAS; (c) Q.-Z. Li, Y. Liu, M.-Z. Li, X. Zhang, T. Qi and J.-L. Li, Org. Biomol. Chem., 2020, 18, 3638–3648 RSC; (d) B. M. Trost and G. Mata, Acc. Chem. Res., 2020, 53, 1293–1305 CrossRef CAS; (e) B. Niu, Y. Wei and M. Shi, Org. Chem. Front., 2021, 8, 3475–3501 RSC; (f) M. M. Zhang, B. L. Qu, B. Shi, W. J. Xiao and L. Q. Lu, Chem. Soc. Rev., 2022, 51, 4146–4174 RSC; (g) Y. You, Q. Li, Y. P. Zhang, J. Q. Zhao, Z. H. Wang and W. C. Yuan, ChemCatChem, 2022, 14, e202101887 CrossRef CAS.
- For recent examples through vinyl cyclic carbonates, see: (a) B. Mao, H. Liu, Z. Yan, Y. Xu, J. Xu, W. Wang, Y. Wu and H. Guo, Angew. Chem., Int. Ed., 2020, 59, 11316–11320 CrossRef CAS PubMed; (b) Y. Zheng, T. Qin and W. Zi, J. Am. Chem. Soc., 2021, 143, 1038–1045 CrossRef CAS PubMed; (c) G. Yang and Y. Zhao, Angew. Chem., Int. Ed., 2021, 60, 12775–12780 CrossRef CAS; (d) J. Liu, L. Yu, C. Zheng and G. Zhao, Angew. Chem., Int. Ed., 2021, 60, 23641–123645 CrossRef CAS; (e) W. Zhang, P. C. Zhang, Y. L. Li, H. H. Wu and J. Zhang, J. Am. Chem. Soc., 2022, 144, 19627–19634 CrossRef CAS PubMed; (f) T. Morita, H. Murakami, Y. Asawa and H. Nakamura, Angew. Chem., Int. Ed., 2022, 61, e2021135 CrossRef.
- For recent examples through vinyl cyclic carbamates, see: (a) V. Garcia-Vazquez, L. Hoteite, C. P. Lakeland, D. W. Watson and J. P. A. Harrity, Org. Lett., 2021, 23, 2811–2815 CrossRef CAS; (b) B. Shi, J. B. Liu, Z. T. Wang, L. Wang, Y. Lan, L. Q. Lu and W. J. Xiao, Angew. Chem., Int. Ed., 2022, 61, e202117215 CrossRef CAS; (c) W. Liu, M. Zang, J. Zhang, Q. Wang, Y. Z. Liu and W. P. Deng, Org. Chem. Front., 2023, 10, 1680–1685 RSC; (d) Z. H. Wang, X. H. Fu, Q. Li, J. Q. Zhao, Y. You, Y. P. Zhang and W. C. Yuan, Org. Lett., 2024, 26, 1589–1594 CrossRef CAS PubMed.
- For recent examples through vinyl lactones, see: (a) L. C. Yang, Y. N. Wang, R. Liu, Y. Luo, X. Q. Ng, B. Yang, Z. Q. Rong, Y. Lan, Z. Shao and Y. Zhao, Nat. Chem., 2020, 12, 860–868 CrossRef CAS PubMed; (b) C. Gao, X. Wang, J. Liu and X. Li, ACS Catal., 2021, 11, 2684–2690 CrossRef CAS; (c) W. Chai, Q. Zhou, W. Ai, Y. Zheng, T. Qin, X. Xu and W. Zi, J. Am. Chem. Soc., 2021, 143, 3595–3603 CrossRef CAS PubMed; (d) K. Li, S. Yang, B. Zheng, W. Wang, Y. Wu, J. Li and H. Guo, Chem. Commun., 2022, 58, 6646–6649 RSC; (e) Y. Liu, Y. He, Y. Liu, K. Wei and W. Guo, Org. Lett., 2022, 24, 2567–2572 CrossRef CAS PubMed; (f) H. Sun, Y. He and W. Guo, Org. Chem. Front., 2022, 9, 4861–4866 RSC; (g) J. Chen, N. Lin, L. Xu, Y. Wang, X. Luo, Y. Liu and W. Deng, Org. Chem. Front., 2024, 11, 1299–1304 RSC.
- For recent examples through acyclic allylic carbonates, see: (a) B. M. Trost and Z. Jiao, J. Am. Chem. Soc., 2020, 142, 21645–21650 CrossRef CAS; (b) Y.-Z. Liu and W.-P. Deng, Angew. Chem., Int. Ed., 2020, 59, 1238–1242 CrossRef CAS; (c) X.-X. Yang and Y.-C. Chen, Angew. Chem., Int. Ed., 2021, 60, 26762–26768 CrossRef CAS PubMed.
- C. Wang, Y. Li, Y. Wu, Q. Wang, W. Shi, C. Yuan, L. Zhou, Y. Xiao and H. Guo, Org. Lett., 2018, 20, 2880–2883 CrossRef CAS PubMed.
- H. I. Ahn, J. U. Park, Z. Xuan and J. H. Kim, Org. Biomol. Chem., 2020, 18, 9826–9830 RSC.
- Z. Zhao, X. X. Yang, G. Y. Ran, Q. Ouyang, W. Du and Y. C. Chen, Org. Lett., 2021, 23, 4791–4795 CrossRef CAS PubMed.
- L. Sun, J. Li, Y. Wu, Y. Li, J. Chen, X. Xia, C. Yuan, H. Guo and B. Mao, Org. Biomol. Chem., 2023, 21, 8107–8111 RSC.
- For selected examples, see: (a) K. Li, S. Zhen, W. Wang, J. Du, S. Yu, Y. Wu and H. Guo, Chem. Sci., 2023, 14, 3024–3029 RSC; (b) Y. Dong, J. Liu, K. Li, S. Han, B. Liang, F. Yang, S. Yu, Y. Wu, C. Zhang and H. Guo, Org. Lett., 2023, 25, 6328–6333 CrossRef CAS PubMed; (c) L. Wang, M. Lu, K. Li, S. Yang, L. Wang and H. Guo, Chem. Commun., 2024, 60, 3729–3732 RSC; (d) W. Wang, Y. Hu, K. Li, J. Xu, C. Zhang, L. Zhou, L. Zhu and H. Guo, ACS Catal., 2024, 14, 9940–9954 CrossRef CAS.
- (a) S. Yang, K. Li, Y. Tang, B. Zheng, W. Wang, Y. Wu, Y. Xiao and H. Guo, Adv. Synth. Catal., 2022, 364, 3967–3972 CrossRef CAS; (b) L. Wang, S. Yang, Y. Tang, K. Li, M. Lu and H. Guo, J. Org. Chem., 2024, 89, 5019–5028 CrossRef CAS PubMed; (c) Y. Dong, S. Yang, Y. Tang, K. Li, J. Liu, F. Yang, C. Zhang and H. Guo, Org. Lett., 2024, 26, 2057–2061 CrossRef CAS PubMed.
- H. Sun, A. Song, S. Gu, Y. Tang, S. Yang, W. Li and H. Guo, J. Org. Chem., 2024, 89, 14141–14150 CrossRef CAS PubMed.
- (a) H. Jia, H. Liu, Z. Guo, J. Huang and H. Guo, Org. Lett., 2017, 19, 5236–5239 CrossRef CAS PubMed; (b) Y. Dong, J. Liu, X. Gao, T. Pan, B. Mao, S. Yu, Y. Wu, C. Zhang and H. Guo, Chin. Chem. Lett., 2023, 34, 108297 CrossRef CAS; (c) Y. Tang, R. Zhang, Y. Dong, S. Yu, Y. Wu and H. Guo, Chem. Commun., 2024, 60, 1436–1439 RSC.
- Crystallographic data for 3aa have been respectively deposited with the Cambridge Crystallographic Data Centre as deposition number CCDC 2355129.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2355129. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ob01611d |
| This journal is © The Royal Society of Chemistry 2025 |