Open Access Article
Open Access ArticleTuning photophysical properties of semiconducting polymer nanoparticles for improved photocatalytic activity†
Jose
Sena-Fernández
ab,
Esther
Rebollar
 c,
Aitana
Hurtado-Mendoza
c,
Aitana
Hurtado-Mendoza
 a,
Timothy J.
Murdoch
d,
Juan Francisco
Vega
a,
Timothy J.
Murdoch
d,
Juan Francisco
Vega
 a,
Ignacio
Martín-Fabiani
a,
Ignacio
Martín-Fabiani
 d,
Tiberio A.
Ezquerra
d,
Tiberio A.
Ezquerra
 a and
Aurora
Nogales
a and
Aurora
Nogales
 *a
*a
aInstituto de Estructura de la Materia, IEM-CSIC, C/Serrano 121, Madrid 28006, Spain. E-mail: aurora.nogales@csic.es
bFaculty of Chemistry, Complutense University of Madrid, 28040 Madrid, Spain
cInstituto de Química Física Blas Cabrera, IQF-CSIC, C/Serrano 119, Madrid 28006, Spain
dDepartment of Materials, Loughborough University, LE11 1RJ Loughborough, UK
First published on 2nd June 2025
Abstract
This study investigates the relation between the optical and structural properties of poly(3-hexylthiophene) nanoparticles with their efficiency as photocatalysts for the degradation of water pollutants. Two nanoparticle preparation methods were investigated. Our results indicate that nanoparticles prepared by the miniemulsion method are more efficient in inducing photodegradation. The higher crystallinity, observed by X-ray scattering experiments and the presence of surfactant in these nanoparticles affect the photophysical properties. The fluorescence lifetime of miniemulsion nanoparticles is shorter than that of nanoparticles prepared by the flash method, favoring charge transfer towards the surface of the nanoparticle. These charges, in the presence of water, produce highly reactive oxygen species, responsible for the pollutant photodegradation.
Introduction
Semiconducting polymers have attracted great attention for materials science applications. They can combine the inherent properties of polymer materials (lightness, flexibility, processability, among others) with electrical conductivity and visible light absorption, due to the presence of conjugated π-bonds in the backbone.1–3 Because of this unique combination of properties, they have been used in organic field effect transistors (OFETs),4 organic light-emitting diodes (OLEDs),4 lasers,5 chemical sensors,6 and even in therapeutic treatments.7–9 Of particular interest is the use of semiconducting polymers in organic photovoltaic devices (OPVs),10,11 where they are used as active materials to convert solar energy into electrical energy. Among the wide variety of semiconducting polymers available nowadays,12 poly(3-hexylthiophene) (P3HT) is commonly chosen as the model polymer to test and study whether traditional concepts on polymer physics also apply to these functional polymers.13,14 P3HT has also been used to prove the applicability of semiconducting polymers in advanced applications other than OPVs.11,15 For example, semiconducting polymers can be used as photocatalysts,16,17 using light to induce the generation and transport of charges to produce chemical reactions that generate highly reactive oxygen species (ROS).18–20 Compared to titanium oxide nanoparticles, that are widely used as low cost photocatalysts,21,22 the large absorption of P3HT in the visible expands its activity as photocatalyst beyond UV range. Besides the applicability of P3HT, this polymer has been deeply studied from a fundamental point of view, therefore constituting an ideal system to correlate changes in physical properties with tunnability in applications. The potential of these highly reactive species to degrade pollutants has boosted research on photocatalysis for environmental applications.23,24 Water pollution caused by organic dyes widely used in the textile industry has become a problem.25,26 The demand for clean and safe drinking water is an environmental must be tackled. In the future, lack of access to this clean water could threaten human health and deplete natural or energy resources.27 Different approaches involving polymeric materials have been taken to tackle the problem of water remediation. Xu et al. fabricated bioinspired absorber gels that undergo a phase transition in the presence of clean water, leaving aside the water pollutants.28 Zhu et al.29 have reported the fabrication of a hybrid Z-scheme photocatalyst with iron nanoparticles (NPs), P3HT and titanium oxide. The ability of semiconducting polymers to produce ROS in the presence of water, enabling them to oxidize or reduce inorganic molecules, has been recently proved.19 However, there are challenges to be solved before these materials can be deployed in real-world applications. The formation of ROS can degrade the semiconducting polymer itself.30,31 When considering the use of semiconducting polymers for water treatment, another issue to overcome is solubility. Most semiconducting polymers, including P3HT, are insoluble in water. However, they can be prepared in the form of water dispersible NPs.32–34 Besides their water dispersibility, processing semiconducting polymers in the form of nanoparticles may enhance their properties compared to bulk materials due to their high surface-to-volume ratio. Also, the photophysical properties of NPs can differ significantly from those of bulk materials. The reduction in particle size can lead to quantum confinement35 and differences in molecular organization,33 resulting in unique optical and electronic behavior.34,36 It has been observed that semiconducting polymer NPs act as efficient light-harvesting agents that transfer the absorbed energy to doped dyes to improve their fluorescence brightness.37 Lifetimes of the different processes that take place following light absorption by semiconducting polymers depend on factors such as morphology,38 crystalline structure, and chemical environment.39 Various competing processes have been identified, such as radiative emission, non-radiative relaxation processes such as charge transfer, and intermolecular interactions such as exciton–exciton interactions.36,39 Understanding the role of each of these processes is critical because excited state deactivation mechanisms can be key to ROS generation.In this work, we investigate the properties of P3HT NPs prepared by different methods, focusing on the photophysical properties, including their absorption/emission spectra and fluorescence lifetimes. We correlate these results with the NPs efficiency to photodegrade a model organic water pollutant. Our results show that tuning the physical properties of the semiconducting polymer NPs enhances their potential in environmental and catalytic applications. This could open new avenues for the development of more efficient water remediation technologies.
Experimental
Materials
The P3HT sample with 97.6% regioregularity, 60.15 kDa weight average molecular weight and a polydispersity index of 2.1 was supplied by Ossila (Batch number M1011). The polymer was used without further purification. Different solvents were used for different types of NP preparations described below. Tetrahydrofurane (THF) (EMPARTA ACS, purity >99.5%, Merck, Darmstadt, Germany) and chloroform (ClCH3) (purity >99.98%, Quimipur SLU, Madrid, Spain) were used. In some preparations, sodium dodecyl sulfate (SDS, from Sigma Aldrich, ACS reagent grade, St Louis, MO, USA) was used as a surfactant. The excess of surfactant was removed by dialysis using a dialysis tubing membrane (Visking DTV, Medicell Int Ltd London, UK). The cutoff range was 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and the tube diameter was 25.5 mm. Methylene blue (methylthioninium chloride, supplied by Merck, purity ≥82%, Mw 319.85 g mol−1, powder) was used as a model dye for photodegradation experiments.
000 g mol−1 and the tube diameter was 25.5 mm. Methylene blue (methylthioninium chloride, supplied by Merck, purity ≥82%, Mw 319.85 g mol−1, powder) was used as a model dye for photodegradation experiments.
Preparation of the nanoparticles
Characterization techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ)/λ, where 2θ is the scattering angle. Azimuthal integration on the 2D images led to 1D scattering patterns, which are presented in this work after background subtraction. The dispersion of NPs in water was drop-cast onto a silicon wafer and allowed to dry for several days.
θ)/λ, where 2θ is the scattering angle. Azimuthal integration on the 2D images led to 1D scattering patterns, which are presented in this work after background subtraction. The dispersion of NPs in water was drop-cast onto a silicon wafer and allowed to dry for several days.
Fluorescence decay lifetimes were obtained by fitting the curves with four exponential decays convoluted with the instrument response function IRF and considering a background (eqn (1)).
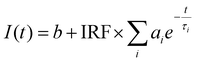 | (1) |
The analysis allowed extraction of representative lifetimes (τi) and amplitudes (ai) of the different decay components.
All samples were measured without any specific pretreatment. Dried nanoparticle suspensions were measured on 0.16–0.19 mm thick coverslips and films were measured on glass slides. The samples were covered so that no external light interfered with the measurements.
Photodegradation tests
A volume of 0.1 mL of NP suspensions with a concentration of P3HT of 0.5 g L−1 was mixed with 1 mL of a solution of MB in water (15 mg L−1). The mixture was poured into a quartz cuvette (100QS, HELLMA Analytics, LineaLab) of 5 mm path length. In this way, UV-VIS experiments to estimate the MB content were performed in the same volume exposed to the lamp light, without the need of extracting aliquots each time. Then it was exposed to a EQ-99X LDLS lamp (Energetiq Technology) located at a distance of 70 cm. The samples were thermostatized at 30 °C during the irradiation time. In each experiment, a control cuvette containing the MB solution without P3HT NPs was also irradiated. Before the photocatalytic degradation experiment, the suspension of MB and the P3HT NPs used as photocatalyst was magnetically stirred in the dark for 30 min to achieve adsorption equilibrium. At the given irradiation time, the samples were studied using the UV–Vis spectrophotometer. The photodegradation efficiency was determined by calculating the MB concentration at a given irradiation time through the UV-Vis absorption peak maximum of MB located at 664 nm, using the Lambert–Beer law (eqn (2)):| A664 = ε664·c·l | (2) |
The degradation efficiency χ(t) can be calculated as:
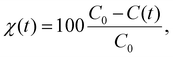 | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, diluted in Milli-Q water to 1/10 and filtered.
000 rpm, diluted in Milli-Q water to 1/10 and filtered.
A gradient consisting of water (eluent A), acetonitrile (eluent B), and HCOOH 5% (eluent C) was used. The elution program started with 2 min isocratic conditions (88% A; 10% B; 2% C), and then the percentage of B was increased to 90% in 10 min, maintaining constant the 2% of eluent C, and consequently, decreasing the percentage of eluent A. These final conditions were kept for 10 min. An injection volume of 100 μL was set for all LC analyses. Detection was done in the electrospray ionization mode. Bruker Compass 1.2 software was employed for LC-MS/MS data acquisition and processing.
Results and discussion
Morphology of the P3HT nanoparticles
Flash and miniemulsion methods produced NPs with a clear tendency toward spherical morphology and very similar sizes as measured by AFM (Fig. 1a and b). Quantitative analysis of the size distribution was performed in several regions of spin coated silicon wafers. The distribution of sizes includes NPs with diameters ranging between 10 nm to 100 nm in both cases, with the higher frequency of diameters around 20 to 30 nm (Fig. 1c). However, the distribution of miniemulsion NPs shows a bimodal shape, with a narrow peak around 20–30 nm in diameter and a broader one around 60 nm. For the case of flash NPs, the distribution is broader and centered around 30 nm.DLS measurements were also performed on the NP water suspensions. The results obtained in terms of diameter by number and polydispersity compared with AFM measurements, are presented in Table 1. Additional details regarding the DLS protocol are included in the ESI.† DLS measurements show larger diameters compared to AFM, with a more significant discrepancy observed for miniemulsion NPs. This difference may be attributed to variations in the hydrodynamic radius between the two types of NPs. Specifically, miniemulsion NPs are stabilized by surfactant molecules, which may influence the dynamics of the NPs in water. In addition, unlike flash NPs, the polydispersity of miniemulsion nanoparticles is found to be negligible in DLS measurements, probably due to the presence of two populations of NPs, one of them with sizes beyond that recorded by the DLS instrument.
| AFM | DLS | ||
|---|---|---|---|
| Diameter (nm) | Diameter (nm) | Polydispersity | |
| Flash | 42 ± 19 | 54 ± 7 | 0.13 ± 1.6 × 10−2 |
| Miniemulsion | 40 ± 20 | 86 ± 2 | 4.7 × 10−2 ± 3.5 × 10−3 |
Crystalline structure of the NPS prepared by miniemulsion and flash methods
WAXS experiments (Fig. 2) of both types of particles show the characteristic features of the form I crystalline phase of P3HT: an intense diffraction maximum at q = 3.8 nm−1 (100), corresponding to the stacking of the main chain/side chain layered structure of the P3HT crystal, with its second-order (200) and third-order (300) diffractions peaks also visible at 7.6 nm−1 and 11.4 nm−1. | ||
| Fig. 2 WAXS intensity of PH3T miniemulsion NPs (blue) and flash NPs (red) deposited on a silicon wafer. Thick black lines correspond to the best fit to the simulated two phase model considering the monoclinic crystalline structure and the amorphous fraction. Thin black lines correspond to the individual Bragg reflections, and dotted line represents the amorphous halo. Further details are included in the ESI.† | ||
The third order is more evident in the case of miniemulsion NPs, indicating a higher degree of crystallinity. It has been reported that the affinity between dodecyl chains of SDS and hexyl chains of P3HT may produce a mixed supramolecular phase between P3HT and SDS, resulting in some modifications in the crystalline structure.34 WAXS results support also this observation. The WAXS curves obtained from each type of NPs have been analysed to obtain the dimensions of the unit cell and the fraction of crystallinity. The protocol for deconvoluting the WAXS data considering the amorphous and crystalline contributions is described in the ESI.† The dimensions of the unit cell for each case and the fraction of crystallinity for each type of NPs are presented in Table S1.† The obtained results show that the unit cell of crystals in miniemulsion NPs has a lower volume than that of flash NPs, indicating a more compact arrangement of the chains in the case of miniemulsion NPs due to increased lamellar stacking. Also, miniemulsion NPs present higher crystallinity and larger crystalline sizes.
Optical properties of the P3HT nanoparticle suspensions
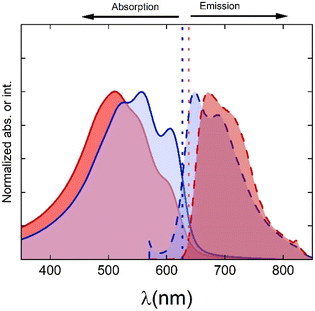
The NP preparation method significantly impacts on the optical properties of the water suspensions. Fig. 3 shows the absorption and emission spectra of water suspensions of P3HT NPs prepared by flash or miniemulsion method. For the case of miniemulsion, the UV-Vis absorption presents three clear maxima, located at 525 nm, 556 nm, and 607 nm, which are associated with the π–π* electronic transitions to different vibronic levels (2–0, 1–0 and 0–0 transitions, respectively).42 The 0–0 and 1–0 transition is also well defined in the emission spectra. The absorption spectra for miniemulsion NPs, shows a ratio A0–0/A0–1 is 0.8, whereas for flash NPs this ratio is around 0.6. According to the description in terms of H- and J-aggregates proposed by Spano et al.43 a higher A0–0/A0–1 ratio corresponds to a higher level of intra-chain order and planarization of the P3HT chains, which is characteristic for J-aggregates. Other signatures of the presence of J-aggregates include the shift towards higher wavelengths of the absorption spectra and the enhancement of 0–0 transition. The absence of well-defined peaks in the optical spectrum for flash-derived NPs suggests lower crystallinity than that of miniemulsion NPs, in agreement with the X ray results. To corroborate this, the absorption spectra were analysed using the modified Frank Condon approximation (see ESI, Fig. S3†). From this analysis, the 0–0 vibronic transition appears at lower energies (E0) for miniemulsion NPs than for flash NPs. Also, the free exciton bandwidth is considerably lower for miniemulsion NPs than for flash NPs (see Tables S2 and S3 of the ESI†). Both aspects are related to a higher planarization of the chains in the aggregates.43 The Frank Condon analysis allows also to estimate the fraction of aggregates in the NPs, resulting in 73% for the miniemulsion process and 60% for the flash NPs, which is qualitatively in agreement with the crystallinity obtained by X ray diffraction.Optical and X ray results suggest that the packing of the chains is less efficient in the case of flash NPs, and therefore, a larger band gap should be expected. Analysis of both absorption spectra by construction of a Tauc plot44 yields similar values for the band gap of both types of NPs, although slightly smaller for miniemulsion NPs (1.95 eV for miniemulsion NPs and 1.97 eV for flash NPs). Tauc plots for both types of NPs are presented in the ESI (Fig. S4†).
However, the bandwidth in the case of miniemulsion NPs is considerably smaller than the one obtained for flash NPs, indicating that in flash NPs, there is a higher chain torsional disorder. To understand the differences observed in optical properties and their relationship with the way the excitation energy flows in the NPs prepared by the different methods, fluorescence lifetime experiments were performed. Fig. 4a shows the fluorescence signal decay after excitation at 640 nm for flash NPs and miniemulsion NPs, respectively. The fluorescence decay for a control P3HT film of around 300 nm in thickness is also presented.
Depending on the system, different number of exponential functions were needed to fit the fluorescence decay curves. The obtained decay times can be grouped in four regions: 10−2 ns time scale, two characteristic times around 10−1 ns and 2 × 10−1 ns, and some longer times around the 1 ns timescale. We will refer to these times as τ4, τ3, τ2, and τ1 respectively, following the notation by Ferreira et al.45 The intensity weighted contribution of each of these decay times is different for each system. Fig. 4b shows the weight of each of the exponential decay as a function of the characteristic decay time for the three systems studied. More details of the fitting parameters are shown in the ESI (Fig. S5 and Tables S4, S5†).
In the P3HT thin film, the shortest lifetime τ4 is absent, and the decay process can be described with contributions of τ3 and τ2, along with a lower amplitude contribution from the process with characteristic time τ1. These results agree with those observed by other groups.39,46,47 However, for the two types of NPs, the shortest time τ4 is the main component. According to the literature τ4 is associated with the transition from a hot photoexcited state to a geometrically relaxed aggregate state.39 Gosh et al. have suggested that, in P3HT NPs in aqueous media, most of the hydrophobic polymer chain coil together.36 In the case of miniemulsion NPs, this hydrophobic–hydrophilic arrangement is enhanced by the presence of the SDS surfactant. In these circumstances, chromophores responsible for the fluorescence are closer to each other than in the case of P3HT thin film, and therefore a higher proportion of aggregates responsible for this transition is expected. The decay characteristic times τ3, τ2 and τ1 observed both in the thin film and flash NPs have been assigned in the literature to the decay of emissive states in P3HT,47 which is consistent with a high density of crystalline aggregates in the material. These states may correspond to excitons confined in crystalline regions, excitons localized in defects or charge-transferred excitons.
When comparing both types of NPs, the main component miniemulsion NPs show only the decay related to the hopping transition to lower energy sites with a conformational planarization of the polymer backbone.46,48 On the contrary, flash NPs exhibit some contributions from decay of emissive states due to exciton recombination, which is consistent with lower crystallinity and higher structural disorder. This structural disorder can limit the efficiency of controlled energy transitions, resulting in a red shift in emission properties (Fig. 3). The absorbed energy in the case of miniemulsion NPs should be dissipated then by other means. The higher efficiency of charge transfer in the miniemulsion would explain the lower weight of τ3, τ2 and τ1 in their fluorescence decay. The reversible formation of a charge transfer complex between polymer and molecular oxygen has been reported in the presence of water.49 This formation is enhanced under visible light illumination. It has been reported that, in electrolyte solutions, P3HT can be polarized by the water environment, lowering the polymer energy levels. Photogenerated electrons are then preferentially localized at the polymer–water interface.50 In miniemulsion P3HT NPs, this effect is further enhanced by the promotion of photogenerated electrons from the inner P3HT core to the outer hydrophilic regions, leading to higher reactivity with the environment. Therefore, photocatalytic differences due to the formation of reactive oxygen species by the two types of NPs in the presence of water could be expected. In the following section, we investigate whether these differences affect the application of the NPs in the photodegradation of water contaminants.
Methylene blue degradation by the presence of P3HT NPs
To prove this idea, photodegradation tests were conducted on water solutions of MB. Fig. 5a and b show UV-Vis spectra of MB solutions exposed to light for different durations in the presence of flash and miniemulsion P3HT NPs. MB solution spectra show an intense peak centered around 664 nm, which is used to estimate the concentration of the studied solutions following eqn (2).The degradation efficiency as a function of exposure time is presented in Fig. 5c in MB solutions in the presence of both types of P3HT NPs. For comparison, the efficiency of MB degradation only by light exposure is also presented.
To ensure that the MB is being degraded by the action of light and that the color decrease observed is not due to MB adsorption on the NPs surface or to the formation of uncoloured derivatives as leuco methylene blue or oxidised methylene blue51 we performed LC-MS analysis of the samples after 240 min irradiation and results are shown in Fig. S6.† Initial LC-MS profile of MB shows a prominent peak at a time of 12.8 min corresponding to m/z 284.2 and assigned to MB molecule. Additional peaks at m/z 270.2 and m/z 227.2 are assigned to azure B and thionin, compounds with one and three fewer methyl groups than MB respectively (see Fig. S7†). When MB alone is exposed to light for 240 min, the MB peak intensity decreases and the intensity of the peak assigned to thionin increases, indicating demethylation cleavage as previously reported in literature.52 In the case of light irradiation of the solutions containing P3HT NPs, the MB peak has disappeared and also the intensity assigned to thionin has significantly decreased, which indicates the almost complete degradation of MB. In addition to the LC-MS analysis of the solutions, the pH was measured initially and after light exposure, and a decrease was observed, which would be in agreement with the mineralisation of MB and the formation of acid species which contribute to the pH reduction.53
Regarding the degradation efficiency, as observed in Fig. 5, there is a clear enhancement when P3HT NPs are present. This effect is stronger in the case of miniemulsion NPs. This result can be correlated with a smaller gap value in this case, increasing the generation of photoexcited electrons. In addition, SDS improves the crystallinity and conformational planarization of the NPs, but may also modify the NP–water interface, facilitating the degradation of MB through increased electron transfer.
The higher crystallinity in the NPs prepared by miniemulsion, confirmed by X-ray, also can favour charge mobility and separation and reduces unwanted exciton recombination and minimise non-radiative losses, thereby facilitating the formation of reactive oxygen species at the NP–water interface is then more efficient for miniemulsion NPs, improving the degradation of MB. In these NPs, our results show that the absorbed energy from light is not dissipated as fluorescence (Fig. 4a), and it appears to be redirected to charge transfer processes, which directly correlates with the higher efficiency of MB photodegradation. The fluorescence decay of the miniemulsion NPs highlights a dominant contribution from charge transfer-related processes (τ4), such as jump transitions to lower-energy sites, either more ordered or less energetic regions. This is because short-lived excitons, which have a greater weight, are likely involved in non-radiative processes that precede the energy conversion into fluorescence. In contrast, in flash-prepared NPs, the greater structural disorder hinders charge separation, leading to exciton recombination and lower photocatalytic performance.
Conclusions
This study highlights the importance of controlling the internal structural, optical, and functional properties of P3HT NPs to optimize their photocatalytic activity through the preparation method.Fluorescence lifetimes of P3HT prepared in the form of NPs by miniemulsion and flash techniques, as well as in thin films, revealed fundamental differences in the decay processes and provided insight into the de-excitation events. The analysis of the fluorescence lifetimes suggests that the deactivation of the excited state in miniemulsion NPs occurs mainly by exciton transition to lower energy states and conformational planarization of the polymer backbone. Meanwhile, flash NPs showed additional contributions from the decay of emissive states due to exciton recombination, similar to what it is observed in P3HT thin films.
Structurally, the miniemulsion NPs were characterized by higher and more homogeneous size and also higher crystallinity, as confirmed by AFM, DLS and X ray diffraction. These observed differences are attributed to the presence of surfactant in miniemulsion NPs. The optical properties of miniemulsion NPs reveal a smaller band gap and more defined bands. The higher crystallinity and surfactant-induced planarity in the miniemulsion NPs could explain these optical properties, which enhance their photodegradation efficiency. The higher crystallinity facilitates charge transfer, a non-radiative process that competes with fluorescence and is associated with the generation of free radicals involved in photodegradation. The flash prepared NPs, on the other hand, show lower band resolution and a red shift that could be due to lower structural organization, resulting in a higher contribution of radiative processes and a lower photodegradation efficiency.
Author contributions
J. S. and A. N. initiated this work. A. N. supervised the method of nanoparticle preparation. A. H. M. carried out the experiments to obtain the absorption coefficient for the contaminant. J. S. and E. R. emission experiments. J. S. performed the AFM and UV-Vis characterization, supervised by A. N. and E. R. J. S., A. H. M. and J. F. V. performed and analysed the DLS experiments. J. S., T. J. M. and I. M. F. performed the FLIM experiments, and together with A. N. analysed them. J. S., E. R., A. N. and T. A. E. performed and analysed the WAXS experiments. J. S. and A. N. wrote the first manuscript with contributions from all authors. A. N. reviewed the final version of the manuscript. All authors participated in the discussion of the results commented on the manuscript.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants PID2019-107514GB-I00, PID2022-138635NB-I00 funded by MCIN/AEI/10.13039/501100011033, by grants TED2021-131914B-I00 and TED2021-129845B-I00 funded by MCIN/AEI/10.13039/501100011033 and by “ERDF A way of making Europe”. JS acknowledge the funding by MCIN/AEI/10.13039/501100011033 through grant number PRE2020-094362 and by the Regional Government of Madrid through the project TEC MATRIX-CM (TEC-2024/TEC-85). The support from the Scientific Network on Photocatalysis OASIS funded by the Consejo Superior de Investigaciones Científicas (CSIC), Spain is also acknowledged. AHM acknowledges funding by the JAE program, CSIC. The authors acknowledge M. Malfois for support in the experiments performed in NCD-SWEET beamline at ALBA synchrotron (Cerdanyola del Vallès, Barcelona, Spain) with the collaboration of ALBA staff.References
- L. Ding, Z.-D. Yu, X.-Y. Wang, Z.-F. Yao, Y. Lu, C.-Y. Yang, J.-Y. Wang and J. Pei, Chem. Rev., 2023, 123, 7421–7497 CrossRef CAS PubMed.
- X. Guo and A. Facchetti, Nat. Mater., 2020, 19, 922–928 CrossRef CAS PubMed.
- A. J. Heeger, Chem. Soc. Rev., 2010, 39, 2354–2371 RSC.
- K. Liu, B. Ouyang, X. Guo, Y. Guo and Y. Liu, npj Flexible Electron., 2022, 6, 1 CrossRef CAS.
- M. Karl, J. M. E. Glackin, M. Schubert, N. M. Kronenberg, G. A. Turnbull, I. D. W. Samuel and M. C. Gather, Nat. Commun., 2018, 9, 1525 CrossRef PubMed.
- U. Lange, N. V. Roznyatovskaya and V. M. Mirsky, Anal. Chim. Acta, 2008, 614, 1–26 CrossRef CAS PubMed.
- W. Li, M. Liang, J. Qi and D. Ding, Macromol. Rapid Commun., 2023, 44, 2300496 CrossRef CAS PubMed.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- Y. Cai, Z. Wei, C. Song, C. Tang, W. Han and X. Dong, Chem. Soc. Rev., 2019, 48, 22–37 RSC.
- G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Moriarty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864–868 CrossRef CAS.
- C. J. Brabec, M. Heeney, I. McCulloch and J. Nelson, Chem. Soc. Rev., 2011, 40, 1185–1199 RSC.
- D. Yang, Z. Li, Z. Li, X. Zhao, T. Zhang, F. Wu, Y. Tian, F. Ye, Z. Sun and X. Yang, Polym. Chem., 2017, 8, 4332–4338 RSC.
- S. Marina, E. Gutierrez-Fernandez, J. Gutierrez, M. Gobbi, N. Ramos, E. Solano, J. Rech, W. You, L. Hueso, A. Tercjak, H. Ade and J. Martin, Mater. Horiz., 2022, 9, 1196–1206 RSC.
- S. X. Drakopoulos, J. Cui, M. Asandulesa, P. W. M. Blom, A. Nogales and K. Asadi, Macromolecules, 2024, 57, 2661–2668 CrossRef CAS.
- S. Ulum, N. Holmes, D. Darwis, K. Burke, A. L. David Kilcoyne, X. Zhou, W. Belcher and P. Dastoor, Sol. Energy Mater. Sol. Cells, 2013, 110, 43–48 CrossRef CAS.
- P. Ramar, B. V. Aishwarya and D. Samanta, Chem. Commun., 2021, 57, 12964–12967 RSC.
- X. Wang, K. Maeda, A. Thomas, K. Takanabe, G. Xin, J. M. Carlsson, K. Domen and M. Antonietti, Nat. Mater., 2009, 8, 76–80 CrossRef CAS PubMed.
- T. Watanabe, A. Kitamura, E. Kojima, C. Nakayama, K. Hashimoto, A. Fujishima, D. Ollis and H. Al-Ekabi, Photocatalytic purification and treatment of water and air, Elsevier, Amsterdam, 1993, p. 747 Search PubMed.
- M. Criado-Gonzalez, C. Marzuoli, L. Bondi, E. Gutierrez-Fernandez, G. Tullii, P. Lagonegro, O. Sanz, T. Cramer, M. R. Antognazza and D. Mecerreyes, Nano Lett., 2024, 24, 7244–7251 CrossRef CAS PubMed.
- M. Zangoli, A. Cantelli, A. Candini, A. Lewinska, F. Fardella, A. Tino, G. Tommasini, M. Wnuk, M. Moschetta, S. Perotto, M. Lucarini, C. Tortiglione, G. Lanzani and F. Di Maria, J. Phys. Chem. C, 2023, 127, 4672–4683 CrossRef CAS.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- C. B. Anucha, I. Altin, E. Bacaksiz and V. N. Stathopoulos, Chem. Eng. J. Adv., 2022, 10, 100262 CrossRef CAS.
- M. A. Oturan and J.-J. Aaron, Crit. Rev. Environ. Sci. Technol., 2014, 44, 2577–2641 CrossRef CAS.
- C. Byrne, G. Subramanian and S. C. Pillai, J. Environ. Chem. Eng., 2018, 6, 3531–3555 CrossRef CAS.
- M. F. Hanafi and N. Sapawe, Mater. Today: Proc., 2020, 31, A141–A150 Search PubMed.
- H. Kumari, S. Suman, R. Ranga, S. Chahal, S. Devi, S. Sharma, S. Kumar, P. Kumar, S. Kumar, A. Kumar and R. Parmar, Water, Air, Soil Pollut., 2023, 234, 349 CrossRef CAS PubMed.
- S. Water, Hygiene and Health (WSH) Team, Progress on household drinking water, sanitation and hygiene 2000–2020: Five years into the SDGs, Report 978 92 4 003084 8, 2020.
- X. Xu, S. Ozden, N. Bizmark, C. B. Arnold, S. S. Datta and R. D. Priestley, Adv. Mater., 2021, 33, 2007833 CrossRef CAS PubMed.
- H. Zhu, L. Gong and Z. Li, Appl. Surf. Sci., 2020, 505, 144639 CrossRef CAS.
- H. Hintz, H. J. Egelhaaf, L. Lüer, J. Hauch, H. Peisert and T. Chassé, Chem. Mater., 2011, 23, 145–154 CrossRef CAS.
- M. Manceau, A. Rivaton, J.-L. Gardette, S. Guillerez and N. Lemaître, Polym. Degrad. Stab., 2009, 94, 898–907 CrossRef CAS.
- K. Landfester, R. Montenegro, U. Scherf, R. Güntner, U. Asawapirom, S. Patil, D. Neher and T. Kietzke, Adv. Mater., 2002, 14, 651–655 CrossRef CAS.
- G. Nagarjuna, M. Baghgar, J. A. Labastide, D. D. Algaier, M. D. Barnes and D. Venkataraman, ACS Nano, 2012, 6, 10750–10758 CrossRef CAS PubMed.
- E. Gutiérrez-Fernández, T. A. Ezquerra, E. Rebollar, J. Cui, S. Marina, J. Martín and A. Nogales, Polymer, 2021, 218, 123515 CrossRef.
- L. E. Brus, J. Chem. Phys., 1984, 80, 4403–4409 CrossRef CAS.
- S. Ghosh, S. Chakraborty, A. Ghosh, K. Marjit, G. Ghosh and A. Patra, J. Phys. Chem. C, 2022, 126, 18177–18187 CrossRef CAS.
- Y. Jin, F. Ye, M. Zeigler, C. Wu and D. T. Chiu, ACS Nano, 2011, 5, 1468–1475 CrossRef CAS PubMed.
- J. A. Labastide, M. Baghgar, I. Dujovne, B. H. Venkatraman, D. C. Ramsdell, D. Venkataraman and M. D. Barnes, J. Phys. Chem. Lett., 2011, 2, 2089–2093 CrossRef CAS.
- P. Parkinson, C. Müller, N. Stingelin, M. B. Johnston and L. M. Herz, J. Phys. Chem. Lett., 2010, 1, 2788–2792 CrossRef CAS.
- M. Sawicki, Publ. Astron. Soc. Pac., 2012, 124, 1208 CrossRef.
- G. Ashiotis, A. Deschildre, Z. Nawaz, J. P. Wright, D. Karkoulis, F. E. Picca and J. Kieffer, J. Appl. Crystallogr., 2015, 48, 510–519 CrossRef CAS PubMed.
- J. Clark, C. Silva, R. H. Friend and F. C. Spano, Phys. Rev. Lett., 2007, 98, 206406 CrossRef PubMed.
- F. C. Spano and C. Silva, Annu. Rev. Phys. Chem., 2014, 65, 477–500 CrossRef CAS PubMed.
- J. Tauc, R. Grigorovici and A. Vancu, Phys. Status Solidi B, 1966, 15, 627–637 CrossRef CAS.
- B. Ferreira, P. F. da Silva, J. S. Seixas de Melo, J. Pina and A. Maçanita, J. Phys. Chem. B, 2012, 116, 2347–2355 CrossRef CAS PubMed.
- N. Banerji, S. Cowan, E. Vauthey and A. J. Heeger, J. Phys. Chem. C, 2011, 115, 9726–9739 CrossRef CAS.
- S. Cook, A. Furube and R. Katoh, Energy Environ. Sci., 2008, 1, 294–299 RSC.
- N. Banerji, S. Cowan, M. Leclerc, E. Vauthey and A. J. Heeger, J. Am. Chem. Soc., 2010, 132, 17459–17470 CrossRef CAS PubMed.
- S. Bellani, D. Fazzi, P. Bruno, E. Giussani, E. V. Canesi, G. Lanzani and M. R. Antognazza, J. Phys. Chem. C, 2014, 118, 6291–6299 CrossRef CAS.
- E. Mosconi, P. Salvatori, M. I. Saba, A. Mattoni, S. Bellani, F. Bruni, B. Santiago-Gonzalez, M. R. Antognazza, S. Brovelli, G. Lanzani, H. Li, J.-L. Brédas and F. De Angelis, ACS Energy Lett., 2016, 1, 454–463 CrossRef CAS.
- A. Mills and J. Wang, J. Photochem. Photobiol., A, 1999, 127, 123–134 CrossRef CAS.
- M. A. Rauf, M. A. Meetani, A. Khaleel and A. Ahmed, Chem. Eng. J., 2010, 157, 373–378 CrossRef CAS.
- A. Krosuri, S. Wu, M. A. Bashir and M. Walquist, J. Water Process Eng., 2021, 40, 101926 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5nr01699a |
| This journal is © The Royal Society of Chemistry 2025 |