Optimizing sodium storage and durability in metal sulfide anodes with a 3D graphene architecture†
Mujtaba
Aminu Muhammad
ab,
Yangjie
Liu
a,
Baffa
Haruna
c,
Ahmed
Abdel-aziz
ac,
Zul
Qarnain
c,
Amir Mahmoud
Makin
c,
Jiaqi
Yu
a,
Bo
Zheng
 d,
Xiang
Hu
d,
Xiang
Hu
 *a and
Zhenhai
Wen
*a and
Zhenhai
Wen
 *a
*a
aCAS Key Laboratory of Design and Assembly of Functional Nanostructures, and Fujian Provincial Key Laboratory of Materials and Techniques toward Hydrogen Energy, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China. E-mail: huxiang@fjirsm.ac.cn; wen@fjirsm.ac.cn
bFujian Science & Technology Innovation Laboratory for Optoelectronic Information of China, Fuzhou, 350100, China
cUniversity of Chinese Academy of Sciences, Beijing, 100049, China
dState Key Laboratory of Clean Energy Utilization, Zhejiang University, China
First published on 26th March 2025
Abstract
Transition metal chalcogenides (TMCs) with a high theoretical capacity are regarded as promising anodes for sodium-ion batteries (SIBs) but encounter several challenges because of the complex conversion process, which leads to numerous side reactions and the inevitable disintegration of active materials, thereby impeding their practical application. In this work, inspired by a three-dimensional (3D) structure design, stable 3D reduced graphene oxide with heteroatom-site coordinated carbon centers (3DNSrGO) is fabricated, which features uniform and abundant nickel sulfide (NiS) particles within the empty spaces, along with sufficient access to the liquid electrolyte, thereby enabling more efficient transfer of sodium ions. Nevertheless, the NiS/3DNSrGO electrode still suffers from unexpected cycling instability and failure issues because of the short-circuiting, resulting from sodium (Na) metal corrosion and the deterioration of the glass fiber (GF) separator. The issue of short cycle life is significantly mitigated at the cell configuration level (inclusion of the polypropylene membrane) by lowering the risks of Na–metal corrosion and protecting the GF membrane. This study holds considerable potential for addressing (1) the growing requirement for efficient and sustainable Na+ host materials and (2) a newfangled approach that optimizes the long-term cycling stability of SIBs via a better cell configuration.
New conceptsThis study addresses the issues of continuous dissolution of anionic species in high-capacity nickel monosulfide and the short cycle life by rationally constructing a 3D reduced graphene oxide (3D-rGO) structure with heteroatom sites (NiS/3DNSrGO) and incorporating a polypropylene (PP) membrane along with glass fiber (GF) in the sodium-ion battery (SIB) cell configuration. The key finding is that the combination of the PP membrane and GF effectively prevents sodium metal corrosion and reduces sodium polysulfide shuttling. Electrochemical studies and ex situ characterization reveal that 3D-rGO enhances internal conductivity, mitigates volume expansion, and improves the contact between the electrolyte and active materials. Specifically, assessments of the half- and full-cell SIB devices show that the NiS/3DNSrGO anode demonstrates excellent rate capability (386 mA h g−1 at 10 A g−1) and high specific capacity (405 mA h g−1 after 2000 cycles at 5 A g−1), along with high energy and power densities. |
1. Introduction
Sodium-ion batteries (SIBs) have received significant research interest over the past two decades, particularly for advanced energy storage solutions, primarily because of the wider distribution and lower cost of sodium (Na) compared to lithium (Li). However, the performance of SIBs, including their rate capability, specific capacity, and cyclic lifespan, is significantly hindered by the larger ionic radius (1.02 Å) and molar weight of Na+ ions.1–3 Consequently, achieving rapid sodium ion reaction kinetics and extending cycling life remain formidable challenges when using cost-effective, high-rate sodium host materials with robust structures, which has prompted extensive research into suitable Na-host electrode materials, particularly for the anode. These challenges have been greatly addressed using carbonaceous materials, such as graphite and hard carbon. For carbonaceous anode materials, Na+ ions are large enough to nestle neatly within the tight lattice structure of the graphite electrode, thus resulting in the low capacity of graphitic carbon when accommodating more Na+ ions (35 mA h g−1), and the Na+//hard carbon cell type shows a limited capacity of less than 250 mA h g−1 and has a rapidly diminishing cycle life over time, which is still unsatisfied to meet the demand of high power and energy density for SIBs.2,3Transition metal chalcogenides (TMCs), exemplified by metal sulfides, are a reputable group of electroactive materials with stable and versatile crystal structures. In particular, nickel monosulfide (NiS) offers widely available resources, multiple electron reactions, high theoretical capacity, and abundant redox centers, which have been utilized in various electrochemical applications, including supercapacitors, electrocatalytic hydrogen generation, and alkali metal batteries.1,4–13 Unfortunately, the instability and limited capacity of nickel sulfide to achieve high-performance and maintain long-term cyclability without structural damage significantly hinder its reliability for successful implementation in alkali-metal ion rechargeable batteries.7,14–23 Various efforts have been made to address issues such as structural failure and nanoparticle aggregation by designing hybrid electrodes, where nanosized active materials are grafted onto conductive carbon matrices like carbon nanofibers, nanotubes, and various porous materials.24–27 Among these, graphene, which is composed of a single layer of carbon atoms arranged in a two-dimensional hexagonal structure, has shown potential due to its exceptional electrical conductivity, stability, flexibility, and ample active surface area.28,29 Consequently, extensive efforts have been made to integrate graphene-based composites with other functionally active nanomaterials to enhance the structural properties of materials designed for various energy applications.30–35 However, graphene-based materials are prone to re-stacking problems driven by the strong π–π interactions and van der Waals forces between graphene sheets, leading to a reduced surface area, lower electrical conductivity, and limited ion accessibility.
Constructing 3D structures is considered an effective approach to enhancing electrode/electrolyte contact, improving the overall structural integrity, and providing additional ion channels, which has generated significant interest in the field of energy storage.36–41 Recently, Thiruppathi et al. reported on 3D reduced graphene oxide (rGO), which demonstrated outstanding electrochemical performance, highlighting the advantages of 3D-rGO over its 2D counterpart.42 The enhanced physicochemical properties make 3D-rGO particularly promising for potential applications in catalysis and energy storage systems. Additionally, a new route was proposed where the use of propylene membranes in cobalt disulfide electrodes can counteract the shuttling of conversion reaction intermediate products (sodium polysulfides) and improve battery longevity.43 The incorporation of heteroatoms into a conductive carbon creates electrochemically active sites and structural defects and donates extra electrons that improve the adsorption of electrolyte ions, thereby increasing the overall conductivity and facilitating faster electron transfer, reducing the diffusion resistance, and enhancing the accessibility of active sites.37,38 From this point, constructing a 3D graphene structure, introducing defects, and incorporating polypropylene membranes are viable strategies to increase the active sites of electrode materials, thereby enhancing sodium ion transport kinetics.
Herein, we report an innovative 3D reduced graphene oxide embedded nickel sulfide (NiS/3DNSrGO) hybrid structure, which features a well-distributed pore network and abundant nitrogen and sulfur bonding (N–C/S–C), designed through a simple hydrothermal process followed by pyrolysis. Benefiting from the 3D graphene structure and the propylene membrane protective barrier, the NiS/3DNSrGO anode demonstrates excellent rate capability (with a specific capacity of 386 at 10 A g−1) and a cycling stability of 405 mA h g−1 after 2000 circulations at 5 A g−1. Furthermore, NVP/C//NiS/3DNSrGO full-cells were successfully assembled, delivering a high-rate specific capacity of 185 mA h g−1 at 2.0 A g−1 and maintaining a decent specific capacity of 202 mA h g−1 at 1 A g−1 even after 200 circulations.
2. Method
2.1 Synthesis of NiS/3DNSrGO
The synthesis process began with preparing 10 mL of graphene oxide (GO) aqueous solution (2 mg mL−1) and dissolving it in 100 mL of deionized water (DIW), which was then sonicated for 4 hours. Next, Ni(NO3)2·6H2O (2 mmol) and CH4N2S (4 mmol) were dispersed into the sonicated GO solution. The resulting precursor solution was heated to 90 °C using an oil bath and kept for 1 hour with mild agitation, while 0.5 g of PVP in aqueous solution was added within 2 minutes (dropwise). After that, the heated solution was sealed in a Teflon-lined autoclave (150 mL), where it was heated continuously in an oven for 24 hours at 180 °C. After cooling to room temperature, the resulting black solution was freeze-dried (2 days) and pyrolyzed at 500 °C under an argon (Ar) flow for 2 hours, yielding the NiS/3DNSrGO hybrid sample.2.2 Synthesis of 3DNSrGO
Initially, 4 mmol of CH4N2S was dispersed into a mixed solution of 20 mL of GO (2 mg mL−1) and 40 mL of DIW and sonicated for 4 hours followed by freeze-drying for one day. The precursor was then pyrolyzed at 700 °C for 2 hours using a reaction tube furnace filled with argon (Ar) gas and maintained under an inert atmosphere throughout the synthesis process.2.3 Synthesis of NiS particles
First, Ni(NO3)2·6H2O (0.8 mmol) and CH4N2S (1.6 mmol) were dispersed into an isopropanol solution (20 mL) under sonication for 0.5 hours, and 15 mL of ethylene glycol was added subsequently. The precursor solution was sealed in a 50 mL Teflon-lined autoclave, where it was heated continuously in an oven for 24 hours at 200 °C. After cooling naturally to room temperature, the product was centrifuged several times with DIW and ethanol absolute and then dried at 70 °C for one day.2.4 Material characterization
Scanning electron microscopy (SEM) images were obtained using a Hitachi SU-8020, while transmission electron microscopy (TEM) analysis was performed using a Tecnai F20. X-ray photoelectron spectroscopy (XPS, ESCALAB 250) was used to confirm the chemical state of elements. The crystalline phase of all prepared samples was examined by XRD, using a Miniflex 600 powder X-ray diffractometer equipped with Cu Kα radiation, following an emission profile of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Kα1
1 Kα1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kα2. Brunauer–Emmett–Teller (BET) analysis and nitrogen adsorption/desorption analysis were performed using an Intelligent Gravimetric Sorption Analyzer (Hiden IGA100B). The Raman spectrum was obtained using a 532 nm micro-Raman spectrometer (LabRAM HR). Thermogravimetric analysis (TGA) and Fourier transform infrared (FTIR) analysis were performed using a thermal analyzer (STA449F3) and an infrared spectrometer (VERTEX 70), respectively.
Kα2. Brunauer–Emmett–Teller (BET) analysis and nitrogen adsorption/desorption analysis were performed using an Intelligent Gravimetric Sorption Analyzer (Hiden IGA100B). The Raman spectrum was obtained using a 532 nm micro-Raman spectrometer (LabRAM HR). Thermogravimetric analysis (TGA) and Fourier transform infrared (FTIR) analysis were performed using a thermal analyzer (STA449F3) and an infrared spectrometer (VERTEX 70), respectively.
2.5 Electrochemical tests
A copper foil was used for coating a homogeneous slurry of active material mixed with carbon super (C65) as conductive carbon and carboxymethyl cellulose (CMC) as a binder in a 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio to prepare a composite anode electrode. The loading of the active substance was approximately 1.0 mg cm−2. The coated active substance was then dried in an electric oven set at 70 °C for 12 hours. For the electrochemical performance evaluation of NiS/3DNSrGO-1, the as-prepared NiS/3DNSrGO anode, sodium (Na) thin film as the counter electrode, and Whatman glass fibers (GF) as the separator were assembled into 2032-type coin cells inside an argon (Ar) filled glovebox (oxygen < 0.5 ppm, water < 0.5 ppm), and 1.0 M NaPF6 salt in diethylene glycol dimethyl ether (DIGLYME) solvent served as the electrolyte. For the electrochemical performance evaluation of NiS/3DNSrGO-2, both polypropylene (PP) membrane and Whatman glass fibers (GF) were used as separators. For full-cell SIBs, the as-prepared NiS/3DNSrGO sample, Na3V2(PO4)3/C, and GF were used as an anode, cathode, and separator, respectively. Battery cycling experiments were conducted on a Neware-battery test system (Wuhan Kingnuo Electronic Co., China) at a constant temperature of 25 °C, with a potential window of 0.01–3.0 V versus Na/Na+. A CHI660E electrochemical workstation was used for the cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests; specifically, the EIS spectra were recorded in the frequency range of 10 MHz to 100 kHz, respectively.
10 ratio to prepare a composite anode electrode. The loading of the active substance was approximately 1.0 mg cm−2. The coated active substance was then dried in an electric oven set at 70 °C for 12 hours. For the electrochemical performance evaluation of NiS/3DNSrGO-1, the as-prepared NiS/3DNSrGO anode, sodium (Na) thin film as the counter electrode, and Whatman glass fibers (GF) as the separator were assembled into 2032-type coin cells inside an argon (Ar) filled glovebox (oxygen < 0.5 ppm, water < 0.5 ppm), and 1.0 M NaPF6 salt in diethylene glycol dimethyl ether (DIGLYME) solvent served as the electrolyte. For the electrochemical performance evaluation of NiS/3DNSrGO-2, both polypropylene (PP) membrane and Whatman glass fibers (GF) were used as separators. For full-cell SIBs, the as-prepared NiS/3DNSrGO sample, Na3V2(PO4)3/C, and GF were used as an anode, cathode, and separator, respectively. Battery cycling experiments were conducted on a Neware-battery test system (Wuhan Kingnuo Electronic Co., China) at a constant temperature of 25 °C, with a potential window of 0.01–3.0 V versus Na/Na+. A CHI660E electrochemical workstation was used for the cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) tests; specifically, the EIS spectra were recorded in the frequency range of 10 MHz to 100 kHz, respectively.
3. Results and discussion
The synthetic composite material is prepared using a wet chemical reaction and subsequent pyrolysis. Initially, graphene oxide (GO) was dissolved in deionized water, where it naturally possesses a negative surface charge due to its oxygen-containing functional groups. The metal and sulfur sources (Ni(NO3)2 and CH4N2S) were then added to the sonicated graphene oxide solution, facilitating the adsorption of Ni2+ onto the GO matrix. The subsequent addition of polyvinylpyrrolidone (PVP) significantly influenced the surface structure of GO, and the size of the resulting nickel monosulfide particles, and contributed to porous formation during pyrolysis. The synthesis of NiS/3DNSrGO was completed with a final heat treatment under an argon flow. Notably, the synthesis process involves three simultaneous steps: (i) during high-temperature treatment, the removal of PVP leads to the formation of distinct three-dimensional pores, (ii) the reduction of GO to rGO, which results in the higher electrical conductivity of 3DrGO, and (iii) the transformation of NiSx to NiS, as illustrated in Fig. 1a.The structural features of the NiS/3DNSrGO system were probed using field emission scanning electron microscopy (FESEM) and transmission electron microscopy (TEM). The FESEM images in Fig. 1b show homogeneous distribution of nickel sulfide (NiS) nanocrystals integrated with N/S-co-doped 3D-reduced graphene oxide (3DNSrGO). The high-resolution FESEM image shown in Fig. 1c confirms that the nanostructured metal sulfides are embedded within the pores of the 3DNSrGO, with a high-surface-area graphitic carbon web connecting the dispersed metal sulfide particles. The TEM images at various microscopic magnifications in Fig. 1d and e clearly demonstrate that the NiS nanocrystals are well encapsulated and interconnected by a multi-channel network of reduced graphene oxide. The higher resolution TEM analysis in Fig. 1f reveals a well-defined crystallographic plane of NiS (101) with an interplanar distance of 0.259 nm. This observed regular pattern of atoms aligned at the lattice structure further validates the structural properties of nickel sulfide, respectively.
The energy dispersive X-ray (EDX) elemental mappings of NiS/3DNSrGO reveal the elemental distribution (Fig. 1g inset), with individual elemental mappings showing carbon (C), nickel (Ni), sulfur (S), and nitrogen (N), which correspond well with the distribution in the NiS/3DNSrGO hybrid. For comparison, the FESEM characterization of 3DNSrGO in Fig. S1 (ESI†) also shows uniform distribution of multi-channel graphitic carbon networks. Therefore, the role of PVP was further investigated, revealing that in the absence of PVP, the NiS/NSrGO consists only of 2D graphene sheets decorated with NiS nanoparticles (Fig. S2, ESI†). Additionally, FESEM images of NiS at various magnifications in Fig. S3 (ESI†) clearly show that the NiS nanocrystals are uniformly prepared, with sizes around 200 to 500 nm. The crystal structures of the developed NiS/3DNSrGO hybrid, NiS, and 3DNSrGO samples were characterized by X-ray diffraction (XRD) measurements (Fig. 2a). The XRD pattern of NiS corresponds to the face-centered cubic (fcc) structure of hexagonal NiS (PDF #77-1624). Notably, the XRD pattern of the NiS/3DNSrGO hybrid shows no significant changes compared to pure NiS, confirming the successful fabrication of the hybrid structure. The XRD pattern of 3DNSrGO also shows two diffraction peaks at 24.8 and 43.0 degrees, corresponding to the (002) and (101) crystal planes of a typical carbon structure. Furthermore, a closer examination of the XRD pattern reveals a diffraction peak above 25° attributed to turbostratic carbon due to the broad nature of the peak.30 The minor peaks at 31°, 44°, and 49° observed in NiS and the hybrid may have originated from trace impurities, intermediate compounds, or byproducts formed during the synthesis process, as materials often retain a small amount of organic solvents used during synthesis. Additionally, the decomposition of any surface-bound functional groups or impurities in the hybrid material may contribute to these minor XRD peaks. However, the intensity is relatively low, suggesting that such phases, if present, are minor and do not influence the intrinsic properties of the as-prepared materials. While we cannot definitively identify the origin of these peaks at this time, the chemical composition of the as-prepared NiS/3DNSrGO composite was confirmed further by performing X-ray photoelectron spectroscopy (XPS) analysis for transparency. The full XPS survey in Fig. 2b reveals that the nickel sulfide hybrid is primarily composed of nickel (Ni), sulfur (S), carbon (C), nitrogen (N), and oxygen (O). All components in the full XPS spectra were fitted using a Lorentzian asymmetric line shape, except for carbon, which was fitted with a customized asymmetric pseudo-Voigt line shape. The high-resolution XPS spectra of Ni 2p for pristine nickel monosulfide, shown in Fig. 2c, indicate the presence of Ni in both the 2+ and 3+ valence states. The observed peaks at 855.6 eV and 856.6 eV signified Ni 2p3/2 and Ni 2p1/2 for Ni2+, while the peaks at 871.9 eV and 874.1 eV are associated with Ni3+, along with two satellite peaks at binding energies (BEs) of 861.4 and 879.4 eV, respectively. These BEs are consistent with those reported in the literature.44–46Fig. 2d displays the high-resolution XPS spectrum of sulfur (S 2p) for the NiS/3DNSrGO hybrid. The broad peaks at 161.16 eV and 162.6 eV all resulted from Ni–S bonds,47 while the distinctive peaks at 163.5 eV and 168.4 eV signify the C–S and S–O bonds, respectively. The C–S and S–O peaks arise from the anionic doping of sulfur atoms into the rGO structure and their reaction with oxygen-containing groups in graphene oxide during high-temperature treatment.15,26,46
The XPS spectra of carbon (C 1s) in Fig. 2e exhibit five prominent peaks. The peaks centered around 284.7 eV corresponded to C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, representing sp2 and Sp3 carbon components. Additional peaks are observed at BEs 285.7, 286.4, 287 eV, and 288 eV belonging to the C–S, C–N, C–O, and carbonyl (C
C bonds, representing sp2 and Sp3 carbon components. Additional peaks are observed at BEs 285.7, 286.4, 287 eV, and 288 eV belonging to the C–S, C–N, C–O, and carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) bonds.48,49 The C–S and N–C bonds in the NiS/3DNSrGO hybrid are attributed to the interactions between heteroatoms and the carbon structure. In Fig. 2f, the nitrogen N 1s spectra reveal two distinct peaks at 398.6 and 400.3 eV, corresponding to pyrrolic and graphitic nitrogen, respectively.46,50 These XPS results indicate the integration of heteroatoms successfully into the GO matrix and the synergistic interaction between nickel monosulfide and 3D-reduced graphene oxide. This is further supported by the Fourier transform infrared (FTIR) results in Fig. 2g, which show specific absorption bands at 692 cm−1 and 1334 cm−1 corresponding to thioether (C–S) and tertiary amine (C–N) bonds. An additional S–O stretch around 1059 cm−1 is attributed to oxidized sulfur bonds. The presence of these heteroatoms, which significantly influence ion transport, helps explain the enhanced electrochemical performance of NiS/3DNSrGO electrodes in alkali metal-ion batteries. The Brunauer–Emmett–Teller (BET) analysis of NiS/3DNSrGO reveals an average surface area of 15.49 m2 g−1, as shown in Fig. 2h. A larger specific surface area enhances the contact between the electrode material and the electrolyte, suggesting that the three-dimensional structure significantly contributes to the electrochemical activity of the highly conductive NiS/3DNSrGO hybrid. The pronounced hysteresis loop in the relative pressure (P/P0) range of 0.5 to 1.0 confirms the presence of mesopores (characteristic of type IV isotherm curves) in the hybrid structure.51 However, the observed incomplete hysteresis loop has also been noted in previous studies.52,53 This phenomenon may be attributed to specific chemical properties of the sample surface, which can cause incomplete separation of gas molecules during adsorption. The average pore diameter of approximately 12.14 nm further supports the presence of mesopores in the NiS/3DNSrGO structure.
O) bonds.48,49 The C–S and N–C bonds in the NiS/3DNSrGO hybrid are attributed to the interactions between heteroatoms and the carbon structure. In Fig. 2f, the nitrogen N 1s spectra reveal two distinct peaks at 398.6 and 400.3 eV, corresponding to pyrrolic and graphitic nitrogen, respectively.46,50 These XPS results indicate the integration of heteroatoms successfully into the GO matrix and the synergistic interaction between nickel monosulfide and 3D-reduced graphene oxide. This is further supported by the Fourier transform infrared (FTIR) results in Fig. 2g, which show specific absorption bands at 692 cm−1 and 1334 cm−1 corresponding to thioether (C–S) and tertiary amine (C–N) bonds. An additional S–O stretch around 1059 cm−1 is attributed to oxidized sulfur bonds. The presence of these heteroatoms, which significantly influence ion transport, helps explain the enhanced electrochemical performance of NiS/3DNSrGO electrodes in alkali metal-ion batteries. The Brunauer–Emmett–Teller (BET) analysis of NiS/3DNSrGO reveals an average surface area of 15.49 m2 g−1, as shown in Fig. 2h. A larger specific surface area enhances the contact between the electrode material and the electrolyte, suggesting that the three-dimensional structure significantly contributes to the electrochemical activity of the highly conductive NiS/3DNSrGO hybrid. The pronounced hysteresis loop in the relative pressure (P/P0) range of 0.5 to 1.0 confirms the presence of mesopores (characteristic of type IV isotherm curves) in the hybrid structure.51 However, the observed incomplete hysteresis loop has also been noted in previous studies.52,53 This phenomenon may be attributed to specific chemical properties of the sample surface, which can cause incomplete separation of gas molecules during adsorption. The average pore diameter of approximately 12.14 nm further supports the presence of mesopores in the NiS/3DNSrGO structure.
Thermogravimetric analyses (TGA) were conducted under an air environment with heating temperatures ranging from 20 to 900 °C (Fig. 2i). The weight loss occurring between 300 °C and 500 °C primarily results from the partial oxidation and degradation of NSrGO, along with the possible decomposition of minor organic residues from the synthesis process. Additionally, the significant weight loss observed after 500 °C in the NiS/3DNSrGO hybrid structure is attributed to the decomposition of NiS into nickel oxide and the consumption of carbon and sulfur components.49 Based on the TGA results, the carbon content in NiS/3DNSrGO was calculated to be 28.3%. This calculation method follows procedures outlined in previous studies.20,24,54
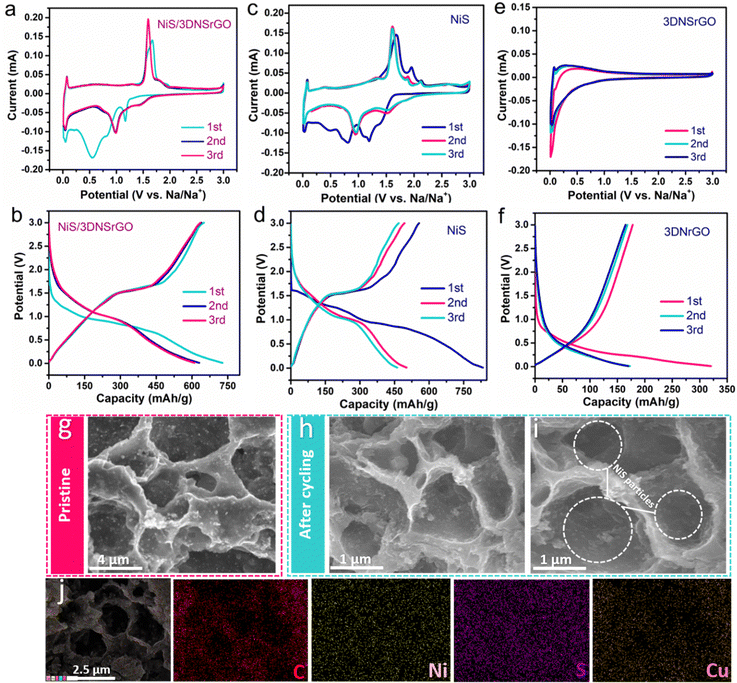
Cyclic voltammetry (CV) tests were performed to comprehend the outstanding electrochemical behavior of the high-capacity nickel sulfide hybrid and understand the effectiveness of the 3DNSrGO engineering for alkali-metal ion storage. Fig. 3a shows the representative first three CV plateaus tested at 0.1 mV s−1 sweep rate (0.01–3.0 V) for the NiS/3DNSrGO electrode. The first CV plateau exhibits a notable reduction peak at 1.16 V and a broad peak close to 0.55 V (discharge process), which is likely to have resulted due to the insertion of Na+, reduction of NiS to Ni3S2 and Ni sequentially, as well as the generation of a solid electrolyte interphase layer (SEI).20,55 The broad peak at 1.67 V (charging process) is attributed to the continuous recombination between metallic Ni and Na2S and the reversible formation of NiS.20 Following the 2nd and 3rd CV scans, the intensity of the oxidation peaks increased (1.59 V) and the main reduction peak underwent a shift to a lower potential of 0.98 V. However, the CV plateaus overlapped and remained steady, indicating an excellent redox reaction of the NiS/3DNSrGO composite electrode. The initial CV curve for the NiS bulk exhibits two reduction peaks situated near 1.19 V and 0.81 V, with an oxidation peak close to 1.67 V, respectively. During the subsequent cycles (second and third), prominent reduction peaks could be identified above 0.9 V (Fig. 3c). The shift of the reduction peaks in the following CV cycles can be regarded as the inevitable SEI layer formation occurring during the initial cycle.56 The oxidation peaks that appeared at a respective voltage of 1.61 V (charging process) could be characterized by the possible formation of NiS.55 As for the 3DNSrGO electrode, Fig. 3e shows two reduction peaks situated at 0.93 V and 0.47 V in the first CV cycle, which disappear in the second and third cycles, indicating Na+ insertion into the 3DNSrGO electrode and generation of the SEI layer on the electrode during the first CV cycle. As for the oxidation process, the broad peak ranging from 0.005 to 0.7 V signifies the extraction of Na+ from the 3DNSrGO electrode. In the subsequent CV scans, a reversible pair of cathodic/anodic peaks at around 0.1 V can be attributed to the reversible sodiation–desodiation into/from 3DNSrGO. It is worth mentioning that all the oxidation peaks shifted only slightly in NiS/3DNSrGO, NiS, and 3DNSrGO, which means that the charging process shares the same Na+ release. However, the CV plateaus for NiS/3DNSrGO exhibit better reversibility than those for NiS and 3DNSrGO electrodes, indicating that NiS/3DNSrGO stands out for its sufficient structural properties and transport of electrolyte ions during the electrochemical redox processes. All the corresponding galvanostatic discharge/charge plateaus of all samples tested at a specific current rate of 0.1 A g−1 are consistent with the corresponding CV results (Fig. 3b, d and f).
Additionally, the high coulombic efficiency (CE) of the NiS/3DNSrGO anode (727/650 mA h g−1) relative to NiS (833/557 mA h g−1) is attributed to the open pores and moderate surface area of NiS/3DNSrGO. These features not only expand the electrochemical active sites but also enhance ion accessibility throughout the redox process. Regarding the detailed intrinsic battery properties, the relatively low Rhigh value of NiS/3DNSrGO (46.1 Ω) indicates that there are faster charge transfer processes at the interface between NiS/3DNSrGO and the electrolyte (as shown in Fig. S4, ESI†), which is beneficial for achieving high-rate performance. On the other hand, the higher Rhigh value of NiS (66.3 Ω) (as shown in the inset of Fig. S4, ESI†) can be attributed to its poor conductivity and insufficient electrode/electrolyte interface. The EIS results provide a baseline, indicating that the lower impedance values of Na//NiS/3DNSrGO generally represent a more favorable Na+ storage process. The results of electrochemical impedance spectroscopy (EIS) suggest that engineering the 3D-graphene-based/NiS hybrid can provide a sufficient electrode interface, reduce the charge transfer resistance of NiS species, and enhance electronic conductivity.46Ex situ FESEM measurements (Fig. 3g–i) reveal the morphology of the pristine and fully charged NiS/3DNSrGO electrodes after the initial 10 cycles at 0.1 A g−1 current rate. The NiS particles, which are still encapsulated by the 3D nanoarchitecture (Fig. 3h and i), likely benefit from the buffering effect of the three-dimensional graphene oxide. The recovery of 3D pores, facilitated by the decomposition of Na2S during the charging process, reduces resistance and supports subsequent cycling. These advantages contribute to the longevity of the batteries during high-rate galvanostatic charge–discharge (GCD) tests. Furthermore, energy-dispersive X-ray spectroscopy (EDS) mapping images clearly show the spatial distribution of Ni, S, C, and Cu in Fig. 3j. The co-localization of Ni and S provides strong evidence supporting the presence of NiS.
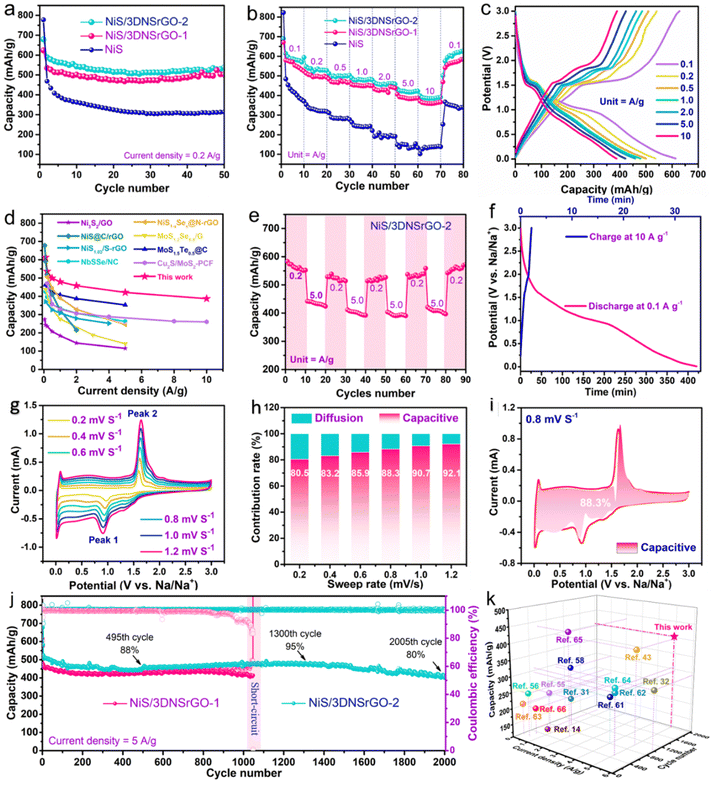
Subsequently, we evaluated the electrochemical properties of the composite electrodes by conducting GCD cycling at various current rates. Fig. 4a presents comparative cycling tests at 0.2 A g−1. The NiS/3DNSrGO electrode shows continuous and stable cycling with a high capacity of 503 mA h g−1 during the 50 cycling. In contrast, for NiS particles, the capacity is 313 mA h g−1 during the initial 50 cycles. The rapid decrease in capacity can be attributed to the limited ion channels and poor conductivity of NiS compared to NiS/3DNSrGO, as well as the possible collapse of NiS particles during the sodiation and desodiation process.30 Perhaps the NSrGO layer plays an essential role in accommodating Na+ and facilitating a smooth redox process compared to bulk NiS. Fig. 4b displays the corresponding rate performances of NiS/3DNSrGO and pure NiS electrodes. The NiS/3DNSrGO electrode demonstrated high-rate performance, which originated from its pseudocapacitive properties and the significant porous 3D carbon matrix with heteroatom sites. Notably, the NiS/3DNSrGO hybrid achieved high capacities at stepwise current densities, respectively (Table S1, ESI†). Due to the multichannel nature of NiS/3DNSrGO, the hybrid exhibits stable and sufficient reversibility at a low current rate of 0.1 A g−1. In contrast, the pure NiS electrode delivers unsatisfactory specific capacity with attenuation, especially at higher current rates. In contrast, the possible aggregation of particles during the sodium insertion/extraction process is likely to have resulted in poor ion transport of the NiS electrode. Moreover, the lack of carbonaceous materials and volume swelling in the electrode lead to insufficient specific capacity at stepwise current rates.
Furthermore, the PP membrane inclusion has led to capacity and cycle stability enhancement of the Na//NiS/3DNSrGO cell (Fig. 4a, inset). The NiS/3DNSrGO-2 electrode with a PP membrane demonstrates continuous and stable cycling, with a capacity of 529 mA h g−1 at 0.2 A g−1 during the initial 50 cycles. In comparison, the capacity of NiS/3DNSrGO-1 without the PP membrane is 503 mA h g−1 during the initial 50 cycles. The rapid decrease in capacity can be ascribed to the loss of conductive active materials in NiS/3DNSrGO-1 compared to NiS/3DNSrGO-2. This enhancement is mainly due to the ability of the PP membrane to suppress the migration of sodium polysulfides (NaPS) toward the sodium metal, minimizing the loss of conductive active material and reducing the risk of sodium metal corrosion/passivation.43 In addition, the inset of Fig. 4b shows the corresponding rate performances of Na//NiS/3DNSrGO-2 and Na//NiS/3DNSrGO-1 batteries. The optimized NiS/3DNSrGO-2 cell noticeably achieves high-rate capacities at a specific current rate, respectively (Table S2, ESI†). Moreover, the polarization between the discharge/charge platforms of the NiS/3DNSrGO-2 cell is very negligible even at stepwise current rates (0.1–10 A g−1) (Fig. 4c). This further explains the usefulness of incorporating the PP membrane, which results in a decrease in electrode polarization and an increase in charge storage capacity. Moreover, when comparing the specific rate capacities of NiS/3DNSrGO-2 with S-doped carbon,56 Se-doped metal sulfide,57–59 Te-doped metal sulfide,60 and several other metal sulfide-based anodes for sodium-ion batteries (SIBs),14,55,61 especially at high specific current rates, the NiS/3DNSrGO-2 anode holds a competitive specific capacity and rate performance for sodium-ion batteries (Fig. 4d).
The excellent pseudocapacitive behavior of NiS/3DNSrGO-2 was also verified by the rate capability test performed at 0.2 and 5 A g−1 (Fig. 4e), which is crucial for practical scalability where the energy demand varies. Besides, it is possible to maintain a discharge capacity of more than 386 mA h g−1 even with a 100-fold increase in the current density (from 100 mA g−1 to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mA g−1) (Fig. 4f). If the Na//NiS/3DNSrGO-2 cell configuration is used to power a small electronic device (such as a mobile phone), it is estimated that the battery could be charged in ∼128 seconds and discharged for approximately 7.0 hours at 100 mA g−1. The CV plateaus in Fig. 4g exhibit better reversibility and a high capacitive ratio is achieved (Fig. 4h) with increasing scan rates from 0.2 to 1.2 mV s−1, respectively. The prominence of the surface-driven capacitive process can be attributed to the well-distributed NiS particles with suitable N–C and S–C bonding properties, as well as the ability of the NiS/3DNSrGO electrode to efficiently accommodate rapid changes during the redox process. Consequently, the non-faradaic (capacitive) process predominates over the faradaic (diffusion) process. Notably, at a sweep rate of 0.8 mV s−1, NiS/3DNSrGO exhibits a significant portion of dominant capacitive behavior (Fig. 4i). These results suggest that the high-rate capacity of NiS/3DNSrGO is primarily derived from its pseudocapacitive behavior.
000 mA g−1) (Fig. 4f). If the Na//NiS/3DNSrGO-2 cell configuration is used to power a small electronic device (such as a mobile phone), it is estimated that the battery could be charged in ∼128 seconds and discharged for approximately 7.0 hours at 100 mA g−1. The CV plateaus in Fig. 4g exhibit better reversibility and a high capacitive ratio is achieved (Fig. 4h) with increasing scan rates from 0.2 to 1.2 mV s−1, respectively. The prominence of the surface-driven capacitive process can be attributed to the well-distributed NiS particles with suitable N–C and S–C bonding properties, as well as the ability of the NiS/3DNSrGO electrode to efficiently accommodate rapid changes during the redox process. Consequently, the non-faradaic (capacitive) process predominates over the faradaic (diffusion) process. Notably, at a sweep rate of 0.8 mV s−1, NiS/3DNSrGO exhibits a significant portion of dominant capacitive behavior (Fig. 4i). These results suggest that the high-rate capacity of NiS/3DNSrGO is primarily derived from its pseudocapacitive behavior.
Fig. 4j shows a long-term cycling performance test. NiS/3DNSrGO-2 can be activated from the initial cycles but there is also a certain degree of capacity decay until the 495th cycle and then it stabilizes with a capacity of 481 mA h g−1 at the 1300th cycle (Fig. 4j inset), demonstrating a notable reduction in the shuttling of NaPSs. Moreover, the PP membrane acts as a protective barrier, lowering the risk of sodium metal corrosion and glass fiber damage, thereby enhancing the overall safety of the cell.43 Consequently, the cycling life of the NiS/3DNSrGO-2 cell significantly extends to more than 2000 cycles at 5 A g−1 (with a specific capacity of 405 mA h g−1), retaining 80% of its capacity throughout the entire cycle. Note that the abrupt short circuit issue in the Na//NiS/3DNSrGO-1 battery is caused by the large and uncontrolled electron flow and the lack of a protective barrier. This leads to overheating and may even damage the NiS/3DNSrGO-1 cell, as evidenced by the repeated experimental results shown in Fig. S5 (ESI†). By comparing the long-term cycling ability of the NiS/3DNSrGO-2 electrode with recently reported metal sulfide-based anode materials for SIBs (Fig. 4k), the NiS/3DNSrGO-2 cell configuration is found to be advantageous and exhibits a truly competitive cycling lifespan.14,31,32,43,55,56,58,61–66
The excellent sodium ion storage can be attributed to the following properties: (i) the increased porosity of the 3DNSrGO architecture enhances the contact between active materials and electrolytes, thereby improving the rate performance of the NiS/3DNSrGO hybrid. (ii) The presence of a graphitic carbon structure boosts internal conductivity, mitigates volume variation, increases the number of active reaction sites, and accelerates charge transfer kinetics. (iii) The doping of heteroatoms (N, S) into the 3DrGO layer enhances ion transport and strengthens the inherent electrical properties, thereby maintaining the overall structural integrity of the electrode material. (iv) The incorporation of a polypropylene membrane effectively addresses cycle life issues at the cell configuration level by reducing NaPS shuttling and mitigating short circuit problems.
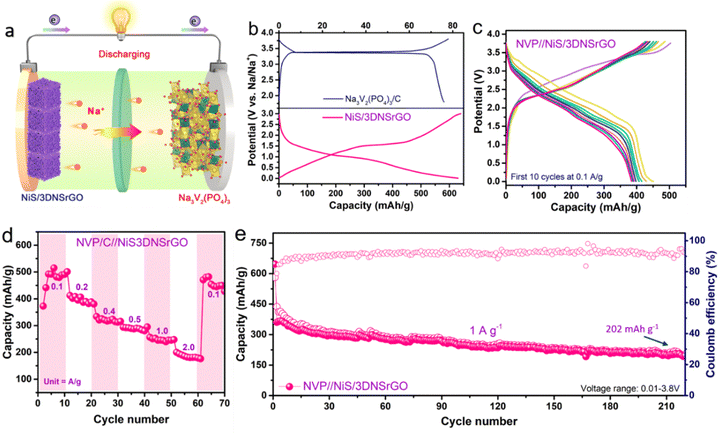
To further validate the outstanding three-dimensional architecture, NiS/3DNSrGO and Na3V2(PO4)3/C were employed as the anode and cathode materials, to test full-cell sodium-ion batteries (SIBs), as illustrated schematically in Fig. 5a. The XRD patterns and morphology of Na3V2(PO4)3/C are shown in Fig. S6 (ESI†). The GCD profiles in Fig. 5b reveal that the reported NVP/C cathode displays a typical flat voltage curve at 0.1 A g−1. After 10 circulations at 0.1 A g−1 (Fig. 5c), the reversible capacity, calculated based on the mass balance of the cathode and anode materials, remains at 423 mA h g−1 within the potential window of 0.01–3.80 V. This demonstrates exceptional Na+ uptake and release in the full-cell SIBs. The rate capability of the NVP/C//NiS/3DNSrGO cell, shown in Fig. 5d, exhibits minimal attenuation with increasing current rates, delivering average reversible capacities of 491, 395, 319, 290, 245, and 185 mA h g−1 from 0.1 to 2 A g−1 current rate, respectively. When the current density is returned to 0.1 A g−1, a reversible capacity of 449 mA h g−1 is retained. Notably, the NVP/C//NiS/3DNSrGO cell delivered a reversible capacity of 202 mA h g−1 even after over 200 cycles at a current density of 1 A g−1 (Fig. 5e), highlighting the promising potential of the NiS/3DNSrGO anode and its application prospects. The excellent sodium-ion storage performance in full cells demonstrates that the engineered 3D architecture with chemically bonded heteroatomic sites offers effective structural properties and stable NiS/3DNSrGO/electrolyte interfaces, facilitating rapid Na+ ion transfer for conversion-type metal sulfide anode materials.
4. Conclusion
In summary, we have developed an advanced anode material for SIBs by constructing a 3D reduced graphene oxide (rGO) structure that encapsulates nickel sulfide (NiS) and is enriched with nitrogen and sulfur (N–C/S–C) bonds. The synthesis method ensured uniform distribution of NiS within the abundant carbon pores. The 3DNSrGO network provided robust mechanical support and facilitated efficient electron transport, enhancing structural properties as well as electrical conductivity of the hybrid material. The resulting NiS/3DNSrGO hybrid, combined with a propylene (PP) membrane, demonstrated exceptional electrochemical performance, including excellent rate capability (with a specific capacity of 386 at 10 A g−1) and prolonged cycling stability (405 mA h g−1 after 2000 cycles at 5 A g−1, respectively). This work underscores the importance of innovative material design in overcoming the challenges associated with sodium-ion storage, particularly through the strategic combination of 3D-structured electrodes and propylene membranes. Our findings not only pave the way for high-performance battery design but also contribute to advancements in the broader field of energy storage technology.Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22225902, U22A20436, and 22209185), the National Key Research & Development Program of China (2022YFE0115900, 2023YFA1507101, and 2021YFA1501500), the Self-deployment Project Research Program of Haixi Institutes, Chinese Academy of Sciences (CXZX-2023-JQ08 and CXZX-2022-GH04), the State Key Laboratory of Clean Energy Utilization (No. ZJUCEU2023003), the China National Postdoctoral Program for Innovative Talents (BX20230365), and the China Postdoctoral Science Foundation (2023M743494 and 381517).References
- Y. Wu, C. Zhang, H. Zhao and Y. Lei, Recent advances in ferromagnetic metal sulfides and selenides as anodes for sodium- and potassium-ion batteries, J. Mater. Chem. A, 2021, 9(15), 9506–9534 RSC.
- S. Qiao, Q. Zhou, M. Ma, H. K. Liu, S. X. Dou and S. Chong, Advanced Anode Materials for Rechargeable Sodium-Ion Batteries, ACS Nano, 2023, 17, 11220–11252 CrossRef CAS PubMed.
- D. Ni, Y. Shen, W. Sun and Q. Wang, Design of 3D topological nodal-net porous carbon for sodium-ion battery anodes, J. Mater. Chem. A, 2022, 10, 7754–7763 RSC.
- M. Aminu Muhammad, Y. Liu, L. Sheng, B. Haruna, X. Hu and Z. Wen, Phase engineering of nickel-based sulfides toward robust sodium-ion batteries, J. Colloid Interface Sci., 2023, 646, 245–253 CrossRef CAS PubMed.
- H. Sun, J. Li, W. Wang, Z. Wang and L. Pan, Facile in situ synthesis of heazlewoodite on nitrogen-doped reduced graphene oxide for enhanced sodium storage, J. Colloid Interface Sci., 2021, 594, 35–46 CrossRef CAS PubMed.
- D. Wang, Z. Xu, Y. Lian, C. Ban and H. Zhang, Nitrogen self-doped porous carbon with layered structure derived from porcine bladders for high-performance supercapacitors, J. Colloid Interface Sci., 2019, 542, 400–409 CrossRef CAS PubMed.
- J. Li, J. Li, Z. Ding, X. Zhang, Y. Li, T. Lu, Y. Yao, W. Mai and L. Pan, In situ encapsulation of Ni3S2 nanoparticles into N-doped interconnected carbon networks for efficient lithium storage, Chem. Eng. J., 2019, 378, 122108 CrossRef CAS.
- Z. Zeng, W. Zhao, S. Yuan, Y. Dong, J. Zhu, F. Jiang, Y. Yang, S. Liu, L. Wang and P. Ge, Advances on Nickel-Based Electrode Materials for Secondary Battery Systems: A Review, ACS Appl. Energy Mater., 2022, 5(7), 9189–9213 CrossRef CAS.
- Y.-X. Wang, W.-H. Lai, Y.-X. Wang, S.-L. Chou, X. Ai, H. Yang and Y. Cao, Sulfur-Based Electrodes that Function via Multielectron Reactions for Room-Temperature Sodium-Ion Storage, Angew. Chem., Int. Ed., 2019, 58(51), 18324–18337 CrossRef CAS PubMed.
- M. Nazari, A. Noori, M. S. Rahmanifar, M. F. El-Kady, N. Hassani, M. Neek-Amal, R. B. Kaner and M. F. Mousavi, Phase-Dependent Energy Storage Performance of the NixSey Polymorphs for Supercapacitor-Battery Hybrid Devices, ACS Appl. Mater. Interfaces, 2022, 14(45), 50900–50912 CrossRef CAS PubMed.
- Y. Sun, K. Xu, Z. Wei, H. Li, T. Zhang, X. Li, W. Cai, J. Ma, H. J. Fan and Y. Li, Strong Electronic Interaction in Dual-Cation-Incorporated NiSe2 Nanosheets with Lattice Distortion for Highly Efficient Overall Water Splitting, Adv. Mater., 2018, 30(35), 1802121 CrossRef PubMed.
- H. Fan, P. Mao, H. Sun, Y. Wang, S. S. Mofarah, P. Koshy, H. Arandiyan, Z. Wang, Y. Liu and Z. Shao, Recent advances of metal telluride anodes for high-performance lithium/sodium–ion batteries, Mater. Horiz., 2022, 9(2), 524–546 RSC.
- O. Maurya, S. Khaladkar, M. R. Horn, B. Sinha, R. Deshmukh, H. Wang, T. Kim, D. P. Dubal and A. Kalekar, Emergence of Ni-Based Chalcogenides (S and Se) for Clean Energy Conversion and Storage, Small, 2021, 17(33), 2100361 CrossRef CAS PubMed.
- Y.-h Zhang, R.-h Liu, L.-j Xu, L.-j Zhao, S.-h Luo, Q. Wang and X. Liu, One-pot synthesis of small-sized Ni3S2 nanoparticles deposited on graphene oxide as composite anode materials for high-performance lithium-/sodium-ion batteries, Appl. Surf. Sci., 2020, 531, 147316 CrossRef CAS.
- X. Chang, Y. Ma, M. Yang, T. Xing, L. Tang, T. Chen, Q. Guo, X. Zhu, J. Liu and H. Xia, In situ solid-state growth of N, S codoped carbon nanotubes encapsulating metal sulfides for high-efficient-stable sodium ion storage, Energy Storage Mater., 2019, 23, 358–366 CrossRef.
- X. Hu, J. Jia, G. Wang, J. Chen, H. Zhan and Z. Wen, Reliable and General Route to Inverse Opal Structured Nanohybrids of Carbon-Confined Transition Metal Sulfides Quantum Dots for High-Performance Sodium Storage, Adv. Energy Mater., 2018, 8(25), 1801452 CrossRef.
- X. Hu, M. Qiu, Y. Liu, J. Yuan, J. Chen, H. Zhan and Z. Wen, Interface and Structure Engineering of Tin-Based Chalcogenide Anodes for Durable and Fast-Charging Sodium Ion Batteries, Adv. Energy Mater., 2022, 12(47), 2202318 CrossRef CAS.
- P. Ge, S. Li, L. Xu, K. Zou, X. Gao, X. Cao, G. Zou, H. Hou and X. Ji, Hierarchical Hollow-Microsphere Metal–Selenide@Carbon Composites with Rational Surface Engineering for Advanced Sodium Storage, Adv. Energy Mater., 2019, 9(1), 1803035 CrossRef.
- B. Liu, Y. Liu, X. Hu, G. Zhong, J. Li, J. Yuan and Z. Wen, N-Doped Carbon Modifying MoSSe Nanosheets on Hollow Cubic Carbon for High-Performance Anodes of Sodium-Based Dual-Ion Batteries, Adv. Funct. Mater., 2021, 31(31), 2101066 CrossRef CAS.
- J. Wu, S. Liu, Y. Rehman, T. Huang, J. Zhao, Q. Gu, J. Mao and Z. Guo, Phase Engineering of Nickel Sulfides to Boost Sodium- and Potassium-Ion Storage Performance, Adv. Funct. Mater., 2021, 31(27), 2010832 CrossRef CAS.
- H. Shan, J. Qin, J. Wang, H. M. K. Sari, L. Lei, W. Xiao, W. Li, C. Xie, H. Yang, Y. Luo, G. Zhang and X. Li, Doping-Induced Electronic/Ionic Engineering to Optimize the Redox Kinetics for Potassium Storage: A Case Study of Ni-Doped CoSe2, Adv. Sci., 2022, 9(18), 2200341 CrossRef CAS PubMed.
- B. Jia, Q. Yu, Y. Zhao, M. Qin, W. Wang, Z. Liu, C.-Y. Lao, Y. Liu, H. Wu, Z. Zhang and X. Qu, Bamboo-Like Hollow Tubes with MoS2/N-Doped-C Interfaces Boost Potassium-Ion Storage, Adv. Funct. Mater., 2018, 28(40), 1803409 CrossRef.
- J. Zhang, P. Cui, Y. Gu, D. Wu, S. Tao, B. Qian, W. Chu and L. Song, Encapsulating Carbon-Coated MoS2 Nanosheets within a Nitrogen-Doped Graphene Network for High-Performance Potassium-Ion Storage, Adv. Mater. Interfaces, 2019, 6(22), 1901066 CrossRef CAS.
- W. Zhao, S. Ci, X. Hu, J. Chen and Z. Wen, Highly dispersed ultrasmall NiS2 nanoparticles in porous carbon nanofiber anodes for sodium ion batteries, Nanoscale, 2019, 11(11), 4688–4695 RSC.
- S. Fan, S. Huang, Y. Chen, Y. Shang, Y. Wang, D. Kong, M. E. Pam, L. Shi, Y. W. Lim, Y. Shi and H. Y. Yang, Construction of complex NiS multi-shelled hollow structures with enhanced sodium storage, Energy Storage Mater., 2019, 23, 17–24 CrossRef.
- H. He, C. Chen, Z. Chen, P. Li, S. Ding, M. Cai and M. Zhang, Ni3S2@S-carbon nanotubes synthesized using NiS2 as sulfur source and precursor for high performance sodium-ion half/full cells, Sci. China Mater., 2020, 63(2), 216–228 CrossRef CAS.
- J. Li, J. Li, D. Yan, S. Hou, X. Xu, T. Lu, Y. Yao, W. Mai and L. Pan, Design of pomegranate-like clusters with NiS2 nanoparticles anchored on nitrogen-doped porous carbon for improved sodium ion storage performance, J. Mater. Chem. A, 2018, 6(15), 6595–6605 RSC.
- M. Park, N. Kim, J. Lee, M. Gu and B.-S. Kim, Versatile graphene oxide nanosheets via covalent functionalization and their applications, Mater. Chem. Front., 2021, 5(12), 4424–4444 RSC.
- E. Yoo, J. Kim, E. Hosono, H.-S. Zhou, T. Kudo and I. Honma, Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries, Nano Lett., 2008, 8(8), 2277–2282 CrossRef CAS PubMed.
- J. Zhu, Y. Li, S. Kang, X.-L. Wei and P. K. Shen, One-step synthesis of Ni3S2 nanoparticles wrapped with in situ generated nitrogen-self-doped graphene sheets with highly improved electrochemical properties in Li-ion batteries, J. Mater. Chem. A, 2014, 2(9), 3142–3147 RSC.
- X. Xu, R. Zhao, W. Ai, B. Chen, H. Du, L. Wu, H. Zhang, W. Huang and T. Yu, Controllable Design of MoS2 Nanosheets Anchored on Nitrogen-Doped Graphene: Toward Fast Sodium Storage by Tunable Pseudocapacitance, Adv. Mater., 2018, 30(27), 1800658 CrossRef PubMed.
- F. Wang, W. Zhang, H. Zhou, H. Chen, Z. Huang, Z. Yan, R. Jiang, C. Wang, Z. Tan and Y. Kuang, Preparation of porous FeS2-C/RG composite for sodium ion batteries, Chem. Eng. J., 2020, 380, 122549 CrossRef CAS.
- S. Jadhav, R. S. Kalubarme, C. Terashima, B. B. Kale, V. Godbole, A. Fujishima and S. W. Gosavi, Manganese dioxide/reduced graphene oxide composite an electrode material for high-performance solid state supercapacitor, Electrochim. Acta, 2019, 299, 34–44 CrossRef CAS.
- T. S. Sunil Kumar Naik, S. Saravanan, K. N. Sri Saravana, U. Pratiush and P. C. Ramamurthy, A non-enzymatic urea sensor based on the nickel sulfide/graphene oxide modified glassy carbon electrode, Mater. Chem. Phys., 2020, 245, 122798 CrossRef CAS.
- X. Mao, X. Gu, S. Wen, L. Zhang, P. Dai, L. Li, D. Liu, D. Li, Z. Li, K. Zhang and X. Zhao, Carbon-coated NiSe nanoparticles anchored on reduced graphene oxide: a high-rate and long-life anode for potassium-ion batteries, Sustainable Energy Fuels, 2021, 5(12), 3240–3246 RSC.
- X. Hu, Y. Li, G. Zeng, J. Jia, H. Zhan and Z. Wen, Three-Dimensional Network Architecture with Hybrid Nanocarbon Composites Supporting Few-Layer MoS2 for Lithium and Sodium Storage, ACS Nano, 2018, 12(2), 1592–1602 CrossRef CAS PubMed.
- J. Yuan, B. Yu, D. Pan, X. Hu, J. Chen, M. Aminua, Y. Liu, L. Sheng, Y. Chen, Y. Wu, H. Zhan and Z. Wen, Universal Source-Template Route to Metal Selenides Implanting on 3D Carbon Nanoarchitecture: Cu2−xSe@3D-CN with Se–C Bonding for Advanced Na Storage, Adv. Funct. Mater., 2023, 2305503 CrossRef CAS.
- J. Li, X. Hu, G. Zhong, Y. Liu, Y. Ji, J. Chen and Z. Wen, A General Self-Sacrifice Template Strategy to 3D Heteroatom-Doped Macroporous Carbon for High-Performance Potassium-Ion Hybrid Capacitors, Nano-Micro Lett., 2021, 13(1), 131 CrossRef CAS PubMed.
- N. B. Trung, T. V. Tam, D. K. Dang, K. F. Babu, E. J. Kim, J. Kim and W. M. Choi, Facile synthesis of three-dimensional graphene/nickel oxide nanoparticles composites for high performance supercapacitor electrodes, Chem. Eng. J., 2015, 264, 603–609 CrossRef CAS.
- W. Wei, S. Yang, H. Zhou, I. Lieberwirth, X. Feng and K. Müllen, 3D Graphene Foams Cross-linked with Pre-encapsulated Fe3O4 Nanospheres for Enhanced Lithium Storage, Adv. Mater., 2013, 25(21), 2909–2914 CrossRef CAS PubMed.
- D. R. Rolison, J. W. Long, J. C. Lytle, A. E. Fischer, C. P. Rhodes, T. M. McEvoy, M. E. Bourg and A. M. Lubers, Multifunctional 3D nanoarchitectures for energy storage and conversion, Chem. Soc. Rev., 2009, 38(1), 226–252 RSC.
- A. R. Thiruppathi, J. van der Zalm, C.-K. Hung and A. Chen, Synthesis and Electrochemical Studies of 3D Reduced Graphene Oxide for Efficient Energy Storage, ACS Appl. Energy Mater., 2023, 6(10), 5486–5497 CrossRef CAS.
- T. Li, B. Wang, H. Song, P. Mei, J. Hu, M. Zhang, G. Chen, D. Yan, D. Zhang and S. Huang, Deciphering the Performance Enhancement, Cell Failure Mechanism, and Amelioration Strategy of Sodium Storage in Metal Chalcogenides-Based Andes, Adv. Mater., 2024, 2314271 CrossRef CAS PubMed.
- S. H. Oh and J. S. Cho, Hierarchical (Ni,Co)Se2/CNT hybrid microspheres consisting of a porous yolk and embossed hollow thin shell for high-performance anodes in sodium-ion batteries, J. Alloys Compd., 2019, 806, 1029–1038 CrossRef CAS.
- J.-S. Park and Y. Chan Kang, Multicomponent (Mo, Ni) metal sulfide and selenide microspheres with empty nanovoids as anode materials for Na-ion batteries, J. Mater. Chem. A, 2017, 5(18), 8616–8623 RSC.
- C. Dong, J. Liang, Y. He, C. Li, X. Chen, L. Guo, F. Tian, Y. Qian and L. Xu, NiS1.03 Hollow Spheres and Cages as Superhigh Rate Capacity and Stable Anode Materials for Half/Full Sodium-Ion Batteries, ACS Nano, 2018, 12(8), 8277–8287 CrossRef CAS PubMed.
- W. Shuang, H. Huang, L. Kong, M. Zhong, A. Li, D. Wang, Y. Xu and X.-H. Bu, Nitrogen-doped carbon shell-confined Ni3S2 composite nanosheets derived from Ni-MOF for high performance sodium-ion battery anodes, Nano Energy, 2019, 62, 154–163 CrossRef CAS.
- J. Yuan, X. Hu, J. Li, Y. Liu, G. Zhong and T. Huang, V2O3 Nanoparticles Confined in High-Conductivity and High-Throughput Carbon Nanofiber Nanohybrids for Advanced Sodium-Ion Capacitors, ACS Appl. Mater. Interfaces, 2021, 13(8), 10001–10012 CrossRef CAS PubMed.
- J. Li, Z. Ding, J. Li, C. Wang, L. Pan and G. Wang, Synergistic coupling of NiS1.03 nanoparticle with S-doped reduced graphene oxide for enhanced lithium and sodium storage, Chem. Eng. J., 2021, 407, 127199 CrossRef CAS.
- W. Li, B. Huang, Z. Liu, J. Yang, Y. Li, S. Xiao, Q. Chen, G. Li, X. Zhao and W. Zhang, NiS2 wrapped into graphene with strong Ni-O interaction for advanced sodium and potassium ion batteries, Electrochim. Acta, 2021, 369, 137704 CrossRef CAS.
- Z. Zhang, Y. Huang, X. Liu, C. Chen, Z. Xu and P. Liu, Zeolitic imidazolate frameworks derived ZnS/Co3S4 composite nanoparticles doping on polyhedral carbon framework for efficient lithium/sodium storage anode materials, Carbon, 2020, 157, 244–254 CrossRef CAS.
- Z. Liu, L. Zhang, L. Sheng, Q. Zhou, T. Wei, J. Feng and Z. Fan, Edge-Nitrogen-Rich Carbon Dots Pillared Graphene Blocks with Ultrahigh Volumetric/Gravimetric Capacities and Ultralong Life for Sodium-Ion Storage, Adv. Energy Mater., 2018, 8(30), 1802042 CrossRef.
- E. Zhang, X. Hu, L. Meng, M. Qiu, J. Chen, Y. Liu, G. Liu, Z. Zhuang, X. Zheng, L. Zheng, Y. Wang, W. Tang, Z. Lu, J. Zhang, Z. Wen, D. Wang and Y. Li, Single-Atom Yttrium Engineering Janus Electrode for Rechargeable Na–S Batteries, J. Am. Chem. Soc., 2022, 144(41), 18995–19007 CrossRef CAS PubMed.
- Y. Liu, J. Li, B. Liu, Y. Chen, Y. Wu, X. Hu, G. Zhong, J. Yuan, J. Chen, H. Zhan and Z. Wen, Confined WS2 Nanosheets Tubular Nanohybrid as High-Kinetic and Durable Anode for Sodium-Based Dual Ion Batteries, ChemSusChem, 2022, e202201200 Search PubMed.
- Z. Song, G. Wang, Y. Chen, Q. Chang, Y. Lu and Z. Wen, Construction of hierarchical NiS@C/rGO heterostructures for enhanced sodium storage, Chem. Eng. J., 2022, 435, 134633 CrossRef CAS.
- J. Li, Z. Ding, J. Li, C. Wang, L. Pan and G. Wang, Synergistic coupling of NiS1.03 nanoparticle with S-doped reduced graphene oxide for enhanced lithium and sodium storage, Chem. Eng. J., 2021, 407, 127199 CrossRef CAS.
- Y. Liu, M. Qiu, X. Hu, J. Yuan, W. Liao, L. Sheng, Y. Chen, Y. Wu, H. Zhan and Z. Wen, Anion Defects Engineering of Ternary Nb-Based Chalcogenide Anodes Toward High-Performance Sodium-Based Dual-Ion Batteries, Nano-Micro Lett., 2023, 15(1), 104 CrossRef CAS PubMed.
- S. Zhang, R. Wang, R. Cao, F. Fang and R. Wu, NiS1−xSex Nanoparticles Anchored on Nitrogen-Doped Reduced Graphene Oxide as Highly Stable Anode for Sodium-Ion Battery, Processes, 2022, 10, 566 CrossRef CAS.
- Y. Zhang, H. Tao, S. Du and X. Yang, Conversion of MoS2 to a Ternary MoS2–xSex Alloy for High-Performance Sodium-Ion Batteries, ACS Appl. Mater. Interfaces, 2019, 11(12), 11327–11337 CrossRef CAS PubMed.
- Y. Liu, X. Hu, J. Li, G. Zhong, J. Yuan, H. Zhan, Y. Tang and Z. Wen, Carbon-coated MoS1.5Te0.5 nanocables for efficient sodium-ion storage in non-aqueous dual-ion batteries, Nat. Commun., 2022, 13(1), 663 CrossRef CAS PubMed.
- N. Shi, B. Xi, M. Huang, X. Ma, H. Li, J. Feng and S. Xiong, Hierarchical Octahedra Constructed by Cu2S/MoS2 ⊂ Carbon Framework with Enhanced Sodium Storage, Small, 2020, 16(23), 2000952 CrossRef CAS PubMed.
- G. Wang, X. Bi, H. Yue, R. Jin, Q. Wang, S. Gao and J. Lu, Sacrificial template synthesis of hollow C@MoS2@PPy nanocomposites as anodes for enhanced sodium storage performance, Nano Energy, 2019, 60, 362–370 CrossRef CAS.
- X. Dong, F. Chen, G. Chen, B. Wang, X. Tian, X. Yan, Y.-X. Yin, C. Deng, D. Wang, J. Mao, S. Xu and S. Zhang, NiS2 nanodots on N,S-doped graphene synthesized via interlayer confinement for enhanced lithium-/sodium-ion storage, J. Colloid Interface Sci., 2022, 619, 359–368 CrossRef CAS PubMed.
- H. Zheng, X. Chen, L. Li, C. Feng and S. Wang, Synthesis of NiS2/reduced graphene oxide nanocomposites as anodes materials for high-performance Sodium and Potassium ion batteries, Mater. Res. Bull., 2021, 142, 111430 CrossRef CAS.
- J. Yan, K. Sang, X. Jiang, Q. Li, F. Jiang and Y. Zhou, Amorphous MoS3-modified porous Co4S3-embedded N,S co-doped carbon polyhedron as new high-capacity and high-rate anode materials for sodium-ion half/full cells, J. Colloid Interface Sci., 2024, 655, 100–109 CrossRef CAS PubMed.
- Y. Jiang, F. Wu, Z. Ye, Y. Zhou, Y. Chen, Y. Zhang, Z. Lv, L. Li, M. Xie and R. Chen, Superimposed effect of hollow carbon polyhedron and interconnected graphene network to achieve CoTe2 anode for fast and ultralong sodium storage, J. Power Sources, 2023, 554, 232174 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nh00653d |
| This journal is © The Royal Society of Chemistry 2025 |