Generation of antibody–drug conjugates by proximity-driven acyl transfer and sortase-mediated ligation†
Zhi-Hui
Cui‡
a,
Hua
Zhang‡
a,
Feng-Hao
Zheng‡
a,
Jun-Hao
Xue
a,
Qing-Hong
Yin
a,
Xiao-Lei
Xie
a,
Yu-Xuan
Wang
a,
Tao
Wang
*b,
Li
Zhou
*c and
Ge-Min
Fang
 *a
*a
aSchool of Life Science, Institutes of Physical Science and Information Technology, Institute of Health Sciences, Anhui University, Hefei, 230601, P. R. China. E-mail: fanggm@ahu.edu.cn
bUniversity of Science and Technology of China, Hefei 230026, P. R. China. E-mail: wangtao15zhiyao@163.com
cAnhui Provincial Peptide Drug Engineering Laboratory, Hefei KS-V Peptide Biological Technology Co., Ltd, P. R. China. E-mail: zhouli@ks-vpeptide.com
First published on 12th November 2024
Abstract
We report a sortase-based site-specific antibody–drug conjugation strategy, which involves an affinity peptide-directed acyl transfer reaction and sortase-mediated peptide ligation. Through the affinity peptide-mediated acyl transfer reaction, an LPXTG-containing peptide is conjugated to a specific Lys side chain of an antibody. Under the assistance of sortase, a protein drug bearing a GG motif reacts specifically with the LPXTG moiety to produce an antibody–drug conjugate. Our strategy for antibody conjugation can be applied not only to chemically synthesized drugs, but also to biologically expressed proteins, and will provide a new sortase-based strategy for the preparation of antibody–drug conjugates.
Introduction
Antibody–drug conjugates (ADCs) have been approved for the treatment of many cancers.1 Conjugation chemistry between the antibody and the drug is crucial to the generation of ADCs.2 In a conventional conjugation strategy, lysine residues of an antibody are often utilized to randomly anchor drugs.3 Lysine-based random conjugation can lead to the formation of heterogeneous ADCs, and there are many disadvantages: (i) the composition of different batches of ADCs may be different, resulting in differential efficacy, and (ii) it is impossible to study the pharmacokinetics of each heterogeneous ADC.4 The narrow therapeutic index is a limitation for the first generation of ADCs. To address this issue, site-specific antibody conjugation has been reported.5 The development of site-selective antibody–drug conjugation strategies could accelerate the discovery and design of new ADCs.6Site-specific antibody–drug conjugates (ADCs) can be obtained in many different ways.7 One direct approach is to fuse peptide-based or protein-based drugs into the antibody sequence via recombinant protein expression.8–11 This approach involves the change in recombinant antibody manufacturing and is not suitable for many drugs that contain unnatural amino acids. To solve this problem, noncanonical amino acids bearing bioorthogonal handles have been incorporated into antibodies through genetic code expansion, enabling the conjugation of drugs by bioorthogonal reactions.12–15 Although this approach has led to several antibody drugs entering clinical trials, the incorporation of noncanonical amino acids requires reprogramming of the cellular translational machinery.16 Besides, the preparation of noncanonical amino acids that contain bioorthogonal handles often involves tedious multi-step chemical synthesis. Endogenous cysteine conjugation is a more convenient and cheaper route, allowing the production of homogeneous ADCs with a drug–antibody ratio (DAR) value of 8.17–20 However, a high DAR could cause the aggregation of many hydrophobic payloads.21,22 By partial disulfide reduction, ADCs with an average DAR of 2–4 can be obtained, but the formation of heterogeneous ADCs is unavoidable.23
Affinity peptide-directed Fc conjugation is a well-known strategy for the preparation of site-specific ADCs.24 This approach utilizes an Fc-binding peptide to transfer a bioorthogonal handle to a specific Lys residue of an off-the-shelf antibody. To date, various bioorthogonal handles have been incorporated into antibodies through the strategy of affinity peptide-directed Fc conjugation, such as sulfhydryl, azide, norbornene, hydroxylamine, etc. (Fig. 1A).25–36 For the conjugation of a drug, the drug has to be modified with a suitable bioorthogonal reactive handle. Notably, Huang et al. developed a traceless one-step site-specific conjugation strategy that avoids the use of bioorthogonal handles.27 They designed a novel thioester-based acyl transfer reagent in which the payload is linked to an Fc-binding peptide via a thioester bond. Guided by the Fc-binding peptide, the attached payload is site-specifically transferred to antibodies via S → N acyl migration. Nevertheless, the attachment of larger payloads such as nanobodies is not easy, because the synthesis of the large thioester-based acyl transfer reagents is a daunting task. With the growing demand for multispecific antibodies in the biopharmaceutical industry, there is a strong need for strategies for site-specific modification of off-the-shelf antibodies with larger payloads.37
Unlike bioorthogonal reactions, enzymatic peptide ligation is performed on natural amino acid sequences, avoiding the use of expensive unnatural amino acids.38–40 A variety of peptide ligases in nature, such as sortase A (SrtA), OaAEP1 and butelase-1, can efficiently and quickly ligate two peptides in a sequence-specific manner.41–48 Conventional ligase-catalyzed antibody modification requires the incorporation of the ligase recognition sequence into antibodies, which is not applicable to off-the-shelf antibodies. Inspired by the affinity peptide-directed Fc conjugation chemistry, we report here a new sortase-based strategy for the generation of site-specific ADCs (Fig. 1B). In our strategy, an Fc-binding peptide is employed to guide the S → N acyl transfer of the pentapeptide recognition sequence (LPSTG) to a site-specific Lys residue. The SrtA-mediated ligation is utilized to connect the GlyGly-containing payload to the LPETG motif to generate antibody–peptide conjugates. The applicability of this strategy has been demonstrated in the preparation of tirzepatide conjugated to bevacizumab. Given that the SrtA recognition sequence can be easily introduced into large peptide or protein payloads, the chemoenzymatic strategy will provide a new ligase-based strategy for the attachment of large payloads to antibodies.
Results and discussion
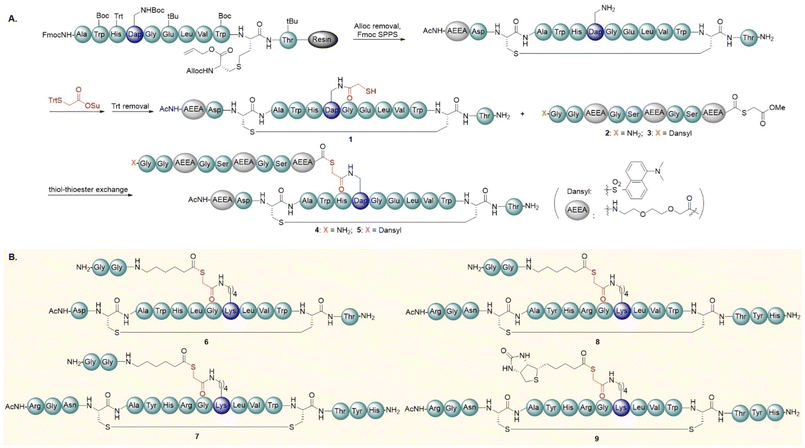
Our study began with the synthesis of the acyl transfer reagents derived from Fc-binding peptides. Although many Fc-binding peptides have been used for affinity peptide-directed antibody modifications, our initial attempts focused on Fc-III because it consists of only 13 amino acid residues and is easily prepared by solid-phase peptide synthesis. Inspired by Chung's study, we decided to replace 6Leu of Fc-III with Dap (Dap: diaminopropionic acid) because the shorter side chain of Dap was shown to be beneficial for transferring payloads to the Lys side chain of the antibodies. The disulfide bond in Fc-III was replaced by the C–S bond to avoid the exchange of the disulfide bond with sulfhydryl during peptide synthesis. The route of the designed affinity-binding peptide is shown in Fig. 2. First, we prepared the disulfide bond mimics of Fc-III by diaminodiacid-based solid phase synthesis.49–55 Then, Trt-protected thioglycolic acid was coupled to the side-chain amino group of Dap. After TFA treatment, the desired 1 was successfully obtained, in which the sulfhydryl group serves as the site to form a thioester bond with the payload.We then installed the sortase recognition sequence (GG) on the side-chain sulfhydryl group of 1. Starting from 2-chlorotritylhydrazine resin, we synthesized N-terminal GG peptide hydrazide.56–59 Peptide hydrazide has been widely used as the surrogate for peptide thioester in chemical protein synthesis.60–65 After NaNO2 oxidation and thiolysis, the peptide hydrazide was converted into the desired 2.66–69 In neutral PBS buffer, 1 reacted with 2 (10.0 equivalents) via thiol–thioester exchange to give the desired 4 (Fig. 2A). Meanwhile, we synthesized peptide 5, in which the N-terminal GG sequence was modified with dansyl chloride. The dye moiety in 5 was used to track the fluorescent antibody product by SDS-PAGE. Commercially available bevacizumab was selected as a model for antibody modification. After incubation with 5 (54 μM, 6 equiv.) in PBS buffer at room temperature for 6 h, the reaction solution was quenched and analysed by SDS-PAGE. As expected, we observed the band of the fluorescent antibody on SDS-PAGE (Fig. S22†). Note that, in the absence of Fc-III, bevacizumab was not fluorescently labelled, indicating that the reaction of bevacizumab with the peptide thioester occurred under the guidance of the Fc-binding peptide.
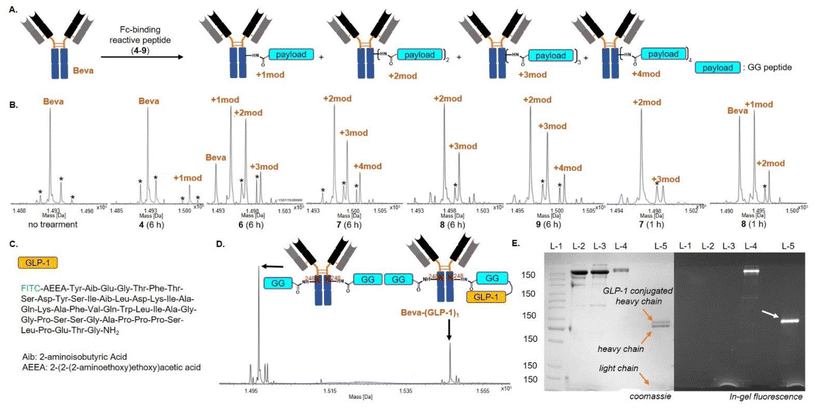
We then performed SrtA-catalyzed antibody modification. Bevacizumab was treated with 4 to afford the GG-labeled bevacizumab. Tirzepatide was selected as the cargo for enzymatic modification of the GG-labeled bevacizumab. A pentapeptide recognition sequence (LPETG) was added to the C-terminus of tirzepatide, and the FITC moiety at its N-terminus was used for fluorescent SDS-PAGE (Fig. 3C). In the presence of SrtA, 4-treated bevacizumab was incubated with different amounts of tirzepatide. By SDS-PAGE analysis, we determined that the optimal conditions for SrtA-catalyzed ligation are 8.0 equiv. of tirzepatide and a reaction time of 1 h (Fig. S25†). After 2 h, we observed a decrease in the fluorescence signal of the tirzepatide-labeled antibody band, which may be due to the SrtA-mediated hydrolysis of the ligation product. Of note, the main band of the antibody did not emit a fluorescent signal (Fig. S25†). To this end, we performed high-resolution mass spectrometry analysis on the GG-labeled bevacizumab that was afforded by 4. Disappointingly, less than 20% of bevacizumab was converted into the GG-labeled bevacizumab (Fig. 3B).
To improve the reaction yield of affinity peptide-directed Fc conjugation, we decided to fine-tune the structure of the Fc-binding peptide. Huang et al. pointed out that the position of the cargo in the Fc-binding peptide has a great influence on the S → N acyl transfer reaction.27 The crystal structure of Fc and the Fc-binding peptide shows that the limited space around 6Leu of Fc-III is not suitable for the accommodation of a larger cargo. To address this problem, we synthesized a new acyl transfer reagent 6 (Fig. 2B). In the structure of 6, 8Glu of Fc-III was mutated to Lys and used as the attachment site for the dipeptide recognition sequence (GlyGly). Meanwhile, we replaced Fc-III with a 17-mer FcBP to produce 7. The FcBP in 7 has been shown to be efficient in site-specific antibody modification. 8 is a thioether bond analogue of 7 and was prepared by diaminodiacid-based solid phase synthesis. Besides, we prepared peptide 9 as a positive control in our study, whose cargo is biotin. Note that 9 has been shown to be highly efficient in modifying antibodies.27
We then performed affinity peptide-directed antibody modification. After incubation with the thioester-based acyl transfer reagent for 6 h, bevacizumab was purified using protein A agarose resin and analysed by mass spectrometry. For the 6-treated sample, a large amount of unmodified bevacizumab remained (Fig. 3B), indicating that the Fc-binding peptide in 6 was not suitable for directing S → N acyl transfer on the antibody. In contrast, only a small amount of unmodified bevacizumab remained in the samples treated with 7 and 8, and bevacizumab labelled with two GG peptides was the major product. According to the crystal structure of Fc and the Fc-binding peptide, Fc-directed acylation tends to occur at Lys248, but may also occur at Lys246.24 The observed bevacizumab labelled with three or four GG peptides may be due to the nonspecific reaction of lysine residues with the acyl transfer reagent. Encouragingly, we found that when the reaction time was shortened to 1 h, bevacizumab treated with 7 was almost converted to the desired antibody containing two GG peptides (Fig. 3B). As a positive control, the labelling efficiency of bevacizumab treated with 9 was similar to that of 7. Unexpectedly, at the 1 hour time point, a significant amount of unmodified bevacizumab still remained in the sample that was treated with 8, suggesting that the subtle modulation of the disulfide bond of FcBP may affect its affinity for Fc.
We then conducted SrtA-catalyzed antibody modification of the GlyGly-labeled bevacizumab. In the presence of SrtA, the GlyGly-labeled bevacizumab reacted with tirzepatide containing C-terminal LPETG (72.0 μM, 8.0 equiv.) to generate the desired bevacizumab conjugated with tirzepatide (Fig. 3E). However, the conversion yield determined by high-resolution mass spectrometry is only about 30%, and bevacizumab was labelled with one tirzepatide molecule instead of two tirzepatide molecules (Fig. 3D). We speculated that the low yield of SrtA-mediated antibody modification could be due to the hydrolysis of the desired product by SrtA. Inspired by the irreversible SrtA-mediated ligation invented by Liu et al., we synthesized a peptide thioester containing a C-terminal GPET motif (Fig. S33†).70,71 Fortunately, the ligation product was not hydrolysed, and the SrtA-mediated modification of the GG-labeled bevacizumab proceeded smoothly. To our disappointment, in the absence of SrtA, we observed the side reactions between bevacizumab and the thioester peptide (Fig. S35†).
In sortase-catalyzed peptide ligation, the N-terminal Gly peptide is usually excessive. The excess N-terminal Gly peptide can compete with water to react with the C-terminal LPSTG peptide, avoiding the sortase-mediated hydrolysis of the ligation product. In contrast, the C-terminal LPSTG peptide can be rapidly hydrolysed by sortase, and thus the excess C-terminal LPSTG peptide cannot effectively suppress sortase-mediated hydrolysis, especially when prolonged ligation time is required. To this end, we decided to modify bevacizumab with an Fc-binding reactive peptide 10 containing an LPSTG motif. The structure of 10 is shown in Fig. 4B, and it is capable of transferring the LPSTG sequence to the specific Lys side chain of bevacizumab through affinity peptide-directed Fc conjugation. After incubation with 10 for 3 h, bevacizumab was labelled with two LPSTG-containing peptides. Importantly, there was little increase in the non-specific modification of bevacizumab even after 6 hours of incubation with 10 (Fig. 4C). This is good for the production of ADCs because the extended reaction time does not result in heterogeneous antibody–drug conjugates. Then, we treated the LPSTG-labelled bevacizumab with GLP-1′ in the presence of SrtA. Encouragingly, about 60% of bevacizumab was labelled with two GLP-1′ molecules (Fig. 4E and F). Moreover, we optimized the strategy of sortase-based antibody modification by using GLP-1′ conjugated 10, as shown in Fig. 4G. Specifically, GLP-1′ was first attached to 10 by the catalysis of sortase, and then the obtained GLP-1′ conjugated 10 was used to modify bevacizumab. To our delight, the efficiency of antibody labeling is further improved, with most of the heavy chains being labelled with GLP-1′ (Fig. 4H). The success of 10-mediated acyl transfer of GLP-1′ to bevacizumab suggests that the affinity peptide-directed Fc conjugation should be suitable for the attachment of large payloads (such as peptide- or DNA-based drugs) to antibodies. Please note that this study focuses on the development of a new sortase-based antibody conjugation strategy, and the bioactivity of the GLP-1′ conjugated bevacizumab still requires further biochemical studies to determine whether the conjugation sites and the used linker affect the activity of bevacizumab and GLP-1′.
Conclusions
To summarize, we report here a sortase-based strategy for the preparation of antibody–drug conjugates. The strategy relies on affinity peptide-directed Fc conjugation chemistry and ligase-mediated peptide ligation. First, under the guidance of an Fc-binding peptide, a SrtA recognition sequence (LPSTG) is transferred to a site-specific Lys residue of the native off-the-shelf antibody. Then, under the catalysis of SrtA, the payload of the GLP-1 peptide is site-specifically conjugated to the engineered LPSTG motif. Notably, our strategy can be used not only for the conjugation of synthetic small molecule drugs, but also for large peptide and protein drugs. Chemoenzymatic antibody modification avoids the use of relatively complex bioorthogonal reactions. Given the existence of many types of ligases in nature, we expect that the concept of sortase-based strategy could be extended to other ligase systems and will provide a new route for the discovery and preparation of multispecific antibody drugs. It should be noted that since each ligase has a different recognition sequence and performs under different reaction conditions, careful optimization is required to achieve the ideal antibody labelling efficiency.Author contributions
The idea of the study was designed by G.-M. Fang, L. Zhou and T. Wang. The procedure for antibody modification was established by Z.-H. Cui. The preparation of the Fc-binding peptide was performed by Z.-H. Cui, H. Zhang, F.-H. Zheng, J.-H. Xue, Q.-H. Yin, X.-L. Xie, and Y.-X. Wang. The manuscript was written and checked by G.-M. Fang, L. Zhou, and T. Wang.Data availability
The supporting data of this study have been included in the ESI.† The synthesis of peptides mentioned in this study, 4, 5, 6, 7, 8, and 9-directed Fc conjugation and SrtA-mediated GLP-1 attachment, and 10-directed Fc conjugation and SrtA-mediated GLP-1 attachment are included in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 22477001 and 22077002). The authors thank the staff for providing technical support with using the facility of the Institute of Health Sciences & Technology, Anhui University.References
- A. Beck, L. Goetsch, C. Dumontet and N. Corvaia, Strategies and challenges for the next generation of antibody-drug conjugates, Nat. Rev. Drug Discovery, 2017, 16, 315–337 CrossRef CAS PubMed.
- J. M. Sasso, R. Tenchov, R. Bird, K. A. Iyer, K. Ralhan, Y. Rodriguez and Q. A. Zhou, The evolving landscape of antibody-drug conjugates: in depth analysis of recent research progress, Bioconjugate Chem., 2023, 34, 1951–2000 CrossRef CAS PubMed.
- A.-D. Guo, D. Wei, H.-J. Nie, H. Hu, C. Peng, S.-T. Li, K.-N. Yan, B.-S. Zhou, L. Feng, C. Fang, M. Tan, R. Huang and X.-H. Chen, Light-induced primary amines and o-nitrobenzyl alcohols cyclization as a versatile photoclick reaction for modular conjugation, Nat. Commun., 2020, 11, 5472 CrossRef CAS PubMed.
- N. C. Reddy, R. Molla, P. N. Joshi, T. K. Sajeev, I. Basu, J. Kawadkar, N. Kalra, R. K. Mishra, S. Chakrabarty, S. Shukla and V. Rai, Traceless cysteine-linchpin enables precision engineering of lysine in native proteins, Nat. Commun., 2022, 13, 6038 CrossRef CAS PubMed.
- P. J. Carter and G. A. Lazar, Next generation antibody drugs: pursuit of the high-hanging fruit, Nat. Rev. Drug Discovery, 2018, 17, 197–223 CrossRef CAS PubMed.
- E. A. Hoyt, P. M. S. D. Cal, B. L. Oliveira and G. J. L. Bernardes, Contemporary approaches to site-selective protein modification, Nat. Rev. Chem., 2019, 3, 147–171 CrossRef CAS.
- S. J. Walsh, J. D. Bargh, F. M. Dannheim, A. R. Hanby, H. Seki, A. J. Counsell, X. Ou, E. Fowler, N. Ashman, Y. Takada, A. Isidro-Llobet, J. S. Parker, J. S. Carroll and D. R. Spring, Site-selective modification strategies in antibody-drug conjugates, Chem. Soc. Rev., 2021, 50, 1305–1353 RSC.
- R. R. Beerli, T. Hell, A. S. Merkel and U. Grawunder, Sortase enzyme-mediated generation of site-specifically conjugated antibody drug conjugates with high in vitro and in vivo potency, PLoS One, 2015, 10, e0131177 CrossRef PubMed.
- Y. Ando, H. Nakazawa, D. Miura, M. Otake and M. Umetsu, Enzymatic ligation of an antibody and arginine 9 peptide for efficient and cell-specific siRNA delivery, Sci. Rep., 2021, 11, 21882 CrossRef CAS.
- D. Schumacher, J. Helma, F. A. Mann, G. Pichler, F. Natale, E. Krause, M. C. Cardoso, C. P. R. Hackenberger and H. Leonhardt, Versatile and efficient site-specific protein functionalization by tubulin tyrosine ligase, Angew. Chem., Int. Ed., 2015, 54, 13787–13791 CrossRef CAS PubMed.
- R. L. Policarpo, H. Kang, X. Liao, A. E. Rabideau, M. D. Simon and B. L. Pentelute, Flow-based enzymatic ligation by sortase A, Angew. Chem., Int. Ed., 2014, 53, 9203–9208 CrossRef CAS.
- P. Agarwal and C. R. Bertozzi, Site-specific antibody-drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development, Bioconjugate Chem., 2015, 26, 176–192 CrossRef CAS PubMed.
- F. Tian, Y. Lu, A. Manibusan, A. Sellers, H. Tran, Y. Sun, T. Phuong, R. Barnett, B. Hehli, F. Song, M. J. DeGuzman, S. Ensari, J. K. Pinkstaff, L. M. Sullivan, S. L. Biroc, H. Cho, P. G. Schultz, J. DiJoseph, M. Dougher, D. Ma, R. Dushin, M. Leal, L. Tchistiakova, E. Feyfant, H.-P. Gerber and P. Sapra, A general approach to site-specific antibody drug conjugates, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1766–1771 CrossRef CAS.
- Y. Wang, J. Zhang, B. Han, L. Tan, W. Cai, Y. Li, Y. Su, Y. Yu, X. Wang, X. Duan, H. Wang, X. Shi, J. Wang, X. Yang and T. Liu, Noncanonical amino acids as doubly bio-orthogonal handles for one-pot preparation of protein multiconjugates, Nat. Commun., 2023, 14, 974 CrossRef CAS.
- J. Li and P. R. Chen, Development and application of bond cleavage reactions in bioorthogonal chemistry, Nat. Chem. Biol., 2016, 12, 129–137 CrossRef CAS PubMed.
- W. Ding, W. Yu, Y. Chen, L. Lao, Y. Fang, C. Fang, H. Zhao, B. Yang and S. Lin, Rare codon recoding for efficient noncanonical amino acid incorporation in mammalian cells, Science, 2024, 384, 1134–1142 CrossRef CAS PubMed.
- E. A. Hoyt, P. M. S. D. Cal, B. L. Oliveira and G. J. L. Bernardes, Contemporary approaches to site-selective protein modification, Nat. Rev. Chem., 2019, 3, 147–171 CrossRef CAS.
- Y. Zhang, X. Zhou, Y. Xie, M. M. Greenberg, Z. Xi and C. Zhou, Thiol specific and tracelessly removable bioconjugation via michael addition to 5-methylene pyrrolones, J. Am. Chem. Soc., 2017, 139, 6146–6151 CrossRef CAS PubMed.
- S. J. Walsh, S. Omarjee, W. R. J. D. Galloway, T. T.-L. Kwan, H. F. Sore, J. S. Parker, M. Hyvonnen, J. S. Carroll and D. R. Spring, A general approach for the site-selective modification of native proteins, enabling the generation of stable and functional antibody-drug conjugates, Chem. Sci., 2019, 10, 694–700 RSC.
- C. E. Stieger, L. Franz, F. Korlin and C. P. R. Hackenberger, Diethynyl phosphinates for cysteine-selective protein labeling and disulfide rebridging, Angew. Chem., Int. Ed., 2021, 60, 15359–15364 CrossRef CAS PubMed.
- P. Ochtrop, J. Jahzerah, P. Machui, I. Mai, D. Schumacher, J. Helma, M.-A. Kasper and C. P. R. Hackenberger, Compact hydrophilic electrophiles enable highly efficacious high DAR ADCs with excellent in vivo PK profile, Chem. Sci., 2023, 14, 2259–2266 RSC.
- T. Journeaux and G. J. L. Bernardes, Homogeneous multi-payload antibody-drug conjugates, Nat. Chem., 2024, 16, 854–870 CrossRef CAS.
- O. Boutureira and G. J. Bernardes, Advances in chemical protein modification, Chem. Rev., 2015, 115, 2174–2195 CrossRef CAS.
- K. Yamada, N. Shikida, K. Shimbo, Y. Ito, Z. Khedri, Y. Matsuda and B. A. Mendelsohn, AJICAP: affinity peptide mediated regiodivergent functionalization of native antibodies, Angew. Chem., Int. Ed., 2019, 58, 5592–5597 CrossRef CAS PubMed.
- T. Fujii, Y. Matsuda, T. Seki, N. Shikida, Y. Iwai, Y. Ooba, K. Takahashi, M. Isokawa, S. Kawaguchi, N. Hatada, T. Watanabe, R. Takasugi, A. Nakayama, K. Shimbo, B. A. Mendelsohn, T. Okuzumi and K. Yamada, AJICAP second generation: improved chemical site-specific conjugation technology for antibody-drug conjugate production, Bioconjugate Chem., 2023, 34, 728–738 CAS.
- W. Shi, F. Tang, J. Ao, Q. Yu, J. Liu, Y. Tang, B. Jiang, X. Ren, H. Huang, W. Yang and W. Huang, Manipulating the click reactivity of dibenzoazacyclooctynes: from azide click component to caged acylation reagent by silver catalysis, Angew. Chem., Int. Ed., 2020, 59, 19940–19944 CrossRef CAS PubMed.
- Y. Zeng, W. Shi, Q. Dong, W. Li, J. Zhang, X. Ren, C. Tang, B. Liu, Y. Song, Y. Wu, X. Diao, H. Zhou, H. Huang, F. Tang and W. Huang, A traceless site-specific conjugation on native antibodies enables efficient one-step payload assembly, Angew. Chem., Int. Ed., 2022, 61, e202204132 CrossRef CAS.
- C. B. Rosen, A. L. B. Kodal, J. S. Nielsen, D. H. Schaffert, C. Scavenius, A. H. Okholm, N. V. Voigt, J. J. Enghild, J. Kjems, T. Torring and K. V. Gothelf, Template-directed covalent conjugation of DNA to native antibodies, transferrin and other metal-binding proteins, Nat. Chem., 2014, 6, 804–809 CrossRef CAS.
- S. Kishimoto, Y. Nakashimada, R. Yokota, T. Hatanaka, M. Adachi and Y. Ito, Site-specific chemical conjugation of antibodies by using affinity peptide for the development of therapeutic antibody format, Bioconjugate Chem., 2019, 30, 698–702 CrossRef CAS PubMed.
- J. Z. Hui, S. Tamsen, Y. Song and A. Tsourkas, LASIC: light activated site-specific conjugation of native IgGs, Bioconjugate Chem., 2015, 26, 1456–1460 CrossRef CAS PubMed.
- J. Ohata and Z. T. Ball, A hexa-rhodium metallopeptide catalyst for site-specific functionalization of natural antibodies, J. Am. Chem. Soc., 2017, 139, 12617–12622 CrossRef CAS PubMed.
- D. Yuan, Y. Zhang, K. H. Lim, S. K. P. Leung, X. Yang, Y. Liang, W. C. Y. Lau, K. T. Chow and J. Xia, Site-selective lysine acetylation of human immunoglobulin G for immunoliposomes and bispecific antibody complexes, J. Am. Chem. Soc., 2022, 144, 18494–18503 CrossRef CAS.
- T. Lee, J. H. Kim, S. J. Kwon, J. W. Seo, S. H. Park, J. Kim, J. Jin, J. H. Hong, H. J. Kang, C. Sharma, J. H. Choi and S. J. Chung, Site-selective antibody-drug conjugation by a proximity-driven S to N acyl transfer reaction on a therapeutic antibody, J. Med. Chem., 2022, 65, 5751–5759 CrossRef CAS PubMed.
- V. Postupalenko, L. Marx, D. Viertl, N. Gsponer, N. Gasilova, T. Denoel, N. Schaefer, J. O. Prior, G. Hagens, F. Levy, P. Garrouste, J.-M. Segura and O. Nyanguile, Template directed synthesis of antibody Fc conjugates with concomitant ligand release, Chem. Sci., 2022, 13, 3965–3976 RSC.
- V. Postupalenko, L. Marx, M. Pantin, D. Viertl, N. Gsponer, G. Giudice, N. Gasilova, M. Schottelius, F. Levy, P. Garrouste, J.-M. Segura and O. Nyanguile, Site-selective template-directed synthesis of antibody Fc conjugates with concomitant ligand release, Chem. Sci., 2023, 15, 1324–1337 RSC.
- M. Tanriver, M. Muuller, M. D. Levasseur, D. Richards, S. Majima, A. DeMello, Y. Yamauchi and J. W. Bode, Peptide-directed attachment of hydroxylamines to specific lysines of IgG antibodies for bioconjugations with acylboronates, Angew. Chem., Int. Ed., 2024, 63, e202401080 CrossRef CAS.
- R. J. Deshaies, Multispecific drugs herald a new era of biopharmaceutical innovation, Nature, 2020, 580, 329–338 CrossRef CAS.
- H. E. Morgan, W. B. Turnbull and M. E. Webb, Challenges in the use of sortase and other peptide ligases for site-specific protein modification, Chem. Soc. Rev., 2022, 51, 4121–4145 RSC.
- Y. Li, J. Heng, D. Sun, B. Zhang, X. Zhang, Y. Zheng, W.-W. Shi, T.-Y. Wang, J.-Y. Li, X. Sun, X. Liu, J.-S. Zheng, B. K. Kobilka and L. Liu, Chemical synthesis of a full-length G-protein-coupled receptor β2-adrenergic receptor with defined modification patterns at the C-terminus, J. Am. Chem. Soc., 2021, 143, 17566–17576 CrossRef CAS PubMed.
- H. Ai, M. Sun, A. Liu, Z. Sun, T. Liu, L. Cao, L. Liang, Q. Qu, Z. Li, Z. Deng, Z. Tong, G. Chu, X. Tian, H. Deng, S. Zhao, J.-B. Li, Z. Lou and L. Liu, H2B lys34 ubiquitination induces nucleosome distortion to stimulate Dot1L activity, Nat. Chem. Biol., 2022, 18, 972–980 CrossRef CAS.
- S. K. Mazmanian, G. Liu, H. Ton-That and O. Schneewind, Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall, Science, 1999, 285, 760–763 CrossRef CAS PubMed.
- M. W.-L. Popp and H. L. Ploegh, Making and breaking peptide bonds: protein engineering using sortase, Angew. Chem., Int. Ed., 2011, 50, 5024–5232 CrossRef CAS.
- Y.-N. Zhang, X.-C. Wan, Y. Tang, Y. Chen, F.-H. Zheng, Z.-H. Cui, Z. Zhou and G.-M. Fang, Employing unnatural promiscuity of sortase to construct peptide macrocycle libraries for ligand discovery, Chem. Sci., 2024, 15, 9649–9656 RSC.
- G. K. T. Nguyen, S. Wang, Y. Qiu, X. Hemu, Y. Lian and J. P. Tam, Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis, Nat. Chem. Biol., 2014, 10, 732–738 CrossRef CAS.
- K. S. Harris, T. Durek, Q. Kaas, A. G. Poth, E. K. Gilding, B. F. Conlan, I. Saska, N. L. Daly, N. L. van der Weerden, D. J. Craik and M. A. Anderson, Efficient backbone cyclization of linear peptides by a recombinant asparaginyl endopeptidase, Nat. Commun., 2015, 6, 10199 CrossRef CAS PubMed.
- R. Yang, Y. H. Wong, G. K. T. Nguyen, J. P. Tam, J. Lescar and B. Wu, Engineering a catalytically efficient recombinant protein ligase, J. Am. Chem. Soc., 2017, 139, 5351–5358 CrossRef CAS.
- X.-C. Wan, Y.-N. Zhang, H. Zhang, Y. Chen, Z.-H. Cui, W.-J. Zhu and G.-M. Fang, Asparaginyl endopeptidase-mediated peptide cyclization for phage display, Org. Lett., 2024, 26, 2601–2605 CrossRef CAS.
- M. Fottner, J. Heimgärtner, M. Gantz, R. Mühlhofer, T. Nast-Kolb and K. Lang, Site-specific protein labeling and generation of defined ubiquitin-protein conjugates using an asparaginyl endopeptidase, J. Am. Chem. Soc., 2022, 144, 13118–13126 CrossRef CAS PubMed.
- Y. Guo, D.-M. Sun, F.-L. Wang, Y. He, L. Liu and C.-L. Tian, Diaminodiacid bridges to improve folding and tune the bioactivity of disulfide-rich peptides, Angew. Chem., Int. Ed., 2015, 54, 14276–14281 CrossRef.
- C.-K. Cui, Y. Guo, Y. He, F.-L. Wang, H.-N. Chang, Y.-J. Wang, F.-M. Wu, C.-L. Tian and L. Liu, Diaminodiacid-based solid-phase synthesis of peptide disulfide bond mimics, Angew. Chem., Int. Ed., 2013, 52, 9558–9562 CrossRef PubMed.
- Q. Qu, S. Gao, F. Wu, M.-G. Zhang, Y. Li, L.-H. Zhang, D. Bierer, C.-L. Tian, J.-S. Zheng and L. Liu, Synthesis of disulfide surrogate peptides incorporating large-span surrogate bridges through a native-chemical-ligation-assisted diaminodiacid strategy, Angew. Chem., Int. Ed., 2020, 59, 6037–6045 CrossRef CAS PubMed.
- C. Zuo, W.-W. Shi, X.-X. Chen, M. Glatz, B. Riedl, I. Flamme, E. Pook, J. Wang, G.-M. Fang, D. Bierer and L. Liu, Chimeric protein probes for C5a receptors through fusion of the anaphylatoxin C5a core region with a small-molecule antagonist, Sci. China: Chem., 2019, 62, 1371–1378 CrossRef CAS.
- Y.-K. Qi, Q. Qu, D. Bierer and L. Liu, A diaminodiacid (DADA) strategy for the development of disulfide surrogate peptides, Chem. – Asian J., 2020, 15, 2793–2802 CrossRef CAS PubMed.
- R. Zhao, P. Shi, J.-B. Cui, C. Shi, X.-X. Wei, J. Luo, Z. Xia, W.-W. Shi, Y. Zhou, J. Tang, C. Tian, M. Meininghaus, D. Bierer, J. Shi, Y. Li and L. Liu, Single-shot solid-phase synthesis of full-length H2 relaxin disulfide surrogates, Angew. Chem., Int. Ed., 2023, 62, e202216365 CrossRef CAS PubMed.
- R. Zhao, P. Shi, J. Y. Chen, S. S. Sun, J. N. Chen, J. B. Cui, F. M. Wu, G. M. Fang, C. L. Tian, J. Shi, D. Bierer, L. Liu and Y. M. Li, Chemical synthesis and biological activity of peptides incorporating an ether bridge as a surrogate for a disulfide bond, Chem. Sci., 2020, 11, 7927–7932 RSC.
- G.-M. Fang, Y.-M. Li, F. Shen, Y.-C. Huang, J.-B. Li, Y. Lin, H.-K. Cui and L. Liu, Protein chemical synthesis by ligation of peptide hydrazides, Angew. Chem., Int. Ed., 2011, 50, 7645–7649 CrossRef CAS PubMed.
- G.-M. Fang, J.-X. Wang and L. Liu, Convergent chemical synthesis of proteins by ligation of peptide hydrazides, Angew. Chem., Int. Ed., 2012, 51, 10347–10350 CrossRef CAS.
- J.-S. Zheng, S. Tang, Y.-C. Huang and L. Liu, Development of new thioester equivalents for protein chemical synthesis, Acc. Chem. Res., 2013, 46, 2475–2484 CrossRef CAS.
- J.-S. Zheng, S. Tang, Y.-K. Qi, Z.-P. Wang and L. Liu, Chemical synthesis of proteins using peptide hydrazides as thioester surrogates, Nat. Protoc., 2013, 8, 2483–2495 CrossRef CAS.
- J.-S. Zheng, M. Yu, Y.-K. Qi, S. Tang, F. Shen, Z.-P. Wang, L. Xiao, L. Zhang, C.-L. Tian and L. Liu, Expedient total synthesis of small to medium-sized membrane proteins via Fmoc chemistry, J. Am. Chem. Soc., 2014, 136, 3695–3704 CrossRef CAS PubMed.
- S. Dong, J.-S. Zheng, Y. Li, H. Wang, G. Chen, Y. Chen, G. Fang, J. Guo, C. He, H. Hu, X. Li, Y. Li, Z. Li, M. Pan, S. Tang, C. Tian, P. Wang, B. Wu, C. Wu, J. Zhao and L. Liu, Recent advances in chemical protein synthesis: method developments and biological applications, Sci. China: Chem., 2024, 67, 1060–1096 CrossRef CAS.
- J.-X. Wang, G.-M. Fang, Y. He, D.-L. Qu, M. Yu, Z.-Y. Hong and L. Liu, Peptide o-aminoanilides as crypto-thioesters for protein chemical synthesis, Angew. Chem., Int. Ed., 2015, 54, 2194–2198 CrossRef CAS.
- M. Pan, S. Gao, Y. Zheng, X. Tan, H. Lan, X. Tan, D. Sun, L. Lu, T. Wang, Q. Zheng, Y. Huang, J. Wang and L. Liu, Quasi-racemic X-ray structures of K27-linked ubiquitin chains prepared by total chemical synthesis, J. Am. Chem. Soc., 2016, 138, 7429–7435 CrossRef CAS.
- W.-W. Shi, C. Shi, T.-Y. Wang, Y.-L. Li, Y.-K. Zhou, X.-H. Zhang, D. Bierer, J.-S. Zheng and L. Liu, Total chemical synthesis of correctly folded disulfide-rich proteins using a removable O-linked β-N-acetylglucosamine strategy, J. Am. Chem. Soc., 2022, 144, 349–357 CrossRef CAS.
- M. Pan, Q. Zheng, T. Wang, L. Liang, J. Mao, C. Zuo, R. Ding, H. Ai, Y. Xie, D. Si, Y. Yu, L. Liu and M. Zhao, Structural insights into Ubr1-mediated N-degron polyubiquitination, Nature, 2021, 600, 334–338 CrossRef CAS.
- B. Zhang, Y. Zheng, G. Chu, X. Deng, T. Wang, W. Shi, Y. Zhou, S. Tang, J.-S. Zheng and L. Liu, Backbone-installed split intein-assisted ligation for the chemical synthesis of mirror-image proteins, Angew. Chem., Int. Ed., 2023, 62, e202306270 CrossRef CAS PubMed.
- H. Ai, G.-C. Chu, Q. Gong, Z.-B. Tong, Z. Deng, X. Liu, F. Yang, Z. Xu, J.-B. Li, C. Tian and L. Liu, Chemical synthesis of post-translationally modified H2AX reveals redundancy in interplay between histone phosphorylation, ubiquitination, and methylation on the binding of 53BP1 with nucleosomes, J. Am. Chem. Soc., 2022, 144, 18329–18337 CrossRef CAS PubMed.
- D.-L. Huang, W.-C. Guo, W.-W. Shi, Y.-P. Gao, Y.-K. Zhou, L.-J. Wang, C. Wang, S. Tang, L. Liu and J.-S. Zheng, Enhanced native chemical ligation by peptide conjugation in trifluoroacetic acid, Sci. Adv., 2024, 10, eado9413 CrossRef CAS PubMed.
- X.-C. Wan, W.-J. Zhu, Y. Chen, Z.-H. Cui, H. Zhang, F.-H. Zheng, Y.-N. Zhang and G.-M. Fang, Thioproline-based oxidation strategy for direct preparation of N-terminal thiazolidine-containing peptide thioesters from peptide hydrazides, Org. Lett., 2024, 26, 5021–5026 CrossRef CAS PubMed.
- Yi.-M. Li, Yi.-T. Li, M. Pan, X.-Qi Kong, Yi.-C. Huang, Z.-Y. Hong and L. Liu, Irreversible site-specific hydrazinolysis of proteins by use of sortase, Angew. Chem., Int. Ed., 2014, 53, 2198–2202 CrossRef CAS PubMed.
- C. Zuo, R. Ding, X. Wu, Y. Wang, G.-C. Chu, L.-J. Liang, H. Ai, Z.-B. Tong, J. Mao, Q. Zheng, T. Wang, Z. Li, L. Liu and D. Sun, Thioester-assisted sortase-A-mediated ligation, Angew. Chem., Int. Ed., 2022, 61, e202201887 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob01624f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |