Sodium trifluoromethanesulfinate-mediated photocatalytic aerobic oxidative esterification of aromatic aldehydes and alcohols†
Yong
Liu
a,
Xianjin
Zhu
a,
Yue
Zhang
a,
Zhengyi
Yi
 a,
Xiaobo
Yang
a,
Xiaobo
Yang
 *b and
Hua
Fu
*b and
Hua
Fu
 *a
*a
aKey Laboratory of Bioorganic Phosphorus Chemistry and Chemical Biology (Ministry of Education), Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China. E-mail: fuhua@mail.tsinghua.edu.cn
bInstitute of Catalysis for Energy and Environment, College of Chemistry and Chemical Engineering, Shenyang Normal University, Shenyang, 110034, P. R. China. E-mail: bxy1223@gmail.com; yangxb@synu.edu.cn
First published on 6th November 2024
Abstract
A sodium trifluoromethanesulfinate-mediated photocatalytic strategy for the aerobic oxidative esterification of aromatic aldehydes and alcohols has been developed, in which the in situ formed pentacoordinate sulfide derived from readily available and inexpensive sodium trifluoromethanesulfinate and oxygen acts as the photocatalyst, and the corresponding aromatic esters were provided in moderate to good yields. The present method is an economical and environmentally friendly protocol.
Introduction
Aromatic esters widely occur in the food industry and pharmaceutical, agrochemical and natural products and they are used as fragrances, drug intermediates, plasticizers, dyestuffs, and polymers.1 Esters are usually prepared via the activation of carboxylic acids and subsequent nucleophilic substitution.2 However, traditional esterification methods usually need various coupling reagents and form undesired side products. Catalytic oxidative coupling of aldehydes and alcohols to target esters under mild conditions is highly desirable in organic synthetic chemistry and industries, and some catalytic oxidative approaches towards esters have been developed.3 In addition, the direct oxidative esterification of aldehyde precursors, such as alkanols4 and alkanes,5 has received increasing attention. However, many of the methods above usually require expensive catalysts, oxidants, dry solvents and other harsh conditions. Therefore, it is necessary to develop a more environmentally benign and lower-cost catalytic protocol.Visible light photocatalysis has attracted great attention for its simplicity, economical nature and reaction novelty,6 and suitable photocatalysts can greatly improve the efficiency of reactions.7 Traditional photocatalysts are usually precious transition-metal complexes8 and elaborate organic dyes.9 Recently, we found that sodium trifluoromethanesulfinate in the presence of light and oxygen could mediate some interesting reactions including selective aerobic oxidations of alkyl arenes10a and alcohols10b and direct oxygen-isotopic labeling of carbonyls in ketones and aldehydes (1) with oxygen-isotopic waters (H218O or H217O).10c Very recently, several research groups applied our photocatalytic systems to realize some useful reactions.11 Herein, we report a sodium trifluoromethanesulfinate-mediated photocatalytic aerobic oxidative esterification of aromatic aldehydes and alcohols (Scheme 1a).
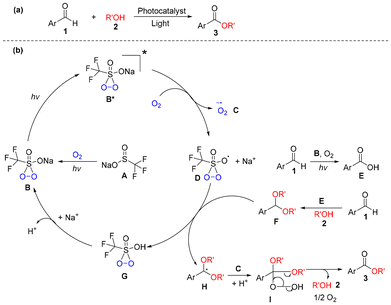 | ||
| Scheme 1 (a) Photocatalytic aerobic oxidation of aromatic aldehyde (1) and alcohol (2) to ester (3). (b) Proposed mechanistic pathway. | ||
In our previous research,10 we found that the in situ produced pentacoordinate sulfide (B) derived from sodium trifluoromethanesulfinate (A) and oxygen could act as the photocatalyst (see Scheme 1b). A detailed description of our proposed photocatalytic cycle is shown in Scheme 1b for the aerobic oxidative esterification of aromatic aldehydes and alcohols. Initial photoexcitation of B would yield B*, and single-electron transfer (SET) of B* to oxygen would form superoxide anion radical C and radical D leaving Na+. Oxidation of aldehyde 1 by oxygen would form acid E in the presence of photocatalyst B and light, and the treatment of aldehyde 1 with alcohol 2 would yield acetal F under the assistance of acid E (Note: AcidEis obtained from the photocatalytic aerobic oxidation of a small amount of aldehyde1 (see Scheme 5)). Transfer of a hydrogen radical in F to D would afford G and radical H, and the exchange of Na+ with the proton in G would regenerate B. Binding of H, C and a proton would provide I, and desorption of 2 and oxygen from I12 would provide ester (3).
Results and discussion
Investigations on reaction conditions
With this mechanistic hypothesis in hand (Scheme 1b), we first chose benzaldehyde (1a) and methanol (2a) as model substrates to optimize the reaction conditions (see Tables S1–S7 in the ESI† for details). The results showed that the conditions using 25 mol% sodium trifluoromethanesulfinate (CF3SO2Na) as the pre-photosensitizer and ethyl acetate as the solvent with the irradiation of a 3 W light emitting diode (LED) bulb (400–405 nm) under an oxygen atmosphere (1 atm) afforded methyl benzoate (3a) in 84% yield (determined by GC-MS) (74% isolated yield) (entry 1 in Table 1). When air replaced oxygen (1 atm), an 81% yield was provided (entry 2). No target product (3a) was observed in the absence of oxygen, light or CF3SO2Na (entries 3–5), which indicated that oxygen, light and CF3SO2Na were indispensable for the reaction. Other sulfur reagents, such as CF3SO3Na, MeSO2Na, EtSO2Na, PhSO2Na or CF3SO2Cl, replaced CF3SO2Na, and no target product was found (entry 6). The effect of solvents was surveyed (entries 7–10), and ethyl acetate was found to be a more suitable solvent than the others. We investigated the wavelength of light and found that wavelength was closely related to the reaction (entries 11–13), and a wavelength of 400–405 nm was optimal. We attempted different amounts of CF3SO2Na (entries 14 and 15) and methanol (entries 16 and 17), and 25 mol% CF3SO2Na and 15 equiv. of methanol were found to be suitable. The volume of ethyl acetate (entries 18 and 19) and the reaction time (entries 20 and 21) were also surveyed, and 0.5 mL of ethyl acetate and 12 h reaction time were found to be better when 0.2 mmol of 1a and 3.0 mmol of methanol (2a) were used as the substrates.| Entry | Variation of the standard conditions | Yieldb (%) |
|---|---|---|
| a Standard conditions: 1a (0.2 mmol), 2a (3.0 mmol), CF3SO2Na (25 mol%), MeCOOEt (0.5 mL), under an O2 atmosphere (1 atm) (the reaction tube was connected to a balloon filled with O2) and light irradiation with a LED (400–405 nm) (3 W) at room temperature (∼25 °C) for 12 h. b The yields were determined by 1H NMR using p-nitrotoluene as the internal standard. c Isolated yield. np = no product. | ||
| 1 | None | 84 (73c) |
| 2 | Air (1 atm) instead of oxygen | 81 |
| 3 | Ar atmosphere instead of oxygen | np |
| 4 | Without light | np |
| 5 | Without CF3SO2Na | np |
| 6 | CF3SO3Na, MeSO2Na, EtSO2Na, PhSO2Na or CF3SO2Cl instead of CF3SO2Na | np |
| 7 | CH3CN instead of MeCOOEt | 59 |
| 8 | CH2Cl2 instead of MeCOOEt | 74 |
| 9 | ClCH2CH2Cl instead of MeCOOEt | 73 |
| 10 | THF instead of MeCOOEt | 76 |
| 11 | LED (420–425 nm) instead of a LED (400–405 nm) | np |
| 12 | LED (380–385 nm) instead of a LED (400–405 nm) | 73 |
| 13 | LED (360–3655 nm) instead of a LED (400–405 nm) | 46 |
| 14 | 10 mol% instead of 15 mol% CF3SO2Na | 36 |
| 15 | 50 mol% instead of 15 mol% CF3SO2Na | 76 |
| 16 | MeOH (10 equiv.) instead of MeOH (15 equiv.) | 75 |
| 17 | MeOH (18 equiv.) instead of MeOH (15 equiv.) | 76 |
| 18 | MeCOOEt (0.25 mL) instead of MeCOOEt (0.5 mL) | 78 |
| 19 | MeCOOEt (0.75 mL) instead of MeCOOEt (0.5 mL) | 79 |
| 20 | 8 h instead of 12 h | 79 |
| 21 | 24 h instead of 12 h | 84 |
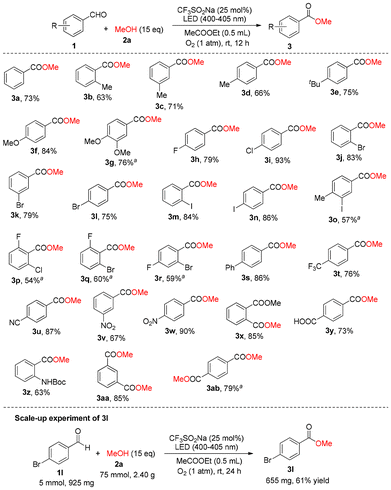
Surveys of the substrate scope of the photocatalytic aerobic oxidative esterification of aromatic aldehydes and alcohols
After obtaining the optimal conditions for this photocatalytic aerobic oxidative esterification of aromatic aldehydes and alcohols, we investigated the scope of substrates. As shown in Scheme 2, various aromatic aldehydes are amenable to this esterification strategy including neutral (3a and 3s, 73% and 86% yields, respectively), electron-rich (3b–3g, 63–84% yields), weak electron-deficient (3h–3r, 54–93% yields), and strong electron-deficient aromatic aldehydes (3t–3x, 67–90% yields). Interestingly, the photocatalytic aerobic oxidative esterification of 4-formylbenzoic acid with methanol provided 4-(methoxycarbonyl)benzoic acid (3y) in 73% yield, in which the previous carboxyl group in 4-formylbenzoic acid was not esterified. Furthermore, we attempted the esterification of N-Boc-2-aminobenzaldehyde, and product 3z was obtained in 63% yield. Two aromatic dialdehydes, 1,3-benzenedialdehyde and 1,4-benzenedialdehyde, were used for this photocatalytic aerobic oxidative esterification, and the corresponding diesters, dimethyl isophthalate (3aa) and dimethyl terephthalate (3ab), were prepared in 85% and 79% yields, respectively. A scale-up production of 3l was performed using 4-bromobenzaldehyde (1l) (5 mmol, 925 mg) and MeOH (2a) (75 mmol, 2.40 g) as the starting materials under the standard conditions with the irradiation of a 3 W LED (400–405 nm) for 24 h, and 655 mg of 3l (61% isolated yield) was obtained. The reactions in Scheme 2 could tolerate various functional groups including C–F, C–Cl, C–Br and C–I bonds, ether, CF3, cyano, nitro, ester, carboxyl and amido groups.As shown in Scheme 3, different alcohols including ethanol (2b) (see 3ac), propan-1-ol (2c) (see 3ad), propan-2-ol (2d) (see 3ae), 2-methylpropan-1-ol (2e) (see 3af), pentan-1-ol (2f) (see 3ag), octanol-1-ol (2g) (see 3ah), octanol-2-ol (2h) (see 3ai) and octanol-3-ol (2i) (see 3aj) are amenable to this photocatalytic aerobic oxidative esterification using 4-bromobenzaldehyde as the partner, and the corresponding esters were provided in 33–74% yields. Therefore, the results above showed that our environmentally friendly photocatalytic aerobic oxidative esterification was effective, economical and practical.
Unfortunately, esterification of some alkyl and heterocyclic aldehydes did not work. The possible reasons are as follows: the electron-donor properties of the alkyls can decrease the reactivity of the alkyl aldehydes and the coordination of the heteroatoms in the heterocyclic aldehydes with the pentacoordinated sulfur photocatalyst can decrease the activity of the photocatalyst.
Mechanistic investigations
To verify the proposed mechanistic pathway of the photocatalytic aerobic oxidative esterification of aromatic aldehydes and alcohols in Scheme 1b, some control experiments were carried out using benzaldehyde (1a) and methanol (2a) as the model substrates: (a) the photocatalytic aerobic oxidative esterification did not occur in the presence of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) as the scavenger under the standard conditions (Scheme 4d), and the results showed that the present aerobic oxidation was a radical process. (b) Treatment of acetal (4a) under the standard conditions provided the corresponding ester (3a) in 67% yield. (c) Oxidation of aldehyde (1a) led to benzoic acid (5) in 71% yield in the absence of alcohol. (d) Treatment of benzoic acid (5) with MeOH could not provide ester under the standard conditions (Scheme 4e), and the results showed that carboxylic acid was not an intermediate in the present photocatalytic esterification.Furthermore, we investigated the influence of oxygen dosage during the formation of acetal (4a) and ester (3a) using benzaldehyde (1a) and methanol (2a) as the model substrates (Table 2). When the amount of oxygen was 1–10 equivalents relative to the amount of 1a, only acetal (4a) was observed and the yields of 4a improved with an increase in oxygen dosage. When the oxygen dosage was increased to 20 equivalents, 4a and 3a were found in 23% and 20% yields, respectively. 4a gradually reduced and ester (3a) gradually increased with a further increase in oxygen dosage. When the amount of oxygen was 40 equivalents, 65% yield of 3a was obtained without the appearance of 4a.
| O2 (equiv.)b | 1 | 2 | 4 | 10 | 20 | 30 | 40 |
|---|---|---|---|---|---|---|---|
| a Standard conditions: benzaldehyde (1a) (0.2 mmol), methanol (2a) (3.0 mmol), CF3SO2Na (25 mol%), MeCOOEt (0.5 mL), O2 (1–40 equiv. relative to the amount of 1a), under light irradiation with a LED (400–405 nm) (3 W) at room temperature (∼25 °C) for 12 h. b See the ESI† for details on the increase of oxygen dosage. c The transformation yields of 3a and 4a were determined by GC-MS. | |||||||
| Yield of 4ac | 9% | 23% | 37% | 40% | 23% | 10% | 0 |
| Yield of 3ac | 0 | 0 | 0 | 0 | 20% | 41% | 65% |
According to the results above, a possible mechanism for the formation of a small amount of acid E in Scheme 1b is proposed in Scheme 5. Treatment of aldehyde 1 with D in Scheme 1b forms radical K freeing G, binding of K with superoxide anion radical C provides L, and the proton transfer from G to L affords peroxy acid (M). Reaction of M with aldehyde 1 yields acid E.
 | ||
| Scheme 5 Proposed mechanistic pathway for the photocatalytic aerobic oxidation of aromatic aldehyde 1 to a small amount of acid E. | ||
On/off experiments with a LED (400–405 nm) (3 W) were performed using 1a and 2a as the substrates under the standard conditions. As shown in Fig. 1, the photocatalytic reaction stopped in the absence of light. This time profile indicated that continuous irradiation with a LED (400–405 nm) is essential for the present photocatalytic transformation. According to the results above, we think that the proposed mechanistic pathway of the photocatalytic aerobic oxidative esterification of aromatic aldehydes in Scheme 1b is reasonable.
 | ||
| Fig. 1 Time profile of the photocatalytic reaction with and without visible light using 1a and 2a as the substrates under the standard conditions. | ||
Finally, our method was used in the synthesis of an analgesic agent, benzocaine (3al).13 As shown in Scheme 6, the photocatalytic aerobic oxidative esterification of tert-butyl (4-formylphenyl)carbamate with ethanol under the standard conditions afforded 3ak in 62% yield, and the deprotection of 3ak gave benzocaine (3al) in 96% yield. The results show that our method for the photocatalytic aerobic oxidative esterification of aromatic aldehydes is very useful in the field of organic synthesis.
Conclusions
We have developed a sodium trifluoromethanesulfinate-mediated photocatalytic strategy for the aerobic oxidative esterification of aromatic aldehydes and alcohols, in which the in situ formed pentacoordinate sulfide derived from sodium trifluoromethanesulfinate and oxygen acts as the photocatalyst, and the corresponding esters were provided in moderate to good yields, and the mechanism of photocatalytic ester formation was investigated well. The present method exhibits the following advantages: readily available and inexpensive sodium trifluoromethanesulfinate as the precursor of a photosensitizer, easy operational reaction conditions, use of environmentally friendly chemicals and high selectivity of the reactions.Data availability
The data supporting this article have been included as part of the article and its ESI.†Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by the Natural Science Foundation of Beijing Municipality (Grant No. 2222011), the Natural Science Foundation of Shenyang (Grant No. 23-503-6-10) and the National Natural Science Foundation of China (Grant No. 22077074).References
- J. N. Junzo Otera, Esterification: Methods, Reactions, and Applications, Wiley-VCH, Weinheim, 2nd edn, 2010 Search PubMed.
- (a) J. F. Wolfe, M. A. Ogliaruso, S. Patai and Z. Rappopory, Synthesis of Carboxylic Acids, Esters, and their Derivatives, Wiley Blackwell, New York, NY, 1991 Search PubMed; (b) R. C. Larock, Comprehensive Organic Transformations: A Guide to Functional Group Preparation, Vol. 2, Wiley Blackwell, New York, NY, 1999 Search PubMed.
- For selected papers, see: (a) Y. Diao, R. Yan, S. Zhang, P. Yang, Z. Li, L. Wang and H. Dong, J. Mol. Catal. A: Chem., 2009, 303, 35–42 CrossRef CAS; (b) R. Gopinath and B. K. Patel, Org. Lett., 2000, 2, 577–579 CrossRef CAS; (c) K. R. Reddy, M. Venkateshwar, U. Maheswari and S. Prashanthi, Synth. Commun., 2009, 40, 186–195 CrossRef; (d) S. Mahmood, T. Li, B.-H. Xu, Y.-F. Guo and S.-J. Zhang, Asian J. Org. Chem., 2017, 6, 768–774 CrossRef CAS; (e) R. Ray, R. D. Jana, M. Bhadra, D. Maiti and G. K. Lahiri, Chem. – Eur. J., 2014, 20, 15618–15624 CrossRef CAS PubMed; (f) A. S. J. Chakravarthy, M. J. Madhura and V. Gayathri, Catal. Lett., 2024, 154, 725–736 CrossRef CAS; (g) M. J. da Silva, C. J. A. Ribeiro and C. B. Vilanculo, Catal. Lett., 2023, 153, 2045–2056 CrossRef CAS; (h) S. Mahmood, T. Li, B.-H. Xu, Y.-F. Guo and S.-J. Zhang, Asian J. Org. Chem., 2017, 6, 768–774 CrossRef CAS; (i) Y.-F. Guo, S. Mahmood, B.-H. Xu, X.-Q. Yao, H.-Y. He and S.-J. Zhang, J. Org. Chem., 2017, 82, 1591–1599 CrossRef CAS PubMed; (j) H. Liu and M. S. Eisen, Organometallics, 2017, 36, 1461–1464 CrossRef CAS.
- For selected papers, see: (a) C. Liu, S. Tang and A. Lei, Chem. Commun., 2013, 49, 1324–1326 RSC; (b) C. Liu, J. Wang, L. Meng, Y. Deng, Y. Li and A. Lei, Angew. Chem., Int. Ed., 2011, 50, 5144–5148 ( Angew. Chem. , 2011 , 123 , 5250–5254 ) CrossRef CAS PubMed; (c) T. Suzuki, T. Matsuo, K. Watanabe and T. Katoh, Synlett, 2005, 1453–1455 CrossRef CAS; (d) N. Merbouh, J. M. Bobbitt and C. Breckner, J. Org. Chem., 2004, 69, 5116–5119 CrossRef CAS; (e) H. Yi, X. Hu, C. Bian and A. Lei, ChemSusChem, 2017, 10, 79–82 CrossRef CAS.
- For selected papers, see: (a) N. Tada, Y. Ikebata, T. Nobuta, S.-i. Hirashima, T. Miura and A. Itoh, Photochem. Photobiol. Sci., 2012, 11, 616–619 CrossRef CAS PubMed; (b) H. Liu, G. Chen, H. Jiang, Y. Li and R. Luque, ChemSusChem, 2012, 5, 1892–1896 CrossRef CAS; (c) S.-i. Hirashima, T. Nobuta, N. Tada, T. Miura and A. Itoh, Org. Lett., 2010, 12, 3645–3647 CrossRef CAS PubMed; (d) L. Zhang, H. Yi, J. Wang and A. Lei, Green Chem., 2016, 18, 5122–5126 RSC.
- (a) A. Bauer, F. Westkämper, S. Grimme and T. Bach, Nature, 2005, 436, 1139–1140 CrossRef CAS PubMed; (b) D. A. Nicewicz and D. W. C. MacMillan, Science, 2008, 322, 77–80 CrossRef CAS; (c) I. Ghosh, T. Ghosh, J. I. Bardagi and B. König, Science, 2014, 346, 725–728 CrossRef CAS PubMed; (d) J. Jin and D. W. C. MacMillan, Nature, 2015, 525, 87–90 CrossRef CAS; (e) E. B. Corcoran, M. T. Pirnot, S. Lin, S. D. Dreher, D. A. DiRocco, I. W. Davies, S. L. Buchwald and D. W. C. MacMillan, Science, 2016, 353, 279–283 CrossRef CAS; (f) M. Silvi, C. Verrier, Y. P. Rey, L. Buzzetti and P. Melchiorre, Nat. Chem., 2017, 9, 868–873 CrossRef CAS PubMed.
- (a) J. M. Narayanam and C. R. J. Stephenson, Chem. Soc. Rev., 2011, 40, 102–113 RSC; (b) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed; (c) J. Twilton, P. Zhang, M. H. Shaw, R. W. Evans and D. W. C. MacMillan, Nat. Rev. Chem., 2017, 1, 0052 CrossRef CAS; (d) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034–10072 CrossRef CAS.
- (a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed; (b) J. C. Tellis, D. N. Primer and G. A. Molander, Science, 2014, 345, 433–436 CrossRef CAS PubMed; (c) C. P. Johnston, R. T. Smith, S. Allmendinger and D. W. C. MacMillan, Nature, 2016, 536, 322–325 CrossRef CAS.
- (a) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed; (b) D. Ravelli, M. Fagnoni and A. Albini, Chem. Soc. Rev., 2013, 42, 97–113 RSC; (c) I. K. Sideri, E. Voutyritsa and C. G. Kokotos, Org. Biomol. Chem., 2018, 16, 4596–4614 RSC.
- (a) X. Zhu, Y. Liu, C. Liu, H. Yang and H. Fu, Green Chem., 2020, 22, 4357–4363 RSC; (b) X. Zhu, C. Liu, Y. Liu, H. Yang and H. Fu, Chem. Commun., 2020, 56, 12443–12446 RSC; (c) X. Zhu, Y. Liu, L. Ou, H. Yang and H. Fu, Chin. Chem. Lett., 2023, 34, 108454 CrossRef CAS.
- (a) Y. Qin, T. Zhang, H. Y. V. Ching, G. S. Raman and S. Das, Chem, 2022, 8, 2472–2484 CrossRef CAS; (b) K.-J. Liu, Z. Wang, L.-H. Lu, J.-Y. Chen, F. Zeng, Y.-W. Lin, Z. Cao, X. Yu and W.-M. He, Green Chem., 2021, 23, 496–500 RSC; (c) P. Xie, C. Xue, C. Wang, D. Du and S. Shi, Org. Chem. Front., 2021, 8, 3427–3433 RSC; (d) Y.-X. Chen, J.-T. He, M.-C. Wu, Z.-L. Liu, K. Tang, P.-J. Xia, K. Chen, H.-Y. Xiang, X.-Q. Chen and H. Yang, Org. Lett., 2022, 24, 3920–3925 CrossRef CAS; (e) C.-L. Zeng, H. Wang, D. Gao, Z. Zhang, D. Ji, W. He, C.-K. Liu, Z. Yang, Z. Fang and K. Guo, Org. Lett., 2022, 24, 3244–3248 CrossRef CAS PubMed; (f) R. Chen, H. Yuan, Y. Wang, H. Chen and Y. Zhang, Organometallics, 2023, 42, 1–5 CrossRef CAS.
- H. Asahara, N. Takao, M. Moriguchi, T. Inoueab and K. Ohkubo, Chem. Commun., 2022, 58, 6176–6179 RSC.
- Y.-T. Xia, X. Wang, M.-H. Su, J.-Y. Zhou, D.-X. Guo, B.-P. Zhang, W.-P. Sun, C. Cui, M. Yan and Y.-X. Li, J. Catal., 2024, 437, 115676 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ob01476f |
| This journal is © The Royal Society of Chemistry 2025 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Methanol (2a) (2.0 mmol).
Methanol (2a) (2.0 mmol).


