Investigating the mechanism of Qifu Yin in ameliorating memory disorders through pseudo-targeted lipidomics†
Fuxia
Zhao
a,
Jing
Wang
a,
Minjun
Wu
a,
Jiaqi
Fan
a,
Shiqi
Liu
b,
Fanying
Deng
a,
Shihui
Wang
a,
Yangang
Cheng
a and
Yan
Wang
 *a
*a
aInstitute of Pharmaceutical and Food Engineering, Shanxi University of Chinese Medicine, 121 Daxue Road, Yuci District, Jinzhong, 030619, China. E-mail: wangyan81823@aliyun.com; wy180119@sxtcm.edu.cn; Tel: +86 13403692396
bSchools of Basic Medical Sciences, Heilongjiang University of Chinese Medicine, Harbin 150040, China
First published on 15th November 2024
Abstract
Memory disorder (MD) is a neurodegenerative disease with an increasing incidence rate that adversely affects the quality of life of patients. Qifu Yin (QFY), a classic traditional Chinese medicine formula used for treating dementia, is known for its neuroprotective properties, although its mechanism of action requires further exploration. In this study, D-galactose combined with aluminum chloride was used to establish an MD rat model, and behavior, histopathology, and related indicators were used to evaluate the pharmacodynamics of the formula in the rats. Furthermore, brain tissues were examined using pseudo-targeted lipidomics analysis, and candidate ion pairs were screened through mass spectrometry using UPLC-Q/Orbitrap HRMS. An sMRM detection method for candidate ion pairs was developed using UHPLC-Q-TRAP-MS/MS and validated. This approach was applied to the lipidomics study of QFY in improving MD. Differential metabolites screened through pseudo-targeted lipidomics were analyzed by employing network pharmacology, and the pathway was verified to explore their mechanism of action. Results demonstrated that QFY could improve memory impairment. A total of 1052 ion pairs were constructed in the pseudo-targeted lipidomics analysis, identifying 33 differential metabolites and 5 metabolic pathways. Furthermore, 31 differential metabolites in MD rats treated with QFY were significantly reversed. Immunohistochemical analysis showed that QFY could inhibit the expression of inflammatory factors. Network pharmacological analysis showed that the calcium signaling pathway was the main signaling pathway, and QFY could significantly reverse the expression levels of mRNA and protein. Thus, QFY can improve memory impairment in rats, which may be related to the regulation of oxidative stress, lipid metabolism disorder and the calcium signaling pathway.
1. Introduction
Memory disorder (MD) is a mild cognitive impairment caused by conditions such as Alzheimer's disease (AD), depression, vascular dementia (VD), cerebral ischemia, and Parkinson's syndrome (PD). The etiology of MD is not fully understood, and numerous hypotheses, including cholinergic system abnormalities, neuronal apoptosis, and oxidative stress, have been proposed. Currently, cholinesterase inhibitors, cerebral metabolism stimulants, neuronal protectants, calcium ion antagonists, and free radical scavengers are commonly used in clinical practice. Although these medications can improve symptoms, they have significant side effects and may lead to drug resistance with long-term use.1 Traditional Chinese medicine (TCM) categorizes MD under the conditions of “forgetfulness,” “stupidity,” and “easy to forget,” attributing its pathogenesis to deficiencies in liver and kidney essence and blood, loss of nourishment in the brain marrow, and dysfunction of the spirit. The disease location is considered to be in the brain, which is closely related to the heart, liver, spleen, and kidneys.2Qifu Yin (QFY), originating from Zhang Jingyue's “Jingyue Quanshu” during the Ming Dynasty, is composed of seven medicinal herbs, namely, ginseng, Angelica sinensis, Rehmannia glutinosa, Atractylodes macrocephala, Polygala tenuifolia, Ziziphus jujuba, and Glycyrrhiza uralensis, and primarily employed to treat dementia because of the deficiency of Qi in the heart, spleen, and kidneys and deficient marrow sea, manifesting as memory decline.3 Modern research indicates that ginsenosides can improve neuronal damage, learning, and memory deficits induced by D-galactose;4 catalpol of Rehmannia glutinosa has neuroprotective effects; and paeoniflorin can ameliorate oxidative damage in the brain.5Angelica, Atractylodes, Polygala, Ziziphus, and Glycyrrhiza have all been shown to improve learning and memory abilities.6–10 QFY also enhanced the learning and memory capabilities in mice with memory consolidation disorders induced by sodium nitrite.11Ginseng, Rehmannia, and Polygala are recorded in the pharmacopeias of Japan, Europe, and Korea, and are used in Japanese medical research to enhance memory.12–14 In Korean medical research, Rehmannia glutinosa has been shown to improve memory and cognitive disorders in rats.15
Lipidomics is the systematic analysis and identification of lipids and their interactions within organisms, tissues, or cells to understand lipid structures and properties, revealing the relationship between lipid metabolism and organ, physiological, and pathological processes. Studies have found that lipid metabolism is associated with neurodegenerative diseases such as AD, VD, cerebral ischemia, and cognitive impairments.16–18 Cerebral ischemia can cause significant increases in the expression of acidic, neutral sphingomyelinases, and phospholipase A2 mRNA, and significant decreases in ceramide synthase 1 and 2 mRNA expression, leading to lipid metabolism disorders.19 VD can downregulate the expression of fatty acid-binding protein 5, peroxisome proliferator-activated receptors, and lipoprotein lipase, causing lipid metabolism disorders.20 AD can increase the levels of ceramides and decrease the levels of phosphatidylinositol in the brains of patients, leading to lipid metabolism disorders.21
This study focused on QFY in an MD rat model established with D-galactose and aluminum chloride, analyzing the therapeutic effects of QFY on MD through the behavioral activities of MD rats, pathological features of their brain tissue, and expression levels of oxidative stress and calcium signaling pathway-related factors in their brain tissue. UPLC-Q/Orbitrap HRMS was used to screen candidate ion pair information, and UHPLC-Q-TRAP-MS/MS was employed to establish a multiple reaction monitoring (MRM) detection method for candidate ion pairs, integrating the established MRM detection methods into sMRM and validating the methodology. This pseudo-targeted approach was applied to the lipidomics study of QFY in improving MD, analyzing differential metabolites in rat brain tissues, and conducting metabolic pathway analysis. The differential metabolites screened by the pseudo-targeted lipidomics were analyzed by network pharmacology, and the network pharmacology pathway was verified by the RT-PCR and western blot techniques, which provided a theoretical basis for comprehensively revealing the mechanism of QFY in improving MD.
2. Materials and methods
2.1. Materials and reagents
Ginseng (batch number: QC20211231), Angelica sinensis (batch number: 20211001), Rehmannia glutinosa (batch number: 210402CP102), Atractylodes macrocephala (batch number: 220302CP071), Ziziphus jujuba (batch number: 220501CP509), Polygala tenuifolia (batch number: 210301CP919), and Glycyrrhiza uralensis (batch number: 2011036)were purchased from Hebei Hanchao Tang Pharmaceutical Co., Ltd (Hebei, China), all authenticated as genuine by Professor Pei Xiangping from Shanxi University of Chinese Medicine, meeting the standards of the 2020 Chinese Pharmacopoeia. D-Galactose (batch number: C12049309) was purchased from Shanghai McLean Biochemical Technology Co., Ltd (Shanghai, China). Aluminum chloride (batch number: 2016122301) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd (Tianjin, China). Ginkgo biloba leaves (batch number: Z20027940) were purchased from Guangxi Liangmianzhen Yikang Pharmaceutical Co., Ltd (Guangxi, China). RNA extraction solution (batch number: G3013) was purchased from Wuhan Servicebio Technology Co., Ltd (Wuhan, China).Eicosapentaenoic acid (EPA) (batch number: 10417-94-4), linoleic acid (LOA) (60-33-3), gamma-linolenic acid (GLA) (506-26-3), alpha-linolenic acid (LA) (463-40-1), arachidonic acid (AA) (506-32-1) and docosahexaenoic acid (DHA) (6217-54-5) were purchased from Anhui Zesheng Technology Co., Ltd (Anhui, China). Oleic acid (112-80-1), Cer (d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18
1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) (5966-28-9), and PC (16
1(9Z)) (5966-28-9), and PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) (26853-31-6) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd (Shanghai, China).
1(9Z)) (26853-31-6) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd (Shanghai, China).
Rat cAMP response element-binding protein (CREB), rat calmodulin (CAM), and rat calcium/calmodulin-dependent protein kinase II (CAMKII) kits (batch numbers: F21253-A, F3130-A, F3112-A, respectively) were purchased from Vankel Biotechnology Co., Ltd (Shanghai, China). The rat calcium ion (Ca2+) kit (batch number: YJ02254) was purchased from Shanghai Source Jujube Biological Technology Center and BCA kit (batch number: PC0020) from Solarbio Science & Technology Co., Ltd (Beijing, China). Superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and catalase (CAT) kits (batch numbers: A001-3-2, A003-1-2, A005-1-2, and A007-1-1, respectively) were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). β-Actin (batch number: GB15001) was purchased from Wuhan Servicebio Technology Co., Ltd (Wuhan, China). Recombinant Anti-NMDAR1 antibody, and anti-BDNF antibody (batch numbers: ab274377 and ab108319, respectively) were from Abcam Company (Cambridge, UK). IL-6, TNF-α, and COX-2 (batch numbers: bs-0782R, bs-10802R and bs-0732R, respectively) were purchased from Beijing Boaosen Biotechnology Co., Ltd. DAB chromogenic solution (batch number: KGB4101) was purchased from Keygen.
A WMT-100 Morris water maze and OFT-100 open field test apparatus were purchased from Chengdu Techman Software Co., Ltd (Chengdu, China). An Axioscope 5 Zeiss fluorescence microscope was purchased from German Zeiss Co., Ltd (Oberkochen, Germany). An ACQUITY UPLC HSS T3 (2.1 × 100 mm, 1.8 μm) chromatography column was purchased from Waters (Massachusetts, USA). An ultra-high performance liquid chromatography-quadrupole/electrostatic field Orbitrap high-resolution mass spectrometer was purchased from Thermo Scientific Thermo Scientific. An ultra-high performance liquid chromatography-triple quadrupole/linear ion trap mass spectrometer was purchased from Shanghai Applied Protein Technology Co., Ltd (Shanghai, China). An ACQUITY UPLC BEH C8 (2.1 × 100 mm, 1.7 μm) chromatography column was purchased from Waters (Massachusetts, USA).
2.2. Experimental animals
Thirty-six SPF Sprague Dawley (SD) rats (weighing 200 ± 20 g, 18 males and 18 females) were purchased from Speyford (Beijing) Biotechnology Co., Ltd, with a certificate of conformity number SCXK (Beijing) 2019-0010. This experiment was approved by the Animal Ethics Committee of Shanxi University of Chinese Medicine (Animal Ethics Number: AWE202209144), and all experiments were conducted according to animal research guidelines. This study was approved by the Institution for the Experiment of Living Subjects. It followed the National Standard of the People's Republic of China on Experimental Animals and Welfare (GB/T 42011-2022). The attachments are as follows.2.3. Preparation of medication
Qifu Yin (consisting of 6 g Ginseng, 9 g Rehmannia glutinosa, 9 g Angelica sinensis, 5 g Atractylodes macrocephala, 3 g roasted Glycyrrhiza uralensis, 6 g Ziziphus jujuba, and 5 g Polygala tenuifolia) was prepared by weighing seven herbs in proportion, soaking in ten times the volume of distilled water for 0.5 h, followed by boiling for 1 h, filtering, and then boiling a second time. The filtrate was collected and concentrated to 2 g mL−1. According to the concentration of high, medium and low test drugs, as follows: high dose test drug (1.72 g mL−1), medium-dose test drug (0.86 g mL−1), low dose test drug (0.43 g mL−1).2.4. Animal grouping, modeling, and medication administration
Thirty-six SPF-grade SD male rats, after one week of acclimatization, were randomly divided into six groups, as follows: control (Con.), model (Mon.), Ginkgo biloba leaf group (FG, 0.15 g kg−1), Qifu Yin low dose (QFY-L, 2.22 g kg−1), medium dose (QFY-M, 4.44 g kg−1), and high dose (QFY-H, 8.88 g kg−1), with six rats in each group. The doses were calculated based on the human equivalent dose for a 60 kg adult and the conversion coefficient for rats of 6.2. All groups, except the control, were administered D-galactose (400 mg kg−1, intraperitoneal injection) and aluminum chloride (200 mg kg−1, orally) daily to induce the model,22–24 while the control group received an equivalent volume of saline and distilled water, respectively, for 60 consecutive days. Medication treatment began on the 30th day of modeling, with administration once daily (as shown in Fig. 1A).2.5. Behavioral experiments
According to the literature,25,26 the Morris water maze (MWM) and open field tests were conducted on days 55–60.2.6. Histopathological analysis of brain tissue
The rapidly extracted brain tissues were separated on ice, with the hippocampal tissues frozen in liquid nitrogen, and then stored at −80 °C. The remaining hippocampal tissues were fixed in 4% paraformaldehyde and prepared using the HE staining method for fixation, dehydration, embedding, and staining. Microscopic observation under a microscope was conducted to examine the morphological changes in the neurons of the rat hippocampus. The brain tissues were also fixed, sectioned, and subjected to Nissl staining, dehydrated in the routine manner, stained with 1% toluidine blue for 40 min, and then examined and photographed under a microscope.2.7. Analysis of oxidative stress and Ca2+ pathway factor expression in brain tissue
Brain tissues from rats were prepared into a 20% homogenate, centrifuged at 3500 rpm for 10 min at 4 °C, and the supernatant was collected. The contents of CAT, SOD, GSH-Px, MDA, CAM, CAMKII, CREB, and Ca2+ in the rat brain tissues were determined according to the instructions of the kit.2.8. Lipidomic analysis of brain tissue
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18
1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)), and PC (16
1(9Z)), and PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) were dissolved in methanol in 5 mL volumetric flasks to concentrations of 8.30 mg mL−1, 9.08 mg mL−1, 8.73 mg mL−1, 0.33 mg mL−1, 72.00 mg mL−1, 85.69 mg mL−1, 89.00 mg mL−1, 0.12 mg mL−1, and 92.00 μg mL−1, respectively.
1(9Z)) were dissolved in methanol in 5 mL volumetric flasks to concentrations of 8.30 mg mL−1, 9.08 mg mL−1, 8.73 mg mL−1, 0.33 mg mL−1, 72.00 mg mL−1, 85.69 mg mL−1, 89.00 mg mL−1, 0.12 mg mL−1, and 92.00 μg mL−1, respectively.
The working solutions of the references were prepared as follows: precise amounts of alpha-linolenic acid, linoleic acid, and oleic acid stock solutions (10 μL each) were taken and made up to volume with methanol in a 10 mL volumetric flask (resulting concentrations were 72.00 μg mL−1, 85.70 μg mL−1, and 89.00 μg mL−1, respectively). The solutions were mixed well and stored in sample vials. Similarly, 100 μL each of arachidonic acid, eicosapentaenoic acid, and gamma-linolenic acid stock solutions were made up to volume with methanol in 10 mL volumetric flasks (concentrations were 82.92 μg mL−1, 90.80 μg mL−1, and 87.30 μg mL−1, respectively). The solutions were mixed well and stored in sample vials. Moreover, 500 μL each of docosahexaenoic acid, Cer (d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18
1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)), and PC (16
1(9Z)), and PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) stock solutions were made up to volume in 10 mL volumetric flasks with methanol (concentrations were 16.50 μg mL−1, 6.00 μg mL−1, and 4.60 μg mL−1, respectively). The solutions were mixed well and stored for further use.
1(9Z)) stock solutions were made up to volume in 10 mL volumetric flasks with methanol (concentrations were 16.50 μg mL−1, 6.00 μg mL−1, and 4.60 μg mL−1, respectively). The solutions were mixed well and stored for further use.
Preparation of the mixed standard solution: 50 μL of each single standard solution (250 μL) for docosahexaenoic acid, 500 μL each for Cer (d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18
1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)), and PC (16
1(9Z)), and PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) was taken in a 4 mL centrifuge tube, added to 950 μL of methanol, mixed well to obtain a total working solution of 2500 μL. The concentrations of alpha-linolenic acid, linoleic acid, oleic acid, arachidonic acid, eicosapentaenoic acid, gamma-linolenic acid, docosahexaenoic acid, Cer (d18
1(9Z)) was taken in a 4 mL centrifuge tube, added to 950 μL of methanol, mixed well to obtain a total working solution of 2500 μL. The concentrations of alpha-linolenic acid, linoleic acid, oleic acid, arachidonic acid, eicosapentaenoic acid, gamma-linolenic acid, docosahexaenoic acid, Cer (d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18
1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)), and PC (16
1(9Z)), and PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) were adjusted to 1.44 μg mL−1, 1.71 μg mL−1, 1.78 μg mL−1, 1.66 μg mL−1, 1.82 μg mL−1, 1.75 μg mL−1, 1.65 μg mL−1, 1.20 μg mL−1, and 0.92 μg mL−1, respectively. This total working solution, with a concentration of 1000C, was sequentially diluted to obtain linear concentration working solutions at 800C, 600C, 200C, 50C, 10C, 2C, 0.4C, and 0.04C levels, with 50C, 200C, and 600C serving as low, medium, and high concentration levels, respectively.
1(9Z)) were adjusted to 1.44 μg mL−1, 1.71 μg mL−1, 1.78 μg mL−1, 1.66 μg mL−1, 1.82 μg mL−1, 1.75 μg mL−1, 1.65 μg mL−1, 1.20 μg mL−1, and 0.92 μg mL−1, respectively. This total working solution, with a concentration of 1000C, was sequentially diluted to obtain linear concentration working solutions at 800C, 600C, 200C, 50C, 10C, 2C, 0.4C, and 0.04C levels, with 50C, 200C, and 600C serving as low, medium, and high concentration levels, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 15 min. The upper organic phase was collected and dried with nitrogen. During the mass spectrometry analysis, 200 μL of mass spectrometry grade methanol solution was added for reconstitution, followed by vortexing and centrifugation at 10
000 rpm and 4 °C for 15 min. The upper organic phase was collected and dried with nitrogen. During the mass spectrometry analysis, 200 μL of mass spectrometry grade methanol solution was added for reconstitution, followed by vortexing and centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and 4 °C for 15 min. The supernatant was taken and subjected to liquid chromatography–mass spectrometry analysis.
000 rpm and 4 °C for 15 min. The supernatant was taken and subjected to liquid chromatography–mass spectrometry analysis.
From the supernatants obtained, 10 μL of each test sample was taken and mixed well to prepare the quality control (QC) sample. One QC sample was inserted after every set of samples to assess the instrument stability during the analysis.
Electrospray ionization-mass spectrometry (ESI-MS) was performed. The positive and negative ions were scanned together. The following spectrometry conditions were applied: a spray voltage of 3.2 kV; flow rate of sheath gas, 40 arb; auxiliary gas flow rate, 5 arb; capillary temperature, 320 °C; auxiliary heating temperature, 350 °C; resolution for the S-lens, 50; scanning range, 100–1000 m/z; and full-width-at-half-maximum resolution (FWHM) of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.
000.
The electrospray ionization (ESI) in positive and negative ion mode scanning, MRM scanning mode, and ion source parameter settings were as follows: ion source temperature 550 °C; ionization voltage 5500 V (positive ion mode), −4500 V (negative ion mode); ion sources Gas 1, Gas 2, and CUR were 50 psi, 55 psi, and 35 psi, respectively. CAD was 7; the DP was 100 V and the collision energy was 50 V.
Principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA) were conducted in SIMCA14.1 software to identify outliers and differentiate metabolites based on the criteria of VIP > 1, P < 0.05, and fold change (FC) > 1.2 or <0.83 criteria. The metabolic pathway analysis was performed on the MetaboAnalyst 6.0 platform (https://www.metaboanalyst.ca/).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200, through DAB color development, hematoxylin counterstaining, transparency, and sealing, and observation under a microscope, using Image J for image analysis.
200, through DAB color development, hematoxylin counterstaining, transparency, and sealing, and observation under a microscope, using Image J for image analysis.
2.9. Network pharmacology analysis
The differential metabolites with a significant callback trend obtained from lipidomics were predicted in the with potential targets predicted using SwissTargetPrediction (https://www.swisstargetprediction.ch/) and SEA database (https://sea.bkslab.org/) to intersect with the “Memory disorder” targets and KEGG pathway enrichment analysis.2.10. Validation by RT-PCR and western blot
Frozen hippocampal tissue was homogenized with RNA extract and centrifuged at low temperature for RNA extraction. The RNA concentration and purity were determined using a NanoDrop 2000, followed by reverse transcription. The reaction conditions were as follows: 95 °C for 30 s pre-denaturation, 95 °C for 15 s denaturation, and 60 °C for 30 s annealing/extension, for 40 cycles. The relative expression levels of BDNF and NMDAR1 mRNA were calculated using the RQ = 2−ΔΔCT method with GADPH as the internal reference. The primer sequences are shown in Table 1.| Primer name | Primer sequences (5′–3′) | Segment size (bp) |
|---|---|---|
| GAPDH | Upstream CTGGAGAAACCTGCCAAGTATG | 138 |
| Downstream GGTGGAAGAATGGGAGTTGCT | ||
| BDNF | Upstream GTGTGACAGTATTAGCGAGTGGG | 221 |
| Downstream ACGATTGGGTAGTTCGGCATT | ||
| NMDAR1 | Upstream AATGCTCCTGCAACCCTCACTT | 209 |
| Downstream GGCTCTGCTCTACCACTCTTTCTAT |
The total protein from the rat hippocampus was extracted with RIPA lysate, and the protein concentration was measured and adjusted using the BCA kit. Next, SDS-PAGE was performed, followed by transfer onto a PVDF membrane. Then, the membrane was blocked at room temperature for 15 min and washed with TBST five times for 5 min each. Diluted primary antibodies (β-actin at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1500, BDNF at 1
1500, BDNF at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000, and NMDAR1 at 1
2000, and NMDAR1 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000) were added and incubated overnight at 4 °C. After washing the membrane again with TBST five times for 5 min each, diluted secondary antibody was added and incubated at room temperature for 1 h. Following another wash, chemiluminescence was developed using ECL, and the gray value of the target band was analyzed using the Image J software.
2000) were added and incubated overnight at 4 °C. After washing the membrane again with TBST five times for 5 min each, diluted secondary antibody was added and incubated at room temperature for 1 h. Following another wash, chemiluminescence was developed using ECL, and the gray value of the target band was analyzed using the Image J software.
2.11. Statistical analysis
All data in this study are presented as mean ± standard deviation (SD). The GraphPad Prism 8.0 (San Diego, California, USA) software was used for plotting and statistical analysis. The two-tailed unpaired T-test was used for comparison between two groups. One-way analysis of variance was used for comparison between multiple groups. The Shapiro-Wilk test was used for the normality test. The Kruskal–Wallis test was used for the nonparametric test. The Brown–Forsythe test was used for the homogeneity of variance test. Welch's ANOVA test was used for heterogeneity of variance. Dunnett's test was used for the multiple comparison analysis to analyze the differences between multiple groups. A p-value < 0.05 was considered statistically significant, whereas a p-value < 0.01 was considered highly statistically significant.3. Results
3.1. Behavioral results
In the Morris water maze test, as the training days increased, the escape latency of the rats in all the groups significantly decreased, with the model group showing the longest latency. Compared with the model group, the escape latency time of the rats in the control group and the QFY group was significantly shortened (see Fig. 1B). In the spatial exploration test, compared with the model group, the retention time, movement distance and number of times of entering the platform in the quadrant of the original platform in the control group were significantly increased (P < 0.05). The medium dose of QFY significantly increased the time spent on the platform (P < 0.05); the low and high doses showed an increasing trend. The positive control group significantly increased the travel distance and platform crossings (P < 0.01), and the time spent in the target quadrant significantly increased (P < 0.05). The low and high doses of QFY significantly increased the travel distance (P < 0.01), and the number of platform crossings significantly increased in all doses of QFY (P < 0.05), as shown in Fig. 1C–F.In the open field test, compared with the model group, the rats in the control group showed strong autonomous movement and exploration ability, the static time and angular time were significantly reduced (P < 0.01), the edge time significantly increased (P < 0.01), and the exercise time showed an increasing trend. The positive control group and QFY in different doses significantly decreased the stationary and corner time (P < 0.01), and significantly increased the movement time and distance (P < 0.01), respectively. The low and high doses of QFY significantly shortened the corner time, and the medium dose significantly shortened it (P < 0.01). The medium dose of QFY significantly increased the border time (P < 0.01), with the high dose showing a significant increase (P < 0.05), and the low dose showing an increasing trend, as shown in Fig. 2A–D.
3.2. Histopathological results
The HE staining results showed that in the control group, the neurons in the hippocampal CA1 and CA3 regions were densely arranged and abundant, with an intact morphology and clear nucleoli. Compared to the control group, the model group showed a decreased number of neurons and loose arrangement, with some neurons exhibiting shrinkage and unclear boundaries. Compared to the model group, the positive control and different doses of QFY improved the neuronal cell density and increased the number of neurons, as shown in Fig. 3. | ||
| Fig. 3 HE staining results of the rats in each group (×200): (A) and (B): CA1, CA3 area (a: Con.; b: Mon.; c: FG; d: QFY-L; e: QFY-M; f: QFY-H). | ||
The Nissl staining results, as shown in Fig. 4, indicated that in the control group, the CA1 and CA3 regions had clear and abundant Nissl bodies, with the pyramidal cells being large and well-arranged, with clear boundaries, intact structure, and numerous Nissl bodies. Compared to the control group, the hippocampal tissue of the model group showed some cell rupture in CA1 and CA3, a decrease in Nissl bodies, and loss of cell nuclei, and some cells appeared shrunken and unclear in structure. Compared to the model group, the positive control and QFY doses increased the number of Nissl bodies in the CA1 and CA3 regions, with cell shrinkage recovery and intact and clear structure.
 | ||
| Fig. 4 Nissl staining results of the rats in each group (×200): (A) and (B): CA1, CA3 area (a: Con.; b: Mon.; c: FG; d: QFY-L; e: QFY-M; f: QFY-H). | ||
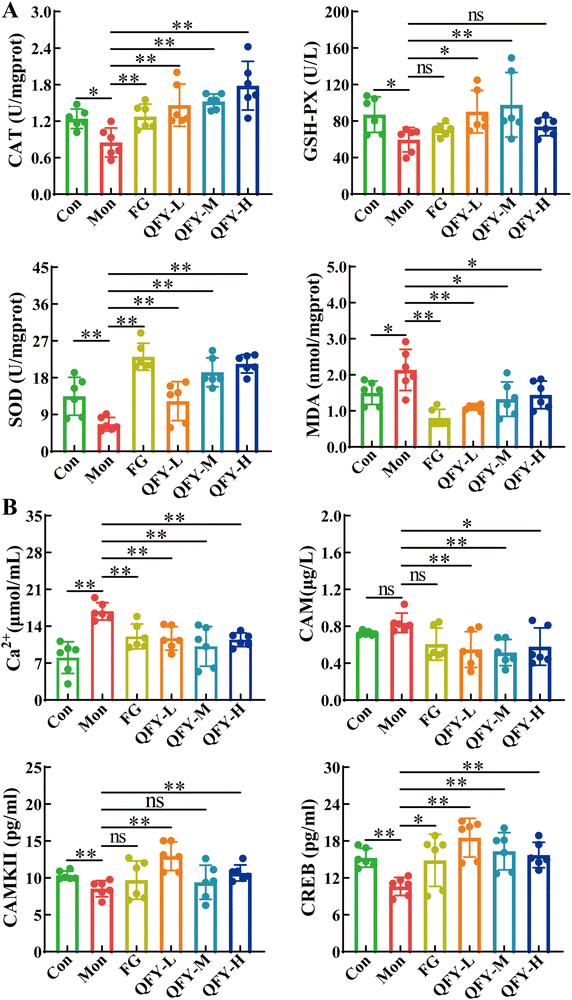
3.3. Oxidative stress and Ca2+ pathway factor expression results
As shown in Fig. 5A, compared with the model group, the levels of CAT and SOD in the brain tissue of the rats in the control group significantly increased (P < 0.01), the level of GSH-Px significantly increased in the control group (P < 0.05), and the content of MDA significantly decreased in the control group (P < 0.01). The positive control and QFY groups showed significantly increased CAT and SOD contents (P < 0.01), with the GSH-Px levels significantly increased in the low and medium doses (P < 0.05), and the positive control and high dose showing an increasing trend. The positive control and low dose groups showed a significantly reduced MDA content (P < 0.01), and the medium and high doses showed a significant reduction (P < 0.05), indicating that QFY can improve memory impairment by regulating oxidative stress.As shown in Fig. 5B, compared with the model group, the Ca2+ level in the brain tissue of the control group significantly decreased (P < 0.01), the level of CAM decreased significantly (P < 0.05), the levels of CAMKII and CREB significantly increased (P < 0.01), and the level of GSH-PX decreased. The positive control and QFY doses significantly reduced the Ca2+ levels (P < 0.01); the low and medium doses of QFY significantly increased (P < 0.01); the high dose significantly increased (P < 0.01), and the positive control showed an increasing trend of CAM levels. The low and high doses of QFY significantly increased the CAMKII levels (P < 0.01); the positive control and medium doses also showed an increasing trend. Different doses of QFY significantly increased the CREB levels (P < 0.01), indicating that QFY can significantly improve the contents of Ca2+ pathway factors in the brain tissue of rats with memory disorders, thereby affecting the pathway mechanism and play a role in improving MD.
3.4. Establishment and methodological validation of the pseudo-targeted lipidomics approach
The candidate ion pair data was obtained from secondary mass spectrometry information using UPLC-Q/Orbitrap HRMS, and a pseudo-targeted detection method including 504 and 548 ion pairs was established under positive and negative ion modes in UHPLC-Q-TRAP-MS/MS.For the feasibility validation of the UHPLC-Q-TRAP-MS/MS pseudo-targeted detection method, a series of methodological validations was conducted using brain tissue QC samples spiked with nine standards including arachidonic acid, eicosapentaenoic acid, gamma-linolenic acid, docosahexaenoic acid, alpha-linolenic acid, linoleic acid, oleic acid, Cer (d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18
1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)), and PC (16
1(9Z)), and PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)).
1(9Z)).
| Name | Q1/Da | Q3/Da | tR/min | Mode | Linearity | LOD (pg mL−1) | LOQ (pg mL−1) | Recovery (%) | Precision (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range (ng mL−1) | r 2 | High | Medium | Low | High | Medium | Low | |||||||
| Linolenic acid | 277.2 | 277.2 | 4.25 | Negative | 0.66–1440 | 0.9971 | 94.8 | 316.2 | 103.08 | 96.29 | 103.22 | 5.71 | 6.99 | 8.71 |
| Linoleic acid | 279.2 | 279.2 | 4.38 | Negative | 0.07–1710 | 0.9914 | 87.4 | 291.1 | 97.89 | 88.11 | 119.03 | 9.59 | 8.95 | 10.71 |
| Eicosapentaenoic acid | 301.2 | 301.2 | 4.24 | Negative | 0.07–1820 | 0.9966 | 123.4 | 411.3 | 105.11 | 96.10 | 103.48 | 5.53 | 7.30 | 7.29 |
| Arachidonic acid | 303.2 | 303.2 | 4.4 | Negative | 0.07–1660 | 0.9929 | 221.4 | 409.1 | 111.23 | 80.70 | 118.38 | 6.27 | 4.80 | 7.31 |
| Oleic acid | 281.2 | 281.2 | 4.61 | Negative | 0.07–1780 | 0.9972 | 7.12 | 23.73 | 85.60 | 81.10 | 99.06 | 7.97 | 9.72 | 10.64 |
| Docosahexaenoic Acid | 327.2 | 327.2 | 3.69 | Negative | 0.07–1650 | 0.9904 | 57.89 | 192.9 | 105.79 | 99.59 | 119.18 | 6.48 | 7.68 | 7.68 |
| γ-Linoleic acid | 277.2 | 277.2 | 4.24 | Negative | 0.08–1750 | 0.9961 | 81.36 | 271.2 | 106.51 | 94.18 | 105.80 | 5.38 | 7.79 | 5.14 |
PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18 0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) 1(9Z)) |
818.6 | 281.2 | 6.56 | Negative | 0.04–920 | 0.9901 | 8.80 | 29.3 | 108.12 | 98.94 | 92.48 | 4.57 | 3.35 | 10.02 |
Cer (d18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/18 1/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1(9Z)) 1(9Z)) |
564.53 | 546.52 | 5.95 | Positive | 0.05–1220 | 0.9931 | 42.11 | 153.8 | 110.80 | 85.52 | 81.33 | 11.38 | 11.59 | 12.67 |
3.5. Pseudo-targeted lipidomics study on the amelioration of memory disorders by QFY
 | ||
| Fig. 6 Extraction ion chromatogram of lipid detection in rat brain tissue samples. (A) Positive ion mode. (B) Negative ion mode. | ||
Similar lipids play a similar role. Firstly, the concentration differences in the lipids in the different groups of rats were analyzed according to lipid categories, and the results are shown in Fig. 7. Compared with the model group, the levels of LPC, LPE and PG in the brain tissue of the rats in the control group significantly decreased (P < 0.01, P < 0.05 and P < 0.05). The levels of FFA, LPC, LPI, SM, TAG and LPE in the brain tissue of the rats in the Qifu Yin group significantly decreased (P < 0.01, P < 0.01, P < 0.01, P < 0.05, P < 0.01, and P < 0.05).
To further differentiate between groups, the supervised method OPLS-DA was used, as shown in Fig. 8B and C. A clear separation was observed among all groups in both the positive and negative ion modes. In the positive ion mode, the parameters of the control group and the model group were R2X of 0.777, R2Y of 0.995, and Q2 of 0.984. The parameters of the QFY-M group and the model group were R2X of 0.618, R2Y of 0.998, and Q2 of 0.965. In the negative ion mode, R2X was 0.63, R2Y was 1, and Q2 was 0.943 in the control group and the model group, and R2X was 0.344, R2Y was 0.999, and Q2 was 0.872 in the QFY-M group, and the model group. R2X and R2Y represent the interpretation rate of the established model to X and Y, and Q2 represents the predictive ability of the model. The larger the Q2, the better the fitting and predictability of the model. Random permutation tests (n = 200) were performed on each model, and the Q2 and Y axes intersected with the negative half-axis, indicating that the model did not appear over-fitting.
| No. | ID | Rt/min | Precursor ion | Product ion | Mode | Mon./Con. | Mon./QFY | No. | ID | Rt/min | Precursor ion | Product ion | Mode | Mon./Con. | Mon./QFY |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | TAG 56:6-FA 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
9.49 | 768.7 | 467.4 | Positive | ↓** | ↑** | 18 | PA (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18 0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6.2 | 673.5 | 281.2 | Negative | ↑** | ↓** |
| 2 | TAG 56:5-FA 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
9.34 | 864.8 | 521.4 | Positive | ↓** | ↑** | 19 | PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18 0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
6.18 | 818.6 | 281.2 | Negative | ↓** | ↑* |
| 3 | TAG 58:7-FA 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
9.51 | 926.8 | 653.5 | Positive | ↓** | ↑** | 20 | PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/20 0/20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
5.78 | 838.6 | 301.2 | Negative | ↑* | ↓** |
| 4 | TAG 42:1-FA 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
8.11 | 970.8 | 623.5 | Positive | ↓** | ↑** | 21 | PC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/16 1/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5.96 | 816.6 | 281.2 | Negative | ↑** | ↓** |
| 5 | TAG 60:11-FA 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
9.21 | 924.8 | 577.5 | Positive | ↓** | ↑** | 22 | PC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/18 2/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
5.82 | 840.6 | 279.2 | Negative | ↓ | ↓** |
| 6 | TAG 56:5-FA 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
9.51 | 738.7 | 465.4 | Positive | ↓** | ↑** | 23 | LPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4.07 | 580.3 | 281.2 | Negative | ↑** | ↓** |
| 7 | TAG 54:4-FA 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
9.45 | 874.8 | 545.5 | Positive | ↓** | ↑** | 24 | LPC (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
4.11 | 630.4 | 331.3 | Negative | ↑** | ↑* |
| 8 | TAG 50:4-FA 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
8.91 | 900.8 | 573.5 | Positive | ↓** | ↑** | 25 | PE (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18 0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
5.69 | 686.5 | 279.2 | Negative | ↓** | ↑** |
| 9 | TAG 56:7-FA 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
9.08 | 844.6 | 571.3 | Positive | ↓** | ↑** | 26 | PE (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2/22 2/22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
5.88 | 786.5 | 327.2 | Negative | ↑* | ↓* |
| 10 | TAG 52:3-FA 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
9.32 | 922.8 | 627.5 | Positive | ↓** | ↑** | 27 | PE (P-16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/16 0/16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
5.30 | 674.5 | 255.2 | Negative | ↓** | ↑** |
| 11 | TAG 52:7-FA 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
9.72 | 950.8 | 603.5 | Positive | ↓** | ↑** | 28 | LPE (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
10.43 | 722.5 | 279.2 | Negative | ↑* | ↓* |
| 12 | TAG 50:5-FA 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
8.64 | 842.6 | 545.3 | Positive | ↓**− | ↑** | 29 | PG (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/20 0/20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) 5) |
7.84 | 767.5 | 301.2 | Negative | ↓* | ↑* |
| 13 | TAG 44:0-FA 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
7.29 | 926.8 | 601.5 | Positive | ↓** | ↑** | 30 | PG (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/20 0/20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
9.51 | 803.5 | 309.3 | Negative | ↓** | ↑* |
| 14 | FFA (20:1) | 4.56 | 309.2 | 309.2 | Negative | ↓** | ↑** | 31 | PG (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/22 0/22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
7.82 | 821.5 | 327.2 | Negative | ↑** | ↓** |
| 15 | FFA (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) 4) |
4.01 | 303.2 | 303.2 | Negative | ↑** | ↓** | 32 | PI (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/22 0/22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
6.36 | 853.5 | 327.2 | Negative | ↑* | ↓** |
| 16 | FFA (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) 6) |
3.95 | 327.2 | 327.2 | Negative | ↑* | ↓** | 33 | PI (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18 0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
5.12 | 833.5 | 279.2 | Negative | ↓** | ↑** |
| 17 | FFA (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :1 :1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ) ) |
5.18 | 365.3 | 365.3 | Negative | ↓** | ↑** |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), LPC (18
1), LPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and LPE (20
1), and LPE (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Arachidonic acid metabolism is affected by metabolites such as arachidonic acid.
5). Arachidonic acid metabolism is affected by metabolites such as arachidonic acid.
Box plots of four key metabolites were created in GraphPad Prism 8.0, comparing differences among the three groups, as shown in Fig. 9C–F. To further show the relationship between different metabolites, KEGG, and related enrichment analysis methods were combined to construct a potentially related metabolic pathway network, as shown in Fig. 9G, which can more intuitively reflect the protective mechanism of QFY on rats with memory impairment by regulating glycerophospholipid metabolism and arachidonic acid metabolism. Combined with Fig. 9D–G, it can be seen that the relative abundance of differential metabolites PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) in the model group increased significantly after QFY treatment, and the abundance of LPC (18
1) in the model group increased significantly after QFY treatment, and the abundance of LPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), LPE (20
1), LPE (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), and arachidonic acid decreased significantly.
5), and arachidonic acid decreased significantly.
3.6. Network pharmacology results
Target prediction was performed on the differentially expressed lipids obtained by pseudo-targeted lipidomics, 337 targets were obtained, 1446 targets were predicted for memory disorder diseases, and 88 targets were intersected by the two. The KEGG pathway enrichment analysis of 88 intersection targets showed that the Rap1 signaling pathway, HIF1 signaling pathway and calcium signaling pathway were the main signaling pathways, as shown in Fig. 11A and B.3.7. RT-PCR and western blot results
To verify the results of the network pharmacology analysis, the calcium signaling pathway, as shown in Fig. 10B, was selected for the RT-PCR and western blot experiments. As shown in Fig. 11C and D, compared to the model group, the expression level of BDNF mRNA in the control and QFY-M group significantly increased (P < 0.01), and the expression level of NMDAR1 mRNA significantly decreased (P < 0.01), suggesting that Qifu Yin may improve memory disorders by modulating the levels of BDNF and NMDAR1 mRNA.The BDNF and NMDAR1 proteins in the Ca2+ pathway were measured. As shown in Fig. 11E and F, compared to the model group, the expression of BDNF protein in the control and QFY-M group significantly increased (P < 0.01), and the expression level of NMDAR1 protein significantly decreased (P < 0.05), indicating that QFY may improve memory disorders by modulating the expression of the BDNF and NMDAR1 proteins.
3.8. Correlation analysis results of Ca2+, CAMII, CREB, CAM, and differential metabolites
A Spearman correlation analysis was conducted between the identified differential metabolites and calcium signaling pathway indicators, as shown in Fig. 11G. Red represents a positive correlation, blue represents a negative correlation, and the deeper the color, the stronger the correlation. The results show a strong correlation between the calcium signaling pathway factors and the differential metabolites identified in lipidomics. The strongest correlations were found with Ca2+ and CREB, followed by CAMII and CAM. Ca2+ and CAM were significantly positively correlated with LPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), LPE (20
1), LPE (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), and arachidonic acid, and significantly negatively correlated with PC (16
5), and arachidonic acid, and significantly negatively correlated with PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); CAMII and CREB showed significant positive correlations with LPC (18
1); CAMII and CREB showed significant positive correlations with LPC (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), LPE (20
1), LPE (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), arachidonic acid, and significant positive correlations with PC (16
5), arachidonic acid, and significant positive correlations with PC (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0/18
0/18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). This analysis indicates the significant relationship between the calcium signaling pathway and identified differential metabolites, underscoring the potential mechanisms through which QFY ameliorates memory disorders by influencing these metabolic and signaling pathways.
1). This analysis indicates the significant relationship between the calcium signaling pathway and identified differential metabolites, underscoring the potential mechanisms through which QFY ameliorates memory disorders by influencing these metabolic and signaling pathways.
4. Discussion
Learning and memory abilities are among the most distinctive physiological characteristics of higher animals and humans. Memory disorders refer to a state in which one cannot remember or recall information or skills, potentially caused by pathological, physiological, or situational reasons, leading to permanent or temporary memory impairment. This study established an MD model using D-galactose combined with aluminum chloride. D-Galactose, a reducing sugar, reacts with amino acids to form advanced glycation end-products with pro-oxidative effects and generates excessive oxygen radicals.27 The accumulation of oxygen radicals can reduce the activity of brain tissue free radical scavenging enzymes, thereby causing cognitive dysfunction in experimental animals.28 Also, aluminum, an accumulative neurotoxin, can accumulate in the body, with the brain being its main site of accumulation. It can accumulate in the hippocampal CA1 and CA3 regions through the blood–brain barrier. Thus, given that the hippocampus is the key brain area for memory regulation, aluminum accumulation in the hippocampus can cause neuronal damage, cognitive dysfunction, and trigger neurodegenerative diseases such as AD and PD.29–32This study observed rat behavior through the Morris water maze and open field experiments. The Morris water maze, a test that forces experimental animals to swim and learn to find a hidden platform in water, is mainly used to test the spatial learning and memory abilities of experimental animals.33 The open field test, an experiment studying spontaneous activity and exploratory behavior of animals, is primarily used for research on neurological diseases.34 The results showed that the learning and memory abilities of the MD rats induced by D-galactose and aluminum chloride significantly declined, while QFY treatment significantly improved their learning and memory abilities.
The hippocampus is the main brain region for learning and memory. Studies have shown that the hippocampal CA1 and CA3 regions play an important role in learning, memory, and cognitive functions.35 The pathological morphological changes revealed that in the MD rats induced by D-galactose and aluminum chloride, the cell layers, neurons, and Nissl bodies in the hippocampal CA1 and CA3 regions were reduced, with some cells showing shrinkage and unclear structure. After treatment with different doses of QFY, the number of cell layers, hippocampal neurons, and Nissl bodies increased, cell shrinkage decreased, and the structure became intact and clear, indicating that Qifu Yin plays a reparative role in hippocampal tissue damage in rats with memory disorders.
Memory disorders can lead to atrophy, degeneration, or necrosis of the hippocampus and cortex, which are processes closely related to oxidative stress.36 Oxidative stress refers to a state of imbalance between oxidation and antioxidation in the body, which can reduce the capacity to clear free radicals, produce reactive oxygen species, and thus cause oxidative damage, leading to learning and memory impairment. CAT, SOD, and GSH-Px are three antioxidant enzymes that play an important role in redox reactions.37 SOD has the effect of anti-oxidation and anti-aging. It can cope with the damage caused by oxidative stress by removing peroxides in the body.38 CAT can avoid the formation of hydroxyl anions that are harmful to cells by eliminating hydrogen peroxide.39 GSH-Px is a peroxidase-degrading enzyme that can block lipid peroxidation and reduce cell membrane peroxidation damage.40 MDA is a product of lipid peroxidation and can reflect the degree of oxidative damage.41 This study found that QFY increased the activity of the CAT, SOD, and GSH-Px antioxidant enzymes in the brain tissue of rats with memory disorders and reduce the content of lipid peroxidation product MDA, indicating that QFY effectively improved the oxidative stress damage in the brain tissue of rats with memory disorders.
The hippocampus, closely associated with memory, is rich in NMDAR1. Studies have found that excessive activation of NMDAR1 can cause Ca2+ overload, leading to increased Ca2+ levels in the intracellular environment, endoplasmic reticulum, and mitochondria. This further regulates the activities of CaM and CaMKII, causing synaptic dysfunction and cell necrosis, leading to memory and cognitive disorders.42–44 Ca2+ is a cationic second messenger involved in neurotransmission and it regulates long-term potentiation (LTP) related to learning and memory patterns. In the body, Ca2+ can bind to CAM to form an active calcium–calmodulin complex (Ca2+/CAM), participating in synaptic function, learning, and memory, activating calmodulin-dependent kinases (CaMK, such as CaMKII and CaMKIV).45 CaMKII, a key factor in axonal regeneration, can mediate various intercellular signals in the short term to promote the learning and memory process, and it is an important kinase in the production of LTP.46 Studies have found that CaMKII is involved in regulating the synthesis and release of neurotransmitters related to memory disorders. It can cause phosphorylation of CREB, and phosphorylated CREB can regulate downstream genes to activate synaptic plasticity genes such as BDNF, playing a neuroprotective role.47–50 CREB is a transcription factor that improves memory damage by regulating synaptic plasticity, mainly affecting long-term memory, and participating in neuronal remodeling, learning, and memory in the mature brain. BDNF is a neurotrophic factor that plays an important role in the formation of learning and memory.51,52 The mechanism is shown in Fig. 12. The results of this study show that QFY can significantly reduce the levels of Ca2+ and CaM in brain tissue, significantly increase the levels of CAMKII and CREB, and significantly reduce the relative expression levels of NMDAR1 mRNA and protein in hippocampal tissue, and significantly increase the relative expression levels of BDNF mRNA and protein. This indicates that QFY may improve memory damage by reducing the levels of Ca2+, CAM, and NMDAR1 and increasing the expression levels of CaMKII, CREB, and BDNF.
Lipids, a type of phospholipid abundantly found in the body, are involved in the biochemical processes of the central nervous system, and lipid metabolism disorders are associated with various neurosystemic diseases such as Alzheimer's disease (AD).53 Glycerophospholipids, vital components of the cell membrane, become dysregulated following neuronal damage, leading to the hydrolysis and generation of fatty acids. Phosphorylcholine (PC), a precursor in acetylcholine synthesis, is closely related to the body's production of free radicals and oxidative stress.54,55 Studies have found that long-term administration of PC can improve learning and memory impairments.56 Lysophosphatidylcholine (LPC), a degradation product of phospholipids, can exacerbate Aβ aggregation, lead to neurotoxicity, and promote the onset of neuroinflammation.57,58 Lysophosphatidylethanolamine (LPE), a biomarker for AD pathology, can facilitate the progression from mild cognitive impairment to AD. Research indicates that reducing the LPC and LPE levels can ameliorate memory disorders.59 In this study, the PC levels increased, while the LPC and LPE levels decreased following QFY treatment, suggesting that its antioxidative and memory improvement effects may be related to the upregulation of PC and downregulation of LPC and LPE.
Unsaturated fatty acids are crucial for the brain, enhancing neural plasticity and playing a significant role in learning and memory.60 Arachidonic acid (AA), a widely distributed polyunsaturated fatty acid within the body and an important inflammatory mediator that participates in the regulation of the body's immune and inflammatory systems,61 can provoke neuroinflammation, increase levels of reactive oxygen species, and exacerbate oxidative stress.62 Studies have found that IL-6, TNF-α, and COX-2 are mainly involved in the inflammatory response to affect nerve cell function and neural plasticity, which can act on neurons, cause neuronal damage and dysfunction, and lead to memory impairment.63 It is further indicated that the disorder of lipid metabolism in MD mice may be related to arachidonic acid metabolism, and QFY can improve memory disorder by inhibiting the inflammatory response. The correlation analysis found a significant positive correlation between calcium ions and AA; AA can mobilize intracellular calcium stores to release Ca2+, and also directly affect other calcium signaling pathways.64 In this experiment, the AA levels increased in the model group rats but decreased after QFY treatment, suggesting that the potential of QFY to improve memory impairment may be related to the regulation of inflammatory mediators such as AA. It was found that lipid metabolism dysregulation occurs in MD, a brain disease where abnormal lipid metabolism in brain tissue can lead to significant biochemical changes within the brain. Intervention with QFY can improve these abnormalities.
5. Conclusion
This study investigated the mechanisms through which QFY improves MD using a pseudo-targeted lipidomic analysis method employing UHPLC-Q-TRAP-MS/MS. The pharmacological research demonstrated that QFY has a beneficial effect on memory impairment in rats with MD. The lipidomics studies showed that MD can cause lipid metabolism disorders, and after QFY treatment, lipids have a tendency to return to normal levels. The network pharmacology, experimental verification, and lipidomics analysis found that the effect of QFY on MD rats may be related to the regulation of the calcium signaling pathway and the improvement of lipid metabolism disorders.Author contributions
Fuxia Zhao conducted the research, analyzed the data, and wrote the paper. Jing Wang and Minjun Wu participated in the experimental and data analysis processes. Jiaqi Fan, Fanying Deng, and Shiqi Liu conducted the experiments. Yan-gang Cheng and Shihui Wang provided experimental instrument guidance and support. Yan Wang made a critical revision of the paper and was responsible for the procedures for obtaining funding.Data availability
All data generated or analyzed during this study are included in this article.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This research was funded by the Research Project Supported by Shanxi Traditional Chinese Medicine Science and Technology Innovation Project (2024kjzy004), Special Plan for Shanxi Provincial Science and Technology Innovation Talent Team (202204051002028), Shanxi University of Traditional Chinese Medicine Science and Technology Innovation Ability Cultivation Plan ‘Taihang Materia Medica’ Special Basic and Applied Research/Genuine Chinese Herbal Medicine Information Database Project (2022PY-TH-15), and Shanxi University of Traditional Chinese Medicine Excellent Innovation Project (2022YS018).References
- J. Y. Dai, J. Zhang, R. D. Chen, B. B. Wu and M. Zhao, J. Basic Chin. Med., 2021, 27(04), 678–682 Search PubMed.
- Q. Q. Sun, F. Xie, L. Yao, Z. M. Lv and M. L. Zhong, J. Shaanxi Univ. Chin. Med., 2023, 46(03), 127–130 Search PubMed.
- H. Y. Li, X. L. Wang, D. M. Qi, X. R. Chen and J. F. Wang, J. Int. Pharm. Res., 2019, 46(12), 885–898 Search PubMed.
- Y. Z. Yang, J. Du, H. F. Qu, J. M. Li, Y. X. Zhang and J. J. Liu, Chin. J. Tissue Eng. Res., 2023, 27(28), 4487–4493 Search PubMed.
- J. H. Zhao, X. Li, W. X. Wu, J. H. Hu, Y. Duan, T. T. Feng, C. C. Fan, Y. C. Ma and X. Fu, Acta Chin. Med. Pharm., 2023, 51(06), 110–114 CAS.
- H. P. Wang, Nat. Prod. R&D, 2023, 34(05), 836–841 Search PubMed.
- C. M. Zhai, Y. F. Xue, P. F. Guo, C. X. Bai, Y. W. Shi, Y. H. Meng and L. Q. Gao, Acta Chin. Med. Pharm., 2021, 49(07), 26–31 CAS.
- X. R. Li, S. M. Chen, W. Y. Chen, J. M. Song and Y. Y. Zhang, Chin. Pharm. J., 2022, 57(01), 15–23 Search PubMed.
- Y. R. Heng, H. Z. Duan, H. Du, Y. Yan and C. H. Du, Chin. Tradit. Herb. Drugs, 2022, 53(21), 6759–6770 CAS.
- Y. Q. Wu, X. C. Xu, H. X. Wei, Q. Li and J. F. Zhang, Clin. Misdiagn. Misther., 2018, 31(07), 25–29 CAS.
- Y. Chen, G. S. Reihan, X. Zhang, S. X. Chen and J. P. Liu, Shaanxi J. Tradit. Chin. Med., 2014, 35(09), 1257–1258 Search PubMed.
- N. T. Rokot, T. S. Kairupan, K. C. Cheng, J. Runtuwene, N. H. Kapantow, M. Amitani, A. Asakawa and A. Inui, J. Evidence-Based Complementary Altern. Med., 2016, 2016, 2614742 CrossRef.
- T. Kuboyama, K. Hirotsu, T. Arai, H. Yamasaki and C. Tohda, Front. Pharmacol., 2017, 8, 805 CrossRef.
- K. Kubota, H. Fukue, H. Sato, K. Hashimoto, A. Fujikane, T. Watanabe, S. Katsurabayashi, M. Kainuma and K. Iwasaki, Front. Pharmacol., 2017, 8, 850 CrossRef.
- B. Lee, I. Shim, H. Lee, H. Lee and D. H. Hahm, J. Microbiol. Biotechnol., 2011, 21(8), 874–883 CrossRef PubMed.
- Y. J. Zeng, S. Cao, N. N. Li, J. Tang and G. X. Lin, Lipids Health Dis., 2023, 22(1), 155 CrossRef CAS PubMed.
- D. Sergi, E. Za, V. Tisato, P. Secchiero, G. Zauli and C. Cervellati., Int. J. Mol. Sci., 2023, 24(5), 4403 CrossRef CAS.
- T. Wang, M. Y. Guan, D. L. Li and Y. Li, Pharmacol. Clin. Chin. Mater. Med., 2022, 38(02), 53–58 Search PubMed.
- S. Barbash, B. P. Garfinkel, R. Mao, A. Simchovitz, B. Nadorp, A. Guffanti, E. R. Bennett, C. Nadeau, A. Türk, L. Paul, T. Reda, Y. Li, A. S. Buchman, D. S. Greenberg, A. Seitz, D. A. Bennett, P. Giavalisco and H. Soreq, Neurobiol. Dis., 2017, 106, 1–13 CrossRef CAS.
- D. D. Chen, Y. Dong and X. D. Yang, Chin. J. Appl. Physiol., 2020, 36(05), 438–443 Search PubMed.
- F. E. Gaamouch, P. Jing, J. Xia and D. Cai, J. Alzheimer's Dis., 2016, 53(1), 15–29 Search PubMed.
- Y. P. Wei, D. Liu, Y. Zheng, H. L. Li, C. S. Hao and W. Q. Yang, Brain Res. Bull., 2017, 134, 262–272 CrossRef CAS PubMed.
- J. S. Yu, H. Y. Kang and J. K. Lee, Brain Sci., 2020, 10(11), 793 CrossRef.
- X. Y. Zhang, L. H. Zhu, H. Yu, G. Ying, Z. Zhu and H. Ren, 2024, 2835, 5612–5617.
- P. Wang, Y. He and L. N. Hou, Chin. J. Gerontol., 2023, 43(19), 4828–4832 CAS.
- J. G. Yang, L. X. Li, Z. H. Li, Y. F. Su, Z. J. Zhang, J. Y. Song, H. H. Zeng, Z. Q. Zhang and J. L. Ma, J. Beijing Univ. Tradit. Chin. Med., 2024, 47(03), 352–363 Search PubMed.
- X. Q. Zheng, W. Wei, H. H. Li, L. L. Ding and X. H. Xue, J. Tradit. Chin. Med., 2024, 65(04), 395–403 Search PubMed.
- R. Bucala and A. Cerami, Adv. Pharmacol., 1992, 23, 1–34 CAS.
- S. C. Ho, J. H. Liu and R. Y. Wu, Biogerontology, 2003, 4(1), 15–18 CrossRef CAS PubMed.
- A. Kaur, K. Joshi, R. W. Minz and K. D. Gill, Toxicology, 2006, 219(1–3), 1–10 CrossRef CAS.
- W. Q. Wu, X. Wang, Q. Xiang, X. Meng, Y. Peng, N. Du, Z. Liu, Q. Sun, C. Wang and X. Liu, Food Funct., 2014, 5(1), 158–166 RSC.
- Z. Cao, X. Yang, H. Zhang, H. Y. Wang, W. Y. Huang, F. B. Xu, C. Zhuang, X. Wang and Y. Li, Chemosphere, 2016, 151, 289–295 CrossRef CAS.
- S. C. Bondy, Toxicology, 2014, 315, 1–7 CrossRef CAS PubMed.
- Q. Huang, G. L. Dai, X. Y. Yang, Y. Cao and W. Z. Ju, China J. Chin. Mater. Med., 2024, 49(07), 1896–1904 Search PubMed.
- H. J. Li, C. K. Li, C. L. Wei and Y. T. Zhnag, Chin. J. Tissue Eng. Res., 2024, 28(31), 4951–4957 Search PubMed.
- D. Hu, Y. J. Cui and J. Zhang, Transl. Neurosc., 2021, 12(1), 237–246 CrossRef CAS.
- Y. Hu, H. Wang, H. Jia, M. Peng, T. Zhu, Y. Liu and J. Wei, Plant Signaling Behav., 2023, 18(1), 2187561 CrossRef PubMed.
- Q. Xu, L. Y. Liu, R. X. Zhang, Z. Yu, F. Hao and J. B. Zhnag, Chin. Acupunct. Moxibustion, 2024, 44(04), 433–440 Search PubMed.
- Y. Yu, F. Bai, W. Wang, Y. Liu, Q. Yuan, S. Qu, T. Zhang, G. Tian, S. Li, D. Li and G. Ren, Pharmacol., Biochem. Behav., 2015, 133, 122–131 CrossRef CAS.
- S. G. Wang, G. W. Su, Q. Zhang, T. T. Zhao, Y. Liu, L. Zheng and M. Zhao, J. Agric. Food Chem., 2018, 66(40), 10617–10627 CrossRef CAS.
- Y. Li, Y. Yu, L. Liu, X. R. Wang and M. W. Kong, J. Beijing Univ. Tradit. Chin. Med., 2023, 46(09), 1250–1257 Search PubMed.
- L. Y. Xue, N. N. Wu, R. R. Feng and L. T. Yin, Chem. Life, 2021, 41(04), 686–692 Search PubMed.
- A. Iyaswamy, A. K. Kammella, C. Thavasimuthu, W. Wankupar, W. Dapkupar, S. Shanmugam, R. Rajan and S. Rathinasamy, J. Food Drug Anal., 2018, 26(2), 903–916 CrossRef CAS PubMed.
- S. C. Liu, W. Y. Hu, W. Y. Zhang, L. Yang, Y. Li, Z. C. Xiao, M. Zhang and Z. Y. He, Psychopharmacology, 2019, 236(9), 2823–2834 CrossRef CAS PubMed.
- T. Yamauchi, Biol. Pharm. Bull., 2005, 28(8), 1342–1354 CrossRef CAS.
- Y. Gao, J. H. Zhu, Y. Zhang, Z. Z. Tian and H. J. Wan, Pharmacol. Clin. Chin. Mater. Med., 2021, 37(04), 8–12 Search PubMed.
- A. Moro, G. M. Woerden, R. F. Toonen and M. Verhage, PLoS Biol., 2020, 18(8), e3000826 CrossRef CAS PubMed.
- X. D. Yan, J. F. Liu, Z. X. Ye, J. H. Huang, F. He, W. Xiao, X. Hu and Z. Luo, PLoS One, 2016, 11(9), e0162784 CrossRef.
- L. M. Mao, D. Z. Jin, X. P. Chu and J. Q. Wang, Shengli Xuebao, 2014, 66(3), 365–372 CAS.
- S. Orrenius, V. Gogvadze and B. Zhivotovsky, Biochem. Biophys. Res. Commun., 2015, 460(1), 72–81 CrossRef CAS PubMed.
- B. L. Hempstead, Trans. Am. Clin. Climatol. Assoc., 2015, 126, 9–19 Search PubMed.
- F. Harrisberger, R. Smieskova, A. Schmidt, C. Lenz, A. Walter, K. Wittfeld, H. J. Grabe, U. E. Lang, P. Fusar-Poli and S. Borgwardt, Neurosci. Biobehav. Rev., 2015, 55, 107–118 CrossRef CAS.
- Y. C. Kao, P. C. Ho, Y. K. Tu, I. M. Jou and K. J. Tsai, Int. J. Mol. Sci., 2020, 21(4), 1505 CrossRef CAS PubMed.
- M. W. Wong, N. Braidy, A. Poljak, R. Pickford, M. Thambisetty and P. S. Sachdev, Alzheimer's Dement, 2017, 13(7), 810–827 CrossRef.
- T. Kawamura, T. Okubo, K. Sato, S. Fujita, K. Goto, T. Hamaoka and M. Iemitsu, Nutrition, 2012, 28(11–12), 1122–1126 CrossRef CAS.
- H. C. Chaung, C. D. Chang, P. H. Chen, C. J. Chang, S. H. Liu and C. C. Chen, Food Chem., 2013, 138(1), 342–347 CrossRef CAS.
- B. Fuchs and J. Schiller, Mini. Rev. Med. Chem., 2009, 9(3), 368–378 CrossRef CAS.
- A. M. Sheikh, M. Michikawa, S. U. Kim and A. Nagai, Neuroscience, 2015, 292, 159–169 CrossRef CAS PubMed.
- D. A. Llano and V. Devanarayan, J. Alzheimer's Dis., 2021, 80(1), 311–319 CAS.
- Q. Qiao, Z. Y. Yu, W. P. Wang, H. F. Ma, Y. R. Sun, Z. G. Li and Z. Q. Zhang, Pharmacol. Clin. Chin. Mater. Med., 2008, 05(01), 1–16 Search PubMed.
- P. C. Calder, Mol. Nutr. Food Res., 2008, 52(8), 885–897 CrossRef CAS.
- P. Toth, A. Csiszar, D. Sosnowska, Z. Tucsek, P. Cseplo, Z. Springo, S. Tarantini, W. E. Sonntag, Z. Ungvari and A. Koller, Br. J. Pharmacol., 2013, 168(8), 1878–1888 CrossRef CAS PubMed.
- L. Yang, R. Y. Zhou, Y. Tong, P. F. Chen, Y. Shen, S. Miao and X. Q. Liu, Neurobiol. Dis., 2020, 140, 104814 CrossRef CAS PubMed.
- K. Yui, M. Koshiba, S. Nakamura and Y. Kobayashi, J. Clin. Psychopharmacol., 2012, 32(2), 200–206 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4mo00141a |
| This journal is © The Royal Society of Chemistry 2025 |