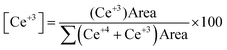Open Access Article
Open Access ArticleOxygen vacancies induced low overpotentials of Ag/CeO2 for electrocatalytic evolution of oxygen and hydrogen†
Ajit Kumar
Dhanka
 a,
Mayank
Tiwari
b,
Prashant Kumar
Bhartiya
c,
Balaram
Pani
a,
Mayank
Tiwari
b,
Prashant Kumar
Bhartiya
c,
Balaram
Pani
 d,
Nityananda
Agasti
d,
Nityananda
Agasti
 *a and
Debabrata
Mishra
*a and
Debabrata
Mishra
 *b
*b
aDepartment of Chemistry, University of Delhi, North Campus, Delhi, 110007, India. E-mail: nnagasti@chemistry.du.ac.in
bDepartment of Physics and Astrophysics, University of Delhi, Delhi, 110007, India
cDelhi School of Climate Change and Sustainability, Institution of Eminence (IOE), University of Delhi, Delhi, 110007, India
dDepartment of Chemistry, Bhashkaracharya College of Applied Sciences, University of Delhi, Dwarka, New Delhi 110075, India
First published on 23rd April 2025
Abstract
Designing efficient catalysts for the evolution of hydrogen and oxygen through electrocatalytic water splitting remains an area of significant interest. Herein, we develop an Ag/CeO2 catalyst that demonstrates a remarkable electrocatalytic performance for hydrogen and oxygen evolution through water splitting. The high catalytic activity can be attributed to the interaction between Ag and CeO2, which increases the oxygen vacancies at the interface. This is substantiated by the results from Raman, X-ray photoelectron, electron paramagnetic resonance and photoluminescence spectroscopy. The reduced photoluminescence intensity validates the effective separation of photogenerated electron–hole pairs due to oxygen vacancies. Besides increasing the oxygen vacancies, Ag enhances light absorption and reduces the band gap of CeO2, which is evident from a remarkable enhancement in the electrocatalytic activity of Ag/CeO2, especially under light illumination, compared to pristine CeO2. Notably, a drastic reduction in overpotential and an increase in current density are observed for Ag/CeO2. For the oxygen evolution reaction, Ag/CeO2 exhibits a reduction of 120 mV in the overpotential and an increase of 19.8 mA cm−2 in the current density with the lowest Tafel slope of 158 mV dec−1 compared to CeO2. For the hydrogen evolution reaction, Ag/CeO2 exhibits a reduction of 130 mV in the overpotential and an increase of 14.1 mA cm−2 in the current density. Considering the results from characterization techniques and electrocatalytic experiments, a plausible mechanism has been proposed for the electrocatalytic performance of the catalyst. This study offers insights into defect-induced ceria-based materials for optimizing and designing effective electrocatalysts for overall water splitting.
1. Introduction
Fossil fuels such as coal, natural gas, and crude oil are essential for meeting energy demands and are used to power various aspects of daily life, agriculture, transportation, and industry. However, the excessive consumption of fossil fuels, due to the combination of rapid economic expansion and an increasing global population, presents a significant threat of future shortages.1 Researchers have predicted that the crude oil may be depleted in 40–50 years,2 coal production will peak by 2042–2062,3 and demand for clean energy is projected to rise 50% by 2030.4 To address these challenges, hydrogen emerges as a viable solution, offering a clean, efficient energy alternative with high energy density and zero carbon emissions.5,6 Steam reforming is a cost-effective way to produce hydrogen; however, it has environmental limitations.7 In contrast, electrochemical water splitting offers a cleaner and more sustainable method for generating hydrogen. Electrochemical water splitting consists of the hydrogen evolution reaction (HER) to produce hydrogen and the oxygen evolution reaction (OER) to generate oxygen.8 High overpotentials, sluggish reaction kinetics, catalyst stability, and the requirement for scalable, inexpensive materials are some of the challenges faced by HER and OER electrocatalysts.9,10 Electrocatalytic water splitting using semiconductor metal oxides as catalysts has gained significant interest.11,12 Oxides of rare earth metals, especially cerium (Ce), have been widely used as catalysts due to their flexible interconversion of the oxidation state (Ce4+ ↔ Ce3+). The conversion between Ce4+ and Ce3+ results in the formation of oxygen vacancies in the lattice structure denoted as Ce4+–Ov–Ce3+ (where Ov represents an oxygen vacancy), an inherent property of CeO2 that makes it a potential redox material for electrocatalytic oxygen evolution reactions (OER).13,14 Moreover, CeO2, being an n-type semiconductor with a band gap of 3.2 eV, acts as a good photocatalyst.15 Its remarkable redox behavior and suitable band gap make it a potential candidate for photoelectrocatalysis. However, there are limitations with ceria due to its wide band gap. Therefore, it is of great importance to develop highly efficient ceria nanostructures for photoelectrocatalytic applications.Recently, the development of ceria-based nanomaterials by modulating the band gap and increasing defects on the ceria surface has been demonstrated as an effective strategy to improve the light-harvesting capability and restrain electron–hole recombination for enhanced photoelectrocatalytic performance.16–18 For example, Ghosh et al. have reported a ceria-based heterojunction catalyst for hydrogen evolution, where the band gap and defects in ceria contributed to enhanced photoelectrocatalytic activity.19 Although efforts were made to prepare ceria-based materials, reports on in-depth characterization of defects in ceria and the mechanistic details highlighting the role of defects in the catalytic performance of the material are limited.20–23 Hence, this work offers a detailed study revealing structural characterization of defects in ceria-based materials and a mechanistic study of the roles of defects in catalytic activity. Besides having good catalytic properties, ceria also acts as a good catalyst support, onto which metal or metal oxide nanoparticles can be incorporated to produce heterojunctions. The introduction of metal nanoparticles onto the surface of a ceria support forms metal–ceria junctions, which can result in a synergistic effect and facilitate interfacial charge transfer, thereby improving photoelectrocatalytic performance. Furthermore, the metal nanoparticles can act as photosensitizers to strengthen the light absorption of the metal–ceria composite. Additionally, the strong metal–support interaction between metal nanoparticles and ceria increases defects on the surface of ceria and reduces the band gap in ceria.
Considering the importance of developing high-performance electrocatalysts based on ceria-based materials, in this report we describe Ag/CeO2 prepared by a solvothermal method, as an effective catalyst for oxygen and hydrogen evolution by electrocatalytic water splitting. The electrocatalytic performance of Ag/CeO2 as a catalyst is found to be distinctly higher than that of pristine CeO2 for electrocatalytic water splitting. The activity of Ag/CeO2 was enhanced remarkably under light illumination. Ag/CeO2 exhibited a 120 mV reduction in the overpotential and a 19.8 mA cm−2 increase in the current density compared to CeO2 for the oxygen evolution reaction. For the hydrogen evolution reaction, Ag/CeO2 exhibited superior activity to CeO2 with an overpotential reduction of 130 mV and a 14.1 mA cm−2 enhancement in the current density. The materials characterization results confirm the role of Ag and Ag/CeO2 (metal–support) interactions in achieving enhanced electrocatalytic performance. Ag nanoparticles improve light absorption and enhance the absorption intensity through the localized surface plasmon resonance (LSPR) effect at the Ag/CeO2 interface. The incorporation of Ag also reduces the band gap of CeO2 to 2.90 eV. Moreover, due to the strong metal–support interaction between Ag and CeO2, there is an increase in surface oxygen vacancies on CeO2. The surface oxygen vacancies trap the photogenerated electrons causing delayed recombination of photogenerated electron and hole pairs. Overall, the interaction between Ag and CeO2 improves the optical properties, reduces the band gap, enhances the charge carrier separation, and restrains the recombination of photogenerated carriers, thereby resulting in an improvement in the overall water splitting process. A plausible mechanism was also proposed to understand the role of the Ag/CeO2 catalyst in electrocatalytic water splitting for hydrogen and oxygen evolution reactions.
2. Experimental section
2.1 Materials
Cerium nitrate hexahydrate (99%) (Ce(NO3)3·6H2O) and sodium hydroxide (98%) (NaOH) were obtained from CDH India; silver nitrate (AgNO3, 98.5%) from Merck; and ethanol (C2H5OH) and methanol (CH3OH) from Sigma-Aldrich, India. Deionized (DI) water was used in the experiments. All chemicals used were of analytical grade and directly used as received without further purification. Ag/CeO2 nanocomposites were synthesized following the literature method.242.2 Preparation of Ag/CeO2
The Ag/CeO2 nanocomposites were prepared via a solvothermal method24,25 (Scheme 1). In a typical synthesis procedure, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 weight ratio of AgNO3 to Ce(NO3)3·6H2O, corresponding to a molar ratio of AgNO3 (0.59 mmol) to Ce(NO3)3 (2.4 mmol), was used. Firstly, 0.1 g of AgNO3 and 1.0 g of Ce(NO3)3·6H2O were dissolved in 50 mL of methanol, followed by the addition of 0.25 g (6.25 mmol) of sodium hydroxide under magnetic stirring for 30 minutes to create a homogenous solution. Furthermore, the resultant reaction mixture was transferred to a 100 ml Teflon-lined stainless-steel autoclave and sealed. Then, the autoclave was heated at 180 °C and maintained at this temperature for 18 hours. The autoclave was left to cool naturally to room temperature. The formed precipitate was collected and washed three to four times with ethanol by centrifugation at 3000 rpm for 10 minutes to remove any residual chemicals from the precipitate. Afterward, the precipitate was heated in an oven at 80 °C for 4 hours and subsequently calcined at 250 °C for 2 hours, yielding a grey color powder of Ag/CeO2 nanocomposites. For comparison, CeO2 nanoparticles were synthesized using a similar procedure but without the addition of AgNO3. No additional reducing agent was used in the preparation of materials. In the presence of NaOH, Ce(NO3)3·6H2O precipitates into Ce(OH)3, which under hydrothermal conditions converts into CeO2. AgNO3 in the presence of NaOH forms Ag2O as an intermediate to form Ag nanoparticles. NaOH facilitates the nucleation of Ag nanoparticles.26
10 weight ratio of AgNO3 to Ce(NO3)3·6H2O, corresponding to a molar ratio of AgNO3 (0.59 mmol) to Ce(NO3)3 (2.4 mmol), was used. Firstly, 0.1 g of AgNO3 and 1.0 g of Ce(NO3)3·6H2O were dissolved in 50 mL of methanol, followed by the addition of 0.25 g (6.25 mmol) of sodium hydroxide under magnetic stirring for 30 minutes to create a homogenous solution. Furthermore, the resultant reaction mixture was transferred to a 100 ml Teflon-lined stainless-steel autoclave and sealed. Then, the autoclave was heated at 180 °C and maintained at this temperature for 18 hours. The autoclave was left to cool naturally to room temperature. The formed precipitate was collected and washed three to four times with ethanol by centrifugation at 3000 rpm for 10 minutes to remove any residual chemicals from the precipitate. Afterward, the precipitate was heated in an oven at 80 °C for 4 hours and subsequently calcined at 250 °C for 2 hours, yielding a grey color powder of Ag/CeO2 nanocomposites. For comparison, CeO2 nanoparticles were synthesized using a similar procedure but without the addition of AgNO3. No additional reducing agent was used in the preparation of materials. In the presence of NaOH, Ce(NO3)3·6H2O precipitates into Ce(OH)3, which under hydrothermal conditions converts into CeO2. AgNO3 in the presence of NaOH forms Ag2O as an intermediate to form Ag nanoparticles. NaOH facilitates the nucleation of Ag nanoparticles.26
 | ||
| Scheme 1 Preparation of Ag/CeO2 nanocomposites by a solvothermal method and their application in water splitting. | ||
2.3 Fabrication of electrodes for electrochemical measurements
The electrodes were fabricated by drop-casting the prepared CeO2 and Ag/CeO2 on nickel foam (NiF). The first electrode was fabricated by drop-casting CeO2 as the active material, polyvinylidene fluoride (PVDF) as the binder, and carbon black as the conducting agent. The ratio of these components was 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, respectively. Similarly, the second electrode was prepared by drop-casting Ag/CeO2 as the active material, PVDF as the binder, and carbon black as the conducting agent in the same ratio. These composites were applied onto a nickel foam using the drop-casting method, covering an area of 1 cm2. Following the application of the coating, the electrodes were placed in a vacuum oven at a temperature of 80 °C for 10 hours to facilitate drying. Subsequently, the electrodes were allowed to cool down naturally until they reached room temperature. The fabricated electrodes were named NiF, NiF/CeO2, and NiF/Ag/CeO2. NiF/CeO2/Light and NiF/Ag/CeO2/Light have been abbreviated to the electrocatalytic activity of NiF/CeO2 and NiF/Ag/CeO2 under light illumination.
10, respectively. Similarly, the second electrode was prepared by drop-casting Ag/CeO2 as the active material, PVDF as the binder, and carbon black as the conducting agent in the same ratio. These composites were applied onto a nickel foam using the drop-casting method, covering an area of 1 cm2. Following the application of the coating, the electrodes were placed in a vacuum oven at a temperature of 80 °C for 10 hours to facilitate drying. Subsequently, the electrodes were allowed to cool down naturally until they reached room temperature. The fabricated electrodes were named NiF, NiF/CeO2, and NiF/Ag/CeO2. NiF/CeO2/Light and NiF/Ag/CeO2/Light have been abbreviated to the electrocatalytic activity of NiF/CeO2 and NiF/Ag/CeO2 under light illumination.
2.4 Electrochemical measurements
Electrochemical measurements including linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), and chronoamperometry were conducted in an alkaline environment using a computerized potentiostat/galvanostat (CS350 Corr-Test electrochemical workstation) within a standard three-electrode electrochemical setup. The counter electrode used was platinum (Pt) and Ag/AgCl was utilized as the reference electrode. The potential values were converted to the reversible hydrogen electrode (ERHE) scale using the Nernst equation.27 All the electrochemical experiments were conducted using a 1 M KOH solution as the electrolyte. The visible light source emitting a wavelength of λ = 405 nm was utilized for illumination during the electrochemical experiments, which was positioned 5 cm away from the working electrode.2.5 Materials characterization
The microstructural and morphological features of the synthesized materials were characterized by multiple techniques. Powder X-ray diffraction (PXRD) was conducted to study the crystalline patterns of the materials using a diffractometer with Cu K radiation (λ = 0.1542 nm) in the 2θ range of 10–80. To study the optical properties of the materials, UV-visible spectra were obtained using a Shimadzu 1800 spectrometer and UV-vis diffuse reflectance spectra (DRS) were obtained using an Agilent Cary 5000. Raman spectroscopy study was carried out using a laser Raman spectrometer, model: inVia II. Field emission scanning electron microscopy (FESEM) using an Oxford-EDX system IE 250 X Max 80 (FEI Quanta 200 F SEM) at a high-resolution with a thermally aided field emission gun (FEG) at an acceleration voltage of Uacc = 0.2−30 kV was conducted to study the morphology and elemental composition of the materials. High-resolution transmission electron microscopy (HRTEM) images of the materials were collected using a TECNAI G20 HR-TEM 200 kV. To assess the surface area and pore characteristics of the synthesized materials, nitrogen adsorption–desorption measurements were carried out using the Autosorb-iQ XR system from Quantachrome Instruments. Prior to analysis, the samples were degassed at 150 °C for 10 hours under a high vacuum of approximately 1 × 10−2 bar to remove any adsorbed impurities. The isotherms were subsequently recorded at 77 K, employing a liquid nitrogen environment to ensure accurate measurements. X-ray photoelectron spectroscopy (XPS) was performed using a Kratos Axis Supra Plus XPS equipped with a monochromatic Al Kα X-ray source (1486.6 eV). The high-resolution data were calibrated using the C 1s peak at a binding energy of 284.5 eV as the standard. The photoluminescence (PL) study was performed at normal temperature on a Horiba Yvon PTI QuantaMaster (8450–11) spectrophotometer with a 375 nm nano-LED as the excitation source. The charge carrier lifetimes were determined by employing time-resolved photoluminescence (TRPL) measurements (in micro- and milliseconds) using a 980 nm laser and a xenon lamp as light sources in modulated mode.3. Results and discussion
3.1 Structure, morphology, and elemental analysis
To understand the formation of the Ag/CeO2 nanocomposite, the optical properties were analyzed by UV-visible spectroscopy and compared with those of pristine CeO2. The absorption bands at wavelengths of 340 nm and 327 nm in the UV-visible absorption spectra (Fig. S1, ESI†) correspond to Ag/CeO2 and CeO2, respectively.28 The absorption band corresponding to Ag/CeO2 suffers a red shift compared to CeO2. The red shift in the absorption band can be attributed to a decrease in the band gap of Ag/CeO2 because of the addition of Ag, suggesting the formation of the composite material.29 To calculate the direct band gap energy (Eg) of Ag/CeO2, UV-vis diffuse reflectance spectra (DRS) were recorded. To mitigate the influence of scattered light, the DRS spectra were transformed using the Kubelka–Munk (K–M) function. The plot of the K–M function versus the energy of light is shown in Fig. S2 (ESI†). From this plot, the band gap energies for Ag/CeO2 were derived. The intercept on the abscissa axis of a linear fit of the Kubelka–Munk function αhv = A(hν − Eg)n/2 corresponds to the Eg values, where Eg, α, A, h, and v represent the band gap, absorption coefficient, a constant, Planck's constant, and light frequency, respectively.30 The DRS spectra reveal band gap energies of 3.25 eV for CeO2 and 2.90 eV for Ag/CeO2, which represent the energy gap between the valence band and conduction band. The reduction in band gap and the shift of the absorption band to a longer wavelength in Ag/CeO2 are attributed to the incorporation of Ag into CeO2 because the addition of Ag facilitates the excitation of electrons from the conduction band to the valence band within the CeO2 lattice.31 The formation of Ag/CeO2 was confirmed by XRD analysis in the 2θ range of 10–80°. The XRD pattern (Fig. 1a) for pristine CeO2 exhibited peaks at 2θ angles of 28.4°, 32.9°, 47.5°, 56.3°, 59.0°, 69.3°, 76.7°, and 78.8°, corresponding to the (111), (200), (220), (311), (222), (400), (331), and (420) planes, respectively. These peaks are indicative of a cubic fluorite structure, consistent with the JCPDS File No. 34-0394.32 Compared to pristine CeO2, the XRD pattern of Ag/CeO2 exhibits additional peaks at 38.5°, 47.7°, 64.9°, and 76.7° corresponding to the (111), (200), (220), and (311) planes of cubic Ag (peaks presented with asterisks)33,34 (JCPDS No. 04-0783). The appearance of these peaks confirms the successful incorporation of Ag into CeO2. This incorporation is further supported by a shift in diffraction peaks to higher angles in Ag/CeO2 compared to pristine CeO2, as shown in Fig. S3 (ESI†). This shift is most noticeable in the prominent (111) diffraction peak of Ag/CeO2.35,36 Additionally, in the diffraction pattern of Ag/CeO2, there is a decrease in the intensity and broadening (Fig. S3, ESI†) of all diffraction peaks corresponding to CeO2, suggesting defect generation in CeO2 due to Ag addition.37 The broadening of peaks can be attributed to the reduction in the crystallite size of CeO2. The average crystallite size, calculated using the Scherrer equation38 (D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ with K = 0.98, λ = 1.54 Å, and β being the full width at half maximum (FWHM) of the (111) peak), was found to be approximately 7 nm for CeO2 and 6 nm for Ag/CeO2.
θ with K = 0.98, λ = 1.54 Å, and β being the full width at half maximum (FWHM) of the (111) peak), was found to be approximately 7 nm for CeO2 and 6 nm for Ag/CeO2.
To further support the formation of Ag/CeO2 nanocomposites and confirm the formation of defects (oxygen vacancies) on the surface of ceria, Raman spectra were recorded in the range of 100–1100 cm−1 (Fig. 1b). The prominent peak at 465 cm−1 in the spectra corresponds to the F2g mode originating from the symmetric stretching of the Ce–O bond.39,40 Compared to pristine CeO2, the Ag/CeO2 composites exhibited a shift towards a lower wave number in the F2g stretching mode, with a peak at 462 cm−1 (Fig. S4a, ESI†). This downshift is due to an increase in oxygen vacancies induced by the incorporation of Ag41 because oxygen vacancies disrupt the Ce–O vibrational unit leading to the downshift of the F2g mode. In the Ag/CeO2 composite, additional peaks appearing with distinct intensities at 255 and 610 cm−1 correspond to the F1u symmetric mode42 and the defect-induced mode (D) due to intrinsic oxygen vacancies.43 The band at 610 cm−1, which is not present in pristine CeO2, shows an increase in oxygen vacancies in Ag/CeO2. An increase in oxygen vacancies in Ag/CeO2 is also inferred from the expansion in the FWHM of the F2g peak compared to that of pristine CeO241 (Fig. 1d and Fig. S4a, ESI†). The increase in oxygen vacancies has been calculated from the ratio of the integrated area of the D peak to the F2g peak (ID/IF2g), which is a key parameter for measuring the relative concentration of oxygen vacancies on the surfaces of CeO2 and Ag/CeO2. The Ag/CeO2 catalyst demonstrates a higher ID/IF2g ratio compared to pristine CeO2, suggesting higher oxygen vacancies in Ag/CeO2 (Fig. S4b, ESI†). Additionally, the incorporation of Ag in the composite is further confirmed by the enhanced the F2g peak intensity, which arises from the strong optical absorption associated to the surface enhanced Raman scattering (SERS) effect.44,45
Furthermore, Ag/CeO2 has been studied by photoluminescence (PL) analysis, which is a technique to ascertain oxygen vacancies and the efficiency of charge carrier trapping in semiconductor materials.46 In this study, PL analysis was utilized to investigate the impact of oxygen vacancies on the separation of photogenerated electron–hole pairs in Ag/CeO2 nanocomposites. In the PL spectra, CeO2 emissions are often attributed to 4f → VB (valence band) transitions, which are strongly influenced by defects like oxygen vacancies in the material.47 The PL spectra of CeO2 and Ag/CeO2 nanocomposites, shown in Fig. 1c, cover the range from 270 to 480 nm. For CeO2, a prominent UV emission peak at 394 nm is observed, corresponding to electron transition from the Ce 4f level to the O 2p level.48 However, with the introduction of Ag, the intensity of this peak decreased substantially along with an additional emission band appearing at 465 nm. The band at 465 nm is mostly associated with the oxygen vacancies with trapped electrons.49 The reduced photoluminescence intensity in Ag/CeO2 suggests improved separation of electron–hole pairs due to the presence of oxygen vacancies, highlighting their role in enhancing charge carrier dynamics and overall material performance. This observation aligns well with the findings reported in previous studies.50
To further evaluate the formation of oxygen vacancies, oxidation states of Ce, and elemental composition, Ag/CeO2 was analyzed by X-ray photoelectron spectroscopy (XPS). The XPS spectra of CeO2 and Ag/CeO2 were carefully compared to understand the effect of Ag on CeO2 in the Ag/CeO2 nanocomposite. The XPS survey spectra (Fig. S5, ESI†) demonstrate the presence of Ce, O and Ag with peaks in the binding energy ranges of 877–922 eV (Ce 3d), 527–536 eV (O 1s), and 364.5–376.1 eV (Ag 3d). The positions of the photoelectron peaks for all the elements are corrected according to the standard carbon (C 1s) peak, which is set at 284.4 eV.
To analyze the oxidation states of cerium in Ag/CeO2, the high-resolution XPS spectra for Ce 3d were deconvoluted over the binding energy range of 877 to 922 eV (Fig. 1d). The XPS spectra of Ce 3d can be fitted according to eight characteristic peaks located at U (881.9 eV), U′ (884.7 eV), U′′ (887.9 eV), U′′′ (897.9 eV), V (900.3 eV), V′ (902.3 eV), V′′ (905.6 eV), and V′′′ (916.2 eV). The peaks U′ and V′ were identified for Ce3+, which are assigned to the oxygen vacancies, and the rest of the peaks V′′′, V′′, V, U′′′, U′′, and U correspond to Ce4+.51 These peaks indicate the presence of both Ce3+ and Ce4+ electronic states on the surface of CeO2. Typically, Ce4+ is the predominant valence state on the surface of ceria, with a smaller proportion of Ce3+. The presence of Ce3+ alongside Ce4+ points to oxygen vacancies within the CeO2 crystals due to their interconversion.52
The surface concentration of Ce3+ in Ag/CeO2 can be determined by calculating the peak integral areas of Ce3+ and Ce4+ using the following formula:43
The Ce3+ content was found to be 17.89% in Ag/CeO2 and 13.92% in CeO2 (Table S1 and Fig. S6, ESI†). The higher Ce3+ content in Ag/CeO2 compared to pristine CeO2 is due to interfacial charge transfer induced by the metal–support interaction between Ag and CeO2. Moreover, the Ce 3d peaks in Ag/CeO2 are shifted towards high binding energies compared to pristine CeO2, which might be a consequence of the interfacial electron transfer from Ag to CeO2 due to the strong interaction between Ag and CeO2.53,54 The Ag/CeO2 interface becomes oxygen vacancy-rich due to this interaction. Additionally, the presence of oxygen vacancies on the surface can be revealed from the XPS spectra for O 1s within the binding energy range of 527 to 536 eV (Fig. 1e). In the deconvoluted XPS spectra for O 1s, the peaks observed at 528.8 eV (OL), 531.3 eV (OV), and 533.1 eV (OC) in Ag/CeO2 correspond to lattice oxygen (Ce4+–O2−), oxygen vacancies (Ce3+–O2−), and surface chemisorbed hydroxyl groups (–OH), respectively. The peak at 531.3 eV is ascribed to oxygen vacancies.41 The presence of Ag is also confirmed by the Ag 3d peaks in the XPS spectra (Fig. 1f). The peaks at 368.2 eV and 374.2 eV, attributed to Ag 3d5/2 and Ag 3d3/2, respectively, suggest the presence of metallic silver in Ag/CeO2. Further analysis of these peaks reveals additional peaks at 366.5 eV and 372.3 eV, which are indicative of Ag+. In a nutshell, the above results confirm the formation of the Ag/CeO2 nanocomposite and also reveal the oxidation states of Ce, the effect of Ag on CeO2, the interaction between Ag and CeO2, and oxygen vacancies at the Ag/CeO2 interface.
The increased oxygen vacancies in Ag/CeO2 were further validated through electron paramagnetic resonance (EPR) analysis performed at room temperature for both pristine CeO2 and Ag/CeO2 samples (Fig. 2a). Compared to pristine CeO2, Ag/CeO2 exhibited more pronounced EPR signals, indicating a higher concentration of oxygen vacancies in the composite. The EPR spectra of Ag/CeO2 revealed distinct g values at 1.93, 1.99, and 2.21, which are characteristic of Ce3+ ions, thereby confirming the presence of oxygen vacancies. Additionally, signals with g values of 2.07 and 2.15 were associated with O2− species and Ce3+–O−–Ce4+ defect sites.55 Collectively, the findings from PXRD, Raman spectroscopy, XPS, and EPR analyses corroborate the enhancement of oxygen vacancies in Ag/CeO2 compared to pristine CeO2. This increase in oxygen vacancies is pivotal for the improved electrocatalytic performance of the Ag/CeO2 composite.
 | ||
| Fig. 2 (a) EPR profiles measured (inset: the calculated g factor value) at room temperature and (b) BET analysis for CeO2 and Ag/CeO2 nanocomposites. | ||
The surface morphology, elemental mapping, and elemental composition of Ag/CeO2 were characterized by field emission scanning electron microscopy (FESEM) analysis with energy dispersive X-ray analysis (EDX). The FESEM image (Fig. 3a) reveals the spherical shapes of Ag/CeO2, while the mapping and EDX (Fig. 3b and c) spectrum verify the presence of Ag, Ce, and O with their elemental distributions being 40.44%, 45.68% and 13.89%, respectively.
 | ||
| Fig. 3 (a) High-magnification FESEM images, (b) EDX elemental mapping, and (c) EDX spectrum of Ag/CeO2 (inset: atomic and weight percentages of elements present in Ag/CeO2 nanocomposites). | ||
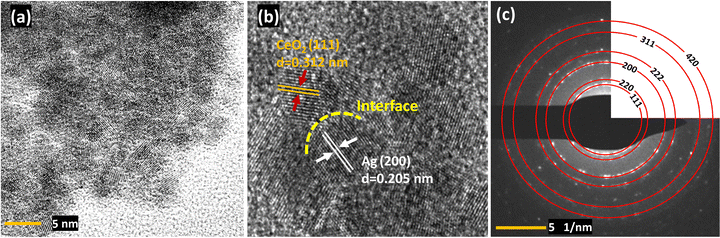
Furthermore, high-resolution transmission electron microscopy (HRTEM) was employed to investigate the nanostructure of Ag/CeO2 (Fig. 4a and Fig. S7, ESI†). HRTEM analysis demonstrates Ag nanoparticles deposited onto the CeO2 surface. The HRTEM image (Fig. 4b) shows Ag (200) and CeO2 (111) planes with interplanar spacings of 0.205 nm and 0.312 nm, respectively. The corresponding selected area electron diffraction (SAED) patterns (Fig. 4c) reveal the polycrystalline nature of Ag/CeO2 with resolved lattice fringes indexed to (111), (220), (200), (222), (311), and (420) lattice planes closely matching those obtained from the XRD pattern of Ag/CeO2 nanocomposites. HRTEM images (Fig. S7, ESI†) reveal that Ag nanoparticles of 4–6 nm size are deposited on CeO2 particles sized 5–8 nm. The particle sizes are consistent with the crystallite sizes calculated using the Scherrer equation from the XRD pattern. Noticeably, Ag and CeO2 are in contact with each other, thereby causing defects at the interface. The existence of an interface is crucial as it promotes the increase in surface oxygen vacancies in CeO2 that can enhance catalysis.
Besides the nanostructure, the specific surface area, pore diameter, and pore volume of the Ag/CeO2 catalyst have significant effects on its catalytic activities. Therefore, the Brunauer–Emmett–Teller (BET) analysis based on the nitrogen (N2) adsorption–desorption experiment was conducted. The N2 adsorption–desorption isotherms (Fig. 2b) show a typical type-IV curve with a fast increase at higher relative pressures (p/p°), indicating the porous structure of the materials.41 For pristine CeO2, the BET-specific surface area, total pore volume, and average pore diameter were measured to be 15.88 m2 g−1, 0.068 cm3 g−1, and 19.30 nm, respectively. However, in Ag/CeO2, the surface area properties significantly change to 42.57 m2 g−1, 1.228 cm3 g−1, and 11.74 nm, respectively. This noticeable change in structural characteristics is attributed to the introduction of Ag on the CeO2 surface, which agrees with the results from the previous studies.56 The porosity and surface area of the material are maintained even after the introduction of Ag.
3.2 Electrochemical measurements (OER and HER)
M + OH− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) M–OH + e− M–OH + e− |
M–OH + OH− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) M–O + H2O + e− M–O + H2O + e− |
M–O + OH− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) M–OOH + e−/2M–O + OH− M–OOH + e−/2M–O + OH− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) 2M–O2 + 2e− 2M–O2 + 2e− |
M–OOH + OH− ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) O2 + H2O + e− + M O2 + H2O + e− + M |
| Oxygen evolution reaction (OER) | |||
|---|---|---|---|
| Catalyst | Overpotential at 10 mA cm−2 (mV) | Tafel slope (mV dec−1) | Current density (mA cm−2) |
| NiF | 730 | 461 | 29 |
| NiF/CeO2, | 620 | 273 | 44.6 |
| NiF/CeO2/Light | 560 | 241 | 49.9 |
| NiF/Ag/CeO2 | 500 | 226 | 64.4 |
| NiF/Ag/CeO2/Light | 450 | 158 | 86.7 |
Here, M represents the active site.
These charge carriers enhance the charge transfer process at the electrode–electrolyte interface, leading to improved reaction kinetics, lower overpotential, and increased current densities.58,59
After investigating the OER performance, the electrocatalytic activity for the hydrogen evolution reaction (HER) of the electrodes, including NiF, NiF/CeO2, NiF/CeO2/Light, NiF/Ag/CeO2, and NiF/Ag/CeO2/Light, was also examined (Table 2). The LSV curves for the HER performance of NiF, NiF/CeO2, NiF/CeO2/Light, NiF/Ag/CeO2, and NiF/Ag/CeO2/Light are shown in Fig. 6a. The overpotentials calculated at a current density of −10 mA cm−2 for NiF, NiF/CeO2, NiF/CeO2/Light, NiF/Ag/CeO2, and NiF/Ag/CeO2/Light were 530 mV, 410 mV, 330 mV, 280 mV, and 240 mV, respectively, and are shown in Fig. 6b. Fig. 6c illustrates the current density at 0.54 V for NiF, NiF/CeO2, NiF/CeO2/Light, NiF/Ag/CeO2, and NiF/Ag/CeO2/Light. The values of current densities for NiF, NiF/CeO2, NiF/CeO2/Light, NiF/Ag/CeO2, and NiF/Ag/CeO2/Light were 10.9 mA cm−2, 21.2 mA cm−2, 25.3 mA cm−2, 35.3 mA cm−2, and 45.7 mA cm−2, respectively (as shown in Fig. 6c). For the HER as well, NiF/Ag/CeO2 exhibited superior activity to NiF/CeO2 with an overpotential reduction of 130 mV to achieve a 14.1 mA cm−2 enhancement in the current density. Furthermore, the overpotential of NiF/CeO2 was reduced by 80 mV, and the current density increased by 4.1 mA cm−2 on illuminating NiF/CeO2 with light. Similarly, for NiF/Ag/CeO2, the overpotential decreased by 40 mV and the current density dramatically increased by 10.4 mA cm−2 under light illumination. Fig. 6d presents the Tafel slope (HER) for NiF, NiF/CeO2, NiF/CeO2/Light, NiF/Ag/CeO2, and NiF/Ag/CeO2/Light. The lowest Tafel slope (HER) was found to be 151 mV dec−1 for NiF/Ag/CeO2 in the presence of light. The reaction mechanism for the HER can be given by the equations:60
| M + H2O + e− → M–Had + OH− |
| 2(M–Had) → 2M + H2 |
| 2(M–Had) + H2O → 2M + 2e− + H2 + H2O |
| Hydrogen evolution reaction (HER) | |||
|---|---|---|---|
| Catalyst | Overpotential at 10 mA cm−2 (mV) | Tafel slope (mV dec−1) | Current density (mA cm−2) |
| NiF | 530 | 461 | 10.9 |
| NiF/CeO2, | 410 | 238 | 21.2 |
| NiF/CeO2/Light | 330 | 219 | 25.3 |
| NiF/Ag/CeO2 | 280 | 169 | 35.3 |
| NiF/Ag/CeO2/Light | 240 | 151 | 45.7 |
The stability of NiF/Ag/CeO2 in the presence of light was examined using the chronoamperometric study at a constant potential (1.56 V vs. RHE) in 1 M KOH solution (see Fig. S8b, ESI†). It was found that NiF/Ag/CeO2 was stable for a time period of 2 hours.
| Catalyst | Reaction | Overpotential (mV) @ 10 mA cm−2 | Electrolyte | Ref. |
|---|---|---|---|---|
| 3D-rGO–CeO2 | HER | 340 | 1 M KOH | 62 |
| CeO2/Co (OH)2 | HER | 317 | 1 M KOH | 63 |
| Ni/ceria | HER | 588 | 1 M KOH | 64 |
| Ni/ceria–rGO | HER | 208 | 1 M KOH | 52 |
| CeO2/Ni/NC | HER | 320 | 1 M KOH | 65 |
| g-C3N4/CeO2/Fe3O4 | HER | 310 | 1 M KOH | 66 |
| Ag/CeO2/Light | HER | 240 | 1 M KOH | This work |
| 2.5-RuO2/CeO2 | OER | 460 | 1 M KOH | 67 |
| 1-RuO2/CeO2 | OER | 350 | 1 M KOH | 53 |
| 5-RuO2/CeO2 | OER | 510 | 1 M KOH | 53 |
| CeO2/Co@NCH | OER | 479 | 1 M KOH | 68 |
| Ag/CeO2/Light | OER | 450 | 1 M KOH | This work |
4. Conclusion
In summary, we studied defects induced in ceria that could enhance electrocatalytic water splitting for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). Ag/CeO2 nanocomposites, prepared via a facile solvothermal method, exhibit outstanding electrocatalytic performance. The achieved high electrocatalytic activity can be ascribed to the interaction between Ag and CeO2, which increases the oxygen vacancies at the interface caused by the reduction of Ce4+ to Ce3+. The oxygen vacancies at the Ag and CeO2 interface facilitate photogenerated electron–hole pair separation, resulting in delayed recombination of electron–hole pairs. Raman spectroscopy, X-ray photoelectron spectroscopy, electron paramagnetic resonance spectroscopy and photoluminescence studies confirm the increase in oxygen vacancies in Ag/CeO2. Besides, the incorporation of Ag onto the surface of CeO2 reduces the band gap and enhances light absorption, thereby contributing to the enhancement of photoelectrocatalytic activity. Compared to pristine CeO2, a remarkable enhancement in the electrocatalytic performance of Ag/CeO2 shows the effect of Ag. The further enhancement of catalytic activity under light illumination reveals the superior photoelectrocatalytic properties of the Ag/CeO2 composite. A drastic reduction in overpotential and an increase in current density are observed for Ag/CeO2. For the oxygen evolution reaction, Ag/CeO2 exhibited a reduction of 120 mV in the overpotential and an increase of 19.8 mA cm−2 in the current density compared to CeO2. For the hydrogen evolution reaction, Ag/CeO2 exhibited a reduction of 130 mV in the overpotential and an increase of 14.1 mA cm−2 in the current density. This work enhances the electrocatalytic performance of Ag/CeO2 by combining oxygen vacancies with light-assisted catalysis. While many studies focus on either doping or structural modifications, our approach leverages both strategies to significantly increase active site density and improve charge transfer. The light activation further reduces the Tafel slope by 30% in the OER and 11% in the HER, boosting the efficiency of both the OER and HER in Ag/CeO2. Furthermore, Ag/CeO2 was found to be stable for a duration of 2 hours under illumination. This synergistic approach can be used for utilizing Ag/CeO2 as a promising material for photoelectrocatalytic water splitting. Moreover, we believe that the present work not only provides detailed insights into the characterization of defective ceria but also opens up possibilities to develop new ceria-based catalysts for efficient electrocatalytic splitting of water.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
Ajit Kumar Dhanka thanks the Council of Scientific & Industrial Research (CSIR) and the University Grants Commission (UGC) for the Junior Research Fellowship (JRF)-16-6(119119 Dec. 2018/19). The authors would also like to acknowledge the University Science Instrumentation Centre (USIC), University of Delhi, Special Center for Nanoscience, Jawaharlal Nehru University (JNU), New Delhi, Central Instrumentation Facility (CRF), Jamia Millia Islamia, New Delhi and the Department of Materials Science and Engineering (DMSE), Indian Institute of Technology (IIT) Delhi, New Delhi, India for the characterization facilities. The TEM facilities at the Sophisticated Analytical Instrumentation Facility (SAIF) in All India Institute of Medical Sciences (AIIMS), New Delhi are gratefully acknowledged for the HRTEM measurements.References
- D. Pimentel, O. Bailey, P. Kim, E. Mullaney, J. Calabrese, L. Walman and X. Yao, Will limits of the earth's resources control human numbers?, Environ. Dev. Sustain., 1999, 1, 19–39, DOI:10.1023/A:1010008112119.
- S. Dale ( 2021). BP statistical review of world energy. BP Plc, London, UK, pp. 14–16. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf Search PubMed.
- G. Maggio and G. Cacciola, When will oil, natural gas, and coal peak?, Fuel, 2012, 98, 111–123, DOI:10.1016/j.fuel.2012.03.021.
- F. Umbach, Global energy security and the implications for the EU, Energy Policy, 2010, 38(3), 1229–1240, DOI:10.1016/j.enpol.2009.01.010.
- L. Xu, S. Wu, X. He, H. Wang, D. Deng, J. Wu and H. Li, Interface engineering of anti-perovskite Ni3FeN/VN heterostructure for high-performance rechargeable zinc–air batteries, Chem. Eng. J., 2022, 437, 135291, DOI:10.1016/j.cej.2022.135291.
- R. M. Navarro, M. A. Pena and J. L. G. Fierro, Hydrogen production reactions from carbon feedstocks: fossil fuels and biomass, Chem. Rev., 2007, 107(10), 3952–3991, DOI:10.1021/cr0501994.
- J. H. Kim, K. Shin, K. Kawashima, D. H. Youn, J. Lin, T. E. Hong and C. B. Mullins, Enhanced activity promoted by CeOx on a CoOx electrocatalyst for the oxygen evolution reaction, ACS Catal., 2018, 8(5), 4257–4265, DOI:10.1021/acscatal.8b00820.
- J. X. Feng, S. H. Ye, H. Xu, Y. X. Tong and G. R. Li, Design and synthesis of FeOOH/CeO2 heterolayered nanotube electrocatalysts for the oxygen evolution reaction, Adv. Mater., 2016, 28(23), 4698–4703, DOI:10.1002/adma.201600054.
- C. Yang, X. Li, M. Li, G. Liang and Z. Jin, Anchoring oxidation co-catalyst over CuMn2O4/graphdiyne S-scheme heterojunction to promote eosin-sensitized photocatalytic hydrogen evolution, Chin. J. Catal., 2024, 56, 88–103, DOI:10.1016/S1872-2067(23)64563-2.
- T. Li, S. Li, Q. Liu, Y. Tian, Y. Zhang, G. Fu and Y. Tang, Hollow Co3O4/CeO2 heterostructures in situ embedded in N-doped carbon nanofibers enable outstanding oxygen evolution, ACS Sustainable Chem. Eng., 2019, 7(21), 17950–17957, DOI:10.1021/acssuschemeng.9b04699.
- L. Bruno, S. Battiato, M. Scuderi, F. Priolo, A. Terrasi and S. Mirabella, Physical insights into alkaline overall water splitting with NiO micro flowers electrodes with ultra-low amount of Pt catalyst, Int. J. Hydrogen Energy, 2022, 47(80), 33988–33998, DOI:10.1016/j.ijhydene.2022.08.005.
- R. Siavash Moakhar, S. M. Hosseini-Hosseinabad, S. Masudy-Panah, A. Seza, M. Jalali, H. Fallah-Arani and M. Saliba, Photoelectrochemical water-splitting using CuO-based electrodes for hydrogen production: a review, Adv. Mater., 2021, 33(33), 2007285, DOI:10.1002/adma.202007285.
- D. J. Li, Z. G. Gu, W. Zhang, Y. Kang and J. Zhang, Epitaxial encapsulation of homodispersed CeO2 in a cobalt–porphyrin network derived thin film for the highly efficient oxygen evolution reaction, J. Mater. Chem. A, 2017, 5(38), 20126–20130, 10.1039/C7TA06580A.
- A. Sivanantham, P. Ganesan and S. Shanmugam, A synergistic effect of Co and CeO2 in nitrogen-doped carbon nanostructure for the enhanced oxygen electrode activity and stability, Appl. Catal., B, 2018, 237, 1148–1159, DOI:10.1016/j.apcatb.2017.08.063.
- S. Yang and Y. Zhang, Spectroscopic ellipsometry study of Mn doped CeO2 thin films prepared by radio-frequency magnetron sputtering, Thin Solid Films, 2022, 760, 139516, DOI:10.1016/j.tsf.2022.139516.
- J. Mazloom, F. E. Ghodsi, F. Z. Tepehan, G. G. Tepehan and I. Turhan, Enhanced lithium electrochemical performance and optical properties of CeO2–SnO2 nanocomposite thin films by transition metal (TM: Ni, Mn, and Co) doping, J. Sol-Gel Sci. Technol., 2018, 86, 51–62, DOI:10.1007/s10971-018-4603-4.
- N. V. Bôas, J. B. S. Junior, L. C. Varanda, S. A. S. Machado and M. L. Calegaro, Bismuth and cerium doped cryptomelane-type manganese dioxide nanorods as bifunctional catalysts for rechargeable alkaline metal-air batteries, Appl. Catal., B, 2019, 258, 118014, DOI:10.1016/j.apcatb.2019.118014.
- S. Dai, E. Montero-Lanzuela, A. Tissot, H. G. Baldoví, H. García, S. Navalón and C. Serre, Room temperature design of Ce (IV)-MOFs: from photocatalytic HER and OER to overall water splitting under simulated sunlight irradiation, Chem. Sci., 2023, 14(13), 3451–3461, 10.1039/D2SC05161C.
- S. Ghosh, S. Pal, M. Biswas, M. Thandavarayan, A. A. Reddy and M. K. Naskar, Dual Active Site Mediated Photocatalytic H2 Evolution through Water Splitting Using CeO2/PPy/BFO Double Heterojunction Catalyst, ACS Appl. Energy Mater., 2024, 7(24), 11453–11465, DOI:10.1021/acsaem.4c00269.
- F. A. C. Oliveira, M. A. Barreiros, A. Haeussler, A. P. Caetano, A. I. Mouquinho, P. M. O. e Silva and S. Abanades, High performance cork-templated ceria for solar thermochemical hydrogen production via two-step water-splitting cycles, Sustainable Energy Fuels, 2020, 4(6), 3077–3089, 10.1039/D0SE00318B.
- P. A. Koyale, S. V. Mulik, J. L. Gunjakar, T. D. Dongale, V. B. Koli, N. B. Mullani and S. D. Delekar, Synergistic enhancement of water-splitting performance using mof-derived ceria-modified g-C3N4 nanocomposites: Synthesis, performance evaluation, and stability prediction with machine learning, Langmuir, 2024, 40(26), 13657–13668, DOI:10.1021/acs.langmuir.4c01336.
- M. Orfila, D. Sanz, M. Linares, R. Molina, R. Sanz, J. Marugán and J. Á. Botas, H2 production by thermochemical water splitting with reticulated porous structures of ceria-based mixed oxide materials, Int. J. Hydrogen Energy, 2021, 46(33), 17458–17471, DOI:10.1016/j.ijhydene.2020.04.222.
- K. Yan, C. Wen, R. Li, B. Zhang, T. Liu, Q. Liu and Z. Zhou, Morphological optimized CeO2 and Cu-doped CeO2 nanocrystals for hydrogen production by solar photo-thermochemical water splitting based on surface photoinduced oxygen vacancies, Appl. Surf. Sci., 2023, 636, 157779, DOI:10.1016/j.apsusc.2023.157779.
- A. Haghighatzadeh, Enhanced third-order optical susceptibility in Ag-doped CeO2 nanostructures under pulsed Nd-YVO4 laser, Opt. Laser Technol., 2020, 126, 106114, DOI:10.1016/j.optlastec.2020.106114.
- X. Miao, H. Yang, J. He, J. Wang and Z. Jin, Adjusting the electronic structure of Keggin-type polyoxometalates to construct S-scheme heterojunction for photocatalytic hydrogen evolution, Acta Phys.-Chim. Sin., 2025, 100051, DOI:10.1016/j.actphy.2025.100051.
- S. Nishimura, D. Mott, A. Takagaki, S. Maenosono and K. Ebitani, Role of base in the formation of silver nanoparticles synthesized using sodium acrylate as a dual reducing and encapsulating agent, Phys. Chem. Chem. Phys., 2011, 13(20), 9335–9343, 10.1039/C0CP02985H.
- Y. Huang, C. F. Yan, C. Q. Guo, Z. X. Lu, Y. Shi and Z. D. Wang, Synthesis of GO-modified Cu2O nanosphere and the photocatalytic mechanism of water splitting for hydrogen production, Int. J. Hydrogen Energy, 2017, 42(7), 4007–4016, DOI:10.1016/j.ijhydene.2016.10.157.
- O. L. Pop, A. Mesaros, D. C. Vodnar, R. Suharoschi, F. Tăbăran, L. Mageruşan and C. Socaciu, Cerium oxide nanoparticles and their efficient antibacterial application in vitro against Gram-positive and Gram-negative pathogens, Nanomaterials, 2020, 10(8), 1614, DOI:10.3390/nano10081614.
- K. W. Aga, M. T. Efa and T. T. Beyene, Effects of sulfur doping and temperature on the energy bandgap of ZnO nanoparticles and their antibacterial activities, ACS Omega, 2022, 7(12), 10796–10803, DOI:10.1021/acsomega.2c00647.
- F. Su, P. Li, J. Huang, M. Gu, Z. Liu and Y. Xu, Photocatalytic degradation of organic dye and tetracycline by ternary Ag2O/AgBr–CeO2 photocatalyst under visible-light irradiation, Sci. Rep., 2021, 11(1), 85, DOI:10.1038/s41598-020-76997-0.
- W. Raza, M. M. Haque and M. Muneer, Synthesis of visible light driven ZnO: Characterization and photocatalytic performance, Appl. Surf. Sci., 2014, 322, 215–224, DOI:10.1016/j.apsusc.2014.10.067.
- L. Zhou, X. Li, Z. Yao, Z. Chen, M. Hong, R. Zhu and J. Zhao, Transition-metal doped ceria microspheres with nanoporous structures for CO oxidation, Sci. Rep., 2016, 6(1), 23900, DOI:10.1038/srep23900.
- N. Agasti and N. K. Kaushik, One pot synthesis of crystalline silver nanoparticles, Am. J. Nanomater, 2014, 2(1), 4–7, DOI:10.12691/ajn-2-1-2.
- M. V. Grabchenko, G. V. Mamontov, V. I. Zaikovskii, V. La Parola, L. F. Liotta and O. V. Vodyankina, The role of metal–support interaction in Ag/CeO2 catalysts for CO and soot oxidation, Appl. Catal., B, 2020, 260, 118148, DOI:10.1016/j.apcatb.2019.118148.
- D. Gao, Y. Zhang, Z. Zhou, F. Cai, X. Zhao, W. Huang and X. Bao, Enhancing CO2 electroreduction with the metal–oxide interface, J. Am. Chem. Soc., 2017, 139(16), 5652–5655, DOI:10.1021/jacs.7b00102.
- Z. Sun, X. Wu, D. Guan, X. Chen, J. Dai, Y. Gu and Z. Shao, One pot-synthesized Ag/Ag-doped CeO2 nanocomposite with rich and stable 3D interfaces and Ce3+ for efficient carbon dioxide electroreduction, ACS Appl. Mater. Interfaces, 2021, 13(50), 59993–60001, DOI:10.1021/acsami.1c19529.
- N. Mahmoudi Khatir, Z. Abdul-Malek, A. K. Zak, A. Akbari and F. Sabbagh, Sol–gel grown Fe-doped ZnO nanoparticles: antibacterial and structural behaviors, J. Sol-Gel Sci. Technol., 2016, 78, 91–98, DOI:10.1007/s10971-015-3922-y.
- T. V. M. Sreekanth, P. C. Nagajyothi, P. Muthuraman, G. Enkhtaivan, S. V. P. Vattikuti, C. O. Tettey and K. Yoo, Ultra-sonication-assisted silver nanoparticles using Panax ginseng root extract and their anti-cancer and antiviral activities, J. Photochem. Photobiol., B, 2018, 188, 6–11, DOI:10.1016/j.jphotobiol.2018.08.013.
- C. Schilling, A. Hofmann, C. Hess and M. V. Ganduglia-Pirovano, Raman spectra of polycrystalline CeO2: a density functional theory study, J. Phys. Chem. C, 2017, 121(38), 20834–20849, DOI:10.1021/acs.jpcc.7b06643.
- N. Agasti, M. A. Astle, G. A. Rance, J. Alves Fernandes, J. Dupont and A. N. Khlobystov, Cerium oxide nanoparticles inside carbon nanoreactors for selective allylic oxidation of cyclohexene, Nano Lett., 2020, 20(2), 1161–1171, DOI:10.1021/acs.nanolett.9b04579.
- C. Akdogan, B. Gökçal, M. Polat, K. Hamaloğlu, C. Kip and A. Tuncel, Porous, oxygen vacancy enhanced CeO2-x microspheres with efficient enzyme-mimetic and photothermal properties, ACS Sustainable Chem. Eng., 2022, 10, 9492–9505, DOI:10.1021/acssuschemeng.2c01981.
- R. Bhargava, J. Shah, S. Khan and R. K. Kotnala, Hydroelectric cell based on a cerium oxide-decorated reduced graphene oxide (CeO2–rG) nanocomposite generates green electricity by room-temperature water splitting, Energy Fuels, 2020, 34(10), 13067–13078, DOI:10.1021/acs.energyfuels.0c02192.
- Z. Wu, M. Li, J. Howe, H. M. Meyer III and S. H. Overbury, Probing defect sites on CeO2 nanocrystals with well-defined surface planes by Raman spectroscopy and O2 adsorption, Langmuir, 2010, 26(21), 16595–16606, DOI:10.1021/la101723w.
- W. Cai, Y. Shi, Y. Zhao, M. Chen, Q. Zhong and Y. Bu, The solvent-driven formation of multi-morphological Ag–CeO2 plasmonic photocatalysts with enhanced visible-light photocatalytic reduction of CO2, RSC Adv., 2018, 8(71), 40731–40739, 10.1039/C8RA08938H.
- A. L. Cámara, V. C. Corberán, A. Martínez-Arias, L. Barrio, R. Si, J. C. Hanson and J. A. Rodriguez, Novel manganese-promoted inverse CeO2/CuO catalyst: In situ characterization and activity for the water-gas shift reaction, Catal. Today, 2020, 339, 24–31, DOI:10.1016/j.cattod.2019.01.014.
- M. M. Khan, S. A. Ansari, D. Pradhan, D. H. Han, J. Lee and M. H. Cho, Defect-induced band gap narrowed CeO2 nanostructures for visible light activities, Ind. Eng. Chem. Res., 2014, 53(23), 9754–9763, DOI:10.1021/ie500986n.
- H. Moreno, G. L. Domingues, M. D. Assis, P. P. Ortega, V. R. Mastelaro, M. A. Ramirez and A. Z. Simões, The relationship between photoluminescence emissions and photocatalytic activity of CeO2 nanocrystals, Inorg. Chem., 2023, 62(10), 4291–4303, DOI:10.1021/acs.inorgchem.2c04411.
- W. Li, S. Xie, M. Li, X. Ouyang, G. Cui, X. Lu and Y. Tong, CdS/CeOx heterostructured nanowires for photocatalytic hydrogen production, J. Mater. Chem. A, 2013, 1(13), 4190–4193, 10.1039/C3TA10394C.
- B. Choudhury, P. Chetri and A. Choudhury, Oxygen defects and formation of Ce3+ affecting the photocatalytic performance of CeO2 nanoparticles, RSC Adv., 2014, 4(9), 4663–4671, 10.1039/C3RA44603D.
- X. J. Wen, C. G. Niu, L. Zhang, C. Liang and G. M. Zeng, A novel Ag2O/CeO2 heterojunction photocatalysts for photocatalytic degradation of enrofloxacin: possible degradation pathways, mineralization activity and an in-depth mechanism insight, Appl. Catal., B, 2018, 221, 701–714, DOI:10.1016/j.apcatb.2017.09.060.
- J. Chen, S. Shen, P. Wu and L. Guo, Nitrogen-doped CeOx nanoparticles modified graphitic carbon nitride for enhanced photocatalytic hydrogen production, Green Chem., 2015, 17(1), 509–517, 10.1039/c4gc01683a.
- P. Dutta, S. Pal, M. S. Seehra, Y. Shi, E. M. Eyring and R. D. Ernst, Concentration of Ce3+ and oxygen vacancies in cerium oxide nanoparticles, Chem. Mater., 2006, 18(21), 5144–5146, DOI:10.1021/cm061580n.
- T. Zheng, S. Chen, J. Qin, F. Yang, J. Shi, Y. Hu and Q. He, Pd/CeO2 Interface with Abundant Oxygen Vacancies for Alkaline Hydrogen Evolution/Oxidation Reaction, ACS Appl. Nano Mater., 2024, 7(2024), 19502–19513, DOI:10.1021/acsanm.4c03414.
- N. Wang, W. Qian, W. Chu and F. Wei, Crystal-plane effect of nanoscale CeO2 on the catalytic performance of Ni/CeO2 catalysts for methane dry reforming, Catal. Sci. Technol., 2016, 6(10), 3594–3605, 10.1039/C5CY01790D.
- L. Wang, Y. Yu, H. He, Y. Zhang, X. Qin and B. Wang, Oxygen vacancy clusters essential for the catalytic activity of CeO2 nanocubes for o-xylene oxidation, Sci. Rep., 2017, 7(1), 1–11, DOI:10.1038/s41598-017-13178-6.
- J. Li, F. Liu and Y. Li, Fabrication of an Ag/Ag2 MoO4 plasmonic photocatalyst with enhanced photocatalytic performance for the degradation of ciprofloxacin, New J. Chem., 2018, 42(14), 12054–12061, 10.1039/C8NJ02327A.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives, Chem. Soc. Rev., 2017, 46(2), 337–365, 10.1039/C6CS00328A.
- X. Wang, Y. Li, X. Guo and Z. Jin, In situ synthesis of Ag/Ag2O on CeO2 for boosting electron transfer in photocatalytic hydrogen production, J. Phys. Chem. C, 2022, 126(31), 13015–13024, DOI:10.1021/acs.jpcc.2c03411.
- P. A. Koyale and S. D. Delekar, A review on practical aspects of CeO2 and its composites for photoelectrochemical water splitting, Int. J. Hydrogen Energy, 2024, 51, 515–530, DOI:10.1016/j.ijhydene.2023.06.251.
- J. Yu, G. Li, H. Liu, L. Zeng, L. Zhao, J. Jia and Y. Hu, Electrochemical flocculation integrated hydrogen evolution reaction of Fe@ N-doped carbon nanotubes on iron foam for ultralow voltage electrolysis in neutral media, Adv. Sci., 2019, 6(18), 1901458, DOI:10.1002/advs.201901458.
- K. Ackland and J. M. D. Coey, Room temperature magnetism in CeO2-A review, Phys. Rep., 2018, 746, 1–39, DOI:10.1016/j.physrep.2018.04.002.
- M. Liu, Z. Ji, X. Shen, H. Zhou, J. Zhu, X. Xie and G. Zhu, An Electrocatalyst for a Hydrogen Evolution Reaction in an Alkaline Medium: Three-Dimensional Graphene Supported CeO2 Hollow Microspheres, Eur. J. Inorg. Chem., 2018, 3952–3959, DOI:10.1002/ejic.201800757.
- M. C. Sung, G. H. Lee and D. W. Kim, CeO2/Co(OH)2 hybrid electrocatalysts for efficient hydrogen and oxygen evolution reaction, J. Alloys Compd., 2019, 800, 450–455, DOI:10.1016/j.jallcom.2019.06.047.
- M. Zhiani and S. Kamali, Synergistic effect of ceria on the structure and hydrogen evolution activity of nickel nanoparticles grown on reduced graphene oxide, J. Mater. Chem. A, 2017, 5(17), 8108–8116, 10.1039/C7TA00146K.
- L. Tian, H. Liu, B. Zhang, Y. Liu, S. Lv, L. Pang and J. Li, Ni and CeO2 nanoparticles anchored on cicada-wing-like nitrogen-doped porous carbon as bifunctional catalysts for water splitting, ACS Appl. Nano Mater., 2021, 5(1), 1252–1262, DOI:10.1021/acsanm.1c03850.
- D. Xiang, Z. Qin, Y. Gan, X. Luo, X. Li, L. Hu and S. Jiao, Interfacial charge density modulation by coupling CeO2 with dual-phase NiS/Ni3S2 to accelerate alkaline water splitting, Mater. Today Chem., 2023, 34, 101791, DOI:10.1016/j.mtchem.2023.101791.
- S. M. Galani, A. Mondal, D. N. Srivastava and A. B. Panda, Development of RuO2/CeO2 heterostructure as an efficient OER electrocatalyst for alkaline water splitting, Int. J. Hydrogen Energy, 2020, 45(37), 18635–18644, DOI:10.1016/j.ijhydene.2019.08.026.
- A. K. Mishra, J. Willoughby, S. L. Estes, K. C. Kohler and K. S. Brinkman, Impact of morphology and oxygen vacancy content in Ni, Fe co-doped ceria for efficient electrocatalyst based water splitting, Nanoscale Adv., 2024, 6(18), 4672–4682, 10.1039/D4NA00500G.
Footnote |
| † Electronic supplementary information (ESI) available: UV-vis spectra, calculated band gaps by UV-vis diffuse reflectance spectroscopy (DRS), XRD peak broadening, Raman peak shifting, XPS spectra with the calculated area of Ce 3d peaks for CeO2 and Ag/CeO2 nanocomposites, low resolution TEM image, calculated average size, and electrochemical impedance spectroscopy (EIS). See DOI: https://doi.org/10.1039/d5ma00321k |
| This journal is © The Royal Society of Chemistry 2025 |