Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Development of a colorimetric sensor utilizing itaconic acid-mediated Griess reaction for sensitive detection of nitrite and nitrate in agricultural products†
Anubhab
Das
,
Sindhu I
Sanakal
,
Gomathi
Sivakumar
,
Anashwara
Babu
and
Samarendra
Maji
 *
*
Department of Chemistry, SRM Institute of Science and Technology, Kattankulathur, 603203, Tamilnadu, India. E-mail: samarenr@srmist.edu.in
First published on 20th June 2025
Abstract
The presence of nitrite (NO2−) and nitrate (NO3−) in agricultural products causes significant health risks, highlighting the need for sensitive and selective detection methods. In this study, we developed a colorimetric sensor based on the Griess assay under organic acidic conditions to detect these compounds, where the formation of a pink color suggests the presence of NO2− and NO3−. We synthesized 4-aminobenzenesulfonohydrazide (C) and 2-(2-(naphthalen-1-ylamino)ethoxy)ethanol (E). N-(1-Naphthyl)ethylenediamine dihydrochloride (F), and commercially available organic dicarboxylic acid “itaconic acid” was used for the investigation. Two chemosensors were designed, one with compounds C and E (known as CE) and another containing compounds C and F (known as CF). These two chemosensors formed pink azo–dyes in the presence of NO2− in acidic conditions (using itaconic acid). The sensing ability was evaluated using UV-spectroscopy, and different sensitivity was demonstrated for the analyte (NO2−). The binding constants for sensors CE and CF were determined to be 5.06 × 10−4 M−1 and 16.7 × 10−4 M−1, respectively, based on the Benesi–Hildebrand (B–H) plot. Under the optimized conditions, chemosensor CE showed a linear range of 0.005–0.421 mM with a detection limit (LOD) of 0.62 mM, while chemosensor CF exhibited a range of 0.01–0.453 mM with a LOD of 0.52 mM. A smartphone-assisted NO2− sensing device was designed based on the RGB value of the generated pink color, which demonstrates the sensor's ability towards real-time detection of NO2− ions. Additionally, the system was extended to paper-based test strips for on-site detection. Our proposed sensor system is also capable of detecting NO3− by using Zn dust as a reducing agent to convert NO3− to NO2−. Real sample testing demonstrated excellent recovery, confirming the potential of this colorimetric sensor for NO2− and NO3− detection in agricultural products.
1. Introduction
Nitrite (NO2−) and nitrate (NO3−) are naturally occurring nitrogen-containing compounds that play crucial roles in the biological nitrogen cycle, ensuring the recycling of nitrogen within ecosystems.1 They are produced through nitrification, a process where nitrifying bacteria first oxidize ammonia (NH3) or ammonium (NH4+) into NO2−, followed by the conversion of NO2− into NO3−. NO3−, being highly soluble and bioavailable, serves as a crucial nitrogen source for plants, which assimilate it to synthesize essential biomolecules such as amino acids, proteins, and nucleotides. In oxygen-deprived environments, NO3− and NO2− undergo denitrification, where denitrifying bacteria convert them back into gaseous nitrogen or nitrous oxide, returning nitrogen to the atmosphere and maintaining ecological balance. These compounds act as intermediates in nitrogen transformations, adapting to environmental conditions to regulate nitrogen availability.2,3 From various sources, NO3− and NO2− can enter the human body via different pathways. Vegetables play a significant role in absorbing these compounds from soil and water, especially in areas where nitrogen-based fertilizers or organic and livestock waste are heavily used.4 Drinking water contaminated with agricultural runoff is another major contributor. Additionally, processed meats like bacon, sausages, and ham often contain added NO2− as a preservative and color stabilizer.5,6 The Joint Food and Agricultural Organization/World Health Organization (WHO) has set an acceptable daily intake (ADI) of 3.7 mg of NO3− ion per kg of body weight and 0.06 mg of NO2− ion per kg of body weight.7,8 To a certain level, NO3− intake is good as it has some positive effects like improving blood flow, reducing blood pressure, and cardio and vasoprotective effects.9–11 NO2− helps to control and inhibit the growth of pathogenic bacteria, e.g., Clostridium botulinum and Listeria monocytogenes.12,13 Besides, it acts as an antioxidant against lipid oxidation as well.14However, excessive exposure to NO2− or NO3− beyond the aforementioned limit can cause significant health risks to humans. In the stomach, NO2− can combine with amines and amides to produce nitrosamines, which are strong carcinogens associated with a higher risk of gastrointestinal cancers.15,16 High levels of NO2− can also cause methemoglobinemia, a condition that impairs the blood's ability to carry oxygen, leading to cyanosis or “blue baby syndrome” in infants.17–19 Prolonged exposure to elevated NO3− and NO2− levels has been associated with adverse effects on thyroid function and a higher risk of cardiovascular diseases, pulse acceleration, muscular tremors, dyspnoea, cyanosis and vomiting, and can even cause death in extreme cases.20 Due to the widespread recognition of these problems, the majority of developed nations have put in place legal frameworks designed to control their levels in food and environmental products. Therefore, the daily routine examination of NO3− or NO2− in agricultural products is necessary for safety and quality management.
Several strategies have been developed to detect NO3− and NO2− accurately due to their environmental and health implications. Scientists have explored a variety of advanced methods, including electrochemical techniques,21,22 which involve measuring the electrical response of NO3− or NO2− in a solution, and potentiometry, which uses ion-selective electrodes to determine their concentrations.23 Conductometry is also a method by which these ions are detected by the changes in electrical conductivity due to the presence of NO3− and NO2−.24 Spectroscopic techniques, such as UV-visible and infrared spectroscopy, enable qualitative and quantitative analysis based on light absorption or emission properties.25,26 Ion chromatography and high-performance liquid chromatography (HPLC) are widely utilized for their ability to separate and quantify NO3− and NO2− in complex mixtures,27,28 while gas chromatography-mass spectrometry (GC-MS) provides high sensitivity and specificity in detection.29 The chemiluminescence method, relying on light emission during chemical reactions, has also been effectively employed.30 Enzyme-based detection methods have gained attention for their specificity, particularly in detecting NO3− through enzymatic reactions.31 Similarly, capillary electrophoresis has proven to be a reliable technique for quantifying both NO3− and NO2− due to its high resolution and efficiency.32 Despite their effectiveness, these techniques have intrinsic limitations, such as high operational costs, time-consuming procedures, and the need for skilled personnel, which hinder their practical application in field settings. Given these challenges, researchers have sought alternative approaches with greater simplicity, sensitivity, and selectivity. Among these, colorimetric detection using the Griess reagent has stood out as a straightforward and widely applied method. This approach, which has been used for over a century, relies on a color change reaction to visually or spectrophotometrically determine NO2− or NO3− concentrations, offering a cost-effective and practical solution for routine analysis.33
The Griess reaction, first reported in 1879, is a well-established chemical method for detecting NO2− and indirectly NO3− in various samples. This reaction employs two reagents, sulfanilamide and N-(1-naphthyl)ethylenediamine dihydrochloride (NED), under acidic conditions. Initially, sulfanilamide reacts with NO2− in the Griess diazotization reaction to form an electrophilic diazonium salt. This intermediate subsequently undergoes an azo coupling reaction with NED to produce an intensely colored azo dye, characterized by the functional group R–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–R′. Due to its simplicity and effectiveness, the Griess assay has remained the most widely used colorimetric method for NO2− detection for over a century.34–38 Capitán-Vallvey and coworkers developed NO2− detecting test strips based on the Griess assay under acidic conditions, demonstrating their application for detecting NO2− in various water sources, including spring, mineral, tap, well, and seawater.39 Wu and coworkers designed a dual-readout sensor by conjugating carbon dots with 3-aminophenol, offering both colorimetric and fluorescent detection for NO2− with detection limits of 10 nM and 2.5 μM, respectively.40 Furthermore, polydimethylsiloxane (PDMS)-based microfluidic devices have been employed to detect NO2− in meat samples with a limit of detection (LOD) of 0.1 mg L−1.41 Detection of NO3− using the Griess assay requires an additional reduction step to convert NO3− into NO2−. This reduction is typically performed using metals such as cadmium (Cd) or zinc (Zn) under acidic conditions, following the reaction mechanism: NO3− + M(s) + 2H+ → NO2− + M2+ + H2O.42 Zn is preferred due to its lower toxicity, affordability, and eco-friendly properties. Murray and coworkers designed a portable NO3− detection system by coupling Zn powder with the Griess assay.43,44 Pai et al. introduced vanadium as a reducing agent for NO3−, combining it with the Griess reagent to design a sensor capable of detecting both NO3− and NO2−, achieving a LOD of 0.2 μM.45 Polymer-based adaptations of the Griess test have also been reported.41,46 A Griess reagent-doped hydrogel test kit and a Zn powder-doped starch film were developed to quantify NO2− and NO3−, exhibiting LODs of 50 μg L−1 for NO2− and 0.32 μg L−1 for NO3−, respectively.37 These advancements demonstrate the versatility and adaptability of the Griess reaction for diverse applications in NO2− and NO3− detection.
N–R′. Due to its simplicity and effectiveness, the Griess assay has remained the most widely used colorimetric method for NO2− detection for over a century.34–38 Capitán-Vallvey and coworkers developed NO2− detecting test strips based on the Griess assay under acidic conditions, demonstrating their application for detecting NO2− in various water sources, including spring, mineral, tap, well, and seawater.39 Wu and coworkers designed a dual-readout sensor by conjugating carbon dots with 3-aminophenol, offering both colorimetric and fluorescent detection for NO2− with detection limits of 10 nM and 2.5 μM, respectively.40 Furthermore, polydimethylsiloxane (PDMS)-based microfluidic devices have been employed to detect NO2− in meat samples with a limit of detection (LOD) of 0.1 mg L−1.41 Detection of NO3− using the Griess assay requires an additional reduction step to convert NO3− into NO2−. This reduction is typically performed using metals such as cadmium (Cd) or zinc (Zn) under acidic conditions, following the reaction mechanism: NO3− + M(s) + 2H+ → NO2− + M2+ + H2O.42 Zn is preferred due to its lower toxicity, affordability, and eco-friendly properties. Murray and coworkers designed a portable NO3− detection system by coupling Zn powder with the Griess assay.43,44 Pai et al. introduced vanadium as a reducing agent for NO3−, combining it with the Griess reagent to design a sensor capable of detecting both NO3− and NO2−, achieving a LOD of 0.2 μM.45 Polymer-based adaptations of the Griess test have also been reported.41,46 A Griess reagent-doped hydrogel test kit and a Zn powder-doped starch film were developed to quantify NO2− and NO3−, exhibiting LODs of 50 μg L−1 for NO2− and 0.32 μg L−1 for NO3−, respectively.37 These advancements demonstrate the versatility and adaptability of the Griess reaction for diverse applications in NO2− and NO3− detection.
In these reported approaches, mineral acid (e.g., HCl) has been used as the source of acid in the Griess test. However, the toxic nature of mineral acid affects the environment. These shortcomings can be overcome by using organic acid. Organic acids’ lower-toxicity and milder activity make them environmentally sustainable and applicable in food and beverage applications. Itaconic acid is a popular organic acid and an unsaturated dicarboxylic acid with a methylene group connected to one carboxyl group. It has huge applications in the agro-based, plastics, textile, paint and pharmaceutical sectors.47
Furthermore, none of the studies examine the use of itaconic acid as the acid source in test strip-based sensors or smartphone-based sensors to cover both NO2− and NO3− in a single report. In our present study, we developed two chemosensors (CE and CF) for the colorimetric detection of NO2− and NO3−. These systems were designed using itaconic acid as the acidic component, and their functionality was based on the principles of the Griess assay. Both sensor systems demonstrated the capability to detect NO2−, with results observable either visually through a distinct color change or quantitatively using UV-visible spectroscopy. To evaluate the practicality of the proposed sensors, trace amounts of NO2− were successfully detected in real-world samples, including various vegetables and lake water, demonstrating the system's reliability and applicability in diverse environments. Additionally, test strips were fabricated by coating the dye onto filter paper, which produced a vivid pink color upon exposure to NO2−, offering a simple and portable detection method. Furthermore, the system was extended for NO3− detection by incorporating a reduction step using Zn dust. In this process, NO3− was reduced to NO2−, enabling the sensor to detect NO3− indirectly while maintaining a high level of responsiveness and accuracy. These results highlight the versatility and effectiveness of the developed systems for NO2− and NO3− sensing in various applications.
2. Experimental
2.1. Materials
All solvents and chemicals used in this work were of analytical grade and used without further purification. N-Acetyl sulfonyl chloride was purchased from Sigma-Aldrich. N-(1-Naphthyl)ethylenediamine dihydrochloride, itaconic acid (99% purity), 4-dimethylaminopyridine (DMAP), dichloromethane, activated basic aluminum oxide, silica gel, acetonitrile, hydrazine hydrate (80%), triethylamine (AR 99.5%), sodium oxalate, sodium hydroxide, sodium carbonate, sodium fluoride, sodium bromide, sodium sulfate, sodium iodide, sodium phosphate tribasic, sodium nitrate, and sodium chloride were obtained from Sisco Research Laboratories Pvt. Ltd (SRL), Maharashtra, India. 1-Naphthylamine was purchased from TCI Chemicals (India) Pvt. Ltd. Sodium nitrite and hydrochloric acid, hexane (Hex), ethyl acetate (EA), and chloroform were obtained from Avantor Performance Materials India Limited, Maharashtra, India. 1-n-Butyl-3-methylimidazoliumtetrafluoroborate (98% purity) ([BMIM]BF4) was sourced from Thermo Fisher Scientific India Pvt. Ltd, Mumbai. Methanol was obtained from Advent Chembio Pvt. Ltd, and Milli-Q water was used throughout the experiments.2.2. Characterization
1H-NMR spectra were recorded on a Bruker Advance 500 MHz spectrometer in chloroform-d (CDCl3), purchased from Eurisotop, at room temperature. Chemical shifts (δ) are reported in parts per million (ppm), relative to CDCl3 at 7.24 ppm. Fourier transform infrared (FT-IR) spectra were acquired using a Shimadzu IR Tracer-100 in the attenuated total reflectance (ATR) mode, covering the 400–4000 cm−1 range. LC-MS analysis was performed on an LC-MS 2020 system equipped with an LC10ADVP Binary pump (Shimadzu, Japan). UV-vis absorption spectra were recorded in water using an Agilent Cary 5000 double-beam UV-vis spectrometer. Ground and excited states were optimized using density functional theory (DFT) with the B3LYP/6-31G and LANL2DZ basis sets. RGB values were calculated using the RGB Detector app on an Android OS to determine the LOD.2.3. Synthesis
Next, compound 4-aminobenzenesulfonohydrazide (C) was prepared as a previously reported method.46 Compound (B) (7.8 g, 0.034 mol) was dissolved in a 25 mL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O and conc. HCl in a round-bottom flask, and the reaction mixture was refluxed for 1.5 h. After cooling to room temperature, the mixture was neutralized by the gradual addition of solid sodium bicarbonate until effervescence ceased. The solution was then placed on ice to facilitate precipitation. The resulting precipitate was filtered and dried until constant weight to get 0.89 g of product with a yield of 14%. 1H NMR of the compound (C) is shown in Fig. S4 (ESI†). 1H NMR (500 MHz, DMSO-d6): 1.77 (m, 3H), 5.93 (s, 2H), 6.56 (d, 2H), 7.43 (d, 2H). FTIR (λmax, cm−1): 686, 1084, 1156, 2900, 3200, 3333, and 3470 (Fig. S5, ESI†). LC-MS (m/z): [M + H]+ = 188 (calculated); 187.6 (observed) (Fig. S6, ESI†).
1 mixture of H2O and conc. HCl in a round-bottom flask, and the reaction mixture was refluxed for 1.5 h. After cooling to room temperature, the mixture was neutralized by the gradual addition of solid sodium bicarbonate until effervescence ceased. The solution was then placed on ice to facilitate precipitation. The resulting precipitate was filtered and dried until constant weight to get 0.89 g of product with a yield of 14%. 1H NMR of the compound (C) is shown in Fig. S4 (ESI†). 1H NMR (500 MHz, DMSO-d6): 1.77 (m, 3H), 5.93 (s, 2H), 6.56 (d, 2H), 7.43 (d, 2H). FTIR (λmax, cm−1): 686, 1084, 1156, 2900, 3200, 3333, and 3470 (Fig. S5, ESI†). LC-MS (m/z): [M + H]+ = 188 (calculated); 187.6 (observed) (Fig. S6, ESI†).
2.4. Preparation of the real sample
The real sample was brought from a nearby supermarket. The sample was prepared according to the procedure mentioned in the literature with a slight modification.50 All vegetables were cleaned properly. The root vegetables were peeled to remove possible traces of soil. The entire mass of the sample was homogenized with a blender. 1.0 g of homogenized sample was extracted with 10 mL MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 1
H2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 extraction solution, followed by ultrasonication for 15 minutes. The extracted solution was diluted over 20 times and filtered through a 0.45 μm syringe filter. The lake water and tap water were directly filtered through a 0.45 μm syringe filter. Finally, real samples were obtained after the stock solution was spiked with various concentrations of NO2−.
1 extraction solution, followed by ultrasonication for 15 minutes. The extracted solution was diluted over 20 times and filtered through a 0.45 μm syringe filter. The lake water and tap water were directly filtered through a 0.45 μm syringe filter. Finally, real samples were obtained after the stock solution was spiked with various concentrations of NO2−.
2.5. Sensing procedure
The NO2− sensing study was conducted at room temperature. A standard stock solution (5 mM) of NO2− was prepared by dissolving the appropriate amount of NO2− salt in an aqueous solution. Interference study was checked by fluoride (F−), chloride (Cl−), bromide (Br−), iodide (I−), oxalate (C2O42−), phosphate (PO43−), carbonate (CO32−), nitrate (NO3−), hydroxide (OH−), and sulfate (SO42−) anions and corresponding stock solutions (5 mM) were prepared. Itaconic acid was used as the acid source. Combinations of sensor compounds, specifically (C) with (E) (sensor CE) and (C) with (F) (sensor CF), were prepared in an aqueous solution (10 mL, 1 mM) by stirring for 30 minutes. A 10 mM itaconic acid solution (10 mL) was also prepared in water. An equivalent mixture of both dyes (0.5 mL) and itaconic acid (0.5 mL) was placed in a cuvette, followed by the addition of 1 mL of water. The UV-vis spectra of this mixture were recorded over a wavelength range of 200 to 800 nm. Following the same procedure, NO3− was detected using Zn dust (1 mg) and 1N HCl (5 μL) as a reducing agent. Here, in addition to itaconic acid, 1N HCl (5 μL) was also used as it helps in the reduction process.3. Results and discussion
3.1. Synthesis and characterization of 4-aminobenzenesulfonylhydrazide (C) and 2-(2-(naphthalen-1-ylamino)ethoxy)ethanol (E)
The compound (C) was synthesized by a two-step procedure as previously reported with slight modifications (Scheme 1).46 The first step is the hydrazination of the starting materials of (A) in the presence of hydrazine hydrate, followed by the deprotection of the N-acetyl group in an acid medium to obtain (C). The structure of (C) was confirmed by 1H NMR spectroscopy (Fig. S4, ESI†), where the multiplet peak arises at δ 1.77 due to three protons present next to the –SO2 group, and the singlet peak at δ 5.93 ppm due to two amine hydrogens next to the phenyl ring. Four aromatic protons are exhibited, corresponding to a doublet peak at δ 6.56 and 7.43 ppm. The deprotection was confirmed by the vanishing of the acetyl (–COCH3) protons’ peak at 2.09 ppm (Fig. S4, ESI†), which was present in Fig. S1 (ESI†). Additionally, the mass m/z peak at 187.7 confirms the formation of (C) (Fig. S7, ESI†).Compound (E) was designed and synthesized via a single-step process. The starting compound (D) was reacted with ligand ethyleneglycolmono-2-chloroethylether to form the mono-substituted product (E), catalyzed by ionic liquid [BMIM]BF4. The formation of the compound was proven by 1H NMR spectroscopy (Fig. S7, ESI†). Four pairs of proton peaks were exhibited at δ 3.45, 3.61, 3.75, and 3.83 ppm, respectively. The seven aromatic protons were observed at δ 7.24–7.84 ppm. The mass m/z peak at 232.15 confirms the successful formation of (E) (Fig. S9, ESI†).
3.2. Colorimetric sensing of NO2−
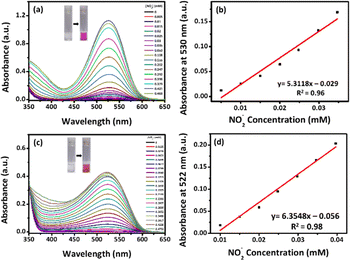
The absorption spectra of the chemosensor (CE) were recorded in the presence of NO2−, as shown in Fig. 1a. No peak was formed at 530 nm in the absence of NO2−. However, after the incubation of NO2−, a new peak at 530 nm was generated due to the formation of the diazo compound, and gradually, the intensity of the peak was increased with the increase of NO2− concentration. The results support the visual observations shown in Fig. S10 (ESI†), demonstrating a color change in the sensor system from colorless to pink. The system has shown an enhancement of absorbance for NO2− concentration in the range of 0.005 to 0.421 mM. However, with the further addition of NO2−, a reduction in absorbance was observed, notably at a concentration around ∼0.47 mM. As indicated in Fig. S11 (ESI†), a pink color solution was observed up to 0.421 mM of NO2− (Fig. S11a, ESI†), followed by a yellow color solution at 0.842 mM. This might be owing to the development of the azo–dye complex, whose concentration increases with the addition of NO2−, and which is thought to be destabilized due to the dye's reduced solubility and the dyes’ predominant hydrophobic interaction in the investigating aqueous medium. The absorption value demonstrated a good linear range of 0.005–0.0347 mM (Fig. 1b). The regression equation is A = 5.3118 (mM) − 0.029 with a correlation coefficient of 0.96. The LOD was calculated using the following equation (eqn (1)) and found to be 0.62 mM.| LOD = 3.3 × σ/S | (1) |
Similarly, the chemosensor (CF) (Fig. 1c) showed a peak at 522 nm upon interaction with NO2−, confirming the formation of the azo dye compound. The results support the visual observations shown in Fig. S12 (ESI†), demonstrating a color change in the sensor system from colorless to pink. The absorbance increased linearly within the range of 0.01–0.0397 mM (Fig. 1d), with the regression equation A = 6.3548 (mM) − 0.056 and a correlation coefficient of 0.98. Similarly, (CF) demonstrated a reduction in absorbance at higher concentrations, particularly at 0.4751 mM, owing to the destabilization at higher concentrations of NO2− as discussed earlier (Fig. S11b, ESI†), where at the 0.88 mM NO2− concentration, the pink color solution turned yellow. The LOD for (CF) was calculated and found to be 0.52 mM.
Furthermore, the binding constant was calculated by using the Benesi–Hildebrand method (B–H plot), where a graph between 1/(A0 − A) and 1/[NO2−] was plotted (Fig. 2) and a linear plot was obtained. The chemosensor (CE) demonstrated a binding constant of 5.06 × 10−4 M−1 with R2 = 0.985 (Fig. 2a) and (CF) exhibited a binding constant value of 16.7 × 10−4 M−1 with R2 = 0.934 (Fig. 2b).
 | ||
| Fig. 2 Benesi–Hildebrand plot of chemosensor (a) (CE) and (b) (CF) upon different concentrations of NO2− (0–0.4 mM). | ||
The temperature and the long-term stability of the chemosensors were checked. The study was done by observing the change in the intensity of the peak that arises for both chemosensors with and without the presence of NO2−. Without the presence of the analyte, chemosensor (CE) exhibited an absorption peak at 324 nm, whereas (CF) showed an absorption peak at 319 nm (Fig. S13, ESI†). The temperature ranging from 25 to 50 °C was selected and the results are depicted in Fig. S14 (ESI†). The outcome demonstrated a slight increase in the intensity for sensor (CE), which may be due to the increase of solubility of the CE, whereas the intensity of sensor (CF) remains almost the same (Fig. S14a and c, ESI†).
The intensity of the nitrite detection peak corresponding to both sensors was reduced with the increase in temperature (Fig. S14b and d, ESI†). A prolonged stability test was done for 8 h (Fig. S15, ESI†). We have noticed some changes of UV-vis absorbance @530 nm for CE whereas, @522 nm for CF at the earlier stage of investigation. After 1 h, an unaltered intensity value was observed from 2–8 h for both sensors, which referred to the negligible change in sensitivity over time (Fig. S15a and c, ESI†). Therefore, we can conclude that both chemosensors were stable at that temperature and over a long time. However, the response in the presence of the analyte (NO2−) needs to be observed quickly, as the intensity of the peak changes with temperature and time.
3.3. Selectivity and interference study
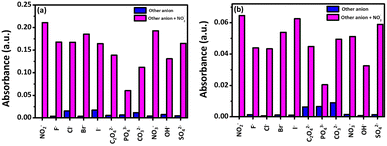
The selective colorimetric response of the proposed sensor systems to NO2− was evaluated by introducing various other anions (F−, Cl−, Br−, I−, C2O42−, PO43−, CO32−, NO3−, OH− and SO42−). Both the chemosensors (CE) and (CF) exhibited high selectivity for NO2− detection. When NO2− was introduced, the chemosensor (CE) displayed a clear peak at 530 nm; however, no significant changes were observed for other anions (Fig. 3a and Fig. S16, ESI†). This was further validated by a histogram plot (Fig. 3b) showing enhanced absorbance at 530 nm exclusively for NO2− and a visible color change to pink, while other anions caused no color change (Fig. S17, ESI†). Similarly, the introduction of NO2− caused a clear peak at 522 nm in the chemosensor (CF), with no discernible alterations for the other anions (Fig. 3c and Fig. S18, ESI†). When NO2− was added, the (CF) chemosensor showed a selective pink color, similar to the (CE) chemosensor, however, other exploring anions did not exhibit any color change (Fig. S19, ESI†). The histogram plot for (CF) (Fig. 3d) confirmed this finding. These findings establish both systems as highly specific for NO2− detection.Colorimetric readouts towards different anions were observed to check the interference study of our proposed sensors (Fig. 4).
Therefore, the competition experiment was carried out in the presence of NO2− (1 equiv.) mixed with other anions (1 equiv.) in aqueous solution. Different types of anions viz. F−, Cl−, Br−, I−, C2O42−, PO43−, CO32−, NO3−, OH− and SO42− were considered in order to investigate the interference study. According to observation, both sensors showed some interfering effects with PO43− and OH−. The interference observed in the proposed colorimetric sensor, particularly with PO43− and OH− ions, is primarily due to their impact on the system's acidity. The Griess reaction, which serves as the basis for the detection mechanism, requires an acidic environment to facilitate the efficient formation of the diazonium intermediate and the subsequent azo dye. OH− ions, being strongly basic, neutralize protons (H+) in the solution, reducing the acidity and hindering the reaction. Similarly, PO43− ions, though weaker bases, act as buffers that partially neutralize the acidic conditions, diluting the concentration of free H+ necessary for the reaction to proceed efficiently.
3.4. NO3− detection
Our proposed sensor system is also capable of detecting NO3− by using Zn dust as a reducing agent to convert NO3− to NO2−, which can then be detected by the proposed chemosensor (Scheme 3). To facilitate this reduction, HCl was used as the acid source, as metal reduction requires a strong acid. Eventually, similar to NO2−, sensor (CE) demonstrated the generation of a peak at 530 nm in the presence of NO3− (Fig. 5a). In contrast, the sensor (CF) exhibited a peak around 522 nm when exposed to NO3− (Fig. 5b).The UV-vis spectra of the sensors at various NO3− concentrations were recorded under optimized conditions, and the LOD was calculated from the plot. For sensor (CE), the absorption at 530 nm increased gradually with NO3− concentration up to 0.412 mM (Fig. 5a), but a further increase in concentration (0.453 mM) led to a decrease in absorbance. A linear relationship was observed between absorption and NO3− concentration in the range of 0.005–0.326 mM (Fig. 5b). The regression equation is A = 1.14244 (mM) − 0.04628, with a correlation coefficient of 0.98. The detection limit for NO3− was found to be 2.89 mM. Although it did not exhibit a linear calibration with NO3− concentration, the sensor (CF) responded to NO3− under similar situations (Fig. 5c and d).
The responsiveness of both sensors to a variety of ambient anions was assessed using colorimetric assays. As depicted in Fig. 6, none of these anions caused a significant change in the sensor (CE) (Fig. 6a and b) and sensor (CF) (Fig. 6c and d). Notably, only the sensor exposed to NO3− exhibited a color change to pink, while the other anions did not induce any observable color shift (Fig. S20 and S21, ESI†). The interference study assessed the sensors’ performance in the presence of other anions by mixing NO3− (1 equiv.) with various anions (1 equiv.) in aqueous solution. The results, shown in Fig. 7, indicated that most anions interfered with NO3− detection. Sensor (CF) (Fig. 7b) was more affected by the presence of these anions than sensor (CE) (Fig. 7a).
 | ||
| Fig. 7 Interference study of sensor (a) (CE) and (b) (CF) (with the presence of Zn dust as reducing agent) in the presence of other anions. | ||
3.5. Sensing mechanism
To investigate the sensing mechanism of the proposed chemosensors, their UV-vis absorption spectra were recorded. As shown in Fig. 8a, sensor (CE) exhibited a strong absorption peak at 530 nm exclusively upon the addition of NO2−, which confirms the formation of an azo compound. A similar spectral behavior was observed for sensor (CF), as represented in Fig. 8b.The basic detection mechanism is based on the well-known Griess reaction.40 In this system, compound (C), which possesses an aromatic amine (–NH2) and a sulfonohydrazide group, reacts with NO2− in the presence of itaconic acid to generate a diazonium salt. This intermediate then undergoes a coupling reaction with either compound (E) or (F) to form a pink-colored azo dye, as shown in Scheme 4.
The structural and electronic properties of compounds (E) and (F) significantly influence their reactivity and thus the sensor performance. Compound (E) contains a bulky naphthyl group and a long ethoxyethanol chain, which introduces substantial steric hindrance around the amino group. This reduces the accessibility of the amine site, leading to diminished diazotization efficiency and a weaker response to NO2−. Conversely, compound (F), with a simpler structure, facilitates a more efficient coupling reaction with the diazonium salt, forming the (CF) azo dye with a more intense colorimetric response. This observation is supported by the lower limit of detection (LOD) and higher binding constant obtained for sensor (CF). DFT calculations further clarify this behavior: compound (F) exhibits a smaller HOMO–LUMO gap (3.05 eV) than (E) (3.07 eV) (Fig. 9), indicating enhanced reactivity and better electron transfer efficiency in F due to its primary amine and naphthyl ring. In contrast, the ethoxyethanol chain in (E) is less electron-donating than (F)'s ethylenediamine moiety (Fig. S22, ESI†), resulting in reduced electron density on the amino group and a larger HOMO–LUMO gap, thereby decreasing its reactivity.
Moreover, ESP (electrostatic potential) mapping (Fig. S22, ESI†) reveals a more concentrated negative potential around the para-nitrogen of compound (F), which enhances its interaction with the diazonium intermediate and stabilizes the transition state during the coupling reaction. On the other hand, the ESP map of (E) shows a more diffuse electron distribution, and the steric bulk of the ethoxyethanol chain further hinders effective coupling. The presence of the ethoxy group also likely introduces electron polarization, withdrawing electron density from the nucleophilic site, and thereby reducing the compound's reactivity. The optimized structure and calculated electrostatic surfaces of the synthesized azo–dyes are depicted in Fig. S23 (ESI†).
The formation of the azo dyes (CE) and (CF) was further confirmed by FT-IR and LC-MS analyses. FT-IR spectra (Fig. S24, ESI†) revealed the disappearance of amidogen vibrations at 3200 and 3343 cm−1 in compound (C)40 after reaction with (E) or (F), accompanied by the appearance of a new weak peak at ∼1460 cm−1 corresponding to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching, confirming azo dye formation.51 The LC-MS spectra (Fig. S25, ESI†) showed peaks at m/z 429.35 (Fig. S25a, ESI†) and 385.35 (Fig. S25b, ESI†), affirming the molecular weights of the (CE) and (CF) azo dyes, respectively.
N stretching, confirming azo dye formation.51 The LC-MS spectra (Fig. S25, ESI†) showed peaks at m/z 429.35 (Fig. S25a, ESI†) and 385.35 (Fig. S25b, ESI†), affirming the molecular weights of the (CE) and (CF) azo dyes, respectively.
For NO3− detection, the sensing mechanism involves its reduction to NO2− using Zn dust, as shown in Scheme 3. Zn acts as the reducing agent and gets oxidized to Zn2+. Again, compound (F) forms a complex with the resultant Zn2+.52 Interestingly, DFT studies demonstrated that both (E) and (F) can coordinate with Zn2+, forming E@Zn and F@Zn complexes that enhance NO3− reduction efficiency (Fig. S26, ESI†). Notably, the E@Zn complexes exhibited lower HOMO–LUMO gaps (4.31 and 4.35 eV) than F@Zn (4.46 eV), indicating higher complex stability. This can be attributed to the bidentate coordination sites provided by (E)—either N, O, or O, O pairs—which interact more strongly with Zn2+ compared to the N,N bidentate site of (F). Literature reports also confirm that Zn–O bonds are more stable than Zn–N bonds.53 Thus, the superior stability of E@Zn complexes increases the availability of NO2− in the medium, enhancing the NO3− sensing capability of (CE). Therefore, between the two azo dye-based sensors, (CF) demonstrated superior sensitivity and selectivity for NO2− detection due to better coupling efficiency and electron transfer, whereas (CE) exhibited enhanced performance in NO3− sensing, primarily due to its stronger and more stable complexation with Zn2+, which facilitated more effective NO3− reduction to NO2−.
3.6. Real-time applications
3.7. Real sample analysis
To check the practicability of the sensor towards NO2−, different types of vegetables such as cabbage, radish, spinach, lettuce, potato, carrot, and capsicum bought from local shops were tested based on colorimetric readout, and the results are listed in Table S1 (ESI†). Lake water and tap water were also taken for analysis. The samples were spiked with NO2− to make them 5 mM concentration and four different volumes (50 μL, 100 μL, 150 μL, and 200 μL) of solution were added into the two different combinations of the sensor system, and their respective absorbance response was noted. The testing results are listed according to the calibration obtained from the respective titration. Here, both the chemosensors demonstrated good recoveries ranging from 93.73% to 106.86%. Hence, both sensors could be used for NO2− detection.Table S2 (ESI†) presents the results of a quantitative estimation of NO3− using our chemosensors in the same set of samples. The samples were spiked with NO3− to make them 5 mM in concentration. A total of three different concentrations of stock solution were added and the UV-vis response was noted for the sensor (CE). The chemosensor (CE) demonstrated a moderate recovery ranging from 73% to 123.2%. Hence, our proposed chemosensor could be used for NO3− sensing. For the present investigation, we used only sensor (CE), as in the interference study, the other system (CF) did not provide satisfactory results.
Overall, our proposed chemosensors demonstrate high sensitivity and selectivity for both nitrite and nitrate detection. A comparative analysis with recently reported sensing systems, summarized in Table S3 (ESI†), highlights the importance of our approach. Although the LOD is moderate, it highlights that the sensing system operates effectively in the presence of an organic acid medium, which distinguishes our system from existing methods. Importantly, the chemosensors enable the detection of not only nitrite but also nitrate through an in situ reduction process using zinc dust. To further demonstrate practical applicability, we developed a smartphone-assisted detection platform and a portable test kit based on these sensors. The system was successfully validated using real-world samples, including various agricultural products and lake water, confirming its potential for field-level applications.
4. Conclusions
In our present study, we successfully developed a pair of colorimetric chemosensors for sensitive and selective detection of NO2− and NO3−, utilizing the well-established Griess assay. NO3− was sensed by converting it to NO2− in the presence of Zn dust. Both chemosensors formed a different azo–dye upon reaction with NO2−, resulting in a clear colorimetric response. Itaconic acid was employed as the acidic medium to facilitate the reaction, ensuring optimal conditions for accurate detection. For NO3− detection, NO3− was efficiently reduced to NO2− using Zn dust in the presence of hydrochloric acid, enabling its indirect quantification via the same azo–dye reaction. The proposed chemosensors exhibited excellent linearity in response to NO2− and NO3− concentrations, with low detection limits highlighting their sensitivity. To explore the practical application of chemosensors, test strips were fabricated using the synthesized compounds used for designing chemosensors. These strips demonstrated distinct color intensities corresponding to varying concentrations of NO2−, and the results were further validated using smartphone-assisted colorimetric analysis for real-time and portable monitoring. The reliability of the developed system was confirmed by testing real samples, including various vegetables and lake water, where the sensor displayed excellent recovery rates. This proves the potential of our sensor for environmental and food safety applications, particularly in detecting NO2− and NO3− in agricultural products.In conclusion, the simplicity, sensitivity, and versatility of the proposed colorimetric sensor, combined with its adaptability to test strip formats and smartphone-assisted analysis, make it a promising tool for on-site detection of NO2− and NO3−. This work not only advances the utility of Griess assay-based detection systems but also opens new paths for the further development of low-cost, portable, and user-friendly devices for monitoring environmental and food contaminants. The use of monomeric itaconic acid opens the pathway to creating a polymer-based sensor system where a more sensitive system can be designed without the addition of external acid.
Author contributions
Anubhab Das: writing – original draft, methodology, investigation, software. Sindhu I Sanakal: formal analysis, investigation, software. Gomathi Sivakumar: formal analysis, investigation, software. Anashwara Babu: formal analysis, investigation, writing – review & editing. Samarendra Maji: supervision, conceptualization, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data used to support the findings of this study are included within the article.Acknowledgements
A. D. and S. S. thank SRM IST for providing a fellowship to support the PhD program. S. M. acknowledges SRMIST for providing a seed grant and the Science and Engineering Research Board (SERB), India for a core research grant (CRG/2021/004203). The authors would like to thank Dr Venkatramaiah Nutalapati for providing UV-vis spectroscopy.References
- L. B. Maia and J. J. G. Moura, How Biology Handles Nitrite, Chem. Rev., 2014, 114, 5273–5357 CrossRef CAS PubMed.
- K. Xiao, R. Tang, J. Wei, J. Wu, Y. Shao, Z. Ma, L. Wang, Z. Hu and Z. Zhou, Achieving stable partial nitrification through synergistic inhibition of free ammonia and salinity on nitrite-oxidizing bacteria, Front. Environ. Sci. Eng., 2025, 19, 42 CrossRef CAS.
- A. Grzyb, A. Wolna-Maruwka and A. Niewiadomska, The Significance of Microbial Transformation of Nitrogen Compounds in the Light of Integrated Crop Management, Agronomy, 2021, 11, 1415 CrossRef CAS.
- M. Giordano, S. Petropoulos and Y. Rouphael, The Fate of Nitrogen from Soil to Plants: Influence of Agricultural Practices in Modern Agriculture, Agriculture, 2021, 11, 944 CrossRef CAS.
- M. H. Shakil, A. T. Trisha, M. Rahman, S. Talukdar, R. Kobun, N. Huda and W. Zzaman, Nitrites in Cured Meats, Health Risk Issues, Alternatives to Nitrites: A Review, Foods, 2022, 11, 3355 CrossRef CAS PubMed.
- M. Karwowska and A. Kononiuk, Nitrates/Nitrites in Food—Risk for Nitrosative Stress and Benefits, Antioxidants, 2020, 9, 241 Search PubMed.
- S. Soni, J. W. Anvil, C. Kurian, P. Chakraborty and K. A. Paari, Food additives and contaminants in infant foods: a critical review of their health risk, trends and recent developments, Food Prod., Process. Nutr., 2024, 6, 63 Search PubMed.
- R. M. Keller, L. Beaver, M. C. Prater and N. G. Hord, Dietary Nitrate and Nitrite Concentrations in Food Patterns and Dietary Supplements, Nutr. Today, 2020, 55, 218–226 Search PubMed.
- N. S. Bryan, S. Ahmed, D. J. Lefer, N. Hord and E. R. von Schwarz, Dietary nitrate biochemistry and physiology. An update on clinical benefits and mechanisms of action, Nitric oxide, 2023, 132, 1–7 CrossRef CAS PubMed.
- M. Norouzzadeh, M. Hasan Rashedi, N. Payandeh, A. Mirdar Harijani and H. Shahinfar, The effects of dietary nitrate on blood pressure and vascular Health: An umbrella review and updated Meta-Analysis and meta-regression, J. Funct. Foods, 2024, 114, 106082 CrossRef CAS.
- M. Volino-Souza, G. V. de Oliveira, V. dos, S. Pinheiro, C. A. Conte-Junior and T. da S. Alvares, The effect of dietary nitrate on macro- and microvascular function: A systematic review, Crit. Rev. Food Sci. Nutr., 2024, 64, 1225–1236 CrossRef PubMed.
- H. N. Rabetafika, A. Razafindralambo, B. Ebenso and H. L. Razafindralambo, Probiotics as Antibiotic Alternatives for Human and Animal Applications, Encyclopedia, 2023, 3, 561–581 CrossRef.
- X. F. Hospital, E. Hierro, J. Arnau, J. Carballo, J. S. Aguirre, M. Gratacós-Cubarsí and M. Fernández, Effect of nitrate and nitrite on Listeria and selected spoilage bacteria inoculated in dry-cured ham, Food Res. Int., 2017, 101, 82–87 CrossRef CAS PubMed.
- C. Loffi, M. Cirlini, N. Cavalca, G. Saccani, R. Virgili, G. Galaverna and T. Tedeschi, Changes in proteolysis and volatile fraction of nitrite-free Italian-type salami modified in formulation and processing, Int. J. Food Sci. Technol., 2024, 59, 5587–5597 CrossRef CAS.
- S. A. Hussain, S. R. Ahmad and H. Fayaz, in Hand Book of Processed Functional Meat Products, Springer Nature Switzerland, Cham, 2024, pp. 365–404 Search PubMed.
- E. F. Bowles, M. Burleigh, A. Mira, S. G. J. Van Breda, E. Weitzberg and B. T. Rosier, Nitrate: “the source makes the poison”, Crit. Rev. Food Sci. Nutr., 2024, 1–27 CrossRef CAS PubMed.
- I. J. Chaudhary, R. Chauhan, S. S. Kale, S. Gosavi, D. Rathore, V. Dwivedi, S. Singh and V. K. Yadav, Groundwater Nitrate Contamination and its Effect on Human Health: A Review, Water Conserv. Sci. Eng., 2025, 10, 33 CrossRef.
- A. C. da, C. Pinaffi-Langley, H. V. M. Nguyen, D. Whitehead, J. M. Roseland, K. C. Heydorn, X. Wu, P. R. Pehrsson, F. A. Hays and N. G. Hord, Nitrate and nitrite quantification in U.S. vegetable-based baby foods and infant formula via ozone chemiluminescence, J. Food Compos. Anal., 2025, 137, 106902 CrossRef.
- A. Viancelli and W. Michelon, Climate Change and Nitrogen Dynamics: Challenges and Strategies for a Sustainable Future, Nitrogen, 2024, 5, 688–701 CrossRef CAS.
- C.-Y. Hou, L.-M. Fu, W.-J. Ju and P.-Y. Wu, Microfluidic colorimetric system for nitrite detection in foods, Chem. Eng. J., 2020, 398, 125573 CrossRef CAS.
- S. S. Paramasivam, S. A. Mariappan, N. K. Sethy and P. Manickam, Enzyme mimetic electrochemical sensor for salivary nitrite detection using copper chlorophyllin and carbon nanotubes-functionalized screen printed electrodes, Mater. Adv., 2023, 4, 6223–6232 RSC.
- P. Lei, N. Wu, Y. Zhou, C. Dong, M. Li and S. Shuang, Simple strategy for dual-responsive ratio electrochemical-colorimetric detection of nitrite in food and environment, Microchim. Acta, 2024, 191, 701 CrossRef CAS PubMed.
- P. J. Sephra., C. Tharini, A. Sachdev and E. Manikandan, An efficient sensing system using ion-selective membrane on Ni2O3/rGO nanocomposite for electrochemical detection of nitrate ions, J. Alloys Compd., 2024, 980, 173414 CrossRef.
- H. Li, Y. Song, B. Zhou and H. Xu, Nitrite: From Application to Detection and Development, Appl. Sci., 2024, 14, 9027 CrossRef CAS.
- M. Darestani-Farahani, F. Ma, V. Patel, P. R. Selvaganapathy and P. Kruse, An ion-selective chemiresistive platform as demonstrated for the detection of nitrogen species in water, Analyst, 2023, 148, 5731–5744 RSC.
- X. Xia, G. Yang, H. Tian, F. Cao, F. Luo and D. Dong, Development of a rapid sensor system for nitrate detection in water using enhanced Raman spectroscopy, RSC Adv., 2025, 15, 5728–5736 RSC.
- J. Lei, H. Zheng, L. Liu and W. Li, Simultaneous determination of six nitroaromatic compounds and three anions in environmental matrices using a liquid chromatography-ion chromatography coupled system, Chin. J. Chromatogr., 2024, 42, 92–98 CrossRef CAS PubMed.
- Y. Mai, A. Ghiasvand, V. Gupta, S. Edwards, S. Cahoon, K. Debruille, I. Mikhail, E. Murray and B. Paull, Application of a portable ion chromatograph for real-time field analysis of nitrite and nitrate in soils and soil pore waters, Talanta, 2024, 274, 126031 CrossRef CAS PubMed.
- N. Luckovitch and E. Pagliano, A reference isotope dilution headspace GC/MS method for the determination of nitrite and nitrate in meat samples, Int. J. Food Sci. Technol., 2020, 55, 1110–1118 CrossRef CAS.
- H. Kodamatani, S. Kubo, A. Takeuchi, R. Kanzaki and T. Tomiyasu, Sensitive Detection of Nitrite and Nitrate in Seawater by 222 nm UV-Irradiated Photochemical Conversion to Peroxynitrite and Ion Chromatography-Luminol Chemiluminescence System, Environ. Sci. Technol., 2023, 57, 5924–5933 CrossRef CAS PubMed.
- M. Zeng, C. Zhang, Q. Yao, J. Jin, T.-X. Ye, X. Chen, Z. Guo and X. Chen, Multifunction nanoenzyme-assisted ion-selective and oxidation catalysis SERS biosensors for point-of-care nitrite testing, Sens. Actuators, B, 2024, 405, 135352 CrossRef CAS.
- Z. Yang, J. Zhang, J. Zhao, W. Zhou, Y. Cheng, Z. Xu, P. Wei, Z. Wang, H. Liang and C. Li, A high-sensitivity lab-on-a-chip analyzer for online monitoring of nitrite and nitrate in seawater based on liquid waveguide capillary cells, Lab Chip, 2024, 24, 3528–3535 RSC.
- K. M. Miranda, M. G. Espey and D. A. Wink, A Rapid, Simple Spectrophotometric Method for Simultaneous Detection of Nitrate and Nitrite, Nitric oxide, 2001, 5, 62–71 CrossRef CAS PubMed.
- D. Giustarini, I. Dalle-Donne, D. Tsikas and R. Rossi, Oxidative stress and human diseases: Origin, link, measurement, mechanisms, and biomarkers, Crit. Rev. Clin. Lab. Sci., 2009, 46, 241–281 CrossRef CAS PubMed.
- V. M. Ivanov, The 125th Anniversary of the Griess Reagent, J. Anal. Chem., 2004, 59, 1002–1005 CrossRef CAS.
- E. García-Robledo, A. Corzo and S. Papaspyrou, A fast and direct spectrophotometric method for the sequential determination of nitrate and nitrite at low concentrations in small volumes, Mar. Chem., 2014, 162, 30–36 CrossRef.
- A. Choodum, J. Tiengtum, T. Taweekarn and W. Wongniramaikul, Convenient environmentally friendly on-site quantitative analysis of nitrite and nitrate in seawater based on polymeric test kits and smartphone application, Spectrochim. Acta, Part A, 2020, 243, 118812 CrossRef CAS PubMed.
- Y.-T. Tai, C.-Y. Cheng, Y.-S. Chen and F.-H. Ko, A hydrogel-based chemosensor applied in conjunction with a Griess assay for real-time colorimetric detection of nitrite in the environment, Sens. Actuators, B, 2022, 369, 132298 CrossRef CAS.
- L. Capitán-Vallvey, R. Avidad, M. Fernández-Ramos, A. Ariza-Avidad and E. Arroyo, Test strip for determination of nitrite in water, Anal. Bioanal. Chem., 2002, 373, 289–294 CrossRef PubMed.
- H. Wu, X. Shen, D. Huo, Y. Ma, M. Bian, C. Shen and C. Hou, Fluorescent and colorimetric dual-readout sensor based on Griess assay for nitrite detection, Spectrochim. Acta, Part A, 2020, 225, 117470 CrossRef CAS PubMed.
- M. K. Morsy, O. M. Morsy, E. M. Abd-Elaaty and R. Elsabagh, Development and Validation of Rapid Colorimetric Detection of Nitrite Concentration in Meat Products on a Polydimethylsiloxane (PDMS) Microfluidic Device, Food Anal. Methods, 2022, 15, 552–564 CrossRef.
- T. J. Chow and M. S. Johnstone, Determination of nitrate in sea water, Anal. Chim. Acta, 1962, 27, 441–446 CrossRef CAS.
- T. Jiwarungrueangkul, O. Kongpuen, M. Yucharoen, C. Sangmanee, D. Tipmanee, T. Areerob and P. Sompongchiyakul, Modification and validation of an analytical method for the simple determination of nitrate in seawater by reduction to nitrite with zinc powder, Mar. Chem., 2023, 251, 104235 CrossRef CAS.
- E. Murray, E. P. Nesterenko, M. McCaul, A. Morrin, D. Diamond and B. Moore, A colorimetric method for use within portable test kits for nitrate determination in various water matrices, Anal. Methods, 2017, 9, 680–687 RSC.
- S.-C. Pai, Y.-T. Su, M.-C. Lu, Y. Chou and T.-Y. Ho, Determination of Nitrate in Natural Waters by Vanadium Reduction and the Griess Assay: Reassessment and Optimization, ACS ES&T Water, 2021, 1, 1524–1532 Search PubMed.
- K. R. Kunduru, A. Basu, T. Tsah and A. J. Domb, Polymer with pendant diazo-coupling functionality for colorimetric detection of nitrates, Sens. Actuators, B, 2017, 251, 21–26 CrossRef CAS.
- N. Devi, S. Singh, S. Manickam, N. Cruz-Martins, V. Kumar, R. Verma and D. Kumar, Itaconic Acid and Its Applications for Textile, Pharma and Agro-Industrial Purposes, Sustainability, 2022, 14, 13777 CrossRef CAS.
- S. V. Bukharov, A. R. Burilov, R. G. Tagasheva, G. N. Nugumanova, E. V. Nikitina and N. A. Mukmeneva, Synthesis and Antibacterial Activity of Sulfanilamides Containing Sterically Hindered Phenol Fragments, Russ. J. Org. Chem., 2021, 57, 1621–1627 CrossRef CAS.
- H. Guo, Y. Zhuang, J. Cao and G. Zhang, Green and Efficient Protocol for the Synthesis of N -(2-Hydroxyethyl)anilines by the Alkylation Reaction in Ionic Liquid, Synth. Commun., 2014, 44, 3368–3374 CrossRef CAS.
- S. Luetic, Z. Knezovic, K. Jurcic, Z. Majic, K. Tripkovic and D. Sutlovic, Leafy Vegetable Nitrite and Nitrate Content: Potential Health Effects, Foods, 2023, 12, 1655 CrossRef CAS PubMed.
- F. Zimmermann, T. Lippert, C. Beyer, J. Stebani, O. Nuyken and A. Wokaun, N
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N Vibrational Frequencies and Fragmentation Patterns of Substituted 1-Aryl-3,3-Dialkyl-Triazenes: Comparison with other High-Nitrogen Compounds, Appl. Spectrosc., 1993, 47, 986–993 CrossRef CAS.
N Vibrational Frequencies and Fragmentation Patterns of Substituted 1-Aryl-3,3-Dialkyl-Triazenes: Comparison with other High-Nitrogen Compounds, Appl. Spectrosc., 1993, 47, 986–993 CrossRef CAS. - M. S. Refat, A. M. A. Adam, T. Sharshar, H. A. Saad and H. H. Eldaroti, Utility of positron annihilation lifetime technique for the assessment of spectroscopic data of some charge-transfer complexes derived from N-(1-Naphthyl)ethylenediamine dihydrochloride, Spectrochim. Acta, Part A, 2014, 122, 34–47 CrossRef CAS PubMed.
- Z.-Q. Liu, Y. M. Ng, P. J. Tiong, R. A. Abu Talip, N. Jasin, V. Y. M. Jong and M. G. Tay, Five-Coordinate Zinc(II) Complex: Synthesis, Characterization, Molecular Structure, and Antibacterial Activities of Bis-[(E)-2-hydroxy-N′-{1-(4-methoxyphenyl)ethylidene}benzohydrazido]dimethylsulfoxidezinc(II) Complex, Int. J. Inorg. Chem., 2017, 2017, 1–8 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Structural characterization of B, C and E; visual color change, UV-vis, temperature stability study, DFT, test-strip, real-time application, and comparison study. See DOI: https://doi.org/10.1039/d5ma00314h |
| This journal is © The Royal Society of Chemistry 2025 |