Endothelial-targeting miR-145 micelles restore barrier function and exhibit atheroprotective effects†
Anisa
Ashraf
a,
Yi
Huang
a,
Auveen
Choroomi
a,
Kyla
Johnson
a,
Jocelynn
Torres
a and
Eun Ji
Chung
 *abcdef
*abcdef
aDepartment of Biomedical Engineering, University of Southern California, Los Angeles, CA 90089, USA. E-mail: eunchung@usc.edu; Fax: +1-213-821-3897; Tel: +1-213-740-2825
bDivision of Vascular Surgery and Endovascular Therapy, Department of Surgery, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA
cMork Family Department of Chemical Engineering and Materials Science, University of Southern California, Los Angeles, CA 90089, USA
dEli and Edythe Broad Center for Regenerative Medicine and Stem Cell Research, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA
eDivision of Nephrology and Hypertension, Department of Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033, USA
fNorris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, CA 90089, USA
First published on 21st March 2025
Abstract
Atherosclerosis remains the leading cause of death worldwide and is characterized by the accumulation of plaque beneath the endothelium. MicroRNA-145-5p (miR-145), which is downregulated in atherosclerosis, has been shown to mitigate plaque development by promoting the contractile vascular smooth muscle cell (VSMC) phenotype. Previously, our lab found that miR-145 micelles conjugated with MCP-1 peptides were able to inhibit atherosclerosis by targeting diseased VSMC via C–C chemokine receptor 2 (CCR2). Diseased endothelial cells similarly express CCR2; however, the impact of miR-145 micelles on endothelial cell function has not been explored. Thus, in this study, the in vitro therapeutic effects of miR-145 micelles in modulating the endothelium during atherosclerosis are evaluated. To that end, the MCP-1 peptide density on the micelle surface was first optimized for activated endothelial cell binding, followed by loading miR-145 into micelles with the optimal MCP-1 ratio. Following characterization, miR-145 micelle treatment of activated endothelial cells resulted in efficient miR-145 transfection, upregulation of atheroprotective genes, and suppression of atherogenic genes. Furthermore, the treatment enhanced the integrity of endothelial tight junctions and reduced monocyte migration. This work establishes miR-145 micelles as an effective nanotherapeutic for restoring endothelial cell health in cardiovascular disease for the first time.
New conceptsThis study introduces a novel mechanism for atherosclerosis therapy by using miR-145 micelles conjugated with MCP-1 peptides to selectively target diseased endothelial cells via C–C chemokine receptor 2 (CCR2). While miR-145 has been extensively explored for its role in vascular smooth muscle cell (VSMC) modulation, its direct therapeutic application in restoring endothelial cell health has remained unexplored. By optimizing MCP-1 peptide density on the micelle surface, we achieved efficient miR-145 delivery to diseased endothelial cells, resulting in the upregulation of atheroprotective genes, suppression of atherogenic pathways, enhanced tight junction integrity, and reduced monocyte migration. By addressing a critical gap in atherosclerosis treatment, our findings not only enhance the understanding of endothelial dysfunction in cardiovascular disease but also establish a framework for the design of nanotherapeutics that integrate precision targeting with genetic modulation, pushing the boundaries of nanomedicine for vascular health. |
1. Introduction
Cardiovascular disease (CVD) remains the leading cause of death worldwide, accounting for nearly one-third of global mortality.1,2 In 2023, over 680![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 deaths in the U.S. were attributed to CVD.3 Atherosclerosis is a chronic, inflammatory disease and the most common underlying pathology of CVD. The disease is defined by vulnerable plaque buildup on arterial walls, which directly contributes to acute cardiac events such as heart attack and stroke.1 Unfortunately, management of atherosclerosis is limited; in severe cases, patients undergo invasive procedures, such as vessel wall supporting stents or the creation of new vessel pathways to bypass plaque-laden regions.4,5 However, despite these interventions, the limitations of current treatment options highlight the need for alternative therapeutic approaches. Statins, the primary pharmacological agents prescribed in atherosclerosis to lower circulating lipids, provide only a 20% reduction in the risk of acute cardiac events over a 5-year period.6,7 Notably, approximately 80% of the 35 million U.S. patients on statins experience a secondary event.6 These limitations highlight the critical need for innovative therapeutic targets and strategies to treat atherosclerosis effectively.
000 deaths in the U.S. were attributed to CVD.3 Atherosclerosis is a chronic, inflammatory disease and the most common underlying pathology of CVD. The disease is defined by vulnerable plaque buildup on arterial walls, which directly contributes to acute cardiac events such as heart attack and stroke.1 Unfortunately, management of atherosclerosis is limited; in severe cases, patients undergo invasive procedures, such as vessel wall supporting stents or the creation of new vessel pathways to bypass plaque-laden regions.4,5 However, despite these interventions, the limitations of current treatment options highlight the need for alternative therapeutic approaches. Statins, the primary pharmacological agents prescribed in atherosclerosis to lower circulating lipids, provide only a 20% reduction in the risk of acute cardiac events over a 5-year period.6,7 Notably, approximately 80% of the 35 million U.S. patients on statins experience a secondary event.6 These limitations highlight the critical need for innovative therapeutic targets and strategies to treat atherosclerosis effectively.
In patients with atherosclerosis, plaque resident cells express significantly lower levels of microRNA-145-5p (miR-145); and patients with CVD also have reduced circulating miR-145 levels compared to healthy individuals.8 miR-145 acts by binding the 3′ untranslated region of mRNA and subjecting it to degradation, inhibiting translation, or translocating to the nucleus and acting as a transcription factor.9 In vascular smooth muscle cells (VSMC), miR-145 lentiviral upregulation was found to increase expression of atheroprotective marker myocardin, reduce expression of atherogenic marker Kruppel-like factor 4 (KLF4), and reduce plaque growth in murine ApoE−/− models of atherosclerosis.10 Previously, therapeutic efficacy of miR-145 for atherosclerosis was demonstrated when delivered via a targeted micelle nanoparticle system.11–20 In this approach, miR-145 was covalently conjugated with DSPE-PEG(2000) to form miR amphiphiles, which were then combined with MCP-1 peptide-containing amphiphiles targeting the CCR2 receptor in VSMC. In an advanced atherosclerotic mouse model, miR-145 micelles reduced plaque size by 43% compared to controls.
While the role of miR-145 in vascular smooth muscle cells (VSMCs) has been well-documented, its potential impact on endothelial cell dysfunction, a key driver of atherosclerosis, remains underexplored. In atherosclerosis, endothelial cells are activated by lipids and the upregulation of inflammatory cues in response to lipids in circulation.21–24 Once activated, atherogenic signaling pathways are initiated for phenotypic switching into a diseased state.25,26 These involve transcription factor NF-κB, and transforming growth factor beta (TGF-β), which suppress tight junction proteins and induce the expression of cellular adhesion molecules, which are implicated in atherosclerosis.27,28 Specifically, endothelial cells (EC) become permeable to circulating lipids, and recruit monocytes into the vessel wall by expression of adhesion molecules, exasperating disease.29 Additionally, as vascular EC also express CCR2,30 targeting EC CCR2 represents a novel therapeutic mechanism for atherosclerosis. Given the high expression of CCR2 in EC within the vascular wall and its upregulation with the progression of atherosclerosis, the therapeutic effects of miR-145 micelles may also extend to vascular EC.30,31 Therefore, it is crucial to investigate how miR-145 micelles influence dysfunctional EC to better understand the extent of their therapeutic potential.
To test our hypothesis, micelles were first synthesized with varying ratios of MCP-1 amphiphiles to determine the formulation with the highest EC targeting capability. Then, miR-145 was loaded into micelles with the optimized MCP-1 ratio. miR-145 micelles were characterized via DLS and gel shift assay to confirm the successful loading of miR-145. Next, TNF-α activated EC were treated with miR-145 micelles to examine their effects on key atheroprotective genes, such as KLF2, and atherogenic genes, such as ACTA2. Finally, the impact of miR-145 micelles on endothelial barrier function and monocyte migration was assessed using a 3D transwell model.
2. Materials & methods
2.1 Peptide and microRNA amphiphile synthesis
Thiolated miR-145-5p mimic (5′-GUCCAGUUUUCCCAGGAAUCCCU-3′, IDT Technologies, Coralville, IA, USA) and MCP-1 peptide (CYNFTNRKISVQRLASYRRITSSK, Biomatik, Canada) were conjugated to DSPE-PEG(2000)-Maleimide (Avanti Lipids, Alabaster, AL, USA), as previously described.11,32–34 Briefly, MCP-1 peptide was incubated in a 10% molar excess of lipid in water at pH 6.5 for 72 hours and purified by reverse-phase high performance liquid chromatography (HPLC) (Shimadzu, Kyoto, Japan) on a C4 column (Phenomenex, Torrance, CA, USA) at 55 °C with 0.1% formic acid in acetonitrile/water mixture. Similarly, miR-145 amphiphile was conjugated to lipid overnight at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of lipid. Successful conjugation of both miR-145 and MCP-1 amphiphiles were verified by matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry (Autoflex speed, Bruker, Billerica, MA, USA) (ESI,† Fig. S1).
1 molar ratio of lipid. Successful conjugation of both miR-145 and MCP-1 amphiphiles were verified by matrix-assisted laser desorption ionization time of flight (MALDI-TOF) mass spectrometry (Autoflex speed, Bruker, Billerica, MA, USA) (ESI,† Fig. S1).
2.2 Micelle synthesis and characterization
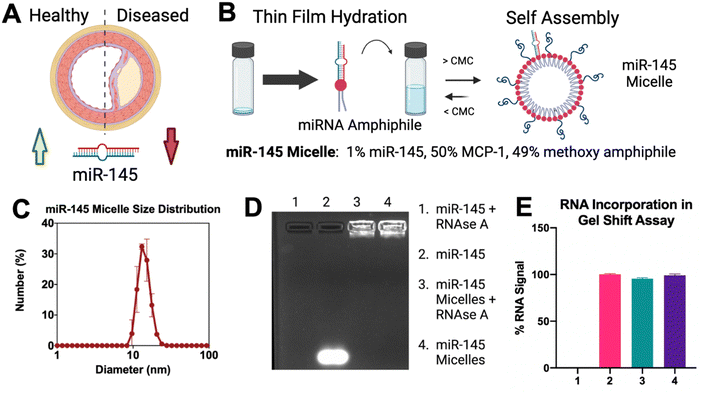
miR-145 micelles were synthesized via self-assembly, as previously described.11 Briefly, micelles consisting of varying molar ratios of MCP-1 amphiphiles (0–75%) were synthesized. All monomers were dissolved in methanol and evaporated with nitrogen to form thin films, and the thin films were vacuum dried overnight at RT. miR-145 amphiphiles were added at a 1% molar ratio upon aqueous rehydration in water or phosphate buffered saline (PBS) and micelles were heated at 60 °C for 30 minutes.2.3 Dynamic light scattering (DLS) and zeta potential measurements
Micelles were synthesized and assessed via DLS and zeta potential measurements. Micelle diameter and zeta potential was measured using Zetasizer Ultra (Malvern Instruments, Malvern, UK) at a micelle concentration of 100 μM in Milli-Q water, PBS or 2% Fetal Bovine Serum (FBS) minimal cell culture media, Opti-MEM, without phenol red (n = 3) immediately after micelles were hydrated from thin films unless otherwise stated (Thermo Fisher Scientific, Waltham, MA, USA).2.4 Transmission electron microscopy (TEM)
Seven microliters of 100 μM micelle solution in Milli-Q water was placed onto a 200-mesh carbon TEM grid (Ted Pella, Redding, CA, USA) for 5 min. The grid was then rinsed with Milli-Q water and stained with a 2% (w/v) uranyl acetate solution (Polysciences, Warrington, PA, USA) for 2 minutes, followed by another rinse with Milli-Q water. After overnight drying at room temperature in the dark, the grid was imaged using a Talos TEM (Thermo Fisher Scientific, Waltham, MA, USA).2.5 Cell culture
Primary human aortic endothelial cells (HAEC) were purchased from ATCC (Manassas, VA, USA) and cultured in human large vessel endothelial cell basal media supplemented with vascular endothelial cell growth factor kit (Thermo Fisher Scientific, Waltham, MA, USA). To activate cells in vitro, HAEC were stimulated with 100 ng/mL of TNF-α (Peprotech) for 24 hours after reaching confluence (ESI,† Fig. S2). Activation was additionally confirmed by RT-qPCR assessing CCR2 and immunocytochemistry assessing VCAM-1. All cells were used under passage 10.2.6 Cell viability
HAEC were seeded at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in a 96-well plate overnight. Cells were then activated with TNF- α for 24 hours. Next, cells were incubated with micelles at 25 or 50 μM for 4 hours, then washed twice with PBS and incubated with fresh media for 20 hours. Cell viability was measured using an MTS assay (((3-(4,5-dimethylthiazol-2-yl))-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, BioVision, Milpitas, CA, USA), following the manufacturer's instructions and measured at an absorbance of 490 nm on a Varioskan LUX plate reader (Thermo Fisher Scientific, Waltham, MA, USA). Cell viability was determined as a percentage compared to PBS-treated controls.
000 cells per well in a 96-well plate overnight. Cells were then activated with TNF- α for 24 hours. Next, cells were incubated with micelles at 25 or 50 μM for 4 hours, then washed twice with PBS and incubated with fresh media for 20 hours. Cell viability was measured using an MTS assay (((3-(4,5-dimethylthiazol-2-yl))-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, BioVision, Milpitas, CA, USA), following the manufacturer's instructions and measured at an absorbance of 490 nm on a Varioskan LUX plate reader (Thermo Fisher Scientific, Waltham, MA, USA). Cell viability was determined as a percentage compared to PBS-treated controls.
2.7 Micelle binding
25 μM rhodamine B labeled micelles containing different molar ratios of MCP-1 were incubated with activated HAEC for 4 hours. After incubation, cells were washed twice with PBS and fixed with 4% (w/v) paraformaldehyde (PFA) for 30 minutes prior to counterstaining with 4′,6-diamidino-2-phenylindole (DAPI). They were then immediately evaluated by fluorescence microscopy at 40× (Leica DMi8 Wetzlar, Germany). Images were quantified for mean fluorescence intensity (MFI) and cell count by ImageJ.2.8 Immunocytochemistry
Cells were washed twice and fixed with 4% (w/v) PFA for 30 minutes at room temperature. Next, they were blocked with 22 mg/mL glycine, 0.1% (v/v) Tween 20, and 1% BSA (v/v) or the 10% (v/v) serum from the host of the secondary antibody. Primary antibodies for CCR2 (Thermo Fisher Scientific, Waltham, MA, USA, 1:100), VCAM-1 (Thermo Fisher Scientific, Waltham, MA, USA, 1:100), ZO-1-AF488 (Thermo Fisher Scientific, Waltham, MA, USA, 1:200) were used and incubated overnight at 4 °C. Secondary antibodies-Goat anti-Rabbit IgG Alexa Fluor™ 594 were used at a 1:500 dilution for 1 hour (Thermo Fisher Scientific, Waltham, MA, USA). Nuclei were counterstained with DAPI (1 μg mL−1) for 5 minutes. Cells were then imaged with fluorescence microscope Leica DMi8 (Wetzlar, Germany) at 10×, or 40× or confocal Nikon AX system at 63× (Tokyo, Japan).For live cell imaging, cells were incubated with Opti-MEM with 10 μg/mL Hoescht 33342 for 10 minutes before Lysotracker green was added at 30 nM and Rhodamine B labelled micelles were added at 25 μM (Thermo Fisher Scientific, Waltham, MA, USA). Micelle uptake was visualized at 37 °C with fluorescence microscope Eclipse Ti Confocal Module C2 over 4 hours (Nikon Instruments, Melville, NY, USA).
2.9 Gene expression via RT-qPCR
After activation, endothelial cells were treated with PBS, miR-145 micelles or MCP-1 micelles (50 μM). RNA was isolated with the miRneasy micro kit (Qiagen, Hilden, DE, USA). cDNA was synthesized with the miRcury LNA RT kit (microRNA) or RT2 First Strand kit (messenger RNA) followed by SYBR Green qPCR, according to manufacturing protocols (Qiagen, Hilden, DE, USA). miR-145 expression was normalized to U6, messenger RNA expression was normalized to GAPDH and expression fold change was calculated using the 2−ΔΔCT method.2.10 Assessing transendothelial integrity
To evaluate transendothelial integrity, HAEC were cultured to confluence in a transwell system. Transendothelial electrical resistance (TEER) was measured using an EndOhm chamber (World Precision Measurements, Sarasota, FL, USA) to confirm monolayer integrity, with confluence indicated by TEER values of 30 Ω cm2. Subsequently, HAEC were stimulated with TNF-α (100 ng/mL) to induce activation, as evidenced by a decrease in TEER. Micelles (25 μM) were applied to the apical compartment for 4 hours, after which the cells were washed twice with PBS and replenished with fresh media. TEER was reassessed 20 hours post-treatment to determine the effects of micelle treatment on endothelial barrier function.2.11 Monocyte migration
24 hours after micelle treatment, HAEC transwells were incubated with 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 THP-1 monocytes (ATCC, Manassas, VA, USA) in the apical compartment in recommended culture conditions of 10% Fetal Bovine Serum (FBS) in Roswell Park Memorial Institute Medium (RPMI) (Thermo Fisher Scientific, Waltham, MA, USA). An additional 10% FBS was added to the basal compartment of each transwell as a chemoattractant for a total of 20% in the basal chamber. After 4 hours, media was collected from the apical and basal compartments, and DNA was quantified by Quant-iT dsDNA PicoGreen reagent according to manufacturer's protocols (Thermo Fisher Scientific, Waltham, MA, USA). Endothelial and media DNA was subtracted from the experimental values from the endothelial cell only transwell measurement. Monocyte migration is represented as the
000 THP-1 monocytes (ATCC, Manassas, VA, USA) in the apical compartment in recommended culture conditions of 10% Fetal Bovine Serum (FBS) in Roswell Park Memorial Institute Medium (RPMI) (Thermo Fisher Scientific, Waltham, MA, USA). An additional 10% FBS was added to the basal compartment of each transwell as a chemoattractant for a total of 20% in the basal chamber. After 4 hours, media was collected from the apical and basal compartments, and DNA was quantified by Quant-iT dsDNA PicoGreen reagent according to manufacturer's protocols (Thermo Fisher Scientific, Waltham, MA, USA). Endothelial and media DNA was subtracted from the experimental values from the endothelial cell only transwell measurement. Monocyte migration is represented as the  .
.
2.12 Statistical analysis
Results are expressed as means ± standard deviation (SD). A two-tailed Student t-test was used to determine statistical significance between two groups, while a one-way analysis of variance (ANOVA) was used to determine statistical significance between more than two groups. A p-value of <0.05 was considered statistically significant and is indicated by a single asterisk (*). Additional p values are indicated as follows: ** for p < 0.01, *** for p < 0.001, ****p < 0.0001. All statistical analyses were conducted using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).3. Results
3.1 Optimizing MCP-1 micelles for activated endothelial cell targeting
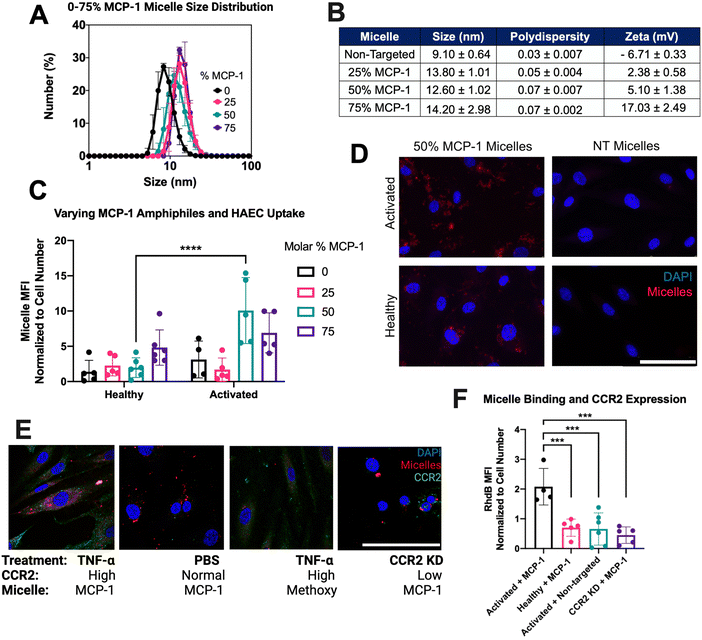
To induce endothelial dysfunction in vitro as found in atherosclerosis, primary human aortic endothelial cells (HAEC) were treated with inflammatory cytokine TNF-α overnight. After treatment, cells were assessed via immunocytochemistry (ICC) and RT-qPCR for CCR2 expression. Activated endothelial cells showed an upregulation of CCR2 as compared to healthy controls (150.4 ± 24.38 vs. 7.44 ± 1.27 by ICC, p < 0.01 and 6.77 ± 0.96 vs. 1.03 ± 0.34 by RT-qPCR p < 0.01, Fig. 1(A)–(C)).To actively target CCR2, the monocyte chemoattractant protein 1 (MCP-1) peptide was utilized as a targeting ligand. MCP-1 is a 76 amino acid long chemokine protein, responsible for recruiting key immune cells.35 MCP-1 has a strong affinity for CCR2 (Kd = 0.77).36 The binding domain of MCP-1 is highlighted in Fig. 1(D) as MCP-1 peptide, a 22 amino acid chain. Therefore, MCP-1 micelles were synthesized as previously reported (Fig. 1(E)).11 After synthesis, the morphology of micelles was confirmed via TEM, which revealed a monodisperse nanoparticle population (Fig. 1(F)).
To determine an optimal targeting ligand density for miR-145 micelles to bind endothelial cells, micelles with MCP-1 amphiphile molar ratio ranging from 0–75% were synthesized. The hydrodynamic diameters of the micelles ranged from 9.10 ± 0.64 to 14.20 ± 2.98 nm (Fig. 2(A) and (B)), and polydispersity remained low for each formulation (PDI < 0.07 ± 0.007), confirming the monodispersity of all micelles. In addition, since the MCP-1 peptide has an overall net positive charge (+2), the surface charge of micelles increased from ∼+2 mV to ∼+17 mV as the MCP-1 amphiphile concentration increased from 25% to 75%, (Fig. 2(B)).35 These micelles were found to be stable in media conditions with non-significant changes to size, PDI, and charge (ESI,† Fig. S5). After 4 hours of incubation, fluorescence imaging showed that only the 50% MCP-1 micelle formulation displayed a significant increase in binding to the activated endothelial cells (1.98 ± 1.40 vs. 9.30 ± 4.33, p < 0.001, Fig. 2(C) and (D)). Further, MCP-1 micelle internalization was monitored over time in activated HAEC and show a significant increase in binding compared to non-targeting micelles and by 4 hours, MCP-1 micelles were able to escape the endosome (ESI,† Fig. S6). Thus, this formulation was found to selectively target activated endothelial cells and chosen for further studies.
To confirm the association of micelle uptake with CCR2 expression, primary HAEC were stained for CCR2 and imaged on a confocal fluorescence microscope. As shown in Fig. 1(E), activated endothelial cells showed the highest uptake of MCP-1 micelles (2.07 ± 0.61) after incubation with 25 μM of 50% MCP-1 micelles for 4 hours, compared to CCR2-knockdown HAEC cells treated with MCP-1 micelles (0.45 ± 0.28, p < 0.001) and healthy controls treated with MCP-1 micelles (0.70 ± 0.29, p < 0.001) (Fig. 2(E) and (F)). Hence, 50% molar MCP-1 micelles were found to interact with the increased expression of CCR2 and demonstrate increased uptake by activated endothelial cells compared to healthy controls.
3.2 Micellar delivery of therapeutic miR-145 to endothelial cells
With the optimization of a CCR2 targeting micelle, therapeutic miR-145 was then incorporated for delivery to endothelial cells (Fig. 3(A)). To develop miR-145 micelles, microRNA-145-5p duplexes were functionalized with a thiol group on the 5′ end of the coding strand and conjugated to DSPE-PEG(2000)-maleimide, as previously described.11 With the addition of miR-145, the hydrodynamic diameter of the micelles remained similar to MCP-1 micelles (14.40 ± 2.07 nm vs. 12.60 ± 1.02 nm), with low polydispersity (PDI < 0.03 ± 0.002) and a near neutral surface charge of 0.52 ± 0.19 mV (Fig. 3(C) and ESI,† Fig. S4), which is consistent with previous studies.11 Next, miR-145 incorporation and protection from circulating RNAse were confirmed via a gel shift assay (Fig. 3(D)). When treated with RNAse A, free miR-145 was degraded, while in micellular formulation, 95.57 ± 0.19% of loaded miR-145 was protected (Fig. 3(E)). Thus, our micelle platform can successfully incorporate and protect therapeutic microRNA.3.3 miR-145 micelles iune the endothelial gene expression for atheroprotection
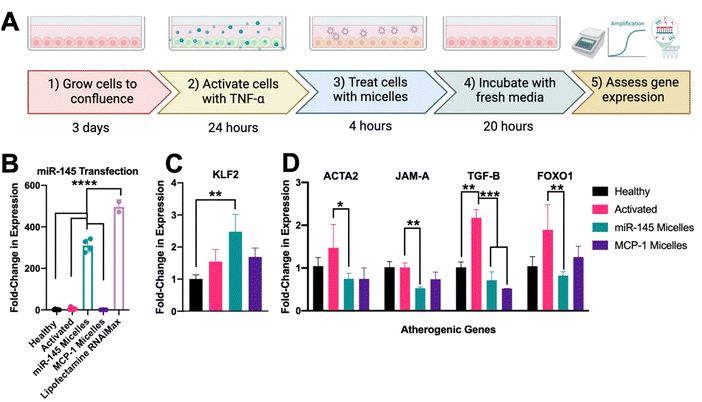
With the incorporation of miR-145 into micelles, micelles maintained targeting and uptake in activated HAEC over 4 hours and they were able to successfully escape the endosome to deliver therapeutic cargo (ESI,† Fig. S6). To assess the efficacy on altering endothelial miR-145 expression, activated and healthy endothelial cells were treated with 50 μM micelles (500 nM dose of miR-145, Fig. 4(A)). As shown in Fig. 4(B), after incubation with miR-145 micelles for 4 hours, endothelial miR-145 expression was increased by 308.90 ± 10.32-fold, as compared to a healthy no-treatment control (1.60 ± 0.16, p < 0.001). In addition, miR-145 micelles showed a comparable ability in promoting miR-145 expression as compared to lipofectamine, the standard method for in vitro transfection, which increased miR-145 expression by 496.43 ± 34.31-fold with an equivalent miR dose (p < 0.001, Fig. 4(D)).To investigate the impact of miR-145 micelles on both atheroprotective and atherogenic genes, the levels of KLF2, ACTA2, JAM-A, TGF-B, and FOXO1 were quantified. As shown in Fig. 4(C), miR-145 micelle treatment increased KLF2 expression by 2.48 ± 0.54-fold compared to activated endothelial controls (1.55 ± 0.38, p < 0.01) and MCP-1 micelle treated controls (1.69 ± 0.28, ns). These findings indicate that miR-145 micelle treatment effectively upregulates the expression of the atheroprotective transcription factor KLF2 in endothelial cells.
While the atheroprotective KLF2 was significantly enhanced by miR-145 micelle treatment, its effects on other critical atherogenic markers were also assessed. ACTA2, for instance, is known to protect vessels through restoration of contractility; however, upregulation of ACTA2 can also cause quiescent endothelial cells to become migratory. As shown in Fig. 4(D), miR-145 micelle treatment significantly reduced expression of ACTA2 to 0.75 ± 0.18-fold compared to activated controls (1.47 ± 0.77, p < 0.05). Similarly, JAM-A, an immunoglobulin adhesion surface protein that promotes monocyte recruitment, inflammation, plaque formation, thrombosis, and plaque rupture, was also significantly downregulated by miR-145 micelles.37–39 As shown in Fig. 4(D), JAM-A expression was reduced to 0.53 ± 0.04 -fold upon miR-145 incubation as compared to activated controls (1.02 ± 0.18, p < 0.01). As shown in Fig. 4(B), activation of endothelial cells resulted in elevated TGF-β expression (2.17 ± 0.33-fold), which was reduced to near-healthy levels (1.02 ± 0.22) following miR-145 micelle treatment (0.72 ± 0.28, p < 0.001). miR-145 micelle treatment reduced FOXO1 expression to 0.83 ± 0.13-fold compared to activated endothelial cells (1.90 ± 0.83, p < 0.01), healthy controls (1.04 ± 0.39, ns), and MCP-1 micelle-treated cells (1.25 ± 0.35, ns, Fig. 4(D)). Overall, these results demonstrate that miR-145 micelle treatment promotes an atheroprotective phenotype and reduces the expression of key markers linked to atherosclerosis progression.
3.4 Rescue of endothelial barrier function by miR-145 micelles
To evaluate the effects of miR-145 micelles on endothelial barrier function, barrier integrity was assessed by transendothelial resistance (TEER) quantification and tight junction protein expression (Fig. 5(A)). After incubation with miR-145 for 4 hours, miR-145 micelle treatment showed a significant improvement in barrier recovery as compared to activated endothelial cells (18.85 ± 6.15% vs. 8.22 ± 6.01%, p < 0.01), confirming that miR-145 micelles promote barrier recovery after cytokine activation (Fig. 5(B)).To further investigate how miR-145 micelles restore endothelial barrier function, the expression of the tight junction protein Zonula Occludens 1 (ZO-1) was analyzed following treatment. Specifically, miR-145 micelle treated cells showed a similar MFI of 185.70 ± 50.84 compared to healthy controls (201.2 ± 71.17, ns), while MCP-1 micelle treatment and activated cells only showed: 82.46 ± 16.03 and 37.76 ± 20.21, respectively, (p < 0.0001 Fig. 5(C) and (D)), confirming that miR-145 micelles were able to rescue ZO-1 expression to that of healthy levels.
3.5 Adhesion molecule and monocyte migration reduction by miR-145 micelles
To evaluate the role of miR-145 micelles in reducing endothelial control of pro-inflammatory cell recruitment, monocyte migration through an in vitro model of the vessel wall and endothelial cell adhesion molecule expression was evaluated. After miR-145 micelle treatment for 4 hours, monocyte migration was significantly lower as compared to activated endothelial cells (25.68 ± 3.97% vs. 45.94 ± 7.22% p < 0.001) and was reduced to similar levels of healthy controls (25.68 ± 3.97% vs. 26.28 ± 4.26%, ns, Fig. 6(A) and (B)). This confirms that miR-145 micelle treatment restores the endothelial barrier and prevents the recruitment of transendothelial monocytes.To understand how endothelial cells may be controlling monocyte recruitment after miR-145 micelle treatment, VCAM-1 expression was evaluated. VCAM-1 is integral in recruiting leukocytes from circulation, allowing them to roll on and transmigrate through the endothelium out of circulation. After treatment by miR-145 micelles for 4 hours, the VCAM-1 expression of activated cells was reduced to similar levels as healthy controls (3.47 ± 0.26 vs. 3.53 ± 0.83, ns, Fig. 6(C) and (D)), confirming miR-145 micelle treatment reduced atherogenic VCAM-1 expression.
4. Discussion and conclusion
CVD remains the leading cause of death in the United States for over 100 years, despite multi-billion investments in the development of novel therapeutics.5 Currently, pharmacological intervention is limited to controlling systemic lipid levels. One area of intervention which could have substantial impact is the use of miRNA to control and revert activated cell fate. These findings align with our previous work on miR-145 micelles in atheroprotection, reinforcing their therapeutic potential in modulating VSMC phenotype to reduce plaque formation. We previously demonstrated that miR-145 micelles restore VSMC contractile markers (ACTA2, Calponin, Myocardin), downregulate atherogenic markers (KLF-4, KLF-5, ELK-1), and reduce plaque formation in vivo.11,40 While previous studies have explored miR-145 as a therapeutic for modulating vascular smooth muscle cell (VSMC) fate, no prior work has investigated its potential to directly reprogram endothelial cells (ECs) through targeted delivery.11 Endothelial activation is a hallmark of atherosclerosis, yet there remains a critical gap in therapies targeting endothelial dysfunction.This study is the first to demonstrate the therapeutic potential of nanoparticle-mediated miR-145 delivery for modulating EC fate, offering a novel strategy for mitigating endothelial dysfunction in atherosclerosis. We found that that MCP-1 micelles can selectively target the activated endothelium while exhibiting minimal targeting to the basal CCR2 expression in healthy controls. First, we established a model of an activated endothelium, which showed increased expression of CCR2, consistent with enhanced CCR2 levels in atherosclerosis patients.30,41 In activated EC, miR-145 micelles promote the expression of atheroprotective genes while suppressing atherogenic genes, which positions miR-145 micelles as a promising candidate for treating dysfunctional endothelial cells.
Specifically, overexpression of transcription factor KLF2 in vascular endothelial cells leads to a decrease in atherosclerotic plaque formation through the control of target genes involved with NF-kB inflammatory pathways.42,43 Here, we showed downregulation of KLF2 by miR-145 micelle treatment. In addition, endothelial TGF-β, a key driver of vascular inflammation and atherosclerosis progression, was markedly suppressed by miR-145 micelle treatment.27 Lastly, miR-145 micelles effectively downregulated FOXO1, a transcription factor associated with the upregulation of cellular adhesion molecules and atherosclerosis progression.44,45 Furthermore, miR-145 micelles increase endothelial tight junction protein expression, reduce cellular adhesion molecule expression, and decrease monocyte migration through the activated endothelium, which could mitigate the progression of atherosclerosis.
While this work is focused on in vitro tuning of endothelial cell fate, changes to EC gene expression has been evaluated in in vivo models. Specifically, EC upregulation of KLF2 was found to increase miR-145 delivery to VSMC through extracellular vesicles to improve VSMC contractility.42 Further, upregulation of miR-145, through direct myocardial injection, downregulated endothelial activation, cell proliferation, and cell migration, hallmarks of atherosclerosis. The authors concluded this was through the downregulation of FOXO1, which we have shown in vitro through miR-145 micelle delivery.46 Building on this work and previous evidence that miR-145 micelles protect and deliver miR-145 to atherosclerotic lesions, we propose that miR-145 micelles can modulate vascular EC fate in vivo, a focus of future studies.
Notably, these micelles have been evaluated for safety through measures of liver and kidney health, weight, anti-PEG-IgG and IgM, and through histological staining of organs, wherein no significant differences were found between treated and untreated groups.11,47,48 Additionally, components of these micelles: RNA, phospholipids of PEG and DSPE are currently FDA-approved in formulations such as Onpattro and the COVID-19 vaccine.49 Thus, the safety of miR-145 micelles and like therapeutics has been well-established.
While our findings are encouraging, several aspects of this study would still need further optimization and exploration. First, endothelial cell gene expression (KLF2, ACTA2, JAM-A, TGF-B, FOXO1) and fate after miR-145 micelle delivery should be validated in an in vivo model of atherosclerosis, where the dynamic interactions among vessel resident cells and circulating cues can be fully captured. Additionally, the in vitro model could be enhanced by incorporating shear flow across the endothelium, as flow plays a crucial role in both atherogenic and atheroprotective signal transduction.21,50 Finally, the impact of miR-145 micelle treatment on monocyte phenotypic switching within the context of atherosclerosis remains unknown. Despite these limitations, we establish new insight into the atheroprotective effects of miR-145 micelles in controlling endothelial cell fate, which represents critical mechanistic insight towards the development of miR-145 micelles as a novel therapeutic for atherosclerosis.
Animal studies
No animal studies were carried out by the authors for this article.Data availability
Data of this paper will be provided upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was support by the University of Southern California and the American Heart Association Transformational Project Award (968730) granted to E.J.C. All figure schemes were made with BioRender.com.References
- S. Barquera, et al., Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease, Arch. Med. Res., 2015, 46(5), 328–338 CrossRef PubMed.
- D. K. Arnett, et al., ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines, Circulation, 2019, 140(11), e596–e646 Search PubMed.
- S. S. Martin, et al., 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association, Circulation, 2024, 149(8), e347–e913 CrossRef PubMed.
- D. S. Schade, K. Gonzales and R. P. Eaton, Stop Stenting; Start Reversing Atherosclerosis, Am. J. Med., 2021, 134(3), 301–303 CrossRef PubMed.
- I. Hetherington and H. Totary-Jain, Anti-atherosclerotic therapies: Milestones, challenges, and emerging innovations, Mol. Ther., 2022, 30(10), 3106–3117 CrossRef CAS PubMed.
- C. Baigent, et al., Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials, Lancet, 2010, 376(9753), 1670–1681 CrossRef CAS PubMed.
- R. Alonso, A. Cuevas and A. Cafferata, Diagnosis and Management of Statin Intolerance, J. Atheroscler. Thromb., 2019, 26(3), 207–215 CrossRef CAS PubMed.
- D. Yang, et al., Endothelial cell-specific deletion of a microRNA accelerates atherosclerosis, Atherosclerosis, 2022, 350, 9–18 CrossRef CAS PubMed.
- J. Faccini, et al., Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease, Sci. Rep., 2017, 7(1), 42916 CrossRef CAS PubMed.
- F. Lovren, et al., MicroRNA-145 targeted therapy reduces atherosclerosis, Circulation, 2012, 126(11_suppl_1), S81–S90 CrossRef CAS PubMed.
- D. D. Chin, et al., miR-145 micelles mitigate atherosclerosis by modulating vascular smooth muscle cell phenotype, Biomaterials, 2021, 273, 120810 CrossRef CAS PubMed.
- L. B. Mlinar, et al., Active targeting of early and mid-stage atherosclerotic plaques using self-assembled peptide amphiphile micelles, Biomaterials, 2014, 35(30), 8678–8686 CrossRef CAS.
- D. D. Chin, et al., Long-term, in vivo therapeutic effects of a single dose of miR-145 micelles for atherosclerosis, Bioact. Mater., 2023, 27, 327–336 CAS.
- N. Patel, et al., Therapeutic Response of miR-145 Micelles on Patient-Derived Vascular Smooth Muscle Cells, Front. Digit. Health, 2022, 4, 836579 CrossRef PubMed.
- K. Y. Chyu, et al., Immunization using ApoB-100 peptide-linked nanoparticles reduces atherosclerosis, JCI Insight, 2022, 7(11), e149741 CrossRef PubMed.
- C. Poon, et al., Hybrid, metal oxide-peptide amphiphile micelles for molecular magnetic resonance imaging of atherosclerosis, J. Nanobiotechnol., 2018, 16(1), 92 CrossRef CAS PubMed.
- Z. Zhou, et al., Targeted polyelectrolyte complex micelles treat vascular complications in vivo, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(50), e2114842118 CrossRef PubMed.
- C. H. Kuo, et al., Inhibition of atherosclerosis-promoting microRNAs via targeted polyelectrolyte complex micelles, J. Mater. Chem. B, 2014, 2(46), 8142–8153 RSC.
- D. D. Chin, et al., Collagenase-cleavable peptide Amphiphile micelles as a novel Theranostic strategy in atherosclerosis, Adv. Ther., 2020, 3(3), 1900196 CrossRef PubMed.
- E. J. Chung, Targeting and therapeutic peptides in nanomedicine for atherosclerosis, Exp. Biol. Med., 2016, 241(9), 891–898 CrossRef CAS PubMed.
- A. Daiber, et al., Targeting vascular (endothelial) dysfunction, Br. J. Pharmacol., 2017, 174(12), 1591–1619 CrossRef CAS PubMed.
- J. F. Bentzon, et al., Mechanisms of plaque formation and rupture, Circ. Res., 2014, 114(12), 1852–1866 CrossRef CAS PubMed.
- I. Pinheiro-de-Sousa, et al., Uncovering emergent phenotypes in endothelial cells by clustering of surrogates of cardiovascular risk factors, Sci. Rep., 2022, 12(1), 1372 CrossRef CAS PubMed.
- J. Mai, et al., An evolving new paradigm: endothelial cells – conditional innate immune cells, J. Hematol. Oncol., 2013, 6(1), 61 CrossRef PubMed.
- A. G. Groenen, et al., Cholesterol efflux pathways, inflammation, and atherosclerosis, Crit. Rev. Biochem. Mol. Biol., 2021, 56(4), 426–439 CrossRef CAS PubMed.
- R. J. Widmer and A. Lerman, Endothelial dysfunction and cardiovascular disease, Glob. Cardiol. Sci. Pract., 2014, 2014(3), 291–308 Search PubMed.
- P. Y. Chen, et al., Endothelial TGF-β signalling drives vascular inflammation and atherosclerosis, Nat. Metab., 2019, 1(9), 912–926 CrossRef CAS PubMed.
- X. Sun, N. Belkin and M. W. Feinberg, Endothelial microRNAs and atherosclerosis, Curr. Atheroscler. Rep., 2013, 15(12), 372 CrossRef PubMed.
- S. R. Botts, J. E. Fish and K. L. Howe, Dysfunctional Vascular Endothelium as a Driver of Atherosclerosis: Emerging Insights Into Pathogenesis and Treatment, Front. Pharmacol., 2021, 12, 787541 CrossRef CAS PubMed.
- K. S. C. Weber, et al., Expression of CCR2 by Endothelial Cells, Arterioscler., Thromb., Vasc. Biol., 1999, 19(9), 2085–2093 CrossRef CAS PubMed.
- S. Irani and G. Shokri, The role of miR-143, miR-145, and miR-590 in expression levels of CD44 and vascular endothelial cadherin in oral squamous cell carcinoma, Middle East J. Cancer, 2019, 10(3), 194–204 CAS.
- L. B. Mlinar, et al., Active targeting of early and mid-stage atherosclerotic plaques using self-assembled peptide amphiphile micelles, Biomaterials, 2014, 35(30), 8678–8686 CrossRef CAS PubMed.
- J. Wang, et al., Design and in vivo characterization of kidney-targeting multimodal micelles for renal drug delivery, Nano Res., 2018, 11(10), 5584–5595 CrossRef CAS.
- Y. Huang, et al., The effect of size, charge, and peptide ligand length on kidney targeting by small, organic nanoparticles, Bioeng. Transl. Med., 2020, 5(3), e10173 CrossRef CAS PubMed.
- S. Singh, D. Anshita and V. Ravichandiran, MCP-1: Function, regulation, and involvement in disease, Int. Immunopharmacol., 2021, 101(Pt B), 107598 CrossRef CAS PubMed.
- B. C. Taylor, et al., Investigating Chemokine Receptor CCR2 Dynamics and Druggability by Ensemble Based Approaches, Biophys. J., 2018, 114(3), 399a CrossRef.
- A. Babinska, et al., The F11 receptor (F11R/JAM-A) in atherothrombosis: overexpression of F11R in atherosclerotic plaques, Thromb. Haemostasis, 2007, 97(02), 272–281 CrossRef CAS.
- A. Babinska, et al., A peptide antagonist of F11R/JAM-A reduces plaque formation and prolongs survival in an animal model of atherosclerosis, Atherosclerosis, 2019, 284, 92–101 CrossRef CAS PubMed.
- M. M. Schmitt, et al., Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis, Circulation, 2014, 129(1), 66–76 CrossRef CAS PubMed.
- D. D. Chin, et al., Long-term, in vivo therapeutic effects of a single dose of miR-145 micelles for atherosclerosis, Bioact. Mater., 2023, 27, 327–336 CAS.
- I. F. Charo and W. Peters, Chemokine receptor 2 (CCR2) in atherosclerosis, infectious diseases, and regulation of T-cell polarization, Microcirculation, 2003, 10(3–4), 259–264 CrossRef CAS PubMed.
- E. Hergenreider, et al., Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs, Nat. Cell Biol., 2012, 14(3), 249–256 CrossRef CAS PubMed.
- S. A. Dabravolski, et al., The Role of KLF2 in the Regulation of Atherosclerosis Development and Potential Use of KLF2-Targeted Therapy, Biomedicines, 2022, 10(2), 254 CrossRef CAS PubMed.
- G. Dong, et al., FOXO1 regulates dendritic cell activity through ICAM-1 and CCR7, J. Immunol., 2015, 194(8), 3745–3755 CrossRef CAS PubMed.
- J.-W. Lee, et al., Protein kinase A-α directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA, J. Biol. Chem., 2011, 286(8), 6423–6432 CrossRef CAS PubMed.
- S. Wu, H. Sun and B. Sun, MicroRNA-145 is involved in endothelial cell dysfunction and acts as a promising biomarker of acute coronary syndrome, Eur. J. Med. Res., 2020, 25(1), 2 CrossRef PubMed.
- N. Trac, et al., MRI Detection of Lymph Node Metastasis through Molecular Targeting of C–C Chemokine Receptor Type 2 and Monocyte Hitchhiking, ACS Nano, 2024, 18(3), 2091–2104 CrossRef CAS PubMed.
- E. J. Chung, et al., Fibrin-binding, peptide amphiphile micelles for targeting glioblastoma, Biomaterials, 2014, 35(4), 1249–1256 CrossRef CAS PubMed.
- Y. Gao, et al., PEGylated therapeutics in the clinic, Bioeng. Transl. Med., 2024, 9(1), e10600 CrossRef CAS PubMed.
- J. J. Wentzel, et al., Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions, Cardiovasc. Res., 2012, 96(2), 234–243 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nh00613e |
| This journal is © The Royal Society of Chemistry 2025 |