Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Highly selective ethanol gas sensor based on CdS/Ti3C2Tx MXene composites†
Ly Tan
Nhiem‡
 a,
Jianbin
Mao‡
b,
Qui Thanh
Hoai Ta
a,
Jianbin
Mao‡
b,
Qui Thanh
Hoai Ta
 *c and
Soonmin
Seo
*c and
Soonmin
Seo
 *b
*b
aFaculty of Chemical and Food Technology, Ho Chi Minh City University of Technology and Education, 01 Vo Van Ngan Street, Linh Chieu Ward, Thu Duc City, Ho Chi Minh City, Vietnam. E-mail: nhiemlt@hcmute.edu.vn
bCollege of BioNano Technology, Gachon University, Gyeonggi 13120, Republic of Korea. E-mail: mg2895852@gmail.com; soonmseo@gachon.ac.kr
cInstitute of Chemical Technology, Vietnam Academy of Science and Technology, 1A TL29 Street, Thanh Loc Ward, District 12, Ho Chi Minh City, 700000, Vietnam. E-mail: tathanhhoaiqui2292@gmail.com
First published on 8th January 2025
Abstract
Sensing of hazardous gases has an important role in ensuring safety in a variety of industries as well as environments. Mainly produced by the combustion of fossil fuels and other organic matter, ethanol is a dangerous gas that endangers human health and the environment. Stability and sensing sensitivity are major considerations when designing gas sensors. Here, a superior ethanol sensor with a high response and fast recovery was synthesized by “wrapping” CdS nanoparticles on metallic Ti3C2Tx MXene using a simple method. CdS nanoparticles were uniformly covered on the Ti3C2Tx MXene surface, forming a “rice crust”-like heterostructure. The sensor displayed good detection of ethanol gas at room temperature. Response signals up to 31% were obtained for ethanol molecules (20 ppm) with quick recovery (41 s). The performance of the ethanol sensor was evaluated across a range of concentrations (5–100 ppm) and relative humidity (60% and 90% RH) at room temperature. Our method could open up a new strategy for the development of ethanol sensors.
1. Introduction
Volatile organic compounds (VOCs) are flammable and poisonous gases. They are found mostly in refineries, mining industries, and our daily lives. Exposure to VOCs by animals and humans can affect the respiratory system. Ethyl alcohol (ethanol) is popularly utilized as a organic solvent in cosmetics, and chemical laboratories.1–4 Ethyl alcohol is a well-known VOC and can be hazardous to consciousness upon heavy exposure, causing dizziness, vomiting, and nausea. Additionally, extreme leakage of ethyl alcohol could cause fires and explosions because of its high flammability. Most detection sensors in the market are costly and consume a lot of power because of the sensor material and design.5,6 Consequently, developing a willingly accessible and low-cost gas detector with a limit of detection towards that of ethyl alcohol is crucial for environmental monitoring. Resistive gas sensors are attractive thanks to their swift and specific determination of toxic gases compared with heavyweight and nonportable systems such as liquid chromatography-mass spectrometry, flue gas analyzers, and gas chromatography-mass spectrometry.7,8Since the first exploration of two-dimensional materials in 2004, graphene has garnered rigorous research curiosity owing to its chemical and physical properties.9 Besides, MXenes could be used as gas sensors owing to their surface functional groups and metallic properties. These multilayer MXenes have a universal formulation of Mn+1XnTz (n = 1–3) whereby M denotes early transition metals (Mo, Ti, V, Nb, Cr), X is nitrogen and/or carbon, and Tz represents the termination groups (F, Cl, Br, I, O, OH).10–14 In 2017, the first report about gas sensing of Ti3C2Tx MXene was published by Lee et al.,15 which displayed p-type sensing properties toward ammonia, acetone, methanol, and ethanol. That report was followed by a speedily increasing number of studies due to their excellent properties, such as electrical conductivity (103–104 S cm−1), large surface area, high hydrophilicity, versatile surface chemistry, and high mechanical stability. Interestingly, the surface termination groups of Ti3C2Tx MXene offer a hydrophilic surface with a greatly negative zeta potential (−30 to −80 mV), which enables effective processing of a combination of Ti3C2Tx MXene with other nanomaterials to form a smart composition.16–19
2D MXene nanosheets are readily restacked after a long time, which results in low sensitivity and stability of gas sensors made of pristine MXene.20 Furthermore, pure Ti3C2Tx-MXene gas sensors are unsatisfactory in terms of the limit of detection and selectivity.21 In order to overcome these problems, the construction of heterostructures has improved the performance of MXene-based gas sensors. Ti3C2Tx MXene is modifiable thanks to its excellent electrical characteristics and its high electronic density in the Fermi energy level. Studies have shown that the construction of heterostructures can effectively increase the number of adsorption sites and improve gas sensing capabilities.22,23 The MXene/In2O3 heterostructure, formed via a simple process, disperses In2O3 on MXene layers, enabling NH3 detection with high sensitivity and selectivity at room temperature.24 The MXene/SnS2 heterojunction sensor exhibits excellent sub-ppm ammonia detection, sensing NH3 down to 10 ppb at room temperature.25 The room temperature sensor has a crucial role because high temperatures impede its usability in wearable applications. Operating at high temperatures results in increased energy consumption when detecting toxic gases at high concentrations. Furthermore, high-temperature operation compromises the nanostructure of the sensing material (thereby reducing its gas sensing efficiency) but also poses challenges in detecting explosive or flammable gases.12,26
Cadmium sulfide (CdS) stands out due to its small bandgap (2.4 eV), efficient absorption of light, and excellent charge separation properties.27,28 Additionally, CdS is stable, non-toxic, and safe.29 As an important member of the II–VI compound family, CdS has been widely applied in various fields and studies, including sensors, lasers, solar cells, photoelectric detection, biomedical tags, photocatalysis, electroluminescent devices, the hydrogen evolution reaction (HER), and photoconductors.30–33 CdS has also been reported in sensor applications for the detection of gases.34,35 For example, Giberti et al. developed a gas sensor using an as-synthesized CdS thick film.36 However, the strong selectivity for alcohols requires a temperature of 300 °C, which is too high for practical applications. The high working temperature, high limit of detection, and poor conductivity of CdS hinder its application in CdS-based gas sensing. Therefore, there is an immediate requirement to develop a CdS-based gas sensor with high selectivity and enhanced responding performance at room temperature. Recently, Guo et al. demonstrated that having many active sites can enhance gas sensing properties by boosting the adsorption capacity for the target gas, thus increasing the affinity for gas molecules.29 According to our previous research, nanostructured semiconductors with mesoporous, nanomesh, and nanowire structures can provide many active sites.37–39 Moreover, semiconductors with narrow energy band gaps (e.g., CdS, CdO) exhibit higher charge carrier concentrations but also lower resistivity, which enhances the adsorption energy of oxidizing gas molecules.40–42
It has been reported that CdS/Ti3C2Tx can be used in the photocatalytic field. A stable Ti3C2Tx MXene/CdS heterojunction catalyst, created via in situ growth of CdS on Ti3C2Tx MXene, enhances hydrogen production by broadening NIR absorption, accelerating photochemical reactions, enabling efficient electron separation, and reducing carrier recombination.43 In another study, it has been demonstrated that grafting MXene with CdS enhances charge separation, optimizes pore structure, and increases surface area, improving photocatalyst-reactant contact.44 In the meantime, ultrathin Ti3C2Tx can reduce CdS aggregation, forming Schottky junctions that boost photocatalytic activity by improving charge separation and electron transfer.45 It also enhances H2O2 selectivity and enables a high-performance photo-Fenton system. In CdS, the sulfur (S) element exhibits low electronegativity, which facilitates the adsorption of oxygen molecules and their subsequent reaction with reactive sulfur species (S2−) to generate a series of ionized oxygen species. Consequently, the activation energy required for the conversion of O2 molecules to these ionized oxygen species at CdS sites is lower than that necessary on metal oxide surfaces. As a result, more ionized oxygen species are generated on CdS structures. It is well established that ionized oxygen species have critical roles in gas sensing reactions. Thus, CdS-based sensors may operate at lower temperatures compared with traditional oxide semiconductor materials.46 However, the combination of Ti3C2Tx MXene and CdS nanomaterials for use as a gas sensor has rarely been reported.
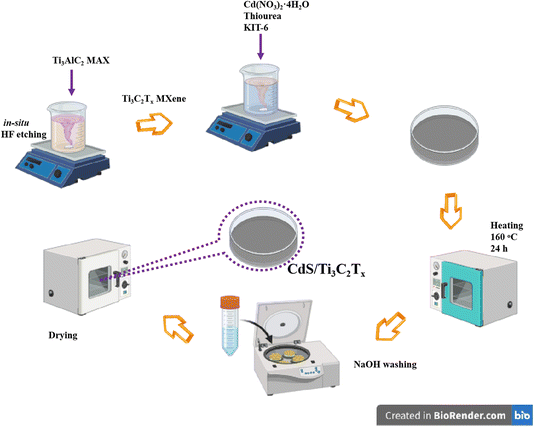
In this research, we synthesized a CdS/Ti3C2Tx composite using Ti3C2Tx MXene and mesoporous CdS nanomaterials with a large surface area and uniform pore size. KIT-6, an ordered mesoporous silica, was chosen as a template because of its bicontinuous mesoporous structure, which belongs to the cubic Ia3d space group.33 This silica consists of enantiomeric pairs of interpenetrating mesoporous networks, all with uniform pore diameters. The three-dimensional (3D) connectivity of the pores make it an ideal template for creating a self-retaining replica.27,39 In addition, the pore diameters can be controlled in the range 4–12 nm by adjusting the synthetic conditions. Overall, the combination of the large pore size and high stability of KIT-6 and 3D interconnected mesopores makes it a highly versatile and effective template for the synthesis of advanced functional materials with “tailored” properties. We examined the VOCs gas-sensing activity of pure Ti3C2Tx MXene and its hybrid structure at room temperature. The mesoporous structure significantly enhanced gas sensing performance by providing a high surface area and interconnected pores that facilitated efficient gas adsorption and diffusion. This structure ensured that target gas molecules could readily access the active sensing sites, thereby increasing sensitivity and enabling fast response and recovery times. In addition, the mesoporous framework supported the formation of nanostructures with optimized morphology, thereby improving the stability and reproducibility of sensor performance. These combined effects make mesoporous materials highly effective for gas-sensing applications. The optimized composite specified a response of 31% at 20 ppm at room temperature with outstanding repeatability. We demonstrated that the mesoporous CdS nanostructures covering Ti3C2Tx strengthened the surface area but also improved electron transfer between composite and gas molecules. Additionally, the plausible sensing mechanism of CdS/Ti3C2Tx sensor was investigated further.
2. Experimental
2.1. Ti3C2Tx MXene preparation
To prepare Ti3C2Tx MXene, the in situ HF etching method was applied. Briefly, two grams of LiF were spread in 50 mL of HCl solution (5.5 M) under vigorous stirring. Subsequently, two grams of Ti3AlC2 MAX phase powder (MilliporeSigma) were progressively placed into the solution in an ice bath to mitigate exothermic reactions. The mixture solution was then kept in a bath at 50 °C for 1 day. The final product was separated by vacuum filtration and washed several times using deionized water until the pH reached around 6–7. The multilayered Ti3C2Tx MXene was dried in a freeze-dryer before being stored for further experiments.2.2. Synthesis of KIT-6
KIT-6 was produced according to the previously reported soft-template method with some modifications. KIT-6 was synthesized hydrothermally, following a procedure documented in previous studies.38 KIT-6 was synthesized by preparing an aqueous solution with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of Pluronic P123 (EO20PO70EO20, MW = 5800) and butanol, maintaining an HCl concentration around 0.5 M at temperatures between 25 and 35 °C. Both tetraethoxysilane (TEOS) and sodium silicate can serve as silica sources. For a typical synthesis using TEOS, we dissolved 6 g of P123 in 217 g of distilled water and added 11.8 g of concentrated HCl (35%). Then, we incorporated 6 g of butanol while stirring at 35 °C. After 1 h of stirring, we added 12.9 g of TEOS at the same temperature. We continued stirring the mixture for 24 h at 35 °C, then heated it for an additional 24 h at 100 °C under static conditions in a sealed polypropylene bottle. The solid product from the hydrothermal treatment was filtered and dried at 100 °C without washing. The template was removed by extracting it in an ethanol solution over 3 days.
1 weight ratio of Pluronic P123 (EO20PO70EO20, MW = 5800) and butanol, maintaining an HCl concentration around 0.5 M at temperatures between 25 and 35 °C. Both tetraethoxysilane (TEOS) and sodium silicate can serve as silica sources. For a typical synthesis using TEOS, we dissolved 6 g of P123 in 217 g of distilled water and added 11.8 g of concentrated HCl (35%). Then, we incorporated 6 g of butanol while stirring at 35 °C. After 1 h of stirring, we added 12.9 g of TEOS at the same temperature. We continued stirring the mixture for 24 h at 35 °C, then heated it for an additional 24 h at 100 °C under static conditions in a sealed polypropylene bottle. The solid product from the hydrothermal treatment was filtered and dried at 100 °C without washing. The template was removed by extracting it in an ethanol solution over 3 days.
2.3. Preparation of mesoporous CdS nanomaterials
We dissolved 0.6 g of Cd(NO3)2·4H2O and 0.2 g of thiourea in 15 mL of ethanol at room temperature, then added 0.8 g of the KIT-6 template to the stirring solution. After evaporating the ethanol, we spread the solid mixture in a Petri dish. The mixture was heated at 160 °C for 24 h in an air atmosphere to form the CdS@KIT-6 intermediate. The silica template was removed by treating the mixture with 50 mL of 2 M NaOH aqueous solution, which served as an etching agent, to obtain mesoporous CdS.2.4. Preparation of CdS/Ti3C2Tx hybrids
We dissolved 0.11 g of Cd(NO3)2·4H2O and 0.03 g of thiourea in 10 mL of ethanol at room temperature, and then added 0.3 g of the KIT-6 template to the stirring mixture. Subsequently, we gradually incorporated the pre-prepared Ti3C2Tx MXene (100, 200, or 300 mg) into the solution and stirred vigorously for 15 min. After evaporating the ethanol, we spread the solid mixture in a Petri dish. The mixture was heated at 160 °C for 24 h in an air atmosphere to form the CdS@KIT-6/Ti3C2Tx intermediate. The silica template was removed by treating the mixture with 50 mL of 2 M NaOH aqueous solution, which acted as an etching agent, to obtain CdS/Ti3C2Tx. The final products were named “CT1” (100 mg), “CT2” (200 mg), and “CT3” (300 mg) (Fig. 1). For comparison, pure mesoporous CdS was also prepared under the same conditions, without the addition of Ti3C2Tx MXene.2.5. Characterization and measurements
The characterization methods employed in this study were: X-ray diffraction (XRD; Rigaku SmartLab) using Cu Kα radiation to analyze sample phase composition; scanning electron microscopy (SEM; Hitachi S-4700) to observe surface chemical characteristics and morphologies; high-performance X-ray photoelectron spectroscopy (XPS; K-Alpha+) for investigating chemical states; and Brunauer–Emmett–Teller (BET) analysis using an ASAP 2020 accelerated surface area and porosimetry system to determine the specific surface areas and pore distributions of the powders. The optical properties of synthesized samples were recorded using UV-vis diffuse reflectance spectroscopy (Jasco V-770).2.6. Gas sensor experiments
The toxic gas sensing activity of the CdS/Ti3C2Tx heterostructure gas sensor was recorded using a homemade system. The as-synthesized samples were dispersed in deionized water at a concentration of 1 mg mL−1 and then drop-coated onto a silicon wafer (1 × 1 cm) with a thickness of approximately 8–10 µm. The sensor wafer was dried overnight to evaporate the solvent and subsequently placed within a stainless-steel chamber connected to a source measure unit (Keysight 34465A). The relative humidity of the gas testing system was calibrated by adjusting the parameters in a humidity meter. The gas concentration was calibrated to the desired level using a mass flow controller and mass flow meter (MFC/MFM) by adjusting the flow rates of synthetic air and the target gas. The diluted gas mixture was subsequently injected into the chamber after thorough mixing. The response of the CdS/Ti3C2Tx heterostructure was calculated as the alteration in the relative resistance in the target gas (Rgas) compared with that in synthetic air (Rair) before and after target gas injection, as follows:3. Results and discussion
3.1. Morphological and component characterization
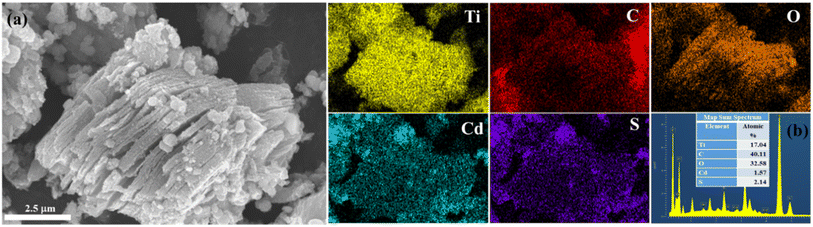
Fig. 2 displays the FE-SEM images of pure Ti3AlC2, Ti3C2Tx, CdS and the CdS/Ti3C2Tx heterostructure. As shown in Fig. 2a, commercial Ti3AlC2, had stacked, laminated and compact structures. After etching with HF acid, the Ti3C2Tx MXene with layered and accordion-like structures was observed in Fig. 2b. Pure CdS showed a spherical granule-like structure with and average diameter of 20 nm (Fig. 2c). With abundant hydrophilic groups, CdS nanoparticles covered readily on the superficial of Ti3C2Tx MXene, further confirming the combination of the intimate interfacial interaction between Ti3C2Tx MXene and CdS (Fig. 2d–f). | ||
| Fig. 2 SEM images of (a) pristine Ti3AlC2, (b) Ti3C2Tx MXene, (c) CdS (the inset figure is the TEM of CdS) and (d–f) the CdS/Ti3C2Tx heterostructure. | ||
Moreover, the results of EDS elemental mapping revealed the uniform distribution of Cd, S, Ti, C, and O elements in the CdS/Ti3C2Tx heterostructure. As shown in Fig. 3a, numerous CdS NPs were decorated on the Ti3C2Tx MXene and a few were agglomerated on the Ti3C2Tx MXene. The direct close interaction between CdS and Ti3C2Tx MXene boosted the fast transfer and migration of generated electrons. As presented in Fig. 3b, a higher oxygen atom content (32.6% atomic%) than that in pristine Ti3C2Tx MXene (14%) was obtained, resulting in more oxygen termination groups, and partial formation of TiO2 during the hydrothermal reaction.
To understand the optical properties, we also obtained the energy band gap of typical samples. Fig. S1† displays the UV-vis diffuse reflectance spectra of selected samples over a wavelength range of 300–800 nm. Notably, an absorption edge peak of CdS was observed at ∼500 nm (Fig. S1a†). In comparison with pristine CdS, the optical absorption edge of the composite exhibited a distinct red shift to ∼550 nm. This 550 nm absorption edge arose from the interband electronic transitions in CdS, indicating that CdS had been decorated and coupled within the composite.47 As shown in Fig. S1b and d,† the estimated energy band gap (Eg) of pure CdS and the composite was 2.14 eV and 1.58 eV, respectively. A composite with a narrow band gap allows for easier excitation of electrons from the valence band (VB) to the conduction band (CB) with lower energy. This implies that less energy is required to trigger the electronic response of a composite to external stimuli, such as gas adsorption, potentially enhancing the sensitivity of the sensor due to the increased responsiveness to environmental changes of the material.40–42
3.2. Structural properties
Fig. 4 illustrates the detailed crystalline phase structures measured by XRD. Fig. 4a clearly shows that the (002) and (004) peaks of the as-prepared Ti3C2Tx MXene were shifted to smaller angles and broadened compared with those of Ti3AlC2 powder, indicating the formation of a laminated microstructure after HF etching.48 The diffraction peak intensity of Ti3C2Tx was significantly lower than that of Ti3AlC2. This disparity may have arisen from differences in the distribution of carbon atoms within the crystal structure and the crystal symmetry of both materials. Additionally, diffraction peaks of the original Ti3AlC2 MAX phase between 35° and 40° vanished, which suggested that Al atoms had been removed. This result also showed that our synthesized Ti3C2Tx had high purity, which is in good agreement with previous reports.49 In the CdS/Ti3C2Tx MXene heterostructure, the diffraction peak of both Ti3C2Tx MXene and mesoporous CdS nanoparticles could be observed. The clear diffraction peaks at 2θ = 26.6°, 30.4°, 44.1°, and 52.5° corresponded to the phases (111), (200), (220) together with (311) planes, and confirmed the existence of lattice plates of cubic zinc blended crystal structure (PDF 01-075-0581).50 In particular, in the CdS/Ti3C2Tx MXene heterostructure, the (002) peak was notably broadened and shifted to a lower angle compared with that in the pure Ti3C2Tx MXene material, indicating an enlarged layer spacing (Fig. 4b). | ||
| Fig. 4 (a) High angle and (b) low angle XRD patterns of Ti3AlC2 powder, Ti3C2Tx MXene, mesoporous CdS nanoparticle, and the CdS/Ti3C2Tx heterostructure. | ||
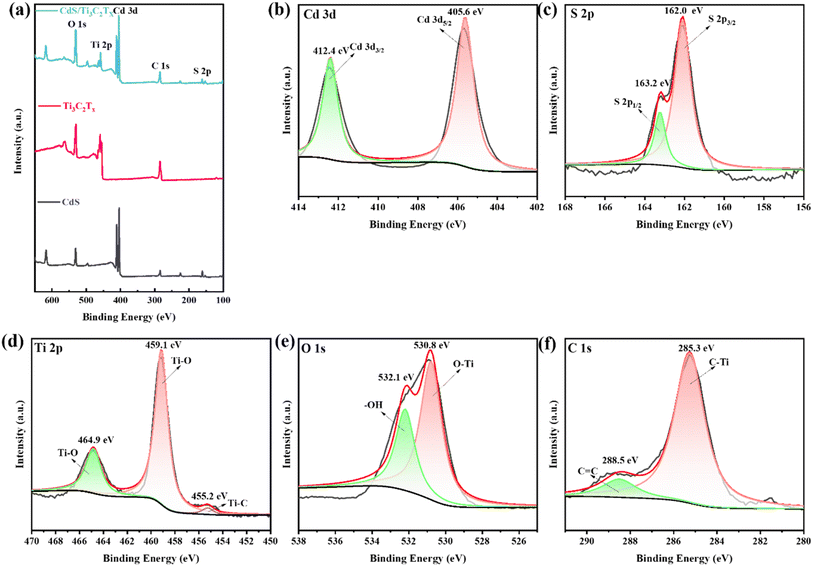
XPS spectra were used to analyze the overall XPS spectra chemical state of the surface as well as the composition of the CdS/Ti3C2Tx heterostructure. The survey spectra of Ti3C2Tx MXene, mesoporous CdS nanoparticle, and CdS/Ti3C2Tx heterostructure are depicted in Fig. 5a. In addition, there were five main chemical elements on the surface, which corresponded to the elements of Cd, S, Ti, O, and C. The highly resolved spectra of the Cd 3d, S 2p, Ti 2p, O 1s and C 1s core levels for the CdS/Ti3C2Tx heterostructure are displayed in Fig. 5b–f. The Cd-3d characteristic peaks located at 405.6 and 412.4 eV belonged to the Cd 3d5/2 and Cd 3d3/2 orbitals (Fig. 5b), respectively, indicating the presence of Cd ions in the +2 oxidation state.51,52 Two significant peaks located at 162.0 and 163.2 eV, which were equal to the binding energy of S 2p3/2 and S 2p1/2 (Fig. 5c), showed the presence of S–S bonding in CdS.44,53 The Ti 2p binding energy exhibited three distinct peaks centered at 455.2 eV, 459.1 eV, and 464.9 eV, which corresponded to Ti–C and Ti–O bonds, respectively, within the Ti 2p domain (Fig. 3d). As shown in Fig. 3e, the O 1s spectrum exhibited two main peaks at 530.8 eV and 532.1 eV, which were attributed to hydroxyl/oxygen-terminated surfaces and in situ oxidized TiO2.54 The spectrum of the C 1s peak showed two distinct peaks at 285.3 eV and 288.5 eV, which corresponded to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–Ti bonds on the surface, respectively, as depicted in Fig. 5f.55,56 The characterization data further indicated fabrication of the CdS/Ti3C2Tx heterostructure.
C and C–Ti bonds on the surface, respectively, as depicted in Fig. 5f.55,56 The characterization data further indicated fabrication of the CdS/Ti3C2Tx heterostructure.
The nitrogen adsorption–desorption isotherms of the as-prepared materials were employed to evaluate the impact of mesoporous CdS nanoparticles on the surface area of Ti3C2Tx MXene. This evaluation aimed to assess potential improvements in gas sensing performance in terms of gas recovery and response time. As illustrated in Fig. 6, the nitrogen sorption isotherms of CdS, and CdS/Ti3C2Tx samples indicated the pore characteristics of these samples with respect to a type-IV isotherm with a long and narrow hysteresis loop, suggesting the presence of uniform mesoporosity.57 The BET surface area of the mesoporous CdS and the CdS/Ti3C2Tx heterostructure was estimated to be 81.1 and 78.4 m2 g−1, respectively. In addition, the average pore width of the mesoporous CdS, and CdS/Ti3C2Tx heterostructure was 8.9, and 6.7 nm, respectively (inset of Fig. 6). These results showed that, after the preparation of mesoporous CdS and Ti3C2Tx MXene as heterojunction materials, a larger pore size and higher specific surface area were observed, which increased the number of active sites and facilitated electron transport.
 | ||
| Fig. 6 Nitrogen adsorption–desorption isotherms were conducted for Ti3C2Tx MXene, mesoporous CdS nanoparticles, and the CdS/Ti3C2Tx heterostructure. Pore-size distributions are depicted in the inset. | ||
3.3. Target gas sensing performance and mechanism
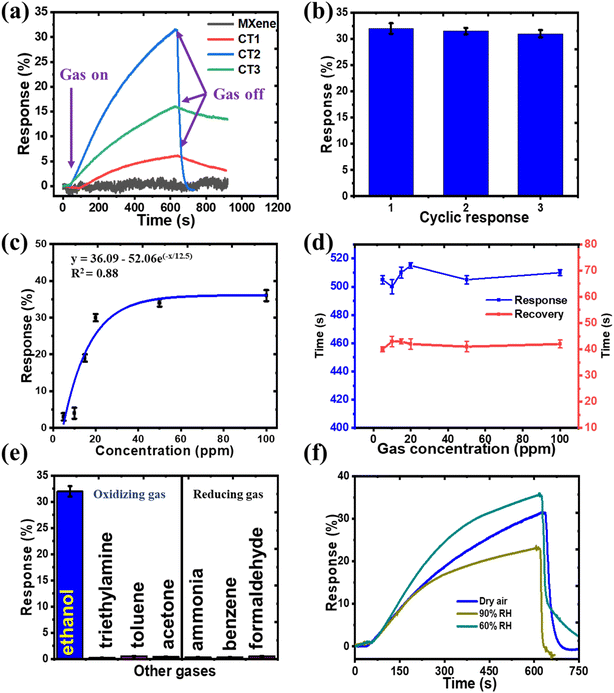
The gas sensing activity of the as-prepared CdS/Ti3C2Tx heterostructure-based sensors was recorded at room temperature by analyzing the resistance variation in target gases and air. First, the synthesis air was used to flow the chamber and obtain a stable condition. Fig. 7 displays the gas response of prepared CdS/Ti3C2Tx-based sensors to different target gas molecules. In Fig. 7a, we analyzed the responses of the as-synthesized CT1, CT2, CT3, and pure Ti3C2Tx MXene to 20 ppm ethanol gas. The CdS/Ti3C2Tx heterostructure displayed an obvious reaction to ethanol, while Ti3C2Tx MXene had no signal as metallic properties. The CT2 heterostructure displayed the highest gas response as compared with that of CT1, CT3, and pure Ti3C2Tx. The repeatability performance of CT2 sensors to 20 ppm ethanol after three cycles was also tested under the same parameter. As revealed in Fig. 7b, the gas responses of the CT2 sample varied slightly and had a similar tendency, which displayed reproducible results.Fig. 7c presents the sensor response curves of CT2 against ethanol concentrations ranging from 5 to 100 ppm. All response signals increased with an increase in ethanol concentration, demonstrating a good correlation. The calculated linear regression coefficient (R2) was ∼0.88, indicating that CT2 was logarithmic in detecting ethanol molecules within this concentration range. Fig. 7d illustrates the response and recovery times as a function of gas concentration, with error bars included. The response time was ∼507 s, compared with a recovery time of 41 s. The relatively long response time could be attributed to the need for ethanol gas to diffuse and undergo subsequent oxidation during sensing. The response time of a sensor was influenced by the number of available reactive sites, which required time to reach the optimal configuration for gas-sensing reactions. In contrast, a faster recovery time was attributed to the porous structure and rapid electron transport of the material, which provided a large surface area for diffusion and facilitated the removal of ethanol molecules under 100% airflow. This phenomenon has also been observed in previous studies.49,58
To further confirm the selectivity of the CdS/Ti3C2Tx heterostructure, the signal histograms of the optimized CT2 sensor for a variety of target gases to 20 ppm are presented in Fig. 7e. The signal responses to different target gases (acetone, toluene, formaldehyde, ammonia, benzene, triethylamine) were negligible. Only the CdS/Ti3C2Tx heterostructure displayed a response to ethanol. These results further confirmed the selectivity of the CdS/Ti3C2Tx sensor to ethanol. Several factors may have contributed to the observed selectivity. Ethanol possesses a hydroxyl (–OH) group and a hydrocarbon chain. Although it is not perfectly symmetrical, it exhibits some symmetry that can influence its interaction with surfaces. The presence of methyl groups affects polarity and induces dipole moments. Ethanol, which primarily contains single bonds, has a lower molecular mass and volatile nature, whereas others contain carbonyl or aromatic structures with double bonds. In contrast, toluene and benzene are nonpolar compounds, resulting in minimal interaction with the sensor surface.59 On the other hand, the greater selectivity of the CdS/Ti3C2Tx composite can be explained via the different unoccupied molecule orbit (LUMO) energies of these gas molecules. The high value of the LUMO energy indicates that a significant amount of energy is required for the interaction between the sensor material and target gas.60,61 The LUMO energy of ethanol (0.12 eV) is the lowest among the gases stated above, which is better for electrons and the reaction between the composite and ethanol molecules.46 Thus, the CdS/Ti3C2Tx composite had excellent selectivity towards ethanol at ambient atmosphere.
Fig. 7f illustrates the response curve of the CdS/Ti3C2Tx composite to ethanol (20 ppm) at various levels of relative humidity. As the humidity increases, the hydroxyl groups in H2O molecules exhibit weak electron-withdrawing behavior, interacting with electrons from the sensor material and enhancing electrical conductivity, which results in an improved response. However, a further increase in humidity leads to a decrease in response, which can be attributed to the H2O poisoning effect. This phenomenon occurs if a significant number of H2O molecules adsorb onto the material, obstructing the available adsorption sites for ethanol.62
Moreover, the ethanol sensing response of our CdS/Ti3C2Tx heterostructures was compared with different MXene composite-based gas sensors: CdS nanostructures, PPy@Ti3C2Tx, W18O49@Ti3C2Tx, MoO3@Ti3C2Tx, and Co3O4@Ti3C2Tx composites. As shown in Table 1, the optimized CT2 did not exhibit the best ethanol sensing response, but it was a promising material for ethanol detection at room temperature.
| Material | Temp. (°C) | Concentration (ppm) | Response (%) | Ref. |
|---|---|---|---|---|
| PPy@Ti3C2Tx | RT | 20 | 16 | 63 |
| CdS film | 250 | 5 | 10 | 36 |
| CdS hierarchical microspheres | 200 | 10 | 8 | 64 |
| 0.5 at% CuO–CdS | 160 | 100 | 7 | 65 |
| CdS quantum dot | 200 | 1000 | 0 | 66 |
| W18O49@Ti3C2Tx | 300 | 20 | 1.67 | 67 |
| MoO3@Ti3C2Tx | 100 | 20 | 39 | 68 |
| Co3O4@Ti3C2Tx | 200 | 50 | 11 | 69 |
| CdS/Ti3C2Tx | RT | 20 | 31 | This work |
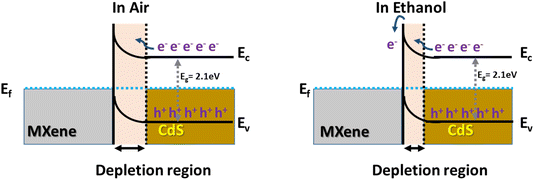
The improved ethanol sensor mechanism of the CdS/Ti3C2Tx composite is displayed schematically in Fig. 8. The p–n junction interface in the composite was formed due to the difference in work functions between n-type CdS (3.2 eV) and p-type Ti3C2Tx MXene (5.9 eV).70 As a result, electrons from CdS tend to migrate to the MXene interface to form a depletion layer. When the CdS/Ti3C2Tx composite is exposed to an air atmosphere, O2 molecules are chemically adsorbed and captured on the surface of the sensor material. In particular, the oxygen molecules were converted into ionized adsorbed oxygen (O−, O2−, and O2−). When the CdS/Ti3C2Tx composite was in contact with and exposed to ethanol molecules, the chemical interaction of ethanol molecules with the ionized adsorbed oxygen could release the generated electron back to the sensor material, resulting in a thinner depletion layer, reduced hole concentration, and increased the resistance of the CdS/Ti3C2Tx composite.64,71–74 This sophisticated route can be explained by the following equation:
| O2 (g) → O2 (ads) |
| O2 (ads) + e− → O2− (ads) |
| O2− (ads) + e− → 2O2− (ads) |
| C2H5OH (gas) + 6O2− → CH3CHO + 3H2O + 12e− |
4. Conclusions
We synthesized VOCs sensors via combining CdS nanostructures with Ti3C2Tx MXene using a simple method. The as-obtained CdS/Ti3C2Tx heterostructures sensor showed a superior sensing activity in the enhanced detection of ethanol gas at room temperature. The CdS/Ti3C2Tx heterostructure presented a good response of 31% towards 20 ppm ethanol, which was superior to that of pure MXene. Moreover, the optimized CdS/Ti3C2Tx heterostructure presented excellent selectivity with fast recovery time to ethanol molecules. The combination effect of CdS and metallic Ti3C2Tx in the composite had a crucial role in ethanol sensing performance. Thus, the optimized CdS/Ti3C2Tx heterostructure provides a potential composite to synthesize and improve superior ethanol sensors.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Vietnam Academy of Science and Technology (THTEXS.02/24-26).References
- B. Han, T. H. Rupam, A. Chakraborty and B. B. Saha, A comprehensive review on VOCs sensing using different functional materials: Mechanisms, modifications, challenges and opportunities, Renewable Sustainable Energy Rev., 2024, 196, 114365 CrossRef CAS.
- S. Sahani, S. J. Park, Y. Myung, T.-H. Pham, T. T. Tung and T. Kim, Enhanced Room-Temperature Ethanol Detection by Quasi 2D Nanosheets of an Exfoliated Polymeric Graphitized Carbon Nitride Composite-Based Patterned Sensor, ACS Omega, 2022, 7, 41905–41914 CrossRef CAS PubMed.
- A. Mangotra and S. K. Singh, Volatile organic compounds: A threat to the environment and health hazards to Living Organisms–A Review, J. Biotechnol., 2024, 382, 51–69 CrossRef CAS.
- Y. Xiang, X. Xie, H. Zhong, F. Xiao, R. Xie, B. Liu, H. Guo, D. Hu, P. Zhang and H. Huang, Efficient Catalytic Elimination of Toxic Volatile Organic Compounds via Advanced Oxidation Process Wet Scrubbing with Bifunctional Cobalt Sulfide/Activated Carbon Catalysts, Environ. Sci. Technol., 2024, 58, 8846–8856 CrossRef CAS PubMed.
- V. Sonde, A. D’souza, R. Tarapore, L. Pereira, M. P. Khare, P. Sinkar, S. Krishnan and C. V Rao, Simultaneous administration of diethylphthalate and ethyl alcohol and its toxicity in male Sprague–Dawley rats, Toxicology, 2000, 147, 23–31 CrossRef CAS PubMed.
- S. C. Bondy, Ethanol toxicity and oxidative stress, Toxicol. Lett., 1992, 63, 231–241 CrossRef CAS.
- R. Guo, H. Wang, R. Tian, D. Shi, H. Li, Y. Li and H. Liu, The enhanced ethanol sensing properties of CNT@ ZnSnO3 hollow boxes derived from Zn-MOF (ZIF-8), Ceram. Int., 2020, 46, 7065–7073 CrossRef CAS.
- H. Roshan and M. H. Sheikhi, A novel room temperature ethanol sensor based on PbS: SnS2 nanocomposite with enhanced ethanol sensing properties, J. Alloys Compd., 2020, 816, 152666 CrossRef.
- K. S. Novoselov, A. K. Geim, S. V Morozov, D. Jiang, Y. Zhang, S. V Dubonos, I. V Grigorieva and A. A. Firsov, Electric field effect in atomically thin carbon films, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- S. Liu, M. Wang, C. Ge, X. Zhang, S. Lei, S. Hussain, M. Wang, G. Qiao and G. Liu, ZnO/Ti3C2 composite with oxygen vacancies and Schottky barrier for effective detection of ppb-level NO2 at room temperature, Appl. Surf. Sci., 2023, 610, 155440 CrossRef CAS.
- M. A. Hope, A. C. Forse, K. J. Griffith, M. R. Lukatskaya, M. Ghidiu, Y. Gogotsi and C. P. Grey, NMR reveals the surface functionalisation of Ti3C2 MXene, Phys. Chem. Chem. Phys., 2016, 18, 5099–5102 RSC.
- L. X. Ou, M. Y. Liu, L. Y. Zhu, D. W. Zhang and H. L. Lu, Recent progress on flexible room-temperature gas sensors based on metal oxide semiconductor, Nano-Micro Lett., 2022, 14(1), 206 CrossRef CAS.
- D. Xiong, X. Li, Z. Bai and S. Lu, Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage, Small, 2018, 14, 1703419 CrossRef.
- Z. Wei, Z. Peigen, T. Wubian, Q. Xia, Z. Yamei and S. ZhengMing, Alkali treated Ti3C2Tx MXenes and their dye adsorption performance, Mater. Chem. Phys., 2018, 206, 270–276 CrossRef CAS.
- E. Lee, A. VahidMohammadi, B. C. Prorok, Y. S. Yoon, M. Beidaghi and D.-J. Kim, Room temperature gas sensing of two-dimensional titanium carbide (MXene), ACS Appl. Mater. Interfaces, 2017, 9, 37184–37190 CrossRef CAS PubMed.
- A. Gentile, C. Ferrara, S. Tosoni, M. Balordi, S. Marchionna, F. Cernuschi, M. Kim, H. Lee and R. Ruffo, Enhanced Functional Properties of Ti3C2Tx MXenes as Negative Electrodes in Sodium‐Ion Batteries by Chemical Tuning, Small Methods, 2020, 4, 2000314 CrossRef CAS.
- C. Ferrara, A. Gentile, S. Marchionna and R. Ruffo, Ti3C2Tx MXene compounds for electrochemical energy storage, Curr. Opin. Electrochem., 2021, 29, 100764 CrossRef CAS.
- M. Benchakar, L. Loupias, C. Garnero, T. Bilyk, C. Morais, C. Canaff, N. Guignard, S. Morisset, H. Pazniak and S. Hurand, One MAX phase, different MXenes: A guideline to understand the crucial role of etching conditions on Ti3C2Tx surface chemistry, Appl. Surf. Sci., 2020, 530, 147209 CrossRef CAS.
- A. Jastrzebska, E. Karwowska, D. Basiak, A. Zawada, W. Ziemkowska, T. Wojciechowski, D. Jakubowska and A. Olszyna, Biological activity and bio-sorption properties of the Ti2C studied by means of zeta potential and SEM, Int. J. Electrochem. Sci., 2017, 12, 2159–2172 CrossRef CAS.
- X. Wu, Y. Gong, B. Yang, Z. Mao, Z. Yan, C. Su, S. Xiong, X. Long and X. Wang, Fabrication of SnO2-TiO2-Ti3C2Tx hybrids with multiple-type heterojunctions for enhanced gas sensing performance at room temperature, Appl. Surf. Sci., 2022, 581, 152364 CrossRef CAS.
- R. Tian, Y. Ding, Q. Wang and P. Song, Designing advanced 2D/2D heterojunctions of MoS2 nanosheets/Ti3C2Tx MXene in gas-sensing applications, Vacuum, 2024, 222, 112991 CrossRef CAS.
- X. Song, T. Liu, K. Gu, Z. Luo and M. Zhang, Highly selective and ultra-sensitive gas sensor based on Fe2O3/Ti3C2Tx MXene heterostructure for ppb-level n-butanol detection, J. Alloys Compd., 2024, 976, 173153 CrossRef CAS.
- B. Sun, F. Qin, L. Jiang, J. Gao, Z. Liu, J. Wang, Y. Zhang, J. Fan, K. Kan and K. Shi, Room-temperature gas sensors based on three-dimensional Co3O4/Al2O3@Ti3C2Tx MXene nanocomposite for highly sensitive NOx detection, Sens. Actuators, B, 2022, 368, 132206 CrossRef CAS.
- Z. Liu, T. He, H. Sun, B. Huang and X. Li, Layered MXene heterostructured with In2O3 nanoparticles for ammonia sensors at room temperature, Sens. Actuators, B, 2022, 365, 131918 CrossRef CAS.
- T. He, S. Sun, B. Huang and X. Li, MXene/SnS2 Heterojunction for Detecting Sub-ppm NH3 at Room Temperature, ACS Appl. Mater. Interfaces, 2023, 15, 4194–4207 CrossRef CAS.
- C. Qi, X. Yan, Y. Wang, Y. Ning, X. Yu, Y. Hou and J. Yu, Flammability limits of combustible gases at elevated temperatures and pressures: recent advances and future perspectives, Energy Fuels, 2022, 36(21), 12896–12916 CrossRef CAS.
- P. Zhang, M. He, W. Teng, F. Li, X. Qiu, K. Li and H. Wang, Ordered mesoporous materials for water pollution treatment: Adsorption and catalysis, Green Energy Environ., 2024, 9(8), 1239–1256 CrossRef CAS.
- S. Hwang, H. Gu, J. L. Young, M. A. Steiner, A. B. Laursen, R. A. Crichton, Y. W. Yeh, P. E. Batson, L. C. Feldman, M. Li, K. Wyatt, A. Safari, T. G. Deutsch, E. Garfunkel and G. C. Dismukes, TiO2/TiN Interface Enables Integration of Ni5P4 Electrocatalyst with a III-V Tandem Photoabsorber for Stable Unassisted Solar-Driven Water Splitting, ACS Energy Lett., 2024, 9, 789–797 CrossRef CAS.
- F. Guo, C. Feng, Z. Zhang, H. Wu, C. Zhang, X. Feng, S. Lin, C. Xu, B. Zhang and H. Bai, A room-temperature and ppb-level NO2 sensor based on n-CdS/p-CuO heterojunction modified with rGO nanosheets, Sens. Actuators, B, 2022, 364, 131898 CrossRef CAS.
- S. Y. Lee, J. Y. Oh, R. P. Patil, M. Kim, J. S. Jang, H. Jin, S. Kim and H. J. Lee, A general guide for adsorption of cadmium sulfide (CdS) quantum dots by successive ionic layer adsorption and reaction (SILAR) for efficient CdS-sensitized photoelectrochemical cells, Appl. Surf. Sci., 2022, 589, 152898 CrossRef CAS.
- R. Balu, A. Panneerselvam, J. R. Rajabathar, G. Devendrapandi, S. Subburaj, S. Anand, U. S. Veerasamy and S. Palani, Synergistic effect of Echinops flower-like Copper sulfide@Cadmium sulfide heterostructure for high-performance all-solid-state asymmetric supercapacitor, J. Energy Storage, 2023, 72, 108447 CrossRef.
- Y. H. Li, L. Cheng, P. F. Liu, L. Zhang, M. Y. Zu, C. W. Wang, Y. H. Jin, X. M. Cao, H. G. Yang and C. Li, Simple Cadmium Sulfide Compound with Stable 95 % Selectivity for Carbon Dioxide Electroreduction in Aqueous Medium, ChemSusChem, 2018, 9, 1421–1425 CrossRef PubMed.
- Q. Rui, J. Gao, Z. Z. Yin, J. Li, W. Cai, D. Wu and Y. Kong, A biodegradable pH and glutathione dual-triggered drug delivery system based on mesoporous silica, carboxymethyl chitosan and oxidized pullulan, Int. J. Biol. Macromol., 2023, 224, 1294–1302 CrossRef CAS PubMed.
- Q. T. H. Ta, L. T. Nhiem, D. T. Y. Oanh, N. H. Hieu and P. K. T. Nguyen, Review on progress in synthesis of CdS‐based composites and their potential applications, Vietnam J. Chem., 2024 DOI:10.1002/vjch.202400155.
- H. Bai, H. Guo, Y. Tan, J. Wang, Y. Dong, B. Liu, Z. Xie, F. Guo, D. Chen, R. Zhang and Y. Zheng, Facile synthesis of mesoporous CdS/PbS/SnO2 composites for high-selectivity H2 gas sensor, Sens. Actuators, B, 2021, 340, 129924 CrossRef CAS.
- A. Giberti, D. Casotti, G. Cruciani, B. Fabbri, A. Gaiardo, V. Guidi, C. Malagù, G. Zonta and S. Gherardi, Electrical conductivity of CdS films for gas sensing: Selectivity properties to alcoholic chains, Sens. Actuators, B, 2015, 207, 504–510 CrossRef CAS.
- J. Mao, L. Zhou, Y. Li, Y. Tao, K. Chai, Y. Shi and W. Xu, Synthesis of MoTe2 nanowire as an efficient hydrogen evolution reaction material, Mater. Lett., 2021, 290, 129471 CrossRef CAS.
- J. Mao, Q. T. H. Ta, N. N. Tri, L. Shou, S. Seo and W. Xu, 2D MoTe2 nanomesh with a large surface area and uniform pores for highly active hydrogen evolution catalysis, Appl. Mater. Today, 2023, 35, 101939 CrossRef.
- W. F. Peng, X. Sun, Y. Ding, P. Liu, Q. Yao and Z. H. Lu, Enhanced Activity of WOx-Promoted PdNi Nanoclusters Confined by Amino-Modified KIT-6 for Dehydrogenation of Additive-Free Formic Acid, ACS Sustain. Chem. Eng., 2023, 11(5), 1898–1908 CrossRef CAS.
- S. Yang, G. Lei, H. Xu, Z. Lan, Z. Wang and H. Gu, Metal oxide based heterojunctions for gas sensors: A review, Nanomaterials, 2021, 11, 1026 CrossRef CAS.
- T. Wang, X. Kou, L. Zhao, P. Sun, C. Liu, Y. Wang, K. Shimanoe, N. Yamazoe and G. Lu, Flower-like ZnO hollow microspheres loaded with CdO nanoparticles as high performance sensing material for gas sensors, Sens. Actuators, B, 2017, 250, 692–702 CrossRef CAS.
- T. Wang, P. Sun, F. Liu and G. Lu, Revealing the correlation between gas selectivity and semiconductor energy band structure derived from off-stoichiometric spinel CdGa2O4, Sens. Actuators, B, 2022, 352, 131039 CrossRef CAS.
- T. Y. Huang, Z. Yang, S. Y. Yang, Z. H. Dai, Y. J. Liu, J. H. Liao, G. Y. Zhong, Z. J. Xie, Y. P. Fang and S. S. Zhang, Construction of 2D/2D Ti3C2Tx MXene/CdS heterojunction with photothermal effect for efficient photocatalytic hydrogen production, J. Mater. Sci. Technol., 2024, 171, 1–9 CrossRef CAS.
- J.-Y. Li, Y.-H. Li, F. Zhang, Z.-R. Tang and Y.-J. Xu, Visible-light-driven integrated organic synthesis and hydrogen evolution over 1D/2D CdS-Ti3C2Tx MXene composites, Appl. Catal., B, 2020, 269, 118783 CrossRef CAS.
- Z. Yang and J. Wang, CdS Nanoparticles Supported on Ti3C2Tx MXene for the Efficient Photocatalytic Production of H2O2: Implications for the Photocatalytic Degradation of Emerging Contaminants, ACS Appl. Nano Mater., 2023, 6, 558–572 CrossRef CAS.
- L. Zhu, C. Feng, F. Li, D. Zhang, C. Li, Y. Wang, Y. Lin, S. Ruan and Z. Chen, Excellent gas sensing and optical properties of single-crystalline cadmium sulfide nanowires, RSC Adv., 2014, 4, 61691–61697 RSC.
- X. Geng, J. You and C. Zhang, Microstructure and sensing properties of CdS-ZnO1−x coatings deposited by liquid plasma spray and treated with hydrogen peroxide solution for nitrogen dioxide detection at room temperature, J. Alloys Compd., 2016, 687, 286–293 CrossRef CAS.
- Y. Yang, L. Shi, Z. Cao, R. Wang and J. Sun, Strain Sensors with a High Sensitivity and a Wide Sensing Range Based on a Ti3C2Tx (MXene) Nanoparticle–Nanosheet Hybrid Network, Adv. Funct. Mater., 2019, 29, 201807882 Search PubMed.
- J. Liang, Y. Han, H. Chen, Y. Zhang and X. Gao, Layered Ti3C2Tx MXene heterostructured with V2O5 nanoparticles for enhanced room temperature ammonia sensing, J. Alloys Compd., 2024, 1010, 177798 CrossRef.
- A. Karkhaneh and M. Marandi, Facile modified chemical precipitation approach for the synthesis of CuIn1−XS2 nanocrystals and their application in high-performance CuIn1−XS2/CdS co-quantum dots sensitized solar cells, J. Mater. Sci.:Mater. Electron., 2024, 35, 847 CrossRef CAS.
- G. Burashev, B. Tatykayev, M. Baláž, N. Khan, A. Jumagazieva, Z. Iskakbayeva, A. Seysembekova, S. Tugelbay, N. Turgynbay and A. Niyazbayeva, The superiority of the photocatalytic and antibacterial performance of mechanochemically synthesized CdS nanoparticles over solvothermal-prepared ones, Semicond. Sci. Technol., 2024, 39, 045006 CrossRef CAS.
- Z. Wei, W. Mao, J. Liu, Y. Xiao, M. Zhu and Y. Tian, CdS nanorods decorated with ultrathin MoS 2 nanosheets for efficient visible-light photocatalytic H 2 production, J. Mater. Sci.:Mater. Electron., 2020, 31, 4574–4581 CrossRef CAS.
- Y. Chen, T. Shan, L. Liu, L. Shen, H. Xue and M.-Q. Yang, Construction of embedded CdS nanosheets@ PEA2PbBr4 nanoplate pn heterojunction photocatalysts with spatial charge transfer for enhanced benzylic C (sp3)-H bond oxidation, Chem. Eng. J., 2024, 480, 148099 CrossRef CAS.
- Y. Liu, H. Zhang, D. Zhu, J. Duan, A. C. Miruka, L. Tang and L. Cai, Enhanced degradation of tetracycline by TiO2@ MXene with peroxydisulfate under visible light irradiation, Sep. Purif. Technol., 2024, 343, 127122 CrossRef CAS.
- Y. Vasseghian, V. D. Doan, T. T. T. Nguyen, T.-T. T. Vo, H. H. Do, K. B. Vu, Q. H. Vu, T. Dai Lam and V. A. Tran, Flexible and high-sensitivity sensor based on Ti3C2–MoS2 MXene composite for the detection of toxic gases, Chemosphere, 2022, 291, 133025 CrossRef PubMed.
- G. Huang, S. Li, L. Liu, L. Zhu and Q. Wang, Ti3C2 MXene-modified Bi2WO6 nanoplates for efficient photodegradation of volatile organic compounds, Appl. Surf. Sci., 2020, 503, 144183 CrossRef CAS.
- T.-L. T. Le, T.-H. T. Le, H. T. Huu, D. T. T. Nu, L. N. Thi, T. T. T. Phan, K. N. Van, P. H. Nguyen and V. Vo, Designing S-scheme of TiO2@ g-C3N4/graphene Heterojunction with enhanced photocatalytic activity under visible light: Experiments and DFT calculations, J. Alloys Compd., 2024, 174716 Search PubMed.
- Z. Wang, T. Han, T. Fei, S. Liu and T. Zhang, Investigation of microstructure effect on NO2 sensors based on SnO2 nanoparticles/reduced graphene oxide hybrids, ACS Appl. Mater. Interfaces, 2018, 10, 41773–41783 CrossRef CAS PubMed.
- P. Dwivedi, S. Das and S. Dhanekar, Wafer-Scale Synthesized MoS2/Porous Silicon Nanostructures for Efficient and Selective Ethanol Sensing at Room Temperature, ACS Appl. Mater. Interfaces, 2017, 9, 21017–21024 CrossRef CAS PubMed.
- L. Liu, S. Shu, G. Zhang and S. Liu, Highly Selective Sensing of C2H6O, HCHO, and C3H6O Gases by Controlling SnO2 Nanoparticle Vacancies, ACS Appl. Nano Mater., 2018, 1, 31–37 CrossRef CAS.
- J. Walker, P. Karnati, S. A. Akbar and P. A. Morris, Selectivity mechanisms in resistive-type metal oxide heterostructural gas sensors, Sens. Actuators, B, 2022, 355, 131242 CrossRef CAS.
- K. Y. Shin, A. Mirzaei, W. Oum, E. B. Kim, H. M. Kim, S. Moon, S. S. Kim and H. W. Kim, Enhanced NO2 gas response of ZnO–Ti3C2Tx MXene nanocomposites by microwave irradiation, Sens. Actuators, B, 2024, 409, 135605 CrossRef CAS.
- G. Wu, H. Du, K. Pakravan, W. Kim, Y. L. Cha, M. Beidaghi, X. Zhang, X. Pan and D.-J. Kim, Wearable room-temperature ethanol sensor based on Ti3C2Tx/Polypyrrole functionalized face mask for drunk driving monitoring, Carbon, 2024, 216, 118565 CrossRef CAS.
- T. Xu, X. Wei, F. Zhao, G. Wang, Z. Deng, J. Zhao and J. Yao, One-step synthesis of CdS hierarchical microspheres and its ethanol sensing property, Appl. Surf. Sci., 2022, 595, 153545 CrossRef CAS.
- N. Zhang, X. Ma, Y. Yin, Y. Chen, C. Li, J. Yin and S. Ruan, Synthesis of CuO-CdS composite nanowires and their ultrasensitive ethanol sensing properties, Inorg. Chem. Front., 2019, 6, 238–247 RSC.
- S. Chakraborty and M. Pal, Improved ethanol sensing behaviour of cadmium sulphide nanoflakes: Beneficial effect of morphology, Sens. Actuators, B, 2017, 242, 1155–1164 CrossRef CAS.
- S. Sun, M. Wang, X. Chang, Y. Jiang, D. Zhang, D. Wang, Y. Zhang and Y. Lei, W18O49/Ti3C2Tx Mxene nanocomposites for highly sensitive acetone gas sensor with low detection limit, Sens. Actuators, B, 2020, 304, 127274 CrossRef CAS.
- S. Zhang, P. Song, J. Sun, Y. Ding and Q. Wang, MoO3/Ti3C2Tx MXene nanocomposites with rapid response for enhanced ethanol-sensing at a low temperature, Sens. Actuators, B, 2023, 378, 133216 CrossRef CAS.
- X. Bu, F. Ma, Q. Wu, H. Wu, Y. Yuan, L. Hu, C. Han, X. Wang, W. Liu and X. Li, Metal-organic frameworks-derived Co3O4/Ti3C2Tx Mxene nanocomposites for high performance ethanol sensing, Sens. Actuators, B, 2022, 369, 132232 CrossRef CAS.
- T. Y. Huang, Z. Yang, S. Y. Yang, Z. H. Dai, Y. J. Liu, J. H. Liao and S. S. Zhang, Construction of 2D/2D Ti3C2Tx MXene/CdS heterojunction with photothermal effect for efficient photocatalytic hydrogen production, J. Mater. Sci. Technol., 2024, 171, 1–9 CrossRef CAS.
- M. Sbeah, A. Zyoud, M. Ishteiwi, M. Hajjyahya, N. Al Armouzi, N. Qamhieh, A. R. Hajamohideen, S. Zyoud, H. H. S. Helal and H. Bsharat, Assessment of flexible pristine CdS film electrodes in photoelectrochemical light-to-electricity conversions, Mater. Chem. Phys., 2023, 293, 126967 CrossRef CAS.
- C. Yue, Z. Zhang, Z. Liu, Y. Mu, Z. Yang, D. Dastan, X.-T. Yin and X. Ma, High-performance ethanol gas sensor with fast response/recovery rate based on the construction of SnO2-CdS heterojunction, J. Alloys Compd., 2024, 981, 173742 CrossRef CAS.
- F. Chen, C. Hong, J. Jiang, Z. Zhang and Q. Zhou, A comparative DFT study on the adsorption properties of lithium batteries thermal runaway gases CO, CO2, CH4 and C2H4 on pristine and Au doped CdS monolayer, Surf. Interfaces, 2024, 46, 104200 CrossRef CAS.
- Y. He, H. Hu, J. Wang, X. Wang, M. Sun, C. Tian and C. Deng, Fabrication of multi-scale CdS/ZnO heteroarchitectures with boosted dual photocatalytic activities for hydrogen generation and organic dye degradation under solar light, Mater. Res. Bull., 2023, 162, 112180 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4na00927d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |